Abstract
Antimicrobials are added to toothpastes to deliver dental plaque/gingivitis benefits. Objectives: This study aimed to evaluate antimicrobial effects of an o-cymen-5-ol/zinc system. Methods:o-Cymen-5-ol and zinc gluconate minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined against Streptococcus mutans, Actinomyces viscosus, Porphyromonas gingivalis, Fusobacterium nucleatum and Candida albicans. Synergy was investigated by checkerboard MIC/MBC; inhibition of P. gingivalis protease activity and S. mutans glycolysis were investigated. Slurried toothpastes containing the system were assessed in kill time assays against S. mutans and E. coli. Results:o-Cymen-5-ol MIC was between 1.7 mM to 3.4 mM; MBC was 3.4 mM to 6.7 mM. Zinc gluconate MIC was 2.8 mM to 11 mM; MBC was between 11 mM and >44 mM. The two agents in solution showed synergy (FICI≤ 0.50) against P. gingivalis and F. nucleatum, with MIC of 0.42 mM/0.69 mM for o-cymen-5-ol/zinc gluconate, respectively. Zinc inhibited glycolysis and protease to a greater degree than o-cymen-5-ol; glycolysis inhibition by the two agents was additive. o-Cymen-5-ol/zinc chloride in toothpaste showed greater effects than placebo (120s log10 kill = 7.35 ± 0.40 and 4.02 ± 0.40, respectively). Conclusions: The zinc/o-cymen-5-ol system has direct antimicrobial effects and inhibits oral disease-related processes. Synergistic effects were seen against anaerobes. A system combining o-cymen-5-ol and zinc shows properties desirable for incorporation in toothpastes.
Key words: Antimicrobial, gingivitis, dental plaque, Toothpaste, zinc, o-cymen-5-ol
INTRODUCTION
The human mouth is sterile at birth, but, rapidly becomes colonised by a diverse microflora1, typically with hundreds of species in any one adult individual’s mouth2 selected from potentially several thousand species found across human populations; the debate continues as to whether such numbers in an individual can comprise many thousands of microbial species3. The oral microflora exists in an intimate relationship with the host, with particular groups of bacteria associated with specific oral habitats4., 5.. In spite of a wide range of dietary and environmental challenges, the oral microflora remains broadly similar across human populations. This stability has been termed microbial homeostasis6., 7., and is assumed to result from the comprehensive biochemical capabilities of the entire oral microflora, together with the intricate web of metabolic interactions between members of the microflora8., 9., 10., 11.. Significant environmental disruption, for example by antibiotics, often allows overgrowth of smaller or insignificant members of the oral microflora, resulting in conditions such as oral candidiasis12., 13.. With these exceptions, the microflora usually exists in a benign and stable relationship with the host. However, the oral microflora is also key to the most common chronic diseases affecting humans, dental caries and gingivitis.
Dental caries is microbially-driven, by generation of lactic and other acids from dietary carbohydrate by a range of microbes, and is associated with excessive and frequent sugar consumption14., 15.. Most attention has focussed on the role of mutans-streptococci, because of their acidogenic, aciduric and adherent properties16 and association in earlier studies with sugar consumption17., 18., 19., 20., and disease susceptibility21. More recent evidence suggests that a large number of microbial taxa other than mutans-streptococci can also generate significant quantities of acid22., 23. and are associated with caries24. The specific bacteria implicated in gingivitis remain unclear. However, it is clear that excessive accumulation of dental plaque and associated by-products at the gum margin produces gingivitis25, which in turn may lead to increased microbial nutrition (via increased gingival crevicular fluid flow, blood etc) and further microbial proliferation. Earlier studies suggested the involvement of general groups of organisms (Gram-negative anaerobes, specific genera from the ‘orange’ or ‘red’ complex)26. More recent studies, however, suggest a much broader aetiology, with greater similarities within a subject than between diseased and non-diseased sites27., 28.. Periodontitis is much rarer than gingivitis, and while initiated by bacterial infection also appears to be linked to immunoinflammatory pathways29, although prolonged pre-existing gingivitis is also considered a risk factor30.
Oral care products are designed to combat the two key microbially-mediated diseases, caries and gingivitis. The beneficial effects of topical fluoride in toothpastes have driven a dramatically reduced caries incidence in the last 30–40 years31. Fluoride appears to reduce caries principally via effects on enamel de- and re-mineralisation32 although it can also have potentially caries-relevant antimicrobial effects, notably on bacterial metabolism, and especially on glycolytic and acid-generating pathways33. Gingivitis, in contrast, may usually be prevented or treated by a diligent oral hygiene regime34. The major beneficial effect of tooth brushing on gingivitis thus rests on the mechanical removal of dental plaque, with this process assisted by toothpaste formulation excipients such as abrasives and surfactants. However, most of the population fail to carry out meticulous oral hygiene, and thus the incorporation of additional antimicrobial actives in toothpastes as an adjunct to mechanical plaque control has been proposed35., 36.. Products must tread a relatively fine line, termed an antimicrobial paradox37, wherein the aim is to suppress the plaque to the extent required to prevent or reduce gingivitis, but not to eliminate the oral microflora or to disrupt it significantly. Such agents must meet a number of important criteria which were recently reviewed38. The most widely used antimicrobial agent in toothpastes is Triclosan. In clinical trials, significant gingivitis benefits were seen when Triclosan was formulated with an additional antimicrobial agent – zinc citrate39, or with a co-polymer, Gantrez40, which improves oral Triclosan retention. Triclosan is, however, currently under review by the US Food & Drug Administration and Environmental Protection Agency41. Stannous fluoride in toothpaste delivers anti-gingivitis efficacy42 and is recognised in the tentative FDA anti-gingivitis monograph43. Stannous fluoride does, however cause staining problems42 and adversely affects the taste of toothpastes. The essential oils combination of eucalyptol, menthol, methyl salicylate and thymol is recognised in the FDA monograph to deliver anti-gingivitis efficacy in mouthwash43, and has also been reported to be effective in toothpaste against gingivitis44. However, thymol in particular, causes a rather polarising, burning sensation, which limits acceptability for family-orientated toothpastes. Zinc salts have been advocated based on a number of beneficial antimicrobial effects the cation can deliver. Zinc inhibits a range of microbial enzyme systems, including a range of glycolytic enzymes in streptococci, with concomitant increased sensitivity of the organisms to acid45. In two typical oral anaerobes, Fusobacterium nucleatum and Prevotella intermedia, a range of enzymatic processes are inhibited by zinc, including catabolism of amino acids, sugars and peptides, as well as oxidative metabolism46. Zinc can also deliver beneficial effects for bad breath: reducing breath concentrations of volatile sulphur compounds (VSCs) associated with halitosis47., 48. as well as having direct49 and indirect46 inhibitory effects on anaerobe mediated-VSC production.
There is thus a clear need for novel antimicrobial systems which overcome some of the issues associated with Triclosan, stannous fluoride and essential oils, and that can deliver antimicrobial effects in a product with a taste profile acceptable to the whole family. This paper discusses the antimicrobial testing carried out on a novel toothpaste system, incorporating o-cymen-5-ol (a tasteless isomer of thymol) and zinc chloride. The agents in this system have been tested, alone and in combination, in a series of standard antimicrobial assays, in simple solutions and in whole toothpaste formulations. Further, the agents have been evaluated for their inhibitory effects on key metabolic processes – acid production by Streptococcus mutans and protease activity of Porphyromonas gingivalis.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Strains were sub-cultured from −80°C or freeze dried stocks. Streptococcus mutans NCTC 10449 and Streptococcus mutans UA159 were then maintained on blood agar at 37°C in an aerobic incubator. Actinomyces viscosus NCTC 10951, Fusobacterium nucleatum NCTC 10562 and Porphyromonas gingivalis ATCC 53978 were maintained on blood agar anaerobically at 37°C. Candida albicans NCPF 3179 was maintained on Sabouraud’s Dextrose agar (Oxoid) at 25°C in an aerobic incubator.
Minimum inhibitory concentration and minimum bactericidal concentration assays
The minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC), were measured using methods described by the National Committee for Clinical Laboratory Standards (NCCLS)50 broth microdilution method with some modifications including: use of flat bottomed 96-well microtitre plates (Corning 3370, FisherScientific, Loughborough, UK), sterile deionised water used as diluent, brain heart infusion broth used in place of Muller-Hinton broth and 100 μl of inoculum added to wells. o-Cymen-5-ol stock solution was prepared at 0.4%w/w (26.67 mM) in 50%v/v DMSO. Zinc gluconate stock solutions were prepared in water. Zinc gluconate was used in simple solution experiments in place of zinc chloride to ensure adequate solubility and stability, and to avoid pH effects, and was made in equivalent zinc molarity to toothpaste concentrations. Briefly, 2-fold serial dilutions of o-cymen-5-ol and zinc gluconate were prepared in sterile deionised water at a volume of 100 μl in the wells of a polystyrene 96-well microtitre plate. This was diluted to give final concentrations of o-cymen-5-ol in MIC wells from 0.0033 mM to 6.66 mM (0.00049 mg/ml to 1 mg/ml). The final concentrations of zinc gluconate ranged from 0.022 mM to 44.02 mM (0.00981 mg/ml to 20.1 mg/ml). The wells were inoculated with 100 μl of bacterial cell suspension in double strength BHI (Merck) at a final concentration of approx. 1 × 106 cells/ml. Control wells of water and uninoculated double strength BHI served as a reference and wells containing test organism in BHI were used as a growth control. The plates were incubated anaerobically for 48 hrs, (for F. nucleatum and P. gingivalis), and aerobically for 24–48 hours, (for S. mutans, A. viscosus and C. albicans), all at 37°C. Growth was assessed by measuring absorbance at 550 nm using a microplate spectrophotometer (SpectraMax M5, Molecular Devices, California, USA) and the lowest concentration inhibiting growth relative to uninoculated control was considered as the MIC. The MBC was measured by taking a 10 μl aliquot from two wells either side of the last well to show growth and plating onto Columbia blood agar (Biomerieux, Basingstoke, UK) followed by appropriate incubation. MBC was considered to be the lowest concentration showing no growth on blood agar after 48 hours incubation.
MIC checkerboard assay
To determine the lowest combinations of o- cymen-5-ol and zinc gluconate that were inhibitory to growth a checkerboard MIC/MBC method was used. Stock solutions were prepared as described above. Two-fold dilution series of o-cymen-5-ol and zinc gluconate at four times final concentration were prepared in sterile deionised water. Fifty microlitres of sterile water was added to wells A-H in columns 1–8 of a 96- well microplate. Fifty microlitres of the first o-cymen-5-ol dilution was added to wells A-H in column 1 and the doubling dilution series continued down to column 8. Zinc gluconate dilutions were added in a similar manner in wells of rows A-H in columns 1–8 to give 64 wells each containing a different concentration of either o-cymen-5-ol or zinc gluconate. The wells were inoculated with the test organism at a cell density of approx 1 × 106 cfu/ml in double strength BHI. Wells in column 11 contained 50 μl of double strength BHI in 150 μl sterile water and were used as a reference. Wells in column 12 contained 50 μl of test organism in double strength BHI and 150 μl sterile water and were used as a growth indicator. Microplates were incubated at 37°C for 24–48 hours and the MIC was determined as above. MIC was defined as the lowest concentration of o-cymen-5-ol and zinc gluconate that inhibited growth of the test organism. The MBC was determined as above by plating a 10 μl aliquot of each test well in each row onto blood agar. The MBC was defined as the lowest combination of o-cymen-5-ol and zinc gluconate that failed to grow on blood agar. Synergy was defined conservatively as Fractional Inhibitory Concentration Index (FICI) ≤ 0.50 according to the criteria described by Odds51 and by Johnson et al.52 where:
In principle, FICI ≤ 1.00 would indicate an increased activity of two agents in combination. However the criteria defined by Odds51 and by Johnson et al.52 also incorporate the need for a two-doubling-dilution difference in activity for both agents to indicate clear synergy.
Antibacterial activity of solutions and whole toothpastes
Toothpastes or solutions formulated to contain o-cymen-5-ol, zinc or the two agents combined were tested for antibacterial activity using the European suspension test53 method with the following modifications; test was scaled down to accommodate a 2 ml deep well plate (Corning Code 3960) using 100 μl artificial saliva as interfering substance, 100 μl bacterial suspension, 800 μl of solution or toothpaste slurry (made to 120% of required final assay concentration) in hard water. o-Cymen-5-ol and zinc gluconate stock solutions were prepared in DMSO and water, respectively as above. Toothpastes were slurried to give dilutions of 1/4 or 1/6, and compared to a placebo product containing neither active but otherwise identical except for the absence of a buffer system. Contact times of 30 seconds and 120 seconds were used to simulate typical contact times during brushing. At these times a 100 μl aliquot of test mixture was taken from the test well and added to 900 μl of neutraliser containing Tryptone Soya Broth, 4% Tween 20 and 0.5% Lecithin (Oxoid, Code BO 1084J, Basingstoke, UK). The test mixture was allowed to neutralise for 10 minutes before being decimally serially diluted in 1% Peptone water with 0.85% Sodium chloride (Oxoid). Dilutions were plated onto Blood Agar number 2 (Oxoid, Basingstoke, UK) and incubated at 37°C anaerobically for 48–72 hours.
Protease inhibition assay
Solutions of o-cymen-5-ol and zinc gluconate were investigated for inhibition of Porphyromonas gingivalis tissue destructive arg-gingipain protease using the method described by Percival and colleagues54 with the following modifications: assay was scaled down to fit in the wells of a 96-well microtitre plate, (Corning) and buffer used was phosphate buffered saline (PBS) (Sigma) at pH 7.49. o-Cymen-5-ol and zinc gluconate stock solutions were prepared in DMSO and water, respectively as above. Test wells of the microplate contained 160 μl of test solution diluted in assay buffer, 40 μl of the synthetic enzyme substrate DL-α-Benzoyl-DL-arginyl-p-nitro-anilide (BAPNA) (Sigma, Product Code B3133) and 20 μl P. gingivalis culture in BHI broth (grown for 48 hours). Controls used the same mixture as above but with test agent replaced with 160 μl of assay buffer. The assay was carried out in triplicate. The increase in absorbance was read every minute for three minutes at a wavelength of 405 nm using a microplate spectrophotometer. Results were presented as percent inhibition of protease enzyme activity [100- (change in OD of test agent/ change in OD of control) x 100].
Glycolysis inhibition assay
Assays of acid production under pH-stat conditions were carried out as described previously45 for thick suspensions of cells of S. mutans UA159. Briefly, for the assays, cells were used from cultures in the early stationary phase of growth in BHI medium with excess glucose so that the cells were fully acid-adapted. Stock solutions were prepared as above. Inhibitory effects on glycolysis of o-cymen-5-ol, zinc chloride and SLS were compared alone and in combination.
RESULTS
MIC and MBC
Data presented in Table 1 show that o-cymen-5-ol MIC values were in the range 1.66 mM and 3.33 mM and MBC between 3.33 mM and 6.66 mM; for zinc gluconate the MIC was between 2.8 mM and 11 mM and MBC between 11.00 mM and > 44.02 mM. Combinations of the two agents in solution showed significant synergistic effects (FICI≤ 0.50) in MIC and MBC tests against oral anaerobic species, with effective MIC values as low as 0.42 mM/0.69 mM for the combination of o-cymen-5-ol/zinc gluconate, respectively.
Table 1.
MIC, MBC & Synergy of o-cymen-5-ol & zinc gluconate
| Bacterial Species & Strain | Individual MIC (mM) | Combination MIC (mM) | FICI§ | Synergy† | |
|---|---|---|---|---|---|
| o-Cymen-5-ol | Zinc gluconate | o-Cymen-5-ol / Zinc gluconate | |||
| S. mutans NCTC 10449 | 1.66 | 2.76 | 0.83/1.38 | 1.00 | No |
| A. viscosus NCTC 10951 | 3.33 | 11.03 | 1.66/5.51 | 1.00 | No |
| F. nucleatum NCTC 10562 | 3.33 | 5.51 | 0.83/1.38 | 0.50 | Yes |
| P. gingivalis ATCC 53978 | 1.66 | 2.76 | 0.42/0.69 | 0.50 | Yes |
| C. albicans NCPF 3179 | 1.66 | 5.51 | ND | -- | -- |
| Bacterial Species & Strain | Individual MBC (mM) | Combination MBC (mM) | FICI§ | Synergy† | |
|---|---|---|---|---|---|
| o-Cymen-5-ol | Zinc gluconate | o-Cymen-5-ol / Zinc gluconate | |||
| S. mutans NCTC 10449 | 3.33 | 11.03 | 1.66/5.51 | 1.00 | No |
| A. viscosus NCTC 10951 | 6.66 | 11.03 | 3.33/5.51 | 1.00 | No |
| F. nucleatum NCTC 10562 | 3.33 | 22.05 | 1.66/1.38 | 0.56 | No |
| P. gingivalis ATCC 53978 | 3.33 | 11.03 | 0.83/2.76 | 0.50 | Yes |
| C. albicans NCPF 3179 | 3.33 | >44.11 | ND | -- | -- |
*for ease of reference concentrations in toothpaste active system are o-cymen-5-ol = 6.66 mM; zinc gluconate (added as zinc chloride) equivalent to 44.11 mM.
FICI = MICo-Cymen-5-ol+Zinc gluconate/MICo-Cymen-5-ol alone + MICZinc gluconate+o-Cymen-5-ol/MICzinc gluconate alone
Antibacterial activity of solutions and whole toothpastes
Solution data are presented for anaerobic species in Figures 1 and 2. Significantly increased antimicrobial activity was seen against P. gingivalis and F. nucleatum for the combination of the three agents than for the individual agents alone. Presumably because of interactions with SLS and flavours, whole toothpastes gave no recoverable counts for these organisms.
Figure 1.
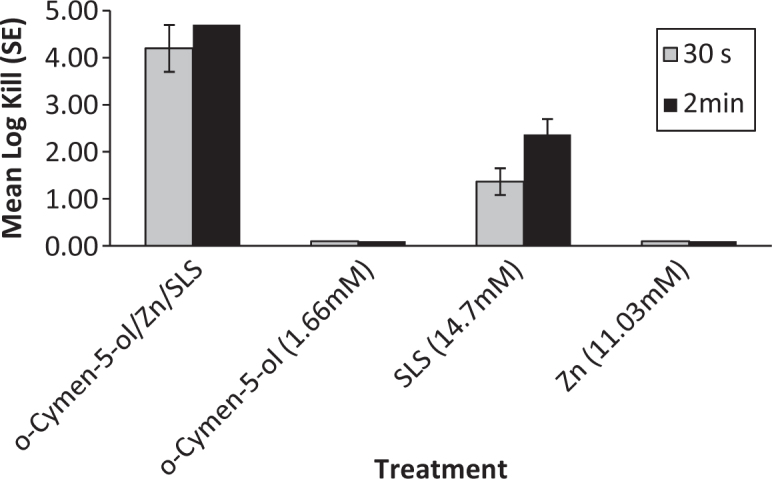
F. nucleatum Solution Kill Time (n = 3).
Figure 2.
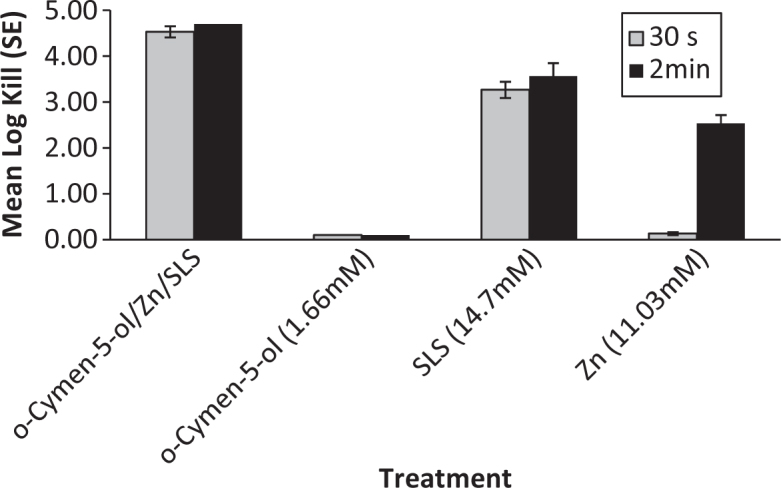
P. gingivalis Solution Kill Time (n = 3).
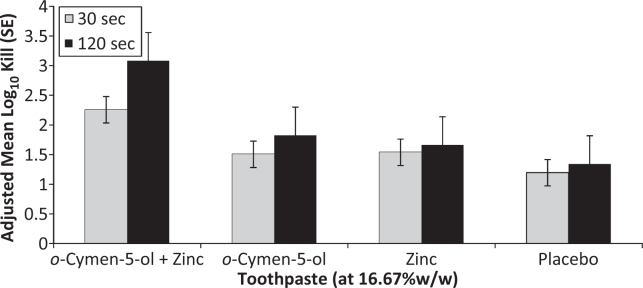
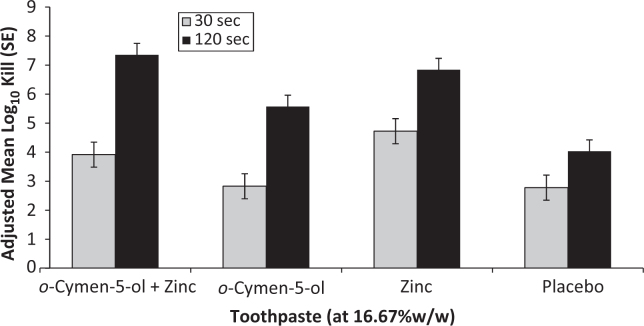
Data comparing the EN1276 kill times versus E. coli and S. mutans for 16.67% w/w dilutions (1/6) of toothpastes are presented in Figures 3 and 4 for two independent experiments on toothpastes. The data were compared by ANOVA with factors for treatment and experimental run. The combination of o-cymen-5-ol and zinc chloride in toothpaste showed greater effects against both S. mutans and E. coli than placebo pastes at both time points. This difference was significant (P < 0.0001) for S. mutans at 120s (log10 kill = 7.35 ± 0.40 versus log10 kill = 4.02 ± 0.40), and significant at both 30s (P = 0.0032) and 120s (P = 0.0183) for E. coli. Zinc only paste showed directionally greater kill than the combination paste at the 30s time point against S. mutans, whereas the combination paste showed directionally better kill at 120s. Data for 25% dilutions showed similar trends, but were less differentiated since the kill was closer to the maximum kill detectable in the test for all pastes.
Figure 3.
E. coli Toothpaste Kill-Time (n = 6).
Glycolysis inhibition
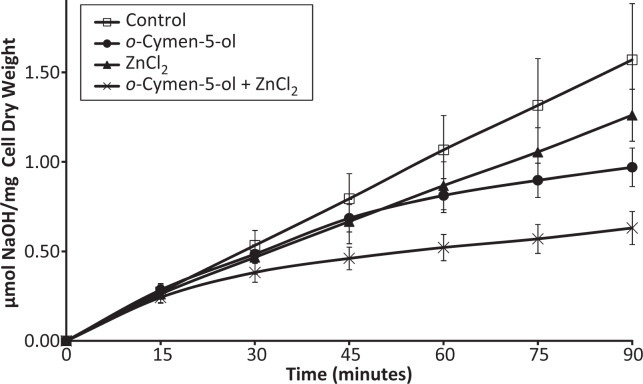
Data on glycolysis inhibition are presented in Figure 5. The agents tested were found to act mainly additively on acid production. For example, the data presented in Figure 5 show that o-cymen-5-ol and ZnCl2 were both inhibitory and, in combination, had additive action. Other combinations of the antimicrobials could have dramatic effects. For example, either 20 mM o-cymen-5-ol or 0.2 mM SLS reduced glycolytic acid production by some 50%; together they totally inhibited the glycolytic process (data not shown).
Figure 5.
Inhibition by 10mM o-Cymen-5-ol & 1 mM Zinc Chloride of S.mutans UA159 Glycolysis (pH 6.0; 1.1 mg/ml dry weight; n = 6). (Error bars = S.E.)
Protease inhibition
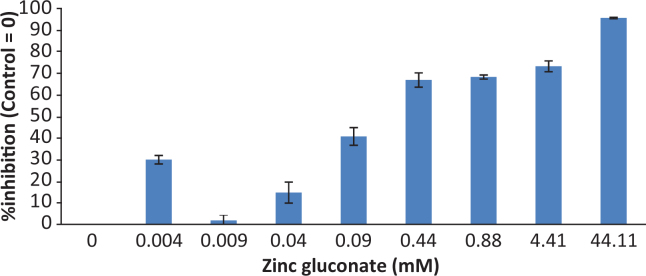
Protease was inhibited in a dose dependent fashion by zinc gluconate, with >50% inhibition of activity at less than 1 mM zinc gluconate (Figure 6). o-Cymen-5-ol showed no inhibitory effect on P. gingivalis protease activity (data not shown).
Figure 6.
Zinc Inhibition of P. gingivalis Protease. (Error bars = S.E.)
DISCUSSION AND CONCLUSIONS
The data presented above describe the wide ranging activity of the key components of the novel system containing o-cymen-5-ol and zinc. The MIC data demonstrate a broad range of activity for inhibition of growth against a representative selection of typical oral micro-organisms. The MIC values all lie in the range between 1/4 and 1/16 of the concentrations of the antimicrobial system in toothpaste, with zinc values generally relatively lower (as a fraction of toothpaste concentrations) than those for o-cymen-5-ol.
Effects of additional antimicrobial agents in toothpaste formulations are often difficult to discern because other formulation components, especially surfactants and to a lesser degree flavour components, also deliver significant effects. In short contact kill time assays of 1/6 toothpaste slurries the effects of the o-cymen-5-ol/zinc chloride system are clear against both S. mutans and E. coli (Figures 3 and 4), in comparison to placebo formulations containing an identical formulation without the system. The combination of agents was superior to the placebo containing neither active, enhancing the antimicrobial effects of the base placebo paste significantly at P < 0.05 for all conditions tested except for S. mutans at 30s, where it was directionally superior. E. coli was included as a comparator in these tests since although it is not directly relevant in the oral cavity, its relative insensitivity to SLS and flavour effects mean it is a useful marker organism for testing antimicrobial additives in whole toothpaste formulations. In the case of S. mutans the differential effect was largely driven by zinc; for E. coli the effects of o-cymen-5-ol were greater. Although the antimicrobial effects of SLS tend to be relatively large in short-contact tests, the significance of SLS effects in vivo are likely to be limited by the relatively short residence time of this agent in the oral cavity55. In contrast, data for zinc show lengthy residence times in saliva and longer still in plaque56, sometimes several hours. There are no direct data currently available for o-cymen-5-ol.
Figure 4.
S. mutans Toothpaste Kill-Time.
The effects of antimicrobial agents at such low concentrations are of great interest, since these are likely to be significant in delivering benefits that span the time periods between tooth brushing events57. Synergy is often discussed in evaluation of combination antimicrobial systems, but is often poorly defined51. We have applied a stringent definition, where the fractional inhibitory concentration index (FICI) must be ≤ 0.50 to indicate synergy51., 52.. This allows for variation in doubling dilution MIC/MBC determination, as well as excluding definitions of merely ‘additive’ effects included in some publications. By this definition, data for the combination of zinc and o-cymen-5-ol show synergy against the anaerobes F. nucleatum and P. gingivalis (4-fold differences in combined MIC compared with MIC for single agent), with no evidence of synergy against S. mutans or A. viscosus. Similarly, FICI for both anaerobes in MBC was lower than for either S. mutans or A. viscosus, although only against P. gingivalis was there evidence of synergy.
Anti-metabolic effects are also likely to be significant in determining the effectiveness of agents in oral care products. Such effects can act both to slow the (re)growth of plaque bacteria after oral care routines, thus reducing the plaque microbial challenge. They can also reduce the production of metabolites which in themselves are deleterious to host tissues. Data presented here shows both glycolytic and proteolytic enzymes can be retarded by one or both of the two agents studied.
Inhibition of the arg-gingipain activity of P. gingivalis extended across a wide concentration range for zinc gluconate, to sub-millimolar concentrations; no significant inhibition by o-cymen-5-ol was observed. This inhibitory effect has been reported briefly previously37. Such sub-MIC effects of antimicrobial agents have been suggested to be an important property of antimicrobial agents in oral care usage58, because they extend the spectrum of potential clinical effect over much greater lengths of time, in some cases potentially extending this as far as the next tooth brushing. At the same time, sub-MIC effects are likely to avoid deleterious effects of more overt antimicrobial action.
Inhibition of glycolytic acid production would reduce the cariogenicity of mutans streptococci and other related organisms involved in caries, and both agents show effects, with an additive effect of the combination. In addition to the data presented here, Burnett et al.49 have shown in both simple and complex microbial model systems that the agents discussed can reduce VSC production.
Taken together, the overt antimicrobial data on simple solutions and toothpastes, together with the data indicating synergistic MIC/MBC effects, as well as inhibition of key bacterial metabolic processes associated with oral diseases suggest a potential antimicrobial basis for the clinical efficacy observed in clinical studies59., 60..
CONFLICT OF INTEREST AND SOURCE OF FUNDING
The work described in this manuscript was funded by GlaxoSmithKline Consumer Healthcare. The author external to GSK received no additional fee for the preparation of this manuscript. The remaining authors are employed by GSK but confirm no potential conflicts of interest.
REFERENCES
- 1.Könönen E. Development of oral bacterial flora in young children. Ann Med. 2000;32:107–112. doi: 10.3109/07853890009011759. [DOI] [PubMed] [Google Scholar]
- 2.Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keijser BJ, Zaura E, Huse SM, et al. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res. 2008;87:1016–1020. doi: 10.1177/154405910808701104. [DOI] [PubMed] [Google Scholar]
- 4.Papaioannou W, Gizani S, Haffajee AD, et al. The microbiota on different oral surfaces in healthy children. Oral Microbiol Immunol. 2009;24:183–189. doi: 10.1111/j.1399-302X.2008.00493.x. [DOI] [PubMed] [Google Scholar]
- 5.Mager DL, Haffajee AD, Socransky SS. Effects of periodontitis and smoking on the microbiota of oral mucous membranes and saliva in systemically healthy subjects. J Clin Periodontol. 2003;30:1031–1037. doi: 10.1046/j.0303-6979.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 6.Marsh PD. Host defenses and microbial homeostasis: role of microbial interactions. J Dent Res. 1989;68:1567–1575. [Google Scholar]
- 7.Marsh PD, Percival RS. The oral microflora--friend or foe? Can we decide? Int Dent J. 2006;56:233–239. doi: 10.1111/j.1875-595x.2006.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 8.Bradshaw DJ, Homer KA, Marsh PD, et al. Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology. 1994;140:3407–3412. doi: 10.1099/13500872-140-12-3407. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson J. Bacterial metabolism in dental biofilms. Adv Dent Res. 1997;11:75–80. doi: 10.1177/08959374970110012001. [DOI] [PubMed] [Google Scholar]
- 10.van der Hoeven JS, van den Kieboom CW, Camp PJ. Utilization of mucin by oral Streptococcus species. Antonie Van Leeuwenhoek. 1990;57:165–172. doi: 10.1007/BF00403951. [DOI] [PubMed] [Google Scholar]
- 11.Wei GX, van der Hoeven JS, Smalley JW, et al. Proteolysis and utilization of albumin by enrichment cultures of subgingival microbiota. Oral Microbiol Immunol. 1999;14:348–351. doi: 10.1034/j.1399-302x.1999.140603.x. [DOI] [PubMed] [Google Scholar]
- 12.Dreizen S. Oral candidiasis. Am J, Med. 1984;77:28–33. [PubMed] [Google Scholar]
- 13.Soysa NS, Samaranayake LP, Ellepola ANB. Antimicrobials as a contributory factor in oral candidosis – a brief overview. Oral Dis. 2008;14:138–143. doi: 10.1111/j.1601-0825.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson BE, Quensel CE, Lanke LS, et al. The Vipeholm dental caries study; the effect of different levels of carbohydrate intake on caries activity in 436 individuals observed for five years. Acta Odontol Scand. 1954;11:232–264. doi: 10.3109/00016355308993925. [DOI] [PubMed] [Google Scholar]
- 15.Zero DT. Sugars - the arch criminal? Caries Res. 2004;38:277–285. doi: 10.1159/000077767. [DOI] [PubMed] [Google Scholar]
- 16.Banas JA. Virulence properties of Streptococcus mutans. Front Biosci. 2004;9:1267–1277. doi: 10.2741/1305. [DOI] [PubMed] [Google Scholar]
- 17.De Stoppelaar JD, Van Houte J, Backer Dirks O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4:114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- 18.Dennis DA, Gawronski TH, Sudo SZ, et al. Variations in microbial and biochemical components of four-day plaque during a four-week controlled diet period. J Dent Res. 1975;54:716–722. doi: 10.1177/00220345750540040401. [DOI] [PubMed] [Google Scholar]
- 19.Minah GE, Lovekin GB, Finney JP. Sucrose-induced ecological response of experimental dental plaques from caries-free and caries-susceptible human volunteers. Infect Immun. 1981;34:662–675. doi: 10.1128/iai.34.3.662-675.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staat RH, Gawronski TH, Cressey DE, et al. Effects of dietary sucrose levels on the quantity and microbial composition of human dental plaque. J Dent Res. 1975;54:872–880. doi: 10.1177/00220345750540042801. [DOI] [PubMed] [Google Scholar]
- 21.Bowden GH, Hardie JM, Fillery ED, et al. In: Proceedings, Methods of Caries Prevention. Bibby BG, Shern RJ, editors. Information Retrieval; London: 1978. Microbial Analyses Related to Caries Susceptibility; pp. 83–97. [Google Scholar]
- 22.Aas JA, Griffen AL, Dardis SR, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407–1417. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi N, Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res. 2008;42:409–418. doi: 10.1159/000159604. [DOI] [PubMed] [Google Scholar]
- 24.Beighton D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol. 2005;33:248–255. doi: 10.1111/j.1600-0528.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 25.Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 26.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar PS, Griffen AL, Moeschberger ML, et al. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–3955. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wade WG. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease. J Clin Periodontol. 2011;38:7–16. doi: 10.1111/j.1600-051X.2010.01679.x. [DOI] [PubMed] [Google Scholar]
- 29.Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. 2008;79:1560–1568. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- 30.Lang NP, Schatzle MA, Löe H. Gingivitis as a risk factor in periodontal disease. J Clin Periodontol. 2009;36(Suppl 10):3–8. doi: 10.1111/j.1600-051X.2009.01415.x. [DOI] [PubMed] [Google Scholar]
- 31.Marinho VC. Evidence-based effectiveness of topical fluorides. Adv Dent Res. 2008;20:3–7. doi: 10.1177/154407370802000102. [DOI] [PubMed] [Google Scholar]
- 32.ten Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontol Scand. 1999;57:325–329. doi: 10.1080/000163599428562. [DOI] [PubMed] [Google Scholar]
- 33.Marquis RE, Clock SA, Mota-Meira M. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev. 2003;26:493–510. doi: 10.1111/j.1574-6976.2003.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 34.Ohrn K, Sanz M. Prevention and therapeutic approaches to gingival inflammation. J Clin Periodontol. 2009;36(Suppl 10):20–26. doi: 10.1111/j.1600-051X.2009.01418.x. [DOI] [PubMed] [Google Scholar]
- 35.Hull PS. Chemical inhibition of plaque. J Clin Periodontol. 1980;7:431–442. doi: 10.1111/j.1600-051x.1980.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 36.Kornman KS. The role of supragingival plaque in the prevention and treatment of periodontal diseases. A review of current concepts. J Clin Periodontol. 1986;21:5–22. [Google Scholar]
- 37.Marsh PD. Microbiological aspects of the chemical control of plaque and gingivitis. J Dent Res. 1992;71:1431–1438. doi: 10.1177/00220345920710071501. [DOI] [PubMed] [Google Scholar]
- 38.Brading MG, Marsh PD. The oral environment: the challenge for antimicrobials in oral care products. Int Dent J. 2003;53:353–362. doi: 10.1111/j.1875-595x.2003.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 39.Stephen KW, Saxton CA, Jones CL, et al. Control of gingivitis and calculus by a dentifrice containing a zinc salt and triclosan. J Periodontol. 1990;61:674–679. doi: 10.1902/jop.1990.61.11.674. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Godoy F, DeVizio W, Volpe AR, Ferlauto RJ, Miller JM. Effect of a triclosan/copolymer/fluoride dentifrice on plaque formation and gingivitis: a 7-month clinical study. Am J Dent. 1990;3:S15–S26. [PubMed] [Google Scholar]
- 41.FDA. Triclosan: What Consumers Should Know. http://www.fda.gov/forconsumers/consumerupdates/ucm205999.htm. 2011
- 42.Perlich MA, Bacca LA, Bollmer BW, et al. The clinical effect of a stabilized stannous fluoride dentifrice on plaque formation, gingivitis and gingival bleeding: a six-month study. J Clin Dent. 1995;6:54–58. Spec No: [PubMed] [Google Scholar]
- 43.Department of Health and Human Services F 21 CFR Part 356 [Docket No. 81N–033P] RIN 0910–AA01 Oral Health Care Drug Products for Over-the-Counter Human Use; Antigingivitis/Antiplaque Drug Products; Establishment of a Monograph. Federal Register. 2003;68:2231–2287. [Google Scholar]
- 44.Celho J, Kohut BE, Mankodi S, et al. Essential oils in an antiplaque and antigingivitis dentifrice: a 6-month study. Am J Dent. 2000;13:5C–10C. [PubMed] [Google Scholar]
- 45.Phan TN, Buckner T, Sheng J, et al. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol Immunol. 2004;19:31–38. doi: 10.1046/j.0902-0055.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 46.Sheng J, Nguyen PT, Marquis RE. Multi-target antimicrobial actions of zinc against oral anaerobes. Arch Oral Biol. 2005;50:747–757. doi: 10.1016/j.archoralbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Young A, Jonski G, Rølla G, et al. Effects of metal salts on the oral production of volatile sulfur-containing compounds (VSC) J Clin Periodontol. 2001;28:776–781. doi: 10.1034/j.1600-051x.2001.280809.x. [DOI] [PubMed] [Google Scholar]
- 48.Newby EE, Hickling JM, Hughes FJ, et al. Control of oral malodour by dentifrices measured by gas chromatography. Arch Oral Biol. 2008;53(Suppl 1):S19–S25. doi: 10.1016/S0003-9969(08)70005-0. [DOI] [PubMed] [Google Scholar]
- 49.Burnett GR, Stephen AS, Pizzey RL, et al. In vitro effects of novel toothpaste actives on components of oral malodour. Int Dent J. 2011;61:67–73. doi: 10.1111/j.1875-595X.2011.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.NCCLS Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline M26A. Approved Guideline. 1999;19:11–13. [Google Scholar]
- 51.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 52.Johnson MD, MacDougall C, Ostrosky-Zeichner L, et al. Combination antifungal therapy. Antimicrob Agents Chemother. 2004;48:693–715. doi: 10.1128/AAC.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.European Committee for Standardisation. European standard EN 1276. Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic, and industrial areas. Test method and requirements (phase 2, step 1). European Committee for Standardisation 1997.
- 54.Percival RS, Marsh PD, Devine DA, Rangarajan M, Aduse-Opoku J, Shepherd P, et al. Effect of temperature on growth, hemagglutination, and protease activity of Porphyromonas gingivalis. Infect Immun. 1999;67:1917–1921. doi: 10.1128/iai.67.4.1917-1921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fakhry-Smith S, Din C, Nathoo SA, et al. Clearance of sodium lauryl sulphate from the oral cavity. J Clin Periodontol. 1997;24:313–317. doi: 10.1111/j.1600-051x.1997.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 56.Cummins D, Creeth JE. Delivery of antiplaque agents from dentifrices, gels, and mouthwashes. J Dent Res. 1992;71:1439–1449. doi: 10.1177/00220345920710071601. [DOI] [PubMed] [Google Scholar]
- 57.Marsh PD. Plaque as a biofilm: pharmacological principles of drug delivery and action in the sub- and supragingival environment. Oral Dis. 2003;9(Suppl 1):16–22. doi: 10.1034/j.1601-0825.9.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 58.Marsh PD. Controlling the oral biofilm with antimicrobials. J Dent. 2010;38(Suppl 1):S11–S15. doi: 10.1016/S0300-5712(10)70005-1. [DOI] [PubMed] [Google Scholar]
- 59.Kakar A, Newby EE, Kakar K, et al. A randomised clinical trial to assess maintenance of gingival health by a novel dentifrice containing 0.1%w/w o-cymen-5-ol and 0.6%w/w zinc chloride. Int Dent J. 2011;61:13–20. doi: 10.1111/j.1875-595X.2011.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kakar A, Newby EE, Ghosh S, et al. A randomised clinical trial to assess maintenance of gingival health by a novel gel to foam dentifrice containing 0.1%w/w o-cymen-5-ol, 0.6%w/w zinc chloride. Int Dent J. 2011;61:21–27. doi: 10.1111/j.1875-595X.2011.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]