Abstract
Nanotechnology has been used for medical applications in several forms, including dental practice with the development of silver nanoparticles (Ag NPs) as a useful tool. The aim of this review was to identify the properties and appliances of Ag NPs in dental practice. Silver compounds and NPs have already been used as dental restorative material, endodontic retrofill cements, dental implants and caries inhibitory solution. Despite the effectiveness that Ag NPs has shown in dental practice, Ag NPs remain a controversial area of research with respect to their toxicity in biological and ecological systems. Therefore any application of Ag NPs in dentistry requires more studies. In order to avoid the toxicity of these materials Ag NPs can be temporarily used in dentistry.
Key words: Silver nanoparticles, dental practice, applications of Ag NPs
INTRODUCTION
Nanotechnology, which concerns structures at the nanometer scale (1–100 nm), is considered as a vital current technology on the 21st century based on its economic and scientific potential. In 2008, the public expenditure on nanotechnology was 430 mmd compared to 25 mmd in 19971., 2.. Nanoparticles (NPs) have a greater surface-to-volume ratio (per unit mass) than non-nanoscale particles of the same material, and therefore are more reactive. Particles smaller than 50 nm are subject to the laws of quantum physics3.
In 2008 and 2009, silver production was 21 300 and 21 400 tons, respectively, according to United States Geological Survey (USGS)4. Over the years, silver compounds and NPs (Figure 1) have exhibited antibacterial activity resulting in the widespread use of silver nanoparticles (Ag NPs) in bedding, washing machines, water purification, toothpaste, shampoo and rinse, nursing bottles, fabrics, deodorants, filters, kitchen utensils, toys and humidifiers5. Furthermore, silver compounds and NPs6 have been studied for dental applications including dental restorative material7, endodontic retrofill cement8, dental implants9, and caries inhibitory solution10. The aim of this brief review was to identify the properties and appliances of Ag NPs in dental practice.
Figure 1.
Scanning Electron Microscope views of Ag NPs. Illustration provided by Professor Raúl Alberto Morales Luckie, Faculty of Chemistry, UAEMex.
ANTIMICROBIAL PROPERTIES
Most of the studies have indicated that silver interacts with sulfhydryl groups of proteins and with DNA, altering hydrogen bonding, respiratory processes, DNA unwinding, cell-wall synthesis and cell division11., 12.. At the macro level, these interactions effectively produce bacterial death13.
It is recognised that Ag NPs have antimicrobial activity against Gram-negative bacteria performing ‘pits’ in the cell wall of the bacteria. Clearly, a membrane with such morphology exhibits a significant increase in permeability, resulting in death of the cell. Overall, silver mainly induces denaturation and oxidisation for cell wall which lead to rupture of the internal cell organelles, resulting in bacterial death14., 15.. Although bacterial cell lysis could be one of the reasons for the observed antibacterial property, NPs also modulate the phosphotyrosine profile of putative bacterial peptides, which could affect bacterial signal transduction and inhibit the growth of the organisms16.
It is worth noting that the antibacterial activity of nanosilver is dominated by silver ions (Ag+ ions) when fine Ag NPs (less than about 10 nm in average diameter) are employed that release high concentrations of these ions. In contrast, when relatively larger Ag NPs are used, the concentration of the released Ag+ ions is lower17. Likewise, Ag NPs with average size 14 ± 6 nm and Ag+ ions such as AgNO3, inhibit the growth of Escherichia coli 55 ± 8% and 100%, respectively (Table 1 shows the antimicrobial effect of Ag NPs).
Table 1.
Antimicrobial properties of Ag NPs
| Gram-positive bacteria | Streptococcus sanguis6 |
| Bacillus subtilis35 | |
| Enterococcus faecalis35 | |
| Staphylococcus aureus36 | |
| Gram-negative bacteria | Escherichia coli36 |
| Salmonella typhimurium35 | |
| Shigella dysenteriae type 138 | |
| Citrobacter sp37 | |
| Pseudomona aeruginosa37 | |
| Pseudomona fluorescens38 | |
| Fungi | Candida albicans39 |
| Fusarium oxysporum37 | |
| Virus | Arenavirus40 |
| Human immunodeficiency virus-142 | |
| Murine norovirus41 | |
| Hepatitis B virus42 |
Furthermore, silver particles are also used as an alternative radiopacifier to get the necessary radiopacity to calcium silicate cements (CSC) and assess the purity of the radiopacifying agents18. These nanomaterials, which can be prepared in a simple and cost-effective manner, may be suitable for the formulation of new types of bactericidal materials13.
CARIES INHIBITORY PROPERTIES
The most common worldwide oral diseases are dental caries and periodontal diseases, 60–90%, according to the World Health Organization (WHO)19. In Mexico, some authors estimate that such diseases affect 90% and 70% of the population, respectively20. In this regard, the use of silver solution, specifically, silver diamine fluoride (Ag [NH3] 2F) has been used as a caries inhibitor. In context, fluoride and silver interact synergistically to form fluorapatite. The first step is the formation of calcium fluoride (CaF2) and silver phosphate (Ag3PO4) in a basic environment, the second reaction is the subsequent dissociation of calcium and fluoride21.
Experimental composite adhesives (ECAs) showed slower bacterial growth than those containing conventional adhesives, suggesting that ECAs can help prevent enamel demineralisation around their surfaces without compromising physical properties22.
RESTORATIVE MATERIALS
Whereas restorative materials with a silver polymer compound have shown effective antimicrobial properties on implant components against Streptococcus sanguis6, silver has been incorporated into glass ionomer cements in order to improve the antibacterial properties, including also, compressive, tensile strength and creep resistance.
Biofilms are surface-adherent populations of microorganisms consisting of cells, water and extracellular matrix material. Nanotechnology is a promising field of science which can guide our understanding of the role of interspecies interaction in the development of biofilm. Streptococcus mutans with other species of bacteria has been known to form dental biofilm. The correlation between genetically modified bacteria Streptococcus mutans and nanoscale morphology has been assessed using atomic force microscopy (AFMi). Occasionally, silver nanofibers have been attached to the implant surfaces to reduce the need of using high doses of antibiotics during the healing period, providing self-cleaning against plaque biofilm9.
THERAPEUTICS
Nanostructures of different sizes, shapes and material properties have many applications in biomedical imaging, clinical diagnostics and therapeutics. In spite of what has been achieved so far, a complete understanding of how cells interact with nanostructures of well-defined sizes, at the molecular level, remains poorly understood.
Gold and Ag NPs coated with antibodies can regulate the process of membrane receptor internalisation. The binding and activation of membrane receptors and subsequent protein expression strongly depend on nanoparticle size. Although all NPs within the 2–100 nm size range alter signaling processes essential for basic cell functions (including cell death) 7, 40- and 50-nm NPs demonstrate the greatest effect. These results show that NPs should no longer be viewed as simple carriers for biomedical applications, but can also play an active role in mediating biological effects. These findings may assist in the design of nanoscale delivery and therapeutic systems and provide insights into nanotoxicity23.
ADVERSE EFFECTS
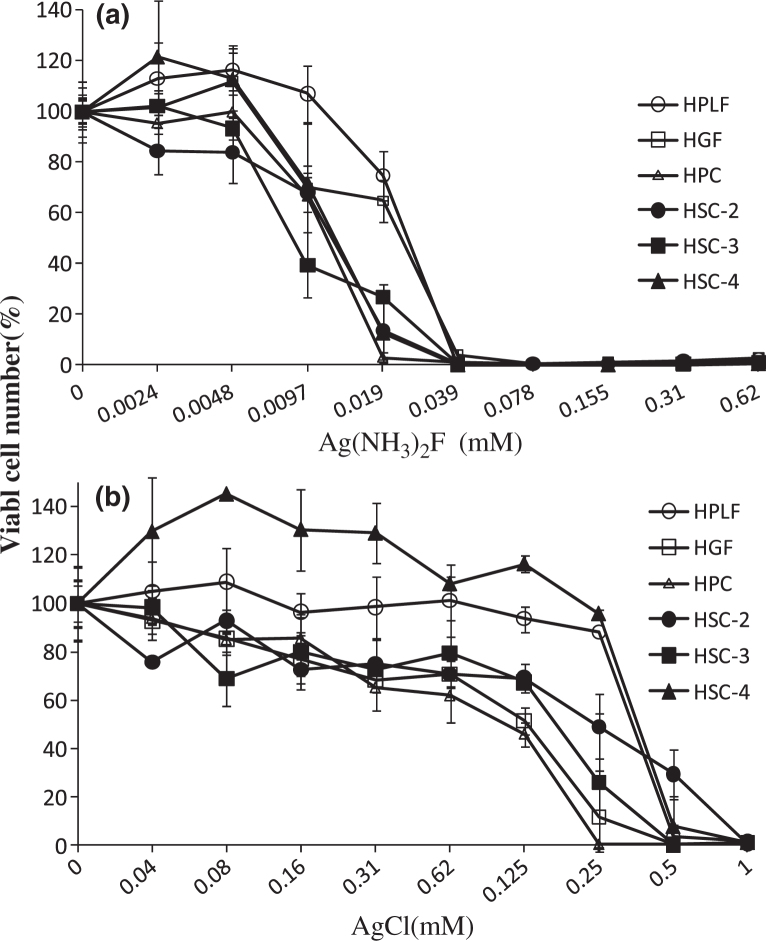
Metal ions are released from casting alloys and cause damage to cell structures and local inflammation. Ag(NH3)2F in contact with Human Gingival Fibroblast (HGF) for only one hour induced irreversible cell death, whereas longer duration of contact with AgCl was necessary to induce this same effect. These data suggest the importance of cautious application of Ag (NH3)2F into the oral cavity24. Ag(NH3)2F shows much more sensibility as a dose-dependent ion against three normal cells and three cancer line cells than AgCl (Figure 2a,b).
Figure 2.
Cytotoxicity of Ag(NH3)F2 and AgCl against six cell lines, respectively. HPLF, human periodontal ligament fibroblast cells; HGF, human gingival fibroblast cells; HPC, human pulp cells and human oral squamous cells carcinoma obtained from different patients (HSC-2, 3, 4) were incubated for 24 h with the indicated concentrations of metal and then the relative viable cell number was determined by MTT method. Each value represents the mean±S.D. of triplicate assays. Reproducible results were obtained in further two independent experiments.
Nanotoxicity is the toxicity imposed by nanomaterials25. The toxic effects of Ag NPs are proportional to the activity of free Ag+ ions released by the NPs26. Although NPs have tremendous potential for a host of applications, their adverse effects on living cells have raised serious concerns for their use in the healthcare and consumer sectors. For example, NPs may be taken up directly into the brain by trans-synaptic transport and Ag NPs can enter via the blood-brain barrier and accumulate in different regions of the brain and this may be beneficial for drug delivery, increasing a risk to the patient. It has also been reported that nanoparticle exposure can induce impairments to normal neuron-microglia microenviroment and even aggravate the process of brain pathology27.
In support of the damage notion, in vitro cell line studies have shown decreased mitochondrial function after exposure to Ag NPs in murine neuroblastoma cells28, hepatic cells29, germline stem cells30, human skin carcinoma31 and human epidermal keratinocytes and fibroblasts32, while in vivo studies showed that exposure to NPs could result in an inflammation, oxidative stress, myocardial infarction and thrombosis33. As mentioned above, NPs could alter the permeability of blood brain barrier34.
Exposure to Ag NPs has been associated with tissue damage especially in liver. In rats, a No Observable Adverse Effect Level (NOAEL) of 30 mg/kg and the Lowest Observable Adverse Effect Level (LOAEL) of 125 mg/kg has been determined for Ag NPs35.
NPs could also damage DNA causing deletions, mutations, single and double strand breakages, adduct formation, and cross linking with proteins. Some studies have confirmed DNA adducts and oxidation and induced DNA fragmentation following exposure to metal oxide NPs. In response to DNA insult, cells attempt to repair damaged DNA but repair failure may lead to cell death (apoptosis) or cell transformation. In the case of severe damage to DNA, cells may die by either necrosis or apoptosis. In this regard, it has been published previously that exposure to certain metal oxide NPs induces apoptosis36.
Corrosion and discolouration of dental materials in contact with Ag NPs may be a concern. On the other hand, antibacterial property carries with it a potential environmental risk once these NPs are discharged into the environment. Of particular concern, Ag+ ions from AgNO3 inhibit the algae’s photosynthesis around 18 times more than Ag NPs. However, over a long period, the NPs are even more toxic than the ions alone37. These environmental concerns have led to a debate among advocacy groups and governments on whether special regulation of nanotechnology is warranted.
CONCLUSION
Despite the effectiveness that Ag NPs have showed in dental practice, Ag NPs remain a controversial area of research with respect to their toxicity in biological and ecological systems38. Therefore any applications of Ag NPs in dentistry requires more study. Initially, in order to avoide the toxicity of these materials we think Ag NPs can be used for temporary periods in the dental field.
REFERENCES
- 1.Harper TE, Holister P. 2nd edn. Cientifica; London: 2003. The Nanotechnology Opportunity Report. [Google Scholar]
- 2.Roco M. Nanoscale science and engineering: unifying and transforming tools. AIChE J. 2004;50:890–897. [Google Scholar]
- 3.Auffan M, Rose J, Bottero JY, et al. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 4.Kelly TD, Matos GR. Historical statistics for mineral and material commodities in the United States. 2010. Available from: http://minerals.usgs.gov/ds/2005/140/. Accessed 31 May 2011, 18:04 PM
- 5.Maynard AD. Woodrow Wilson International Center for Scholars; Washington: 2006. Nanotechnology: A Research Strategy for Addressing Risk. Available from: http://www.tinhoahoc.com/Nanotechnology/RiskRelatedReseach_Maynard_7-06-Final.pdf. Accessed 31 May 2011, 18:04 PM. [Google Scholar]
- 6.Slenters TV, Hauser-Gerspach I, Daniels AU, et al. Silver coordination compounds as light-stable, nano-structured and anti-bacterial coatings for dental implant and restorative materials. J Mater Chem. 2008;18:5359–5362. [Google Scholar]
- 7.Jia H, Hou W, Wei L, et al. The structures and antibacterial properties of nano-SiO2 supported silver/zinc-silver materials. Dent Mater. 2008;24:244–249. doi: 10.1016/j.dental.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Pissiotis E, Spangberg L. Reaction of bony tissue to implanted silver glass ionomer and a reinforced zinc oxide-eugenol cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:623–629. doi: 10.1067/moe.2000.105173. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh FA, Barakat NA, Kanjwal MA, et al. Electrospun titanium dioxide nanofibers containing hydroxyapatite and silver nanoparticles as future implant materials. J Mater Sci Mater Med. 2010;21:2551–2559. doi: 10.1007/s10856-010-4102-9. [DOI] [PubMed] [Google Scholar]
- 10.Swift EJ., Jr In vitro caries-inhibitory properties of a silver cermet. J Dent Res. 1989;68:1088–1093. doi: 10.1177/00220345890680060601. [DOI] [PubMed] [Google Scholar]
- 11.Lansdown AB. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol. 2006;33:17–34. doi: 10.1159/000093928. [DOI] [PubMed] [Google Scholar]
- 12.Oppermann RV, Johansen JR. Effect of fluoride and non-fluoride salts of copper, silver and tin on the acidogenicity of dental plaque in vivo. Scand J Dent Res. 1980;88:476–480. doi: 10.1111/j.1600-0722.1980.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 13.Wu MY, Suryanarayanan K, van Ooij WJ, et al. Using microbial genomics to evaluate the effectiveness of silver to prevent biofilm formation. Water Sci Technol. 2007;55:413–419. doi: 10.2166/wst.2007.285. [DOI] [PubMed] [Google Scholar]
- 14.Lara HH, Ixtepan-Turrent L, Garza-Trevino EN, et al. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J Nanobiotechnology. 2010;8:15. doi: 10.1186/1477-3155-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Shrivastava S, Bera T, Singh SK, et al. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano. 2009;3:1357–1364. doi: 10.1021/nn900277t. [DOI] [PubMed] [Google Scholar]
- 17.Sotiriou GA, Pratsinis SE. Antibacterial activity of nanosilver ions and particles. Environ Sci Technol. 2010;44:5649–5654. doi: 10.1021/es101072s. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri J, Gandolfi MG. Evaluation of the radiopacity of calcium silicate cements containing different radiopacifiers. Int Endod J. 2010;43:21–30. doi: 10.1111/j.1365-2591.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. 2011. Available from: http://www.who.int/mediacentre/factsheets/fs318/en/index.html. Accessed 31 May 2011, 18:04 PM
- 20.de la Fuente-Hernández J, González de Cosío M, Ortega-Maldonado M, et al. [Dental decay and tooth loss at the high school level in Mexican students] Salud Publica Mex. 2008;50:235–240. [PubMed] [Google Scholar]
- 21.Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: a caries ‘silver-fluoride bullet’. J Dent Res. 2009;88:116–125. doi: 10.1177/0022034508329406. [DOI] [PubMed] [Google Scholar]
- 22.Ahn SJ, Lee SJ, Kook JK, et al. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 2009;25:206–213. doi: 10.1016/j.dental.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Jiang W, Kim BY, Rutka JT, et al. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 24.Contreras RG, Sakagami H, Nakajima H, et al. Type of cell death induced by various metal cations in cultured human gingival fibroblasts. In Vivo. 2010;24:513–517. [PubMed] [Google Scholar]
- 25.Fadeel B, Garcia-Bennett AE. Better safe than sorry: understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv Drug Deliv Rev. 2010;62:362–374. doi: 10.1016/j.addr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Park EJ, Yi J, Kim Y, et al. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol In Vitro. 2010;24:872–878. doi: 10.1016/j.tiv.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Liu ZW, Allaker RP, et al. A review of nanoparticle functionality and toxicity on the central nervous system. J R Soc Interface. 2010;7(Suppl. 4):S411–S422. doi: 10.1098/rsif.2010.0158.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrand AM, Braydich-Stolle LK, Schlager JJ, et al. Can silver nanoparticles be useful as potential biological labels? Nanotechnol. 2008;19:104–119. doi: 10.1088/0957-4484/19/23/235104. [DOI] [PubMed] [Google Scholar]
- 29.Hussain SM, Hess KL, Gearhart JM, et al. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19:975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 30.Braydich-Stolle L, Hussain S, Schlager JJ, et al. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci. 2005;88:412–419. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora S, Jain J, Rajwade JM, et al. Cellular responses induced by silver nanoparticles: in vitro studies. Toxicol Lett. 2008;179:93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Burd A, Kwok CH, Hung SC, et al. A comparative study of the cytotoxicity of silver-based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen. 2007;15:94–104. doi: 10.1111/j.1524-475X.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 33.Kumar CSSR. Wiley-VCH; LA, USA: 2006. Nanomaterials: toxicity, health and environmental issues; p. 96. 306–308. [Google Scholar]
- 34.Zhao Y, Nalwa HS. American Scientific Publishers; Stewenson Ranch, USA: 2006. Nanotoxicology. [Google Scholar]
- 35.Kim YS, Song MY, Park JD, et al. Subchronic oral toxicity of silver nanoparticles. Part Fibre Toxicol. 2010;7:20. doi: 10.1186/1743-8977-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang YW, Wu C, Aronstam RS. Toxicity of Transition Metal Oxide Nanoparticles: recent Insights from in vitro Studies. Materials. 2010;3:4842–4859. doi: 10.3390/ma3104842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarro E, Piccapietra F, Wagner B, et al. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol. 2008;42:8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- 38.Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89:1175–1186. doi: 10.1177/0022034510377794. [DOI] [PubMed] [Google Scholar]
- 39.Sadhasivam S, Shanmugam P, Yun K. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B Biointerfaces. 2010;81:358–362. doi: 10.1016/j.colsurfb.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 40.Speshock JL, Murdock RC, Braydich-Stolle LK, et al. Interaction of silver nanoparticles with Tacaribe virus. J Nanobiotechnology. 2010;8:19. doi: 10.1186/1477-3155-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De GB, Sintubin L, Baert L, et al. Biogenic silver for disinfection of water contaminated with viruses. Appl Environ Microbiol. 2010;76:1082–1087. doi: 10.1128/AEM.02433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Sun RW, Chen R, et al. Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther. 2008;13:253–262. [PubMed] [Google Scholar]