Abstract
Purpose: The purpose of this study was to determine if a Health Coaching (HC) approach compared with formal health education (HE) resulted in better health outcomes among type II diabetes (T2DM) patients in improving glycaemic control and oral health, by use of clinical and subjective outcome measures. Methods: The study is part of a prospective intervention among randomly selected T2DM patients (n = 186) in Istanbul, Turkey. The data analysed were clinical [glycated haemoglobin (HbA1C), clinical attachment loss (CAL)] and psychological measures [tooth-brushing self efficacy (TBSES)]. Data were collected initially and at the end of intervention. Participants were allocated randomly to HC (intervention) (n = 77) and HE (control) (n = 111) groups. Results: At baseline, there was no statistical difference between HC and HE regarding clinical and psychological measures, (P > 0.05). At post-intervention the HC group had significantly lower HBA1C and CAL (reduction: 7%, 56%) than the HE group (reduction: HbA1C 0%; CAL 26%), (P ≤ 0.01). Similarly, HC group, compared with HE group, had better TBSES (increase: 61% vs. 25%) and stress (reduction: 16% vs. 1%), (P ≤ 0.01). Among high-risk group patients, the HC patients had significant improvements compared with the HE group (reduction: HbA1C 16% vs. 5%; CAL 63% vs. 18%; stress 39% vs. 2%; fold increase: TBSES 6.6 vs. 3.6) (P ≤ 0.01). Conclusions: The present findings may imply that HC has a significantly greater impact on better management of oral health and glycaemic control than HE. It is notable that the impact was more significant among high-risk group patients, thus HC may be recommended especially for high-risk group patients.
Key words: Type II Diabetes Mellitus, health promotion, health coaching, health education, high risk
INTRODUCTION
The World Dental Federation (FDI) and International Diabetes Federation (IDF) signed a joint declaration in 2007 stating the urgent need1: (1) to include prevention of oral disease and promotion of oral health as an essential component of diabetes management; (2) to initiate research leading to evidence-based treatment strategies to improve the health and oral health of diabetes patients.
A common-risk factor approach to promote better oral health and successful diabetes management are proposed as urgent needs by both the World Health Organisation (WHO)2 and IDF1., 2., 3.; behavioural interventions are highly recommended to meet this need4. This is vitally important because about 40% of the deaths attributable to type 2 diabetes mellitus (T2DM) are preventable by improving lifestyles4. However, to our knowledge, there has not been a behavioural intervention focusing on a common risk factor approach to promote better oral health and diabetes-related quality of life.
Learning to perform oral- and diabetes self-care activities and integrate these health behaviours in daily life, in the face of other responsibilities and life stresses, is psychologically complex and burdensome5., 6.. Acute and chronic diabetes complications and oral health problems can negatively affect the persons’ wellbeing and ability to function. People differ in their appraisal of, and ability to effectively cope with, the demands of diabetes self-care management. Therefore, owing to the complexity of making behavioural changes, oral health- and diabetes-care professionals require, in addition to teaching skills, a good understanding of the psychosocial impact of diabetes on daily living and knowledge of behavioural sciences in order to enhance people’s ability to cope5., 6., 7.. Therefore, there is a need for behavioural interventions that can:
-
•
Guide health professionals on how to motivate the patients to adjust health behaviours
-
•
Be adjusted to real-life settings and daily life of patients
-
•
Speak for transformation of health knowledge by education into health behaviour8.
Health Coaching (HC), is the most recent behavioural approach that facilitates individuals in transforming their cognitive and emotional functioning to adopt positive health behaviours, by the use of setting of personal goals and specific action plans. HC, one of the most effective behavioural techniques, is directly associated with positive lifestyle outcomes (smoking cessation, obesity and diabetes management)9., 10., 11., 12., 13.. Studies comparing the impact of HC with formal health education (HE) are scarce in the field of diabetes management and, to our knowledge, none have been published in dentistry.
The aim of this study was to determine if a HC approach resulted in better health outcomes, compared with formal HE, among T2DM patients in improving glycaemic control and oral health, by use of clinical and subjective outcome measures.
METHODS
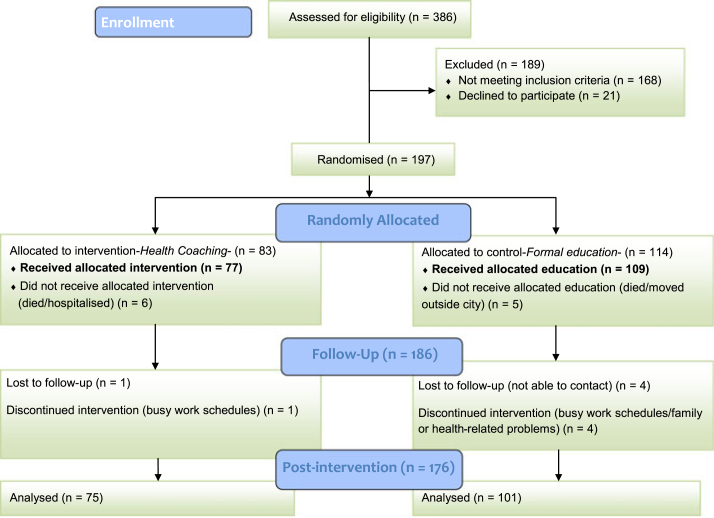
The present study is part of a prospective intervention study among T2DM patients (n = 186), randomly selected from the outpatient clinics of two hospitals in Istanbul, Turkey (Figure 1). The power and sample size was explained previously14. Eligibility criteria were: (1) confirmed T2DM; (2) 30- to 65-year-olds with at least four functional teeth and (3) no psychological treatment and hospitalisation.
Figure 1.
Schematic representation of the recruitment of patients.
Ethical approval and written permission were granted by the Ministry of Health, in Istanbul, Turkey to conduct the study. The methodology of the study was explained previously14. The study was conducted in full accordance with the World Medical Association Declaration of Helsinki.
Of the patients participating (n = 186), 96% attended the clinical examinations (baseline visit, n = 179; final visit, n = 176) and more than 90% filled in the questionnaires (baseline visit, n = 179; final visit: 168), (Figure 1). All patients provided basic socio-economic information themselves and biomedical records were obtained from the hospitals, at baseline. Of 186 participants, the drop out rate was 7% (n = 10) and the corresponding figure for the participants who did not regularly participate in all sessions was 13% (n = 24).
Back translation to and from Turkish was done for health behaviour questionnaires by two native speakers to ensure comparability with the original forms in English.
Procedure and randomisation
At the baseline visit, participants provided informed consent and filled in questionnaires (including demographic background, psychosocial and behavioural variables). Subsequently, all participants were invited for baseline oral examination, which was run by two calibrated examiners. Following the oral examination, participants were randomly allocated to either HC (intervention, n = 77) or formal oral health education (control, n = 109) group by a researcher who was blinded to outcome measures. The study included two phases (10-month initiation and maintenance, 6-month follow-up). During the 10-month intervention, participants were invited for free periodontal cleaning and three seminars about oral health and diabetes management. At the end of the 6-month follow-up phase, the same outcome measures were obtained.
The content and the design of HC and HE are described in detail elsewhere15. The HC approach in the study originally stems from coaching that is internationally accredited and uses specific psychological techniques16 including neuro-linguistic programming (NLP)17 and self-efficacy18. The HC approach focuses on empowerment of patients for daily diabetes- and oral health-related practices (compliance with healthy diet, regular physical activity and daily toothbrushing), building up health-related capacity building skills (self-efficacy, self-esteem) and taking responsibility for one’s own health.
A coach with a dental professional background (AB Cinar) provided HC intervention. Participants randomised to the HC had face-face sessions with the coach (five or six) and three or four phone-coaching sessions based on patient’s achievement of goals and need for support, over the intervention period. The primary method is that patients set up the goal and an action plan, focusing on improvement of lifestyle and clinical measures, under the supervision of the coach. Each coaching session, as the foundation for the next coaching session, was used for subsequent monitoring of patients’ progress towards the achievement of their target goal.
In the formal HE group (control), participants randomised to the HE group received standard lifestyle advice referring to oral health-care practices, diet and physical exercise. One dentist provided HE. At the initial session, within 2 weeks following the baseline oral examination, patients’ knowledge about these main areas of health were assessed by individual sessions. This was followed by two face-face and four phone sessions during the study period. All these sessions were supported by education, by a dietician and/or diabetes nurse in outpatient clinics.
The data in the present study come from the clinical measurements and self-assessed questionnaires that were collected initially and at the end of intervention.
Oral health management
At baseline, oral examinations were performed, including number of teeth lost and clinical attachment loss (CAL). The latter is the distance from the cemento-enamel junction (CEJ) in an apical direction to the base of the pocket/sulcus. All examinations were carried out by two dentists (I. Oktay and A. Beklen) by Michigan-0 probe. Examiners had previous experience in dental public health and periodontology, respectively, and were calibrated at measurement of CAL. Intraclass and interclass k value was 0.85. The detailed clinical examination is described elsewhere14. For further analysis, the high-risk CAL group was defined as the patients having ‘attachment loss >4 mm’ at baseline; high periodontal destruction is generally characterised with clinical attachment loss more than 4 mm19.
The tooth-brushing self-efficacy scale (TBSES)5., 20., 21., 22. was used to assess individual’s belief in his/her competency to brush his/her teeth daily across different challenging situations by the question ‘How sure that you can brush your teeth?’ TBSES consisted of eight items on a five-point Likert scale (0 = not sure at all to 5 = absolutely sure). The design and validity-reliability measures of the scale have been described previously20., 21.. For further analysis, sum scores for the TBSES were categorised into three equal groups by taking the 33% percentiles as the cut-off points separately for HC and HE groups; those who reported ≤33% of the total sum score (HC 12.4 vs. HE 10.3) were defined as high-risk group for TBSES.
Diabetes management
Information regarding HbA1C (glycated haemoglobin expressed as the percentage of haemoglobin that is exposed to glucose), fasting blood glucose and cholesterol levels were taken from the latest medical records at the hospital. Taking the target levels (HbA1C < 6.5%, fasting blood glucose <110 mg/dl, high-density lipoprotein (HDL) >39 mg/dl, and low-density lipoprotein (LDL) <95 mg/dl)23 as the cut-points, respective variables taken from the most recent health records were dichotomised as ‘favourable’ = 0 and ‘unfavourable’ = 1. For further analysis, the patients having HBA1C ≥ 8% were defined as a high-risk group23.
Body mass index (BMI), the proportion body fat measured by the Tanita TBF-300-A Body Composition Analyser and Scale (Tanita Europe BV, Amsterdam, the Netherlands); The methodology is described elsewhere14. According to the current WHO BMI cut-off points24, BMI ≥ 30 was categorised as obese, namely high risk, for further analysis in the study.
The Problem Areas in Diabetes Scale (PAID)25 has been widely adopted as a measure of psychosocial adjustment specific to diabetes26., 27.. The modified PAID scale used in the present study is a 13-item questionnaire, rated on a five-point Likert scale (0 = not at all to 5 = completely). The scale was used to assess a range of elements of diabetes-related psychosocial distress (e.g. ‘I have control over my diabetes’) by asking ‘Tell us how strongly you agree or disagree with the statement’. The original PAID has 20 items, however seven items were extracted for the modified PAID after being tested by the pilot study as they had low item total correlation and factor loading. The Cronbach correlation coefficient measure (α = 0.86) was good. Further, split-half internal consistency was applied to test the reliability of the modified PAID (0.84, equal-length Spearman–Brown) with the correlation between the two halves r = 0.72. For further analysis, sum scores for the modified PAID scale were categorised into three equal groups by taking the 33% percentiles as the cut-off points separately for HC and HE groups; those who reported ≤33% of the total sum score (HC:37 vs. HE:36) were defined as a high-risk group for modified PAID scale.
Stress was assessed by a single question from WHO Quality of Life Measure28, by asking ‘How often do you feel hopeless, depressive or anxious?’ Answer ranged on a five-point Likert scale (0 = never to 5 = always).
Data analysis
Statistical analyses were performed using SPSS v.17 (SPSS, Chicago, IL, USA). For assessment of correlation and baseline similarities/differences between HC and HE groups, respectively, Spearman rank correlation and independent sample t test were used for assessment. Time-by-group interaction effects were measured with repeated-measures analysis of variance (anova) procedures using time as the within-subjects factor (pre-intervention vs. post-intervention) and group as the between-subjects factor (coaching vs. control). Paired-sample t tests were used for normally distributed data to assess change over time for each group alone. Statistical significance was set at a P <0.05 for each test.
RESULTS
Patients in the HC group had 13.1 ± 21.8 years of clinically diagnosed diabetes and it was 10.8 ± 12.2 for the HE group (P > 0.05). At baseline there were no statistical differences between the HC and HE groups on the clinical and psychological measures (P > 0.05) (Table 1). The HC group had unfavourable HbA1C (6.5%≤) at 73% and CAL at 89%. The corresponding figures for the HE group were 71% and 91%.
Table 1.
Baseline characteristics of health coaching (n = 77) and health education (n = 109) groups by favourable biological and psychological measures
| n | HC Group (%) | n | ED Group (%) | |
|---|---|---|---|---|
| HbA1C ≥ 6.5% | 18 | 27 | 26 | 29 |
| Fasting blood glucose ≥ 110 mg | 12 | 17 | 16 | 18 |
| HDL cholesterol (mg/dl) >39 | 52 | 75 | 64 | 79 |
| Body fat (within ideal range) | 22 | 33 | 37 | 39 |
| BMI < 30 | 9 | 14 | 16 | 17 |
| Mean CAL ≤ 4 mm | 8 | 11 | 9 | 9 |
| TBSES ≥ mean | 37 | 49 | 49 | 50 |
| PAID ≥ mean | 42 | 57 | 53 | 55 |
| Stress (never/rare) | 18 | 24 | 35 | 37 |
HbA1C, glycated haemoglobin; HDL, high-density lipoprotein; BMI, body mass index; CAL, clinical attachment loss; TBSES, tooth-brushing self efficacy; PAID, Problem Areas in Diabetes Scale.
At post-intervention there was a significant reduction at HbA1C (−7%) and CAL (50%) among HC. Reduction was also significant for CAL among the HE group (−26%), (P < 0.05), (Table 2). The group differences over time were significant for HbA1C, CAL in favour of HC group, (P < 0.05). During the intervention period, the HC group almost maintained body fat (P > 0.05) whereas HE group had an increased amount of body fat (P < 0.05).
Table 2(a–b).
Between- and within-group differences from baseline to 16 months
| (a) Clinical parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Health coaching |
Formal education |
Difference between groups by time§ | |||||||||
| Baseline |
Post-intervention | Change % | P‡ | Baseline |
Post-intervention | Change % | P‡ | ||||
| Clinical parameters | n* | % or mean ± SD | % or mean ± SD | n* | % or mean ± SD | % or mean ± SD | |||||
| HbA1C | 70 | 7.5 ± 1.5% | 6.9 ± 1.3 | −7 | 0.001 | 92 | 7.8 ± 1.6% | 7.8 ± 1.6% | † | NS | 0.004 |
| Fasting blood glucose | 75 | 164.3 ± 67.6 | 144.7 ± 53.2 | −12 | 0.009 | 92 | 170.1 ± 71.9 | 159.5 ± 57.3 | −6.2 | NS | NS |
| HDL cholesterol (mg/dl) | 72 | 49.1 ± 13.8 | 48.6 ± 17.8 | −2.4 | NS | 91 | 48.5 ± 11.4 | 45.5 ± 9.3 | −6.2 | 0.001 | NS |
| Body fat | 66 | 27.8 ± 11.5 | 28.3 ± 11.6 | 3.6 | NS | 66 | 27.5 ± 12.8 | 30.6 ± 15.2 | 11 | 0.004 | 0.04 |
| BMI | 67 | 30.1 ± 5.3 | 34.0 ± 32.3 | 13.3 | NS | 99 | 31.0 ± 6.1 | 30.8 ± 6.6 | † | NS | NS |
| CAL | 77 | 2.2 ± 1.2 | 1.1 ± 0.8 | −50 | 0.001 | 97 | 2.3 ± 1.2 | 1.7 ± 1.5 | −26 | 0.001 | 0.004 |
| Total number of teeth lost | 69 | 6.4 ± 5.8 | 6.8 ± 6.4 | 6 | 0.06 | 77 | 8.5 ± 6.5 | 9.1 ± 6.9 | 7 | 0.003 | NS |
| (b) Psychological variables | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Health coaching |
Formal education |
Difference between groups by time** | |||||||||
| Baseline |
Post-intervention | Change % | P¶ | Baseline |
Post-intervention | Change % | P¶ | ||||
| Psychological parameters | n | % or mean ± SD | % or mean ± SD | n | % or mean ± SD | % or mean ± SD | |||||
| TBSES | 71 | 18.3 ± 11.9 | 29.4 ± 8.6 | 61 | 0.001 | 70 | 16.7 ± 11.8 | 20.9 ± 11.4 | 25 | 0.002 | 0.001 |
| PAID | 68 | 39.8 ± 10.5 | 49.8 ± 11.2 | 25 | 0.001 | 64 | 38.9 ± 13.5 | 45.8.±14.9 | 18 | 0.003 | NS |
| Feeling stressed (never/rare) | 75 | 24% | 40% | 16 | 0.01 | 77 | 37% | 38% | 1 | NS | 0.01 |
HbA1C, glycated haemoglobin; HDL, high-density lipoprotein; BMI, body mass index; CAL, clinical attachment loss; TBSES, tooth-brushing self efficacy; PAID, Problem Areas in Diabetes Scale.
The total number for each variable differs because the same participants did not answer all the questions; n for each variable represents paired matches.
Percentages ≤ 1.
Paired t-test stratified by within each group.
Repeated measures anova between two groups.
Two-related samples Wilcoxon test within each group.
Repeated measures anova between two groups.
Both the HC and HE groups significantly improved diabetes-self management beliefs (PAID) over time (P < 0.01) and there was no statistically significant difference between these groups (P > 0.05) (Table 2). The HC group had significantly greater improvement in tooth-brushing self-efficacy beliefs (TBSES, 61%) compared with the HE group (25%, P = 0.001).
Among high-risk group patients, the reduction in HbA1C (16%) and CAL (63%) was highly significant compared with those in the HE group (5% vs. 18%), (P ≤ 0.03, (Table 3). It was striking that HC group had a 6.6-fold increase at mean TBSES compared with the HE group, which had a 3.3-fold mean increase (P = 0.001). The HE patients neither in general nor in risk groups had better stress management over time (P > 0.05); however, the HC patients had less stress over time (P < 0.05).
Table 3.
For selected variables, changes by 16 months within study groups for participants who were high-risk at baseline
| Health coaching |
Formal education |
Difference between groups by time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Post-intervention | Change% | P | Baseline |
Post-intervention | Change% | P | ||||
| n | % or mean ± SD | % or mean ± SD | n | % or mean ± SD | % or mean ± SD | ||||||
| Clinical parameters | |||||||||||
| HbA1C ≥ 8% | 21 | 9.4 ± 1.2% | 7.9 ± 0.7 | −16 | 0.001 | 92 | 9.5 ± 1.2% | 8.97 ± 1.5% | −5 | 0.01 | 0.003 |
| BMI ≥ 30 | 29 | 34.9 ± 4.1 | 34.8 ± 4.2 | * | NS | 38 | 35.9 ± 4.1 | 35.9 ± 4.8 | * | NS | NS |
| CAL > 4 mm | 8 | 4.9 ± 0.9 | 1.8 ± 1.3 | −63 | 0.004 | 9 | 4.99 ± 0.9 | 4.07 ± 2.3 | −18 | NS | 0.03 |
| Psychological parameters | |||||||||||
| TBSES* | 24 | 4.4 ± 3.8 | 26.7 ± 11.6 | 507 | 0.001 | 27 | 3.7 ± 3.4 | 13.3 ± 10.6 | 259 | 0.001 | 0.001 |
| PAID* | 24 | 28.5.±7.8 | 43.8 ± 13.5 | 54 | 0.017 | 27 | 26.2 ± 9.7 | 43.8 ± 12.9 | 67 | 0.001 | NS |
| Feeling stressed† | 23 | 31% | 40% | 29 | 0.004 | 22 | 23% | 25% | 2 | NS | 0.01 |
HbA1C, glycated haemoglobin; BMI, body mass index; CAL, clinical attachment loss; TBSES, tooth-brushing self efficacy; PAID, Problem Areas in Diabetes Scale.
For TBSES and PAID, the high-risk group was identified as those who responded at ‘≤33% percentile’ of the total sum score; the analysis performed separately for each group.
For feeling stressed, the high-risk group defined as those who felt stressed ‘always and/or mostly’.
DISCUSSION
To our knowledge, this is the first randomised controlled trial to analyse the effectiveness of an individualised HC intervention, compared with education, on oral health and diabetes. It targets internal motivation by linking behavioural goals to patients’ values and personal vision of health. Improvements were observed in HbA1C, CAL, TBSES, PAID and stress.
The results of this study indicate that the HC intervention resulted in diabetes- and oral health-related improvements (HbA1C, fasting blood glucose, CAL) over the 16 months of the study. The HBA1C levels of the HC group reduced by 7%, whereas the HE group’s HbA1C levels remained same. The reduction in HbA1C is in line with the results of some recent studies and contradicts others. Whittemore et al. found that the improvement in HbA1C in a coaching group was not significantly different from that in an education group over a 3-months period13. Conversely, Wolever et al. (2010) found a significant impact of coaching intervention compared with an education group at reducing HbA1C levels29. The coaching approach stems from motivational interviewing (MI)30 focusing on personal empowerment to adopt healthy behaviours. Recent studies to improve HbA1C levels by MI have a range of different results. Chen et al. found that patients having MI for 3 months significantly reduced their HbA1C whereas those in the control group, provided with usual care, had no significant change31. In a US study of female T2DM patients, a two-session MI intervention was delivered within an 18-month, multidisciplinary behavioural weight-loss programme22. Participants showed clinically and statistically significant improvement in glycaemic control for the MI group (HbA1C, −0.8%) compared with the control group (HbA1C,−0.5%) at 6-month follow-up; however, mean HbA1C returned to baseline levels at the 12-month follow-up time-point and remained so at the end of the 18-month intervention32. However, Welch et al. found that MI was less effective than education33. They claimed that the results could reflect an inability of the educators both in the MI and non-MI groups to work with the self-efficacy perceptions of patients. All these studies vary in design, population type/size and duration, and some are unclear in terms of content or the number of sessions applied during the study period. Therefore, it is difficult to draw definitive conclusions when referring to the findings of the present study. However, in general, it is evident that coaching intervention has a higher significant impact on reduction of HbA1C compared with the education group.
A Cochrane review reported that psychological interventions, in particular MI, may lead to significant improvements in periodontal health by altering health behaviours at clinical settings34., 35.. However, such interventions are scarce in dentistry. The individually tailored oral health educational programme of Jonsson and his colleagues, based on MI30 and Social Cognitive Theory28, was more efficacious in improving periodontal health compared with an education group36. Similar results have been found by other individually tailored psychological interventions based on Social Cognitive Theory and MI37., 38., 39.. The findings of the present study are in line with these recent studies that coaching intervention, based on MI, Social Cognitive Theory, and NLP has significantly greater impact on the reduction of clinical attachment loss scores compared with an education group.
The studies that improved HbA1C and periodontal disease have in common the self-efficacy component as part of interventions29., 30., 36., 37., 39.. Chen et al. found that self-efficacy and HbA1C improved among intervention group patients receiving self-efficacy integrated MI30. Kakudate et al. (2009) has shown that a behavioural cognitive method is more effective than traditional oral hygiene instruction in terms of improving self-efficacy and oral hygiene37. In line with these studies, glycaemic control in an integrative health coaching intervention for psychosocial factors29 and periodontal health in a patient-empowerment based intervention39 were significantly more successful compared with education groups. Supported by the findings of these studies, the current study, integrating self-efficacy with the HC intervention, significantly reduced HbA1C and improved periodontal health over the study period. The perception of self-efficacy plays a crucial role in adoption, maintenance and improvement of health behaviours as people engage in activities that they believe they can manage but avoid the ones that they perceive as more than they can cope with40. As stated by Philippot et al.39, better results can be obtained if patients’ sense of self-efficacy is developed through their own direct experience, by observing the effects of their behaviour on periodontitis symptoms. As these effects can be experienced in a shorter period of time compared with the impact on general health, such as weight loss and reduction in HbA1C, improvement at tooth brushing self-efficacy can be an initiating step in improvement of health. This may need to be taken into consideration, especially when targeting health promotion programmes for the patients in high-risk groups. In the present study, self-efficacy integrated HC had a dramatically greater impact in improving clinical and psychological measures compared with the education group and the general HC group. There is limited knowledge on how to encourage patients to take responsibility for their own oral health36 and diabetes care41, in particular by improving self-efficacy. There is a need for further studies to explore how patients in high-risk groups, in terms of oral health and diabetes, can be supported and motivated to take action to improve their health.
Self-management support, defined as the ‘systematic provision of education and supportive interventions to increase patients’ skills and confidence in managing their health conditions’ has been shown to improve diabetes-related clinical outcomes42. HC targets the improvement of self-management skills by empowering patients within the health-care setting and in their daily lives43. Within the health-care setting, empowerment is characterised by highlighting the pros and cons of personal decisions, asking open-ended questions, providing information about diabetes and oral health care, and collaboratively setting up goals and developing action plans to adjust healthy lifestyles. In their daily lives, empowered patients are more likely to adhere to treatment plans and engage in lifestyle changes to effectively manage their chronic conditions44., 45.. There is growing evidence that primary care clinicians are unable to provide all needed preventive and chronic care support alone. It would require an estimated 21.7 hours per day for a clinician to meet the chronic, preventive and acute care needs of a panel of 2,500 patients46., 47., 48.. In addition, according to a WHO report, because of its chronic nature, the severity of its complications and the means required to control them, diabetes is a costly disease not only for the affected individual and his/her family, but also for the health authorities. The total health-care costs of a person with diabetes in the USA are between two and three times those for people without the condition. Diabetes has direct (hospital services, physician services, laboratory tests and the daily management of diabetes) and indirect costs (loss of work hours, premature retirement/death) to society. Lifestyle modifications (appropriate diet and increased physical activity and a consequent reduction of weight), supported by a continuous education programme have been used to achieve a reduction of almost two-thirds in the progression to diabetes in China, USA and Finland49. The WHO underline that this type of measure is not easy, but is likely to be cost effective if it can be implemented on a population scale. In contrast, despite great achievements, oral diseases are still among the most important aspect of global oral health and traditional treatment of oral disease is extremely costly – the fourth most expensive disease to treat in most industrialised countries50. There is a need for common risk approach based on behavioural interventions to reduce of the cost of these diseases1., 2., 3.. Providing HC training to physicians and dentists at internationally accredited standards may seem costly at first sight but this cost is probably less than the direct and indirect costs of these diseases. Another aspect is that improving doctor–patient communication skills of physicians and dentists by providing education about the principles of HC will contribute to increased patient compliance. In that case, there may need to be specialisation among medical care professionals (such as psychologists or behavioural therapists) as professional health coaches. This may appear not to be cost effective but in the longer term, considering the high costs of diabetes and oral diseases and their complications, this approach may both reduce the costs and improve the quality of lives. However, all these approaches require further research in the field.
A limitation of the present study is the small sample size. However, it is within the range of sample sizes of the studies in the field, which measure the impact of behavioural interventions for HbA1C21., 31., 33. and periodontal health34. Another limitation is that the interaction between clinical and psychological variables has not been assessed further. However, the aim of the present study was to evaluate the impact of HC compared with HE on diabetes management and oral health among T2DM patients. Even if the sample used is not representative of the general population of T2DM patients in Turkey, the study can be a model for further studies as it is an intervention focusing on a common risk factor approach for diabetes and oral health management, to our knowledge for the first time.
The strengths of this study include the theoretical background of HC approach used, strong support from the medical team of the hospitals (physicians, nurses) and low patient resistance to research activities.
CONCLUSION
Multifaceted interventions are recommended to address the complexity of chronic disease management, in particular diabetes and oral health. It is evident that health promotion for diabetes should go far beyond diet, physical activity and foot care education. As psychosocial markers play a significant role in both oral health and diabetes, there is a need for behavioural interventions examining the assessment and improvement of these markers. Self-efficacy, namely personal belief in one’s own capacity to perform a specific kind of action, can be seen as a trigger to adopt and improve healthy behaviours. The findings of the present study show that self-efficacy integrated HC improved oral health and diabetes in terms of both clinical and psychological parameters. This may highlight that there is a need for further research to assess the impact of similar interventions on diabetes and oral health management, in order that a common HC-based health promotion can be set up for T2DM patients to improve their quality of life.
Acknowledgements
We express our deepest thanks to Prof. Nazif Bagriacik (Head, Turkish Diabetes Association) and Associate Prof. Mehmet Sargin and Head Diabetes Nurse Sengul Isik (Diabetes Unit, S.B. Kartal Research and Education Hospital) for all their support and help during the research. We thank Prof. Aytekin Oguz for his help in the preparation of the documents for ethical permission. We also thank Prof. I Oktay and periodontologist Dr A. Beklen for clinical oral examinations. We also express our thanks to Duygu Ilhan for training and for her support in clinical examinations. We also thank ZENDIUM for oral health care kits, SPLENDA (TR) for the promotional tools, ChiBall World Pty Ltd for exercising chi-balls, and to IVOCLAR Vivadent, Plandent, Denmark for provision of CRT kits. Many thanks are extended to our patients for their participation and cooperation, and as well to the staff at Diabetes Unit, S.B. Kartal Research and Education Hospital for their kind help and support.
The research is part of an international project that has two phases. The Turkish phase is presented here and is supported by FDI, and the International Research Fund of University of Copenhagen.
Conflict of interest
None declared.
REFERENCES
- 1.IDF. Diabetes and oral health Available from: http://www.idf.org/guidelines/diabetes-and-oral-health. [Accessed June 5 2013]; 2007
- 2.Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century – the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31(Suppl 1):3–23. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 3.IDF. International Curriculum for Diabetes Health Professional Education Module. http://www.idf.org/webdata/docs/Curriculum_Final%20041108_EN.pdf. [Accessed June 5 2013]; 2008
- 4.WHO. Available from: http://www.who.int/chp/chronic_disease_report/turkey.pdf. [Accessed June 5 2013]; 2010
- 5.Cinar AB. University of Helsinki; Helsinki: 2008. Preadolescents and Their Mothers as Oral Health-Promoting Actors: Non-biologic Determinants of Oral Health among Turkish and Finnish Preadolescents. Doctorate Thesis. [Google Scholar]
- 6.IDF. International Curriculum for Diabetes Health Professional Education Module 1-4: Psychosocial and behavioural Approaches. Available from: http://www.idf.org/publications/international-curriculum-diabetes-health-professional-education. [Accessed June 5 2013]
- 7.Minet L, Møller S, Vach W et al. Mediating the effect of self-care management intervention in type 2 diabetes: a metaanalysis of 47 randomised controlled trials. Patient Educ Couns 2009. doi:10.1016/j.pec.2009.09.33. [DOI] [PubMed]
- 8.Danish Health Technology Assessment . Danish Health Technology Assessment; 2005. TYPE 2 DIABETES: Health Technology Assessment of screening, diagnosis and treatment. publication no.7. [Google Scholar]
- 9.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005;18:CD001292. doi: 10.1002/14651858.CD001292.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Bacon L, Stern JS, Van Loan MD, et al. Size acceptance and intuitive eating improve health for obese, female chronic dieters. J Am Diet Assoc. 2005;105:929–936. doi: 10.1016/j.jada.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Sarvestani RS, Jamalfard MH, Kargar M, et al. Effect of dietary behaviour modification on anthropometric indices and eating behaviour in obese adolescent girls. J Adv Nurs. 2009;65:1670–1675. doi: 10.1111/j.1365-2648.2009.05029.x. [DOI] [PubMed] [Google Scholar]
- 12.Whittemore R, D’Eramo Melkus G, Grey M. Metabolic control, self-management and psychosocial adjustment in women with type 2 diabetes. J Clin Nurs. 2005;14:195–203. doi: 10.1111/j.1365-2702.2004.00937.x. [DOI] [PubMed] [Google Scholar]
- 13.Whittemore R, Melkus GD, Sullivan A, et al. A nurse-coaching intervention for women with type 2 diabetes. Diabetes Educ. 2004;30:795–804. doi: 10.1177/014572170403000515. [DOI] [PubMed] [Google Scholar]
- 14.Cinar AB, Oktay I, Schou L. Relationship between oral health, diabetes management and sleep apnea. Clin Oral Invest. 2013;17:967–974. doi: 10.1007/s00784-012-0760-y. [DOI] [PubMed] [Google Scholar]
- 15.Cinar AB. In: Periodontal Diseases – A Clinician’s Guide [online book] Manakil J, editor. InTECH; 2012. One for All™: how to tackle with diabetes, obesity and periodontal diseases. Available from: http://www.intechopen.com/books/show/title/periodontal-diseases-a-clinician-s-guide. [Accessed April 29 2013] [Google Scholar]
- 16.O’Connor J, Lages A. Element; London: 2004. Coaching with NLP: How to be a Master Coach. [Google Scholar]
- 17.Tosey P, Mathison J. Centre for Management Learning and Development, School of Management, University of Surrey; UK: 2006. Introducing Neuro-Linguistic Programming Surrey. Available from: http://www.som.surrey.ac.uk/NLP/Resources/IntroducingNLP.pdf. [Accessed April 29 2013] [Google Scholar]
- 18.Bandura A. W.H. Freeman and Company; New York: 1997. Self-Efficacy: The Exercise of Control; p. 604. [Google Scholar]
- 19.Parameter on chronic periodontitis with slight to moderate loss of periodontal support. J Periodontol. 2000;71(5 Supplement):353–355. doi: 10.1902/jop.2000.71.5-S.853. [DOI] [PubMed] [Google Scholar]
- 20.Cinar AB, Kosku N, Sandalli N, et al. Self-efficacy perspective on oral health among Turkish preadolescents. Oral Health Prev Dent. 2005;4:209–215. [PubMed] [Google Scholar]
- 21.Cinar AB, Oktay I, Schou L. Self-efficacy perspective on oral health behaviour and diabetes management. Oral Health Prev Dent. 2012;10:379–387. [PubMed] [Google Scholar]
- 22.Cinar AB, Tseveenjav B, Murtomaa H. Oral health-related self-efficacy beliefs and toothbrushing: Finnish and Turkish pre-adolescents’ and their mothers’ responses. Oral Health Prev Dent. 2009;7:173–181. [PubMed] [Google Scholar]
- 23.International Diabetes Federation Clinical Guidelines Task Force [2005] Global Guideline for Type 2 Diabetes; Chapter 6: Glucose control levels and Chapter 12: Cardiovascular risk protection. International Diabetes Federation 2005
- 24.WHO. BMI classification. Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html:bmi [Accessed August 24 2013]
- 25.Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale. An evaluation of its clinical utility. Diabetes Care. 1997;20:760–766. doi: 10.2337/diacare.20.5.760. [DOI] [PubMed] [Google Scholar]
- 26.Welch G, Weinger K, Anderson B, et al. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med. 2003;20:69–72. doi: 10.1046/j.1464-5491.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 27.Snoek FJ, Pouwer F, Welch GW, et al. Diabetes-related emotional distress in Dutch and US diabetic patients: cross-cultural validity of the problem areas in diabetes scale. Diabetes Care. 2000;23:1305–1309. doi: 10.2337/diacare.23.9.1305. [DOI] [PubMed] [Google Scholar]
- 28.WHO. WHO Quality of Life-BREF (WHOQOL-BREF). Available from: http://www.who.int/substance_abuse/research_tools/whoqolbref/en/index.html. [Accessed August 24 2013]; 2004
- 29.Wolever RQ, Dreusicke M, Fikkan J, et al. Integrative health coaching for patients with type 2 diabetes: a randomized clinical trial. Diabetes Educ. 2010;36:629–639. doi: 10.1177/0145721710371523. [DOI] [PubMed] [Google Scholar]
- 30.Miller R, Rollnick S. Guilford Press; New York: 2002. Motivational Interviewing – Preparing People for Change; p. 428. [Google Scholar]
- 31.Chen SM, Creedy D, Lin HS, et al. Effects of motivational interviewing intervention on self-management, psychological and glycemic outcomes in type 2 diabetes: a randomized controlled trial. Int J Nurs Stud. 2012;49:637–644. doi: 10.1016/j.ijnurstu.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 32.West DS, DiLillo V, Bursac Z, et al. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30:1081–1087. doi: 10.2337/dc06-1966. [DOI] [PubMed] [Google Scholar]
- 33.Welch G, Zagarins SE, Feinberg RG, et al. Motivational interviewing delivered by diabetes educators: does it improve blood glucose control among poorly controlled type 2 diabetes patients? Diabetes Res Clin Pract. 2011;91:54–60. doi: 10.1016/j.diabres.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renz A, Newton T, Robinson PG et al. Psychological interventions to improve adherence to oral hygiene instructions in adults with periodontal diseases. Cochrane Database Syst Rev 2007; 2. Art. No.:CD005097, doi:10.1002/14651858.pub2. [DOI] [PubMed]
- 35.Yevlahova D, Satur J. Models for individual oral health promotion and their effectiveness: a systematic review. Aust Dent J. 2009;54:190–197. doi: 10.1111/j.1834-7819.2009.01118.x. [DOI] [PubMed] [Google Scholar]
- 36.Jönsson B, Ohrn K, Lindberg P, et al. Evaluation of an individually tailored oral health educational programme on periodontal health. J Clin Periodontol. 2010;37:912–919. doi: 10.1111/j.1600-051X.2010.01590.x. [DOI] [PubMed] [Google Scholar]
- 37.Kakudate N, Morita M, Sugai M, et al. Systematic cognitive behavioral approach for oral hygiene instruction: a short-term study. Patient Educ Couns. 2009;74:191–196. doi: 10.1016/j.pec.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Almomani F, Williams K, Catley D, et al. Effects of an oral health promotion program in people with mental illness. J Dent Res. 2009;88:648–652. doi: 10.1177/0022034509338156. [DOI] [PubMed] [Google Scholar]
- 39.Philippott P, Lenoir N, D’Hoore W, et al. Improving patients’ compliance with the treatment of periodontitis: a controlled study of behavioural intervention. J Clin Periodontol. 2005;32:653–658. doi: 10.1111/j.1600-051X.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- 40.Bandura A. WH Freeman and Company; New York, USA: 1997. Self-efficacy: The Exercise of Control; pp. 79–160. 279-313. [Google Scholar]
- 41.Krichbaum K, Aarestad V, Buethe M. Exploring the connection between self-efficacy and effective diabetes self-management. Diabetes Educ. 2003;29:653–662. doi: 10.1177/014572170302900411. [DOI] [PubMed] [Google Scholar]
- 42.Norris SL, Engelgau MM, Narayan KMV. Effectiveness of self-management training in type 2 diabetes. Diabetes Care. 2001;24:561–587. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 43.Bennett HD, Coleman EA, Parry C, et al. Health coaching for patients. Fam Pract Manag. 2010;17:24–2943. [PubMed] [Google Scholar]
- 44.Hibbard JH, Mahoney ER, Rock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42:1143–1463. doi: 10.1111/j.1475-6773.2006.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osterberg L, Blaschke T. Adherence to medication. NEJM. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 46.Østbye T, Yarnall KSH, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3:209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yarnall KSH, Pollak KI, Østbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willard-Grace R, DeVore D, Chen EH, et al. Thom DHBMC Fam Pract. The effectiveness of medical assistant health coaching for low-income patients with uncontrolled diabetes, hypertension, and hyperlipidemia: protocol for a randomized controlled trial and baseline characteristics of the study population. BMC Fam Pract. 2013;23:14–27. doi: 10.1186/1471-2296-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diabetes: the cost of diabetes. Available from: http://www.who.int/mediacentre/factsheets/fs236/en/. [Accessed June 5 2013]; 2013
- 50.What is the burden of oral disease?. Available from: http://www.who.int/oral_health/disease_burden/global/en/. [Accessed June 5 2013]; 2013