Abstract
Objectives: This study aimed to appraise, within the context of tooth caries, the current clinical evidence and its risk for bias regarding the effects of xylitol in comparison with sorbitol. Methods:: Databases were searched for clinical trials to 19 March 2011. Inclusion criteria required studies to: test a caries-related primary outcome; compare the effects of xylitol with those of sorbitol; describe a clinical trial with two or more arms, and utilise a prospective study design. Articles were excluded if they did not report computable data or did not follow up test and control groups in the same way. Individual dichotomous and continuous datasets were extracted from accepted articles. Selection and performance/detection bias were assessed. Sensitivity analysis was used to investigate attrition bias. Egger’s regression and funnel plotting were used to investigate risk for publication bias. Results:: Nine articles were identified. Of these, eight were accepted and one was excluded. Ten continuous and eight dichotomous datasets were extracted. Because of high clinical heterogeneity, no meta-analysis was performed. Most of the datasets favoured xylitol, but this was not consistent. The accepted trials may be limited by selection bias. Results of the sensitivity analysis indicate a high risk for attrition bias. The funnel plot and Egger’s regression results suggest a low publication bias risk. External fluoride exposure and stimulated saliva flow may have confounded the measured anticariogenic effect of xylitol. Conclusions:: The evidence identified in support of xylitol over sorbitol is contradictory, is at high risk for selection and attrition bias and may be limited by confounder effects. Future high-quality randomised controlled trials are needed to show whether xylitol has a greater anticariogenic effect than sorbitol.
Key words: Xylitol, sorbitol, caries, systematic review
INTRODUCTION
Polyols, also called sugar alcohols, are reported to be anticariogenic when used as alternatives to dietary sugars, especially sucrose1. This effect has been ascribed to the difficulty that microorganisms have in fermenting polyols into acids. Polyols are therefore classified as hypo- or non-acidogenic2. One well-known polyol is xylitol, a 5-carbon sugar alcohol that occurs widely in nature and is regarded as having characteristics as a dietary sweetener similar to those of sucrose3., 4.. As well as the general anticariogenic property it shares with other polyols, xylitol is believed to have specific anticariogenic effects that may give it a special role in the prevention and therapy of dental caries5. These specific anticariogenic effects have been concluded from laboratory observations. These observations comprise: a complete lack of fermentation by cariogenic bacteria (unlike other polyols, such as sorbitol, which can be fermented to a small extent)6; reduction of dental plaque7, and inhibition of mutans streptococci growth8. These observations lend plausibility to the hypothesis that xylitol acts as an active anticariogenic agent9. Clinical confirmation of such a hypothesis would necessarily include evidence that xylitol, in comparison with other common polyols (i.e. sorbitol), has superior effects in the prevention and therapy of caries that are at least equal to those of topical fluoride application, the current reference standard.
The use of sorbitol as a control intervention in a comparison with xylitol is justified because sorbitol is the polyol most commonly used as an alternative to dietary sugars10. A number of narrative reviews of clinical trials comparing xylitol with sorbitol have been published2., 4., 9., 11.. Their conclusions vary in whether they support4., 9., 11. or refute2 the hypothesis that xylitol has preventive and therapeutic effects in caries that are superior to those of sorbitol. In addition, two systematic reviews have evaluated the clinical evidence regarding the use of polyols in relation to dental caries12., 13.. The first systematic review, by Lingström et al.12, focused on the general role of dietary factors in caries prevention, including xylitol. This review was unable to verify the hypothesis that xylitol has a superior role as a sugar substitute12. The second systematic review, by Deshpande and Jadad13, appraised evidence for the impact of polyol-containing chewing gums on dental caries. It concluded that consistent evidence favoured the use of polyol-containing chewing gum over no chewing gum13. Whereas the Lingström et al. review12 employed a qualitative synthesis of the results of the trials reviewed, Deshpande and Jadad13 included a meta-analysis with a random-effects model, as well as a sensitivity analysis of the data they extracted. Neither of these systematic reviews focused on the direct comparison of the preventive and therapeutic effects in caries of xylitol with those of sorbitol. Both reviews also failed to investigate the influence of systematic error and risk for bias on the clinical evidence identified.
Against such a background, the aim of this systematic review was to provide a quantitative, in-depth appraisal of the current clinical evidence and its risk for bias and systematic error regarding the preventive and therapeutic effects in caries of xylitol in comparison with those of sorbitol. Its objective was to resolve the issue of whether xylitol is superior to sorbitol in its assumed anticariogenic (preventive and therapeutic) effects.
METHODS
Systematic search strategy
PubMed was systematically searched for articles reporting on clinical trials published up to 19 March 2011 using the string of MeSH search terms, with Boolean operators: ((‘Xylitol’[Mesh]) AND ‘Sorbitol’[Mesh]) AND ‘Dental Caries’[Mesh]. A subsequent search using the string of English text terms ‘Xylitol AND Sorbitol’ was conducted in the databases: BBO (Bibliografia Brasileira de Odontologia); Biomed Central; Cochrane Library; Directory of Open Access Journals; LILACS (Literatura Latino-Americana e do Caribe em Ciências da Saúde); Open-J-Gate; OpenSIGLE; Sabinet; Science-Direct; Scielo, and Scirus (Medicine). The non-English databases BBO, LILACS and Scielo were also searched using the term ‘Xilitol’. In addition, the journal Dental Abstracts and the International Association for Dental Research (IADR) online abstract submission site were searched for suitable abstracts of non-published studies. References of all included articles were checked for further potentially relevant trials.
On the basis of their listed titles and abstracts, articles from the search results were selected for review according to their compliance with inclusion criteria that required the trials to have:
-
•
Tested caries-related primary outcomes (trials that reported on surrogate endpoints such as bacterial counts, pH-values of saliva or plaque, salivary flow or mother-to-child transmission were not included)
-
•
Compared the effects of xylitol with those of sorbitol
-
•
Represented a clinical trial with two or more arms that included test and control groups
-
•
Used a prospective study design.
If only a relevant title without a listed abstract was available, a full copy of the article was assessed for inclusion.
Article review
Only articles that complied with the inclusion criteria were reviewed further. Full copies of articles were reviewed independently by both reviewers (SM and VY). Articles were excluded if:
-
•
They did not report computable data, dichotomous or continuous, for each treatment group
-
•
They did not follow up test and control groups in the same way.
Disagreements between reviewers were resolved through discussion and consensus.
Extraction of data from accepted trials
The outcome measures considered were caries prevention and therapy. Two reviewers (SM and VY) independently extracted data from the accepted trials. Individual dichotomous datasets (DS), consisting of the number of evaluated units (N) and the number of units (tooth surfaces and/or patients) with caries (n), as well as individual continuous datasets, consisting of the number of evaluated units (N), the mean value per group and the standard deviation (SD) were extracted for both types of materials in the control and test groups. If SDs were not reported, they were calculated from 95% confidence intervals (CIs). Disagreements between reviewers during data extraction were resolved through discussion and consensus.
Statistical analysis
RevMan Version 4.2 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) was used in the analysis of individual datasets. Differences in treatment groups were computed on the basis of relative risk (RR), with 95% CIs for each dichotomous dataset and on the basis of mean differences (MDs), with 95% CIs for each continuous dataset. Meta-analysis was considered only for clinically homogeneous datasets.
Quality of studies and assessment of potential risk for bias
Both reviewers conducted the quality assessment independently. Disagreements between reviewers were resolved through discussion and consensus. Criteria for quality assessment of trials are listed in Table 1. Quality assessment of accepted trials was undertaken on the basis of the availability of evidence indicating the successful prevention of selection and detection or performance bias from the start to end of each trial. If a trial merely reported that randomisation was conducted, reported only the name of the randomisation method used or included a detailed description of the randomisation process without providing any evidence that randomisation was indeed effective throughout the trial, this was regarded as inadequate14., 15..
Table 1.
Criteria for assessing the quality of trials
| Score | Criteria | Impact on risk for bias |
|---|---|---|
| Randomisation and concealment | ||
| A | Randomisation: details of any adequate type of allocation method that generates random sequences with the patient as unit of randomisation are reported* Concealment: the trial provides evidence† that concealment was indeed effective and that the random sequence could not have been observed or predicted throughout the duration of the trial |
Doubt may still exist as to whether the trial results are influenced by selection bias, but no indication can be found from the trial report to support such doubt |
| B | Randomisation: details of any adequate type of allocation method that generates random sequences with the patient as unit of randomisation are reported* Concealment: the trial reports on any adequate method to prevent direct observation‡ and prediction§ of the allocation sequence and sequence generation rules |
Despite the implementation of a method considered able to prevent the unmasking of the concealed allocation sequence through direct observation and prediction, there are reasons to expect that the concealed allocation sequence may have been unmasked during the course of the trial |
| C | Randomisation: details of any adequate type of allocation method that generates random sequences with the patient as unit of randomisation are reported* Concealment: the trial reports on any adequate method to prevent direct operator observation of allocation sequence and sequence generation rules‡ However, the allocation sequence and sequence generation may have been sufficiently predicted |
Despite the implementation of a method considered able to prevent the unmasking of the concealed allocation sequence through direct observation, there are reasons to expect that operators could have predicted the concealed allocation sequence |
| D | Randomisation: details of any adequate type of allocation method that generates random sequences with the patient as unit of randomisation are reported* Concealment: the trial report does not include information on how the allocation of random sequence was concealed The allocation could have been directly observed and/or predicted |
Despite the theoretical chance for each patient to be allocated to either treatment group, operator knowledge of the allocation sequence may have led to patient allocation that favoured the outcome of one type of treatment over the other |
| 0 | The trial does not comply with criteria A–D | No guarantee of equal chance for patients to be allocated to either treatment group; thus allocation may have favoured the outcome of one type of treatment over the other |
| Baseline data for randomised trials | ||
| A | Baseline data collected before randomisation and reported for both treatment groups Data show no significant differences between groups |
Evidence is given that randomisation has led to equal groups, suggesting little risk for selection bias |
| B | Baseline data collected before randomisation and reported for both treatment groups Data show significant differences between groups but have been statistically adjusted appropriately |
Differences have been adjusted; thus the influence of possible selection bias appears to be reduced |
| C | Baseline data collected before randomisation and reported for both treatment groups Data show significant differences between the groups without being statistically adjusted |
Reported differences may reflect ineffective randomisation and thus indicate risk for selection bias |
| 0 | Trial does not comply with criteria A–C | No evidence is given on whether randomisation has indeed led to equal groups with differences beyond chance; thus differences may exist indicating selection bias |
| Blinding/masking | ||
| A | The trial reports on any type of method that is known to prevent the patient AND operator AND evaluator from discerning whether patients have been allocated to the test or control group (blinding/masking) The trial reports a process with which the effect of blinding/masking was evaluated, as well as the results of such evaluation |
Evidence is given that the trial results may not have been influenced by detection/performance bias that may have favoured the outcome of one type of treatment over the other |
| B | The trial reports on any type of method that is known to prevent the patient AND operator AND evaluator from discerning whether patients have been allocated to the test or control group (blinding/masking) The trial report does not give reason for doubt that patient allocation to either the test or control group has been unmasked throughout the duration of the trial |
Doubt may still exist as to whether the trial results are influenced by detection/performance bias, but no indication can be found in the trial report to support such doubt However, no evaluation of the blinding/masking effect has been included in the trial and thus no evidence for lack of bias is given |
| C | The trial reports on any type of method that is known to prevent the patient AND operator AND evaluator from discerning whether patients have been allocated to the test or control group (blinding/masking) The trial report gives reason for doubt that patient allocation to either the test or control group has been unmasked throughout the duration of the trial |
Despite the implementation of a method considered able to prevent unmasking, there are reasons to expect that operators or patients could have discovered the allocation |
| 0 | No process reported or implemented able to blind/mask patients AND operators as to whether patients were allocated to the test or control group (It is insufficient to report that blinding/masking occurred without reporting the details of the process) |
Knowledge about patient allocation may have caused patients or operators to act in a way that may have favoured the outcome of one type of treatment over the other |
| Loss to follow-up | ||
| A | Available case analysis, loss to follow-up reported per treatment group Subsequent sensitivity analysis does not indicate a possible risk for bias effect |
The trial allows the extraction of evidence that loss to follow-up has not favoured the outcome of one type of treatment over the other |
| B | Available case analysis, loss to follow-up reported per treatment group Subsequent sensitivity analysis indicates a possible risk for bias effect |
The trial allows the assessment of the risk that loss to follow-up may have favoured the outcome of one type of treatment over the other |
| 0 | The trial does not report the number of included participants per treatment group at baseline or give any indication that would allow the rate of loss to follow-up per treatment group to be ascertained | The trial carries an unknown risk that loss to follow-up may have favoured the outcome of one type of treatment over the other |
| Trial outcome | ||
| 0 | The trial reports on secondary or surrogate outcomes as endpoints | Even if the surrogate results would highly correlate with primary (i.e. clinical) outcomes, they cannot serve as valid replacements and should be considered for hypothesis development only |
| A | The trial reports on primary outcomes as endpoints | Primary outcomes may provide evidence for hypothesis testing |
Excluded are types of allocation methods that are considered inadequate, such as: cluster randomisation; fixed block randomisation with block size 2; minimisation; alternation; randomisation of teeth; use of date of birth or patient record number; ‘quasi’-randomisation, and split-mouth design.
For example, by reporting results of the Berger–Exner test or any other statistical tests that show that covariates of compared groups were similar at baseline.
For example, by opening the opaque envelope, obtaining allocation from tables, by computer generation or from other sources.
For example, central randomisation, sequence allocation by other than operator; excluding varied block randomisation.
When possible, sensitivity analysis was performed using RevMan Version 4.2 in order to investigate potential risk for attrition bias in trials. Results of continuous datasets were investigated by assuming a worst-case scenario for participants who were lost to follow-up. Such a worst-case scenario was constructed by increasing the mean caries increment by the SD value for the number of participants reported as lost to follow-up in the xylitol (test) group and reducing the mean caries increment by the SD value for the number of participants reported as lost to follow-up in the sorbitol (control) group. These were combined with the patient number (N), mean values and SDs established for participants in both groups for whom follow-up data were available and an adjusted MD (95% CI) and P-value computed. Any changes in statistical significance (P < 0.05) between the adjusted MD and the MD established for participants who were not lost to follow-up were regarded as indicators of risk for attrition bias.
To investigate publication bias, a funnel plot was generated, using suitable datasets from the included clinical trials. The standard error (SE) of the MDs was plotted on the y-axis, and the MD on the x-axis, using mix Version 1.7 meta-analysis software16. In addition, Egger’s linear regression method17 was used to calculate an intercept with a 95% CI, with statistical significance set at α = 0.05.
Any potential confounder influence on the reported trial results was investigated through the use of a directed acyclic graph (DAG)18., 19..
RESULTS
Literature search
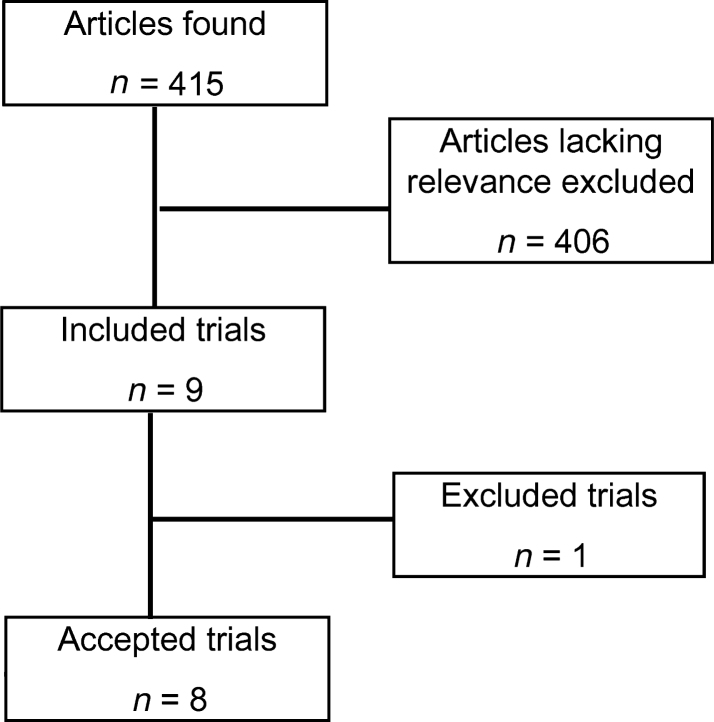
Figure 1 provides information on the number of articles identified by the search strategy. Nine articles were initially included20., 21., 22., 23., 24., 25., 26., 27., 28.. Of these, eight were accepted20., 21., 22., 23., 24., 25., 26., 27. and one was excluded28. The reason for exclusion was lack of computable data (no SD of investigated endpoint per group was reported). Table 2, Table 3, Table 4 describe the characteristics of the included articles and the datasets derived from the results presented in each of them. All eight articles were published in English, in peer-reviewed journals listed in PubMed and reported on findings from six separate clinical trials conducted in Belize City22., 26.; Dayton, Ohio25; Kaunas, Lithuania24, Marshall Islands20; Michigan21 and Stann Creek, Belize23., 26., 27.. All trials followed a parallel-group study design: four used sugar-free chewing gum as the mode of xylitol and sorbitol application (Belize City22., 26.; Kaunas, Lithuania24; Michigan21; Stann Creek, Belize23., 26., 27.); one used syrup (Marshall Islands20) and one trial gave participants the choice of alternating between chewing gum and dragees (pastilles) (Dayton, OH25).
Figure 1.
Flow diagram of trial selection. n, number of trials; DS, dataset number.
Table 2.
Details of accepted studies: design; location; application; dosage, and regime
| Study | DS | Study design | Place of study | Application | Xylitol group | Sorbitol group | ||
|---|---|---|---|---|---|---|---|---|
| Dosage | Regime | Dosage | Regime | |||||
| Milgrom et al.20 | 01 | PG | Marshall Islands | Syrup | 4.00 g xylitol, 2.00 g sorbitol | 2× daily xylitol, 1× daily sorbitol | 2.00 g sorbitol, 2.67 g xylitol | 2× daily sorbitol |
| 02 | PG | Marshall Islands | Syrup | 2.67 g xylitol, 2.00 g sorbitol | 3× daily xylitol, 1× daily sorbitol | 2.00 g sorbitol, 2.67 g xylitol | 1× daily xylitol | |
| Mäkinen et al.21 | 03 | PG | Michigan, USA | Chewing gum | 1.3 g pellet gum, 65% xylitol | 5× daily for 5 (10–20) min | 1.3 g pellet gum, 65% sorbitol | 5× daily for 5 (10–20) min |
| Mäkinen et al.22 | 04 | PG | Belize City | Chewing gum | Pellet gum, 65% xylitol | 5× daily for 5 min | Pellet gum, 65% sorbitol | 5× daily for 5 min |
| Mäkinen et al.26 | 07 | PG | Belize City | Chewing gum | 10.7 g pellet gum, 100% xylitol | 3× daily for 5 min | 10.7 g pellet gum, 100% sorbitol | 3× daily for 5 min |
| 08 | PG | Belize City | Chewing gum | 10.7 g pellet gum, 100% xylitol | 5× daily for 5 min | 10.7 g pellet gum, 100% sorbitol | 5× daily for 5 min | |
| 09 | PG | Belize City | Chewing gum | 10.7 g stick gum, 100% xylitol | 3× daily for 5 min | 10.7 g stick gum, 100% sorbitol | 3× daily for 5 min | |
| 10 | PG | Belize City | Chewing gum | 10.7 g stick gum, 100% xylitol | 5× daily for 5 min | 10.7 g stick gum, 100% sorbitol | 5× daily for 5 min | |
| Mäkinen et al.26 | 11 | PG | Stann Creek, Belize | Chewing gum | 10.7 g pellet gum, 100% xylitol | 5× daily for 5 min | 10.7 g pellet gum, 100% sorbitol | 5× daily for 5 min |
| 12 | PG | Stann Creek, Belize | Chewing gum | 10.7 g stick gum, 100% xylitol | 5× daily for 5 min | 10.7 g stick gum, 100% sorbitol | 5× daily for 5 min | |
| Mäkinen et al.23 | 05 | PG | Stann Creek, Belize | Chewing gum | 10.7 g pellet gum, 100% xylitol | 5× daily for 5 min | 10.7 g pellet gum, 100% sorbitol | 5× daily for 5 min |
| 06 | PG | Stann Creek, Belize | Chewing gum | 10.7 g stick gum, 100% xylitol | 5× daily for 5 min | 10.7 g stick gum, 100% sorbitol | 5× daily for 5 min | |
| Hujoel et al.27 | 13 | PG | Stann Creek, Belize | Chewing gum | 10.7 g pellet gum, 100% xylitol | 5× daily for 5 min | 10.7 g pellet gum, 100% sorbitol | 5× daily for 5 min |
| 14 | PG | Stann Creek, Belize | Chewing gum | 10.7 g stick gum, 100% xylitol | 5× daily for 5 min | 10.7 g stick gum, 100% sorbitol | 5× daily for 5 min | |
| Machiulskiene et al.24 | 15 | PG | Kaunas, Lithuania | Chewing gum | Pellet gum, 589 mg xylitol | 5× daily for 10 min | Pellet gum, 589 mg sorbitol | 5× daily for 10 min |
| 16 | PG | Kaunas, Lithuania | Chewing gum | Pellet gum, 589 mg xylitol | 5× daily for 10 min | Pellet gum, 589 mg sorbitol | 5× daily for 10 min | |
| 17 | PG | Kaunas, Lithuania | Chewing gum | Pellet gum, 589 mg xylitol | 5× daily for 10 min | Pellet gum, 589 mg sorbitol | 5× daily for 10 min | |
| Mäkinen et al.25 | 18 | PG | Dayton, OH, USA | Dragees or gum | 8.5 g xylitol per day | 3–5 dragees per day (3 min) or 5× gum daily after meals (15 min) | 8.5 g sorbitol per day | 3–5 dragees per day (3 min) or 5× gum daily after meals (15 min) |
DS, dataset number; PG, parallel group.
Table 3.
Details of accepted studies: number at baseline; number evaluated; number of events; means, and loss to follow-up
| Study | DS | Xylitol group | Sorbitol group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | N | n | Mean | SD | LtF | BL | N | n | Mean | SD | LtF | ||
| Milgrom et al.20 | 01 | 35 | 33 | Nil | 0.6 | 1.1 | 2 | 32 | 29 | Nil | 1.9 | 2.4 | 3 |
| 02 | 33 | 32 | Nil | 1 | 1.4 | 1 | 32 | 29 | Nil | 1.9 | 2.4 | 3 | |
| Mäkinen et al.21 | 03 | 60 | 37 | Nil | 0.01 | 3.4 | 23 | 65 | 33 | Nil | 0.6 | 5.7 | 32 |
| Mäkinen et al.22 | 04 | 125 | 95 | Nil | −0.8 | 0.5 | 30 | 129 | 120 | Nil | 3.8 | 0.4 | 9 |
| Mäkinen et al.26 | 07 | Nil | 75 | 10 | Nil | Nil | Nil | Nil | 157 | 17 | Nil | Nil | Nil |
| 08 | Nil | 120 | 32 | Nil | Nil | Nil | Nil | 157 | 17 | Nil | Nil | Nil | |
| 09 | Nil | 133 | 20 | Nil | Nil | Nil | Nil | 157 | 17 | Nil | Nil | Nil | |
| 10 | Nil | 176 | 43 | Nil | Nil | Nil | Nil | 157 | 17 | Nil | Nil | Nil | |
| Mäkinen et al.26 | 11 | Nil | 337 | 94 | Nil | Nil | Nil | Nil | 571 | 85 | Nil | Nil | Nil |
| 12 | Nil | 529 | 138 | Nil | Nil | Nil | Nil | 705 | 108 | Nil | Nil | Nil | |
| Mäkinen et al.23 | 05 | Nil | 36 | Nil | 15.5 | 1.08* | Nil | Nil | 60 | Nil | 20.67 | 0.62* | Nil |
| 06 | Nil | 90 | Nil | 24.2 | 0.48* | Nil | Nil | 63 | Nil | 36.4 | 0.72* | Nil | |
| Hujoel et al.27 | 13 | Nil | 2,746 | 32 | Nil | Nil | Nil | Nil | 4,129 | 87 | Nil | Nil | Nil |
| 14 | Nil | 5,234 | 72 | Nil | Nil | Nil | Nil | 4,088 | 83 | Nil | Nil | Nil | |
| Machiulskiene et al.24 | 15 | 126 | 107 | Nil | 5.5 | 0.52† | 19 | 118 | 105 | Nil | 3 | 0.45† | 13 |
| 16 | 126 | 99 | Nil | 8.1 | 0.62† | 27 | 118 | 68 | Nil | 9 | 0.80† | 50 | |
| 17 | 125 | 99 | Nil | 3.2 | 0.3† | 26 | 114 | 71 | Nil | 3.4 | 0.48† | 43 | |
| Mäkinen et al.25 | 18 | 41 | 41 | Nil | 2.6 | 0.55* | 0 | 42 | 42 | Nil | 13.7 | 2.64* | 0 |
SD calculated from 95% confidence interval (asymmetric).
SD calculated from 95% confidence interval (symmetric).
DS, dataset number; BL, number at baseline; N, number evaluated; n, number of events; SD, standard deviation; LtF, lost to follow-up; Nil, no information provided.
Table 4.
Details of accepted studies: outcome measure; evaluation criteria; evaluation method; dentition; fluoride exposure, and follow-up period
| Article | DS | Outcome measure | Evaluation criteria | Evaluation method | Dentition | Fluoride exposure | Follow-up period |
|---|---|---|---|---|---|---|---|
| Milgrom et al.20 | 01 | Prevention: number of decayed teeth | Cavitated carious lesion | Clinical exam: dental mirror, artificial light | Primary | None | 10.5 months |
| 02 | Prevention: number of decayed teeth | Cavitated carious lesion | Clinical exam: dental mirror, artificial light | Primary | None | 10.5 months | |
| Mäkinen et al.21 | 03 | Prevention: DMFS increment | WHO codes 2–4 | Clinical exam: dental mirror, artificial light, sharp probe | Permanent | Topical | 20 months |
| Mäkinen et al.22 | 04 | Prevention: DMFS increment | WHO codes 2–4 | Clinical exam: dental mirror, artificial light, sharp probe | Permanent | Toothpaste | 40 months |
| Mäkinen et al.26 | 07 | Therapy: rehardening of carious lesions | Arrested dentine caries with/without pulp involvement | Clinical exam: dental mirror, artificial light, sharp probe | Permanent | Toothpaste | 40 months |
| 08 | Therapy: rehardening of carious lesions | Arrested dentine caries with/without pulp involvement | Clinical exam: dental mirror, artificial light, sharp probe | Permanent | Toothpaste | 40 months | |
| 09 | Therapy: rehardening of carious lesions | Arrested dentine caries with/without pulp involvement | Clinical exam: dental mirror, artificial light, sharp probe | Permanent | Toothpaste | 40 months | |
| 10 | Therapy: rehardening of carious lesions | Arrested dentine caries with/without pulp involvement | Clinical exam: dental mirror, artificial light, sharp probe | Permanent | Toothpaste | 40 months | |
| Mäkinen et al.26 | 11 | Therapy: rehardening of carious lesions | Arrested dentine caries with/without pulp involvement | Clinical exam: dental mirror, artificial light, sharp probe | Primary, first permanent molars | Toothpaste | 18 months |
| 12 | Therapy: rehardening of carious lesions | Arrested dentine caries with/without pulp involvement | Clinical exam: dental mirror, artificial light, sharp probe | Primary, first permanent molars | Toothpaste | 18 months | |
| Mäkinen et al.23 | 05 | Prevention: caries increment | WHO codes 2–4 | Clinical exam: dental mirror, artificial light, sharp probe | Primary | Toothpaste | 24 months |
| 06 | Prevention: caries increment | WHO codes 2–4 | Clinical exam: dental mirror, artificial light, sharp probe | Primary | Toothpaste | 24 months | |
| Hujoel et al.27 | 13 | Prevention: caries onset on previously caries-free surfaces at risk | WHO codes 2 and higher | Clinical exam: dental mirror, artificial light, sharp probe | Permanent | Toothpaste | 5 years |
| 14 | Prevention: caries onsets on previously caries-free surfaces at risk | WHO codes 2 and higher | Clinical exam: dental mirror, artificial light, sharp probe | Permanent | Toothpaste | 5 years | |
| Machiulskiene et al.24 | 15 | Prevention: DMFS increment | All stages of carious lesion | Clinical exam: dental mirror, artificial light, sharp probe | Permanent | Water < 0.2 ppm | 24 months |
| 16 | Prevention: DMFS increment | All stages of carious lesion | Clinical exam: dental mirror, artificial light, sharp probe | Permanent | Water < 0.2 ppm | 3 years | |
| 17 | Prevention: DMFS increment | All stages of carious lesion | X-Ray: Bitewing | Permanent | Water < 0.2 ppm | 3 years | |
| Mäkinen et al.25 | 18 | Prevention: SGRSC lesion onset per 1,000 surfaces per year | Soft, brown/yellow | Clinical exam: dental mirror, artificial light, sharp probe | Root surfaces | Nil | 1.8 years |
DS, dataset number; DMFS, decayed/missing/filled surface increment; SGRSC, supragingival root surface caries; WHO, World Health Organization.
All trials investigated the ability of xylitol to prevent caries in comparison with that of sorbitol. Two trials also investigated a possible therapeutic effect on carious lesions (Belize City26; Stann Creek, Belize26).
Dataset extraction and analysis
Ten continuous20., 21., 22., 23., 24., 25. and eight dichotomous26., 27. datasets were extracted from the eight accepted articles (Tables 5 and 6). Of these, seven continuous (DS 01, 04–06, 16–18) and six dichotomous (DS 08, 10–14) datasets were in favour of xylitol; two continuous (DS 02, 03) and two dichotomous (DS 07, 09) datasets showed no difference between xylitol and sorbitol, and one continuous dataset indicated in favour of sorbitol (DS 15). All datasets differed from one another in at least one clinical characteristic in terms of: type of data; mode of application; application regime; dosage; outcome measure; evaluation criteria and method; type of dentition, or length of follow-up period (Tables 2 and 4). Therefore, no meta-analysis of datasets was conducted. The results of the dichotomous datasets in favour of xylitol suggest that:
Table 5.
Results per extracted dataset for continuous data
| Study | DS | Continuous data | LtF corrected | ||||
|---|---|---|---|---|---|---|---|
| MD | 95% CI | P-value | MD | 95% CI | P-value | ||
| Milgrom et al.20 | 01 | −1.30 | −2.25 to −0.35 | 0.007* | −0.42 | −1.30 to 0.46 | 0.35 |
| 02 | −0.90 | −1.01 to 0.21 | 0.08 | −0.04 | −0.98 to 0.90 | 0.93 | |
| Mäkinen et al.21 | 03 | −0.59 | −2.82 to 1.64 | 0.60 | 3.63 | 2.43–4.83 | <0.00001† |
| Mäkinen et al.22 | 04 | −4.60 | −4.72 to −4.48 | <0.00001* | −4.31 | −4.41 to −4.21 | <0.00001* |
| Mäkinen et al.23 | 05 | −5.15 | −5.54 to −4.76 | <0.00001* | |||
| 06 | −12.23 | −12.43 to −12.03 | <0.00001* | ||||
| Machiulskiene et al.24 | 15 | 2.50 | 2.37–2.63 | <0.00001† | 2.24 | 2.34–2.45 | <0.00001† |
| 16 | −0.90 | −1.13 to −0.67 | <0.00001* | −0.43 | −0.58 to −0.28 | <0.00001* | |
| 17 | −0.20 | −0.33 to −0.07 | 0.002* | 0.04 | −0.04 to 0.12 | 0.34 | |
| Mäkinen et al.25 | 18 | −11.10 | −11.92 to −10.28 | <0.00001* | |||
DS, dataset; LtF, lost to follow-up; MD, mean difference; 95% CI, 95% confidence interval.
Difference statistically significant in favour of xylitol.
Difference statistically significant in favour of sorbitol.
Table 6.
Results per extracted dataset for dichotomous data
| Study | DS | Dichotomous data | ||
|---|---|---|---|---|
| RR | 95% CI | P-value | ||
| Mäkinen et al.26 | 07 | 1.23 | 0.59–2.56 | 0.58 |
| 08 | 2.46 | 1.44–4.22 | 0.001* | |
| 09 | 1.39 | 0.76–2.54 | 0.29 | |
| 10 | 2.26 | 1.34–3.79 | 0.002* | |
| 11 | 1.87 | 1.44–2.43 | <0.00001* | |
| 12 | 1.7 | 1.36–2.13 | <0.00001* | |
| Hujoel et al.27 | 13 | 0.55 | 0.37–0.83 | 0.004* |
| 14 | 0.68 | 0.50–0.93 | 0.01* | |
DS, dataset; RR, relative risk; 95% CI, 95% confidence interval.
Difference statistically significant in favour of xylitol.
-
•
Chewing 10.5 g of gum containing 100% xylitol five times daily for 5 min per time is associated with a 3.5-times (250%) greater rehardening of soft brownish discoloured carious lesions in permanent teeth after 40 months than occurs if the same gum is used with sorbitol (DS 08, 10)
-
•
Chewing 10.5 g of gum containing 100% xylitol five times daily for 5 min per time is associated with a 2–3-times (100–200%) greater rehardening of soft brownish discoloured carious lesions in primary teeth and first permanent molars after 18 months than occurs if the same gum is used with sorbitol (DS 11, 12)
-
•
Chewing 10.5 g of gum containing 100% xylitol for 5 min five times daily for 2 years is associated with 50–70% fewer caries lesions on previously caries-free surfaces of permanent teeth after 5 years than if the same gum was used with sorbitol (DS 13, 14).
The results of the continuous datasets in favour of xylitol suggest that:
-
•
A twice daily intake of syrup with 400 g xylitol plus a once daily intake of syrup with 200 g sorbitol is associated with a lower mean increment of cavitated caries lesions in permanent teeth after 10.5 months than occurs with a twice daily intake of syrup with 200 g sorbitol plus a once daily intake of syrup with 2.67 g xylitol (DS 01)
-
•
Chewing gum with 65% xylitol for 5 min five times daily is associated with a lower mean decayed/missing/filled surface (DMFS) increment after 40 months than occurs if the same gum is chewed with sorbitol (DS 04)
-
•
Chewing 10.5 g of gum with 100% xylitol five times daily for 5 min each time is associated with a lower mean caries increment in primary teeth after 24 months than occurs if the same gum is chewed with sorbitol (DS 05, 06)
-
•
Chewing gum with 589 mg xylitol five times daily for 10 min each time is associated with a lower mean DMFS increment after 3 years than occurs if the same gum is chewed with sorbitol (DS 16, 17)
-
•
A daily intake of 8.5 g xylitol, either through using three to five dragees (5 min) and/or chewing gum five times per day for 5 min, is associated with a lower mean number of supragingival root surface caries lesions after 1.8 years than occurs if 8.5 g sorbitol is used (DS 18).
By contrast, no difference between xylitol and sorbitol was found:
-
•
In the rehardening of lesions in permanent teeth after 40 months, if 10.5 g of gum containing 100% xylitol was chewed for 5 min three times daily (DS 07, 09)
-
•
In the mean increment of cavitated caries lesions in permanent teeth after 10.5 months, after a thrice daily intake of syrup with 2.67 g xylitol plus a once daily intake of syrup with 200 g sorbitol (DS 02)
-
•
In the DMFS increment after 20 months if 1.3 g of gum containing 65% xylitol was chewed for 10–20 min five times daily (DS 03).
Moreover, a lower DMFS increment after 24 months was observed if gum with 589 mg sorbitol was chewed for 10 min five times daily than was observed if the same gum was used with xylitol (DS 15).
Quality assessment of trials and risk for bias
Risk for selection, detection and performance bias
The results of the quality assessment regarding selection and detection or performance bias are shown in Table 7. None of the accepted trials reported sufficient details of any randomisation process that would have given each patient the same chance of being allocated to the xylitol or the sorbitol group and ensured that direct observation and prediction of the allocation sequences was successfully prevented.
Table 7.
Results of quality assessment of trials and datasets according to criteria defined in Table 1
| Study | DS | Selection bias* | Detection/performance bias* | Attrition bias* | Run-in phase* | Trial outcome* | |
|---|---|---|---|---|---|---|---|
| Randomisation | Baseline data | Blinding/masking | LtF | ||||
| Milgrom et al.20 | 01 | D | 0 | B | B | 0 | A |
| 02 | D | 0 | B | A | 0 | A | |
| Mäkinen et al.21 | 03 | 0 | 0 | B | B | A | A |
| Mäkinen et al.22 | 04 | 0 | C | B | A | A | A |
| Mäkinen et al.26 | 07 | 0 | C | B | 0 | A | A |
| 08 | 0 | C | B | 0 | A | A | |
| 09 | 0 | C | B | 0 | A | A | |
| 10 | 0 | C | B | 0 | A | A | |
| Mäkinen et al.26 | 11 | 0 | C | B | 0 | A | A |
| 12 | 0 | C | B | 0 | A | A | |
| Mäkinen et al.23 | 05 | 0 | C | B | 0 | A | A |
| 06 | 0 | C | B | 0 | A | A | |
| Hujoel et al.27 | 13 | 0 | C | B | 0 | A | A |
| 14 | 0 | C | B | 0 | A | A | |
| Machiulskiene et al.24 | 15 | 0 | C | B | A | A | A |
| 16 | 0 | C | B | A | A | A | |
| 17 | 0 | C | B | B | A | A | |
| Mäkinen et al.25 | 18 | 0 | A | B | A | A | A |
A–D and 0, see criteria defined in Table 1.
DS, dataset; LtF, lost to follow-up.
Only the Marshall Islands trial20 reported details of random allocation by stating that block randomisation was used. However, no information was given on how the allocation of the random sequence was concealed in order to prevent its direct observation or accurate prediction. Additionally, this trial included a 3-week run-in period preceding randomisation, during which all participants received doses of xylitol20. All other trials either conducted cluster randomisation of schools instead of participants (Lithuania24) or reported the systematic assignment of participants to either group without randomisation (Belize City22., 26.; Michigan21; Stann Creek, Belize23., 26., 27.; Dayton, OH25).
In order to indicate whether both groups had similar characteristics and were thus essentially comparable, only two trials reported baseline data per group: in one trial (Lithuania) baseline data showed that groups differed significantly (P < 0.02) in the age of participants and total number of tooth surfaces included24. The baseline data in the other trial (Dayton, OH), including mean age of participants, mean number of active lesions, mean number of root surfaces at risk and mean number of drugs used daily, showed no statistically significant differences between the two groups (P > 0.05)25. The trial in Belize City did report baseline data22., 26.. However, these baseline data were not related to the outcome data (prevented DMFS increment, number of rehardened carious lesions) extracted from this trial for review.
All of the accepted trials reported on methods used for preventing participants, operators and evaluators from discerning which treatment group participants had been allocated to. However, no evaluation regarding the success of the blinding and masking was included in any trial.
Risk for attrition bias
Sensitivity analysis regarding whether the number of patients lost to follow-up would have had any influence on the results was possible for only seven continuous datasets (DS 01–04, 15–17) reported in four articles20., 21., 22., 24.. For all other datasets, the information reported on the number of participants at baseline or number of participants lost to follow-up at the time of assessment was insufficient to enable sensitivity analysis. The new adjusted MD (95% CI) and P-values of the datasets analysed are shown in Table 3.
Of the seven datasets, the conclusions of three (DS 01, 03, 17) would change if a worst-case scenario was assumed for all participants lost to follow-up in the xylitol and sorbitol groups:
-
•
DS 01: a twice daily intake of syrup with 400 g xylitol plus a once daily intake of syrup with 200 g sorbitol would not be associated with a lower mean increment of cavitated caries lesions in permanent teeth after 10.5 months20
-
•
DS 03: chewing 1.3 g of 65% sorbitol gum for 10–20 min five times daily would be associated with a lower mean DMFS increment after 20 months than if the same gum was used with xylitol21
-
•
DS 17: chewing gum with 589 mg xylitol for 10 min five times daily would not be associated with a lower mean DMFS increment after 3 years25.
Risk for publication bias
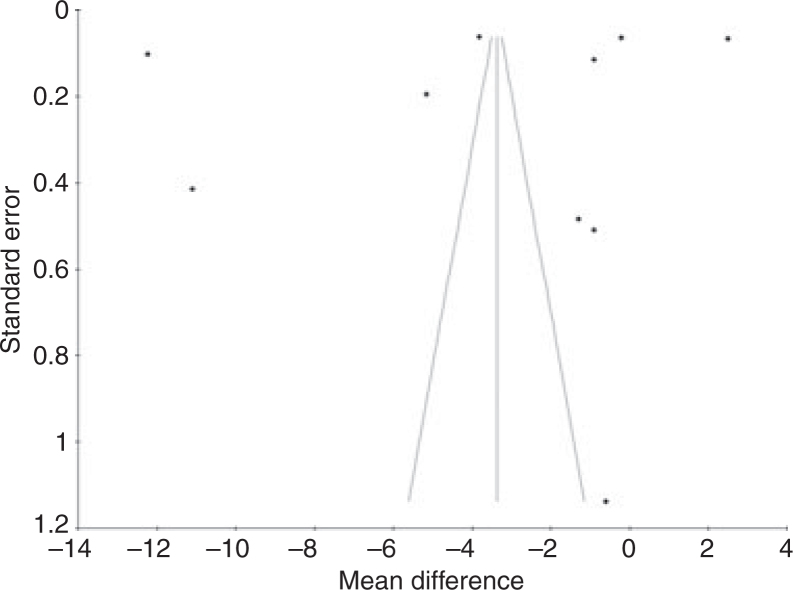
Because of the small number of dichotomous datasets available, publication bias was investigated using only the 10 continuous datasets by funnel plot and Egger’s regression. The generated funnel plot (Figure 2) showed an even distribution that did not suggest publication bias. Egger’s linear regression method for the same datasets showed an intercept of − 15.36 (95% CI − 69.53 to 38.8; P = 0.53).
Figure 2.
Funnel plot of dataset results (test for publication bias).
Risk for confounding
A directed acyclic graph was used to explore the influences of confounding factors within the accepted trials on the measured preventive effect of xylitol ([E] in Figure 3). Directed acyclic graphs have been developed to graphically evaluate causal effects and to identify multiple confounders within a causal system18., 19.. These graphs display a web of causation and consist of variables represented by alphabet letters (A, B, …) and arrows that represent direct causal links between these variables.
Figure 3.
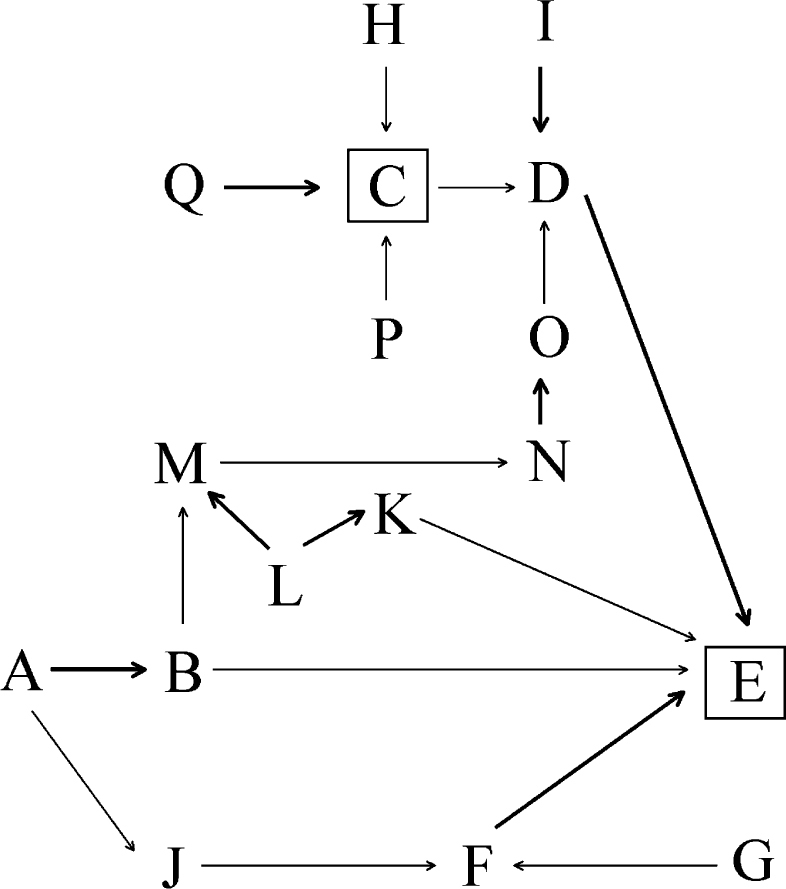
Directed acyclic graph: investigation of bias and confounding factors. A, group selection; B, group characteristics; C, actual affect of xylitol; D, caries; E, measured effect of xylitol; F, subjects evaluated, n; G, loss to follow-up; H, time; I, fluoride effect; J, subjects at baseline, n; K, evaluation process; L, foreknowledge; M, group behaviour; N, gum chewing; O, saliva flow effect; P, application regime effect; Q, dosage effect. [A→B], selection bias risk; [F→E], attrition bias risk; [I→D], fluoride effect confounder risk; [L→M/L→K] detection/performance bias risk; [N→O], gum chewing confounder risk.
The constructed DAG shown in Figure 3 reflects the causal connection of selection, detection or performance and attrition bias effects on the measured outcome [E]. The measured outcome [E] is affected by selection bias through the form of group selection [A] via its influence on group characteristics [B]. The measured outcome [E] is further affected by attrition bias if loss to follow-up [G] alters the number of evaluated subjects [F], which then, in turn, may no longer be representative of the combined characteristics of the original group of subjects [J] selected at baseline. Foreknowledge [L] amongst trial participants about who has been selected to which treatment group may alter group behaviour [M], which may affect the level of gum chewing [N] which, in turn, can be linked to the effect that chewing has on saliva flow [N] and its subsequent anticariogenic impact [O] (= performance bias). Such foreknowledge [L] amongst trial evaluators may affect the evaluation process by favouring one treatment group over another [K], and thus directly affect the measured outcome [E] (= detection bias).
In addition to selection, detection or performance and attrition bias, the DAG in Figure 3 shows the influence of further factors, such as dosage [Q], application regime [P] and time [H], on the actual preventive effect of xylitol [C] and thus, on the number of actually prevented or treated caries lesions [D]. As well as the actual preventive effect of xylitol [C], the number of actually prevented or treated caries lesions [D] is potentially also affected by the preventive effect of fluoride [I] and the anticariogenic impact of stimulated saliva flow [O]. In addition, the type and process of caries measurement may affect the degree to which the measured preventive effect of xylitol [E] is represented [D].
DISCUSSION
Quantitative synthesis in systematic reviews allows for the detection of statistically significant (P < 0.05) treatment effects and higher precision in effect estimation29. This provides a more objective assessment of the currently available evidence. In this case, the presumed anticariogenic effect of xylitol in comparison with that of sorbitol was investigated.
Systematic literature search
This systematic review employed a broad search strategy with very unrestrictive inclusion and exclusion criteria for trials. This resulted in the acceptance of any investigation that compared the clinical efficacy of xylitol with that of sorbitol in caries prevention and therapy. Unlike common recommendations for the conduct of systematic reviews29, the decision not to restrict acceptance was based on criteria related to the internal validity of trials. This highly minimised the exclusion of trials from review, thus allowing for the inclusion or coverage of the widest possible range of available information on this topic. Nevertheless, only eight of the articles found could be accepted (Figure 1). The number of trials with actual relevance to the review topic (n = 6) was even lower. Although no restrictions on the search were made in terms of language of publication, no articles published in languages other than English were found. All of these articles were listed in PubMed, indicating that the search in all other sources did not yield any additional results. This suggests that there is a general lack of available trials covering this topic and that the adoption of a broad search strategy with very unrestrictive inclusion and exclusion criteria for trials was appropriate. Such a search strategy assisted in the composition of a comprehensive overview of current evidence on the topic, as well as in subjecting the found evidence to:
-
•
A detailed analysis of available trial results
-
•
An in-depth evaluation of the validity of these results in light of potential risk for bias and confounders.
This two-point approach made it possible to report on the status of currently available evidence and to use the conclusions as a basis for recommendations concerning the need for further research on this topic.
Trial results
Most of the extracted datasets showed in favour of xylitol (Tables 5 and 6). Nevertheless, the results are inconsistent and conflicting. Rehardening of significantly more caries lesions (DS 08, 10) was not observed after the same type of xylitol gum was chewed three times instead of five times per day (DS 07, 09). This suggests that the process of gum chewing may play an important role in the therapeutic effect originally observed. The chewing of 65% xylitol gum five times per day was observed to have a preventive effect against caries after 40 months (DS 04), but not after 20 months although chewing time in the latter case was twice as long (DS 03). This suggests the influence of a time factor. Similarly, chewing gum containing 589 mg sorbitol over a 2-year period was observed to be more effective in preventing caries than chewing 589 mg xylitol gum according to the same chewing regime (DS 15). However, in the same trial, 589 mg xylitol gum was observed to be more preventive than sorbitol after 3 years (DS 16, 17). In addition, the three times per day intake of 2.67 g xylitol syrup over 10.5 months (DS 02) was no more effective in preventing caries than a twice daily intake of 4.00 g (DS 01). Here, a dosage effect regardless of the frequency of intake may be assumed.
Alternatively, rather than reflecting differences in the assumed effects of gum chewing, time or dosage, the inconsistencies observed in the results may reflect either chance (in the absence of any actual effect of xylitol over sorbitol) or systematic error (bias).
Risk for selection, detection and performance bias
Quality assessment showed that the findings of all of the accepted trials appeared to be limited by risk for selection bias (Table 7).
It has been emphasised that selection bias can only be successfully prevented if the allocation sequence remains truly random and free from potential interference throughout the trial14., 15.. Thus, it is important that trials should include an effective process for concealing the random allocation sequence and that, by the end of each trial, this process should indeed have prevented the direct observation and prediction of the random sequence allocation14., 15.. Quality assessment in terms of the internal validity of trials should, therefore, be a measure of the result of random sequence allocation and allocation concealment, not only of its being reported.
All of the trials accepted in this systematic review failed to report evidence of the successful concealment of the random sequence allocation. Only one article described the inclusion of a reasonable form of random sequence generation20. However, the same trial also employed a run-in phase before randomisation. A run-in phase is considered to be a pre-trial stage in which, for example, all potential subjects receive the test treatment; subsequently, only those who responded well to the test treatment are randomly allocated to either the control or the test group15. Such a practice would effectively exclude some potential candidates from the randomisation process and would favour the type of treatment used during the run-in phase over the other in the subsequent trial30. A treatment effect overestimation of 54% caused by lack of allocation concealment has been reported31. Under a condition of a 50% overestimation, the actual result for a test treatment would be a 20% higher RR (1.20) in comparison with the control, whereas the trial report would claim a 20% lower RR (0.80). This fact adds perspective regarding the seriousness of such overestimation. Thus, the true result of a trial would constitute the complete opposite of the reported result and therefore all trial results identified in this systematic review must be interpreted with caution.
Despite the lack of adequate randomisation, one trial provided evidence that its two treatment groups did not differ significantly in selected covariables (mean age of participants, mean number of active lesions, mean number of root surfaces at risk, mean number of drugs used daily) at baseline25. However, the lack of significant differences in these covariables does not serve as evidence that the groups did not differ in other unknown covariables and thus does not refute the risk related to selection bias in this trial.
All trials reported on adequate methods for blinding participants and evaluators to the knowledge of which participants received xylitol or sorbitol. Thus, the risk for detection and performance bias may be small. There is, nonetheless, a risk that the treatment allocation could have been unmasked during the trials as none of the articles reported on the methods used to monitor blinding and the results of these. This type of risk has been quantified as potentially causing overestimation of the true treatment effect by 53%31.
Risk for attrition and publication bias
Attrition bias may be introduced when participants who have been allocated to a treatment group are excluded from the final data analysis32. The most common reason for such exclusion is that participants are lost to follow-up because they become unavailable for data collection. If the number of participants lost to follow-up and the number of participants included at baseline per intervention group is reported, sensitivity analysis can be used in order to quantitatively assess whether the loss to follow-up would have influenced the results of the data analysis. Of the articles accepted in this review, only four20., 21., 22., 24. reported sufficient information to subsequently allow for the quantitative investigation of attrition bias risk for seven (DS 01–04, 15–17) of the 18 datasets. This means that the validity of the remaining 11 dataset results should be regarded with caution as it remains unclear whether or not these may have been limited by potential attrition bias. Sensitivity analysis results of the seven datasets that could be investigated show that three datasets (DS 01, 03, 17) would lead to different conclusions if a worst-case scenario were assumed. These conclusions further contradict the results reported in favour of xylitol. As the true results for participants who were lost to follow-up remain unknown, the results of the assumed worst-case scenario cannot be accepted as evidence for clinical consideration. Nonetheless, the sensitivity analysis results provide reason for doubting the validity of the MDs provided by the reported datasets (DS 01, 03, 17) and thus for doubting the value of their evidence for considering xylitol more anticariogenic than sorbitol.
Publication bias was investigated by generating a funnel plot (Figure 2) and by using Egger’s regression. This type of bias is present when the results of published research differ from those of all previous studies 33. Funnel plots are scatter graphs showing the size of studies, expressed in their SE, on the y-axis (large studies on top, small studies at the bottom) and the effect size observed in these studies on the x-axis. As this review used only continuous datasets to do this, the effect size in the funnel plot is expressed as the MD. The effect sizes of larger studies tend to cluster near the mean. Small studies have effect sizes that are dispersed across a wider range. The results of both types of studies, plotted on a scatter graph, give the shape of an inverted, in the absence of publication bias, symmetrical funnel34. Publication bias affects a funnel plot by concentrating studies to one side only (asymmetry). Such asymmetry is created when particular smaller studies are published only when they show an effect that is larger than average. However, if the number of studies (n) is < 10, any asymmetry may reflect chance and not publication bias35. For that reason, the decision was made to plot results of the 10 extracted dichotomous datasets as units of investigation. These are not all independent from the published trials and this forms a departure from the common application of funnel plots in investigating for publication bias. Despite this departure, the use of datasets (instead of published trials) will also indicate potential publication bias when only datasets that show a larger than average effect are published and other datasets are not. Although in this review the funnel plot covering continuous data did not show an asymmetrical spread, its spread of dataset results appears rather random and does not show the tendency of larger trials to cluster near the mean. This suggests that there are too few data available on the topic to allow a reliable test for publication bias. The calculated non-significant intercept using Egger’s regression appears to confirm the observations from the funnel plot of a more symmetric rather than asymmetric spread of trial results. Thus, both the funnel plot and Egger’s regression result suggest that risk for publication bias may be low. However, because of the lack of sufficient data, this conclusion should be regarded with caution and, if necessary, revised in the future once more trials on this topic are available.
Risk for confounding
In addition to the risk for biases, the DAG in Figure 3 also suggests an influence on the measured anticariogenic effect of xylitol [E] during the trials by the factors: dosage [Q]; application regime [P]; time (duration of intervention) [H]; fluoride exposure [I], and stimulated saliva flow [O].
A higher concentration of xylitol might be assumed to lead to a larger anticariogenic (preventive/therapeutic) effect [Q→C]. The result of DS 01 concerning the intake of 4.00 g instead of 2.67 g xylitol in syrup supports this assumption. However, this evidence for a dose–response effect may be disregarded on the basis of the high risk for selection and attrition bias. No clear direction may be ascertained from the available evidence regarding the influence of different application regimes [P→C] (Tables 2, 5 and 6). The effect shown in favour of xylitol for chewing gum two more times daily [five times daily (DS 08, 10) vs. three times daily (DS 07, 09)] may be attributed to the higher frequency of saliva stimulation caused by chewing plus differences in characteristics of the xylitol and sorbitol groups (selection bias risk), rather than to any assumed anticariogenic effect of xylitol alone. The effect of time [H→C] in the form of a longer application of xylitol as the sole cause of any anticariogenic effect appears weak. The evidence from DS 04 suggests that a 20-month longer period of application would affect a reversal, from ‘sorbitol’s being more effective than xylitol after 20 months (DS 03)’ to ‘xylitol’s being more effective than sorbitol after 40 months’. Time would not only have bearing on the effect of xylitol (in this case by increasing its assumed anticariogenic effect), but would also affect other factors (i.e. by increasing the anticariogenic effects of saliva stimulation or fluoride exposure). In addition, such causalities need to be considered under the category of potential differences between the xylitol and sorbitol groups in their characteristics (selection bias risk) because such differences may have promoted or inhibited the impact of any factor to different degrees in the xylitol and sorbitol groups over time.
The largest potential confounder risk affecting the measured anticariogenic effect of xylitol [E] during the trials may originate from fluoride exposure [I→D→E] and stimulated saliva flow [O→D→E], both combined with the high risk for selection bias [A→B→E] (Figure 3). Potential access to fluoride sources was reported in the results of 15 of the 18 extracted datasets and 16 of the 18 datasets were extracted from trials that used chewing gum as the mode of application (Tables 2 and 4). As none of the accepted trials describe adequate randomisation, differences in group characteristics may have led to participants’ responding differently, either to fluoride exposure or to saliva flow stimulation through gum chewing. Both effects are anticariogenic and, under the condition of high selection bias risk, may have confounded the measured anticariogenic effect of xylitol [E].
Recommendations for further research
Evidence from systematic reviews can only be as good as the quality of the trials reviewed. The trials accepted in this quantitative systematic review are at high risk for selection and attrition bias, as well as for potential confounder effects caused by fluoride exposure and saliva stimulation. All the accepted trials may have sufficiently controlled the potential risk for performance and detection bias. However, evidence that such bias control was indeed effective throughout the duration of the trials is missing. There is weak evidence that the overall results on this topic have not been influenced by publication bias. Therefore, the results should be regarded with caution and require verification. Future high-quality randomised controlled trials (RCTs) are needed. Such trials should adopt a parallel group design that allows the use of randomisation and allocation concealment methods which can effectively prevent the direct observation and prediction of the random allocation sequence. The inclusion of tests (such as the Berger–Exner test) is suggested in order to enable trialists to investigate whether selection bias has been introduced into their studies14., 15.. Where bias risk has been found, it may be statistically adjusted14 and these outcomes should be included in the final trial report. In order to ensure that lack of blinding may not have led to the favouring of one treatment over the other, trials should adopt and report procedures within their methodology and, consequently, be able to provide quantitative evidence that the established trial results were not affected by performance and detection bias. In order to enable sensitivity analysis regarding the potential risk for attrition bias, the number of participants in each intervention group at baseline, as well as the numbers of those lost to follow-up, should be clearly reported. It is further recommended that future RCTs should base their reporting on the CONSORT statement36.
CONCLUSIONS
Xylitol has been assumed to have specific anticariogenic properties. Any evidence in support of such an assumption would necessarily include a clear demonstration of the superior effects of xylitol in caries prevention and therapy in comparison with those of sorbitol. The aim of this quantitative systematic review was to appraise the evidence in comparisons of the effects of xylitol and sorbitol. The results of this systematic literature search with broad inclusion and exclusion criteria indicate a general lack of trials covering this topic. Moreover, the evidence found in support of xylitol over sorbitol is contradictory, contains a high risk for selection and attrition bias and may be limited by confounder effects. Future high-quality RCTs are needed to provide conclusive evidence on this topic.
Acknowledgement
The authors wish to thank Professor Paul Fatti from the School of Statistics and Actuarial Science, University of the Witwatersrand, for his valuable advice during the writing of this paper.
Conflicts of interest
None declared.
REFERENCES
- 1.Imfeld T. Efficacy of sweeteners and sugar substitutes in caries prevention. Caries Res. 1993;27(Suppl 1):50–55. doi: 10.1159/000261603. [DOI] [PubMed] [Google Scholar]
- 2.Van Loveren C. Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Res. 2004;38:286–293. doi: 10.1159/000077768. [DOI] [PubMed] [Google Scholar]
- 3.Burt BA. Xylitol chewing gum/pastilles and reduction of the risk of tooth decay. EFSA J. 2008;852:1–15. [Google Scholar]
- 4.Burt BA. The use of sorbitol- and xylitol-sweetened chewing gum in caries control. J Am Dent Assoc. 2006;137:190–196. doi: 10.14219/jada.archive.2006.0144. [DOI] [PubMed] [Google Scholar]
- 5.Tanzer JM. Xylitol chewing gum and dental caries. Int Dent J. 1995;45:65–76. [PubMed] [Google Scholar]
- 6.Grenby TH, Phillips A, Mistry M. Studies of the dental properties of lactitol compared with five other bulk sweeteners in vitro. Caries Res. 1989;23:315–319. doi: 10.1159/000261199. [DOI] [PubMed] [Google Scholar]
- 7.Topitsoglou V, Birkhed D, Larsson LA, et al. Effect of chewing gums containing xylitol, sorbitol or a mixture of xylitol and sorbitol on plaque formation, pH changes and acid production in human dental plaque. Caries Res. 1983;17:369–378. doi: 10.1159/000260690. [DOI] [PubMed] [Google Scholar]
- 8.Vadeboncoeur C, Trahan L, Mouton C, et al. Effect of xylitol on the growth and glycolysis of acidogenic oral bacteria. J Dent Res. 1983;62:882–884. doi: 10.1177/00220345830620080601. [DOI] [PubMed] [Google Scholar]
- 9.Mäkinen KK. Sugar alcohols, caries incidence, and remineralisation of caries lesions: a literature review. Int J Dent 2010: 981072. [DOI] [PMC free article] [PubMed]
- 10.Chandler N. Sorbitol – safe for teeth. N Z Dent J. 1992;88:66. [PubMed] [Google Scholar]
- 11.Gales MA, Nguyen TM. Sorbitol compared with xylitol in prevention of dental caries. Ann Pharmacother. 2000;34:98–100. doi: 10.1345/aph.19020. [DOI] [PubMed] [Google Scholar]
- 12.Lingström P, Holm AK, Mejàre I, et al. Dietary factors in the prevention of dental caries: a systematic review. Acta Odontol Scand. 2003;61:331–340. doi: 10.1080/00016350310007798. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande A, Jadad AR. The impact of polyol-containing chewing gums on dental caries: a systematic review of original randomised controlled trials and observational studies. J Am Dent Assoc. 2008;139:1602–1614. doi: 10.14219/jada.archive.2008.0102. [DOI] [PubMed] [Google Scholar]
- 14.Berger VW. John Wiley & Sons; Chichester: 2005. Selection Bias and Covariate Imbalances in Randomised Clinical Trials; pp. 9–15. [Google Scholar]
- 15.Berger VW, Alperson SY. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4:79–88. doi: 10.2174/157488709788186021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bax L, Yu LM, Ikeda N, et al. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearl J. Causal diagrams for empirical research. Biometrika. 1995;82:669–710. [Google Scholar]
- 19.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 20.Milgrom P, Ly KA, Tut OK, et al. Xylitol paediatric topical oral syrup to prevent dental caries: a double-blind randomised clinical trial of efficacy. Arch Pediatr Adolesc Med. 2009;163:601–607. doi: 10.1001/archpediatrics.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mäkinen KK, Mäkinen PL, Pape HR, Jr, et al. Conclusion and review of the Michigan Xylitol Programme (1986–1995) for the prevention of dental caries. Int Dent J. 1996;46:22–34. [PubMed] [Google Scholar]
- 22.Mäkinen KK, Bennett CA, Hujoel PP, et al. Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res. 1995;74:1904–1913. doi: 10.1177/00220345950740121501. [DOI] [PubMed] [Google Scholar]
- 23.Mäkinen KK, Hujoel PP, Bennett CA, et al. Polyol chewing gums and caries rates in primary dentition: a 24-month cohort study. Caries Res. 1996;30:408–417. doi: 10.1159/000262352. [DOI] [PubMed] [Google Scholar]
- 24.Machiulskiene V, Nyvad B, Baelum V. Caries preventive effect of sugar-substituted chewing gum. Community Dent Oral Epidemiol. 2001;29:278–288. doi: 10.1034/j.1600-0528.2001.290407.x. [DOI] [PubMed] [Google Scholar]
- 25.Mäkinen KK, Pemberton D, Mäkinen PL, et al. Polyol-combinant saliva stimulants and oral health in Veterans Affairs patients – an exploratory study. Spec Care Dentist. 1996;16:104–115. doi: 10.1111/j.1754-4505.1996.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 26.Mäkinen KK, Mäkinen PL, Pape HR, Jr, et al. Stabilisation of rampant caries: polyol gums and arrest of dentine caries in two longterm cohort studies in young subjects. Int Dent J. 1995;45:93–107. [PubMed] [Google Scholar]
- 27.Hujoel PP, Mäkinen KK, Bennett CA, et al. The optimum time to initiate habitual xylitol gum-chewing for obtaining longterm caries prevention. J Dent Res. 1999;78:797–803. doi: 10.1177/00220345990780031301. [DOI] [PubMed] [Google Scholar]
- 28.Petersson LG, Birkhed D, Gleerup A, et al. Caries-preventive effect of dentifrices containing various types and concentrations of fluorides and sugar alcohols. Caries Res. 1991;25:74–79. doi: 10.1159/000261346. [DOI] [PubMed] [Google Scholar]
- 29.Cochrane Collaboration . Cochrane Collaboration; Oxford: 2006. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6; pp. 97–99. [Google Scholar]
- 30.Berger VW, Vali B. Intent-to-randomise corrections for missing data resulting from run-in selection bias in clinical trials for chronic conditions. J Biopharma Stat. 2011;21:263–270. doi: 10.1080/10543406.2011.550107. [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Jüni P, Bartlett C, et al. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7:1–76. [PubMed] [Google Scholar]
- 32.Pocock SJ. John Wiley & Sons; Chichester: 1983. Clinical Trials. A Practical Approach; pp. 182–186. [Google Scholar]
- 33.Rothstein HR, Sutton AJ, Borenstein M. In: Publication Bias in Meta-Analysis – Prevention, Assessment and Adjustment. Rothstein HR, Sutton MJ, Borenstein M, editors. John Wiley & Sons; Chichester: 2005. Publication bias in meta-analysis; pp. 1–7. [Google Scholar]
- 34.Rothstein HR, Sutton AJ, Borenstein M. In: Publication Bias in Meta-Analysis – Prevention, Assessment and Adjustment. Rothstein HR, Sutton MJ, Borenstein M, editors. John Wiley & Sons; Chichester: 2005. Software for publication bias; pp. 193–220. [Google Scholar]
- 35.Lau J, Ioannidis JP, Terrin N, et al. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.3736/jcim20100702. [DOI] [PubMed] [Google Scholar]