Abstract
Objectives: To evaluate the efficacy of a novel toothpaste containing zinc ions and o-cymen-5-ol to reduce volatile sulfur compounds (VSCs) in in vitro models and to elucidate the mode of action for any activity observed. Methods: Three models were employed, a chemical neutralisation model to evaluate the chemical reactivity of toothpaste slurries to VSCs, a biofilm perfusion model to measure activity in an orally-relevant biofilm and a planktonic bacterial model to measure antimicrobial effects. Results: The models showed that zinc ions were able to react chemically with hydrogen sulfide to remove this odorous component of halitotic breath. This activity was confirmed within a complex biofilm model, with over 90% of hydrogen sulfide removed from perfusate gas by a slurry of the test toothpaste. Conclusions: This work provides a mode of action for the clinically observed reduction in VSCs seen for up to 12 hours post brushing with this novel toothpaste.
Key words: Halitosis, o-cymen-5-ol, volatile sulfur compounds, zinc
INTRODUCTION
It is now generally accepted that oral malodour primarily originates from bacterial putrefaction of proteinaceous and other compounds within the oral cavity,1 particularly by anaerobic bacteria including Fusobacterium nucleatum, Porphyromonas gingivalis, Prevotella intermedia, and Tanerella forsythensis.2., 3., 4., 5. These bacteria are located throughout the oral cavity, but are found in particularly high numbers sub-gingivally and on the tongue,6., 7. especially towards the tongue posterior.8 The odours that these bacteria produce include amines such as putrescine and cadaverine and fatty acids such as butyrate and valerate. However, the most significant contributors to oral malodour are the volatile sulfur compounds (VSCs) such as hydrogen sulfide, methanethiol and dimethylsulfide,9., 10. with hydrogen sulfide the most concentrated odour on the breath of halitosis sufferers.6
Although management of halitosis is an active area of research, objective, clinical measurement of halitosis, and thereby determination of treatment efficacy, is currently not possible. Organoleptic/hedonic assessment is often employed and clearly has the benefit of relevance and simplicity, however the human nose is not linear in response to malodour concentration11 and measurement is often complicated by the flavours that are routinely added to healthcare products. Measurement of VSC concentration on the breath is therefore routinely used as a surrogate measurement, and given the prevalence of VSCs this would appear to be appropriate. Indeed clinical measurement of VSCs by techniques such as the Halimeter,12 the OralChroma13., 14., 15. and gas chromatography (GC)16 have long been used to provide clinical evidence of efficacy of treatments. Moreover, Rosenberg et al.17., 18. have demonstrated a correlation between organoleptic assessment of halistosis and clinically measured VSC concentrations. Effective treatments to control oral malodour include mechanical means, such as tongue scraping and brushing,19 and antimicrobials such as chlorhexidine,20 cetylpyridinium chloride,21 essential oils22 and zinc ions.16 Zinc-containing products in particular appear to be highly effective, possibly because they are dual active, being both antimicrobial23., 24. and chemically reactive to VSCs.25
Halitosis models are commonly employed, both to understand better the microbiology behind halitosis and to measure the efficacy of actives. Such models include planktonic bacterial models from individual cultured species26 and saliva27 as well as more sophisticated biofilm models based upon constant depth film fermentors,28 flat beds29 and perfusion devices.29., 30., 31. Of particular value is the Sorbarod biofilm-perfusion model of Spencer et al.30., 32. based upon the use of a highly porous cellulosic substrate (the Sorbarod) that is inoculated with tongue-derived bacteria and perfused with a cysteine-rich media and air. This model is particularly robust, lending itself to testing with whole product, such as toothpaste slurries, and benefits from realistic conditions in terms of relevancy of the organisms and perfusion with air rather than anaerobic gas.
In this work, three different models have been utilised to measure the effectiveness and understand the mode of action of a novel toothpaste containing the actives o-cymen-5-ol and zinc chloride.
MATERIALS AND METHODS
VSC determination
Concentrations of VSCs were measured using gas chromatography (GC). The GC (Agilent 6890N; Agilent Technologies, Edinburgh, UK) was equipped with a sulfur-specific flame photometric detector and a Chromosil 330 packed column running isothermally at 60 °C with helium carrier gas at a flow-rate of 45 ml/min. Sample gases were introduced either through a gas sampling valve fitted with a 0.1 ml sulfinert-treated sample loop (for Perfusion Biofilm Model and Chemical Neutralisation Model) or, for the Bacterial Headspace Model, through 0.1 ml headspace injection via an autosampler (CTC Combi PAL; LEAP Technologies, Carrboro, NC, USA). The GC was calibrated before use with a gas standards generator (Kin-tek 491M; Kin-tek Laboratories Inc, La Marque, TX, USA) containing hydrogen sulfide and methanethiol permeation tubes.
Bacterial Headspace Model
Sterile BHI broth (Oxoid, Basingstoke, UK) (37 g/l) was inoculated with Porphyromonas gingivalis W50 (ATCC 53978) from a stock culture that was maintained anaerobically on Columbia Blood Agar (bioMérieux, Basingstoke, UK) at 37 °C. The inoculated BHI broth was incubated at 37 °C for 48 hrs under anaerobic conditions (80% N2; 10% H2 and 10% CO2), then cells were harvested by centrifugation for 15 minutes at 3,000 g and 20 °C and washed by adding 20 ml sterile Phosphate Buffered Saline (PBS; pH 7.2 ± 0.2) to the bacterial pellet and vortexing for 15s. This step of centrifugation and washing was repeated twice before re-suspending the harvested bacteria in 20 ml of PBS. The bacterial concentrate thus obtained was then diluted (if necessary) in PBS to achieve an optical density at 550 nm of 0.10 cm−1. The bacterial suspension (0.5 ml) was incubated with gentle shaking for one hour at 37 °C in sealed 10 ml vials with 0.5 ml of 0.5% w/v of either Cysteine or Methionine (both supplied by Sigma, Poole, UK) and test agent (0.5 ml). 1 ml of absolute ethanol was then injected into the vials immediately after the incubation period to arrest bacterial metabolism of the amino acid. All vials were then subjected to GC headspace analysis and the VSC concentration presented as the mean ± standard error of triplicate vials obtained on three separate occasions.
Perfusion Biofilm Model
The Perfusion Biofilm Model (PBM) was based on the previously described model by Spencer et al.30. Briefly, bacteria obtained from a tongue scraping were inoculated onto a Sorbarod device that was perfused with air and media to form a confluent biofilm (see Figure 1 for a schematic of the Sorbarod apparatus). The Sorbarod (Ilacon, Tonbridge, UK) is an inert, porous substrate similar to a cigarette filter, with high surface area that provides a suitable substrate for growing high-density biofilms. Bacteria from the tongue of a single donor were obtained using a tongue scraper (Whatman® Omniswab) and suspended in 10 ml of peptone water (Oxoid) supplemented with 4.12% w/v brain heart infusion (BHI) broth (Oxoid), 0.0008% w/v dithiothreitol (DTT) (Sigma), 0.005% w/v L-cysteine (Sigma) and 0.00005% w/v haemin (Sigma). 1.5 ml of suspension was used to inoculate each of five Sorbarods which were then left undisturbed at 37 °C for two days to enable colonisation of the Sorbarods. The Sorbarods were then perfused with medium (0.74% w/v BHI, 0.01% w/v L-cysteine, 0.0017% w/v DTT and 0.0001% w/v haemin) at a rate of 36 ml/hour and sterile laboratory air at 13.75 ml/min, both delivered via peristaltic pumps, and allowed to equilibrate for 12 hours. Three pre-treatment baseline measurements (at times -10, -5 and 0 minutes) of hydrogen sulfide and methanethiol concentrations in the perfusate air were then taken, each in triplicate, using gas chromatography. At time 0, air and media flow were stopped, 1 ml of active solution was injected onto the Sorbarod and held in contact with the biofilm for one minute, after which flow was restored. Perfusate air and perfusate media were then sampled at regular time intervals. All VSC determinations were performed from three separate perfusate air samples at each time point. VSC concentration data was normalised to time zero equalling 100%, and was the mean of two separate experiments. Microbial analysis of the perfusate media was performed by serial decimal dilutions of a 100 μl aliquot from the perfusate sample up to 1 in 107, firstly in a neutraliser (4% Tween20 and 0.5% Lecithin in Tryptone Soya Broth (Oxoid)) which was allowed to neutralise for 10 mins before continuing the remaining dilutions in BHI broth. Duplicate 100 μl aliquots from the dilutions 10−5, 10−6 and 10−7 were spread on Blood Agar plates and incubated anaerobically for 72 hours at 37 °C after which the numbers of bacterial colonies were counted to determine the colony forming units (CFU) per ml of perfusate.
Figure 1.
Schematic of the perfusion biofilm model.
Chemical Neutralisation Model
A dynamic, real-time model for studying the reaction between VSCs and metal ions was designed based upon bubbling VSCs through test solutions. A gas standards generator (Kin-tek 491M) was used both to calibrate the GC and to create a nitrogen gas stream containing hydrogen sulfide and methanethiol at approximately 1.0–1.5ppm, a concentration approximately double that of severe halitosis sufferers.33 The gas stream was flowed continuously at 360ml min−1 and was delivered to the base of a 50 ml sealed chamber through a narrow-bore tube. The gas exited the chamber at the top where it was analysed for VSCs by GC. Sample solutions could be delivered into the chamber, whereupon the gas stream bubbled through the solutions. In the experiments a gas stream was passed through the empty chamber for an equilibration period of at least ten minutes; 10 ml of test agent was then injected into the chamber and the VSC stream allowed to bubble through the test solution. VSC concentrations in this exiting gas stream were determined by the GC methodology every minute. All experiments were performed at room temperature (18–21 °C) and the data presented are the mean ± standard error of two separate experiments.
Sample preparation
The test toothpaste (containing 0.1% w/w o-cymen-5-ol and 0.6% w/w zinc chloride in a 0.204% w/w sodium fluoride silica base) and placebo toothpaste (identical to test paste but without o-cymen-5-ol, zinc chloride and buffer system) were prepared as 25%w/w slurries in deionised water. For the work in the chemical neutralisation model 1% v/v Antifoam A (Sigma) was also added to prevent overfoaming of the test slurries. Solutions of zinc chloride (for the chemical neutralisation model) and zinc gluconate (for the bacterial headspace model) were prepared at 11 mM, equivalent to the concentration of zinc in a 25% slurry of the test toothpaste. Similarly o-cymen-5-ol solutions were prepared at 1.66 mM, representative of the concentration of this agent in a 25% slurry of the test toothpaste.
RESULTS
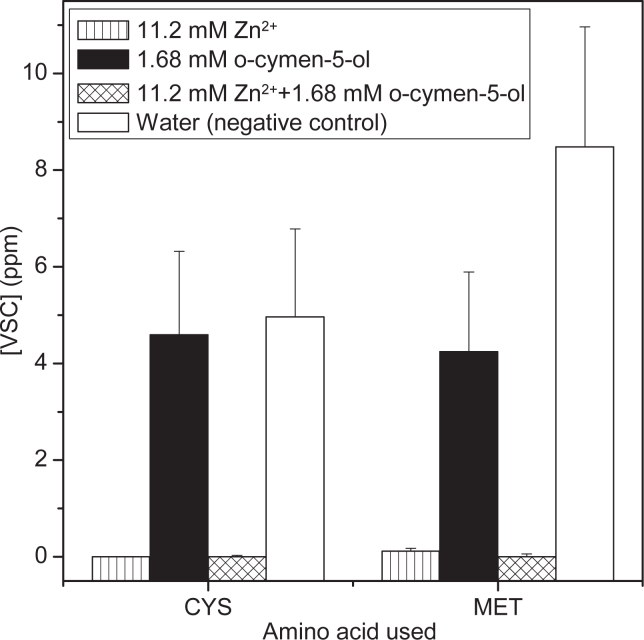
Treatment of P. gingivalis with zinc ions, o-cymen-5-ol or a combination of these two actives in the bacterial headspace model at concentrations of one quarter of those found in the experimental paste resulted in near complete elimination of both hydrogen sulfide and methanethiol when incubated with Cys or Met respectively when zinc ions were present (Figure 2). o-Cymen-5-ol alone did not appear to significantly reduce hydrogen sulfide generation from Cys, although methanethiol production from Met was reduced compared to the water control.
Figure 2.
Chart showing the effect of aqueous solutions of actives on the gases derived in the headspace above P. gingivalis incubated with Cysteine or Methionine. Note the [VSC] refers to either [H2S] for Cys incubation or [CH3SH] for Met incubation. Error bars are standard errors from nine separate measurements.
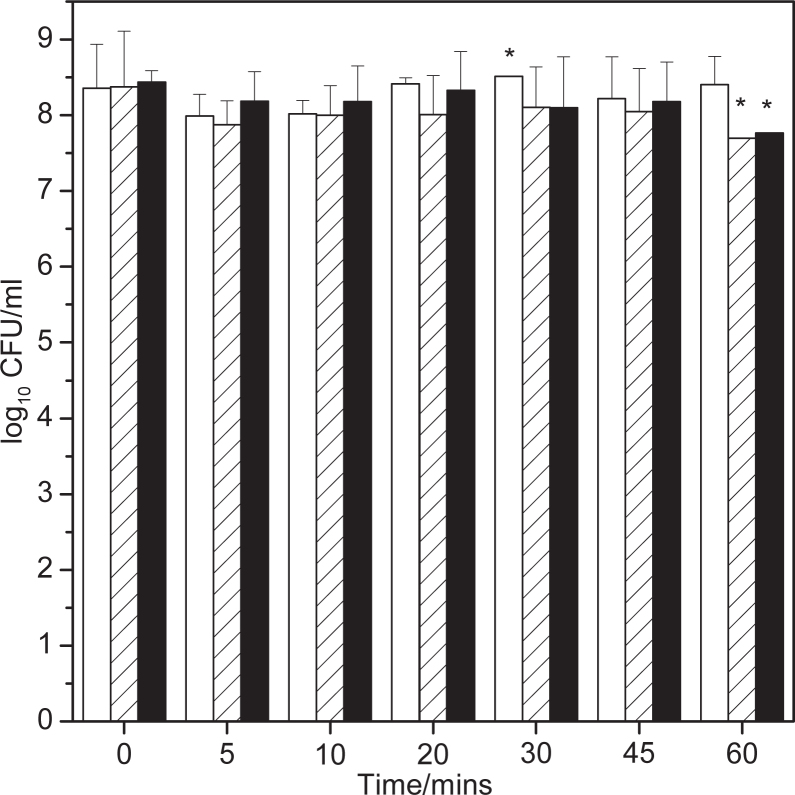
In the perfusion biofilm model, addition of the placebo paste as a 25% w/w slurry resulted in a transient drop in the presence of hydrogen sulfide in the perfused air (Figure 3). Presumably this drop was either due to the moderate antimicrobial effects of the surfactant (sodium dodecyl sulfate) and flavour present in the paste,34 or the physical disruption to the biofilm caused by flushing the biofilm with surfactant. However, both the test paste slurry and the 11 mM zinc solution showed a pronounced drop in the hydrogen sulfide concentration in the perfused air, to essentially zero ppm at five minutes post-addition with clear reduction in comparison with placebo paste still being evident one hour post-addition. In contrast, the effects of actives on methanethiol production (Figure 4) were less dramatic, with only a marginal reduction from the test paste compared to placebo. Analysis of the numbers of viable bacteria in the effluent medium (Figure 5) show that there were no significant reductions in bacterial numbers from any of the tests at any time point.
Figure 3.
Plot showing hydrogen sulfide concentration as a function of time in the perfusion biofilm model. Square data from a 25% w/w slurry of test paste, circles from a 25% w/w slurry of placebo paste and triangles from 11 mM ZnCl2. Error bars are standard errors.
Figure 4.
Plot showing methanethiol concentration as a function of time in the perfusion biofilm model. Square data from a 25% w/w slurry of test paste, circles from a 25% w/w slurry of placebo paste and triangles from 11 mM ZnCl2. Error bars are standard errors.
Figure 5.
Bar chart showing bacterial counts on the perfused media from the Sorbarod model as a function of time. Data is a mean of two independent experiments (except asterisked data where one of the two samples were lost during analysis) and error bars are the standard deviation. White bar is treatment with a 25% w/w slurry of test paste, hatched bar is from a 25% w/w slurry of placebo paste and black bar is 11 mM ZnCl2 solution.
In the chemical neutralisation model, the presence of zinc, either from 25% w/w slurry of the test paste or from 11 mM zinc solution, reduced the concentration of the hydrogen sulfide in the bubbled gas stream from 1.1 ppm to 0.2 or 0.4 ppm respectively (Figure 6). Placebo paste treatment produced only a moderate drop in hydrogen sulfide concentration immediately after introduction into the gas stream, however this effect was transient and small compared to the effects of zinc ions and test paste. In contrast, no effects were seen on the concentration of methanethiol by treatment with any of the test agents in the chemical neutralisation model (Figure 7).
Figure 6.
Plot showing hydrogen sulfide concentration as a function of time in the chemical neutralisation model. Square data from a 25% w/w slurry of test paste, circles from a 25% w/w slurry of placebo paste and triangles from 11 mM ZnCl2. Error bars are standard errors.
Figure 7.
Plot showing methanethiol concentration as a function of time in the chemical neutralisation model. Square data from a 25% w/w slurry of test paste, circles from a 25% w/w slurry of placebo paste and triangles from 11 mM ZnCl2. Error bars are standard errors.
DISCUSSION
The data from the chemical neutralisation model clearly showed that both the test paste containing zinc chloride and o-cymen-5-ol and the zinc solution were highly effective at removing hydrogen sulfide, presumably by means of a chemical reaction between bisulfide and zinc ions as previously postulated by Jonski et al.27. o-Cymen-5-ol did not remove either hydrogen sulfide or methanethiol (data not shown) therefore this activity can be positively ascribed to the activity of the zinc ions within the test paste, although the test paste does show better activity than the molar equivalent zinc solution, possibly due to the formulation excipients enhancing the effects of the zinc ion. Methanethiol concentration was found not to be reduced in this model, possibly since the contact time between the methanethiol (within a bubble) and the test solution is very short, being the time taken for the bubble to rise to the surface of the solution.
Whilst the chemical neutralisation model showed that it was possible for zinc ions and the test paste to react with hydrogen sulfide in a non-biological system, it does not necessarily follow that this same activity would be observed in the complexity of a biofilm system. However, the perfusion biofilm model data clearly showed not only that the test paste and the zinc solution were capable of considerably reducing hydrogen sulfide emanating from the biofilm, but also that the methanethiol produced by the biofilm was moderately reduced by the test paste. It is possible that the zinc ions within this model have a longer contact time with the methanethiol, allowing the reaction to occur. Indeed previous studies where zinc had been in contact with methanethiol for prolonged periods of time, showed that zinc could remove methanethiol.27 The fact that the numbers of viable bacteria in the effluent from the biofilm do not decrease after addition of the test agents suggests that they were only moderately antimicrobial to the biofilm bacteria in this model. The activity therefore observed in this model can be ascribed primarily to the chemical reactivity of zinc to the VSCs. The prolonged nature of the activity (>1 hour) against hydrogen sulfide suggests that zinc is strongly retained within the biofilm. This is mirrored by clinical measurement where 16–20 ppm zinc within dental plaque has been reproducibly observed post brushing with zinc-containing toothpastes,35., 36., 37. with elevated concentrations of zinc found in saliva for many hours post use.36., 38., 39., 40.
The bacterial headspace model was utilised to measure the effects of o-cymen-5-ol and zinc ions on planktonic suspensions of bacteria, in this case P. gingivalis, an organism that is widely present in the oral environment and that produces a wide range of malodours.2 In this model it was observed that o-cymen-5-ol at the concentrations used in this model (representative of quarter strength found in the test toothpaste) did not prevent P. gingivalis from producing hydrogen sulfide, although methanethiol production was reduced by half. Zinc ions or o-cymen-5-ol in combination with zinc reduced hydrogen sulfide production to effectively zero. This can be explained by zinc chemically reacting with the hydrogen sulfide produced by metabolism of cysteine to neutralise the odour. P. gingivalis produced methanethiol following incubation of methionine, which again was neutralised by zinc ions. In this case o-cymen-5-ol was able to inhibit production of methanethiol, despite not being able to chemically react with methanethiol. This suggests that o-cymen-5-ol may be able to reduce VSCs by other means, possibly by interference with bacterial enzymes, or direct anti-microbial effects.34
In conclusion, the ability of zinc ions to be strongly retained within oral biofilms and subsequently to react with hydrogen sulfide therefore appears to be the predominant mechanism for the long-term clinical reduction of VSCs following use of zinc-containing pastes.41., 42. Together, these data show a likely mode of action for how toothpaste actives can have a pronounced effect on odours present in breath from halitosis sufferers.
CONFLICT OF INTEREST AND SOURCE OF FUNDING
The work described in this manuscript was funded by GlaxoSmithKline Consumer Healthcare. The authors are employed by GSK but confirm no potential conflicts of interest.
REFERENCES
- 1.Delanghe G, Ghyselen J, Bollen C, et al. An inventory of patients’ response to treatment at a multidisciplinary breath odor clinic. Quintessence Int. 1999;30:307–310. [PubMed] [Google Scholar]
- 2.Kleinberg I, Codipilly DM. In: Bad Breath: Research Perspectives. Rosenberg M, editor. Ramot Publishing; Tel Aviv: 1997. The Biological Basis of Oral Malodor Formation; pp. 13–40. [Google Scholar]
- 3.De Boever EH, De Uzeda M, Loesche WJ. Relationship between volatile sulfur compounds, BANA-hydrolyzing bacteria and gingival health in patients with and without complaints of oral malodor. J Clin Dent. 1994;4:114–119. [PubMed] [Google Scholar]
- 4.Koshimune S, Awano S, Gohara K, et al. Low salivary flow and volatile sulfur compounds in mouth air. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:38–41. doi: 10.1016/s1079-2104(03)00162-8. [DOI] [PubMed] [Google Scholar]
- 5.Spratt DA, Pratten J. Biofilms and the oral cavity. Rev Env Sci Biotechnol. 2003;2:109–120. [Google Scholar]
- 6.Lee CH, Kho HS, Chung SC, et al. The relationship between volatile sulfur compounds and major halitosis-inducing factors. J Periodontol. 2003;74:32–37. doi: 10.1902/jop.2003.74.1.32. [DOI] [PubMed] [Google Scholar]
- 7.Sanz M, Roldan S, Herrera D. Fundamentals of breath malodour. J Contemp Dental Pract. 2001;2:1–13. [PubMed] [Google Scholar]
- 8.Allaker RP, Waite RD, Hickling J, et al. Topographic distribution of bacteria associated with oral malodour on the tongue. Arch Oral Biol. 2008;53:S8–S12. doi: 10.1016/S0003-9969(08)70003-7. [DOI] [PubMed] [Google Scholar]
- 9.Tonzetich J, Richter VJ. Evaluation of volatile odoriferous components of saliva. Arch Oral Biol. 1964;9:39–45. doi: 10.1016/0003-9969(64)90042-1. [DOI] [PubMed] [Google Scholar]
- 10.Tonzetich J. Direct gas chromatographic analysis of sulphur compounds in mouth air in man. Arch Oral Biol. 1971;16:587–597. doi: 10.1016/0003-9969(71)90062-8. [DOI] [PubMed] [Google Scholar]
- 11.Greenman J, El Maaytah M, Duffield J, et al. Assessing the relationship between concentrations of malodor compounds and odor scores from judges. J Am Dent Assoc. 2005;136:749–757. doi: 10.14219/jada.archive.2005.0258. [DOI] [PubMed] [Google Scholar]
- 12.Bosy A, Kulkarni GV, Rosenberg M, et al. Relationship of oral malodor to periodontitis: evidence of independence in discrete subpopulations. J Periodontol. 1994;65:37–46. doi: 10.1902/jop.1994.65.1.37. [DOI] [PubMed] [Google Scholar]
- 13.Van Den Velde S, Quirynen M, Van Hee P, et al. Halitosis associated volatiles in breath of healthy subjects. J Chromatogr B Anal Technol Biomed Life Sci. 2007;853:54–61. doi: 10.1016/j.jchromb.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Tangerman A, Winkel EG. The portable gas chromatograph OralChroma: a method of choice to detect oral and extra-oral halitosis. J Breath Res. 2008;2:17010. doi: 10.1088/1752-7155/2/1/017010. [DOI] [PubMed] [Google Scholar]
- 15.Tsai CC, Chou HH, Wu TL, et al. The levels of volatile sulfur compounds in mouth air from patients with chronic periodontitis. J Periodontal Res. 2008;43:186–193. doi: 10.1111/j.1600-0765.2007.01011.x. [DOI] [PubMed] [Google Scholar]
- 16.Newby EE, Hickling JM, Hughes F, et al. Control of oral malodour by dentifrices measured by gas chromatography. Arch Oral Biol. 2008;53:519–525. doi: 10.1016/S0003-9969(08)70005-0. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg M, Kulkarni GV, Bosy A, et al. Reproducibility and sensitivity of oral malodor measurements with a portable sulphide monitor. J Dent Res. 1991;70:1436–1440. doi: 10.1177/00220345910700110801. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg M, Septon I, Eli I, Bar-Ness R, et al. Halitosis measurement by an industrial sulphide monitor. J Periodontol. 2011;62:487–489. doi: 10.1902/jop.1991.62.8.487. [DOI] [PubMed] [Google Scholar]
- 19.Outhouse TL, Al Alawi R, Fedorowicz Z et al. Tongue scraping for treating halitosis. Cochrane Database Syst Rev 2006 2: Art No. CD005519. [DOI] [PubMed]
- 20.Rosenberg M, Gelernter I, Barki M, et al. Day-long reduction of oral malodor by a two-phase oil: Water mouthrinse as compared to chlorhexidine and placebo rinses. J Periodontol. 1992;63:39–43. doi: 10.1902/jop.1992.63.1.39. [DOI] [PubMed] [Google Scholar]
- 21.Kim SE, Shim KM, Yoo KH, et al. The effect of cetylpyridinium chloride on halitosis and periodontal disease-related parameters in dogs. Biotechnol Bioprocess Eng. 2008;13:252–255. [Google Scholar]
- 22.Hur MH, Park J, Maddock-Jennings W, et al. Reduction of mouth malodour and volatile sulphur compounds in intensive care patients using an essential oil mouthwash. Phytother Res. 2007;21:641–643. doi: 10.1002/ptr.2127. [DOI] [PubMed] [Google Scholar]
- 23.Phan TN, Buckner T, Sheng J, et al. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol Immunol. 2004;19:31–38. doi: 10.1046/j.0902-0055.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 24.Sheng J, Nguyen PTM, Marquis RE. Multi-target antimicrobial actions of zinc against oral anaerobes. Arch Oral Biol. 2005;50:747–757. doi: 10.1016/j.archoralbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Young A, Jonski G, Rølla G, et al. Effects of metal salts on the oral production of Volatile Sulfur-containing Compounds VSC. J Clin Periodontol. 2001;28:776–781. doi: 10.1034/j.1600-051x.2001.280809.x. [DOI] [PubMed] [Google Scholar]
- 26.Bradshaw DJ, Perring KD, Cawkill PM, et al. Creation of oral care flavours to deliver breath-freshening benefits. Oral Dis. 2005;11:75–79. doi: 10.1111/j.1601-0825.2005.01098.x. [DOI] [PubMed] [Google Scholar]
- 27.Jonski G, Young A, Waler SM, Rolla G. Insoluble zinc, cupric and tin pyrophosphates inhibit the formation of volatile sulphur compounds. Eur J Oral Sci. 2004;112:429–432. doi: 10.1111/j.1600-0722.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 28.Pratten J, Pasu M, Jackson G, et al. Modelling oral malodour in a longitudinal study. Arch Oral Biol. 2003;48:737–743. doi: 10.1016/s0003-9969(03)00154-7. [DOI] [PubMed] [Google Scholar]
- 29.Thorn RMS, Greenman J. A novel in vitro flat-bed perfusion biofilm model for determining the potential antimicrobial efficacy of topical wound treatments. J Appl Microbiol. 2009;107:2070–2079. doi: 10.1111/j.1365-2672.2009.04398.x. [DOI] [PubMed] [Google Scholar]
- 30.Spencer P, Greenman J, McKenzie C, et al. In vitro biofilm model for studying tongue flora and malodour. J Appl Microbiol. 2007;103:985–992. doi: 10.1111/j.1365-2672.2007.03344.x. [DOI] [PubMed] [Google Scholar]
- 31.Greenman J, McKenzie C, Saad S, et al. Effects of chlorhexidine on a tongue-flora microcosm and VSC production using an in vitro biofilm perfusion model. J Breath Res. 2008;2:046005. doi: 10.1088/1752-7155/2/4/046005. [DOI] [PubMed] [Google Scholar]
- 32.Taylor B, Greenman J. Modelling the effects of pH on tongue biofilm using a sorbarod biofilm perfusion system. J Breath Res. 2010;4:017107. doi: 10.1088/1752-7155/4/1/017107. [DOI] [PubMed] [Google Scholar]
- 33.Van Den Velde S, Van Steenberghe D, Van Hee P, et al. Detection of odorous compounds in breath. J Dent Res. 2009;88:285–289. doi: 10.1177/0022034508329741. [DOI] [PubMed] [Google Scholar]
- 34.Pizzey RL, Marquis RE, Bradshaw DJ. Antimicrobial effects of o-cymen-5-ol and zinc, alone and in combination in simple solutions and toothpaste formulations. Int Dent J. 2011;61:33–40. doi: 10.1111/j.1875-595X.2011.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Creeth JE, Abraham PJ, Barlow JA, et al. Oral delivery and clearance of antiplaque agents from Triclosan-containing dentifrices. Int Dent J. 1993;43:387–397. [PubMed] [Google Scholar]
- 36.Lynch RJM. Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int Dent J. 2011;61:46–54. doi: 10.1111/j.1875-595X.2011.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saxton CA, Harrap GJ, Lloyd AM. The effect of dentifrices containing zinc citrate on plaque growth and oral zinc levels. J Clin Periodontol. 1986;13:301–306. doi: 10.1111/j.1600-051x.1986.tb02226.x. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert RJ. The oral clearance of zinc and triclosan after delivery from a dentifrice. J Pharm Pharmacol. 1987;39:480–483. doi: 10.1111/j.2042-7158.1987.tb03425.x. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert RJ, Ingram GS. The oral disposition of zinc following the use of an anticalculus toothpaste containing 0.5% zinc citrate. J Pharm Pharmacol. 1988;40:399–402. doi: 10.1111/j.2042-7158.1988.tb06303.x. [DOI] [PubMed] [Google Scholar]
- 40.Harrap GJ, Best JS, Saxton CA. Human oral retention of zinc from mouthwashes containing zinc salts and its relevance to dental plaque control. Arch Oral Biol. 1984;29:87–91. doi: 10.1016/0003-9969(84)90110-9. [DOI] [PubMed] [Google Scholar]
- 41.Navada R, Kumari H, Le S, Zhang J. Oral malodor reduction from a zinc-containing toothpaste. J Clin Dent. 2008;19:69–73. [PubMed] [Google Scholar]
- 42.Payne D, Gordon JJ, Nisbet S, et al. A randomised clinical trial to assess control of oral malodour by a novel dentifrice containing 0.1%w/w o-cymen-5-ol, 0.6%w/w zinc chloride. Int Dent J. 2011;61:21–27. doi: 10.1111/j.1875-595X.2011.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]