Abstract
Objectives: To assess the ability of a 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice to control oral malodour compared to a sodium fluoride control dentifrice. Design: Following baseline measurement of oral volatile sulfur compounds (VSCs), the subjects brushed twice daily for 1 week with either the test or control dentifrice. The VSC concentration in breath samples was monitored up to 12 hours post-treatment, by gas chromatography (GC). Results: 75 subjects were included in the efficacy analysis. Relative to the sodium fluoride control dentifrice group the o-cymen-5-ol/ zinc chloride/ sodium fluoride dentifrice exhibited statistically significant reductions (P < 0.0001) in hydrogen sulfide, methyl mercaptan and total measured VSCs immediately and after 1, 2, 3 and 12 (overnight) hours post-treatment. Conclusion: The results of the present clinical study demonstrated that the use of the 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice over a one week period provided a statistically significant benefit in controlling oral malodour for up to 12 hours post-treatment compared to a sodium fluoride control dentifrice.
Key words: Gas chromatography, oral malodour, volatile sulfur compounds
INTRODUCTION
Oral malodour or halitosis is a common condition affecting up to 50% of the population1., 2. and it can significantly impact normal social interactions3. Halitosis can be classified into three categories: genuine halitosis, pseudo-halitosis and halitophobia4. Pseudo-halitosis and halitophobia are conditions where the patients believe they have oral malodour but there is no evidence to support this belief. Genuine halitosis (malodour) predominantly originates from within the oral cavity5., 6., 7. and can be further sub-divided into malodour of physiological or pathological origin. Physiological malodour is primarily due to the bacterial metabolism of salivary proteins, retained food debris and sloughed oral mucosa to yield amines, fatty acids and volatile sulfur compounds (VSCs). Oral malodour can also be due to extrinsic factors, such as tobacco, food and drink. Another component of genuine oral malodour is pathological malodour, caused by disease or disorders within the body e.g. post nasal drip, diabetes mellitus and xerostomia. Treatment of oral malodour, with the exception of genuine physiological oral malodour, requires management by a doctor, dentist or psychologist8. The treatment of physiological oral malodour focuses on improving the sufferer’s oral hygiene5., 8., 9., 10.. This may include instruction by the dentist or dental hygienist to help improve the sufferer’s oral hygiene, e.g. cleaning technique and the use of antimicrobial oral care products such as dentifrices and mouthwashes to control the oral bacteria involved in the breakdown of salivary proteins and food debris. Active ingredients used in the management of physiological oral malodour include botanical extracts, cetylpyridinium chloride, chlorhexidine, chlorine dioxide, essential oils, hydrogen peroxide, triclosan and zinc salts11. The efficacy of these compounds is due to their antimicrobial activity12., 13., 14. although the zinc salts can also act via a chemical neutralisation mechanism15., 16.. Oral malodour can be assessed subjectively by odour judges or measured indirectly by the analysis of breath samples for VSCs, in particular hydrogen sulfide and methyl mercaptan which account for about 90% of the VSCs found in breath samples17 and are strongly correlated with the organoleptic assessment of oral malodour18. Trained odour judges can detect differences both in the intensity and composition of the breath odour; however, there are difficulties obtaining duplicate samples and with reproducibility between odour judges19. Oral VSCs can be measured by the Oral Chroma™20., 21., 22. (Oral Chroma™ is a registered trademark of the Abilit Corporation, Osaka 542-0081), Halimeter®23 (Halimeter® is a registered trademark of Interscan Corporation, Chatsworth CA 913132496) or by analysing breath samples by gas chromatography (GC). As previously reported there is a significant discrepancy between results obtained from a Halimeter® and by GC analysis24 and these authors recommend that if precise knowledge of VSCs is required then breath samples should be analysed by GC. The measurement of VSCs by GC was first reported by Tonzetich in 197117, however, the methodology has since been refined and in this study breath samples were analysed using the methodology described by Newby et al.25.
The objectives of this clinical study were to assess the ability of a 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice to control oral malodour compared to a sodium fluoride control dentifrice. The combination of o-cymen-5-ol and zinc chloride has been shown to have antimicrobial activity26 and the zinc chloride also has odour neutralisation activity15., 16.. This product was used twice daily for one week and then the concentrations of hydrogen sulfide and methyl mercaptan in breath samples were measured immediately and at 1, 2, 3 and 12 hours after brushing with the dentifrice.
MATERIALS AND METHODS
Study design
This was a single centre, examiner blind, two way crossover study in healthy adult volunteers conducted at Intertek 4-Front Research, Ellesmere Port, UK to assess the ability of 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/sodium fluoride dentifrice to control oral malodour compared to a sodium fluoride control dentifrice. The study protocol and consent form was reviewed and approved by the Manchester Consumer Healthcare Research Ethics Committee. After providing written informed consent, 89 adult subjects were screened and 78 subjects were randomised to treatment. The study commenced in August 2010 and was completed in November 2010.
Male and female subjects were enrolled into the study based on the following criteria:
-
•
Subjects had to be at least 18 years of age and in good general health
-
•
Subjects had good oral health with at least 20 natural uncrowned teeth
-
•
Subjects had to have a reproducible level of hydrogen sulfide (> 300 parts per billion (ppb) by GC analysis) on at least 3 separate occasions.
Subjects were excluded from the study if they:
-
•
Were pregnant or breast feeding
-
•
Had diabetes mellitus, evidence or recent history of bronchitis, tonsillitis or sinusitis, a significant autoimmune or infectious disease, such as hepatitis, tuberculosis, HIV positive or AIDS, any infectious disease, respiratory infection, oesophageal reflux, colds, flu, sore throat or any condition which could be transmitted in saliva or salivary aerosols, or severe xerostomia
-
•
Had known or suspected intolerance or hypersensitivity to oral care products, orthodontic or prosthetic appliances, including dental implants
-
•
Had undergone dental professional cleaning within three weeks prior to the screening visit
-
•
Had used chlorhexidine containing mouthwashes, used Colgate® Total within seven days prior to treatment, or had used antibiotics within 14 days prior to treatment.
Following screening, eligible subjects underwent a minimum two day wash in period. Subjects were requested to avoid spicy or odorous foods 24 hours prior to assessments and to refrain from consuming any substance by mouth, except water, from 11 pm the evening prior to the assessments. On Day 1 of each treatment period, subjects provided three samples of mouth air at baseline, which had to have a mean hydrogen sulfide concentration of greater than 300 ppb. If they failed to produce a baseline sample meeting this criterion, they were permitted to return on two further occasions. If they failed to produce a baseline sample meeting this criterion on the third occasion they were withdrawn from the study. Eligible subjects were randomised on Day 1 of treatment period 1 and then brushed with their dispensed study treatment under supervision at the study site. Subjects were instructed to wet their toothbrush head with tap water, apply a strip of dentifrice to cover the head of their toothbrush and then brush in their usual manner for 1 timed minute. The subjects then brushed at home with their study treatment twice daily for seven days.
On Day 8 of each treatment period, subjects returned to the site in the morning with their treatment. Subjects provided three samples of mouth air for the 12 hour (overnight) evaluation of breath odour. Subjects then brushed with their study treatment for 1 timed minute, swirled the dentifrice slurry around their mouth for a further 10 seconds before expectorating. The subjects then wiped any excess dentifrice from the outside of their mouth with a damp paper towel before providing 3 samples of mouth air immediately, and at 1 hour, 2 hours and 3 hours post-treatment.
There was a washout period of 7 – 21 days between the two treatment periods.
Randomisation and blinding procedure
The randomisation schedule was generated using a computerised randomisation generator and provided to the site by the Biostatistics Department of the sponsor. Subjects were randomly assigned to one of two treatment groups. Randomisation numbers were assigned chronologically as subjects were randomised to treatment. The study staff who dispensed the treatment were provided with a randomisation schedule that did not contain the treatment identities. The dental examiners at site and the study statistician, data management staff and other employees of the sponsor who might have influenced study outcomes were blinded to the allocation of treatment to subjects. The study treatments were both white dentifrices provided in plain white tubes with study label detailing the treatment codes and instructions for use to ensure the subject was blinded to the treatment identity.
Analysis of breath samples
Subjects were instructed to keep their mouth closed for 60 seconds and to breathe normally through their nose. After 60 seconds, the subjects inserted a fluorinated ethylene propylene (FEP) tubing mouthpiece between closed lips to a comfortable position above the back half of the tongue. The subjects then took a deep breath through the nose and held their breath for 5 seconds at which point a 20 mL sample of breath was collected via a 20 mL syringe. Breath samples were analysed for hydrogen sulfide and methyl mercaptan concentration by gas chromatography with flame photometric detection (FPD) within 30 minutes of collection.
Statistical methods
A sample size of 70 subjects was required to detect a mean difference of 0.114 log ppb (standard deviation (SD) of differences = 0.3336 log ppb), 0.120 log ppb (within subject SD = 0.3285 log ppb), and 0.113 log ppb (within subject SD = 0.3240 log ppb) in change from baseline in hydrogen sulfide, methyl mercaptan and VSC (based on a log10 scale) with 80% power. To allow for withdrawals from the study approximately 85 subjects were randomised.
The data value for each VSC parameter (hydrogen sulfide concentration, methyl mercaptan concentration and total VSC) was log transformed (log10) prior to conducting within- and between-treatment analyses. For total VSC, the hydrogen sulfide and methyl mercaptan concentrations were summed together and then log transformed. For each VSC parameter the comparison between study treatments with respect to the mean change in log concentration from baseline to each post-treatment time point was performed using an analysis of covariance (ANCOVA) with factors for treatment, treatment period, subject (as a random effect) and baseline value as a covariate. Treatment differences and 95% confidence intervals (CIs) were presented. All tests were two-sided and performed at the 5% significance level.
RESULTS
Eighty nine adult subjects were screened, 78 subjects were randomised to treatment and 75 (96.2%) were included in the efficacy analysis based on the Intent to Treat (ITT) population. Ten of the randomised subjects did not complete the study; one was due to an adverse event, one to protocol violation and the remaining eight for ‘other’ reasons. The baseline demographics are summarised in Table 1.
Table 1.
Demographics of intent to treat (ITT) study population and baseline VSC concentrations*
| Overall (N = 78) | |
|---|---|
| Sex N (%) | |
| Male | 18 (23.1) |
| Female | 60 (76.9) |
| Race N (%) | |
| American Indian or Alaska Native | 0 |
| Asian | 1 (1.3) |
| Black or African American | 1 (1.3) |
| Native Hawaiian or other Pacific Islander | 0 |
| White | 76 (97.4) |
| Multiple | 0 |
| Age (Years) | 46.3 (12.21) |
| Baseline VSC concentrations (log ppb) | |
| Hydrogen Sulfide | |
| Test - 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice | 2.74 (0.223) |
| Reference - Sodium fluoride dentifrice | 2.70 (0.266) |
| Methyl Mercaptan | |
| Test - 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice | 2.32 (0.275) |
| Reference - Sodium fluoride dentifrice | 2.34 (0.249) |
Data are means (SD) or numbers (%).
‘Baseline’ is the sample prior to first treatment on day 1 of each treatment period and is the mean concentration of 3 samples.
Comparison of VSC concentrations
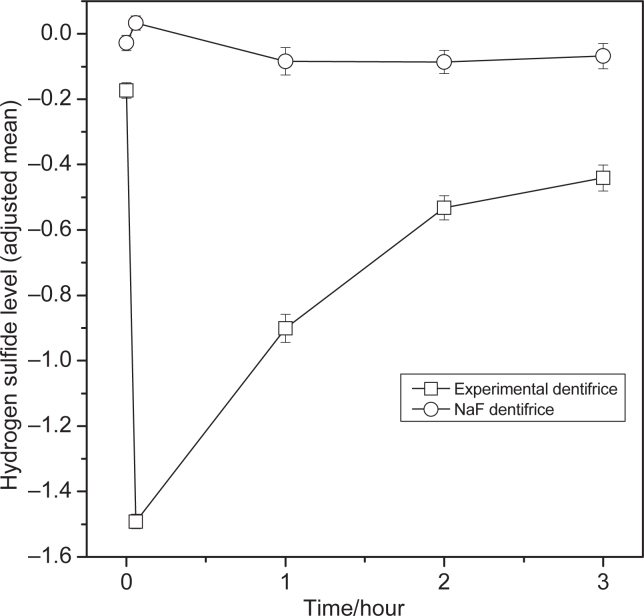
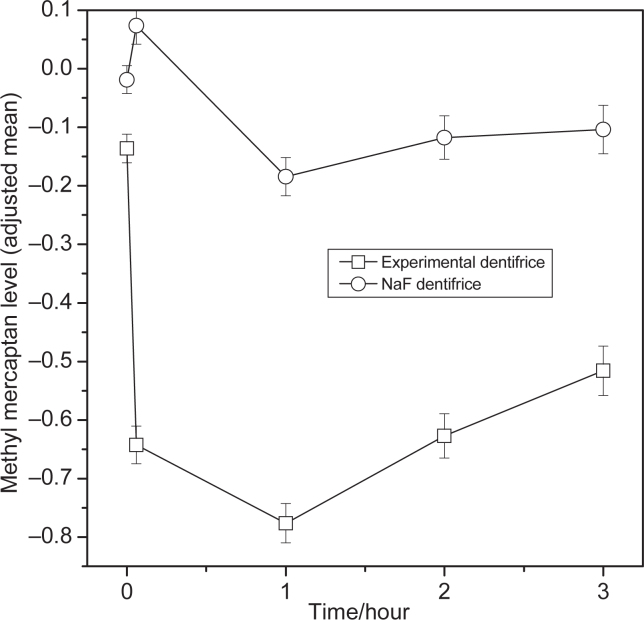
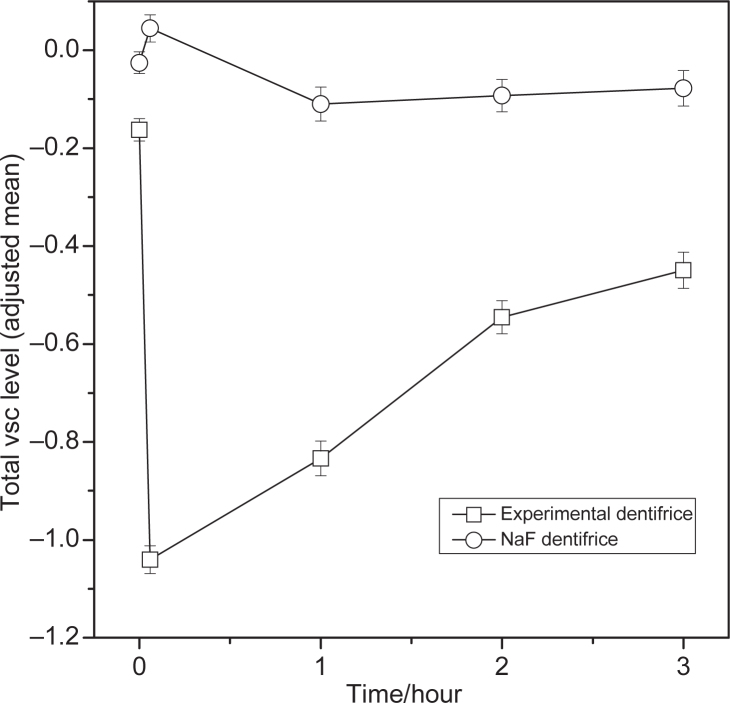
Within treatment comparisons versus baseline for hydrogen sulfide, methyl mercaptan, and total VSCs showed significant differences from baseline for the 0.1%o-cymen-5-ol/ 0.6% zinc chloride dentifrice at all the time points (Tables 2, 3 and 4). The effect of the 0.1%o-cymen-5-ol/ 0.6% zinc chloride dentifrice is shown in Figures 1, 2 and 3. Compared to baseline, the experimental dentifrice showed a reduction in concentration of hydrogen sulfide, methyl mercaptan and total VSC ranging from a 97% reduction in hydrogen sulfide immediately after brushing to a 33% reduction 12 hours post brushing, a 77% reduction in methyl mercaptan immediately after brushing to a 27% reduction 12 hours post brushing, and a 91% reduction in total VSCs immediately after brushing to a 31% reduction 12 hours post brushing.
Table 2.
Summary of within treatment efficacy results for hydrogen sulfide levels (log scale) – ITT population
| Timepoint | Treatment | Hydrogen Sulfide – within treatment comparison | |||
|---|---|---|---|---|---|
| Adjusted LS Mean ± SE1 | 95% CI1 | P-value1 | % Change from baseline2 (95% CI) | ||
| 12 hour Overnight | Test | −0.17 ± 0.024 | (−0.22, −0.13) | <0.0001 | −32.989 (−39.84, −25.35) |
| Reference | −0.03 ± 0.023 | (−0.07, 0.02) | 0.2315 | −6.232 (−15.65, 4.24) | |
| Immediately | Test | −1.49 ± 0.022 | (−1.53, −1.45) | <0.0001 | −96.776 (−97.08, −96.44) |
| Reference | 0.03 ± 0.022 | (−0.01, 0.08) | 0.1210 | 8.037 (−2.05, 19.16) | |
| 1 Hour | Test | −0.90 ± 0.043 | (−0.99, −0.82) | <0.0001 | −87.453 (−89.68, −84.74) |
| Reference | −0.08 ± 0.042 | (−0.17, −0.001) | 0.0473 | −17.662 (−32.05, −0.23) | |
| 2 Hour | Test | −0.53 ± 0.037 | (−0.61, −0.46) | <0.0001 | −70.655 (−75.19, −65.29) |
| Reference | −0.09 ± 0.036 | (−0.16, −0.01) | 0.0182 | −18.035 (−30.47, −3.37) | |
| 3 Hour | Test | −0.44 ± 0.039 | (−0.52, −0.36) | <0.0001 | −63.777 (−69.70, −56.70) |
| Reference | −0.07 ± 0.038 | (−0.14, 0.01) | 0.0799 | −14.471 (−28.22, 1.91) | |
1Obtained from ANCOVA with factors for treatment, period, subject (random effect) and baseline (logged) as covariate.
2% Change from baseline is based on antilog value of adjusted LS Mean. A negative difference indicates a reduction.
Test - 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice.
Reference - Sodium fluoride dentifrice.
Table 3.
Summary of within treatment efficacy results for methyl mercaptan levels (log scale) – ITT population
| Timepoint | Treatment | Methyl Mercaptan – within treatment comparison | |||
|---|---|---|---|---|---|
| Adjusted LS Mean ± SE1 | 95% CI1 | P-value1 | % Change from baseline2 (95% CI) | ||
| 12 hour Overnight | Test | −0.14 ± 0.024 | (−0.18, −0.09) | <0.0001 | −26.935 (−34.62, −18.34) |
| Reference | −0.02 ± 0.024 | (−0.07, 0.03) | 0.4362 | −4.210 (−14.10, 6.81) | |
| Immediately | Test | −0.64 ± 0.032 | (−0.71, −0.58) | <0.0001 | −77.228 (−80.33, −73.64) |
| Reference | 0.07 ± 0.032 | (0.01, 0.14) | 0.0209 | 18.508 (2.65, 36.81) | |
| 1 Hour | Test | −0.78 ± 0.034 | (−0.84, −0.71) | <0.0001 | −83.258 (−85.63, −80.49) |
| Reference | −0.18 ± 0.033 | (−0.25, −0.12) | <0.0001 | −34.564 (−43.66, −24.00) | |
| 2 Hour | Test | −0.63 ± 0.038 | (−0.70, −0.55) | <0.0001 | −76.396 (−80.14, −71.94) |
| Reference | −0.12 ± 0.037 | (−0.19, −0.04) | 0.0020 | −23.689 (−35.58, −9.60) | |
| 3 Hour | Test | −0.52 ± 0.042 | (−0.60, −0.43) | <0.0001 | −69.519 (−74.83, −63.08) |
| Reference | −0.10 ± 0.041 | (−0.19, −0.02) | 0.0130 | −21.279 (−34.77, −5.00) | |
1Obtained from ANCOVA with factors for treatment, period, subject (random effect) and baseline (logged) as covariate.
2% Change from baseline is based on antilog value of adjusted LS Mean. A negative difference indicates a reduction.
Test - 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice.
Reference - Sodium fluoride dentifrice.
Table 4.
Summary of within treatment efficacy results for total VSC levels (log scale) – ITT population
| Timepoint | Treatment | Total VSC – within treatment comparison | |||
|---|---|---|---|---|---|
| Adjusted LS Mean ± SE1 | 95% CI1 | P-value1 | % Change from baseline2 (95% CI) | ||
| 12 hour Overnight | Test | −0.16 ± 0.023 | (−0.21, −0.12) | <0.0001 | −31.169 (−37.97, −23.62) |
| Reference | −0.03 ± 0.022 | (−0.07, 0.02) | 0.2530 | −5.749 (−14.89, 4.37) | |
| Immediately | Test | −1.04 ± 0.029 | (−1.10, −0.98) | <0.0001 | −90.890 (−92.00, −89.62) |
| Reference | 0.04 ± 0.028 | (−0.01, 0.10) | 0.1124 | 10.849 (−2.42, 25.92) | |
| 1 Hour | Test | −0.83 ± 0.036 | (−0.90, −0.76) | <0.0001 | −85.338 (−87.53, −82.76) |
| Reference | −0.11 ± 0.035 | (−0.18, −0.04) | 0.0020 | −22.323 (−33.71, −8.98) | |
| 2 Hour | Test | −0.55 ± 0.034 | (−0.61, −0.48) | <0.0001 | −71.530 (−75.59, −66.80) |
| Reference | −0.09 ± 0.033 | (−0.16, −0.03) | 0.0060 | −19.149 (−30.46, −6.00) | |
| 3 Hour | Test | −0.45 ± 0.037 | (−0.52, −0.38) | <0.0001 | −64.458 (−69.96, −57.95) |
| Reference | −0.08 ± 0.036 | (−0.15, −0.01) | 0.0333 | −16.417 (−29.13, −1.43) | |
1Obtained from ANCOVA with factors for treatment, period, subject (random effect) and baseline (logged) as covariate.
2% Change from baseline is based on antilog value of adjusted LS Mean. A negative difference indicates a reduction.
Test - 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice.
Reference - Sodium fluoride dentifrice.
Figure 1.
Change from baseline of hydrogen sulfide levels (log scale (base: 10)) by treatment (adjusted mean ± standard error (SE))– ITT population. Error bars represent between-subject standard error. Experimental Dentifrice (Test) - 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice. NaF dentifrice (Reference) - Sodium fluoride dentifrice. The first point is the 12 hour (overnight) time-point, the second point is the immediately after treatment (+5 min).
Figure 2.
Change from baseline of methyl mercaptan levels (log scale (base: 10)) by treatment (adjusted mean ± SE)– ITT population. Error bars represent between-subject standard error. Experimental Dentifrice (Test) - 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/sodium fluoride dentifrice. NaF dentifrice (Reference) - Sodium fluoride dentifrice. The first point is the 12 hour (overnight) time-point, the second point is the immediately after treatment (+5 min).
Figure 3.
Change from baseline of total VSC (hydrogen sulfide + methyl mercaptan) levels (log scale (base: 10)) by treatment (adjusted mean ± SE)– ITT population. Error bars represent between-subject standard error. Experimental Dentifrice (Test) - 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice. NaF dentifrice (Reference) - Sodium fluoride dentifrice. The first point is the 12 hour (overnight) time-point, the second point is the immediately after treatment (+5 min).
The 0.1%o-cymen-5-ol/ 0.6% zinc chloride dentifrice was found to be statistically significantly superior to the reference dentifrice with respect to the mean hydrogen sulfide, methyl mercaptan, and total VSC levels (Table 5), at all time points.
Table 5.
Summary of between treatment comparisons of change from baseline in hydrogen sulfide levels, methyl mercaptan and total VSC (log scale) – ITT population
| Timepoint | Difference1 (95% CI) | P-value2 | % Difference3 (95% CI) |
|---|---|---|---|
| Hydrogen Sulfide (Test vs. Reference) | |||
| 12 hour Overnight | −0.15 (−0.20, −0.09) | <0.0001 | −28.5 (−37.53, −18.24) |
| Immediately | −1.53 (−1.59, −1.46) | <0.0001 | −97.0 (−97.41, −96.57) |
| 1 Hour | −0.82 (−0.94, −0.70) | <0.0001 | −84.8 (−88.42, −79.95) |
| 2 Hour | −0.45 (−0.54, −0.35) | <0.0001 | −64.2 (−71.40, −55.18) |
| 3 Hour | −0.37 (−0.47, −0.28) | <0.0001 | −57.6 (−65.96, −47.31) |
| Methyl Mercaptan (Test vs. Reference) | |||
| 12 hour Overnight | −0.12 (−0.18, −0.06) | 0.0003 | −23.7 (−33.88, −12.01) |
| Immediately | −0.72 (−0.79, −0.64) | <0.0001 | −80.8 (−83.83, −77.16) |
| 1 Hour | −0.59 (−0.68, −0.50) | <0.0001 | −74.4 (−79.24, −68.46) |
| 2 Hour | −0.51 (−0.60, −0.42) | <0.0001 | −69.1 (−74.97, −61.78) |
| 3 Hour | −0.41 (−0.51, −0.32) | <0.0001 | −61.3 (−69.03, −51.59) |
| Total VSCs (Test vs. Reference) | |||
| 12 hour Overnight | −0.14 (−0.19, −0.08) | <0.0001 | −27.0 (−35.94, −16.75) |
| Immediately | −1.09 (−1.16, −1.01) | <0.0001 | −91.8 (−93.04, −90.30) |
| 1 Hour | −0.72 (−0.82, −0.63) | <0.0001 | −81.1 (−84.95, −76.33) |
| 2 Hour | −0.45 (−0.54, −0.37) | <0.0001 | −64.8 (−71.13, −57.04) |
| 3 Hour | −0.37 (−0.46, −0.28) | <0.0001 | −57.5 (−65.22, −48.01) |
1Difference in LS Mean of first named treatment minus second named treatment. A negative difference favors the first name treatment.
2Obtained from ANCOVA with factors for treatment, period, subject (random effect) and baseline (logged) as covariate.
3Difference in the geometric mean concentration. First named treatment-relative to baseline, expressed as percentage of the second named treatment-relative to baseline. A negative value indicates more favourable findings for the first-mentioned treatment.
Test - 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice.
Reference - Sodium fluoride dentifrice.
Safety results
There were a total of 31 treatment-emergent AEs reported for 26 subjects, 19 non-oral and 12 oral. One oral AE (tingling of lips) was associated with the test dentifrice while two other oral AEs (dry mouth and sore gums) were associated with the reference dentifrice. All of the oral AEs were mild in nature. There were no serious adverse events.
DISCUSSION
This single centre, examiner blind, two way crossover study in healthy adult volunteers assessed the ability of 0.1% w/w o-cymen-5-ol/ 0.6% w/w zinc chloride/ sodium fluoride dentifrice to control oral malodour compared to a sodium fluoride control dentifrice. The study was designed to include a population that had genuine physiological malodour, determined by consistent levels of VSCs in baseline breath samples. It allowed the subjects to use the product in their normal manner and did not impose artificial routines or practices with respect to oral hygiene or the ingestion of food and drink. However, subjects were requested to abstain from smoking or eating spicy or odorous foods for 24 hours prior to the assessments to minimise the impact of extrinsic factors on their oral malodour.
This study required the accurate, objective measurement of oral malodour to enable comparison of the efficacy of different products. The flavours present in oral healthcare products impact the organoleptic assessment of oral malodour therefore, as hydrogen sulfide and methyl mercaptan account for approximately 90% of the VSCs found in breath samples, GC was selected as the most appropriate technique to assess the ability of these products to control oral malodour.
Treatments for oral malodour include control of oral bacteria and/or neutralisation of the VSCs produced by the breakdown of salivary proteins and food debris. The antimicrobial activity of the o-cymen-5-ol/ zinc chloride system used in the test dentifrice has been reported by Pizzey et al.26 and, in addition, the zinc chloride has known odour neutralising properties15., 16.. The results of this study have shown that this combination, when used twice daily in the test dentifrice, was able to reduce and control the levels of both hydrogen sulfide and methyl mercaptan over a 12 hour period more effectively than the sodium fluoride control product.
CONCLUSION
The results of the present clinical study demonstrated that the use of the 0.1%o-cymen-5-ol/ 0.6% zinc chloride dentifrice over a one week period provided a statistically significant benefit in controlling oral malodour up to 12 hours post-treatment compared to a sodium fluoride control dentifrice.
CONFLICT OF INTEREST AND SOURCE OF FUNDING
The work described in this manuscript was funded by GlaxoSmithKline Consumer Healthcare. The authors external to GSK received no additional fee for the preparation of this manuscript. The remaining authors are employed by GSK but confirm no potential conflicts of interest.
REFERENCES
- 1.Hughes FJ, McNab R. Oral malodour-a review. Arch Oral Biol. 2008;53(Suppl 1):S1–S7. doi: 10.1016/S0003-9969(08)70002-5. [DOI] [PubMed] [Google Scholar]
- 2.Porter SR, Scully C. Oral malodour (halitosis) Br Med J. 2006;333:632–635. doi: 10.1136/bmj.38954.631968.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Settineri S, Mento C, Gugliotta SC, Saitta A, Terranova A, Trimarchi G, et al. Self-reported halitosis and emotional state: impact on oral conditions and treatments. Health Qual Life Outcomes. 2010;8:34. doi: 10.1186/1477-7525-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murata T, Yamaga T, Iida T, et al. Classification and examination of halitosis. Int Dent J. 2002;52(Suppl 3):181–186. doi: 10.1002/j.1875-595x.2002.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg M. Diagnosis and treatment of bad breath. Cosmetics & Toiletries Magazine. 1996;111:85–90. [Google Scholar]
- 6.Tonzetich J. Production and origin of oral malodor: A review of mechanisms and methods of analysis. J Periodontol. 1977;48:13–20. doi: 10.1902/jop.1977.48.1.13. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg M. The science of bad breath. Sci Am. 2002;286:72–79. doi: 10.1038/scientificamerican0402-72. [DOI] [PubMed] [Google Scholar]
- 8.Yaegaki K, Coil JM. Examination, classification, and treatment of halitosis; clinical perspectives. J Can Dent Assoc. 2000;66:257–261. [PubMed] [Google Scholar]
- 9.Lee PP, Mak WY, Newsome P. The aetiology and treatment of oral halitosis: an update. Hong Kong Med J. 2004;10:414–418. [PubMed] [Google Scholar]
- 10.Association Report: ADA Council on Scientific Affairs Oral Malodor. J Am Dent Assoc. 2003;134:209–214. doi: 10.14219/jada.archive.2003.0135. [DOI] [PubMed] [Google Scholar]
- 11.Lourith N, Kanlayavattanakul M. Oral malodour and active ingredients for treatment. Int J Cosmet Sci. 2010;32:321–329. doi: 10.1111/j.1468-2494.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho MD, Tabchoury CM, Cury JA, et al. Impact of mouthrinses on morning bad breath in healthy subjects. J Clin Periodontol. 2004;31:85–90. doi: 10.1111/j.0303-6979.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 13.Lobene RR, Kashket S, Soparkar PM, et al. The effect of cetylpyridinium chloride on human plaque bacteria and gingivitis. Pharmacol Ther Dent. 1979;4:33–47. [PubMed] [Google Scholar]
- 14.Jenkins S, Addy M, Newcombe RG. A comparison of cetylpyridinium chloride, triclosan and chlorhexidine mouthrinse formulations for effects on plaque regrowth. J Clin Periodontol. 1994;21:441–444. doi: 10.1111/j.1600-051x.1994.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 15.Young A, Jonski G, Rølla G, et al. Effects of metal salts on the oral production of Volatile Sulfur-containing Compounds (VSC) J Clin Periodontol. 2001;28:776–781. doi: 10.1034/j.1600-051x.2001.280809.x. [DOI] [PubMed] [Google Scholar]
- 16.Burnett GR, Stephen AS, Pizzey RL, et al. In vitro assessment of oral malodour reduction by a novel toothpaste. Int Dent J. 2011;61:67–73. doi: 10.1111/j.1875-595X.2011.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonzetich J. Direct gas chromatographic analysis of sulphur compounds in mouth air in man. Arch Oral Biol. 1971;16:587–597. doi: 10.1016/0003-9969(71)90062-8. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg M, Kulkarni GV, Bosy A, et al. Reproducibility and sensitivity of oral malodor measurements with a portable sulphide monitor. J Dent Res. 1991;70:1436–1440. doi: 10.1177/00220345910700110801. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg M, McCulloch CA. Measurement of oral malodor: current methods and future prospects. J Periodontol. 1992;63:776–782. doi: 10.1902/jop.1992.63.9.776. [DOI] [PubMed] [Google Scholar]
- 20.Van Den Velde S, Quirynen M, Van Hee P, et al. Halitosis associated volatiles in breath of healthy subjects. J Chromatogr B Anal Technol Biomed Life Sci. 2007;853:54–61. doi: 10.1016/j.jchromb.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 21.Tangerman A, Winkel EG. The portable gas chromatograph OralChroma: a method of choice to detect oral and extra-oral halitosis. J Breath Res. 2008;2:17010. doi: 10.1088/1752-7155/2/1/017010. [DOI] [PubMed] [Google Scholar]
- 22.Tsai CC, Chou HH, Wu TL, et al. The levels of volatile sulfur compounds in mouth air from patients with chronic periodontitis. J Periodontal Res. 2008;43:186–193. doi: 10.1111/j.1600-0765.2007.01011.x. [DOI] [PubMed] [Google Scholar]
- 23.Bosy A, Kulkarni GV, Rosenberg M, et al. Relationship of oral malodor to periodontitis: evidence of independence in discrete subpopulations. J Periodontol. 1994;65:37–46. doi: 10.1902/jop.1994.65.1.37. [DOI] [PubMed] [Google Scholar]
- 24.Furne J, Majerus G, Lenton P, et al. Comparison of volatile sulfur compound concentrations measured with a sulfide detector vs. gas chromatography. J Dent Res. 2002;81:140–143. [PubMed] [Google Scholar]
- 25.Newby EE, Hickling JM, Hughes F, et al. Control of oral malodour by dentifrices measured by gas chromatography. Arch Oral Biol. 2008;53(suppl. 1):S19–S25. doi: 10.1016/S0003-9969(08)70005-0. [DOI] [PubMed] [Google Scholar]
- 26.Pizzey RL, Marquis RE, Bradshaw DJ. Antimicrobial effects of o-cymen-5-ol and zinc, alone & in combination in simple solutions and toothpaste formulations. Int Dent J. 2011;61:33–40. doi: 10.1111/j.1875-595X.2011.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]