Abstract
Zinc is an essential trace element. In the mouth, it is present naturally in plaque, saliva and enamel. Zinc is formulated into oral health products to control plaque, reduce malodour and inhibit calculus formation. It has good oral substantivity, and elevated concentrations can persist for many hours in plaque and saliva following delivery from mouthrinses and toothpastes. Although low concentrations of zinc can both reduce enamel demineralisation and modify remineralisation, during caries clinical trials, the addition of zinc to fluoride toothpastes has not affected their ability to reduce caries. Mechanistic studies may help explain this apparent contradiction. Zinc is readily desorbed from hydroxyapatite by calcium, which is plentiful in plaque and saliva. Where crystal-growth sites remain occupied by zinc despite this, they may simply be ‘over-grown’ by remineralisation initiated at unoccupied sites. Further, under certain conditions, low concentrations of zinc can enhance remineralisation of enamel lesions, by retarding lesion arrestment. Although this may help to explain the apparent lack of an overall zinc effect on caries, it seems unlikely that any negative effects would be countered exactly by positive effects. Further mechanistic studies, complementing well-designed in vitro and in situ caries studies, should lead to further understanding of the zinc-enamel interactions relevant to demineralisation and remineralisation.
Key words: Oral health, zinc, enamel, hydroxyapatite, caries
INTRODUCTION
Zinc is an essential trace element and is found in tissues throughout the body, approaching iron in its relative abundance1. The human body contains about 2g of zinc, of which approximately 60% is found in muscle tissue, 30% in bone and 5% in skin2. It is essential for growth and development in humans3 and has diverse roles, being a critical component of several hundred enzymes and proteins4. Uptake and release of zinc are mediated by the bone reservoir5. It is found in foods including meat, cereals, milk and milk products6. Zinc is implicated in biomineralisation, where it stimulates both bone growth and mineralisation7., 8. and influences osteoclast activity9., 10.. Zinc-doped hydroxyapatite (HA) may improve bone formation around implant materials11., 12., 13.. Retardation of bone growth in animals is commonly associated with conditions linked to zinc deficiency14., 15., 16., 17., 18. and reduced bone density has been linked to a zinc-deficient diet19., 20..
While the effects of zinc on calculus and plaque-growth have been reviewed extensively, its interaction with the dental hard-tissues and possible role in de- and remineralisation have received less attention. The aim of this review is to summarise data regarding the oral disposition of zinc, before and after the use of zinc-containing toothpastes and rinses, the interactions of zinc with enamel and its analogues, and hence discuss the possible effects of zinc on caries.
ZINC IN THE MOUTH
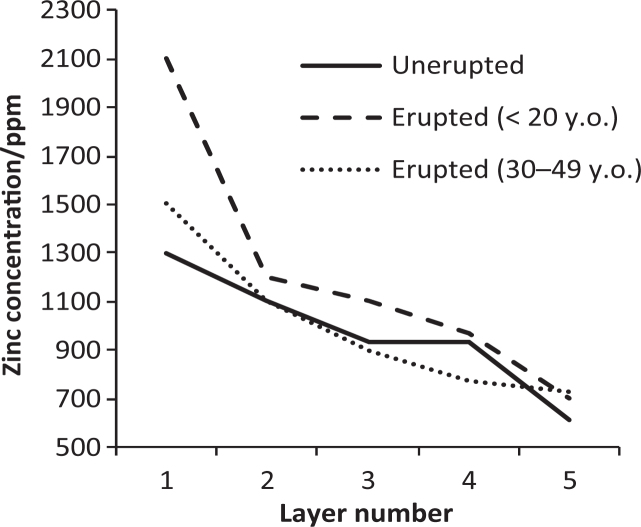
Zinc is ubiquitous in the body and it is naturally present in saliva21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31., 32., 33. and the teeth34., 35., 36., 37., 38.. Table 1 summarises reported background zinc concentrations for saliva. (Although the SI units are moles/m3, values for zinc and other species, for example fluoride, are often reported in parts per million (ppm); hence ppm is used to facilitate comparison.) Relatively large amounts of zinc are incorporated into enamel prior to eruption, but after eruption, zinc concentration at the surface of the teeth apparently increases further (Figure 1)34, suggesting that some incorporation does occur during post-eruptive exposure to the oral fluids. It seems probable that this is facilitated by many sub-clinical caries events, in a similar way to the post-eruptive uptake of fluoride, where repeated de- and remineralising challenges re-model the outermost layer of enamel39. This extra zinc is apparently lost over the following two to three decades (Figure 1), again in a similar fashion to fluoride40.
Table 1.
Some reported values for background concentrations of zinc in saliva and plaque, with standard deviations in brackets (except * where ranges are quoted). Values for ‘dry’ plaque have been reduced sevenfold to facilitate comparison41., 42., 43.
| Authors | Salivary Zn conc/ppm | Authors | Plaque Zn conc/ppm |
|---|---|---|---|
| Kim et al. | 0.0135 (0.0122) | Schafer et al. | 15.2 (13.0) |
| Burguera-Pascu et al. | 0.055 (0.017) | Hall et al. | 31.6 (4.70) |
| Menegário et al. | 0.127 (0.0744) | Günbay et al. | 6.41 (0.687) |
| Watanabe et al. | 0.080 (0.043) | Creeth et al. | 19.5 (2.70) |
| Özdemir et al. | 0.17 (0.176) | Dobl et al. | 18.6 (11.2) 17.9 (10.6) |
| Kuraner et al. | 0.112 (0.048) | Gilbert and Ingram | < 40 |
| Gilbert et al., Gilbert and Ingram | < 0.200 | Saxton et al. | 15.9 (pooled sample) |
| Sighinolfi et al. | 0.0465 (0.0152) | Harrap et al. | 17.4 (5.86) |
| Tan-Walker and Gilbert | 0.150 (0.06) 0.190 (0.06) | Afseth et al. | 7.86 (2.29) |
| Harrap et al. | 0.244 (0.0883) | Afseth J. | < 10.7 |
| Klinger et al. | 0.0654 (0.0418-0.0100)* 0.079 (0.0347-0.178)* | Schamschula et al. | 17.1 (12.3) |
| Henkin et al. | 0.051 (0.014) | Schamschula et al | 13.4 (7.46) – 15.5 (6.71) |
Figure 1.
Zinc concentration in outer enamel. Layers relate to successive biopsies, each deeper than the previous34. The apparent increase in layer 1 relatively soon after eruption, in subjects less than 20 years old, was absent in subjects aged 30-49 years old.
Zinc is also found naturally in dental plaque, but comparison between reported values is not straightforward, as concentrations for ‘wet’ or ‘dry’ plaque are reported. However, assuming that drying increases the apparent concentration sevenfold41., 42., 43., values are broadly similar29., 31., 44., 45., 46., 47., 48., 49., 50., 51., 53. (Table 1). Zinc is taken up by the salivary pellicle29., 54. and it seems likely that the oral mucosa is the most important oral reservoir46., 55., 56., though insufficient data exist to support this proposition conclusively.
ZINC APPLIED FROM TOOTHPASTES AND RINSES
Zinc is added to toothpastes and mouth rinses, as an anti-bacterial agent to help to control plaque, to reduce oral malodour and to reduce calculus formation through crystal-growth modification/inhibition (discussed below)57., 58., 59.. To exert these effects, it must be present at the site of action at an effective concentration for sufficient time. Accordingly, zinc pharmacokinetic data for both plaque and saliva, following zinc delivery from these vehicles, have been well reported22., 25., 28., 29., 30., 31., 44., 45., 50., 56.. Despite differences in experimental protocols, for example different zinc salts, doses and rinsing regimes, all of which may affect zinc delivery and release, some general trends can be discerned.
Following application, relatively large amounts of the applied zinc dose are retained in the mouth, with reported values typically between about 15-40%28., 29., 30., 46., 56.. Good oral substantivity was confirmed by Gilbert and Ingram29, who reported that when zinc was applied from a toothpaste, of the remaining 30% retained in the mouth, only a further 5.7% was removed by rinsing three times.
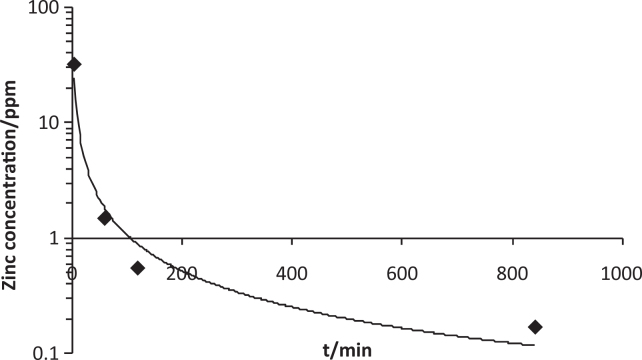
Zinc is cleared from saliva bi-modally57, with relatively high post-application concentrations falling rapidly over 30-60 min, after which low concentrations, significantly elevated when compared to baseline, may persist for many hourse.g.28., 29., 30., 31. (Figure 2). This trend reflects rapid clearance of loosely-bound zinc followed by slower clearance of more firmly-bound zinc57, as is the case for fluoride. A similar trend is seen in plaque, but elevated concentrations can persist for at least 12 hours after application44., 45., 50.. Repeated application of zinc has shown that a build-up effect occurs in plaque45., 50., as for fluoride41.
Figure 2.
Clearance of zinc from saliva.
Pharmacokinetic data relating to zinc concentrations in plaque-fluid following application are apparently absent from the dental literature. We were able to locate only a value measured 1 hour after application from a mouthwash49. It is reasonable to assume, however, that the clearance profile will be broadly similar to that seen in plaque and saliva. A statistically-significant correlation was reported between concentrations of another divalent metal cation, magnesium, in plaque-fluid and saliva, which lends some support to this assumption60 although differences in dissociation constants61 should also be considered. However, it has been reported that most of the zinc in plaque is associated with the particulate phase49, with only 1% of the total amount present in the plaque-fluid, and the relative distribution would be very likely to change during a cariogenic challenge.
Zinc competes with calcium for bacterial binding-sites in model biofilms and it has been proposed that half of the bound zinc would be released under cariogenic conditions, through, for example, protonation of carboxylate and phosphate groups in bacterial lipoteichoic acid61. Circadian variations in concentration exist62, further complicating the matter.
DIETARY ZINC, ENAMEL ZINC CONTENT AND CARIES
Increased caries incidence was reported when rats were fed a zinc-deficient diet63., 64., although in the latter study the co-administration of vitamin B6 with the zinc makes estimation of any zinc effect difficult. However, while transient sub-clinical zinc deficiency is relatively common, prolonged chronic deficiency, as was the case in the rat studies, is rare in humans65., 66.. Further, caution should be exercised when extrapolating the findings from studies of caries in rodents, as salivary pH tends to be higher than in human saliva67, concentrations of calcium and phosphate may differ, and differences have been noted in some other factors which can influence caries68. Nonetheless, zinc distribution in rat enamel does at least seem to be similar, with higher concentrations reported near the enamel surface69. Studies into the effect of zinc concentrations in human enamel and caries incidence have failed to show a consistent, significant correlation70., 71.. One factor which has been proposed as a confounding influence is that zinc is a common component of dental restorations, leading to contamination in subjects who are, or have been, by definition caries-prone71.
EFFECTS OF ZINC ON FLUORIDE EFFICACY AND EFFECTIVENESS IN USE
Zinc, along with other metal cations, has long been associated with reductions in enamel solubility34., 72., 73. and can also modify crystal-growth of the calcium phosphates implicated in remineralisation (discussed below). Therefore, it has the potential to influence the dynamic de/remineralisation balance in the mouth.
However, data from pH-cycling studies where zinc and fluoride were delivered from a toothpaste and both de- and remineralisation were alternated do not suggest an overall zinc effect74., 75., 76.. Increased remineralisation after application of dipping solutions containing zinc has been reported77., 78. but the presence of strontium, also capable of affecting enamel de- and remineralisation79 was a confounding factor and the role of zinc cannot easily be deduced. A reduction in enamel demineralisation with the use of a zinc-containing fluoride toothpaste was reported during an in situ study. Lesions in the zinc/fluoride group did not demineralise significantly when compared to baseline, whereas those in the fluoride control group did74. However, it was concluded that this could not be attributed wholly to direct interaction with the enamel substrate, and may have been the result of anti-bacterial effects to some extent. Churchley et al. report a similar anti-demineralisation effect in this issue80.
However, zinc had no effect on the anti-caries effect of fluoride during a rat-caries study81 and in a three-year caries clinical trial (CCT), the addition of zinc to fluoride toothpastes, containing 1,000, 1,500 and 2,500 ppm fluoride (as sodium monofluorophosphate (SMFP)), had no effect on caries, either positive or detrimental, at any of the fluoride concentrations82 even though use of the zinc-containing toothpastes reduced calculus during the same trial83. During a subsequent CCT, no significant difference in anti-caries effectiveness was observed, between two zinc-containing fluoride toothpastes and a non-zinc fluoride control formulation84.
While zinc has anti-microbial properties, no consistent link has yet been established between anti-microbial efficacy and caries reductions85, except in the rather extreme case of, for example, use of chlorhexidine86, or in the complete absence of the microflora in gnotobiotic rats87.
Given zinc’s potential to affect both de- and remineralisation, this apparent lack of a consistent effect of zinc on caries would seem to be contradictory.
ZINC, FLUORIDE DELIVERY AND DE/REMINERALISATION; MECHANISTIC STUDIES
CCTs are considered to be the ultimate predictor of anti-caries effectiveness, and in vitro and in situ studies can be useful pre-clinical screening tools. In most cases, however, net changes are only quantified after many applications of active agents and many de- and remineralisation events88. Thus is it difficult to assess the effect of an agent, in this case zinc, on fluoride delivery or efficacy, and here, mechanistic studies can be useful.
Fluoride-uptake (from SMFP) to enamel lesions was reduced by 60% in the presence of zinc citrate89. It was suggested that zinc may have reacted with phosphates in the enamel lesions and that subsequent precipitation blocked pores at the surface of the lesion, or that zinc-MFP complexes may have inhibited uptake. Subsequently, however, fluoride-uptake to enamel lesions from a zinc/SMFP toothpaste was shown to be substantially greater than from its control, although no difference was seen when a sound enamel substrate was used74, and zinc citrate in a sodium fluoride toothpaste had no effect on fluoride-uptake to lesions75. Precipitation seems an unlikely explanation prima facie, as the experimental solutions were, in both cases, highly under-saturated with respect to both HA and zinc phosphate, based on respective –log10(solubility-product constants) of 58.490 and 35.291, but it is possible that localised supersaturation, and therefore thermodynamically favourable conditions for precipitation, may have existed in the unstirred solutions. In contrast, in solutions supersaturated with respect to HA, a combination of zinc and fluoride enhanced remineralisation of enamel lesions92 when compared to fluoride alone, probably by retarding lesion arrestment, i.e. the opposite of the surface-blocking effect mentioned above. A very similar effect has been reported for salivary macromolecules93.
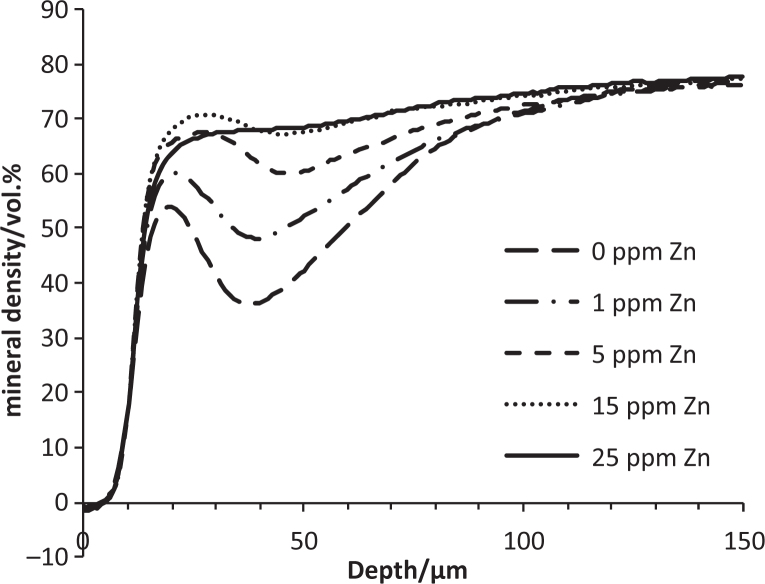
Data from a pilot study into the effect of physiologically-relevant zinc concentrations30, added to acid-gel demineralising systems, showed that zinc reduced demineralisation markedly (Figure 3). Significant reductions in both integrated mineral-loss and R-values94 were seen in all groups where zinc was added, and in both cases, 25ppm Zn = 15ppm Zn = 5ppm Zn < 1 ppm Zn < 0ppm Zn (p < 0.05, Fisher’s LSD multiple range test). Enamel is not stoichiometric HA but contains impurities associated with its solubility and which are lost during early demineralisation95., 96., 97.. One possibility is that zinc retarded the dissolution of the more soluble mineral phases, leading to a reduction in R-value. Related to this was the suggestion that an increase in R-value with increasing time in demineralising systems was caused by preferential dissolution of more soluble mineral88. Further, during a recent intra-oral de/remineralisation study, lesions with low-R values underwent net demineralisation, whereas high-R lesions with a similar integrated mineral-loss values underwent net remineralisation, and a similar preferential dissolution effect was proposed98. The observation that the acidified gels were supersaturated with respect to Hopeite is worthy of note (see ‘interactions with HA’ below).
Figure 3.
Mean mineral-density profiles for lesions formed in the presence and absence of zinc in vitro.
REDUCTION OF HA SOLUBILITY
Enamel is a defective form of HA, and HA has often been used as an enamel analogue during mechanistic de- and remineralisation studies99., 100.. In simple terms, zinc can interact with HA by adsorption onto crystal surfaces100., 101., 102. and/or incorporation into the crystal lattice103., 104..
Reductions in the solubility of apatites effected by metal ions are widely documented beyond the dental literature105., 106., 107., but the mechanism is still unclear. Adsorption at high-energy ‘kink’ sites is one possibility108. Alternatively, the precipitation of Hopeite, observed during zinc-adsorption studies109, may be implicated, and data from some studies suggest that above 1ppm, Hopeite dominates the surface-properties of HA under certain conditions110. Zinc uptake was reported to have continued beyond the point of complete monolayer coverage with increasing solution concentration, and the proposed explanation was the precipitation of Hopeite once surface adsorption was complete, again a concentration-dependent effect. Other reports tend to support an adsorption mechanism. Zinc was readily displaced from HA by calcium in an equimolar fashion100. More recently111, HA specimens were exposed to a demineralising solution, whose zinc concentration was increased and then decreased. As zinc concentration increased, dissolution decreased but when the zinc concentrations was subsequently reduced, dissolution rates quickly increased again. This lack of a zinc ‘memory’ in the HA specimens used also suggests an adsorption mechanism. However, other factors101, such as changes in pH during reactions112, and use or otherwise of pre-equilibrated113 systems, can modify zinc/HA interactions. The two mechanisms are apparently not mutually exclusive and it is likely that both are implicated in reduced solubility of HA and apatites, in the presence of zinc, to a greater or lesser extent.
MODIFICATION OF CALCIUM PHOSPHATE CRYSTAL-GROWTH
The ability of zinc to modify the crystal-growth of orally-relevant calcium phosphates has been exploited to control dental calculus, and is reviewed extensively elsewhere114. Briefly, zinc can influence precipitation of the calcium phosphates relevant to remineralisation. It can affect the crystallinity of apatite precipitated de novo and can inhibit the crystal-growth not only of HA but also of two HA precursors, di-calcium phosphate dihydrate (DCPD) and octacalcium phosphate (OCP)115., 116., 117.. Some effects are concentration-dependent. Inhibition of crystal-growth in HA, OCP and DCPD was reported below a pH-threshold, above which ACP or Zn-substituted TCP were formed114. In general, it has been concluded that zinc promotes the deposition of the more soluble calcium phosphate phase114. However, it has been shown that while PRP-3, a proline-rich phosphoprotein found in saliva, reduced HA crystal-growth to undetectable amounts in the absence of fluoride, with the addition of 1ppm fluoride, crystal-growth could be measured readily. It was proposed that even at maximum PRP-3 coverage, some crystal-growth sites remained uncovered118 and that this would ultimately allow ‘overgrowth’ of occupied growth sites. A similar mechanism was proposed for zinc74. Calcium readily displaces zinc from HA100 and reduces adsorption of zinc by some HA precursors119, potentially enhancing this effect by increasing the number of growth-sites unoccupied by zinc.
INCORPORATION INTO HA
It has been predicted that zinc replaces calcium in the HA lattice120, preferentially in the Ca(II) position121., 122., 123., 124., and that for fluorapatite, the ‘columnar’ Ca (I) position would be preferred121, although recently it has been suggested that simple ionic exchange mechanisms might be too simplistic125. It adopts tetrahedral-coordination as might be expected122., 126.. Some ‘relaxation’ of the surrounding oxygens is reported, made possible by the smaller ionic radius of zinc, when compared to calcium127., 128.. Covalent zinc-oxygen bonds are predicted124, whereas bonds in HA are ionic in nature107. When zinc is incorporated into both carbonated and non-carbonated HA then its solubility is generally reduced106., 129..
Reduced acid reactivity in zinc-substituted carbonated apatites129, a closer analogue to human enamel than non-carbonated HA130, may be linked to the reduction of paracrystalline disorder131, the production of larger crystals during precipitation132 and the formation of crystals with fewer structural defects129 than in non zinc-substituted carbonated apatites.
An inverse relationship between enamel fluoride and enamel carbonate contents has long been suggested133. However, a combination of zinc and fluoride was even more effective at reducing carbonate-induced crystal-structural disorder in the HA lattice, when compared to fluoride alone, or to other combinations of fluoride and metal ions132.
However, given concentrations found in the mouth, it is not clear if sufficient zinc would be incorporated to affect enamel solubility markedly. Carious enamel lesions remineralised under simulated plaque-fluid conditions, in the presence of zinc and fluoride, contained relatively little zinc92, and in the region of maximum remineralisation, only background concentrations, suggesting displacement of zinc by calcium. Other authors have suggested that zinc was incorporated into enamel during remineralisation in situ, but again, the increase in zinc concentration was modest134.
SUMMARY AND CONCLUSIONS
Zinc is ubiquitous in the body and is found naturally in plaque, saliva and the teeth. It has been suggested that the acquisition of zinc by enamel, following the eruption of the teeth, is a part of post-eruptive maturation135. Zinc has been formulated into oral-health products to give a range of benefits. It can reduce dental calculus formation, control plaque and reduce oral malodour. It has good oral substantivity and is retained in plaque and saliva for many hours after application, and a build-up effect occurs in plaque with repeated application.
While it can reduce the solubility of both enamel and HA, and retard/inhibit remineralisation, no net effect has been reported for caries during in vitro and in situ caries studies, or during CCTs. Mechanistic studies are useful where fundamental understanding is the aim. The findings from these studies, where zinc was of interest, suggest that while it can affect remineralisation, both displacement of adsorbed zinc by calcium, and over-growth of newly-deposited mineral from sites unoccupied by zinc, may counter potential negative effects. Further, in lesions, it may retard fluoride-induced lesion-arrest, allowing more complete lesion consolidation via remineralisation at greater depth.
During mechanistic studies, both chemical and physical physiological parameters, for example zinc, fluoride, calcium and phosphate concentrations, possible inhibition of diffusion by plaque and diffusion into, out of and within lesions should be considered before attempting to extrapolate findings to the clinical setting. Thus, a degree of caution should be exercised and these studies should complement, rather than be substituted for, properly-designed designed in vitro and in situ studies.
Regarding the clinical effects of zinc on de- and remineralisation, it seems unlikely that potentially beneficial effects, such as reductions in solubility and enhanced/prolonged lesion porosity to mineral ingress, will counter exactly any possible negative effects. Further work, with mechanistic studies complementing well-designed in vitro and in situ caries studies, should lead to further understanding of the zinc-enamel interactions relevant to demineralisation and remineralisation.
CONFLICT OF INTEREST AND SOURCE OF FUNDING
The work described in this manuscript was funded by GlaxoSmithKline Consumer Healthcare. The author is employed by GSK but confirms no potential conflicts of interest.
REFERENCES
- 1.Christianson DW. Structural biology of zinc. Adv Protein Chem. 1991;42:281–355. doi: 10.1016/s0065-3233(08)60538-0. [DOI] [PubMed] [Google Scholar]
- 2.Thomas B, Bishop J. 4th ed. Blackwell Publishing; Oxford: 2007. Manual of dietetic practice; pp. 209–210. [Google Scholar]
- 3.Burch RE, Hahn HK, Sullivan JF. Newer aspects of the roles of zinc, manganese and copper in human nutrition. Clin Chem. 1975;21:501–520. [PubMed] [Google Scholar]
- 4.Hao Q, Maret W. Imbalance between pro-oxidant and pro-antioxidant functions of zinc in disease. J Alzheimers Dis. 2005;8:161–170. doi: 10.3233/jad-2005-8209. [DOI] [PubMed] [Google Scholar]
- 5.Windisch W. Homeostatic reactions of quantitative zinc metabolism on deficiency and subsequent repletion with Zn in Zn-65-labeled adult rats. Trace Elem Electrolytes. 2001;18:122–128. [Google Scholar]
- 6.Henderson L, Irving K, Gregory J, et al. The Stationery Office; London: 2003. National diet and nutrition survey: adults aged 19 to 64 years. Volume 3: vitamin and mineral intake and urinary analyses; pp. 75–126. [Google Scholar]
- 7.Yamaguchi M, Oishi H, Suketa Y. Stimulatory effect of zinc on bone formation in tissue culture. Biochem Pharmacol. 1987;15:4007–4012. doi: 10.1016/0006-2952(87)90471-0. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi M, Oishi H, Suketa Y. Zinc stimulation of bone protein synthesis in tissue culture. Activation of aminoacyl-tRNA synthetase. Biochem Pharmacol. 1988;37:4075–4080. doi: 10.1016/0006-2952(88)90098-6. [DOI] [PubMed] [Google Scholar]
- 9.Kishi S, Yamaguchi M. Inhibitory effect of zinc compounds on osteoclast-like cell formation in mouse marrow cultures. Biochem Pharmacol. 1994;48:1225–1230. doi: 10.1016/0006-2952(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi M, Uchiyama S. Receptor activator of NF-κB ligand-stimulated osteoclastogenesis in mouse marrow culture is suppressed by zinc in vitro. Int J Mol Med. 2004;14:81–85. [PubMed] [Google Scholar]
- 11.Grandjean-Laquerriere A, Laquerriere P, Jallot E, et al. Influence of the zinc concentration of sol-gel derived zinc substituted hydroxyapatite on cytokine production by human monocytes in vitro. Biomaterials. 2006;27:3195–3200. doi: 10.1016/j.biomaterials.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Jallot E, Nedelec JM, Grimault AS, et al. STEM and EDXS characterisation of physico-chemical reactions at the periphery of sol-gel derived Zn-substituted hydroxyapatites during interactions with biological fluids. Colloids Surf B Biointerfaces. 2005;42:205–210. doi: 10.1016/j.colsurfb.2005.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii E, Ohkubo M, Tsuru K, et al. Selective protein adsorption property and characterization of nano-crystalline zinc-containing hydroxyapatite. Acta Biomater. 2006;2:69–74. doi: 10.1016/j.actbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Leek JC, Vogler JB, Gershwin ME, et al. Studies of marginal zinc deprivation in rhesus monkeys. V. Fetal and infant skeletal effects. Am J Clin Nutr. 1984;40:1203–1212. doi: 10.1093/ajcn/40.6.1203. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh HS, Navia JM. Zinc deficiency and bone formation in guinea pig alveolar implants. J Nutr. 1980;110:1581–1588. doi: 10.1093/jn/110.8.1581. [DOI] [PubMed] [Google Scholar]
- 16.Oner G, Bhaumick B, Bala RM. Effect of zinc deficiency on serum somatomedin levels and skeletal growth in young rats. Endocrinology. 1984;114:1860–1863. doi: 10.1210/endo-114-5-1860. [DOI] [PubMed] [Google Scholar]
- 17.Cunha Ferreira RM da, Marguiequi IM, Elizaga IV. Teratogenicity of zinc deficiency in the rat: study of the fetal skeleton. Teratology. 1989;39:181–194. doi: 10.1002/tera.1420390210. [DOI] [PubMed] [Google Scholar]
- 18.Hurley LS. Teratogenic aspects of manganese, zinc and copper nutrition. Physiol Rev. 1981;61:249–295. doi: 10.1152/physrev.1981.61.2.249. [DOI] [PubMed] [Google Scholar]
- 19.Ryz NR, Weiler HA, Taylor CG. Zinc deficiency reduces bone mineral density in the spine of young adult rats: a pilot study. Ann Nutr Metab. 2009;54:218–226. doi: 10.1159/000224627. [DOI] [PubMed] [Google Scholar]
- 20.Scrimgeour AG, Stahl CH, McClung JP, et al. Moderate zinc deficiency negatively affects biomechanical properties of rat tibiae independently of body composition. J Nutr Biochem. 2007;18:813–819. doi: 10.1016/j.jnutbio.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Kim YJ, Kim YK, Kho H-S. Effects of smoking on trace metals in saliva. Oral Disease. 2010;16:823–830. doi: 10.1111/j.1601-0825.2010.01698.x. [DOI] [PubMed] [Google Scholar]
- 22.Burguera-Pascu M, Rodríguez-Archilla A, Burguera JL, et al. Flow injection on-line dilution for zinc determination in human saliva with electrothermal atomic absorption spectrometry detection. Anal Chim Acta. 2007;600:214–220. doi: 10.1016/j.aca.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Menegário AA, Packer AP, Giné MF. Determination of Ba, Cd, Cu, Pb and Zn in saliva by isotope dilution direct injection inductively coupled plasma mass spectrometry. Analyst. 2001;126:1363–1368. doi: 10.1039/b102638k. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe M, Asatsuma M, Ikui A, et al. Measurements of several metallic elements and matrix metalloproteinases (MMPs) in saliva from patients with taste disorder. Chem Senses. 2005;30:121–125. doi: 10.1093/chemse/bji007. [DOI] [PubMed] [Google Scholar]
- 25.Özdemir A, Sayal A, Akca E, et al. The determination of salivary zinc level following delivery from zinc-containing toothpaste. Tr J Med Sci. 1998;28:281–283. [Google Scholar]
- 26.Kuraner T, Beksac MS, Kayakirilmaz K, et al. Serum and parotid saliva testosterone, calcium, magnesium, and zinc levels in males, with and without periodontitis. Biol Trace Elem Res. 1991;31:43–49. doi: 10.1007/BF02990358. [DOI] [PubMed] [Google Scholar]
- 27.Sighinolfi GP, Gorgoni C, Bonori O, et al. Comprehensive determination of trace elements in human saliva by ETA-AAS. Mikrochim Acta. 1989;1:171–179. [Google Scholar]
- 28.Gilbert RJ, Tan-Walker RLB, van der Ouderaa FJG. Delivery of zinc and triclosan to micro-reservoirs of anti-bacterial activity. J Dent Res. 1989;68:1706–1707. (Sp Iss) [Google Scholar]
- 29.Gilbert RJ, Ingram GS. The oral disposition of zinc following the use of an anti-calculus toothpaste containing 0.5% zinc citrate. J Pharm Pharmacol. 1988;40:399–402. doi: 10.1111/j.2042-7158.1988.tb06303.x. [DOI] [PubMed] [Google Scholar]
- 30.Tan-Walker RLB, Gilbert RJ. Oral delivery of zinc from slurries and separated supernatant fractions of dentifrices. J Dent Res. 1989;68(Sp Iss):1708–1709. [Google Scholar]
- 31.Harrap GJ, Best JS, Saxton CA. Human oral retention of zinc from mouthwashes containing zinc salts and its relevance to dental plaque control. Arch Oral Biol. 1984;29:87–91. doi: 10.1016/0003-9969(84)90110-9. [DOI] [PubMed] [Google Scholar]
- 32.Klinger G, Dawczynski H, Tennigkeit E. Concentration of Na, K, Ca, Mg and Zn in human submandibular saliva. Laryngol Rhinol Otol. 1980;59:279–281. [PubMed] [Google Scholar]
- 33.Henkin RI, Mueller CW, Wolf RO. Estimation of zinc concentration of parotid saliva by flameless atomic absorption spectrophotometry in normal subjects and in patients with idiopathic hypogeusia. J Lab Clin Med. 1975;86:175–180. [PubMed] [Google Scholar]
- 34.Brudevold F, Steadman LT, Spinelli MA, et al. A study of zinc in human teeth. Arch Oral Biol. 1963;8:135–144. doi: 10.1016/0003-9969(63)90051-7. [DOI] [PubMed] [Google Scholar]
- 35.Thylstrup A, Fejerskov O. 2nd ed. Munksgaard; Copenhagen: 1999. Caries chemistry and fluoride – mechanism of action. Textbook of clinical cariology; pp. 231–258. [Google Scholar]
- 36.Williams RAD, Elliott JC. Churchill Livingstone; Edinburgh London and New York: 1989. Basic and applied dental biochemistry; pp. 342–382. [Google Scholar]
- 37.Anttila A. Proton-induced X-ray emission analysis of Zn, Sr and Pb in human deciduous tooth enamel and its relationship to dental caries scores. Arch Oral Biol. 1986;31:723–726. doi: 10.1016/0003-9969(86)90003-8. [DOI] [PubMed] [Google Scholar]
- 38.Frank RM, Sargentini-Maier ML, Turlot JC, et al. Zinc and strontium analyses by energy dispersive X-ray fluorescence in human permanent teeth. Arch Oral Biol. 1989;34:593–597. doi: 10.1016/0003-9969(89)90012-5. [DOI] [PubMed] [Google Scholar]
- 39.Fejerskov O, Larsen MJ, Richards A, Baelum V. Dental tissue effects of fluoride. Adv Dent Res. 1994;8:15–31. doi: 10.1177/08959374940080010601. [DOI] [PubMed] [Google Scholar]
- 40.Weatherell JA, Robinson C, Hallsworth AS. Changes in the fluoride concentration of the labial enamel surface with age. Caries Res. 1972;6:312–324. doi: 10.1159/000259810. [DOI] [PubMed] [Google Scholar]
- 41.Duckworth RM, Morgan SN, Murray AM. Fluoride in saliva and plaque following use of fluoride-containing mouthwashes. J Dent Res. 1987;66:1730–1734. doi: 10.1177/00220345870660120701. [DOI] [PubMed] [Google Scholar]
- 42.Agus HM, Un PS, Cooper MH, et al. Ionized and bound fluoride in resting and fermenting dental plaque and individual human caries experience. Arch Oral Biol. 1980;25:517–522. doi: 10.1016/0003-9969(80)90063-1. [DOI] [PubMed] [Google Scholar]
- 43.Tatevossian A. Distribution and kinetics of fluoride ions in the free aqueous and residual phases of human dental plaque. Arch Oral Biol. 1978;23:893–898. doi: 10.1016/0003-9969(78)90293-5. [DOI] [PubMed] [Google Scholar]
- 44.Schäfer F, Adams SE, Nicholson JA, et al. In vivo evaluation of an oral health toothpaste with 0.1% vitamin E acetate and 0.5% sunflower oil (with vitamin F) Int Dent J. 2007;57:119–123. [Google Scholar]
- 45.Hall PJ, Green AK, Horay CP, et al. Plaque antibacterial levels following controlled food intake and use of a toothpaste containing 2% zinc citrate and 0.3% Triclosan. Int Dent J. 2003;53:379–384. doi: 10.1111/j.1875-595x.2003.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 46.Creeth JE, Abraham PJ, Barlow JA, et al. Oral delivery and clearance of antiplaque agents from Triclosan-containing dentifrices. Int Dent J. 1993;43:387–397. [PubMed] [Google Scholar]
- 47.Günbay S, Biçakçi N, Parlak H, et al. The effect of zinc chloride dentifrices on plaque-growth and oral zinc levels. Quintessence Int. 1992;23:619–624. [PubMed] [Google Scholar]
- 48.Dobl P, Gabsch HC, Nossek H. The zinc concentration in the dental plaque after zinc chloride mouth rinsing in relation to previous mouth rinsings with different pH values. Zahn-Mund-Kieferheilkd-Zentralbl. 1990;78:593–596. [PubMed] [Google Scholar]
- 49.Saxton CA, Harrap GJ, Lloyd AM. The effect of dentifrices containing zinc citrate on plaque growth and oral zinc levels. J Clin Periodontol. 1986;13:301–306. doi: 10.1111/j.1600-051x.1986.tb02226.x. [DOI] [PubMed] [Google Scholar]
- 50.Afseth J, Oppermann RV, Rølla G. Accumulation of Cu and Zn in human dental plaque in vivo. Caries Res. 1983;17:310–314. doi: 10.1159/000260682. [DOI] [PubMed] [Google Scholar]
- 51.Afseth J. Some aspects of the dynamics of Cu and Zn retained in plaque as related to their effect on plaque pH. Scand J Dent Res. 1983;91:169–174. doi: 10.1111/j.1600-0722.1983.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 52.Schamschula RG, Agus H, Bunzel M, et al. The concentration of selected major and trace minerals in human dental plaque. Arch Oral Biol. 1977;22:321–325. doi: 10.1016/0003-9969(77)90030-9. [DOI] [PubMed] [Google Scholar]
- 53.Schamschula RG, Adkins BL, Barmes DE, et al. Caries experience and the mineral content of plaque in a primitive population in New Guinea. J Dent Res. 1977;56:C62–C70. doi: 10.1177/002203457705600319011. (Sp Iss C) [DOI] [PubMed] [Google Scholar]
- 54.Gilbert RJ, Watson GK. The tooth surface as a reservoir of anti-microbial activity. J Dent Res. 1986;65:787. [Google Scholar]
- 55.Gilbert RJ, Baehni P. Uptake and desorption of triclosan and zinc gingival tissue. J Dent Res. 1986;65:787. [Google Scholar]
- 56.Afseth J, Helgeland K, Bonesvoll P. Retention of Cu and Zn in the oral cavity following rinsing with aqueous solutions of copper and zinc salts. Scand J Dent Res. 1983;91:42–45. doi: 10.1111/j.1600-0722.1983.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 57.Cummins D, Creeth JE. Delivery of anti-plaque agents from dentifrices, gels and mouthwashes. J Dent Res. 1992;71:143–149. doi: 10.1177/00220345920710071601. [DOI] [PubMed] [Google Scholar]
- 58.Young A, Jonski G, Rölla G. Inhibition of orally produced volatile sulfur compounds by zinc, chlorhexidine or cetylpyridinium chloride-effect of concentration. Eur J Oral Sci. 2003;111:400–404. doi: 10.1034/j.1600-0722.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 59.Segreto V A, Collins EM, D’Agostino R, et al. Anti-calculus effect of a dentifrice containing 0.5% zinc citrate dihydrate. Community Dent Oral Epidemiol. 1991;19:29–31. doi: 10.1111/j.1600-0528.1991.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka M, Matsunaga K, Kadoma Y. Correlation in inorganic ion concentration between saliva and plaque fluid. J Med Dent Sci. 2000;47:55–59. [PubMed] [Google Scholar]
- 61.Rose RK. Competitive binding of calcium, magnesium and zinc to Streptococcus sanguis and purified S.sanguis cell walls. Caries Res. 1996;30:71–75. doi: 10.1159/000262139. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka M, Matsuda M. Concentration and individual variation of inorganic ions in unstimulated whole saliva. J Stomatol Soc Japan. 2000;67:46–51. doi: 10.5357/koubyou.67.46. [DOI] [PubMed] [Google Scholar]
- 63.Cerklewski FL. Effect of suboptimal zinc nutrition during gestation and lactation on rat molar tooth composition and dental caries. J Nutr. 1981;111:1780–1783. doi: 10.1093/jn/111.10.1780. [DOI] [PubMed] [Google Scholar]
- 64.Rapisarda E, Longo A. Effects of zinc and vitamin B 6 in experimental caries in rats. Minerva Stomatol. 1981;30:317–320. [PubMed] [Google Scholar]
- 65.Wuehler SE, Peerson JM, Brown KH. Use of national food balance data to estimate the adequacy of zinc in national food supplies: methodology and regional estimates. Publ Health Nutr. 2005;8:812–819. doi: 10.1079/phn2005724. [DOI] [PubMed] [Google Scholar]
- 66.Fischer WF, Black RE. Zinc and the risk for infectious disease. Ann Rev Nutr. 2004;24:255–275. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- 67.Ericsson Y. Some differences between human and rodent saliva of probable importance for the different specie reactions to cariogenic and cariostatic agents. Arch Oral Biol. 1962;7:327–336. [Google Scholar]
- 68.Cole MF, Bowen WH. In: Animal models in cariology. Tanzer JM, editor. IRL; Washington D.C and London: 1980. Organic and inorganic composition of saliva in certain experimental animals; pp. 357–366. [Google Scholar]
- 69.Navia J M. University of Alabama Press; Tuscaloosa: 1977. Animal models in caries research. [Google Scholar]
- 70.Hsieh S, Al-Hayali RN, Navia JM. In: >Trace Elements and Dental Diseases. Curzon MEJ, Cuttress TW, editors. Wright; Boston: 1983. Zinc; p. 199. [Google Scholar]
- 71.Anttila A. Proton-induced X-ray emission analysis of Zn, Sr and Pb in human deciduous tooth enamel and its relationship to dental caries scores. Arch Oral Biol. 1986;31:723–726. doi: 10.1016/0003-9969(86)90003-8. [DOI] [PubMed] [Google Scholar]
- 72.Abdullah AZ, Strafford SM, Brookes SJ, et al. The effect of copper on demineralization of dental enamel. J Dent Res. 2006;85:1011–1015. doi: 10.1177/154405910608501107. [DOI] [PubMed] [Google Scholar]
- 73.Dedhiya MG, Young F, Higuchi WI. Mechanism for the retardation of the acid dissolution rate of hydroxapatite by strontium. J Dent Res. 1973;52:1097–1109. doi: 10.1177/00220345730520051901. [DOI] [PubMed] [Google Scholar]
- 74.Ten Cate JM. The caries-preventive effect of a fluoride dentifrice containing triclosan and zinc citrate, a compilation of in vitro and in situ studies. Int Dent J. 1993;43:407–413. [PubMed] [Google Scholar]
- 75.Laucello M, Noel N, Lynch RJM, et al. The anti-caries efficacy of a silica-based fluoride toothpaste containing 0.75% Zinc Citrate, 0.3% Triclosan, Vitamin E and sunflower oil. Int Dent J. 2007;57:145–149. [Google Scholar]
- 76.Torrado A, Valiente M, Zhang W, et al. Remineralization potential of a new toothpaste formulation: an in vitro study. J Contemp Dent Pract. 2004;5:18–30. [PubMed] [Google Scholar]
- 77.Featherstone JDB, Rodgers BE, Smith MW. Physicochemical requirements for rapid remineralisation of early carious lesions. Caries Res. 1981;15:221–235. doi: 10.1159/000260518. [DOI] [PubMed] [Google Scholar]
- 78.Ten Cate JM, Shariati M, Featherstone JDB. Enhancement of (salivary) remineralization by ‘dipping’ solutions. Caries Res. 1985;19:335–341. doi: 10.1159/000260864. [DOI] [PubMed] [Google Scholar]
- 79.Featherstone JDB, Shields CP, Khademazad B, et al. Acid reactivity of carbonated apatites with strontium and fluoride solutions. J Dent Res. 1983;62:1049–1053. doi: 10.1177/00220345830620100801. [DOI] [PubMed] [Google Scholar]
- 80.Churchley D, Newby CS, Lynch RJM, et al. Protection against enamel demineralisation using sodium fluoride toothpastes containing zinc chloride. Int Dent J. 2011;16:55–59. doi: 10.1111/j.1875-595X.2011.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ingram GS, Baker G, Best S, et al. The influence of zinc citrate in a fluoride dentifrice on rat caries and fluoride content of molar enamel in vivo. J Dent Res. 1984;63:497. [Google Scholar]
- 82.Stephen KW, Creanor SL, Russell JI, et al. A 3-year oral health dose-response study of sodium monofluorophosphate dentifrices with and without zinc citrate: anti-caries results. Community Dent Oral Epidemiol. 1988;16:321–325. doi: 10.1111/j.1600-0528.1988.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 83.Stephen KW, Burchell CK, Huntington E, et al. In vivo anticalculus effect of a dentifrice containing 0.5% zinc citrate trihydrate. Caries Res. 1987;21:380–384. doi: 10.1159/000261043. [DOI] [PubMed] [Google Scholar]
- 84.Ripa LW, Leske GS, Triol CW, et al. Clinical study of the anticaries efficacy of three fluoride dentifrices containing anticalculus ingredients: three-year (final) results. J Clin Dent. 1990;2:29–33. [PubMed] [Google Scholar]
- 85.Scheie AA, Petersen FC. In: >Dental caries: the disease and its clinical management. 2nd ed. Fejerskov O, Kidd EAM, editors. Blackwell Munksgaard; Oxford: 2008. Antimicrobials in caries control; pp. 265–277. [Google Scholar]
- 86.van Rijkom HM, Truin GJ, van‘t Hof MA. A meta-analysis of clinical studies on the caries-inhibiting effect of chlorhexidine treatment. J Dent Res. 1996;75:790–795. doi: 10.1177/00220345960750020901. [DOI] [PubMed] [Google Scholar]
- 87.Orland FJ, Blayney JR, Harrison RW, et al. Use of the germ-free animal technic in the study of experimental dental caries. J Dent Res. 1954;33:147–174. doi: 10.1177/00220345540330020201. [DOI] [PubMed] [Google Scholar]
- 88.Lynch RJM, ten Cate JM. The effect of lesion characteristics at baseline on subsequent de- and remineralisation behaviour. Caries Res. 2006;40:530–535. doi: 10.1159/000095653. [DOI] [PubMed] [Google Scholar]
- 89.Mellberg JR, Chomicki WG. Effect of zinc citrate on fluoride uptake by artificial caries lesions. J Dent Res. 1983;62:145–147. doi: 10.1177/00220345830620021201. [DOI] [PubMed] [Google Scholar]
- 90.McDowell H, Gregory TM, Brown WE. Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25, and 37°C. J Res Natl Bur Stand. 1977;81:273–281. [Google Scholar]
- 91.Nriagu JO. Solubility equilibrium constant of α-hopeite. Geochim Cosmochim Acta. 1973;37:2357–2361. [Google Scholar]
- 92.Lynch RJM, Churchley DR, Cooper L, et al. Effect of zinc and fluoride on the remineralisation of early carious lesions under simulated plaque-fluid conditions. Caries Res. 2011;45:313–322. doi: 10.1159/000324804. [DOI] [PubMed] [Google Scholar]
- 93.Fujikawa H, Matsuyama K, Uchiyama A, et al. Influence of salivary macromolecules and fluoride on enamel lesion remineralization in vitro. Caries Res. 2008;42:37–45. doi: 10.1159/000111748. [DOI] [PubMed] [Google Scholar]
- 94.Arends J, Christoffersen J. The nature of early caries lesions in enamel. J Dent Res. 1986;65:2–11. doi: 10.1177/00220345860650010201. [DOI] [PubMed] [Google Scholar]
- 95.Hallsworth AS, Weatherell JA, Robinson C. Loss of carbonate during the first stages of enamel caries. Caries Res. 1973;7:345–348. doi: 10.1159/000259857. [DOI] [PubMed] [Google Scholar]
- 96.Weatherell JA, Robinson C, Hiller CR. Distribution of carbonate in thin sections of dental enamel. Caries Res. 1968;2:1–9. doi: 10.1159/000259538. [DOI] [PubMed] [Google Scholar]
- 97.Shellis RP, Wilson RM. Apparent solubility distributions of hydroxyapatite and enamel apatite. J Colloid Interface Sci. 2004;278:325–332. doi: 10.1016/j.jcis.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 98.Lippert F, Lynch RJM, Eckert GJ, et al. In situ fluoride response of caries lesions with different mineral distributions at baseline. Caries Res. 2011;45:47–55. doi: 10.1159/000323846. [DOI] [PubMed] [Google Scholar]
- 99.Kosoric J, Hector M P, Anderson P. The influence of proteins on demineralization kinetics of hydroxyapatite aggregates. J Biomed Mater Res A. 2010;94:972–977. doi: 10.1002/jbm.a.32759. [DOI] [PubMed] [Google Scholar]
- 100.Ingram GS, Horay CP, Stead WJ. Interaction of zinc with dental mineral. Caries Res. 1992;26:248–253. doi: 10.1159/000261447. [DOI] [PubMed] [Google Scholar]
- 101.Lee YJ, Elzinga EJ, Reeder RJ. Sorption mechanisms of zinc on hydroxyapatite: systematic uptake studies and EXAFS spectroscopy analysis. Environ Sci Technol. 2005;39:4042–4048. doi: 10.1021/es048593r. [DOI] [PubMed] [Google Scholar]
- 102.Stötzel C, Müller FA, Reinert F, et al. Ion adsorption behaviour of hydroxyapatite with different crystallinities. Colloids Surf B Biointerfaces. 2009;74:91–95. doi: 10.1016/j.colsurfb.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 103.Li M, Xiao X, Liu R, et al. Structural characterization of zinc-substituted hydroxyapatite prepared by hydrothermal method. J Mater Sci Mater Med. 2008;19:797–803. doi: 10.1007/s10856-007-3213-4. [DOI] [PubMed] [Google Scholar]
- 104.Mayer I, Apfelbaum F, Featherstone JDB. Zinc ions in synthetic carbonated hydroxyapatites. Arch Oral Biol. 1994;39:87–90. doi: 10.1016/0003-9969(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 105.Daabees N, El-Khordagui LK, Shams Eldeen MA, et al. Dissolution Behaviour of Carbonated Apatites in the Presence of Some Ions. Drug Devel Ind Pharm. 1988;14:1855–1875. [Google Scholar]
- 106.Costa AM, Rossi AM, Soares GA. Effect of zinc content on in vitro dissolution of non-calcinated and calcinated Zn-containing hydroxyapatite. Acta Microscopica. 2003;12(Supp C):49–50. [Google Scholar]
- 107.Valsami-Jones E, Ragnarsdottir KV, Putnis A, et al. The dissolution of apatite in the presence of aqueous metal cations at pH 2-7. Chem Geol. 1998;151:215–233. [Google Scholar]
- 108.Williams RAD, Elliott JC, editors. Basic and applied dental biochemistry. Churchill Livingstone; Edinburgh London and New York: 1989. Chemistry of the calcium phosphates; pp. 318–341. [Google Scholar]
- 109.Dybowska A, Manning DAC, Collins MJ, et al. An evaluation of the reactivity of synthetic and natural apatites in the presence of aqueous metals. Sci Total Natural Environ. 2009;407:2953–2965. doi: 10.1016/j.scitotenv.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 110.Fuierer T A, LoRe M, Puckett SA, et al. A mineralization adsorption and mobility study of hydroxyapatite surfaces in the presence of zinc and magnesium ions. Langmuir. 1994;10:4721–4725. [Google Scholar]
- 111.Lingawi H, Barbour M, Lynch RJM, et al. Effect of zinc ions (Zn+2) on hydroxyapatite dissolution kinetics studied using scanning microradiography. Caries Res. 2011;45:195. [Google Scholar]
- 112.Lusvardi G, Menabue L, Saladini M. Reactivity of biological and synthetic hydroxyapatites towards Zn(II) ion, solid-liquid investigation. J Mater Sci Mater Med. 2002;13:91–98. doi: 10.1023/a:1013655123340. [DOI] [PubMed] [Google Scholar]
- 113.Xu Y, Schwartz FW, Traina SJ. Sorption of Zn2+ and Cd2+ on hydroxyapatite surface. Environ Sci Technol. 1994;28:1219–1228. doi: 10.1021/es00057a015. [DOI] [PubMed] [Google Scholar]
- 114.LeGeros RZ, Bleiwas CB, Retino M, et al. Zinc effect on the in vitro formation of calcium phosphates: relevance to clinical inhibition of calculus formation. Am J Dent. 1999;12:65–71. [PubMed] [Google Scholar]
- 115.LeGeros RZ, Taheri MH, Quirolgico G et al. Formation and stability of apatites; effect of some cationic substituents. Proc 2nd international conference on phosphorus compounds. Boston: 1980. pp 41–54.
- 116.LeGeros RZ. In: Monographs in oral science, Vol 15. Myers HM, editor. Karger; Basel: 1991. Calcium phosphates in oral biology and medicine; pp. 1–201. [PubMed] [Google Scholar]
- 117.LeGeros RZ. Effect of zinc on the formation of calcium phosphates in vitro. Caries Res. 1997;31:434–440. [Google Scholar]
- 118.Margolis HC, Varughese K, Moreno E. Effect of fluoride on crystal growth of calcium apatites in the presence of a salivary inhibitor. Calcif Tiss Int. 1982;34:S33–40. [PubMed] [Google Scholar]
- 119.Davey HP, Embery G, Cummins C. Interaction of zinc with a synthetic calcium phosphate mineral. Caries Res. 1997;31:434–440. doi: 10.1159/000262435. [DOI] [PubMed] [Google Scholar]
- 120.Mayer I, Apfelbaum F, Featherstone JDB. Zinc ions in synthetic carbonated apatites. Arch Oral Biol. 1994;39:87–90. doi: 10.1016/0003-9969(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 121.Tamm T, Peld M. Computational study of cation substitutions in apatites. J Solid State Chem. 2006;179:1581–1587. [Google Scholar]
- 122.Tang Y, Chappell HF, Dove MT, et al. Zinc incorporation into hydroxylapatite. Biomaterials. 2009;30:2864–2872. doi: 10.1016/j.biomaterials.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 123.Terra J, Jiang M, Ellis DE. Characterization of electronic structure and bonding in hydroxyapatite: Zn substitution for Ca. Philos Mag A. 2002;82:2357–2377. [Google Scholar]
- 124.Matsunaga A. First-principles study of substitutional magnesium and zinc hydroxyapatite and octacalcium phosphate. J Chem Phys. 2008;128:245101. doi: 10.1063/1.2940337. [DOI] [PubMed] [Google Scholar]
- 125.Matsunaga A. Mechanism of incorporation of zinc into hydroxyapatite. Acta Biomaterialia. 2010;6:2289–2293. doi: 10.1016/j.actbio.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 126.Roe RR, Pang YP. Zinc’s exclusive tetrahedral coordination governed by its electronic structure. J Mol Mod. 1999;5:134–140. [Google Scholar]
- 127.Atkins PW. 4th ed. Oxford University Press; Oxford: 1993. Physical Chemistry; pp. 933–969. [Google Scholar]
- 128.Cotton FA, Wilkinson G. 5th ed. Wiley Interscience; New York, Chichester, Brisbane, Toronto, Singapore: 1988. Advanced Inorganic Chemistry; pp. 1385–1388. [Google Scholar]
- 129.Mayer I, Featherstone JDB. Dissolution studies of Zn-containing carbonated hydroxyapatites. J Cryst Growth. 2000;219:98–101. [Google Scholar]
- 130.Featherstone JDB, Mayer I, Driessens FCM, et al. Synthetic apatites containing Na, Mg, and CO3 and their comparison with tooth enamel mineral. Calcif Tissue Int. 1983;35:169–171. doi: 10.1007/BF02405026. [DOI] [PubMed] [Google Scholar]
- 131.Nelson DGA, Featherstone JDB, Duncan JF, et al. Paracrystalline disorder of biological and synthetic carbonate-substituted apatites. J Dent Res. 1982;61:1274–1281. doi: 10.1177/00220345820610111301. [DOI] [PubMed] [Google Scholar]
- 132.Featherstone JDB, Nelson DGA. The effect of fluoride, zinc, strontium, magnesium and iron on the crystal-structural disorder in synthetic carbonated apatites. Aust J Chem. 1980;33:2363–2368. [Google Scholar]
- 133.Nikiforuk G, McLeod IM, Burgess RC, et al. J Dent Res. 1962;44:1477. [Google Scholar]
- 134.Matsunaga T, Ishizaki H, Tanabe S, et al. Synchrotron radiation microbeam X-ray fluorescence analysis of zinc concentration of remineralised enamel in situ. Arch Oral Biol. 2009;54:420–423. doi: 10.1016/j.archoralbio.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 135.Fang MM, Lei KY, Kilgore LT. Effects of zinc deficiency on dental caries in rats. J Nutr. 1980;110:1032–1036. doi: 10.1093/jn/110.5.1032. [DOI] [PubMed] [Google Scholar]