Abstract
Objectives: This study was carried out to assess the associations between oral diseases and specifically oral health-related quality of life (OHRQoL) in a nationally representative sample of 12-year-old children in Thailand in order to classify children according to their levels of risk for adverse OHRQoL and to apply findings to formulate proposals for oral health goals. Methods: Oral examinations and OHRQoL interviews using the Child–Oral Impacts on Daily Performances (Child-OIDP) schedule were conducted in 1,100 children as part of the Thailand National Oral Health Survey. The severity of oral impacts was categorised according to their ‘intensity’. Associations of oral diseases and condition-specific (CS) OHRQoL impacts were investigated. Results: Oral impacts were prevalent. Most were of low intensity; these were excluded from the analysis used to develop goals. At the threshold of moderate/high intensity, CS impacts attributable to dental caries, edentulous areas, periodontal disease and discolouration were reported by 18.5%, 0.2%, 8.7% and 2.6% of participants, respectively. Missing teeth was significantly related to CS impacts. Condition-specific impacts were 1.6 times and three to four times more likely to affect children with one decayed tooth and two or more decayed teeth, respectively. Children with gingivitis or calculus in three or more sextants were twice as likely and children with calculus and gingivitis in three or more sextants were 3.5 times more likely to report CS impacts. Based on these findings, these recommendations are proposed: all 12-year-old children should have fewer than two untreated decayed teeth; 60% of 12-year-old children should not have periodontal disease in any form in more than two sextants, and the proportion of 12-year-old children with calculus with gingivitis in three or more sextants should not exceed 5%. Conclusions: Measures of oral health and goals for oral health in children should include measures of OHRQoL.
Key words: Calculus, dental caries, dental health surveys, gingivitis, goals, quality of life
INTRODUCTION
Although the importance of sociodental measures of oral health was highlighted 35 years ago with the recognition that ‘the greatest contribution of dentistry is to the improvement of quality of life’1, very few national goals for oral health include such measures. Sociodental measures, later termed oral health-related quality of life (OHRQoL), emphasise that oral health should not be defined as the absence of oral disease, but should be considered in terms of physical, psychological and social well-being in relation to oral status2. Therefore, oral health goals, in relation to both dental disease prevention and treatment, should not be limited to the improvement of clinical oral conditions only, but should include goals that refer to people’s ability to perform the activities of daily life3. At an individual level, OHRQoL indicators are frequently used in addition to clinical indicators to evaluate treatment outcomes in patients4. At a population level, some countries have included the assessment of OHRQoL in national oral health surveys5., 6., 7., 8., 9. and proposals to integrate OHRQoL into the oral health needs assessments of populations have been made10., 11., 12.. However, little oral health service planning for populations includes sociodental measures of oral health13. In 2003 the World Dental Federation (FDI), World Health Organization (WHO) and International Association for Disability and Oral Health (IADR) proposed global oral health goals for the year 202014 that include some sociodental measures. The FDI/WHO/IADR document was intended to serve as a guideline for oral health planners in the development of goals and plans for oral health by suggesting a range of possible aspects of oral health that should be taken into consideration in oral health service planning. Among them was the improvement of OHRQoL or the minimising of impacts of oral diseases on physical, psychological and social aspects of life14. However, these sociodental goals are very general and are not defined in measurable terms. Although the guideline encouraged local action, such abstract ideas have never been transformed into measurable goals for oral health service planning in any country.
Thailand published national oral health goals for the years 2000 and 2005 using only clinical parameters obtained from national surveys conducted in 1994 and 2000, respectively15. For the 12-year-old population, Thailand aimed to reduce the mean score on the decayed, missing and filled teeth (DMFT) index from 1.6 (in 1994) to 1.5 (in 2000), and to increase the average number of periodontally healthy sextants from 1.4 (in 1994) to three (in 2000). After the national survey in 2000, oral health goals for the 12-year-old population in 2005 were to achieve a mean DMFT score of 1.5 (the figure at that time was 1.6), to increase the percentage of caries-free subjects from 42% to 45%, and to increase the percentage of children with no periodontal disease from 23% to 25%. In 2007, national oral health goals for the year 2020 were published16. Periodontal disease was not included for 12-year-olds, but was for other older groups. The prevalence of dental caries was not cited in the goals because levels were considered to be stable; mean DMFT scores had remained stable at 1.5–1.6 since 1984. The only goal for 12-year-olds was that all children should have all of their first molar teeth. The ultimate goals of oral health, namely, to improve QoL, were based on a theoretical framework of connections between oral health status, oral health care and social concern. However, although the ultimate oral health goals declared that there should be improvements in QoL, Thai oral health goals considered only clinical data16.
There is a link between clinical dental status and QoL. Studies in a number of countries report that in school-age populations, dental caries is significantly associated with OHRQoL17., 18., 19., 20., 21., 22., 23.. Poor oral hygiene and severe periodontitis negatively affect OHRQoL24., 25., whereas general periodontal disease does not19., 23., 25., 26.. Although there has been much research on OHRQoL over some 25 years, no study has assessed associations between specific oral disease and specific OHRQoL in a nationally representative sample.
As the Thai oral health goals considered only clinical data and their ultimate goal was to improve QoL, although the latter was not quantified, the objectives of this study were to assess the associations between oral diseases and specific OHRQoL in a nationally representative sample of 12-year-old Thai children in order to classify children according to their levels of risk for adverse OHRQoL and to apply the findings to formulate proposals for oral health goals for 12-year-old Thai children.
MATERIALS AND METHODS
National oral health survey
The sixth Thailand national oral health survey was conducted in 2007 to assess oral status and oral health behaviours in the Thai population classified into seven age groups27. A stratified multi-stage method was used for sample selection. The country was divided into five strata comprising, respectively, Bangkok and four regions (north, south, central and northeast). There were 52 survey sites (48 in the regions and four in Bangkok). Within each selected site, samples were randomly drawn from the register of citizens. Sample size within each stratum at each stage of selection (country, region, province, district, sub-district) was calculated taking into account the proportion of the population in the particular stratum to constitute an equal probability sample. The total sample size was 2,200. Full details of the sample procedures have been described elsewhere27., 28..
Children were orally examined by trained and calibrated community dentists for dental caries, missing teeth and dental fluorosis and periodontal conditions using WHO guidelines29. The Community Periodontal Index (CPI)30 was used to assess periodontal conditions in six mouth sextants: upper right; upper anterior; upper left; lower left; lower anterior, and lower right. The components of the CPI were scored separately: a score of 1 referred to gingival bleeding without calculus; a score of 2 referred to calculus without gingival bleeding, and a score of 5 referred to calculus with bleeding. A total of 10% of children were re-examined to ensure the reliability of data. The intraclass correlation coefficient (ICC) was 0.857. In addition to clinical data, sociodemographic information on sex, region, area of residence and school type was recorded.
Oral health-related QoL survey
A cross-sectional survey on OHRQoL in 12-year-old children was conducted on subsamples of the national oral health survey sample. The protocol was approved by the Ethics Committee of Chulalongkorn University. The study was conducted in full accordance with the World Medical Association Declaration of Helsinki. The sample represented half of the national survey sample: two of the four provinces within each region were randomly selected and two of the four sub-districts in Bangkok were randomly selected. All children in the selected provinces or sub-districts were recruited as study participants. Written information on this study together with a consent form was sent to the guardians of all participants. All guardians gave their consent and returned signed consent forms. The sample size in this study was 1,100.
Study participants were interviewed by trained and calibrated interviewers about their OHRQoL using the Thai version of the Child–Oral Impacts on Daily Performances (Child-OIDP) questionnaire31. The index assesses oral impacts during the past 3 months in relation to the performance of eight daily activities: (i) eating; (ii) speaking; (iii) cleaning teeth; (iv) demonstrating emotional stability; (v) relaxing/sleeping; (vi) smiling without feeling embarrassment; (vii) studying, and (viii) participating in social contact. For each activity, a frequency score (in the range of 0–3) and severity score (in the range of 0–3) were recorded. If any impact was detected, the child was asked to report any oral conditions he or she perceived to be the main causes of oral impacts.
Data analysis
spss Version 16.0 (SPSS, Inc., Chicago, IL, USA) was used for data analysis. Data were doubly entered to ensure the validity of data transfer. Tooth status was analysed in terms of the presence or not of dental caries (DMFT score) and its components (DT, MT and FT scores), as well as fluorosis. Data on periodontal disease were analysed for overall CPI and for CPI components (gingivitis without calculus = 1, calculus without gingivitis = 2, calculus with gingivitis = 5). The presence of periodontal disease was indicated by scores of 1, 2 or 5 for these three conditions, respectively. In addition, occurrences of gingivitis without or with calculus (scores of 1 or 5, respectively), and calculus without or with gingivitis (scores of 2 or 5, respectively) were analysed.
Oral health-related QoL was analysed in relation to overall oral impacts and condition-specific (CS) impacts. Condition-specific impacts were impacts caused by specific oral conditions, identified by taking into account the oral conditions children perceived to be the main causes of impacts. A range of all of the most common perceived causes of oral impacts has been reported28. Eight groups of CS impacts were identified: (i) dental caries, when the main causes of oral impacts were perceived to be toothache, a sensitive tooth, a hole in a tooth, a broken filling or toothache after a filling; (ii) periodontal disease, when the main causes were perceived to be inflamed gums, pain in the gums, tartar or bad breath; (iii) edentulous area, when the main cause was perceived to be tooth space attributable to the extraction of a permanent tooth; (iv) oral lesions, when the main causes were perceived to be an oral ulcer or other oral lesions such as herpes, and dry or cracked lips; (v) discolouration, when the main cause was perceived to be the colour of a tooth or teeth; (vi) malocclusion, when the main cause was perceived to be the position of a tooth or teeth; (vii) trauma-related dental injuries, when the main cause was perceived to be a fractured tooth or teeth, and (viii) natural processes, when the main causes were perceived to be related to tooth space attributable to an unerupted permanent tooth, and an exfoliating primary tooth and an erupting permanent tooth.
The prevalences and severity of overall impacts and CS impacts were calculated. As well as using an oral impact score, the Child-OIDP system allows for alternative methods of presenting the severity of impacts32. ‘Intensity’ of impacts was used in this study. This refers to the most severe impact on any of the eight daily activities. From the data collected, performance scores were calculated by multiplying the frequency score by the severity score. The sum of eight activity scores results in the total impact score. There are seven possible performance scores (0, 1, 2, 3, 4, 6 and 9) for each activity. ‘Intensity’ is defined by the highest performance score of the eight activities and is classified into six levels: none (no impact); very low (highest performance score = 1); low (highest performance score = 2); moderate (highest performance score = 3 or 4); severe (highest performance score = 6), and very severe (highest performance score = 9). The purpose of using the concept of intensity is to focus on the most severe impact on any activity regardless of the number of activities that are affected32. A total of 10% of participants were randomly selected for re-interview. The ICC was 0.863.
The associations of oral diseases with OHRQoL were investigated using the chi-squared, chi-squared for trend and Fisher’s exact tests, and logistic regressions. Oral diseases were independent categorical variables predicting the presence or absence of CS impacts. Sociodemographic variables (sex, region and area of residence) and other oral diseases were controlled for in multiple logistic regressions. Statistical significance was indicated by a P-value of < 0.05.
Panel of dental public health professionals
The findings of this study were sent to a panel of dental public health professionals that included academics and dentists employed by the Dental Health Division, Ministry of Public Health. The significance of the findings was discussed and potential additional oral health goals were proposed. In addition to the specific, measurable, attainable, realistic and time-bound (SMART) concept for general considerations of goal setting33, we also considered how the proposed goals complied with the published Thailand Oral Health Goals 2020 and current strategies and plans for the provision of oral health services.
RESULTS
Analysis of the overall situation
A total of 1,063 12-year-old children (96.6% response rate) completed the interviews on OHRQoL and underwent oral examinations. Half (49.6%) were male, 90.3% attended public schools and 43.0% resided in municipal areas (Table 1). The prevalence of caries was 58.8%; the mean ± standard deviation (SD) DMFT score was 1.6 ± 2.1. Mean scores for DT, MT and FT were 0.9 ± 1.6, 0.07 ± 0.3 and 0.7 ± 1.3, respectively. Periodontal disease was the most prevalent (79.3%) dental disease. Gingivitis without calculus was noted in 57.8% of children, whereas calculus without and with gingivitis was observed in 33.8% and 33.2%, respectively. Fluorosis was detected in 4.0% of children (Table 1). The prevalence of oral impacts was very high (85.1%). However, most (50.0%) impacts were of very low (14.8%) to low (35.2%) intensity (Table 2). Condition-specific impacts attributed to dental caries were the most prevalent (47.7%), followed by CS impacts attributed to periodontal disease (26.0%) and oral lesions (25.8%). Most CS impacts were of very low or low intensity (Table 2).
Table 1.
Descriptive analysis of sociodemographic characteristics and oral status of 1,063 12-year-old Thai children
| Characteristics | Values |
|---|---|
| Sex, % | |
| Male | 49.6 |
| Female | 50.4 |
| Region, % | |
| Bangkok | 14.0 |
| Central | 20.7 |
| North | 20.2 |
| South | 16.0 |
| Northeast | 29.1 |
| Area of residence, % | |
| Municipal | 43.0 |
| Rural | 57.0 |
| School type, % | |
| Public | 90.3 |
| Private | 9.7 |
| Oral status | |
| Incidence of caries (DMFT > 0), % | 58.8 |
| DMFT scores, range | 0–17 |
| DMFT score, mean ± SD | 1.6 ± 2.1 |
| Untreated decay (DT > 0), % | 41.9 |
| DT scores, range | 0–17 |
| DT score, mean ± SD | 0.9 ± 1.6 |
| Missing teeth due to caries (MT > 0), % | 5.9 |
| MT scores, range | 0–4 |
| MT score, mean ± SD | 0.07 ± 0.3 |
| Filled teeth (FT > 0), % | 29.4 |
| FT scores, range | 0–10 |
| FT score, mean ± SD | 0.7 ± 1.3 |
| Periodontal disease (1,2,5)*, % | 79.3 |
| Sextants, range | 0–6 |
| Sextants, mean ± SD | 2.7 ± 2.1 |
| Gingivitis, no calculus (1)*, % | 57.8 |
| Sextants, range | 0–6 |
| Sextants, mean ± SD | 1.4 ± 1.6 |
| Calculus, no gingivitis (2)*, % | 33.8 |
| Sextants, range | 0–5 |
| Sextants, mean ± SD | 0.6 ± 1.0 |
| Calculus with gingivitis (5)*, % | 33.2 |
| Sextants, range | 0–6 |
| Sextants, mean ± SD | 0.7 ± 1.3 |
| Fluorosis, % | 4.0 |
DMFT, decayed, missing and filled teeth; DT, decayed teeth; MT, missing teeth; FT, filled teeth; SD, standard deviation.
1 = gingivitis without calculus; 2 = calculus without gingivitis; 5 = calculus with gingivitis.
Table 2.
Classification of levels of intensity of overall and condition-specific (CS) impacts on 1,063 12-year-old Thai children
| % | Intensity levels | ||||||
|---|---|---|---|---|---|---|---|
| None | Very low | Low | Moderate | Severe | Very severe | ||
| Overall oral impacts | 85.1 | 14.9 | 14.8 | 35.2 | 19.1 | 13.0 | 3.0 |
| CS impacts attributed to: | |||||||
| Caries | 47.7 | 52.3 | 9.5 | 19.7 | 9.2 | 7.7 | 1.6 |
| Periodontal diseases | 26.0 | 74.0 | 7.0 | 10.3 | 5.4 | 2.8 | 0.5 |
| Edentulism | 0.4 | 99.6 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 |
| Oral lesions | 25.8 | 74.2 | 4.1 | 12.8 | 5.8 | 2.7 | 0.4 |
| Discolouration | 8.2 | 91.8 | 2.4 | 3.2 | 1.5 | 0.9 | 0.2 |
| Malocclusion | 11.8 | 88.2 | 2.4 | 4.4 | 1.9 | 2.3 | 0.8 |
| Traumatic dental injuries | 1.5 | 98.5 | 0.3 | 0.5 | 0.4 | 0.2 | 0.1 |
| Natural processes | 10.2 | 89.8 | 1.3 | 4.3 | 2.4 | 1.7 | 0.5 |
Identification of oral impact thresholds and associated oral diseases
As oral impacts on 12-year-old children were very common, very low- and low-intensity impacts were excluded from the analysis as these were considered to fall outwith the goals for oral health services. Oral impacts of moderate or higher intensity were considered as sociodental public health problems among 12-year-old Thai children. At the moderate/higher intensity threshold, the percentage of overall oral impacts was 35.1%. Condition-specific impacts attributed to dental caries, edentulous areas, periodontal disease and discolouration were present in 18.5%, 0.2%, 8.7% and 2.6%, respectively, of children. The relationships between moderate/higher CS impacts attributed to these four oral conditions and actual oral diseases were investigated (Table 3). There were significant trends in increasing moderate/higher CS impacts attributed to caries and increases in the number of untreated decayed teeth (DT) (P < 0.001). No significant difference in moderate/higher CS impacts attributed to caries emerged between participants with and without filled teeth. Having missing teeth (MT) was significantly related to moderate/higher CS impacts attributed to caries (P < 0.001) and to impacts attributed to the presence of an edentulous area (P < 0.01). With reference to periodontal disease, we found significant increases in the proportions of children with moderate/higher CS impacts attributed to periodontal disease in line with increasing numbers of sextants with periodontal disease (P < 0.01), gingivitis (P < 0.001), calculus (P < 0.01) and calculus with bleeding (P < 0.001). Having more sextants with gingivitis without calculus or calculus without gingivitis was not significantly related to moderate/higher CS impacts attributed to periodontal disease. Moderate/higher CS impacts attributed to tooth discolouration were not significantly related to dental fluorosis (Table 3).
Table 3.
Comparisons of percentages of children with moderate to very severe condition-specific (CS) impacts between groups with different levels of oral disease among 1,063 12-year-old Thai children
| Oral disease | % | Moderate to very severe CS impacts, % | |||
|---|---|---|---|---|---|
| Caries | Edentulism | Periodontal disease | Discolouration | ||
| Study sample | 100 | 18.5 | 0.2 | 8.7 | 2.6 |
| Untreated decay | |||||
| None | 58.1 | 12.0 | |||
| 1 tooth | 19.4 | 19.9 | |||
| 2 teeth | 11.0 | 29.1 | |||
| ≥ 3 teeth | 11.5 | 39.3* | |||
| Missing teeth | |||||
| None | 94.1 | 17.1 | 0.0 | ||
| ≥ 1 tooth | 5.9 | 41.3* | 3.2† | ||
| Filled teeth | |||||
| None | 70.6 | 17.3 | |||
| ≥ 1 tooth | 29.4 | 21.5 (NS) | |||
| Periodontal disease (1,2,5)‡ | |||||
| None | 20.7 | 5.5 | |||
| 1–2 sextants | 30.0 | 6.6 | |||
| ≥ 3 sextants | 49.3 | 11.4† | |||
| Gingivitis (1,5)‡ | |||||
| None | 32.8 | 5.7 | |||
| 1–2 sextants | 30.0 | 6.3 | |||
| ≥ 3 sextants | 37.2 | 13.4* | |||
| Calculus (2,5)‡ | |||||
| None | 46.4 | 6.7 | |||
| 1–2 sextants | 32.8 | 7.7 | |||
| ≥ 3 sextants | 20.8 | 14.9† | |||
| Gingivitis without calculus (1)‡ | |||||
| None | 42.2 | 7.3 | |||
| 1–2 sextants | 36.6 | 10.0 | |||
| ≥ 3 sextants | 21.2 | 9.3 (NS) | |||
| Calculus without gingivitis (2)‡ | |||||
| None | 66.2 | 8.8 | |||
| 1–2 sextants | 28.1 | 9.1 | |||
| ≥ 3 sextants | 5.7 | 6.6 (NS) | |||
| Calculus with gingivitis (5)‡ | |||||
| None | 66.8 | 6.6 | |||
| 1–2 sextants | 22.6 | 8.3 | |||
| ≥ 3 sextants | 10.6 | 23.0* | |||
| Fluorosis | |||||
| No | 96.0 | 2.6 | |||
| Yes | 4.0 | 2.4 (NS) | |||
*P <0.001; †P <0.01.
1 = gingivitis without calculus; 2 = calculus without gingivitis; 5 = calculus with gingivitis.
Chi-squared test for missing teeth with CS impacts relating to caries, filled teeth, fluorosis.
Chi-squared for trend test for untreated decay and periodontal diseases.
Fisher’s exact test for missing teeth with CS impacts relating to the presence of an edentulous area.
Multivariate logistic regressions were performed to ascertain the associations between oral diseases and moderate/higher CS impacts (Table 4). After adjusting for sex, region, area of residence and the presence of other oral diseases, children with one untreated decayed tooth were 1.6 times [95% confidence interval (CI) 1.0–2.4] more likely to experience moderate/higher CS impacts attributed to caries compared with those with no decayed teeth. The likelihood of experiencing such impacts increased to 2.7 times (95% CI 1.7–4.3) in children with two decayed teeth and to 3.9 times (95% CI 2.5–6.2) in those with three or more decayed teeth. No regression analysis of moderate/higher CS impacts attributed to edentulism was performed because the prevalence of such impacts was very low (0.2%). Instead, moderate/higher CS impacts attributed to caries were regressed on missing teeth because the loss of teeth attributable to caries reflects the progression of untreated dental caries. Indeed, children with missing teeth as a result of caries were 2.6 times (95% CI 1.5–4.5) more likely to experience CS impacts attributed to caries. Periodontal disease, gingivitis, calculus and calculus with gingivitis occurring in one or two sextants of the mouth did not significantly increase the likelihood of experiencing moderate/higher CS impacts attributed to periodontal disease, whereas the occurrence of any of these four periodontal conditions in three or more sextants was significantly more likely to cause such impacts. The likelihood of such impacts was twice as high in the presence of the periodontal diseases gingivitis and calculus, but increased to 3.5 times as high (95% CI 2.0–6.2) in the presence of calculus with gingivitis (Table 4).
Table 4.
Logistic regressions of moderate to very severe condition-specific (CS) impacts of oral diseases in 1,063 12-year-old Thai children
| Oral disease | Moderate to very severe CS impacts, % | |||
|---|---|---|---|---|
| Caries | Periodontal disease | |||
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Untreated decay | ||||
| None | 1 | 1 | – | – |
| 1 tooth | 1.8 (1.2–2.8)† | 1.6 (1.0–2.4)‡ | – | – |
| 2 teeth | 3.0 (1.9–4.8)* | 2.7 (1.7–4.3)* | – | – |
| ≥ 3 teeth | 4.8 (3.1–7.4)* | 3.9 (2.5–6.2)* | – | – |
| Missing teeth | ||||
| None | 1 | 1 | – | – |
| ≥ 1 tooth | 3.4 (2.0–5.8)* | 2.6 (1.5–4.5)† | – | – |
| Periodontal disease (1,2,5)§ | ||||
| None | – | – | 1 | 1 |
| 1–2 sextants | – | – | 1.2 (0.6–2.5) | 1.2 (0.6–2.5) |
| ≥ 3 sextants | – | – | 2.2 (1.2–4.2)‡ | 1.9 (1.0–3.8)‡ |
| Gingivitis (1,5)§ | ||||
| None | – | – | 1 | 1 |
| 1–2 sextants | – | – | 1.1 (0.6–2.1) | 1.0 (0.5–1.9) |
| ≥ 3 sextants | – | – | 2.5 (1.5–4.4)† | 2.2 (1.3–3.9)† |
| Calculus (2,5)§ | ||||
| None | – | – | 1 | 1 |
| 1–2 sextants | – | – | 1.2 (0.7–2.0) | 1.2 (0.7–2.1) |
| ≥ 3 sextants | – | – | 2.4 (1.5–4.1)† | 2.2 (1.3–3.7)† |
| Calculus with gingivitis (5)§ | ||||
| None | – | – | 1 | 1 |
| 1–2 sextants | – | – | 1.3 (0.7–2.2) | 1.1 (0.6–2.0) |
| ≥ 3 sextants | – | – | 4.2 (2.5–7.2)* | 3.5 (2.0–6.2)* |
*P < 0.001; †P < 0.01; ‡P < 0.05.
1 = gingivitis without calculus; 2 = calculus without gingivitis; 5 = calculus with gingivitis.
OR, odds ratio; 95% CI, 95% confidence interval.
Adjusted analyses were controlled for sex, region, area of residence and other oral diseases.
The presence of calculus with gingivitis in three or more sextants was strongly significantly associated with moderate/higher CS impacts (P < 0.001). The likelihood of experiencing such impacts increased by 3.5 times (Table 4). By contrast, the presence of gingivitis alone or calculus alone was not significantly associated with CS impacts. It appears that the significant associations found between CS impacts and overall periodontal disease, gingivitis and calculus were related to the strong effect of including calculus with gingivitis among these three conditions. The condition considered to represent periodontal disease and associated with moderate/higher CS impacts was calculus in combination with gingivitis in three or more sextants.
Levels of oral disease classifications and distributions
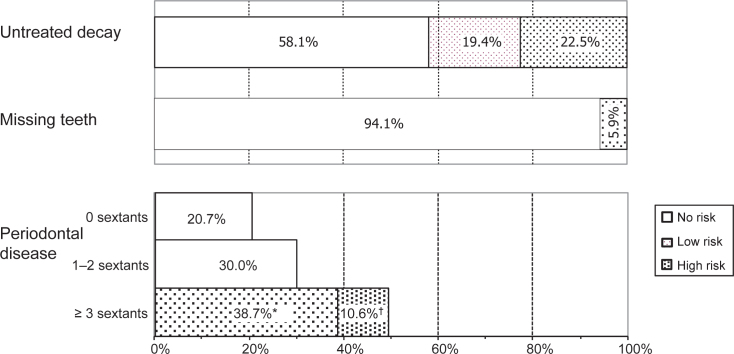
Based on the present findings, oral diseases, namely, untreated decay, missing teeth and periodontal disease, were classified into three levels of risk based on their associations with moderate/higher CS impacts. The three levels were: (i) no risk; (ii) low risk, and (iii) high risk (Figure 1).
Figure 1.
Proportions of 12-year-old Thai children categorised by risk for moderate to very severe oral health-related impacts on quality of life according to the presence of untreated decayed and missing teeth and periodontal disease (n = 1,063). *Fewer than three sextants with calculus with gingivitis. †Three or more sextants with calculus with gingivitis. No risk: no untreated decay; no missing teeth; periodontal disease in fewer than three sextants. Low risk: untreated decay in one tooth; periodontal disease in three or more sextants, but calculus with gingivitis in fewer than three sextants. High risk: untreated decay in two or more teeth; loss of one or more teeth; periodontal disease in three or more sextants with calculus with gingivitis.
No risk
Children in the ‘no-risk’ category included those with no untreated decayed teeth, no missing teeth and no more than two sextants with periodontal disease in any form (gingivitis and/or calculus). Children at this level are unlikely to experience moderate/higher degree oral impacts.
Low risk
Children in the ‘low-risk’ category included those with one untreated decayed tooth and three or more sextants with periodontal disease in any form (gingivitis and/or calculus), but fewer than three sextants with calculus with gingivitis. Children at this level have a small chance of experiencing moderate/higher oral impacts.
High risk
Children in the ‘high-risk’ category included those with two or more untreated decayed teeth, one or more missing teeth, and three or more sextants with calculus with gingivitis. Children in this category have a significant likelihood of experiencing moderate/higher oral impacts.
The proportions of children in the three risk-based categories are shown in Figure 1. Nearly a quarter (22.5%) of 12-year-old children were at high risk for moderate/higher oral impacts caused by untreated decayed teeth, whereas 19.4% were at low risk. A total of 5.9% were at high risk because of missing teeth, and 10.6% were at high risk and 38.7% at low risk because of periodontal disease.
Proposed additional Thai oral health goals
The oral health goals for Thailand for the year 2020 state that all 12-year-old children should have all their first molar teeth (no loss of teeth caused by dental caries)16. This goal is supported by findings from this study that a child with one or more missing teeth is significantly more likely to have moderate/higher CS impacts attributable to tooth loss. Moreover, tooth loss attributable to caries reflects the progression of untreated decay and increases the likelihood of moderate/higher CS impacts attributable to dental caries by 2.6 times. Other than missing teeth, no further oral diseases are mentioned in the Thai goals16.
The mean DMFT score of 12-year-old Thai children in the present national survey was relatively low (1.6). The majority of the DMFT score referred to the DT component (0.9). Although the average DT score of 0.9 was lower than those of the low- and high-risk thresholds (DT scores of 1 and 2, respectively), 19.4% of 12-year-old children had one untreated decayed tooth (low risk) and 22.5% had two or more (high risk). For high-risk children, the likelihood of experiencing moderate/higher oral impacts increases by as much as three to four times. Therefore, based on the present findings, goals for the improvement of children’s QoL should focus on reducing the prevalence of untreated decayed teeth by expanding oral health services. The goal should be to firstly reduce the percentage of children in the high-risk group by using a population-based approach34. The additional proposed goal based on the QoL measure refers to the aim that all 12-year-old children should have fewer than two untreated decayed teeth.
The former Thai goal for gingival health for the year 2005 stipulated that 25% of children should have healthy periodontium or no sextant with periodontal disease15. However, the present study found that having one or two sextants with periodontal disease did not significantly increase the likelihood of experiencing moderate/higher oral impacts compared with the healthy periodontium group, whereas children with three or more sextants were a little more likely to experience moderate/higher impacts. Therefore, these children should be categorised within the low-risk group. Moreover, the conditions of ‘gingivitis alone’ and ‘calculus alone’ did not increase the likelihood of moderate/higher oral impacts. The moderate/higher impacts were actually related to the occurrence of calculus with gingivitis in three or more sextants of the mouth, here defined as indicating high risk. The reduction of gingivitis does not depend on oral health services alone, but mainly on children’s ability to maintain good daily oral health practices. In the current study, 10.6% of Thai children were categorised as belonging to the high-risk group, 38.7% to the low-risk group and 50.7% to the no-risk group. Therefore, we suggest the goal should be to increase the no-risk and decrease the high-risk groups. The goals proposed stipulate that 60% of 12-year-old children should not have periodontal disease in any form in more than two sextants, and that the proportion of 12-year-old children with calculus with gingivitis in three or more sextants should not exceed 5%.
DISCUSSION
This study proposes oral health goals that include sociodental measures based on findings of associations between oral diseases and oral impacts and the identification of risk levels for specific oral diseases according to their associations with moderate/higher degree oral impacts. The study set out to provide information supporting the use of QoL measures in setting national goals for oral health in Thailand in particular, but also in other countries in general. Although clinical oral health indicators and the OHRQoL indicator represent two different concepts in the assessment of oral health, they are interdependent. There are associations between oral diseases and QoL1., 2., 3.. Therefore, this study attempted to investigate such links and, subsequently, to integrate the two concepts as such integration has practical implications for oral health service planning. Some oral conditions, such as calculus and dental fluorosis, are not oral diseases. However, oral health services usually define dental public health problems according to existing oral conditions detected by professionals using professional criteria5., 6., 7., 8., 9., 15., 27.. Therefore, this study included all oral conditions assessed by professionals as oral diseases using normative criteria. Thereafter, we investigated their associations with subjective OHRQoL.
Because overall impacts were very prevalent among 12-year-old Thai children (85.1%), some low-intensity impacts were excluded. Impacts of moderate intensity and above were suggested as the threshold. This was consistent with the guideline on developing goals for oral health for 2020 reported by Hobdell et al.14 They recommended that a reduction in the prevalence of moderate and severe social impacts on daily activities should be one of the oral health goals.14 Furthermore, the moderate level was not arbitrarily defined by using a cut-off point from a range of total impact scores. The Child-OIDP and OIDP system facilitates the categorising of oral impacts into different levels of severity by using the ‘intensity’ rather than the impact score28., 32.. Moreover, the Child-OIDP and OIDP were designed to assess CS impacts in order to provide more accurate results of analyses of associations between oral impacts and specific oral diseases. Oral diseases are categorised by oral health services as specific diseases, not overall diseases. Therefore, using CS impacts is more appropriate than using overall measures of OHRQoL as CS impacts relate to specific dental conditions. In the present study, the incidence of moderate/higher overall impacts was 35.1%, whereas those of CS impacts for dental caries and periodontal disease were 18.5% and 8.7%, respectively. Proposals made here are limited to reductions in the moderate/higher CS impacts attributed to dental caries and periodontal disease, rather than reductions in overall impacts because overall impacts include impacts attributable to other oral conditions, such as recurrent aphthous ulcers and malocclusion28, which were not included in the clinical examination. The percentages of children experiencing moderate/higher CS impacts attributable to recurrent aphthous ulcers and malposed teeth were considerable, at 8.9% and 5.0%, respectively (Table 2).
Although associations between oral disease and OHRQoL have been investigated17., 18., 19., 20., 21., 24., 25., 26., 35., 36., very few studies have used CS impacts22., 23.. Moreover, the thresholds levels of disease that are significantly related to impacts have not previously been reported. The findings of this study of associations between dental caries and OHRQoL were consistent with those of previous studies in that dental caries, in terms of DT scores, was significantly related to OHRQoL17., 18., 19., 20., 21., 22., 23., whereas DMFT was not35., 36.. In addition, this study found that a DT score of 1 was significantly related to moderate/higher CS impacts attributable to dental caries (P < 0.05) and that the likelihood of experiencing impacts increased to 1.6 times that of the group with no untreated decayed teeth. However, other oral conditions, such as oral ulcers and exfoliating primary teeth, that may cause oral pain, confound the perceptions of children in terms of relating the causes of impacts to caries. Therefore, we categorised children with a DT score of 1 within a low-risk group. A DT score of 2 increased the likelihood of experiencing moderate/higher CS impacts by 2.7 times, and a DT score of ≥ 3 increased it up to 3.9 times. Therefore, a DT score of 2 was defined as the threshold for categorisation within the high-risk group. No previous study has reported the association between OHRQoL and missing teeth in children. This study found that the loss of teeth caused by caries was significantly associated with moderate/higher CS impacts. However, the significance of moderate/higher CS impacts attributed to edentulous areas should be viewed with caution as its prevalence (0.2%) was very low. The loss of teeth as a result of caries reflects untreated decay and therefore was highly significantly related to moderate/higher CS impacts attributed to dental caries.
The findings of this study in relation to gingivitis compare positively with those of other studies reporting significant associations between OHRQoL and poor oral hygiene and severe ulcerative gingivitis24., 25., but not with the more common periodontal diseases of gingivitis and calculus19., 23., 25., 26.. However, no previous study investigated associations of OHRQoL with different levels of common periodontal diseases. We found that having three or more sextants with periodontal disease, gingivitis and calculus, significantly increased the chance of experiencing moderate/higher CS impacts, whereas having up to two sextants affected did not. More importantly, no previous study has differentiated the impacts of gingivitis alone, calculus alone and calculus with gingivitis. By exploring the associations between CS impacts and periodontal disease, as well as the components of the latter, this study revealed that calculus with gingivitis was the only periodontal component to be significantly associated with CS impacts and indicated significant associations between overall periodontal disease, gingivitis and calculus, and CS impacts. The finding suggests that gingivitis occurring without calculus is unlikely to be associated with moderate/higher CS impacts. Calculus without gingivitis may cause appearance-related oral impacts if it is present in the anterior segments. This is not common in 12-year-old children. In the light of these findings, and the fact that gingivitis and calculus do not invariably progress to destructive periodontal disease37., 38., the presence of gingival conditions that were not significantly associated with oral impacts were not included in the risk groups.
The link between oral diseases and OHRQoL is not straightforward and is influenced by various individual and socioenvironmental factors39. Therefore, the associations between certain oral diseases and OHRQoL, as well as the thresholds identified for categorisation within the various risk groups in this study, may not be universally applicable to other populations. Thus, a situation analysis to establish oral health goals should be conducted locally for each country, as the FDI/WHO/IADR guidelines14 propose. This will identify thresholds and proposed goals that are actually relevant to the local population under its particular socioenvironmental circumstances. Although this study is limited by its use of cross-sectional data for classifying levels of risk, we suggest that the present study can be cited as an example of the use of OHRQoL data to establish a population’s oral health service goals and its dental care needs. It is strongly recommended that countries, particularly those that already include OHRQoL assessments in their national surveys, should make more use of their OHRQoL data by integrating them into the analyses used for goal setting and assessing treatment needs based on sociodental concepts of needs10., 11., 12.. If the oral health goals proposed by this study are applied, further longitudinal study will be required to test the validity of such goals. A longitudinal study design and time series data will also be necessary to better classify and validate the categorisation of risk groups according to different levels of oral disease in order to better formulate oral health goals.
The oral health goals proposed here were compatible with the oral health goals declared in Thailand for 2020. Currently, these aims include the retention of all first molars until the age of at least 12 years16. Our findings suggest that children with any loss of teeth attributable to caries will be more likely to experience moderate/higher CS impacts attributable to edentulism and to dental caries. Moreover, this study proposes two additional goals in relation to dental caries and periodontal disease. The fact that the prevalence of dental caries has been low and remained stable, as indicated by a mean DMFT index of 1.6 in 12-year-old children, since 198415 may reflect relative success in controlling the disease, as a result of which oral health planners have not focused attention on further reducing the incidence and impact of caries. This study revealed that even with the relatively low level of caries in Thailand, the presence of untreated decayed teeth significantly increased the likelihood of experiencing moderate/higher impacts, particularly in children with two or more untreated decayed teeth, in whom the likelihood of experiencing moderate/higher impacts increased by three to four times that of children without untreated decay. This suggests that the scope of oral health services regarding dental caries should be extended from the reduction of DMFT to also cover the reduction of DT.
In addition to controlling the prevalence of disease, dental services should aim to reduce the impacts of existing diseases. Early treatment is recommended to avoid the loss of teeth attributable to caries and the subsequent increase in the likelihood of oral impacts. Furthermore, there are oral health inequalities in the distribution of DT scores. Reducing inequalities in oral health should therefore be included within oral health goals40., 41.. The periodontal disease-related goals proposed by this study differ, in terms of numbers of diseased sextants, from those stipulated in the previous Thailand national oral health goals15. Based on clinical data, the previous national goal was to increase the percentage of children with healthy periodontium or no diseased sextants15. The inclusion of OHRQoL data in the development of these goals refines the proposal to increase the percentage of children with fewer than three periodontally diseased sextants. A further goal is to reduce the percentage of children categorised as being at high risk according to the presence of three or more sextants with calculus and gingivitis.
The present findings on oral health differ from those derived using a disease-oriented approach. Based on clinical data, periodontal disease was previously specified as the most important oral disease among this age group as a result of its high prevalence and the stable low prevalence of dental caries15. However, our findings suggest that dental caries remains the most important oral disease among 12-year-old Thais because the prevalence of moderate/higher CS impacts attributable to dental caries is high (18.5%) and that of moderate/higher CS impacts attributable to periodontal disease is much lower (8.7%). In addition, nearly a quarter of Thai children were found to be at high risk for dental caries, whereas only 10.6% were at high risk for periodontal disease.
Acknowledgements
We thank the World Dental Federation (FDI)/Unilever Live.Learn.Laugh programme for funding the survey on oral health-related quality of life through the Dental Association of Thailand.
Conflicts of interest
None declared.
REFERENCES
- 1.Cohen K, Jago JD. Toward the formulation of socio-dental indicators. Int J Health Serv. 1976;6:681–687. doi: 10.2190/LE7A-UGBW-J3NR-Q992. [DOI] [PubMed] [Google Scholar]
- 2.Sheiham A. Oral health, general health and quality of life. Bull World Health Organ. 2005;83:644–645. [PMC free article] [PubMed] [Google Scholar]
- 3.Slade GD. University of North Carolina; Chapel Hill, NC: 1997. Measuring Oral Health and Quality of Life. [Google Scholar]
- 4.Jankauskiene B, Narbutaite J. Changes in oral health-related quality of life among children following dental treatment under general anaesthesia. A systematic review. Stomatologija. 2010;12:60–64. [PubMed] [Google Scholar]
- 5.Kelly M, Steele J, Nuttall N, et al. The Stationery Office; London: 2000. Adult Dental Health Survey: oral health in the United Kingdom 1998. [Google Scholar]
- 6.Sanders AE, Slade GD, Lim S, et al. Impact of oral disease on quality of life in the US and Australian populations. Community Dent Oral Epidemiol. 2009;37:171–181. doi: 10.1111/j.1600-0528.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John MT, Hujoel P, Miglioretti DL, et al. Dimensions of oral health-related quality of life. J Dent Res. 2004;83:956–960. doi: 10.1177/154405910408301213. [DOI] [PubMed] [Google Scholar]
- 8.Aromaa A, Koskinen S. KTL (National Public Health Institute); Helsinki: 2004. Health and Functional Capacity in Finland. Baseline Results of the Health 2000 Health Examination Survey; pp. 62–66. [Google Scholar]
- 9.Bae KH, Kim C, Paik DI, et al. A comparison of oral health-related quality of life between complete and partial removable denture-wearing older adults in Korea. J Oral Rehabil. 2006;3:317–322. doi: 10.1111/j.1365-2842.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- 10.Gherunpong S, Tsakos G, Sheiham A. A socio-dental approach to assessing children’s oral health needs: integrating and oral health-related quality of life (OHRQoL) measure into oral health service planning. Bull World Health Organ. 2006;84:36–42. doi: 10.2471/blt.05.022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gherunpong S, Tsakos G, Sheiham A. A socio-dental approach to assessing the orthodontic needs of children. Eur J Orthod. 2006;28:393–399. doi: 10.1093/ejo/cji114. [DOI] [PubMed] [Google Scholar]
- 12.Sheiham A, Tsakos G. In: Community Oral Health. Pine CM, Harris R, editors. Quintessence Books; London: 2007. Oral health needs assessment; pp. 59–79. [Google Scholar]
- 13.Rozier RG, Pahel BT. Patient- and population-reported outcomes in public health dentistry: oral health-related quality of life. Dent Clin North Am. 2008;52:345–365. doi: 10.1016/j.cden.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Hobdell M, Petersen PE, Clarkson J, et al. Global goals for oral health 2020. Int Dent J. 2003;53:285–288. doi: 10.1111/j.1875-595x.2003.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 15.Dental Health Division . Department of Health, Ministry of Public Health; Nonthaburi: 2002. Report on the Fifth National Oral Health Survey of Thailand (2000–2001) [Google Scholar]
- 16.Yongvanichkarn B, Prasertsom P, Jirapongsa W, et al. War Veterans Organization Office of Printing Mill; Bangkok: 2007. Thailand Oral Health Goal 2020; pp. 2–3. [Google Scholar]
- 17.Do LG, Spencer AJ. Evaluation of oral health-related quality of life questionnaires in a general child population. Community Dent Health. 2008;25:205–210. [PubMed] [Google Scholar]
- 18.Baker SR, Mat A, Robinson PG. What psychosocial factors influence adolescents’ oral health? J Dent Res. 2010;89:1230–1235. doi: 10.1177/0022034510376650. [DOI] [PubMed] [Google Scholar]
- 19.Nurelhuda NM, Ahmed MF, Trovik TA, et al. Evaluation of oral health-related quality of life among Sudanese schoolchildren using Child-OIDP inventory. Health Qual Life Outcomes. 2010;8:152. doi: 10.1186/1477-7525-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piovesan C, Antunes JLF, Guedes RS, et al. Impacts of socioeconomic and clinical factors on child oral health-related quality of life (COHRQoL) Qual Life Res. 2010;19:1359–1366. doi: 10.1007/s11136-010-9692-7. [DOI] [PubMed] [Google Scholar]
- 21.Page LAF, Thomson WM, Mohamed R, et al. Performance and cross-cultural comparison of the short-form version of the CPQ11–14 in New Zealand, Brunei and Brazil. Health Qual Life Outcomes. 2011;9:40. doi: 10.1186/1477-7525-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mbawalla HS, Mtaya M, Masaku JR, et al. A discriminative ability of the generic and condition-specific Child–Oral Impacts on Daily Performances (Child-OIDP) by the Limpopo-Arusha School Health (LASH) Project: a cross-sectional study. BMC Pediatr. 2011;11:45. doi: 10.1186/1471-2431-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsakos G, Gherunpong S, Sheiham A. Can oral health-related quality of life measures substitute for normative needs assessments in 11 to 12-year-old children? J Public Health Dent. 2006;66:263–268. doi: 10.1111/j.1752-7325.2006.tb04079.x. [DOI] [PubMed] [Google Scholar]
- 24.Lopez R, Baelum V. Oral health impact of periodontal diseases in adolescents. J Dent Res. 2007;86:1105–1109. doi: 10.1177/154405910708601116. [DOI] [PubMed] [Google Scholar]
- 25.Marshman Z, Rodd H, Stern M, et al. An evaluation of the child perceptions questionnaire in the UK. Community Dent Health. 2005;22:151–155. [PubMed] [Google Scholar]
- 26.Robinson PG, Nalweyiso N, Busingye J, et al. Subjective impacts of dental caries and fluorosis in rural Uganda children. Community Dent Health. 2005;22:231–236. [PubMed] [Google Scholar]
- 27.Dental Health Division . Department of Health, Ministry of Public Health; Nonthaburi: 2008. Report on the Sixth National Oral Health Survey of Thailand. [Google Scholar]
- 28.Krisdapong S, Sheiham A, Tsakos G. Oral health-related quality of life of 12- and 15-year-old Thai children: findings from a national survey. Community Dent Oral Epidemiol. 2009;37:509–517. doi: 10.1111/j.1600-0528.2009.00503.x. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization . 4th edn. WHO; Geneva: 1997. Oral Health Surveys. Basic Methods. [Google Scholar]
- 30.Ainamo J, Barmes D, Beagrie G, et al. Development of the WHO community periodontal index of treatment needs (CPITN) Int Dent J. 1982;32:281–293. [PubMed] [Google Scholar]
- 31.Gherunpong S, Tsakos G, Sheiham A. Developing and evaluating an oral health-related quality of life index for children: the Child-OIDP. Community Dent Health. 2004;21:161–169. [PubMed] [Google Scholar]
- 32.Gherunpong S, Tsakos G, Sheiham A. The prevalence and severity of oral impacts on daily performances in Thai primary school children. Health Qual Life Outcomes. 2004;2:57. doi: 10.1186/1477-7525-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev. 1981;70:35–36. [Google Scholar]
- 34.Batchelor P, Sheiham A. The limitations of a ‘high-risk’ approach for the prevention of dental caries. Community Dent Oral Epidemiol. 2002;30:302–312. doi: 10.1034/j.1600-0528.2002.00057.x. [DOI] [PubMed] [Google Scholar]
- 35.Brown A, Al-Khayal Z. Validity and reliability of the Arabic translation of the child oral health-related quality of life questionnaire (CPQ(11–14)) in Saudi Arabia. Int J Paediatr Dent. 2006;16:405–411. doi: 10.1111/j.1365-263X.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 36.Kolawole KA, Otuyemi OD, Oluwadaisi AM. Assessment of oral health-related quality of life in Nigerian children using the child perception questionnaire (CPQ 11–14) Eur J Paediatr Dent. 2011;21:55–59. [PubMed] [Google Scholar]
- 37.Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. 2008;79:1560–1568. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- 38.Sheiham A. Is the chemical prevention of gingivitis necessary to prevent severe periodontitis? Periodontol 2000. 1997;15:15–24. doi: 10.1111/j.1600-0757.1997.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 39.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 40.Sheiham A, Alexander D, Cohen L, et al. Global oral health inequalities: task group implementation and delivery of oral health strategies. Adv Dent Res. 2011;23:259–267. doi: 10.1177/0022034511402084. [DOI] [PubMed] [Google Scholar]
- 41.Petersen PE, Kwan S. Equity, social determinants and public health programmes – the case of oral health. Community Dent Oral Epidemiol. 2011;39:481–487. doi: 10.1111/j.1600-0528.2011.00623.x. [DOI] [PubMed] [Google Scholar]