Abstract
Objectives: To assess the ability of 0.1%w/w o-cymen-5-ol/ 0.6%w/w zinc chloride gel to foam dentifrice to maintain gingival health compared to a sodium fluoride control dentifrice. Design: Following a baseline examination, subjects went through a regimen to bring them to a high level of gingival health. This involved a professional dental prophylaxis supported by oral hygiene instruction prior to commencing study treatment. Subjects brushed twice daily for 12 weeks with either the test or control dentifrice. Examinations for gingival inflammation (MGI), bleeding and plaque were performed after 12 weeks. Results: 205 subjects were included in the efficacy analysis. Relative to the sodium fluoride/ silica control dentifrice group the o-cymen-5-ol/ zinc chloride gel to foam dentifrice exhibited statistically significant reductions (p < 0.0001) in MGI, bleeding and plaque of 32.2%, 26.3% and 20.7% respectively after 12 weeks. Conclusion: The results of the present clinical study demonstrate that the use of the 0.1%w/w o-cymen-5-ol/ 0.6%w/w zinc chloride gel to foam dentifrice over a 12 week period provides a statistically significant benefit in maintaining gingival health compared to a sodium fluoride control dentifrice.
Key words: Gingival health, gel to foam dentifrice, zinc chloride
INTRODUCTION
This clinical study is a second clinical investigation to assess the ability of a dentifrice containing 0.1%w/w o-cymen-5-ol and 0.6%w/w zinc chloride to maintain gingival health following a 14 day pre-experimental phase of dental prophylaxis and oral hygiene instruction prior to study treatment compared to a regular sodium fluoride/ silica based dentifrice. The first clinical study1 evaluated a paste dentifrice containing 0.1%w/w o-cymen-5-ol and 0.6%w/w zinc chloride, while this clinical investigation evaluates a similar formulation in a gel to foam format. The gel to foam format contains an elevated surfactant level and generates greater foam volume compared to the control dentifrice through evaporation of the post-foaming agent, isopentane. In addition, the ability of the test gel to foam dentifrice to control dental plaque was assessed. The unique combination of o-cymen-5-ol and zinc chloride has resulted in a spectrum of antimicrobial activity greater than for each of the ingredients alone2. The product was used twice daily and assessments of gingival inflammation, bleeding and plaque were conducted after 12 weeks.
MATERIALS AND METHODS
Study design
This was a single centre, examiner blind, two arm, parallel group, randomised and stratified clinical study in healthy adult volunteers conducted at Global Health Research Group, New Delhi, India to assess the ability of a gel to foam dentifrice containing 0.1%w/w o-cymen-5-ol and 0.6%w/w zinc chloride to maintain gingival health over a 12 week period compared to a control sodium fluoride/ silica dentifrice. The study protocol and consent form were reviewed and approved by the Institutional Ethics Committee for Global Health Research Group. After providing written informed consent, 322 healthy adult subjects between the ages of 18–65 years were screened in August 2010, with 234 subjects randomised to study treatment. The study completed in December 2010. Subjects were enrolled into the study if they had:
-
•
Good oral health with at least 20 natural teeth and had mild to moderate gingivitis at baseline (mean whole mouth Modified Gingival Index (MGI) score 1.50–2.50). Additionally they would have to demonstrate a reduction in MGI and a reduction or no change in bleeding during the pre-experimental phase.
Subjects were excluded from the study if they:
-
•
Were pregnant or breast feeding or were smokers.
-
•
Had dental conditions requiring immediate treatment, sensitivity to oral care products, active caries, severe periodontitis or gingivitis, partial dentures, orthodontic appliances or fixed retainers or restorations in a poor state of repair.
Subjects were also screened for the use of any systemic medications which would have an effect on gingival conditions (e.g. antibiotics, calcium channel blockers, ibuprofen or aspirin therapy) within 14 days of any gingivitis assessment.
The study was conducted in two phases: a 14 day pre-experimental phase and a 12 week experimental period with a total duration of 14 weeks. Following an initial screening visit, at the start of the pre-experimental phase (Pre-Prophy Baseline visit) subjects underwent oral soft tissue (OST), gingival inflammation using the Modified Gingival Index (MGI)3, bleeding using the Bleeding Index (BI)4 and plaque using the six site Turesky Modification of the Quigley Hein Index (TPI)5 examinations (having abstained from brushing for a 12 hour period prior to the visit). To obtain optimum gingival health, subjects received a thorough professional dental prophylaxis. Afterwards, subjects received thorough professional instruction in oral hygiene which included the use of a medium bristled manual toothbrush (Aquafresh® Clean Control®) (Aquafresh and Clean Control are registered trademarks of GlaxoSmithKline group of companies) with a sodium fluoride/ silica toothpaste (Colgate® Herbal, Indian marketed product) (Colgate Herbal is a registered trademark of Colgate-Palmolive). They were instructed to use these products brushing twice daily at home for the next 14 days. Written instructions, a timer and a diary card to record brushing occasions were provided. Subjects returned to the site after seven days (two hours since their last brushing) for an additional visit at which they received an OST assessment followed by brushing under supervision and subsequent plaque disclosure. A qualified dental professional highlighted areas missed during brushing and reviewed the dental hygiene brushing instructions and removed residual plaque.
Subjects returned to site 14 days after the Pre-Prophylaxis Baseline visit for the randomisation visit (after abstaining from brushing for 12 hours) and underwent OST, MGI, BI and TPI assessments. Only subjects who demonstrated a decrease in MGI and a decrease or no change in bleeding over the two week period since the Pre-Prophy Baseline visit were entered into the experimental phase of the study. Subjects were stratified according to their MGI score from the Pre-Prophy Baseline Visit and randomly assigned either the gel to foam dentifrice containing 0.1%w/w o-cymen-5-ol and 0.6%w/w zinc chloride in a 0.204%w/w sodium fluoride/ silica base or a 0.204%w/w sodium fluoride/ silica base dentifrice. Subjects underwent a professional dental polishing with their assigned treatment and entered into the experimental phase. Subjects were instructed to brush twice daily (Aquafresh Clean Control flat trim toothbrush) at home for one timed minute with their assigned treatment using a strip of toothpaste that covered the head of the study toothbrush. Treatment compliance was assessed from diaries that the subjects completed each time they brushed at home throughout the study.
At six weeks subjects returned to the site for an OST examination and re-supply of study products. At 12 weeks after randomization subjects returned with overnight plaque and underwent OST, MGI, BI and TPI assessments.
Randomisation and blinding procedure
The randomisation schedule was generated using a computerised randomisation generator and provided to the site by the Biostatistics Department of the sponsor. Subjects were randomly assigned to one of two treatment groups according to the method of permuted blocks using a fixed block size of four. Subjects were stratified according to their Pre-Prophy Baseline MGI score into one of two strata: Low MGI (1.50–<2.00) and High MGI (≥2.00). Randomisation numbers were assigned within each strata chronologically as subjects were randomised to treatment. Study staff dispensing the treatment were blinded to the treatment identities. The examiners and the study statistician, data management staff and other employees of the Sponsor who may influence study outcomes were blinded to the treatment allocation of subjects. The study treatments were provided with white study labels detailing the treatment code and instructions for use however, as the gel to foam dentifrice is supplied in an aerosol can and the control toothpaste is supplied in a tube it was not possible to conceal product identity from the subject. Subjects were re-supplied with study treatment and a new toothbrush at the six week visit.
Modified Gingival Index
The MGI3 is a non-invasive evaluation of early visual changes in severity and extent of gingivitis. The MGI was assessed by a single examiner grading facial and lingual surfaces at two sites on each tooth (papillae and margin) using standard dental illumination under office conditions. The MGI scoring system was as follows:
0 = absence of inflammation
1 = mild inflammation; slight change in colour, little change in colour; little change in texture of any portion of the marginal or papillary gingival unit
2 = mild inflammation; criteria as above but involving the entire marginal or papillar gingival unit
3 = moderate inflammation; glazing, redness, oedema, and/or hypertrophy of the marginal or papillary gingival unit
4 = severe inflammation; marked redness, oedema and/or hypertrophy of the marginal or papillary gingival unit, spontaneous bleeding, congestion, or ulceration.
Bleeding Index
The Bleeding Index (modified Index of Saxton4) was performed by a single examiner using a colour coded periodontal probe. The probe was engaged approximately 1 millimetre (mm) into the gingival crevice. A moderate pressure was used whilst sweeping along the sulcular epithelium gingiva.
The BI scoring system was as follows:
0 = no bleeding after 30 seconds
1 = bleeding upon probing after 30 seconds
2 = immediate bleeding observed
Dental plaque assessment
A single dental examiner used a six-site modification of the Turesky Modification of the Quigley Hein Index5 to assess plaque on all gradable teeth. Only natural teeth (excluding third molars) were assessed.
The plaque was disclosed using 5 ml of a dye solution (Red Cote®) (Red Cote is a registered trademark of Butler) for 10 seconds followed by rinsing with 10 ml of water for 10 seconds. Each tooth was divided into six areas: mesiofacial, facial, distofacial, mesiolingual, lingual and distolingual surfaces.
Disclosed plaque was scored as follows:
Score Description
-
0
No plaque
-
1
Slight flecks of plaque at the cervical margin of the tooth
-
2
A thin continuous band of plaque (1 mm or smaller) at the cervical margin of the tooth
-
3
A band of plaque wider than 1 mm but covering less than 1/3 of the area
-
4
Plaque covering at least 1/3 but less than 2/3 of the area
-
5
Plaque covering 2/3 or more of the crown of the tooth.
Statistical methods
To detect a between treatment difference in MGI of 0.09 (SD = 0.19), with a two-sided 5% significance level and a power of 90%, a sample size of 90 subjects per group was required. To allow for withdrawals from the study, approximately 115 subjects per group were randomised.
The MGI and BI were calculated taking the average over all tooth sites for a subject. Teeth which were missing or not gradable were excluded from all calculations.
The MGI and BI were compared between treatments using a mixed model (MM) analysis of covariance. The statistical model included factors for treatment group and the pre-prophy baseline and randomisation baseline levels of MGI and BI as covariates. The gingival strata level was not included as the actual baseline level was included as a covariate.
The Turesky modification of the Quigley Hein Index (overall) was calculated taking the average over all tooth sites for a subject. The interproximal scores were calculated in the same way as for the overall scores but just based on the mesiofacial, distofacial, mesiolingual and distolingual surfaces. Teeth which were missing or not gradable were excluded from all calculations.
The plaque and interproximal plaque were compared between treatments using a MM ANCOVA analysis. The statistical model included treatment group and strata level of gingival index as factors, and the pre-prophy baseline and randomisation baseline whole mouth plaque score as covariates.
The margin and papillae scores for MGI and BI were based on the mean scores just from the gingival margin areas (facial and lingual) and the papillae areas (facial and lingual). The MGI and BI (for margin and papillae regions separately) were compared between treatments using a MM analysis. The statistical model included factors for treatment group and the pre-prophy baseline and randomisation baseline levels of MGI and BI (whole mouth scores) as covariates.
RESULTS
Three hundred and twenty two adult subjects were screened, with 234 subjects randomised to study treatment. A total of 205 subjects were included in the efficacy analysis which was based on the Intent to Treat (ITT) population. Twenty nine of the randomised subjects did not complete the study as they were lost to follow up.
Demographics
The baseline demographics for the study population are summarised in Table 1 and show that the two treatment groups were balanced with respect to age, sex and Pre-Prophy Baseline MGI scores of the subjects.
Table 1.
Demographics of intent to treat study population
| Treatment | Number of Subjects | Age (years) | |||||
|---|---|---|---|---|---|---|---|
| Male | Female | Overall | MGI Score* (1.5–<2.00) | MGI Score* (≥2.00) | Mean | Range | |
| 0.1% o-cymen-5-ol/ 0.6%Zinc chloride (ZnCl2) | 22 | 78 | 100 | 15 | 85 | 30.9 | 18–50 |
| 0.204% Sodium Fluoride | 15 | 90 | 105 | 12 | 93 | 30.1 | 18–56 |
MGI score at Pre-Prophy Baseline visit.
Pre-experimental phase
The purpose of the pre-experimental phase was to bring subjects to their optimum gingival health prior to commencing study treatment. MGI, BI and TPI all showed significant reductions between the pre-prophylaxis visit and the randomisation visit (Table 2). There was an 84.7% reduction in MGI, a reduction of 66.2% in BI and a 61.9% reduction in TPI. All these reductions were statistically significant (p < 0.0001).
Table 2.
Summary of changes during pre-experimental phase (Pre-Prophy Baseline to Randomisation Visits)
| Index | Mean Score (±SD) | Comparison* | |||
|---|---|---|---|---|---|
| Pre-Prophy Baseline (N = 205) | Randomisation (N = 205) | Mean Difference (%) | 95% CI | P-value | |
| Modified Gingival Index | 2.09 ± 0.122 | 0.32 ± 0.160 | −1.77 (84.7%) | −1.79, −1.75 | <0.0001 |
| Bleeding Index | 0.68 ± 0.177 | 0.23 ± 0.073 | −0.45 (66.2%) | −0.47, −0.42 | <0.0001 |
| Plaque Index | 3.39 ± 0.341 | 1.29 ± 0.492 | −2.10 (61.9%) | −2.17, −2.03 | <0.0001 |
Comparison based on model-adjusted means.
Experimental phase
Comparison of Mean Modified Gingival Index scores
The pre-prophylaxis baseline MGI scores for the 205 subjects included in the efficacy analysis are presented in Table 3. The control dentifrice group had a mean MGI score of 2.09 while the o-cymen-5-ol/ zinc chloride dentifrice group had a score of 2.08. The mean MGI scores for the two dentifrice groups after 12 weeks dentifrice use are also presented in Table 3. The control dentifrice group had a mean MGI score of 0.63, while the o-cymen-5-ol/ zinc chloride dentifrice group had a mean MGI score of 0.43 after 12 weeks of product use. This MGI score was 32.2% lower compared to the control dentifrice and statistically significant (p < 0.0001).
Table 3.
Summary of Modified Gingival Index over time
| Time point | Mean Modified Gingival Index (±SD) | Between Treatment Comparison* | |||
|---|---|---|---|---|---|
| 0.1% o-cymen-5-ol/ 0.6%ZnCl2 (N = 100) | 0.204% Sodium Fluoride (N = 105) | Mean Difference (%) | 95% CI | P-value | |
| Pre-Prophy Baseline | 2.08 ± 0.126 | 2.09 ± 0.119 | |||
| Randomisation | 0.32 ± 0.156 | 0.32 ± 0.165 | |||
| Week 12 | 0.43 ± 0.193 | 0.63 ± 0.206 | −0.20 (−32.2%) | −0.25, −0.15 | <0.0001 |
0.1%w/w o-cymen-5-ol/ 0.6%w/w zinc chloride (ZnCl2) gel to foam dentifrice vs 0.204%w/w sodium fluoride dentifrice, a negative difference favours the experimental dentifrice. Comparison based on model-adjusted means.
The MGI scores were separately analysed to evaluate the margin and papillae, this is presented in Table 4. The results closely mirrored the overall mean scores indicating that the severity of the inflammation was similar in both the margin and papillae sites.
Table 4.
Summary of Modified Gingival Index Margin and Papillae scores
| Timepoint | Margin or papillae | Mean Modified Gingival Index (±SD) | |
|---|---|---|---|
| 0.1% o-cymen-5-ol/0.6%ZnCl2 (N = 100) | 0.204% Sodium Fluoride (N = 105) | ||
| Pre-Prophy Baseline | Margin | 2.08 ± 0.121 | 2.09 ± 0.116 |
| Papillae | 2.08 ± 0.131 | 2.10 ± 0.124 | |
| Randomisation | Margin | 0.35 ± 0.150 | 0.34 ± 0.162 |
| Papillae | 0.29 ± 0.164 | 0.29 ± 0.170 | |
| Week 12* | Margin | 0.45 ± 0.190 | 0.65 ± 0.204 |
| Papillae | 0.40 ± 0.200 | 0.61 ± 0.213 | |
Between treatment differences based on model-adjusted means were statistically significant (p < 0.0001) in favour of 0.1%w/w o-cymen-5-ol/ 0.6%w/w zinc chloride (ZnCl2) gel to foam dentifrice for margin and papillae.
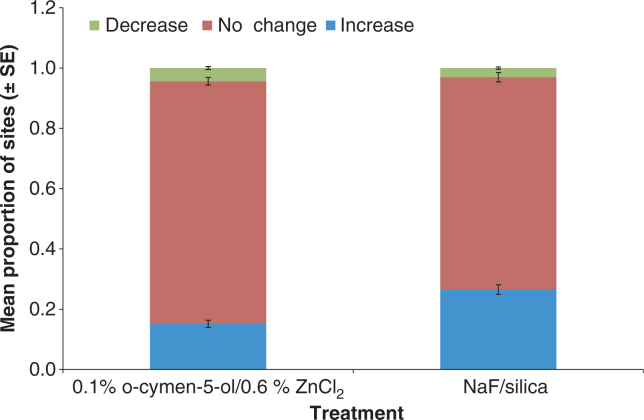
The MGI scores were evaluated to determine the number of individual sites that increased, decreased or showed no change in MGI from the randomisation visit at week 12. This is illustrated in Figure 1 as the relative proportion of sites showing increase, decrease or no change in MGI for each treatment.
Figure 1.
Proportion of tooth sites that showed increase, no change and decrease in MGI after 12 weeks.
Comparison of Bleeding Index scores
The pre-prophylaxis baseline BI scores for the 205 subjects included in the efficacy analysis are presented in Table 5. The o-cymen-5-ol/ zinc chloride dentifrice and the control dentifrice group both had a mean BI score of 0.68. The mean BI scores for the two dentifrice groups after 12 weeks dentifrice use are also presented in Table 5. The control dentifrice group had a mean BI score of 0.47, while the o-cymen-5-ol/ zinc chloride dentifrice group had a mean BI score of 0.35 after 12 weeks. This BI score was 26.3% lower compared to the control dentifrice and statistically significant (p < 0.0001).
Table 5.
Summary of Bleeding Index over time
| Time point | Mean Bleeding Index (±SD) | Between Treatment Comparison* | |||
|---|---|---|---|---|---|
| 0.1% o-cymen-5-ol/0.6%ZnCl2 (N = 100) | 0.204% Sodium Fluoride (N = 105) | Mean Difference (%) | 95% CI | P-value | |
| Pre-Prophy Baseline | 0.68 ± 0.165 | 0.68 ± 0.189 | |||
| Randomisation | 0.24 ± 0.073 | 0.23 ± 0.073 | |||
| Week 12 | 0.35 ± 0.151 | 0.47 ± 0.175 | −0.12 (−26.3%) | (−0.17, −0.08) | <0.0001 |
0.1%w/w o-cymen-5-ol/0.6%w/w zinc chloride (ZnCl2) gel to foam dentifrice vs 0.204%w/w sodium fluoride dentifrice, a negative difference favours the experimental dentifrice. Comparison based on model-adjusted means.
The BI scores were separately analysed to evaluate the margin and papillae, this is presented in Table 6. There were lower BI scores at the papillae sites than the margin sites. The between treatment differences were statistically significant in both cases.
Table 6.
Summary of Bleeding Index Margin and Papillae scores
| Time point | Margin or papillae | Mean Modified Gingival Index (±SD) | |
|---|---|---|---|
| 0.1% o-cymen-5-ol/0.6%ZnCl2 (N = 100) | 0.204% Sodium Fluoride (N = 105) | ||
| Pre-Prophy Baseline | Margin | 0.73 ± 0.172 | 0.73 ± 0.192 |
| Papillae | 0.62 ± 0.186 | 0.63 ± 0.205 | |
| Randomisation | Margin | 0.27 ± 0.078 | 0.27 ± 0.076 |
| Papillae | 0.20 ± 0.078 | 0.19 ± 0.080 | |
| Week 12* | Margin | 0.37 ± 0.155 | 0.49 ± 0.171 |
| Papillae | 0.32 ± 0.154 | 0.44 ± 0.186 | |
Between treatment differences based on model-adjusted means were statistically significant (P < 0.0001) in favour of 0.1%w/w o-cymen-5-ol/ 0.6%w/w zinc chloride (ZnCl2) dentifrice for margin and papillae.
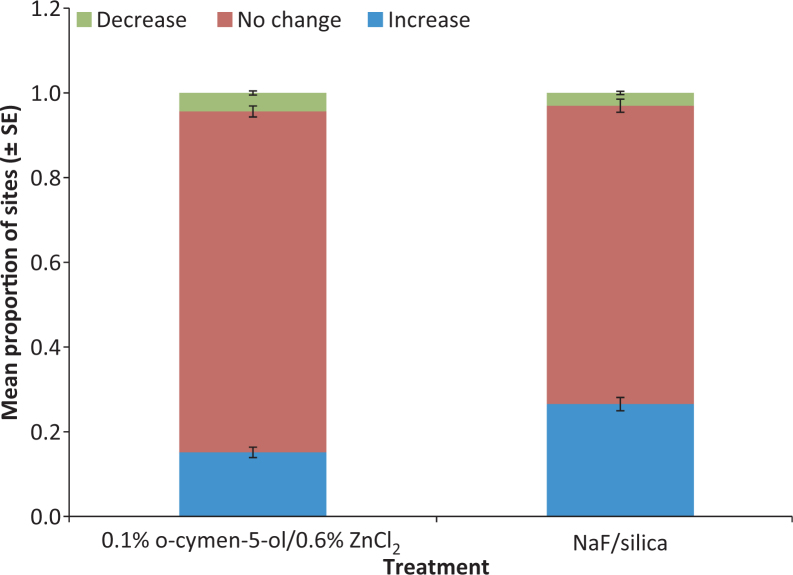
The BI scores were evaluated to determine the number of individual sites that increased, decreased or showed no change in BI from the randomisation visit at week 12. This is illustrated in Figure 2 as the relative proportion of sites showing increase, decrease or no change in BI for each treatment.
Figure 2.
Proportion of tooth sites that showed increase, no change and decrease in Bleeding Index after 12 weeks.
Comparison of Plaque Index scores
The pre-prophy baseline TPI scores for the 205 subjects included in the efficacy analysis are presented in Table 7. The o-cymen-5-ol/ zinc chloride dentifrice group and the control dentifrice group both had a mean TPI score of 3.39. The mean TPI scores for the two dentifrice groups after 12 weeks dentifrice use are also presented in Table 6. The control dentifrice group had a mean TPI score of 1.64, while the o-cymen-5-ol/ zinc chloride dentifrice group had a mean TPI score of 1.35 after 12 weeks. This TPI score was 20.7% lower compared to the control dentifrice. The reduction was statistically significant (p < 0.0001).
Table 7.
Summary of Overall Plaque Index over time
| Time point | Mean Overall Plaque Index (±SD) | Between Treatment Comparison* | |||
|---|---|---|---|---|---|
| 0.1% o-cymen-5-ol/0.6%ZnCl2 (N = 100) | 0.204% Sodium Fluoride (N = 105) | Mean Difference (%) | 95% CI | P-value | |
| Pre-Prophy Baseline | 3.39 ± 0.348 | 3.39 ± 0.337 | |||
| Randomisation | 1.32 ± 0.504 | 1.26 ± 0.482 | |||
| Week 12 | 1.35 ± 0.501 | 1.64 ± 0.487 | −0.34 (−20.6%) | −0.42, −0.26 | <0.0001 |
0.1%w/w o-cymen-5-ol/0.6%w/w zinc chloride (ZnCl2) dentifrice vs 0.204%w/w sodium fluoride dentifrice, a negative difference favours the experimental dentifrice. Comparison based on model-adjusted means.
The TPI scores were separately analysed to evaluate interproximal plaque (analysis of the mesiofacial, distofacial, mesiolingual and distolingual surfaces only) this is presented in Table 8. The results for the interproximal plaque scores were similar to the overall plaque scores indicating that control of dental plaque is present over all sites.
Table 8.
Summary of Interproximal Plaque Index over time
| Time point | Mean Interproximal Plaque Index (±SD) | Between Treatment Comparison* | |||
|---|---|---|---|---|---|
| 0.1% o-cymen-5-ol/0.6%ZnCl2 (N = 100) | 0.204% Sodium Fluoride (N = 105) | Mean Difference (%) | 95% CI | P-value | |
| Pre-Prophy Baseline | 3.40 ± 0.353 | 3.40 ± 0.339 | |||
| Randomisation | 1.33 ± 0.504 | 1.26 ± 0.483 | |||
| Week 12 | 1.35 ± 0.500 | 1.64 ± 0.487 | −0.34 (−20.7%) | −0.42, −0.26 | <0.0001 |
0.1%w/w o-cymen-5-ol/0.6%w/w zinc chloride (ZnCl2) dentifrice vs 0.204%w/w sodium fluoride dentifrice, a negative difference favours the experimental dentifrice. Comparison based on model-adjusted means.
Safety results
A total of 14 subjects reported 14 treatment-emergent adverse events. There was one oral adverse event and 13 non oral adverse events. None of the adverse events were serious and none of the subjects withdrew from the study due to an adverse event.
DISCUSSION
This single centre, examiner blind, two arm, parallel group, randomised and stratified clinical study evaluated the ability of a gel to foam dentifrice containing 0.1%w/w o-cymen-5-ol and 0.6%w/w zinc chloride to maintain gingival health when used as part of an oral hygiene regimen over a 12 week period compared to a control sodium fluoride/ silica dentifrice. The study was conducted in a population with mild to moderate gingivitis typical of the general population. Results of this study indicate that the pre-experimental phase where professional dental prophylaxis and oral hygiene instruction were administered was highly effective in reducing levels of gingival inflammation and bleeding to a level of health (mean MGI and BI scores of <1.0) prior to product administration. This study design enabled assessment of the ability of the test product to maintain this level of gingival health over the test period. It is difficult to achieve the level of plaque control administered from professional prophylaxis, therefore it is not unexpected to notice that both the test and control groups showed an increase in gingival inflammation, plaque and bleeding during the study relative to the healthy state observed at randomisation after the subjects had received a complete dental prophylaxis, additional polishing and individualised oral hygiene instruction. The test gel to foam dentifrice containing 0.1%w/w o-cymen-5-ol and 0.6%w/w zinc chloride maintained the level of gingival health at randomisation to a greater extent than the control dentifrice after 12 weeks twice daily use. This is further illustrated in Figures 1 and 2 where the proportion of individual sites in the mouth that either increased, decreased or showed no change in MGI or BI were compared (where no change or decrease indicates maintenance of health). These results indicate that this antimicrobial activity of the formulation as reported by Pizzey2 does control dental plaque and enhance the ability of the gel to foam dentifrice to maintain gingival health over the 12 week test period compared to a regular sodium fluoride dentifrice. The results from this study show similar benefits in maintenance of gingival health for an 0.1%w/w o-cymen-5-ol/ 0.6%w/w zinc chloride dentifrice in a gel to foam format as those observed for a 0.1%w/w / 0.6%w/w zinc chloride dentifrice1.
CONCLUSION
The results of the present clinical study demonstrate that the use of the 0.1%w/w o-cymen-5-ol/ 0.6%w/w zinc chloride gel to foam dentifrice over a 12 week period provides a statistically significant benefit in maintaining gingival health compared to a regular sodium fluoride control dentifrice.
CONFLICT OF INTEREST AND SOURCE OF FUNDING
The work described in this manuscript was funded by GlaxoSmithKline Consumer Healthcare. The authors external to GSK received no additional fee for the preparation of this manuscript. The remaining authors are employed by GSK but confirm no potential conflicts of interest.
REFERENCES
- 1.Kakar A, Newby E, Kakar K, et al. A randomised clinical trial to assess maintenance of gingival health by a novel dentifrice containing 0.1%w/w o-cymen-5-ol, 0.6%w/w zinc chloride. Int Dent J. 2011;61:13–20. doi: 10.1111/j.1875-595X.2011.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pizzey R, Bradshaw DJ, Marquis R. Antimicrobial effects of o-Cymen-5-ol and zinc, alone and in combination in simple solutions and toothpaste formulations. Int Dent J. 2011;61:33–44. doi: 10.1111/j.1875-595X.2011.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobene RR. A Modified Gingival Index for Use in Clinical Trials. Clin Prev Dent. 1986;8:3. [PubMed] [Google Scholar]
- 4.Saxton C, Van der Ouderara FGJ. The effect of a dentifrice containing zinc citrate and triclosan on developing gingivitis. J Periodont Res. 1989;24:75. doi: 10.1111/j.1600-0765.1989.tb00860.x. [DOI] [PubMed] [Google Scholar]
- 5.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of Vitamin C. J Periodontol. 1970;41:41–44. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]