Abstract
Aim: To evaluate the ability of two experimental toothpastes containing 0.1%w/w o-cymen-5-ol, 0.6%w/w ZnCl2 and 0.320%w/w NaF to reduce demineralisation of sound human enamel compared with control toothpastes. Methods: Study 1: Specimens were treated with toothpaste slurries, followed by alternating periods in demineralising and neutral solutions. Demineralisation was assessed using surface microhardness (SMH). Study 2: Specimens were subjected to a 14 day cycling regime of alternating demineralisation / remineralisation with two toothpaste treatments per day, before and after demineralisation. Demineralisation was assessed by cross-sectional microhardness and mineral loss (ΔZ) was calculated. Test toothpastes were a) 0%w/w or 0.002%w/w NaF placebo, b) 0.055%w/w or 0.149%w/w NaF (dose response), c) 0.320%w/w NaF marketed product, d & e) 0.1%w/w o-cymen-5-ol, 0.6%w/w ZnCl2 and 0.320%w/w NaF (experimental toothpastes). Results: Study 1: Mean ± SE % of baseline hardness values were a) 48.0 ± 2.1a, b) 66.7 ± 1.7b, c) 82.9 ± 1.9c, d) 91.7 ± 1.4d and e) 94.6 ± 2.1d. Study 2: Mean ± SE ΔZ values were a) 2114 ± 187a, b) 1206 ± 132b, c) 303 ± 89c, d) 19 ± 73c, and e) −10 ± 55c. Letters represent different statistical groupings (P < 0.05). Conclusion: In study 1, both experimental toothpastes were statistically superior to the marketed product and in study 2; they were at least as effective as the marketed product at reducing caries lesion development.
Key words: Demineralisation, Zinc
INTRODUCTION
Fluoride (F) is a well established anti-caries agent that works by protecting teeth against demineralisation and enhancing remineralisation.1 Additionally, it has been shown in vitro to interfere with the metabolism of plaque bacteria and so may help inhibit plaque acid production.2 Therefore, when incorporating other ingredients into oral care products it is important to ensure that the anti-caries efficacy of that product is not compromised.
Zinc is frequently incorporated into oral care products, especially toothpastes and mouthwashes to inhibit plaque growth3 and reduce malodour.4 In addition, zinc is used as an anti-calculus agent as it can inhibit the crystal growth of different calcium phosphate species often found in calculus, for example brushite, octacalcium phosphate and carbonated hydroxyapatite.5
A number of in vitro de- and remineralisation studies have shown that the addition of zinc citrate did not affect the anti-caries efficacy of either sodium monofluorophosphate (SMFP) or sodium fluoride (NaF) containing toothpastes.6., 7. While some authors have reported that in vitro enamel fluoride uptake (EFU) into incipient caries lesions was enhanced in the presence of the zinc,6 others have reported either no effect, or a negative effect.8 However, in a rat caries study, a zinc citrate / SMFP toothpaste gave identical levels of caries reduction and improved EFU compared to an SMFP only control.9 A caries clinical trial in children which investigated the effect of using toothpastes containing 1,000, 1,500 and 2,500 ppm F (as SMFP) with and without zinc citrate found that the ability of fluoride to reduce caries incidence was unaffected by zinc.10
Given the potential of zinc to affect both de- and remineralisation, this apparent lack of a consistent effect on caries would seem contradictory. Therefore the aim of this study was to evaluate the ability of two experimental fluoride (as NaF) toothpaste formulations containing 0.1%w/w o-cymen-5-ol and 0.6%w/w ZnCl2 to reduce caries lesion development in sound human enamel compared with a commercially available NaF toothpaste. Low fluoride and non-fluoride control formulations were also tested to ensure the validity of the in vitro models.
MATERIALS AND METHODS
Two experimental toothpastes (ET1 and ET2) contained 0.1%w/w o-cymen-5-ol, 0.6% w/w zinc chloride and 0.320% w/w NaF (equivalent to 1,450 ppm F). Both experimental formulations were the same except that ET2 contained high cleaning silicas. The NaF control was a 0.320% w/w NaF (equivalent to 1,450 ppm F) toothpaste (Study 1: Aquafresh Fresh & Minty®, GlaxoSmithKline, UK; Study 2: Aquafresh Mild & Minty®, GlaxoSmithKline, UK). For both studies, the fluoride free placebo was essentially the same formulation as the NaF control except it contained either no fluoride or 0.002% w/w NaF (equivalent to 10ppm F) and either a 0.055% w/w NaF (equivalent to 250ppm F) or 0.149% w/w NaF (equivalent to 675ppm F) toothpaste was used as a matched dose response control. All toothpastes used silica as an abrasive.
STUDY 1: DEMINERALISATION STUDY
The model used was a modification of the one previously reported by Laucello et al.7
Specimen preparation
Caries-free human molars free from discernible cracks or surface imperfections were used in this study. Specimens were cut into 2 × 2 mm sections and mounted in acrylic resin. The topsides of the specimens were ground using 400-grit silicon carbide wet/dry grinding paper until most of the tooth surface was flattened and then serially polished using 1200, 2500-grit papers (Buehler) followed by 1 μm diamond polishing suspension.
The baseline hardness of the sound enamel specimens was determined using a Knoop diamond indenter. The hardness of each specimen was determined from the average of 10 individual measurements made centrally on each specimen using 100 g weight and a dwell time of 20 seconds. Only specimens with a Knoop hardness number (KHN) ranging from 300 to 350 kgf mm−2 were accepted into the study. Specimens were divided randomly into five treatment groups (n = 10) per group.
pH cycling regime
Specimens were subjected to the following pH cycling regime:
Step 1: Five minute treatment with the toothpaste slurries (one part by weight toothpaste : three parts by weight of deionised water).
Step 2: Specimens were rinsed thoroughly with deionised water and placed for 30 minutes in a demineralisation solution containing 50 mM acetic acid, 1.5 mM calcium chloride dihydrate, 0.9 mM potassium dihydrogen orthophosphate, 130 mM potassium chloride, pH adjusted to 5.0 using KOH. The demineralisation solution was replaced each cycle.
Step 3: Specimens were removed from demineralising solution, rinsed in deionised water, and immersed for 5 minutes in a neutral solution containing 20 mM HEPES, 1.5 mM calcium chloride dihydrate, 0.9 mM potassium dihydrogen orthophosphate, 130 mM potassium chloride, pH adjusted to 7.0 using KOH. This solution was replaced for each cycle.
Step 4: Steps 2 and 3 were repeated a further 11 times.
At the end of the cycling period, the average Knoop hardness numbers (KHN) for each specimen were determined as described above. This data were used to calculate the % of baseline specimen hardness for each treatment group using Equation 1:
STUDY 2: DEMINERALISATION/REMINERALISATION STUDY
The model used was a modification of the pH cycling model described by Featherstone et al.11
Specimen preparation
Caries-free human teeth (erupted third molars, molars and pre-molars) free from discernible cracks or surface imperfections were used in this study. The roots were removed and the enamel surface was lightly abraded with 600 grit, flat silicon carbide wet/dry grinding paper (Buehler), following the shape of the tooth for 30 seconds to remove any surface debris or stain. The entire enamel surface except for one area measuring approximately 4 × 5 mm on a flat, clean surface of the enamel was covered with an acid resistant nail polish (Cover Girl Red Revolution). The specimens were randomly divided into five treatment groups (n = 20 per group).
pH cycling regime
Specimens were subjected to the following pH cycling regime which was repeated daily on consecutive days for a total of 14 days interrupted by two weekends when specimens were stored at 37 °C in remineralisation solution (total study duration was nearly three weeks).
The detailed cycling procedure was as follows:
Step 1: One minute treatment with toothpaste slurries (one part by weight toothpaste: three parts by weight of water); approximately 4 ml per tooth specimen.
Step 2: Specimens were rinsed thoroughly with deionised water and placed for 6 hours in a demineralisation solution at 37 °C containing 2.0 mM dibasic calcium phosphate, 75 mM acetic acid and adjusted to pH 4.5 with NaOH (40 ml per tooth). The demineralisation solution was reused for 2–3 days of treatment. Fresh solutions were prepared at the beginning of each 5 days of treatment.
Step 3: Specimens were removed from demineralising solution, rinsed in deionised water, and immersed for 1 minute in the toothpaste slurry.
Step 4: After treatment, specimens were rinsed thoroughly with deionised water and placed for 18 h in a remineralisation solution at 37 °C containing 1.5 mM calcium nitrate tetrahydrate, 0.9 mM dibasic potassium phosphate, 150 mM potassium chloride 20 mM cacodylate buffer, adjusted to pH 7.0 with HCl (20 ml per tooth). The remineralisation solution was replaced every 2 days.
After 14 days of pH cycling, specimens were sectioned longitudinally through the lesion and mounted in a cold set acrylic resin which covered all surfaces except the cut face. The cut faces were polished with 600 grit silicon carbide paper and then serially polished with a 9, 3, and 1 μm lapping film. Cross-sectional microhardness was performed at 12.5, 25, 37.5, 50, 62.5, 75 and 87.5 μm below the surface (into the depth of the lesion) using a Knoop diamond with 10 g of weight and a dwell time of 15 seconds. Additional indents were made at 100, 150, 200, 250, and 300 μm below the surface using a 50 g weight and a dwell time of 15 seconds. These procedures were a modification of those described by Featherstone et al.12 and ten Cate et al.13.
The relationship between Knoop Hardness Number (KHN) and volume percent mineral has been previously described.12 All volume percent mineral values collected were normalised using the underlying sound enamel (the volume percent mineral values at 150, 200, 250, and 300 μm were used to calculate an average volume percent mineral for sound enamel). Each volume percent mineral value for each series of indents per specimen was normalised, either up or down, so that the average of 150 through 300 μm was 85%. After normalisation, the area under the curve was determined using the trapezoid method which uses the average volume percent mineral value of two adjacent indents and the distance between the indents. The area under the curve was subtracted from the sound enamel value to achieve a mineral loss compared to sound enamel, or ΔZ value (units: vol% x μm) for each series of indents on each specimen. A comparison of the ΔZ values between treatment groups was used to assess the ability of the treatment to inhibit caries lesion development.
Statistical analysis
Statistical analyses were conducted using an analysis of variance model (Sigma Stat software, version 3.1 and OriginPro, version 8.1 SR1). Where significant differences were found, additional pair-wise comparisons were performed using a Tukeys HSD test (P < 0.05).
RESULTS
Study 1: Demineralisation Study
The results for the demineralisation study are shown in Table 1. All fluoride containing treatments were able to significantly reduce demineralisation compared to the fluoride free placebo control. A statistically significant fluoride dose response was observed within this study which demonstrates the validity of this model. In addition, both experimental toothpastes provided statistically superior protection against enamel demineralisation compared to the marketed toothpaste. The order of protection was as follows: ET 1 = ET 2 > NaF control > dose response > placebo.
Table 1.
Knoop hardness data for enamel specimens (n = 10). Letter superscripts represent the different statistical groupings
| Treatment Toothpaste | Hardness | % of Baseline Hardness | |
|---|---|---|---|
| Baseline KNH | Post-cycling KHN | ||
| 0 ppm F (placebo) | 331.7 (4.7) | 159.2 (7.2) | 48.0 (2.1)a |
| 675 ppm F (Dose Response) | 330.6 (4.7) | 221.0 (8.0) | 66.7 (1.7)b |
| 1,450 ppm F (NaF Control) | 328.4 (4.8) | 272.6 (9.0) | 82.9 (1.9)c |
| 0.1%w/w o-cymen-5-ol, 0.6%w/w ZnCl2, 1,450 ppm F (Experimental Toothpaste 1 (ET1)) | 329.6 (4.7) | 312.1 (7.5) | 94.6 (1.4)d |
| 0.1%w/w o-cymen-5-ol, 0.6%w/w ZnCl2, 1,450 ppm F (Experimental Toothpaste 2 (ET2)) | 332.8 (5.0) | 305.4 (8.9) | 91.7 (2.1)d |
Note: Standard error in brackets
Study 2: Demineralisation / Remineralisation Study
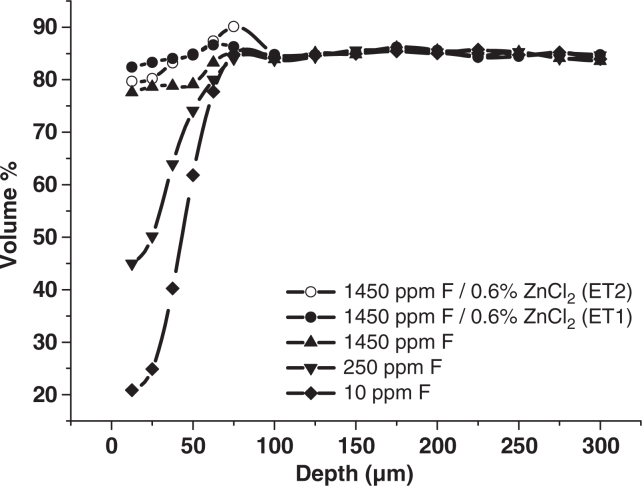
The results of the demineralisation / remineralisation study are shown in Table 2. The data show that all treatments were better at promoting resistance to caries lesion development compared to the fluoride-free placebo. A statistically significant fluoride dose response was observed within this study. In addition, the two experimental toothpastes containing 0.1%w/w o-cymen-5-ol, 0.6%w/w ZnCl2 and 1,450ppm F were directionally superior to the marketed toothpaste. The mineral profiles in Figure 1 show that some hypermineralisation had occurred in the deeper parts of the lesions in the groups treated with the two experimental toothpastes, however, it was more prominent in the ET2 group.
Table 2.
ΔZ values calculated from cross-sectional microhardness data (n = 20). The letter superscripts represent the different statistical groupings
| Treatment Toothpaste | ΔZ (vol% x μm) |
|---|---|
| 10 ppm F (placebo) | 2114 (187)a |
| 675 ppm F (Dose Response) | 1206 (132)b |
| 1,450 ppm F (NaF Control) | 303 (89)c |
| 0.1%w/w o-cymen-5-ol, 0.6%w/w ZnCl2, 1,450 ppm F (ET1) | 19 (73)c |
| 0.1%w/w o-cymen-5-ol, 0.6%w/w ZnCl2, 1,450 ppm F (ET2) | −10 (55)c |
Note: Standard error in brackets
Figure 1.
Average volume % mineral profiles for each treatment group (n = 20).
DISCUSSION
Zinc and other metal cations have been reported to reduce enamel solubility.14., 15., 16. However, they can also modify the crystal-growth of calcium phosphate species implicated in remineralisation. This means that they have the potential to influence the dynamic balance between de- and remineralisation in the mouth. In study 1, the reduction in demineralisation observed when specimens were treated with the zinc-containing toothpastes confirms the ability of zinc to reduce enamel demineralisation. However, this zinc effect was not observed in a similar pH-cycling study.7 Whilst formulation differences may account for this observation, in study 1, the acidic solutions were refreshed before each cycle, possibly maintaining a higher degree of undersaturation with respect to enamel than would have been the case if the same solutions had been used throughout the cycling phase. Enamel dissolution products would have gradually reduced the degree of undersaturation as cycling progressed, making treatment effects harder to discern. Further, a single application of toothpaste was employed, rather than multiple applications, again mitigating in favour of demineralisation.
Although still optimised for the study of net demineralisation, the cycling regime in study 2 was intended to simulate more closely the de- and remineralising events experienced in the oral cavity and hence the enamel specimens spent a substantial proportion of the cycling phase in a remineralising solution. This model has been previously shown to reproduce results found in vivo during one month of demineralisation around orthodontic appliances.17
In study 2, no significant benefit was seen for the toothpastes containing both zinc and fluoride, compared with the fluoride-only control toothpaste. The difference from study 1 may lie in the fact that the enamel specimens were not abraded and this would likely have made them less prone to demineralisation. Outer enamel acquires relatively large amounts of fluoride during post-eruptive maturation,18 and becomes considerably less porous than underlying enamel.19., 20..
The use of cross-sectional microhardness allowed mineral distribution, as well as net mineral loss, to be measured, and hypermineralisation was observed in the deeper parts of the enamel specimens treated with the zinc containing experimental toothpastes. A similar effect was reported for pre-formed enamel lesions21 where, under relatively constant-composition remineralising conditions, substantial lesion-body remineralisation was observed in the presence of zinc and fluoride, whereas lesions in the presence of fluoride alone apparently arrested. The authors proposed that zinc, acting as a crystal-growth inhibitor, retarded fluoride-induced lesion arrest. It has recently been reported that lesions remineralised in the presence of zinc do so preferentially in the deeper regions,22 and in the current study, extended maintenance of porosity may have allowed the lesions which formed during the cycling phase to remineralise more fully, leading to the observed hypermineralisation.
A number of plausible explanations for the lack of an overall effect of zinc on the ability of fluoride to reduce caries have been proposed. For instance, it has been reported that concentrations of calcium similar to those in saliva displaced pre-adsorbed zinc from hydroxyapatite.23 Additionally, not all of the crystal growth sites are likely to be affected by zinc; therefore, in the presence of fluoride, overgrowth of the inhibitor covered regions of the crystals may occur.6 As discussed above, zinc has the potential to delay fluoride induced arrest and therefore maintain surface zone porosity to facilitate greater lesion body remineralisation.21
Whilst the mechanisms of zinc induced reductions in enamel solubility have received little attention, possible explanations can be deduced from work with other metal species and from the non-dental literature. Dedhiya et al.15 have reported that HA dissolution rates in the presence of strontium was governed by a calcium-strontium apatite complex at the hydroxyapatite surface of the formula Ca6 Sr4 (PO4)6 (OH)2 which controlled the dynamic conditions at the crystal-solvent interface. Studies on the use of copper for inhibiting HA dissolution have suggested that precipitation of an acid-insoluble copper phosphate protective layer on the enamel surface is a possible mechanism.16 With reference to zinc, it has been reported that Hopeite (Zn3(PO4)2.4H2O) precipitation can occur,24 or alternatively, that zinc adsorbed onto apatite may prevent the formation of ‘dissolution nuclei’ on the surface of HA.25 Given that low concentrations of zinc can both modify or inhibit remineralisation, but can also reduce dissolution markedly, the lack of an apparent effect is intriguing and further work should lead to greater understanding of the possible role of zinc in caries.
CONCLUSION
Two experimental toothpastes containing 0.1%w/w o-cymen-5-ol, 0.6%w/w ZnCl2 and 1,450 ppm F have been evaluated for their ability to reduce early caries lesion development. Our findings show that in study 1, both experimental toothpastes were statistically superior to a marketed toothpaste containing 1,450 ppm F; in study 2 they were at least as effective as the marketed toothpaste.
CONFLICT OF INTEREST AND SOURCE OF FUNDING
The work described in this manuscript was funded by GlaxoSmithKline Consumer Healthcare. The authors external to GSK received no additional fee for the preparation of this manuscript. The remaining authors are employed by GSK but confirm no potential conflicts of interest.
REFERENCES
- 1.Marinho VC, Higgins JP, Sheiham A, et al. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2003;(1):CD002278. doi: 10.1002/14651858.CD002278. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marquis RE, Clock SA, Mota-Meira M. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev. 2003;26:493–510. doi: 10.1111/j.1574-6976.2003.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 3.Saxton CA, Harrap GJ, Lloyd AM. The effect of dentifrices containing zinc citrate on plaque growth and oral zinc levels. J Clin Periodontol. 1986;13:301–306. doi: 10.1111/j.1600-051x.1986.tb02226.x. [DOI] [PubMed] [Google Scholar]
- 4.Jonski G, Young A, Waler SM, et al. Insoluble zinc, cupric and tin pyrophosphates inhibit the formation of volatile sulphur compounds. Eur J Oral Sci. 2004;112:429–432. doi: 10.1111/j.1600-0722.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 5.LeGeros RZ, Bleiwas CB, Retino M, et al. Zinc effect on the invitro formation of calcium phosphates: Relevance to clinical inhibition of calculus formation. Am J Den. 1999;12:65–71. [PubMed] [Google Scholar]
- 6.ten Cate JM. The caries preventative effect of a fluoride dentifrice containing Triclosan and zinc citrate, a compilation of in vitro and in situ studies. Int Dent J. 1993;43:401–413. [PubMed] [Google Scholar]
- 7.Laucello M, Noel N, Ferro R, et al. The anti-caries efficacy of a silica-based fluoride toothpaste containing zinc citrate, triclosan, vitamin E and sunflower oil. Int Dent J. 2007;57:145–149. [Google Scholar]
- 8.Mellberg JR, Chomicki WG. Effect of zinc citrate on fluoride uptake by artificial caries lesions. J Dent Res. 1983;62:145–147. doi: 10.1177/00220345830620021201. [DOI] [PubMed] [Google Scholar]
- 9.Ingram GS, Baker AG, Best JS, et al. The influence of zinc citrate in a fluoride dentifrice on rat caries and fluoride content of molar enamel in vivo. J Dent Res. 1984;63:497. [Google Scholar]
- 10.Stephen KW, Creanor SL, Russell JI, et al. A 3-year oral health dose-response study of sodium monofluorophosphate dentifrices with and without zinc citrate: anti-caries results. Community Dent Oral Epidemiol. 1988;16:321–325. doi: 10.1111/j.1600-0528.1988.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 11.Featherstone JDB, O’Reilly MM, Shariati M, et al. In: Factors Relating to Demineralisation and Remineralisation of the Teeth. Leach SA, editor. IRL Press Ltd; Oxford: 1986. Enhancement of remineralization in vitro and in vivo; pp. 23–34. [Google Scholar]
- 12.Featherstone JDB, ten Cate JM, Shariati M, et al. Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res. 1983;17:385–391. doi: 10.1159/000260692. [DOI] [PubMed] [Google Scholar]
- 13.ten Cate JM, Shariati M, Featherstone JDB. Enhancement of salivary remineralization by ‘dipping’ solutions. Caries Res. 1985;19:335–341. doi: 10.1159/000260864. [DOI] [PubMed] [Google Scholar]
- 14.Brudevold FL, Steadman T, Spinelli MA, et al. A study of zinc in human teeth. Arch Oral Biol. 1963;8:135–144. doi: 10.1016/0003-9969(63)90051-7. [DOI] [PubMed] [Google Scholar]
- 15.Dedhiya MG, Young F, Higuchi WI. Mechanism for the retardation of the acid dissolution rate of hydroxyapatite by strontium. J Dent Res. 1973;52:1097–1109. doi: 10.1177/00220345730520051901. [DOI] [PubMed] [Google Scholar]
- 16.Brookes SJ, Shore RC, Robinson C, et al. Copper ions inhibit the demineralisation of human enamel. Arch Oral Biol. 2003;48:25–30. doi: 10.1016/s0003-9969(02)00162-0. [DOI] [PubMed] [Google Scholar]
- 17.O’Reilly MM, Featherstone JDB. De- and remineralisation around orthodontic appliances: an in vivo study. Am J Orthod. 1987;92:33. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 18.Fejerskov O, Nyvad B, Kidd EAM. In: Dental caries: the disease and its clinical management. 2nd ed. Fejerskov O, Kidd EAM, editors. Blackwell Munksgaard; Oxford: 2008. Pathology in Dental Caries; pp. 19–48. [Google Scholar]
- 19.Imanishi H, Nishino M. Post eruptive maturation of immature young permanent enamel. J Int Ass Dent Child. 1983;14:49–54. [PubMed] [Google Scholar]
- 20.Fejerskov O, Josephsen K, Nyvad B. Surface ultrastructure of unerupted mature human enamel. Caries Res. 1984;18:302–314. doi: 10.1159/000260781. [DOI] [PubMed] [Google Scholar]
- 21.Lynch RJM, Churchley DR, Cooper L, et al. Effect of zinc and fluoride on the remineralisation of early carious lesions under simulated plaque-fluid conditions. Caries Res. 2011;45:313–322. doi: 10.1159/000324804. in press. [DOI] [PubMed] [Google Scholar]
- 22.Lippert F, Zero DR. Dose-response effects of zinc and fluoride on caries lesion remineralisation. IADR 2011 Abstract 1548 [DOI] [PubMed]
- 23.Ingram GS, Horay CP, Stead WJ. Interaction of zinc with dental mineral. Caries Res. 1992;24:248–255. doi: 10.1159/000261447. [DOI] [PubMed] [Google Scholar]
- 24.Dybowska A, Manning DAC, Collins MJ, et al. An evaluation of the reactivity of synthetic and natural apatites in the presence of aqueous metals. Sci Total Natural Environ. 2009;407:2953–2965. doi: 10.1016/j.scitotenv.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 25.Lingawi H, Barbour M, Lynch RJM, et al. Effect of zinc ions (Zn+2) on hydroxyapatite dissolution kinetics studied using scanning microradiography. Caries Res. 2011;45:195. [Google Scholar]