Abstract
Traumatic experiences during childhood can have profound effects on stress sensitive brain structures (e.g., amygdala and hippocampus) and the emergence of psychiatric symptoms. Recent theoretical and empirical work has delineated dimensions of trauma (i.e., threat and deprivation) as having distinct neural and behavioral effects, although there are few longitudinal examinations. A sample of 243 children and adolescents were followed for three time points, with each assessment approximately 1 year apart (ages 9–15 years at Time 1; 120 males). Participants or their caregiver reported on youths' threat exposure, perceived stress (Time 1), underwent a T1‐weighted structural high‐resolution MRI scan (Time 2), and documented their subsequent psychiatric symptoms later in development (Time 3). The primary findings indicate that left amygdala volume, in particular, mediated the longitudinal association between threat exposure and subsequent internalizing and externalizing symptomatology. Greater threat exposure related to reduced left amygdala volume, which in turn differentially predicted internalizing and externalizing symptoms. Decreased bilateral hippocampal volume was related to subsequently elevated internalizing symptoms. These findings suggest that the left amygdala is highly threat‐sensitive and that stress‐related alterations may partially explain elevated psychopathology in stress‐exposed adolescents. Uncovering potential subclinical and/or preclinical predictive biomarkers is essential to understanding the emergence, progression, and eventual targeted treatment of psychopathology following trauma exposure.
Keywords: adolescence, amygdala, brain development, brain structure, trauma
The left amygdala mediated the longitudinal association between threat exposure and subsequent internalizing and externalizing symptomatology in youths. Greater threat exposure related to reduced left amygdala volume, which in turn differentially predicted internalizing and externalizing symptoms. Similarly, decreased bilateral hippocampal volume was related to subsequently elevated internalizing symptoms.
1. INTRODUCTION
The developing brain is exquisitely sensitive to traumatic experiences, with major structural alterations observed and documented for decades (Teicher & Samson, 2016). However, research mapping the sequalae of childhood trauma to specific structural alterations has suffered from several methodological limitations including cross‐sectional designs, adults retrospectively reporting on childhood trauma, and smaller sample sizes. In addition, historically, the literature has focused more on general exposure to trauma or childhood maltreatment (i.e., abuse and neglect). More recently, the field has adopted approaches that seek to identify the specificity of subtypes of trauma (e.g., institutional rearing, subtypes of child abuse and neglect) in altering youth brain development. In so doing, research has revealed differences in brain structure that may be attributable to the timing, severity, and type of childhood trauma experienced (Cassiers et al., 2018; Cohodes et al., 2020; Edmiston, 2011; Johnson et al., 2016; Teicher et al., 2016).
A recent theoretical model has proposed that characterizing childhood trauma by dimensions of deprivation or threat may offer a more mechanistic and hypothesis‐driven approach to understanding distinct impacts of childhood trauma on the developing brain and patterns of psychopathology (dimensional model of adversity and psychopathology (DMAP); McLaughlin et al., 2014). Specifically, threat experiences are those characterized by the threat of physical harm and/or actual harm (e.g., physical abuse, exposure to domestic, and/or neighborhood violence), which are expected to alter social‐affective processing and the underlying neural structures. In contrast, deprivation experiences are defined by a dearth of cognitive and/or social inputs from the caregiving environment (e.g., physical neglect, poverty), which are expected to alter cognitive processing and the supporting neural structures. Growing empirical support for the DMAP model suggests that exposure to threat versus deprivation differentially impacts neurobiological pathways toward psychopathology. For example, evidence suggests that youths who have been exposed to threat (e.g., physical abuse, sexual abuse) evince smaller hippocampal and amygdala volumes, which may confer vulnerability to subsequent depression (Weissman et al., 2020). There is also evidence that young children with greater deprivation exposure exhibit poorer cognitive control skills, while children with greater threat exposure exhibit poorer discrimination between threat and safety cues in a fear learning paradigm (Machlin et al., 2019).
Another important facet of trauma that is poorly understood, particularly in developmental populations, is the extent to which perceptions of stress modulate the impact of trauma on brain structure and subsequent psychopathology. Recent empirical and theoretical work has highlighted this, suggesting that one of the flaws of adversity research is that researchers tend to determine which events are stressful to youth (Danese & Widom, 2020; Smith & Pollak, 2021). Thus, investigating how youth perceive stressful events is paramount to understanding whether and how perceptions of stress are consequential to an individuals' neurobiology, perhaps above and beyond features of the event itself. Indeed, psychological perceptions of stress are known to be related to both physiological responses to stress and brain structure, particularly in neural regions affected by stress (Hashimoto et al., 2015). In the present study, the goal was two‐fold: a) evaluate whether associations between threatening experiences and subsequent psychopathology are mediated by brain structure in a longitudinal design and b) assess whether perceptions of stress moderate associations between experiences of threat and neural alterations. To this end, we focused on two regions that are highly susceptible to stress hormones (McGaugh & Roozendaal, 2002; Phelps et al., 2004) and appear to be uniquely sensitive to threatening experiences, the amygdala and hippocampus (e.g., Foell et al., 2019; Lambert et al., 2017).
Studies on the effects of trauma on amygdala and hippocampal volumes have been largely inconsistent, with reports of smaller, larger, or no volumetric differences (for reviews, see Hanson & Nacewicz, 2021; Teicher et al., 2016; Teicher & Khan, 2019). There are also mixed reports regarding specific hemispheric effects of stress on the amygdalae and hippocampi (Dannlowski et al., 2012; Teicher et al., 2012; Teicher & Khan, 2019). Focusing on pediatric studies, exposure to deprivation (i.e., poverty and/or neglect) has been linked with smaller (Ellwood‐Lowe et al., 2018; Herzog et al., 2020; Luby et al., 2013), larger (Noble et al., 2012; Roth et al., 2018; Tottenham et al., 2010), and no differences in amygdala or hippocampal volumes (Gold et al., 2016; King et al., 2019). Similarly, exposure to threatening experiences (e.g., abuse, community violence) has been linked with smaller amygdala and hippocampal volumes (Hanson et al., 2015; Lee et al., 2018; Weissman et al., 2020), as well as no differences in amygdala or hippocampal volumes (Butler et al., 2018; Saxbe et al., 2018). In the case of the amygdala, mixed findings may be due to samples capturing snapshots of the inverted‐U growth pattern of the amygdala at different time points (Hanson & Nacewicz, 2021); thus younger, preadolescent samples may show increases in amygdala volume related to trauma, followed by decreases in volume during adolescence. In the hippocampus, there has been some suggestion that greater levels of stress earlier in life may induce hippocampal atrophy but not necessarily later in childhood (Humphreys et al., 2019).
Taken together, the existing literature is inconclusive and has not yet evaluated whether perceptions of stress play a key role in the extent to which traumatic experiences impact highly stress‐sensitive brain structures, and portend longer‐term mental health outcomes (i.e., internalizing, externalizing, and dysregulation). Moreover, there has been a lack of longitudinal studies investigating these effects, making it difficult to infer mechanisms, and examine targeted effects of specific types of trauma (i.e., threatening experiences, DMAP) in larger cohorts. Therefore, the current study sought to fill these gaps by evaluating how exposure to threatening experiences during late childhood/early adolescence (Time 1) and subsequent psychopathology symptoms later in adolescence (Time 3) are mediated by interim amygdala and hippocampal volumes (Time 2). Moreover, we examined whether perceived stress (Time 1) moderates associations between threat exposure (Time 1) and amygdala/hippocampal volumes (Time 2).
2. MATERIALS AND METHODS
2.1. Participants
A sample of 243 typically developing children and adolescents ages 9–15 were recruited to participate in the Developmental Chronnecto‐Genomics study (meanage = 11.73 years, SD = 1.78; 120 males) (Stephen et al., 2021). The study was multisite, with 135 participants recruited at the University of Nebraska Medical Center (UNMC) and 108 participants from the Mind Research Network (MRN) for the initial study assessment. Participants were invited back to participate annually for 3 years. Of the full sample, 177 returned for time/year 2 and 141 returned for time/year 3 of data collection. Inclusion criteria included English as a primary language, ages 9–15, and participant and parent willingness to assent/consent, respectively. Exclusion criteria determined via parent report were as follows: inability to assent/consent, history of developmental delays and/or psychiatric disorders, history of neurological disorders (e.g., epilepsy), history of concussion or head injury, pregnancy, prenatal exposure to drugs, use of medications known to affect brain function, and magnetic resonance imaging (MRI) contraindications (e.g., orthodontia, metallic foreign bodies). All parents and youth provided written consent or assent, respectively, prior to participating in the study. The appropriate institutional review boards for both study sites approved all study procedures.
2.2. Structural neuroimaging acquisition and processing
Participants underwent a structural T1‐weighted MRI scan during each visit (i.e., three scans total). Children recruited at UNMC were scanned using a Siemens 3 T Skyra scanner (N = 112) or a Siemens 3 T Prisma Fit scanner (N = 19), and those at MRN were scanned using a Siemens 3 T TIM Trio (N = 108). Structural T1‐weighted MR images at both data collection sites were acquired with a 32‐channel head coil and an MPRAGE sequence with the following parameters: TR = 2400 ms; TE = 1.94 ms; flip angle = 8°; field of view = 256 mm; slice thickness = 1 mm (no gap); base resolution = 256; 192 slices; voxel size = 1 × 1 × 1 mm. The T1‐weighted structural brain images of all participants were processed using FreeSurfer version 5.3 (http://surfer.nmr.mgh.harvard.edu). Cortical thickness and subcortical volume estimates were computed for the 70 Desikan‐Killiany atlas regions. We followed the ENIGMA protocol for quality assurance, including performing visual checks on all segmentations (http://enigma.usc.edu/protocols/imaging-protocols) and checking for motion artifacts. No segmentations were flagged at this stage of processing. Histograms of all regional values were computed for visual inspection; all data were clean and met criteria for inclusion in further analyses. Here, we focused on bilateral amygdala and hippocampus subcortical volumes from participants' Time 2 MRI. Left and right volumes were modeled separately for each region, given hypotheses and discrepancies in the existing literature regarding stress and laterality (Teicher & Khan, 2019).
2.3. Trauma history profile
During participants' first visit, they completed the self‐report Trauma History Profile (THP), which was derived from the UCLA PTSD Reaction Index for DSM IV (Steinberg et al., 2004) and assessed a variety of trauma types and events. Participants endorsed whether or not they experienced different types of trauma in their lifetime (No = 0, Yes = 1). The individual items from this scale were submitted to an exploratory factor analysis (EFA) to determine the extent that items coalesced onto a latent factor representing a particular type of trauma. Results from the EFA are reported below.
2.4. Perceived stress
To assess participants' subjective experience of stress in the past month (at Time 1), we used the Perceived Stress survey from the NIH Toolbox (Salsman et al., 2013). Participants 12 and older provided self‐report (n = 34) and the remainder had their parents complete the perceived stress survey on their behalf (n = 146). Example items included: “How often have you felt nervous and ‘stressed’?” and “how often have you felt confident about your ability to handle your personal problems?” Participants responded using a Likert scale where Never = 1 and Very Often = 5.
2.5. Child Behavior Checklist
During participants' third visit, a caregiver completed the Child Behavior Checklist (CBCL; Achenbach et al., 2001) to assess their child's internalizing and externalizing behaviors over the past 6 months. In addition to examining the internalizing and externalizing profiles, we also computed the dysregulation profile, which is a summed score of the attention, aggression, and anxious/depressed subscales. Raw scores for all scales were used in our models.
2.6. Data analytic plan
We began by running descriptive statistics on demographics and all variables of interest in SPSS version 25. Variables entered into subsequent models were examined for violations of normality (i.e., skewness and kurtosis) and were transformed according to their distribution type (e.g., positive vs. negative skew). We also tested whether there were any systematic differences in those who did or did not complete all three waves of data collection. Next, we fit several structural equation models (SEM) to estimate whether left and right amygdala and hippocampus at Time 2, respectively, mediated the association between reported threatening experiences at Time 1 and internalizing, externalizing, and dysregulation symptomology at Time 3. In tandem, we tested whether perceived stress at Time 1 moderated associations between experienced threat and amygdala/hippocampal volume at Time 2.
Before fitting the final models, we iteratively tested which covariates to include (e.g., sex, age, site, and total intracranial volume [TIV]). To compare across models, we inspected whether absolute fit indices such as Akaike's information criterion (AIC) and Bayesian information criterion (BIC) were decreasing in value to indicate model improvement, with a difference of >10 within each metric indicating superior model fit. In the current analysis, we primarily used BIC to determine the model fit. All models were tested in Mplus Version 7.4 (Muthén & Muthén, 2015). After determining which covariates yielded the best model fit, we tested whether perceived stress moderated the relationship between threat exposure and bilateral amygdala and hippocampal volumes separately. All mediation models were bootstrapped with 1000 iterations bias‐corrected bootstrapping for the expected nonparametric distributions of the interaction between a × b (i.e., threat × perceived stress) (MacKinnon et al., 2004). Finally, we examined the mediation effect of bilateral amygdala and hippocampal volumes at Time 2 on the association between threat exposure at Time 1 and symptomology at Time 3 (i.e., indirect effects of threat on symptomology via amygdala or hippocampal volume). All symptomology scales (i.e., internalizing, externalizing, and dysregulation profiles derived from the CBCL), as well as perceived stress, trauma exposure, and their interactions were permitted to freely correlate. All parameters were freely estimated.
We examined the goodness of fit for each model using standard criteria (Hu & Bentler, 1999). Specifically, we evaluated models for root mean square error of approximation (RMSEA) < 0.06, standardized root mean square residual (SRMR) < 0.08, and comparative fit index (CFI) > 0.95. We also examined the χ 2 test of model fit, where a nonsignificant result indicates good model fit. Based upon best statistical practices, we evaluated significance of indirect effects using bootstrapped confidence intervals (Hayes, 2009, 2018; MacKinnon et al., 2000; Mallinckrodt et al., 2006; Rucker et al., 2011; Zhao et al., 2010). In other words, significance of the mediation effect was not limited to significance of the a, b, or c paths.
2.7. Statistical power
Regarding statistical power, it is important to note that the core model leverages a multivariate linear regression approach with seven variables estimated in a sample of 243 participants. Recent approaches to estimating power in such models have focused on the number of parameters estimated in the model, and the effect sizes of yielded estimates in the model solution (Iacobucci, 2010; Kim, 2006; Marsh et al., 2004; Wolf et al., 2013). With respect to the number of parameters estimated, one must include more observations than there are estimated parameters in order for the model to be identified. In our case, the model is identified given that we have a sample size of 243 youths, and we have a total of 44 free parameters in the model. With respect to effect sizes, the smallest effect of interest in our final model was the estimate from left amygdala volume to externalizing symptoms (β = .11), which is a small effect size. Using G*Power we computed the minimum sample size to detect a statistically significant effect of this size in the framework of a multivariate linear regression with seven predictors. The total sample size required was 74 participants with power at 0.80, and 98 participants with power at 0.90. Thus, we have compelling support from the data and from the literature that our model design and sample size are sufficiently powered to test our hypotheses.
2.8. Missing data
Of the 243 children recruited at Time 1, not all participants had a structural scan at Time 2, and fewer provided CBCL at Time 3 (reported in Table 1). We conducted each SEM with and without missing data estimation using full‐information maximum likelihood (FIML) and the same conclusions were reached. Therefore, in order to reduce potential bias from data missing at random, we report results using FIML estimation.
TABLE 1.
Demographic variables for the full sample and comparisons by data collection site
| Full sample (n = 243) | Site 1 (UNMC 1) (n = 135) | Site 2 (MRN) (n = 108) | Comparison | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % | χ 2 | p |
| Sex, male | 120 | 49.38 | 63 | 47.70 | 57 | 52.78 | 0.61 | .44 |
| Race | 9.89 | .08 | ||||||
| AI/AN | 7 | 2.90 | 1 | 0.70 | 6 | 5.60 | ||
| Asian | 1 | .40 | 1 | 0.70 | 0 | 0 | ||
| B/AA | 7 | 2.90 | 5 | 3.70 | 2 | 1.90 | ||
| White | 197 | 81.10 | 106 | 78.5 | 91 | 84.30 | ||
| More than 1 race | 18 | 7.40 | 12 | 8.90 | 6 | 5.60 | ||
| Not reported | 13 | 5.30 | 10 | 7.40 | 3 | 2.80 | ||
| Ethnicity | 25.22 | <.01 | ||||||
| Latinx | 54 | 22.20 | 14 | 10.40 | 40 | 37.00 | ||
| Not Latinx | 184 | 75.70 | 117 | 86.70 | 67 | 62.00 | ||
| Not reported | 5 | 2.10 | 4 | 3.0 | 1 | 0.90 | ||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | t | p | |
|---|---|---|---|---|---|---|---|---|
| Age T1 | 11.73 (1.78) | 9–15 | 11.70 (1.69) | 9–15 | 11.76 (1.88) | 9–15 | 0.24 | .81 |
| Age T2 | 12.96 (1.81) | 10–17 | 12.88 (1.69) | 9–15 | 13.04 (1.95) | 10–17 | 0.71 | .48 |
| Age T3 | 13.86 (1.75) | 11–17 | 13.96 (1.93) | 11–17 | 13.79 (1.61) | 11–16 | 0.63 | .53 |
| Stress T1 | 23.15 (7.98) | 11–62 | 23.07 (7.89) | 11–62 | 23.28 (8.18) | 12–56 | 0.50 | .62 |
| Threat T1 | 0.93 (1.24) | 0–6 | 1.10 (1.32) | 0–5 | 0.79 (1.16) | 0–6 | 1.43 | .15 |
| L Amygdala T2 | 1576.29 (329.17) | 410.40–3307.00 | 1628.26 (395.10) | 426.40–3307.00 | 1536.20 (263.63) | 410.40–2025.90 | 0.28 | .78 |
| R Amygdala T2 | 1575.73 (355.11) | 216.20–3056.40 | 1588.34 (393.48) | 216.20–3056.40 | 1566.00 (325.04) | 289.70–2190.30 | 0.31 | .76 |
| L Hippocampus T2 | 4288.71 (586.77) | 1839.90–7732.90 | 4245.07 (722.36) | 1839.90–7732.90 | 4322.38 (458.58) | 3185.90–5806.50 | 2.88 | < .01 |
| R Hippocampus T2 | 4318.11 (605.38) | 1917.30–8064.00 | 4289.58 (743.61) | 1917.30–8064.00 | 4340.13 (476.91) | 2630.00–5487.00 | 1.11 | .27 |
| Internalizing T3 | 5.02 (5.57) | 0–29 | 4.67 (5.41) | 0–28 | 5.43 (5.77) | 0–29 | 0.78 | .44 |
| Externalizing T3 | 3.48 (4.24) | 0–21 | 3.25 (3.79) | 0–14 | 3.75 (4.73) | 0–21 | 0.67 | .50 |
| Dysregulation T3 | 6.63 (6.26) | 0–28 | 6.33 (6.23) | 0–28 | 6.97 (6.32) | 0–28 | 0.57 | .57 |
Note: Brain measures are reported in volume mm3. Age at each timepoint is reported in years. Internalizing, externalizing, and dysregulation are all raw scores from the Child Behavior Checklist. To evaluate potential study site effects, χ 2 analyses were conducted for binary variables and independent samples t‐tests were conducted for continuous variables.
Abbreviations: AI/AN, American Indian/Alaska Native; B/AA, Black, African American; L, left; R, right; T, time.
3. RESULTS
3.1. Trauma history profile EFA
Eleven of the 12 items from the trauma history profile (THP) were submitted to the EFA, as one item asked about “other” trauma experienced, we were unable to discern the specific type of trauma experienced. Of the 11 remaining items, a component structure was revealed whereby seven items loaded onto component 1, explaining 21% of the variance (see Table 2). One item (“someone close to me died”) was excluded because it was straddling Components 1 and 3, making it unclear which component it belonged to. Thus, we excluded this item, which resulted in six items total. Variance explained by the remaining components dropped off precipitously. We interpret that the six items from Component 1 represent exposure to physically threatening experiences (Table 2). Therefore, for each participant, we summed these items to create the “Threat Index” (range = 0–6). Reliability for this scale was acceptable (α = .62).
TABLE 2.
Exploratory factor analysis component loadings
| Item | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| Saw someone who was beaten up, shot at, or killed | 0.750 | −0.108 | −0.227 |
| Saw a family member being hit, punched, kicked | 0.667 | 0.067 | −0.351 |
| Was hit, punched, kicked very hard (not play fighting) | 0.647 | 0.170 | 0.319 |
| Was beaten up, shot at, or threated to be hurt badly in school, neighborhood or town | 0.581 | 0.164 | 0.419 |
| See or hear about the violent death or serious injury of a loved one or friend | 0.546 | −0.282 | −0.364 |
| In a bad accident, like a serious car accident or fall | 0.187 | 0.608 | 0.075 |
| Someone close died | 0.159 | −0.531 | 0.143 |
| In a place where a war was going on | 0.163 | 0.012 | 0.599 |
| Had a painful or scary medical treatment | 0.247 | −0.324 | −0.148 |
| In a natural disaster | 0.292 | −0.268 | 0.398 |
| Saw a dead body (not at a funeral) | 0.217 | 0.514 | −0.281 |
| Eigenvalue | 2.34 | 1.24 | 1.23 |
| % of Total variance | 21% | 11% | 11% |
| Total variance | 43% | ||
Note: Bolded component loading values indicate which component each item loaded onto.
3.2. Descriptive statistics and covariates
Descriptive statistics and demographic variables for the full sample and separately by site are reported in Table 1. The data collection sites were well matched on most demographic characteristics except for ethnicity. The MRN site had a greater proportion of Latinx identifying participants than the UNMC site. Moreover, participants from the MRN site had larger left hippocampal volumes compared to participants from the UNMC site, which was no longer significant once volumes were adjusted for total intracranial volume (TIV). There were no other systematic differences by site in brain structure measures examined here. Moreover, these differences are before accounting for TIV in the final data models. In addition, there were no significant differences between those who completed all 3 years of data collection and those who did not (Table S1).
Several variable distributions had violations of normality, which were transformed using either square root or natural log. Specifically, perceived stress, threat, and the CBCL measures were transformed prior to data modeling. Based upon decreasing BIC, comparison of model fit statistics for different covariates revealed that including age, site, and TIV yielded the best model fit in all models (i.e., left and right amygdala and hippocampus) (Table 3). Sex was ultimately excluded from the final models because the addition of TIV resulted in sex no longer accounting for a significant amount of variance. Therefore, in what follows, we report results from models with age, site, and TIV as covariates. Full reporting of correlations between all study variables of interest can be found in Table S2. An example of the full moderated mediation model is illustrated in Figure 1. All final models for each region had excellent fit (i.e., Model 3 in each region in Table 3). In what follows, we report results for each region.
TABLE 3.
Model fit comparison for covariate variables
| Model | AIC | BIC | Adjust BIC | χ 2 | RMSEA | CFI | SRMR |
|---|---|---|---|---|---|---|---|
| Left amygdala | |||||||
| Model 1 | 1378.98 | 1550.88 | 1392.41 | p = .30 | 0.03 | 0.999 | 0.03 |
| Model 2 | 940.82 | 1084.24 | 922.98 | p = .21 | 0.05 | 0.994 | 0.05 |
| Model 3 | 935.25 | 1058.98 | 919.86 | p = .38 | 0.02 | 0.998 | 0.05 |
| Right amygdala | |||||||
| Model 1 | 1400.24 | 1572.15 | 1413.66 | p = .25 | 0.04 | 0.998 | 0.03 |
| Model 2 | 965.85 | 1109.27 | 948.01 | p = .17 | 0.05 | 0.993 | 0.05 |
| Model 3 | 960.83 | 1084.56 | 945.44 | p = .29 | 0.04 | 0.997 | 0.05 |
| Left hippocampus | |||||||
| Model 1 | 1518.11 | 1690.02 | 1531.55 | p = .25 | 0.04 | 0.998 | 0.03 |
| Model 2 | 1042.75 | 1186.18 | 1024.92 | p = .18 | 0.05 | 0.994 | 0.04 |
| Model 3 | 1036.70 | 1160.43 | 1021.31 | p = .40 | 0.02 | 0.999 | 0.04 |
| Right hippocampus | |||||||
| Model 1 | 1528.95 | 1700.85 | 1542.38 | p = .25 | 0.04 | 0.998 | 0.03 |
| Model 2 | 1053.23 | 1196.65 | 1035.39 | p = .24 | 0.05 | 0.996 | 0.04 |
| Model 3 | 1048.55 | 1172.29 | 1033.16 | p = .47 | 0.00 | 1.00 | 0.04 |
Note: Model 1 = moderated mediation (MM) model with sex, age at Time 1, and site as covariates—site was only a covariate for the respective brain volume (i.e., left amygdala, right amygdala, left hippocampus, right hippocampus); Model 2 = MM model with sex, age at Time 1, site, and total intracranial volume (TIV) at Time 2 as covariates—TIV was only a covariate for the respective brain volume; Model 3 = MM model with age at Time 1, site, and TIV at Time 2.
Abbreviations: AIC, Akaike's information criterion; BIC, Bayesian information criterion; CFI, comparative fit index; RMSEA, root mean square error of approximation; SRMR, standardized root mean square residual.
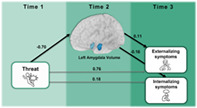
FIGURE 1.
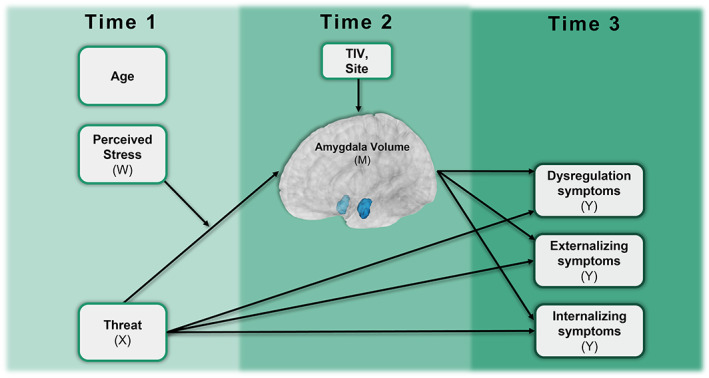
Full moderated mediation model. Moderated mediation model with amygdala volume (Time 2) as an example of mediating the relationship with reported threat exposure from the trauma history profile (Time 1) and internalizing, externalizing, and/or dysregulation symptoms (Time 3) measured via the Child Behavior Checklist (CBCL). Perceived stress at Time 1 from the NIH toolbox serves as a moderator on the association between threat exposure and amygdala volume. Covariates included total intracranial volume (TIV) and site at Time 2 for amygdala volume and age at Time 1 for all variables.
3.3. Moderation results
Full results for the moderation analyses are reported in Tables S3–S6. Briefly, there were no moderation effects of perceived stress on the relationship between threat exposure and any of the brain structures examined (bilateral amygdala and hippocampus). However, across all regions, perceived stress at Time 1 was a significant predictor of externalizing behaviors at Time 3, with greater stress predicting greater number of reported externalizing behaviors. In addition, in both the right and left hippocampi, reductions in volume at Time 2 predicted greater levels of internalizing behaviors at Time 3. Although sex was excluded from the final models since it did not explain a significant amount of variance, we have reported models that include sex as an additional covariate in the supplement (Tables S7–S10) to inform future studies. Given that we did not uncover moderation effects, we also report model fit comparisons for the moderated mediation and the mediation only models in the supplement (Table S11). Moreover, to ensure that results did not differ on the basis of informant type on the perceived stress measure (i.e., parent‐ vs. self‐report), we conducted our analyses with parental‐report only (i.e., n = 146), which yielded comparable results to the full sample model. Specifically, similar to the model including the full sample, parental‐report of their child's stress at Time 1 predicted externalizing behaviors at Time 3 (β = .38, b = 1.07, p = .002).
3.4. Mediation results
3.4.1. Left amygdala
Left amygdala volume at Time 2 mediated the relationship between threat exposure at Time 1 and internalizing behaviors at Time 3 (b = 0.14; 95% CI [0.19, 0.28]) (Figure 2). There was no direct relationship between threat exposure and internalizing behaviors (b = −0.90; 95% CI [−5.86, 2.03]). Likewise, left amygdala volume mediated the relationship between threat exposure and externalizing behaviors (b = −0.10; 95% CI [−1.49, −0.04]) and there was no direct relationship between threat exposure and externalizing behaviors (b = 0.71; 95% CI [−2.84, 3.66]). Figure 3 illustrates the associations between left amygdala volume and internalizing and externalizing behaviors. Notably, individuals who were high in internalizing or high in externalizing were largely nonoverlapping individuals, which is illustrated in Figure S1. Left amygdala volume did not mediate associations between threat exposure and dysregulation at Time 3 (b = −0.03; 95% CI [−0.74, 0.10]) and there was no direct effect of threat exposure on dysregulation (b = −0.29; 95% CI [−6.69, 3.45]).
FIGURE 2.
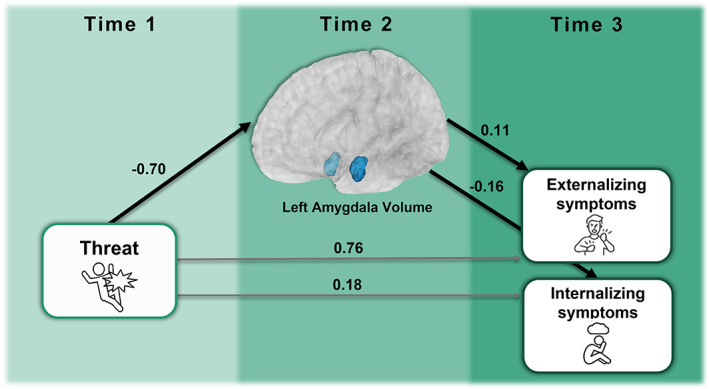
Mediation model results. Results of the mediation model show that the left amygdala volume at Time 2 mediated associations between threat exposure at Time 1 and externalizing as well as internalizing symptoms at Time 3. All estimates shown are standardized. Although individual path estimates were not statistically significant at p < .05, the fully mediated model was statistically significant (lines in black), with no direct relationship between threat and externalizing or internalizing symptoms at Time 3 (lines in grey). Note that covariates are not depicted here, but that age at Time 1 was covaried for each variable and total intracranial volume (TIV) as well as site at Time 2 were covariates for left amygdala volume at Time 2. The 3D rendering of the left amygdala from the FSAverage segmentation is displayed in blue on the MNI152 brain template.
FIGURE 3.
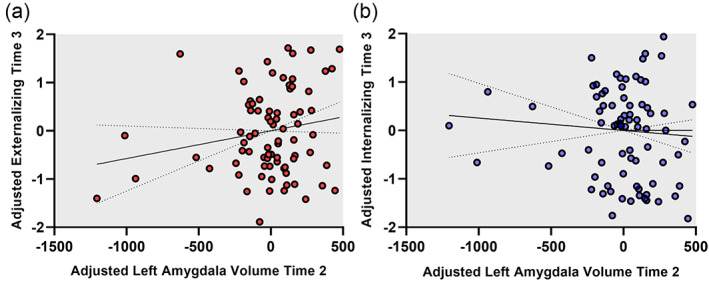
Associations between left amygdala volume and psychopathology symptoms. Scatterplots demonstrating relationships between left amygdala volume at Time 2 and externalizing (a) and internalizing (b) symptoms at Time 3. Left amygdala volume values were adjusted by regressing out the effects of all variables in the full moderated mediation model (i.e., age at Time 1, study site, total intracranial volume [TIV], threat, perceived stress, and their interaction). Similarly, the internalizing and externalizing were both adjusted by regressing out the effects estimated in the full model (i.e., age at Time 1, threat, stress, and their interaction).
3.4.2. Right amygdala
Right amygdala volume at Time 2 did not mediate associations between threat exposure at Time 1 and internalizing (b = 0.30; 95% CI [−0.17, 0.81]), externalizing (b = −0.22; 95% CI [−1.27, 0.11]), or dysregulation behaviors at Time 3 (b = −0.03; 95% CI [−0.82, 0.14]). Moreover, there were no direct associations between threat exposure and internalizing (b = −1.22; 95% CI [−6.59, 4.73]), externalizing (b = 0.91; 95% CI [−4.41, 6.06]), or dysregulation behaviors (b = −0.30; 95% CI [−7.67, 6.32]).
3.4.3. Left hippocampus
Left hippocampal volume at Time 2 did not mediate associations between threat exposure at Time 1 and internalizing (b = −0.06; 95% CI [−0.98, 0.26]), externalizing (b = −0.01; 95% CI [−0.28, 0.42]), or dysregulation behaviors at Time 3 (b = −0.01; 95% CI [−0.57, 0.12]). In addition, threat exposure at Time 1 did not have a direct effect on internalizing (b = −0.95, 95% CI [−4.64, 4.03]), externalizing (b = 0.64, 95% CI [−2.93, 5.05]), or dysregulation at time 3 (b = −0.33, 95% CI [−4.78, 5.67]).
3.4.4. Right hippocampus
Right hippocampal volume at Time 2 did not mediate associations between threat exposure at Time 1 and internalizing (b = 0.08; 95% CI [−0.31, 0.56]), externalizing (b = 0.01; 95% CI [−0.12, 0.74]), or dysregulation behaviors at Time 3 (b = 0.02; 95% CI [−0.11, 0.29]). Threat exposure at Time 1 did not have a direct effect on internalizing (b = −1.07; 95% CI [−4.43, 4.86]), externalizing (b = 0.55; 95% CI [−2.82, 4.74]), or dysregulation at time 3 (b = −0.40; 95% CI[−4.66, 5.47]).
4. DISCUSSION
Traumatic experiences have been linked to a myriad of effects on youth brain structure and psychopathology (Teicher et al., 2016). In one of only very few studies of its kind (also see Weissman et al., 2020), we employed a longitudinal design to interrogate the extent to which threat exposure is linked to the structure of two highly stress‐sensitive brain regions (i.e., amygdala and hippocampal) and in turn, subsequent psychopathology symptoms. In addition, we sought to disentangle whether perceptions of stress moderated the impact of threat exposure on amygdala and hippocampal volumes. We report three key findings from the present study. First, although we did not find that perceived stress moderated the association between threat and amygdala or hippocampal volume, we did find that greater perceived stress predicted more externalizing (but not internalizing) symptoms at Time 3 in all models. This finding is supported by prior literature and it may be indicative of poorer coping strategies following trauma, which confers elevated risk for externalizing symptoms, in particular (Modecki et al., 2017). This result will need to be further examined in future studies to determine the extent to which perceptions of stress coupled with coping strategies play a role in conferring risk for externalizing symptoms, and how those effects may be mediated by underlying stress‐sensitive neural structures. Second, our mediation model results suggest that the left amygdala specifically mediates the effect of threat experiences on subsequent internalizing and externalizing symptoms during adolescence. Interestingly, the left amygdala differentially mediated internalizing and externalizing outcomes such that smaller amygdala volumes were related to increased internalizing behaviors at Time 3, while greater amygdala volumes at Time 2 were related to increased externalizing behaviors at Time 3. Finally, decreased volume in left and right hippocampi at Time 2 predicted increased internalizing symptoms at Time 3. Indeed, prior cross‐sectional work has shown that reduced hippocampal volume is related to elevated internalizing symptoms during childhood and adolescence (Koolschijn et al., 2013).
Building on existing behavioral literature that points to general increases in psychopathology following threat exposure (McLaughlin et al., 2020), the present study provides a pivotal clue in how risk for distinct types of psychopathology is likely mediated through specific structural characteristics of the amygdala. By and large, existing longitudinal pediatric studies of the effects of threat exposure on structural brain development have yielded evidence for accelerated cortical thinning hypotheses (e.g., Colich et al., 2020). However, literature regarding threat‐related effects on the amygdala and hippocampus have been inconclusive, with some work showing decreases in volume (Badura‐Brack et al., 2020; Hanson et al., 2015; Lee et al., 2018; Saxbe et al., 2018; Weissman et al., 2020) or no effects (Butler et al., 2018; Saxbe et al., 2018). With the exception of a few studies (Saxbe et al., 2018; Weissman et al., 2020), most studies examining threat exposure have been conducted in cross‐sectional designs, often with smaller sample sizes that could hinder the ability to model different patterns of symptomatology simultaneously. Those longitudinal studies have typically been limited to two time points of data collection, limiting conclusions that can be made with respect to cascading effects on risk for psychopathology. Thus, the current study contributes to delineating distinctive brain‐mediated pathways toward internalizing and externalizing psychopathology. Taken together, our study supports the notion that there are decreases in left amygdala volume following threat exposure and smaller bilateral hippocampal volumes are longitudinally predictive of internalizing symptoms during adolescence. The hippocampal findings are in line with prior reports of smaller hippocampal volumes in youth with anxiety and depressive disorders (Gold et al., 2017; Henje Blom et al., 2015; Jaworska et al., 2016; MacMaster et al., 2014) as well as youth with subclinical internalizing symptomatology (Merz, He, & Noble, 2018). Thus, given the longitudinal nature of the current study, it may be that youth who go on to develop internalizing disorders evince smaller hippocampal volumes preclinically.
The present study is also consistent with prior work demonstrating that externalizing behaviors in adolescence are related to larger amygdala volumes (Saxbe et al., 2018), and that internalizing symptoms are related to smaller amygdala volumes (Merz, Tottenham, & Noble, 2018; Weissman et al., 2020). The current study adds to existing findings by highlighting that the left amygdala volume, in particular, differentially mediates associations between earlier threat exposure and internalizing or externalizing symptomology outcomes. This laterality effect stands in contrast to previous theoretical work positing that the left and right amygdala encodes different types of threat (Teicher et al., 2012). That is, work has recently proposed that the right amygdala is sensitized to physically harmful threats and the left amygdala is more sensitized to threats of inadequate care (Teicher & Khan, 2019). In our study, we attempted to capture a measure of physical harm or threat of physical harm, but future work will need to more thoroughly tease apart these two forms of threat and their laterality effects on the amygdalae. It is worth noting that this laterality finding is, to some extent, supported by previous work showing that threat‐exposed children exhibit greater anticorrelated functional connectivity between the left (not the right) amygdala and ventromedial prefrontal cortex and medial orbitofrontal connectivity, which was related to greater externalizing and internalizing symptomatology, respectively (Peverill et al., 2019). Findings from the ABCD youth study have also suggested differential associations between negative life events and connectivity trajectories for left and right amygdala, such that a greater number of stressful events is related to weaker positive connectivity between the left amygdala and the cingulo‐opercular network, which in turn predicted reduced internalizing symptomology (Brieant et al., 2021). Conversely, more stressful events were related to greater negative connectivity between the right amygdala and cingulo‐opercular network, which predicted reduced internalizing symptomology. Although not specific to threat experiences per se, these findings support the notion that left and right amygdala may be differentially sensitized and may transmit information about threatening experiences in a lateralized manner. Another related line of inquiry that is sorely needed is more fine‐grained analysis of how the volume of amygdalae subnuclei may differentially mediate longitudinal associations between dimensions of trauma and psychopathology outcomes (for an elegant cross‐sectional example, see Oshri et al., 2019). It may be that some laterality effects induced by threat‐related trauma are attributable to specific, lateralized subnuclei.
Although the current study has many strengths, several limitations must be acknowledged. First, this study examined only threat experiences, as we did not have measures of deprivation (e.g., physical neglect, food insecurity, poverty measures). In future studies, it would be ideal to characterize experiences of deprivation and threat in the same sample, as other researchers have begun to do (Everaerd et al., 2016; Machlin et al., 2019). Following such an approach would allow further delineation of the ways in which these different dimensions of trauma confer differential and/or overlapping risk toward psychopathology. Second, it is important to note that the perceived stress measure collected in this study was not in direct reference to the threat experiences measured. To better capture this, subsequent work would benefit from evaluating youths' perceived stress levels in response to traumatic experiences. Moreover, the perceived stress measure was based upon either parent report for children under 12 or self‐report for participants 12 years and older. Although these measures have high internal consistency (Cronbach's α = .89 and .87 for child and parent report, respectively; Salsman et al., 2013), it may be the case that parent and child report capture different aspects of children's stress. Thus, future work should evaluate perceptions of stress with consistent reporter types (i.e., self, parent, or combined). Third, the current study was focused on typical development and was not oversampled for traumatic experiences. High‐risk samples that have been explicitly collected for the purposes of prospectively mapping the neurodevelopmental effects of childhood trauma are greatly needed. That said, it is also useful to understand subclinical, or even preclinical, processes that may underlie neurobiological responses to trauma; uncovering potential subclinical and/or preclinical biomarkers is essential to understanding the emergence, progression, and eventual targeted treatment of psychopathology that may follow trauma exposure.
5. CONCLUSIONS
The present study offers a novel investigation of how threat exposure during development may confer elevated risk for psychopathology, which is likely mediated through structural characteristics of the amygdala. Here, we employed a longitudinal design to demonstrate that the left amygdala volume mediates the association between threat exposure and internalizing as well as externalizing symptoms in later adolescence. Findings suggest that greater exposure to threatening experiences is related to subsequent reductions in left amygdala volume; left amygdala volume, in turn, is longitudinally associated with elevated internalizing and externalizing symptoms. These findings build upon and extend previous work revealing that specific dimensions of trauma induce alterations to stress‐sensitive brain structures and subsequent psychopathology. Importantly, this work suggests that the left amygdala is highly threat‐sensitive and stress‐specific alterations likely portend elevated psychopathology during adolescence.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation (#1539067 to Tony W. Wilson, Yu‐Ping Wang, Julia M. Stephen, and Vince D. Calhoun), the National Institutes of Health (R01MH121101, R01MH116782, R01MH118013, and P20‐GM144641 to Tony W. Wilson; R01EB020407 and R01MH118695 to Vince D. Calhoun), and At Ease, USA. Funding agencies had no part in the study design or the writing of this report.
Picci, G. , Taylor, B. K. , Killanin, A. D. , Eastman, J. A. , Frenzel, M. R. , Wang, Y.‐P. , Stephen, J. M. , Calhoun, V. D. , & Wilson, T. W. (2022). Left amygdala structure mediates longitudinal associations between exposure to threat and long‐term psychiatric symptomatology in youth. Human Brain Mapping, 43(13), 4091–4102. 10.1002/hbm.25904
Funding information National Institutes of Health, Grant/Award Numbers: P20‐GM144641, R01EB020407, R01MH116782, R01MH118013, R01MH118695, R01MH121101; National Science Foundation, Grant/Award Number: 1539067
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the principal investigators upon reasonable request.
REFERENCES
- Achenbach, T. M. , Dumenci, L. , & Rescorla, L. A. (2001). Ratings of relations between DSM‐IV diagnostic categories and items of the CBCL/6‐18, TRF, and YSR. University of Vermont Research Center for Children, Youth, & Families. [Google Scholar]
- Badura‐Brack, A. S. , Mills, M. S. , Embury, C. M. , Khanna, M. M. , Klanecky Earl, A. , Stephen, J. M. , Wang, Y.‐P. , Calhoun, V. D. , & Wilson, T. W. (2020). Hippocampal and parahippocampal volumes vary by sex and traumatic life events in children. Journal of Psychiatry and Neuroscience, 45(4), 288–297. 10.1503/jpn.190013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieant, A. E. , Sisk, L. M. , & Gee, D. G. (2021). Associations among negative life events, changes in cortico‐limbic connectivity, and psychopathology in the ABCD study. Developmental Cognitive Neuroscience, 52, 101022. 10.1016/j.dcn.2021.101022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, O. , Yang, X.‐F. , Laube, C. , Kühn, S. , & Immordino‐Yang, M. H. (2018). Community violence exposure correlates with smaller gray matter volume and lower IQ in urban adolescents. Human Brain Mapping, 39(5), 2088–2097. 10.1002/hbm.23988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiers, L. L. M. , Sabbe, B. G. C. , Schmaal, L. , Veltman, D. J. , Penninx, B. W. J. H. , & Van Den Eede, F. (2018). Structural and functional brain abnormalities associated with exposure to different childhood trauma subtypes: A systematic review of neuroimaging findings. Frontiers in Psychiatry, 9, 329. 10.3389/fpsyt.2018.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohodes, E. M. , Kitt, E. R. , Baskin‐Sommers, A. , & Gee, D. G. (2020). Influences of early‐life stress on frontolimbic circuitry: Harnessing a dimensional approach to elucidate the effects of heterogeneity in stress exposure. Developmental Psychobiology, 63(2), 153–172. 10.1002/dev.21969 [DOI] [PubMed] [Google Scholar]
- Colich, N. L. , Rosen, M. L. , Williams, E. S. , & McLaughlin, K. A. (2020). Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta‐analysis. Psychological Bulletin, 146(9), 721–764. 10.1037/bul0000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese, A. , & Widom, C. S. (2020). Objective and subjective experiences of child maltreatment and their relationships with psychopathology. Nature Human Behaviour, 4(8), 811–818. 10.1038/s41562-020-0880-3 [DOI] [PubMed] [Google Scholar]
- Dannlowski, U. , Stuhrmann, A. , Beutelmann, V. , Zwanzger, P. , Lenzen, T. , Grotegerd, D. , Domschke, K. , Hohoff, C. , Ohrmann, P. , Bauer, J. , Lindner, C. , Postert, C. , Konrad, C. , Arolt, V. , Heindel, W. , Suslow, T. , & Kugel, H. (2012). Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry, 71(4), 286–293. 10.1016/j.biopsych.2011.10.021 [DOI] [PubMed] [Google Scholar]
- Edmiston, E. E. (2011). Corticostriatal‐limbic Gray matter morphology in adolescents with self‐reported exposure to childhood maltreatment. Archives of Pediatrics & Adolescent Medicine, 165(12), 1069–1077. 10.1001/archpediatrics.2011.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood‐Lowe, M. E. , Humphreys, K. L. , Ordaz, S. J. , Camacho, M. C. , Sacchet, M. D. , & Gotlib, I. H. (2018). Time‐varying effects of income on hippocampal volume trajectories in adolescent girls. Developmental Cognitive Neuroscience, 30, 41–50. 10.1016/j.dcn.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerd, D. , Klumpers, F. , Zwiers, M. , Guadalupe, T. , Franke, B. , van Oostrom, I. , Schene, A. , Fernández, G. , & Tendolkar, I. (2016). Childhood abuse and deprivation are associated with distinct sex‐dependent differences in brain morphology. Neuropsychopharmacology, 41(7), 1716–1723. 10.1038/npp.2015.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foell, J. , Palumbo, I. M. , Yancey, J. R. , Vizueta, N. , Demirakca, T. , & Patrick, C. J. (2019). Biobehavioral threat sensitivity and amygdala volume: A twin neuroimaging study. NeuroImage, 186, 14–21. 10.1016/j.neuroimage.2018.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, A. L. , Sheridan, M. A. , Peverill, M. , Busso, D. S. , Lambert, H. K. , Alves, S. , Pine, D. S. , & McLaughlin, K. A. (2016). Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. Journal of Child Psychology and Psychiatry, 57(10), 1154–1164. 10.1111/jcpp.12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, A. L. , Steuber, E. R. , White, L. K. , Pacheco, J. , Sachs, J. F. , Pagliaccio, D. , Berman, E. , Leibenluft, E. , & Pine, D. S. (2017). Cortical thickness and subcortical gray matter volume in pediatric anxiety disorders. Neuropsychopharmacology, 42(12), 2423–2433. 10.1038/npp.2017.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , & Nacewicz, B. M. (2021). Amygdala allostasis and early life adversity: Considering excitotoxicity and inescapability in the sequelae of stress. Frontiers in Human Neuroscience, 15, 624705. 10.3389/fnhum.2021.624705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , Nacewicz, B. M. , Sutterer, M. J. , Cayo, A. A. , Schaefer, S. M. , Rudolph, K. D. , Shirtcliff, E. A. , Pollak, S. D. , & Davidson, R. J. (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4), 314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, T. , Takeuchi, H. , Taki, Y. , Sekiguchi, A. , Nouchi, R. , Kotozaki, Y. , Nakagawa, S. , Miyauchi, C. M. , Iizuka, K. , Yokoyama, R. , Shinada, T. , Yamamoto, Y. , Hanawa, S. , Araki, T. , Hashizume, H. , Kunitoki, K. , & Kawashima, R. (2015). Neuroanatomical correlates of the sense of control: Gray and white matter volumes associated with an internal locus of control. NeuroImage, 119, 146–151. 10.1016/j.neuroimage.2015.06.061 [DOI] [PubMed] [Google Scholar]
- Hayes, A. F. (2009). Beyond baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76(4), 408–420. 10.1080/03637750903310360 [DOI] [Google Scholar]
- Hayes, A. F. (2018). Causal steps, confounding, and causal order. In Introduction to mediation, moderation, and conditional process analysis: A regression‐based approach (2nd ed., pp. 113–146). Guildford Press. [Google Scholar]
- Henje Blom, E. , Han, L. K. M. , Connolly, C. G. , Ho, T. C. , Lin, J. , LeWinn, K. Z. , Simmons, A. N. , Sacchet, M. D. , Mobayed, N. , Luna, M. E. , Paulus, M. , Epel, E. S. , Blackburn, E. H. , Wolkowitz, O. M. , & Yang, T. T. (2015). Peripheral telomere length and hippocampal volume in adolescents with major depressive disorder. Translational Psychiatry, 5(11), e676. 10.1038/tp.2015.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog, J. I. , Thome, J. , Demirakca, T. , Koppe, G. , Ende, G. , Lis, S. , Rausch, S. , Priebe, K. , Müller‐Engelmann, M. , Steil, R. , Bohus, M. , & Schmahl, C. (2020). Influence of severity of type and timing of retrospectively reported childhood maltreatment on female amygdala and hippocampal volume. Scientific Reports, 10(1), 1903. 10.1038/s41598-020-57490-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, L. , & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Humphreys, K. L. , King, L. S. , Sacchet, M. D. , Camacho, M. C. , Colich, N. L. , Ordaz, S. J. , Ho, T. C. , & Gotlib, I. H. (2019). Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Developmental Science, 22(3), e12775. 10.1111/desc.12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci, D. (2010). Structural equations modeling: Fit indices, sample size, and advanced topics. Journal of consumer psychology, 20(1), 90–98. [Google Scholar]
- Jaworska, N. , Yücel, K. , Courtright, A. , MacMaster, F. P. , Sembo, M. , & MacQueen, G. (2016). Subgenual anterior cingulate cortex and hippocampal volumes in depressed youth: The role of comorbidity and age. Journal of Affective Disorders, 190, 726–732. 10.1016/j.jad.2015.10.064 [DOI] [PubMed] [Google Scholar]
- Johnson, S. B. , Riis, J. L. , & Noble, K. G. (2016). State of the art review: Poverty and the developing brain. Pediatrics, 137(4), e20153075. 10.1542/peds.2015-3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. S . (2006). Effects of a voluntary summer reading intervention on reading achievement: Results from a randomized field trial. Educational Evaluation and Policy Analysis, 28(4), 335–355. 10.3102/01623737028004335 [DOI] [Google Scholar]
- King, L. S. , Humphreys, K. L. , Camacho, M. C. , & Gotlib, I. H. (2019). A person‐centered approach to the assessment of early life stress: Associations with the volume of stress‐sensitive brain regions in early adolescence. Development and Psychopathology, 31(02), 643–655. 10.1017/S0954579418000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn, P. C. M. P. , van Ijzendoorn, M. H. , Bakermans‐Kranenburg, M. J. , & Crone, E. A. (2013). Hippocampal volume and internalizing behavior problems in adolescence. European Neuropsychopharmacology, 23(7), 622–628. 10.1016/j.euroneuro.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Lambert, H. K. , Sheridan, M. A. , Sambrook, K. A. , Rosen, M. L. , Askren, M. K. , & McLaughlin, K. A. (2017). Hippocampal contribution to context encoding across development is disrupted following early‐life adversity. The Journal of Neuroscience, 37(7), 1925–1934. 10.1523/JNEUROSCI.2618-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. W. , Yoo, J. H. , Kim, K. W. , Kim, D. , Park, H. , Choi, J. , Teicher, M. H. , & Jeong, B. (2018). Hippocampal subfields volume reduction in high schoolers with previous verbal abuse experiences. Clinical Psychopharmacology and Neuroscience, 16(1), 46–56. 10.9758/cpn.2018.16.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby, J. , Belden, A. , Botteron, K. , Marrus, N. , Harms, M. P. , Babb, C. , Nishino, T. , & Barch, D. (2013). The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatrics, 167(12), 1135–1142. 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlin, L. , Miller, A. B. , Snyder, J. , McLaughlin, K. A. , & Sheridan, M. A. (2019). Differential associations of deprivation and threat with cognitive control and fear conditioning in early childhood. Frontiers in Behavioral Neuroscience, 13, 80. 10.3389/fnbeh.2019.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon, D. P. , Krull, J. L. , & Lockwood, C. M. (2000). Equivalence of the mediation, confounding and suppression effect. Prevention Science, 1(4), 9–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon, D. P. , Lockwood, C. M. , & Williams, J. (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research, 39(1), 99–128. 10.1207/s15327906mbr3901_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster, F. P. , Carrey, N. , Langevin, L. M. , Jaworska, N. , & Crawford, S. (2014). Disorder‐specific volumetric brain difference in adolescent major depressive disorder and bipolar depression. Brain Imaging and Behavior, 8(1), 119–127. 10.1007/s11682-013-9264-x [DOI] [PubMed] [Google Scholar]
- Mallinckrodt, B. , Abraham, W. T. , Wei, M. , & Russell, D. W. (2006). Advances in testing the statistical significance of mediation effects. Journal of Counseling Psychology, 53(3), 372–378. 10.1037/0022-0167.53.3.372 [DOI] [Google Scholar]
- Marsh, H. W. , Hau, K. T. , & Wen, Z. (2004). In search of golden rules: Comment on hypothesis‐testing approaches to setting cutoff values for fit indexes and dangers in overgeneralizing Hu and Bentler's (1999) findings. Structural equation modeling, 11(3), 320–341. [Google Scholar]
- McGaugh, J. L. , & Roozendaal, B. (2002). Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology, 12(2), 205–210. 10.1016/S0959-4388(02)00306-9 [DOI] [PubMed] [Google Scholar]
- McLaughlin, K. A. , Colich, N. L. , Rodman, A. M. , & Weissman, D. G. (2020). Mechanisms linking childhood trauma exposure and psychopathology: A transdiagnostic model of risk and resilience. BMC Medicine, 18(1), 96. 10.1186/s12916-020-01561-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. A. , Sheridan, M. A. , & Lambert, H. K. (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz, E. C. , He, X. , & Noble, K. G. (2018). Anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage: Clinical, 20, 243–251. 10.1016/j.nicl.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz, E. C. , Tottenham, N. , & Noble, K. G. (2018). Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. Journal of Clinical Child & Adolescent Psychology, 47(2), 312–323. 10.1080/15374416.2017.1326122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modecki, K. L. , Zimmer‐Gembeck, M. J. , & Guerra, N. (2017). Emotion regulation, coping, and decision making: Three linked skills for preventing externalizing problems in adolescence. Child Development, 88(2), 417–426. 10.1111/cdev.12734 [DOI] [PubMed] [Google Scholar]
- Muthén, L. K. , & Muthén, B. O. (2015). Mplus user's guide (7th ed.). Muthén & Muthén. [Google Scholar]
- Noble, K. , Grieve, S. , Korgaonkar, M. , Engelhardt, L. , Griffith, E. , Williams, L. , & Brickman, A. (2012). Hippocampal volume varies with educational attainment across the life‐span. Frontiers in Human Neuroscience, 6, 307. 10.3389/fnhum.2012.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri, A. , Gray, J. C. , Owens, M. M. , Liu, S. , Duprey, E. B. , Sweet, L. H. , & MacKillop, J. (2019). Adverse childhood experiences and amygdalar reduction: High‐resolution segmentation reveals associations with subnuclei and psychiatric outcomes. Child Maltreatment, 24(4), 400–410. 10.1177/1077559519839491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peverill, M. , Sheridan, M. A. , Busso, D. S. , & McLaughlin, K. A. (2019). Atypical prefrontal‐amygdala circuitry following childhood exposure to abuse: Links with adolescent psychopathology. Child Maltreatment, 24(4), 411–423. 10.1177/1077559519852676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps, E. A. , Delgado, M. R. , Nearing, K. I. , & LeDoux, J. E. (2004). Extinction learning in humans: Role of the amygdala and vmPFC. Neuron, 43(6), 897–905. 10.1016/j.neuron.2004.08.042 [DOI] [PubMed] [Google Scholar]
- Roth, M. C. , Humphreys, K. L. , King, L. S. , & Gotlib, I. H. (2018). Self‐reported neglect, amygdala volume, and symptoms of anxiety in adolescent boys. Child Abuse & Neglect, 80, 80–89. 10.1016/j.chiabu.2018.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker, D. D. , Preacher, K. J. , Tormala, Z. L. , & Petty, R. E. (2011). Mediation analysis in social psychology: Current practices and new recommendations. Social and Personality Psychology Compass, 5(6), 359–371. 10.1111/j.1751-9004.2011.00355.x [DOI] [Google Scholar]
- Salsman, J. M. , Butt, Z. , Pilkonis, P. A. , Cyranowski, J. M. , Zill, N. , Hendrie, H. C. , Kupst, M. J. , Kelly, M. A. R. , Bode, R. K. , Choi, S. W. , Lai, J.‐S. , Griffith, J. W. , Stoney, C. M. , Brouwers, P. , Knox, S. S. , & Cella, D. (2013). Emotion assessment using the NIH toolbox. Neurology, 80(11), S76–S86. 10.1212/WNL.0b013e3182872e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe, D. , Khoddam, H. , Piero, L. D. , Stoycos, S. A. , Gimbel, S. I. , Margolin, G. , & Kaplan, J. T. (2018). Community violence exposure in early adolescence: Longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Developmental Science, 21(6), e12686. 10.1111/desc.12686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. E. , & Pollak, S. D. (2021). Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspectives on Psychological Science, 16(1), 67–93. 10.1177/1745691620920725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, A. M. , Brymer, M. J. , Decker, K. B. , & Pynoos, R. S. (2004). The University of California at Los Angeles post‐traumatic stress disorder reaction index. Current Psychiatry Reports, 6(2), 96–100. 10.1007/s11920-004-0048-2 [DOI] [PubMed] [Google Scholar]
- Stephen, J. M. , Solis, I. , Janowich, J. , Stern, M. , Frenzel, M. R. , Eastman, J. A. , Mills, M. S. , Embury, C. M. , Coolidge, N. M. , Heinrichs‐Graham, E. , Mayer, A. , Liu, J. , Wang, Y. P. , Wilson, T. W. , & Calhoun, V. D. (2021). The developmental Chronnecto‐genomics (dev‐CoG) study: A multimodal study on the developing brain. NeuroImage, 225, 117438. 10.1016/j.neuroimage.2020.117438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , Anderson, C. M. , & Polcari, A. (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences, 109(9), E563–E572. 10.1073/pnas.1115396109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , & Khan, A. (2019). Childhood maltreatment, cortical and amygdala morphometry, functional connectivity, laterality, and psychopathology. Child Maltreatment, 24(4), 458–465. 10.1177/1077559519870845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , & Samson, J. A. (2016). Annual research review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry, 57(3), 241–266. 10.1111/jcpp.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , Samson, J. A. , Anderson, C. M. , & Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652–666. 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- Tottenham, N. , Hare, T. A. , Quinn, B. T. , McCarry, T. W. , Nurse, M. , Gilhooly, T. , Millner, A. , Galvan, A. , Davidson, M. C. , Eigsti, I.‐M. , Thomas, K. M. , Freed, P. J. , Booma, E. S. , Gunnar, M. R. , Altemus, M. , Aronson, J. , & Casey, B. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1), 46–61. 10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman, D. G. , Lambert, H. K. , Rodman, A. M. , Peverill, M. , Sheridan, M. A. , & McLaughlin, K. A. (2020). Reduced hippocampal and amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma. Depression and Anxiety, 37(9), 916–925. 10.1002/da.23062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, E. J. , Harrington, K. M. , Clark, S. L. , & Miller, M. W. (2013). Sample size requirements for structural equation models: An evaluation of power, bias, and solution propriety. Educational and psychological measurement, 73(6), 913–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Lynch, J. G. , & Chen, Q. (2010). Reconsidering baron and Kenny: Myths and truths about mediation analysis. Journal of Consumer Research, 37(2), 197–206. 10.1086/651257 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the principal investigators upon reasonable request.


