Abstract
The burden of health-care costs relative to gross domestic product in Japan is increasing. A large percentage (7.6% in 2009) of the Japanese gross domestic product has been spent on health care, and this percentage has been increasing annually. Soaring health-care costs have been recognised as a serious social problem. In this study, we attempted to estimate the relationship between periodontal disease and health-care costs. Subjects consisted of teachers and staff members (35 men, 26 women; mean age, 45 ± 9 years) from two high schools. The salivary levels of lactate dehydrogenase and haemoglobin were adopted as biomarkers to assess periodontal disease. After salivary tests, data for the health-care costs over the subsequent 6 months were provided by the mutual association of the public schools on an individual basis. Curve-fit estimations were then performed where health-care costs were used as a dependent variable and age or salivary levels of haemoglobin or lactate dehydrogenase were used as independent variables. However, no good fitness was obtained. Subsequently, multilayer perceptron neural networks were applied. With the neural networks, good fitness was obtained by using lactate dehydrogenase as an independent variable. The results of this study show that oral health, particularly periodontal disease, is correlated with total health-care costs. The data presented in this study suggests that, from the perspective of both oral and systemic health, oral health can be a signpost in well-being and health promotion.
Key words: Salivary test, health-care cost, haemoglobin, lactate dehydrogenase, metabolic syndrome
INTRODUCTION
The burden of health-care costs relative to gross domestic product (GDP) in Japan is worsening. A large percentage (7.6% in 2009) of the Japanese GDP has been spent on health care and this percentage has been increasing annually. Soaring health-care costs have been recognised as a serious social problem.
The number of individuals suffering from chronic lifestyle-related diseases such as diabetes, cerebral apoplexy, cardiovascular diseases and cancers has been increasing as a result of an ageing society, and this is particularly apparent in Japan.
Various lifestyle-related diseases are caused by low-grade chronic persistent inflammation1., 2.. Metabolic syndrome and periodontal disease have been suggested to cause chronic lifestyle-related diseases3., 4., 5., 6., 7., 8..
Many studies have discussed the relationships between metabolic syndrome and health-care costs. For example, a 2-year study that compared the annual health-care costs of those with or without diabetes found both higher health-care utilisation and significantly greater expenditure ($5,732 versus $3,581 per year)9. In this study, we attempted to estimate health-care costs arising from periodontal conditions by using salivary testing.
METHODS
Saliva test
In order to assess periodontal disease, we developed a saliva test.
In this study, lactate dehydrogenase (LD) and haemoglobin (Hb) were adopted for use as biomarkers in order to assess periodontal disease10. Five-minute stimulated saliva was obtained by chewing gum base without any flavourful or fragrant ingredients. Samples were transported to a clinical laboratory and kept at 4° C until use. The salivary levels of LD and Hb were measured using commercially available kits. The kits used in this study were LD (L type Wako LDH J; Wako Pure Chemical Industries, Osaka, Japan), developed for routine blood tests, and Hb (Saliva Hemo plus; Alfresa Pharma, Osaka, Japan).
Subjects
Subjects were 61 individuals (35 men, 26 women; mean age 45 ± 9 years) who worked as teachers and support staff at two high schools in Ehime prefecture in Japan. This prefecture is located in the southwest of Japan. Informed written consent was obtained from all subjects. The study was approved by the Ethics Committee of Tsurumi University, School of Dental Medicine (approval number: 430) and was conducted in accordance with the Helsinki Declaration.
Data collection on health-care costs
After performing salivary tests, data on health-care costs over the subsequent 6 months were provided by the mutual association of public schools on an individual basis. Individual names were concealed to preserve anonymity and the data on health-care costs and salivary tests were connected by mutual association.
Data analysis
Curve-fit estimations were carried out using health-care costs as a dependent variable and age or salivary levels of Hb or LD as the independent variables. The models applied in this study were: linear, Y = b0 + (b1 × t); logarithmic, Y = b0 + (b1 × ln(t)); Inverse, Y = b0 + (b1/t); quadratic, Y = b0 + (b1 × t) + (b2 × t); cubic, Y = b0 + (b1 × t) + (b2 × t) + (b3 × t); compound, ln(Y) = ln(b0) + (ln(b1)t); power, ln(Y) = ln(b0) + (b1 × ln(t)); S-curve, ln(Y) = b0 + (b1/t); Growth, ln(Y) = b0 + (b1 × t); exponential, ln(Y) = ln(b0) + (b1 × t); and logistic, ln(1/y–1/u) = ln (b0) + (ln(b1) × t).
The fitness of the data for these models was evaluated by P-values and R2. A P-value of <0.05 was considered to be statistically significant.
Multilayer perceptron neural networks were also applied using health-care costs as dependent variable, and age and salivary levels of Hb or LD as independent variables. Analyses were carried out using IBM SPSS Statistics Ver 19.0 and PASW Modeler Ver 14.0 (IBM SPSS, Tokyo, Japan).
RESULTS
Descriptive analyses of the subjects participating in this study are shown in Table 1. We produced 11 curve estimation regression models for medical expenses, with age, Hb and LD as independent variables. The summaries of these models are shown in Table 2 and scatter plots of the models, including raw data, are shown in Figure 1a for age, Figure 1b for Hb and Figure 1c for LD, respectively. Among the 33 models shown in Table 2 and Figure 1 11 showed statistical significance. However, for estimation of medical expenses, statistically significant correlations were obtained with age as an independent variable; the fitness of the models was insufficient for estimation when evaluated by R2.
Table 1.
Descriptive analyses of subjects
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Age | 45 | 9 | 23 | 63 |
| Haemoglobin (Hb) | 4.84 | 7.60 | 0 | 31 |
| Lactate dehydrogenase (LD) | 378 | 283 | 46 | 1804 |
| Medical Expense (JPY) | 1390 | 2085 | 0 | 9840 |
Table 2.
Summaries of curve estimation regression models
| Model | Age | Hb | LD | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter estimation | R2 | P | Parameter estimation | R2 | P | Parameter Estimation | R2 | P | |||||||||||
| Intercept | b1 | b2 | b3 | Intercept | b1 | b2 | b3 | Intercept | b1 | b2 | b3 | ||||||||
| Linear | Y = b0 + (b1 × t) | −1794.7 | 71.4 | 0.09 | 0.02 | 1631 | −49.6 | 0.03 | 0.16 | 1602.8 | −0.6 | 0.01 | 0.56 | ||||||
| Logarithmic | Y = b0 + (b1 × ln(t)) | −10081 | 3037.2 | 0.09 | 0.02 | 1453.3 | −191.2 | 0.02 | 0.26 | 1318.3 | 12.7 | 0 | 0.97 | ||||||
| Inverse | Y = b0 + (b1/t) | 4132 | −117106 | 0.09 | 0.02 | 1110.7 | 172 | 0.01 | 0.36 | 1586.7 | −46570 | 0.01 | 0.56 | ||||||
| Quadratic | Y = b0 + (b1 × t) + (b2 × t2) | −5528.6 | 248.3 | −2 | 0.1 | 0.05 | 1669.5 | −76.5 | 1.1 | 0.03 | 0.37 | 1251.7 | 1 | −0.0011 | 0.01 | 0.66 | |||
| Cubic | Y = b0 + (b1 × t) + (b2 × t2) + (b3 × t3) | 28252.4 | −2277.7 | 58.6 | −0.5 | 0.16 | 0.02 | 1552.2 | 70.7 | −14 | 0.4 | 0.04 | 0.5 | 502.3 | 6.5 | −0.0103 | 0.000004 | 0.03 | 0.65 |
| Compound | ln(Y) = ln(b0) + (ln(b1)t) | 2 | 1.1 | 0.05 | 0.08 | 188.9 | 0.9 | 0.05 | 0.1 | 268.3 | 1 | 0.03 | 0.17 | ||||||
| Power | ln(Y) = ln(b0) + (b1 × ln(t)) | 0.00003 | 4 | 0.06 | 0.06 | 127.8 | −0.3 | 0.02 | 0.32 | 1181.7 | −0.4 | 0.01 | 0.55 | ||||||
| S-curve | ln(Y) = b0 + (b1/t) | 8.5 | −159 | 0.06 | 0.07 | 4.3 | 0.3 | 0.01 | 0.4 | 4.9 | −39.3 | 0 | 0.77 | ||||||
| Growth | ln(Y) = b0 + (b1 × t) | 0.7 | 0.1 | 0.05 | 0.08 | 5.2 | −0.1 | 0.05 | 0.1 | 5.6 | 0 | 0.03 | 0.17 | ||||||
| Exponential | ln(Y) = ln(b0) + (b1 × t) | 2 | 0.1 | 0.05 | 0.08 | 188.9 | −0.1 | 0.05 | 0.1 | 268.3 | 0 | 0.03 | 0.17 | ||||||
| Logistic | ln(1/y–1/u) = ln (b0) + (ln(b1) × t) | 0.9 | 0.9 | 0.06 | 0.05 | 0.004 | 1.1 | 0.05 | 0.09 | 0.003 | 1 | 0.03 | 0.21 | ||||||
Figure 1.
Scatter plots of curve estimation models. Medical expenses were plotted against age (a), haemoglobin (Hb) (b) and lactate dehydrogenase (LD) (c).
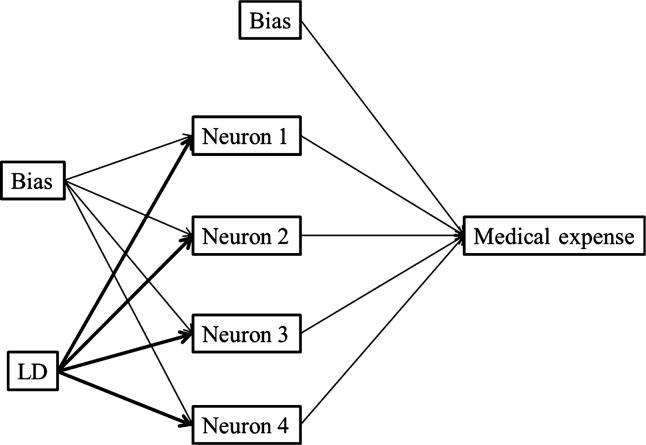
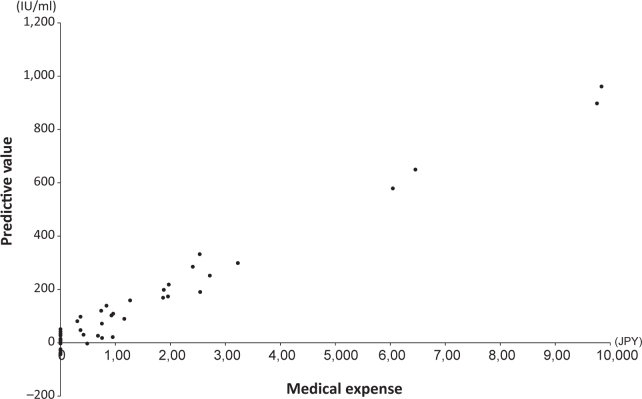
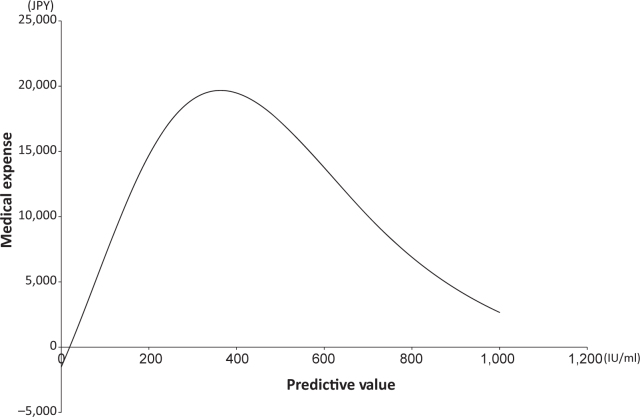
We then attempted to estimate the medical expenses based on a neural network, which is a non-linear regression method. We constructed three models for estimating medical expenses with age, Hb and LD as independent variables. The best fit was produced with LD, as shown in Figure 2. By applying these models we created scatter plots of predictive values against medical expenses (Figure 3). Among the three independent variables, sufficient fitness was obtained with LD used as an independent variable. Figure 4 shows a simulation of estimated medical expenses based on a neural network. We used LD as the independent variable.
Figure 2.
Prediction of medical expenses by neural network model. The neural network comprised three models for the estimation of medical expenses based on lactate dehydrogenase (LD).
Figure 3.
Scatter plot of predictive values against medical expenses determined by neural network. Lactate dehydrogenase was used as an independent variable.
Figure 4.
Simulation of medical expenses estimated by neural network. Lactate dehydrogenase was used as an independent variable.
DISCUSSION
Owing to recent advances in the knowledge of risk factors related to lifestyle diseases, oral condition (particularly periodontal disease) has been found to be clearly related to lifestyle diseases8. Among the risk factors, related genes, ethnicity and ageing are uncontrollable, whereas factors such as periodontal disease and smoking are controllable11., 12.. Periodontal disease is also thought to be a risk factor in diabetes3., 4., 5. and obesity13, and has been suggested to be a risk factor in some cancers14., 15., 16., 17., 18.. These diseases adversely affect quality of life. As a result of recent advances in clinical dental practice, periodontal disease can now be controlled. The results of this study suggest that controlling oral health contributes to reducing total health-care costs.
A neural network is an information processing paradigm inspired by biological nervous systems. The key element of a neural network is the novel structure of the information processing system, which is composed of highly interconnected processing elements working in union to solve specific problems. A neural network is configured for a specific application, such as pattern recognition or data classification, through a learning process. Neural networks, with their remarkable ability to derive meaning from complex or imprecise data, can be used to extract patterns and detect trends that are too complex to be noticed by standard statistical methods.
Neural networks take a different approach to problem solving than do standard statistical methods. Conventional computers use an algorithmic approach whereas neural networks learn by example: they cannot be programmed to perform a specific task. Thus, some tasks are suited to an algorithmic approach and others are suited to neural networks. The data on health-care costs are complex because they reflect a large variety of diseases. Therefore, the data may not fit with curve estimation but sufficient fitness and prediction can be obtained with a neural network.
One important role of oral health is to contribute to wellbeing. The data presented in this study suggest that oral health may be used as a signpost for wellbeing and health promotion, and that it is related to both oral and systemic health. However, the present study was limited by the small sample size and, therefore, a further study with a larger sample will be necessary to confirm these findings.
Conflict of interest
None declared.
Acknowledgement
This work was supported by JSPS KAKENHI Grant Number 24659938.
REFERENCES
- 1.Kolb H, Mandrup-Poulsen T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia. 2010;53:10–20. doi: 10.1007/s00125-009-1573-7. [DOI] [PubMed] [Google Scholar]
- 2.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–192. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 4.Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991;62:123–131. doi: 10.1902/jop.1991.62.2.123. [DOI] [PubMed] [Google Scholar]
- 5.Kaur G, Holtfreter B, Rathmann W, et al. Association between type 1 and type 2 diabetes with periodontal disease and tooth loss. J Clin Periodontol. 2009;36:765–774. doi: 10.1111/j.1600-051X.2009.01445.x. [DOI] [PubMed] [Google Scholar]
- 6.Buhlin K, Hultin M, Norderyd O, et al. Risk factors for atherosclerosis in cases with severe periodontitis. J Clin Periodontol. 2009;36:541–549. doi: 10.1111/j.1600-051X.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey LL, Fu R, Buckley DI, et al. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008;23:2079–2086. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchetti E, Monaco A, Procaccini L, et al. Periodontal disease: the influence of metabolic syndrome. Nutr Metab (Lond) 2012;9:88. doi: 10.1186/1743-7075-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudreau DM, Malone DC, Raebel MA, et al. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord. 2009;7:305–314. doi: 10.1089/met.2008.0070. [DOI] [PubMed] [Google Scholar]
- 10.Nomura Y, Shimada Y, Hanada N, et al. Salivary biomarkers for predicting the progression of chronic periodontitis. Arch Oral Biol. 2012;57:413–420. doi: 10.1016/j.archoralbio.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Fardal Ø, Johannessen AC, Linden GJ. Tooth loss during maintenance following periodontal treatment in a periodontal practice in Norway. J Clin Periodontol. 2004;31:550–555. doi: 10.1111/j.1600-051X.2004.00519.x. [DOI] [PubMed] [Google Scholar]
- 12.Axelsson P, Nyström B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol. 2004;31:749–757. doi: 10.1111/j.1600-051X.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 13.de Castilhos ED, Horta BL, Gigante DP, et al. Association between obesity and periodontal disease in young adults: a population-based birth cohort. J Clin Periodontol. 2012;39:717–724. doi: 10.1111/j.1600-051X.2012.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abnet CC, Kamangar F, Dawsey SM, et al. Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand J Gastroenterol. 2005;40:681–687. doi: 10.1080/00365520510015430. [DOI] [PubMed] [Google Scholar]
- 15.Abnet CC, Qiao YL, Mark SD, et al. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control. 2001;12:847–854. doi: 10.1023/a:1012290009545. [DOI] [PubMed] [Google Scholar]
- 16.Hujoel PP, Drangsholt M, Spiekerman C, et al. An exploration of the periodontitis–cancer association. Ann Epidemiol. 2003;13:312–316. doi: 10.1016/s1047-2797(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 17.Michaud DS, Joshipura K, Giovannucci E, et al. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99:171–175. doi: 10.1093/jnci/djk021. [DOI] [PubMed] [Google Scholar]
- 18.Stolzenberg-Solomon RZ, Dodd KW, Blaser MJ, et al. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am J Clin Nutr. 2003;78:176–181. doi: 10.1093/ajcn/78.1.176. [DOI] [PubMed] [Google Scholar]