Abstract
Background
Use of muscle relaxants is rapidly increasing in the USA. Little is understood about the role of drug interactions in the known association between muscle relaxants and unintentional traumatic injury, a clinically important endpoint causing substantial morbidity, disability, and death.
Objective
We examined potential associations between concomitant drugs (i.e., precipitants) taken with muscle relaxants (affected drugs, i.e., objects) and hospital presentation for unintentional traumatic injury.
Methods
In a series of self-controlled case series studies, we screened to identify drug interaction signals for muscle relaxant + precipitant pairs and unintentional traumatic injury. We used Optum’s de-identified Clinformatics® Data Mart Database, 2000–2019. We included new users of a muscle relaxant, aged 16–90 years, who were dispensed at least one precipitant drug and experienced an unintentional traumatic injury during the observation period. We classified each observation day as precipitant exposed or precipitant unexposed. The outcome was an emergency department or inpatient discharge diagnosis for unintentional traumatic injury. We used conditional Poisson regression to estimate rate ratios adjusting for time-varying confounders and then accounted for multiple estimation via semi-Bayes shrinkage.
Results
We identified 74,657 people who initiated muscle relaxants and experienced an unintentional traumatic injury, in whom we studied concomitant use of 2543 muscle relaxant + precipitant pairs. After adjusting for time-varying confounders, 16 (0.6%) pairs were statistically significantly and positively associated with injury, and therefore deemed signals of a potential drug interaction. Among signals, semi-Bayes shrunk, confounder-adjusted rate ratios ranged from 1.29 (95% confidence interval 1.04–1.62) for baclofen + sertraline to 2.28 (95% confidence interval 1.14–4.55) for methocarbamol + lamotrigine.
Conclusions
Using real-world data, we identified several new signals of potential muscle relaxant drug interactions associated with unintentional traumatic injury. Only one among 16 signals is currently reported in a major drug interaction knowledge base. Future studies should seek to confirm or refute these signals.
1. Introduction
Muscle relaxants are commonly used in the USA. Rates of muscle relaxant prescribing (and therapy continuations) in ambulatory care settings increased from 15 to 30 per 1000 office visits between 2005 and 2016 [1]. Muscle relaxants have been linked to adverse drug events (ADEs) such as unintentional traumatic injury [2, 3], a substantial cause of morbidity, disability, and death [4]. Adverse drug events, particularly dizziness, drowsiness, and anticholinergic effects, are consistently reported with their use [5, 6] and increase patients’ risk of injuries including falls and motor vehicle crash [2, 7–10]. The risk can be exacerbated in the presence of concomitant drugs that may alter their pharmacokinetic or pharmacodynamic properties [11]. Yet, little is known about which drugs co-administered with muscle relaxants have the potential to increase the risk of injuries in real-world settings.
Few studies have examined associations between drugs taken concomitantly with muscle relaxants and risk of injury. An analysis of US Department of Transportation data found that the combined use of muscle relaxants, narcotics, and anti-anxiety drugs resulted in a potentiating effect causing extreme disorientation [12]. The US Food and Drug Administration and Centers for Disease Control and Prevention warned about concurrent use of central nervous system (CNS)-depressing drugs, including muscle relaxants, with opioids resulting in notably slowed or difficult breathing, altered mental states affecting driving ability, and death [13, 14]. Muscle relaxant users often have multiple health conditions [15] which can increase the possibility of drug–drug interactions (DDIs) with hundreds of other commonly used drugs. Identifying potential DDI signals via screening studies can provide a framework for future etiologic research and interventional approaches to minimize patient harm. Given the limited real-world evidence concerning muscle relaxant DDIs, we examined potential associations between concomitant drugs (i.e., affecting drugs, i.e., precipitants) taken with muscle relaxants (affected drugs, i.e., objects) and hospital presentation for unintentional traumatic injury.
2. Methods
2.1. Data Source
We used Optum’s de-identified Clinformatics® Data Mart Database data from 1 May, 2000 through 30 June, 2019. Optum includes longitudinal enrollment and healthcare billing data from > 71 million commercially insured and Medicare Advantage beneficiaries of the largest US-based private health insurer by market share. Data domains include patient demographics, enrollment, outpatient claims, inpatient claims, ambulatory prescription drug dispensings, and laboratory results (the latter for a subset of beneficiaries).
2.2. Study Design
We conducted semi-automated, high-throughput, pharmacoepidemiologic screening analyses using a series of self-controlled case series (SCCS) studies to examine potential associations between concomitant drugs (i.e., precipitants) taken with muscle relaxants (i.e., objects) and hospital presentation for unintentional traumatic injury. We selected a self-controlled design because it: (a) controls for both measured and unmeasured static confounders, as comparisons are made within an individual; (b) is computationally efficient, as it only includes people who experience the outcome of interest; (c) permits adjustment for time-varying confounders; and (d) has been used extensively for DDI screening studies. [16–18]
2.3. Observation Period
We created observation periods comprising new users of the object drugs. Eligible people: (a) received one or more dispensed muscle relaxant prescription (i.e., object drugs); (b) were continuously enrolled in medical and pharmacy benefits during a 183-day period preceding each first muscle relaxant dispensing (i.e., baseline period); (c) were new users of a muscle relaxant defined by the absence of prior use during the baseline period; (d) were aged 16–90 years; (e) were dispensed one or more overlapping precipitant drug during their observation period; and (f) experienced an unintentional traumatic injury (people without this outcome of interest do not contribute to an SCCS analysis) [Fig. S1 of the Electronic Supplementary Material [ESM]). We excluded adults aged older than 90 years because age is capped at 90 years in the Optum database. We excluded children aged younger than 16 years because (a) the youngest legal driving age in the USA is 16 years; (b) growth and development cause changes in pharmacokinetic processes including absorption, distribution, metabolism, and excretion [19]; (c) differences have been found between adults and the pediatric population in phase I (involving structural modification of the drug molecule) and phase II metabolic enzymes (consisting of drug molecule conjugation with another) [20]; and (d) development can alter response and action of a drug [21].
The date study subjects met inclusion criteria was assigned as the cohort entry date. Included people were allowed to contribute more than one treatment episode as long as they re-met criterion ‘b’ in Fig. S2 of the ESM. To minimize the potential for reverse causality (i.e., injury outcome leading to a muscle relaxant dispensing), we considered the drug dispensing date as the drug fill date + 1 day.
The observation period began when inclusion criteria were met (Fig. 1) and ended at the earliest of: (a) muscle relaxant discontinuation (i.e., defined as the end date based on days’ supply); (b) switch between muscle relaxants; (c) health plan disenrollment (permitting a 45-day maximum enrollment gap); and (d) end of study period (i.e., 30 June, 2019). Unlike with a cohort study, an SCCS design does not censor upon occurrence of an outcome. Within each observation period, we identified candidate precipitants. We classified the observation period into focal and referent windows. Focal windows (i.e., precipitant-exposed periods) were observation days defined by concomitant use of the muscle relaxant and candidate precipitant. Referent windows (i.e., precipitant-unexposed periods) were observation days defined by use of the muscle relaxant alone. Thus, if a person did not refill a subsequent prescription for a candidate precipitant, but remained exposed to the muscle relaxant, such days were classified as referent days. If a person did not refill a subsequent prescription for the muscle relaxant, observation time was censored upon depletion of the days’ supply.
Fig. 1.
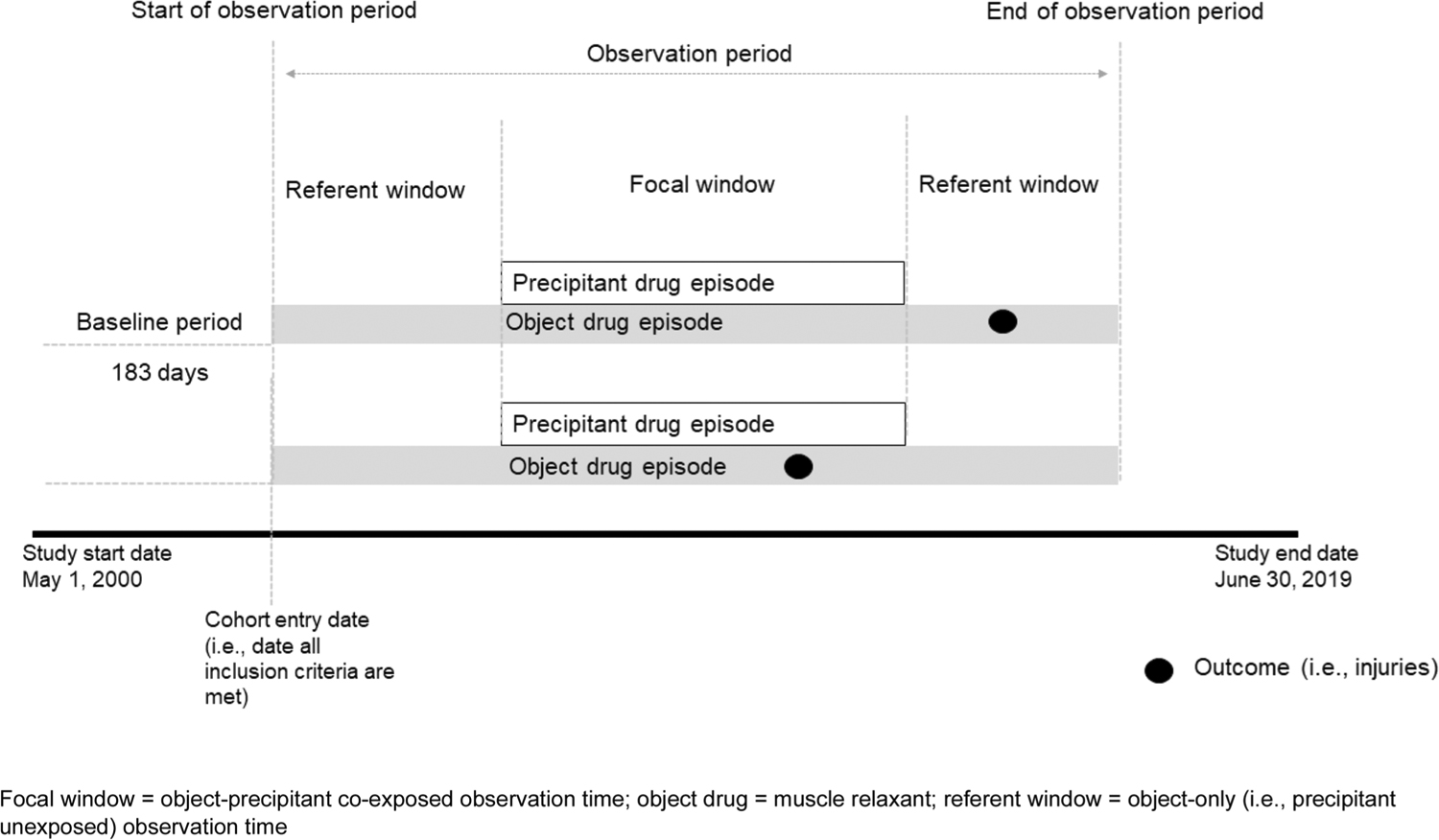
Study design
2.4. Exposure Definition
2.4.1. Object Drugs (i.e., Muscle Relaxants)
We used pharmacy claims to define new use muscle relaxant episodes (i.e., devoid of prior use during a 183-day lookback period). Muscle relaxants included baclofen, carisoprodol, chlorzoxazone, cyclobenzaprine, dantrolene, metaxalone, methocarbamol, orphenadrine, and tizanidine. We considered people continuously exposed if they refilled prescriptions without exceeding a grace period between consecutive refills. We calculated the length of the grace period as days’ supply × 0.20.
2.4.2. Precipitant Drugs (i.e., Co-Administered Drugs)
Within the cohorts of muscle relaxant new users experiencing injury, we identified exposure to precipitants defined as orally administered drugs frequently dispensed with muscle relaxants. The definition for frequent was met if at least five patients were dispensed a given precipitant during the observation period. We considered people exposed to the precipitant if they continued to refill their prescriptions without a gap between contiguous refills.
2.5. Outcome Definition
The primary outcome of interest was unintentional traumatic injury defined by International Classification of Diseases codes present in any position on emergency department claims or principal position on inpatient claims (International Classification of Diseases, Ninth Revision codes: fracture, 800–829, dislocation, 830–839, sprain/strain, 840–848, intracranial injury, 850–854, internal injury of thorax, abdomen or pelvis, 860–869, open wound, 870–897, injury to blood vessels, 900–904, crushing injury, 925–929, injury to nerves or spinal cord, 950–957, certain traumatic complications and unspecified injuries, 958–959). International Classification of Diseases, Tenth Revision codes included: injuries to specific body parts (S00–S99 with a seventh character modifier of A, B, or C only; excluding superficial injuries indicated by S00, S10, S20, S30, S40, S50, S60, S70, S80, or S90), unspecified multiple injuries (T07 with a seventh character modifier of A only), injury of unspecified body region (T14 with a seventh character modifier of A only), and traumatic compartment syndrome (T79.A1–T79. A9 with a seventh character modifier of A only). A detailed description of outcomes definitions is listed in Table S1 of the ESM. This definition was adapted from that used by the American College of Surgeons’ National Trauma Data Standard. Our definition excluded late effects of poisonings, toxic effects, and other external causes, superficial injury, contusion with intact skin surface, effects of a foreign body entering through an orifice, and burns [22]. Secondary outcomes included typical hip fracture and motor vehicle crash while the person was driving. We defined typical hip fracture by International Classification of Diseases codes present in the principal position on inpatient claims, excluding pathologic and atypical hip fractures. We defined motor vehicle crash as unintentional traumatic injury (see primary outcome definition earlier) plus an external cause of injury code for unintentional traffic or non-traffic accident. We excluded crashes of a self-inflicted, assault, or undetermined manner (consistent with the Centers for Disease Control and Prevention’s injury mortality framework) [Table S1 of the ESM].
2.6. Time-Varying Covariates
We included the following time-varying covariates (assessed each observation day): (a) muscle relaxant average daily dose defined by most recent prescription; (b) ever prior injury diagnosis defined by inpatient or outpatient claims (any position); and (c) follow-up time, defined as month since cohort entry.
2.7. Statistical Analysis
We summarized characteristics of people under study by the muscle relaxant object drug of interest. Within each cohort, we compared outcome occurrences during focal vs referent windows. We used conditional Poisson regression models (xtpoisson with fe option, Stata version 16) to estimate rate ratios (RRs) adjusting for time-varying covariates (Sect. 2.6), then accounted for multiple estimation via semi-Bayes shrinkage [23, 24]. The primary dependent variable was the outcome (e.g., unintentional traumatic injury) and the primary independent variable was exposure status (precipitant exposed vs precipitant unexposed). We deemed a potential DDI as a signal if it had a statistically significant increase in the rate of unintentional traumatic injury.
We conducted a prespecified sensitivity analysis dropping the use of semi-Bayes shrinkage. Further, we compared potential DDIs signals identified in the current study to those documented in two drug interaction knowledge bases: Micromedex (IBM Watson Health, Cambridge, MA, USA) and Facts & Comparisons eAnswers (Wolters Kluwer Clinical Drug Information, Inc., Alphen aan den Rijn, The Netherlands). All analyses were conducted using SAS 9.4 and STATA version 16.
2.8. Institutional Review Board Approval and Role of Funding Source
The University of Pennsylvania’s Institutional Review Board approved this research as protocol #831486. The US National Institutes of Health had no input on the conduct or interpretation of this research.
3. Results
3.1. Characteristics of the Study Population
We identified 74,657 people (range: 42 for dantrolene to 35,940 for cyclobenzaprine) who initiated muscle relaxants, experienced an unintentional traumatic injury, and met study inclusion criteria (Table 1), in sum contributing ≥ 5 million person-days. People included in the cohorts had a median age that ranged between 47 and 65 years and were mostly female, Caucasian, and resided in the South Atlantic USA. The most common injuries were dislocation/sprain/strain, certain traumatic complications and unspecified injuries, and fractures, although the distribution differed by category of muscle relaxant (i.e., antispastic vs antispasmodic). The median observation time ranged from 13 days for metaxalone and orphenadrine to 104 days for dantrolene. Forty (dantrolene) to 88% (metaxalone) of people who initiated a muscle relaxant and experienced an unintentional traumatic injury had only one prescription dispensing for the muscle relaxant. For the secondary outcomes, cohorts of new users of muscle relaxants with a typical hip fracture were relatively small and ranged from two for dantrolene to 542 for cyclobenzaprine (Table S2 of the ESM). Similarly, cohorts of new users of muscle relaxants with motor vehicle crash ranged from one for dantrolene to 336 for cyclobenzaprine.
Table 1.
Characteristics of cohorts of new users of muscle relaxants with unintentional traumatic injuries, by object drug of interest
| Baclofen n = 7981 | Carisoprodol n = 5726 | Chlorzoxazone n = 629 | Cyclobenzaprine n = 35,940 | Dantrolene n = 42 | Metaxalone n = 4626 | Methocarbamol n = 6054 | Orphenadrine n = 1492 | Tizanidine n = 12,167 | |
|---|---|---|---|---|---|---|---|---|---|
| Days of observation period, median (Q1–Q3) per person | 37.0 (28.0–123.0) | 37.0 (13.0–70.0) | 19.0 (13.0–37.0) | 19.0 (13.0–37.0) | 104.0 (37.0–256.0) | 13.0 (10.0–37.0) | 19.0 (10.0–37.0) | 13.0 (10.0–35.0) | 37.0 (23.0–109.0) |
| Days of observation, sum | 994,372 | 613,874 | 31,119 | 1,549,774 | 9731 | 128,846 | 266,560 | 51,624 | 1,385,662 |
| Demographics | |||||||||
| Age in years, median (Q1–Q3) | 65.0 (53.4–74.5) | 49.5 (39.7–60.1) | 51.0 (39.9–62.9) | 51.8 (39.8–64.8) | 55.1 (43.1–66.6) | 47.0 (37.2–57.9) | 51.7 (39.6–63.8) | 47.9 (36.7–60.5) | 61.6 (50.4–71.9) |
| Female, % | 66.4 | 59.4 | 61.4 | 59.9 | 50.0 | 59.4 | 59.3 | 56.2 | 67.8 |
| Race, % | |||||||||
| Caucasian | 64.2 | 67.5 | 66.8 | 68.1 | 64.3 | 69.3 | 70.2 | 67.8 | 65.5 |
| African American | 12.6 | 9.5 | 11.6 | 10.2 | 11.9 | 6.7 | 9.7 | 9.0 | 14.6 |
| Hispanic | 10.5 | 7.9 | 6.8 | 8.3 | 9.5 | 6.8 | 7.9 | 7.1 | 7.1 |
| Asian | 1.2 | 1.7 | 1.6 | 1.8 | 0.0 | 1.4 | 1.8 | 1.1 | 1.1 |
| Unknown | 11.5 | 13.4 | 13.2 | 11.6 | 14.3 | 15.8 | 10.4 | 15.1 | 11.7 |
| Area of residence, by geographic division, % | |||||||||
| New England | 3.1 | 2.8 | 1.6 | 4.1 | 2.4 | 3.1 | 4.1 | 2.7 | 2.1 |
| Middle Atlantic | 5.1 | 3.1 | 4.3 | 5.4 | 4.8 | 6.7 | 3.6 | 3.4 | 3.8 |
| East North Central | 12.2 | 8.3 | 24.0 | 17.8 | 9.5 | 18.2 | 16.2 | 26.8 | 15.4 |
| West North Central | 8.0 | 5.5 | 10.0 | 10.6 | 26.2 | 8.2 | 8.7 | 12.7 | 8.3 |
| South Atlantic | 32.6 | 26.9 | 24.0 | 25.8 | 33.3 | 29.0 | 23.1 | 26.6 | 32.8 |
| East South Central | 5.8 | 5.8 | 7.2 | 3.8 | 2.4 | 4.7 | 6.9 | 4.5 | 8.2 |
| West South Central | 12.5 | 18.1 | 17.5 | 13.8 | 7.1 | 15.8 | 14.5 | 11.7 | 15.7 |
| Mountain | 10.2 | 13.5 | 7.2 | 9.6 | 7.1 | 8.5 | 10.0 | 9.3 | 8.4 |
| Pacific | 10.2 | 15.3 | 4.1 | 8.7 | 7.1 | 5.7 | 12.5 | 2.2 | 5.2 |
| Unknown | 0.3 | 0.7 | 0.2 | 0.4 | 0.0 | 0.1 | 0.4 | 0.1 | 0.2 |
| Unintentional traumatic injuries, sum | 8978 | 6133 | 693 | 38,856 | 46 | 4772 | 6545 | 1548 | 13,738 |
| Type of injury, % | |||||||||
| Fracture | 28.7 | 18.7 | 14.0 | 16.7 | 23.9 | 13.4 | 19.1 | 15.8 | 25.3 |
| Dislocation/sprain/strain | 36.9 | 64.9 | 65.5 | 68.7 | 41.3 | 76.2 | 67.3 | 72.9 | 44.7 |
| Intracranial injury | 4.4 | 3.1 | 2.5 | 2.3 | 8.7 | 2.3 | 2.7 | 2.5 | 3.6 |
| Internal injury of thorax, abdomen, or pelvis | 2.0 | 1.4 | 1.3 | 1.3 | 0.0 | 1.1 | 1.6 | 1.6 | 1.4 |
| Open wound | 14.5 | 9.5 | 10.4 | 6.9 | 15.2 | 6.1 | 7.1 | 6.6 | 12.9 |
| Injury to blood vessels | 0.2 | 0.3 | 0.4 | 0.1 | 0.0 | 0.0 | 0.2 | 0.1 | 0.2 |
| Crushing injury | 0.2 | 0.2 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 |
| Injury to nerves or spinal cord | 0.9 | 1.1 | 1.0 | 0.8 | 4.3 | 0.9 | 1.1 | 1.4 | 0.8 |
| Certain traumatic complications, unspecified injuries, and other specified injuries | 46.7 | 24.2 | 24.1 | 21.0 | 41.3 | 13.3 | 20.7 | 17.5 | 41.8 |
Q quartile
3.2. Primary Outcome: Potential DDI Signals for Traumatic Injury
Table S3 of the ESM summarizes the prevalence of time-varying covariates during observation periods. Summaries of unadjusted and adjusted signals, with and without semi-Bayes shrinkage, for candidate-interacting precipitants and muscle relaxants for an unintentional traumatic injury are listed in Table S4 of the ESM and Fig. 2. Confounder-adjusted analyses after semi-Bayes shrinkage are summarized in Table 2. In short, we identified 16 DDI signals: five for baclofen (among 396 precipitants examined); two for carisoprodol (among 357); two for chlorzoxazone (among 128); four for methocarbamol (among 323); and three for tizanidine (among 428). See further details in Sect. 4.3. We identified no DDI signals for cyclobenzaprine (among 465), dantrolene (among 13), metaxalone (among 268), or orphenadrine (among 165). Table S4 of the ESM summarizes confounder-adjusted RRs after semi-Bayes shrinkage for muscle relaxants with opioids, including statistically significant and non-significant interactions.
Fig. 2.
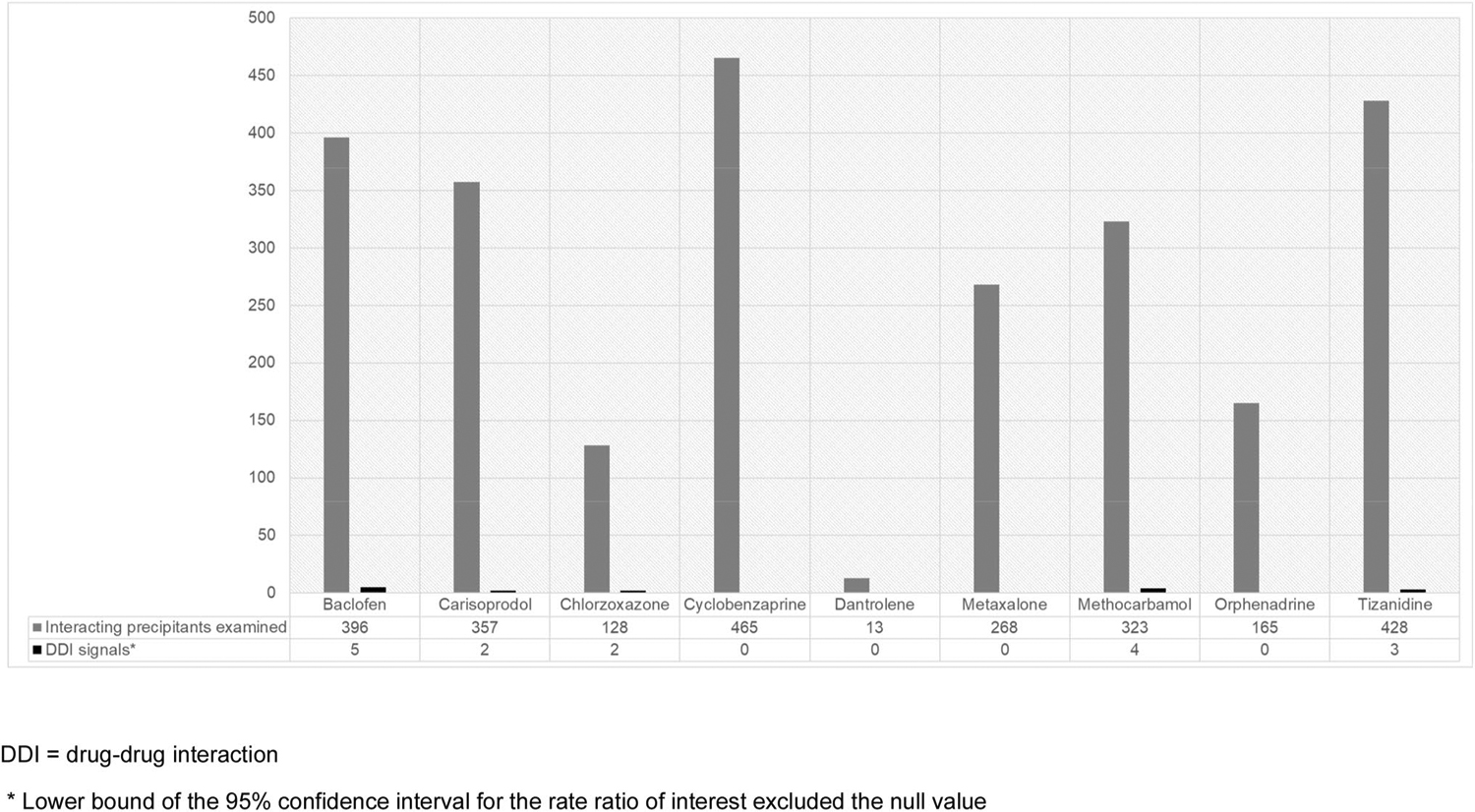
Counts of interacting precipitants examined and confounder-adjusted drug–drug interaction signals (after semi-Bayes shrinkage) that were associated with an increased rate of unintentional traumatic injuries, by muscle relaxant object drug
Table 2.
Confounder-adjusted RRs after semi-Bayes shrinkage for muscle relaxant drug interaction signals, given statistically significantly increased rates of unintentional traumatic injury, by therapeutic category of the precipitant drug
| Object drug | Precipitant drug | Precipitant drug therapeutic categorya | Adjusted RR | 95% CI | |
|---|---|---|---|---|---|
| Baclofen | Morphineb | CNS | 1.46 | 1.13 | 1.87 |
| Sertraline | CNS | 1.29 | 1.04 | 1.62 | |
| Atropine | GI | 1.70 | 1.05 | 2.74 | |
| Diphenoxylate | GI | 1.79 | 1.10 | 2.91 | |
| Sitagliptin | Endocrine and metabolic | 1.67 | 1.07 | 2.60 | |
| Carisoprodol | Varenicline | CNS | 2.11 | 1.18 | 3.75 |
| Lansoprazole | GI | 1.72 | 1.02 | 2.90 | |
| Chlorzoxazone | Cephalexin | Anti-infective | 2.03 | 1.00 | 4.13 |
| Methylprednisolone | Endocrine and metabolic | 1.76 | 1.02 | 3.06 | |
| Methocarbamol | Lamotrigine | CNS | 2.28 | 1.14 | 4.55 |
| Ondansetron | CNS | 1.49 | 1.08 | 2.05 | |
| Levothyroxine | Endocrine and metabolic | 1.43 | 1.05 | 1.94 | |
| Dexlansoprazole | GI | 2.03 | 1.01 | 4.10 | |
| Tizanidine | Amiodarone | CV | 1.65 | 1.04 | 2.62 |
| Digoxin | CV | 1.91 | 1.13 | 3.23 | |
| Oxybutynin | Renal and genitourinary | 1.51 | 1.12 | 2.02 |
CI confidence interval, CNS central nervous system, CV cardiovascular, GI gastrointestinal, RR rate ratio
RRs > 2.00 were bolded to highlight N = 4 potential signals that may warrant particular attention in future etiologic work
Per Facts and Comparisons eAnswers (Wolters Kluwer N.V., Alphen aan den Rijn, The Netherlands)
Drug interaction with impact on object documented in Micromedex
3.3. Primary Outcome: Detail on the Signaling Object + Precipitant Pairs
Baclofen: Interacting precipitants included morphine (RR: 1.46; 95% confidence interval [CI] 1.13–1.87), sertraline (RR 1.29; 95% CI 1.04–1.62), atropine (RR 1.70; 95% CI 1.05–2.74), diphenoxylate (RR 1.79; 95% CI 1.10–2.91), and sitagliptin (RR 1.67; 95% CI 1.07–2.60). Carisoprodol: Interacting precipitants included varenicline (RR 2.11; 95% CI 1.18–3.75) and lansoprazole (RR 1.72; 95% CI 1.02–2.90). Chlorzoxazone: Interacting precipitants included cephalexin (RR 2.03, 95% CI 1.00–4.13) and methylprednisolone (RR 1.76; 95% CI 1.02–3.06). Methocarbamol: Interacting precipitants included lamotrigine (RR 2.28; 95% CI 1.14–4.55) ondansetron (RR 1.49; 95% CI 1.08–2.05), levothyroxine (RR 1.43, 95% CI 1.05–1.94), and dexlansoprazole (RR 2.03; 95% CI 1.01–4.10). Tizanidine: Interacting precipitants included amiodarone (RR 1.65; 95% CI 1.04–2.62), digoxin (RR 1.91; 95% CI 1.13–3.23), and oxybutynin (RR 1.51; 95% CI 1.12–2.02). Only one of these 16 signals (6.3%) is currently reported in the two examined major drug interaction knowledge bases.
3.4. Secondary Outcomes: Potential DDI Signals for Typical Hip Fracture and Motor Vehicle Crash While the Subject was Driving
Table S3 of the ESM summarizes time-varying covariates included in the secondary analyses. Confounder-adjusted analyses, after semi-Bayes shrinkage, for typical hip fracture and motor vehicle crash yield no DDI signals (Table S5 of the ESM).
4. Discussion
In this population-based screening study, we identified 16 potential DDI signals with muscle relaxants that were associated with an increased rate of unintentional traumatic injuries. This represents 0.6% of the 2543 muscle relaxant-precipitant pairs examined. These signals included precipitants in the following therapeutic classes: CNS (31%), gastrointestinal (25%), endocrine and metabolic (19%), cardiovascular (13%), anti-infective (6%), and renal and genitourinary (6%). The vast majority of these signals (94%) were not documented in either of two major drug interaction knowledge bases. Our findings utilizing semi-automated high-throughput screening methods are not intended to provide strong evidence for causation, but rather to generate hypotheses to inform future etiologic studies.
Unintentional traumatic injury is a major source of emergency department visits, hospitalization, and work loss [25]. Over 170,000 Americans die each year from unintentional traumatic injury [25]. Despite the high burden, few studies have examined potential DDIs of muscle relaxants. The US Department of Transportation reported that the combined use of muscle relaxants, narcotics, and anti-anxiety drugs resulted in a potentiating effect causing extreme disorientation [12]. The US Food and Drug Administration and Centers for Disease Control and Prevention warned about concurrent use of opioids with muscle relaxants, which depress the CNS [13]. Yet, studies of DDIs involving muscle relaxants that may increase injury rates are scarce. This lack of evidence is concerning as polypharmacy is common among users of muscle relaxants [15]. Further, studies report that muscle relaxant use has increased in recent years [1, 26]. From 2005 to 2018, there was an increase in the use of baclofen, tizanidine, and methocarbamol, but a decline in carisoprodol and metaxalone use. Cyclobenzaprine accounted for ~ 50% of muscle relaxant prescriptions.
Among our 16 potential DDI signals, only one is currently documented in Micromedex’s interaction knowledge base (i.e., concern for CNS and respiratory depression with baclofen + morphine). We found that concomitant use was associated with an increased rate of injuries (adjusted RR 1.5; 1.13–1.87); the magnitude of this association is broadly consistent with our recent findings for injury among opioid users with vs without co-exposure to a muscle relaxant (adjusted RRs, range 1.8–2.5) [27]. Drug–drug interactions between muscle relaxants and opioids are biologically plausible given additive pharmacodynamic (e.g., CNS effects such as disorientation, sedation, and altered mental states) and/or pharmacokinetic effects (e.g., alteration of cytochrome P450 [CYP] 2D6 and CYP3A4 hepatic enzymes). Opioid metabolism commonly involves these hepatic pathways [28]. Our inability to replicate findings for other muscle relaxant-opioid pairs (e.g., methocarbamolcodeine, orphenadrine-oxycodone) is likely due to limited statistical precision.
We identified several new signals for injury with muscle relaxants + certain CNS-active precipitant drugs, including sertraline (antidepressant), varenicline (smoking cessation aid), lamotrigine (anticonvulsant), and ondansetron (antiemetic). Use of the selective serotonin reuptake inhibitor (SSRI) antidepressant sertraline with baclofen was associated with a 1.3-fold (1.04–1.62) increase in the rate of injury, a finding supported by a plausible additive or synergistic CNS depression effect. A recent retrospective cohort study of Medicare patients reported a higher hazard ratio with concurrent use of muscle relaxants + SSRIs vs SSRI use alone (hazard ratio = 2.9, 0.93–8.95 vs. 2.3, 2.2–2.4, respectively) [29]. Sertraline is an SSRI inhibitor that can cause fatigue and drowsiness and has been linked to an increased risk of fractures in older adults [30]. Selective serotonin reuptake inhibitors as a class can cause drowsiness, impaired level of alertness and neuromuscular function, and an increased risk of recurrent falls [31]. Potential DDI signals between antidepressants and muscle relaxants were noted in our recently published work (adjusted RR range, 1.1–1.9) [18]. Baclofen is a gamma-aminobutyric acid (GABA) agonist and may produce sedation due to inhibition of dopaminergic systems. A study reported a higher risk of hospitalization with a fall, fracture, and hypotension among users of baclofen (vs non-users). [32]
The increased injury rate with carisoprodol–varenicline (2.11, 1.18–3.75) can partially be explained by rare, although serious, psychiatric side effects of varenicline including hallucinations and altered alertness [33]. In 2008, post-marketing reports of serious psychiatric adverse events including traffic accidents and suicide in varenicline-treated patients led to a boxed warning on a drug label [34, 35], although emerging data later resulted in the warning’s removal [36]. Carisoprodol is a centrally acting muscle relaxant that is metabolized mainly by CYP2C19 to its active metabolite meprobamate [37]. Studies suggested that the effect of carisoprodol is mainly attributed to meprobamate, which modulates GABA receptors and probably contributes to its abuse potential [38]. Similarly, varenicline stimulates the release of GABA in the basal forebrain and hippocampus [39]. The interaction of the two drugs can be potentially explained by the binding of GABA to many neurons leading to a sedative effect. We found an increased injury rate with methocarbamol-lamotrigine (2.28, 1.14–4.55) and methocarbamol-ondansetron (1.49, 1.08–2.05). The mechanisms of methocarbamol and lamotrigine are not fully understood. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults suggested avoiding the use of methocarbamol due to anticholinergic adverse effects, sedation, and an increased risk of fractures. Ondansetron can also cause drowsiness and sedation through its selective antagonist effect on 5-HT3 serotonin receptors [40].
We identified several other muscle relaxant DDIs with precipitants in the following therapeutic categories: gastrointestinal, cardiovascular, endocrine/metabolic, anti-infective, and renal/genitourinary. These potential signals are biologically plausible. For example, the increase in injury rates by 1.7-fold with atropine and 1.8-fold with diphenoxylate can be explained by their CNS-depressant effects and anticholinergic properties. The increase in injury rate with carisoprodol–lansoprazole (1.72, 1.02–2.90) and methocarbamol-dexlansoprazole (2.03, 1.01–4.10) can be explained by the direct neuromuscular blocking action of proton pump inhibitors as reported in an animal-based study [41]. We also found signals of potential DDIs between tizanidine with amiodarone (1.65, 1.04–2.62) and digoxin (1.91, 1.13–3.23). Due to hepatic metabolism of tizanidine via CYP1A2, concomitant administration of inhibitors of cytochrome P450 such as anti-arrhythmic drugs (e.g., amiodarone, propafenone) may impair drug elimination leading to an increased risk of ADEs [42, 43]. Amiodarone and its metabolite N,N-dide-sethylamiodarone are major contributors to DDIs that arise as a consequence of inhibition of CYP1A2 [44]. Increased injury rates with tizanidine and digoxin can be explained by an additive slow heart rate effect leading to syncope. Digoxin was also previously found to be associated with an increase in fall-related injuries. [45]
We also found signals of increased injury rates among users of baclofen-sitagliptin (1.7, 1.07–2.60), explainable by the potential additive effect between baclofen-related hemodynamic complications, including severe bradycardia and hypotension [46], and the potential hypotensive effect of sitagliptin [47]. We also found increased injury rates with chlorzoxazone-cephalexin (2.0, 1.00–4.13). Oxybutynin is on the Beers list of potentially harmful drugs in the elderly, has anticholinergic CNS effects, and can cause confusion, hallucination, and sedation. A retrospective study of Veterans Affairs long-term care residents reported an increased risk of hip fracture and any fracture among new users of immediate-release oxybutynin as compared with nonusers (hip fracture: hazard ratio = 3.67, 95% CI 1.46–9.34, “any” fracture: 2.64, 95% CI 1.37–1.50) [48]. We are unaware of mechanisms to support the following signals: chlorzoxazone-methylprednisolone and methocarbamol-levothyroxine (1.4, 1.05–1.94). With respect to the former two pairs, it is possible that clinicians prescribed an antibiotic and/or glucocorticoid in response to an injury that did not yet present to the emergency department, and therefore we cannot rule out reverse causation.
Our study has strengths. First, we used the SCCS design, which inherently controls for measured and unmeasured static confounders. Second, we used a semi-Bayes shrinkage method to minimize the false-positive rate. Third, the large sample size allowed us to study a wide range of muscle relaxant and precipitant drugs. Our study also has notable limitations. First, we based our exposure definition on dispensed prescriptions, which may not reflect consumption. There is the potential for misclassification of observation time and exposure status as muscle relaxants and certain precipitants may be taken as needed (i.e., pro re nata). Exposure misclassification is possible if drugs obtained over the counter or through alternative health insurance coverage (e.g., governmental) affected the rates of injury. Second, the SCCS design is susceptible to reverse causation if physicians prescribed precipitants to treat early symptoms of injury leading to inflated RRs in the focal window. The design can also lead to biased estimates if having injuries affects the likelihood of future exposure to the precipitant of interest. Third, our small sample size of users of muscle relaxants with the secondary outcomes (i.e., falls and motor vehicle crashes) hampered our ability to identify potential signals. Fourth, the SCCS design is most robust when the focal window (defined by exposure to the precipitant drug) is short relative to the observation period (defined by exposure to the muscle relaxant). Thus, the relatively short median observation periods for metaxalone and orphenadrine make it likely that focal windows represented a substantial proportion (or the entirety) of observation time; this would have minimized our power to identify statistically significant associations. In fact, we identified no signals for these two muscle relaxants. While dantrolene had a very long observation period and therefore avoided this concern, its analysis lacked power because of the very few events among users. This only permitted us to study a few co-dispensed precipitant drugs for dantrolene. Similarly, we identified no signals for this muscle relaxant. Further, violation of the event-independent exposure assumption can lead to upward bias if the injury event decreased the likelihood of subsequent exposure to the precipitant (shorter referent window) or downward bias if it increases subsequent exposure to the precipitant (longer referent window). If injury events increase the probability of subsequent exposure to the object drug, the estimates would be biased toward the null. Fifth, we focused on DDI pairs and did not examine higher order drug interactions (e.g., triplets), which may be of interest given the high prevalence of polypharmacy among people using muscle relaxants. Sixth, although we adjusted for several time-varying confounders including average daily dose, prior injury diagnosis, and follow-up time, we were not able to adjust for changes in mobility or functioning (which may contribute to risk of falls) or practitioner reasoning for prescribing muscle relaxants because of the lack of information on these variables. The SCCS design innately controls for confounders that are stable over time (e.g., chronic comorbidities). Residual confounding from co-prescribed drugs or acute comorbidities is possible. Finally, our findings may not be generalizable to patients with government-provided insurance or those without health coverage.
5. Conclusions
In this population-based analysis, we utilized the SCCS design as a screening method to detect potential DDIs with muscle relaxants. Our analysis identified one anticipated and several new signals of muscle relaxant DDIs associated with unintentional traumatic injury. Future studies should seek to confirm or refute these potential interactions.
Supplementary Material
Key Points.
There is limited population-based evidence concerning muscle relaxant drug interactions. This is concerning given a national trend of increasing muscle relaxant use and frequent concomitant use with other central nervous system-depressing drugs, especially but not solely among older adults.
Using real-world data of a large US-based health insurer, we identified several new signals of potential drug–drug interactions with muscle relaxants that increased the rate of unintentional traumatic injury.
Funding
The US National Institutes of Health supported this work (R01AG060975, R01DA048001, R01AG064589, and R01AG025152).
Conflict of interest
GKD receives funding from the American Society of Hematology and the National Institutes of Health. CEL is an Executive Committee Member of the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training. The Center receives funds from Pfizer and Sanofi to support pharmacoepidemiology education. CEL recently received honoraria from the American College of Clinical Pharmacy Foundation and the University of Florida College of Pharmacy. CEL receives travel support from John Wiley and Sons. CEL is a Special Government Employee of the US Food and Drug Administration; he consults for their Reagan-Udall Foundation. CEL’s spouse is employed by Merck; neither she nor CEL holds stock in the company. JRH is a coauthor and publisher of The Top 100 Drug Interactions: A Guide to Patient Management and a consultant to Urovant Sciences and Seegnal US. WBB serves on multiple data safety monitoring boards for Genentech. SD has research funding from GlaxoSmithKline. SH has consulted for multiple pharmaceutical companies, and directs the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training. CMB, EKA, SC, MMM, TAM, DWO, TPPN, SES, and DJW declare no competing interests for this work.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s40263-022-00909-1.
Code availability Codes are available upon request from the corresponding author.
Ethics approval At the University of Pennsylvania, studies using Optum are deemed exempt by the Institutional Review Board (protocol #819924).
References
- 1.Soprano SE, Hennessy S, Bilker WB, Leonard CE. Assessment of physician prescribing of muscle relaxants in the United States, 2005–2016. JAMA Netw Open. 2020;3(6): e207664. 10.1001/jamanetworkopen.2020.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spence MM, Shin PJ, Lee EA, Gibbs NE. Risk of injury associated with skeletal muscle relaxant use in older adults. Ann Pharmacother. 2013;47(7–8):993–8. 10.1345/aph.1R735. [DOI] [PubMed] [Google Scholar]
- 3.Billups SJ, Delate T, Hoover B. Injury in an elderly population before and after initiating a skeletal muscle relaxant. Ann Pharmacother. 2011;45(4):485–91. 10.1345/aph.1P628. [DOI] [PubMed] [Google Scholar]
- 4.Norton R, Hyder AA, Bishai D, Peden M, et al. Unintentional injuries. In: Jamison DT, Breman JG, Measham AR, et al. , editors. Disease control priorities in developing countries. 2nd ed. Washington, DC: World Bank; 2006. [Google Scholar]
- 5.See S. Choosing a skeletal muscle relaxant. Am Family Physician. 2008;78(3):365–70. [PubMed] [Google Scholar]
- 6.Zacny JP, Paice JA, Coalson DW. Characterizing the subjective and psychomotor effects of carisoprodol in healthy volunteers. Pharmacol Biochem Behav. 2011;100(1):138–43. 10.1016/j.pbb.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez CA, Mortensen EM, Makris UE, et al. Association of skeletal muscle relaxers and antihistamines on mortality, hospitalizations, and emergency department visits in elderly patients: a nationwide retrospective cohort study. BMC Geriatr. 2015;15:2. 10.1186/1471-2318-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bramness JG, Skurtveit S, Mørland J, Engeland A. The risk of traffic accidents after prescriptions of carisoprodol. Accid Anal Prev. 2007;39(5):1050–5. 10.1016/j.aap.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Logan BK, Case GA, Gordon AM. Carisoprodol, meprobamate, and driving impairment. J Forensic Sci. 2000;45(3):619–23. [PubMed] [Google Scholar]
- 10.Golden AG, Ma Q, Nair V, Florez HJ, Roos BA. Risk for fractures with centrally acting muscle relaxants: an analysis of a national medicare advantage claims database. Ann Pharmacother. 2010;44(9):1369–75. 10.1345/aph.1P210. [DOI] [PubMed] [Google Scholar]
- 11.Moody DE, Fu Y, Fang WB. Inhibition of in vitro metabolism of opioids by skeletal muscle relaxants. Basic Clin Pharmacol Toxicol. 2018;123(3):327–34. 10.1111/bcpt.12999. [DOI] [PubMed] [Google Scholar]
- 12.LeRoy Aida A, Morse M Lee. Multiple medications and vehicle crashes: analysis of databases. Washington DC: U.S. Department of Transportation; 2008. [Google Scholar]
- 13.FDA drug safety communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. Silver Spring (MD): US Food and Drug Administration; 2016. [Google Scholar]
- 14.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain: United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 15.Kitzman P, Cecil D, Kolpek JH. The risks of polypharmacy following spinal cord injury. J Spinal Cord Med. 2017;40(2):147–53. 10.1179/2045772314Y.0000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou M, Leonard CE, Brensinger CM, et al. Pharmacoepidemiologic screening of potential oral anticoagulant drug interactions leading to thromboembolic events. Clin Pharmacol Ther. 2020;108(2):377–86. 10.1002/cpt.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard CE, Zhou M, Brensinger CM, et al. Clopidogrel drug interactions and serious bleeding: generating real-world evidence via automated high-throughput pharmacoepidemiologic screening. Clin Pharmacol Ther. 2019;106(5):1067–75. 10.1002/cpt.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard CE, Brensinger CM, Acton EK, et al. Population-based signals of antidepressant drug interactions associated with unintentional traumatic injury. Clin Pharmacol Ther. 2021;110(2):409–23. 10.1002/cpt.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Anker J, Reed MD, Allegaert K, Kearns GL. Developmental changes in pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2018;58(Suppl. 10):S10–25. 10.1002/jcph.1284. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez E, Perez R, Hernandez A, Tejada P, Arteta M, Ramos JT. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics. 2011;3(1):53–72. 10.3390/pharmaceutics3010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephenson T. How children’s responses to drugs differ from adults. Br J Clin Pharmacol. 2005;59(6):670–3. 10.1111/j.1365-2125.2005.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sears JM, Bowman SM, Rotert M, Hogg-Johnson S. A new method to classify injury severity by diagnosis: validation using workers’ compensation and trauma registry data. J Occup Rehabil. 2015;25(4):742–51. 10.1007/s10926-015-9582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland S. A semi-Bayes approach to the analysis of correlated multiple associations, with an application to an occupational cancer-mortality study. Stat Med. 1992;11(2):219–30. 10.1002/sim.4780110208. [DOI] [PubMed] [Google Scholar]
- 24.Strömberg U. Empirical Bayes and semi-Bayes adjustments for a vast number of estimations. Eur J Epidemiol. 2009;24(12):737–41. 10.1007/s10654-009-9393-0. [DOI] [PubMed] [Google Scholar]
- 25.National Center for Injury Prevention and Control. WISQARS: leading causes of death reports, 1981–2019, United States. https://wisqars.cdc.gov:8443/costT/ProcessPart1FinishOutServlet. Updated 2014 [Accessed 3 Dec 2021].
- 26.Li Y, Delcher C, Reisfield GM, Wei YJ, Brown JD, Winterstein AG. Utilization patterns of skeletal muscle relaxants among commercially insured adults in the United States from 2006 to 2018. Pain Med. 2021;22(10):2153–61. 10.1093/pm/pnab088. [DOI] [PubMed] [Google Scholar]
- 27.Leonard CE, Brensinger CM, Pham Nguyen TP, et al. Screening to identify signals of opioid drug interactions leading to unintentional traumatic injury. Biomed Pharmacother. 2020;130: 110531. 10.1016/j.biopha.2020.110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emeny RT, Chang C, Skinner J, et al. Association of receiving multiple, concurrent fracture-associated drugs with hip fracture risk. JAMA Netw Open. 2019;2(11): e1915348. 10.1001/jamanetworkopen.2019.15348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343: d4551. 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcum ZA, Perera S, Thorpe JM, et al. Antidepressant use and recurrent falls in community-dwelling older adults: findings from the Health ABC Study. Ann Pharmacother. 2016;50(7):525–33. 10.1177/1060028016644466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muanda FT, Blake PG, Weir MA, et al. Association of baclofen with falls and fractures in patients with CKD. Am J Kidney Dis. 2021;78(3):470–3. [DOI] [PubMed] [Google Scholar]
- 33.Pfizer Laboratories Div Pfizer Inc. Chantix: varenicline tartrate tablet, film coated. Updated 2019. http://www.labeling.pfizer.com. Accessed 02 Jan 2022.
- 34.Kuehn BM. Varenicline gets stronger warnings about psychiatric problems, vehicle crashes. JAMA. 2009;302(8):834. 10.1001/jama.2009.1153. [DOI] [PubMed] [Google Scholar]
- 35.Gunnell D, Irvine D, Wise L, Davies C, Martin RM. Varenicline and suicidal behaviour: a cohort study based on data from the general practice research database. BMJ. 2009;339: b3805. 10.1136/bmj.b3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molero Y, Lichtenstein P, Zetterqvist J, Gumpert CH, Fazel S. Varenicline and risk of psychiatric conditions, suicidal behaviour, criminal offending, and transport accidents and offences: population based cohort study. BMJ. 2015;350: h2388. 10.1136/bmj.h2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalén P, Alvan G, Wakelkamp M, Olsen H. Formation of meprobamate from carisoprodol is catalysed by CYP2C19. Pharmacogenetics. 1996;6(5):387–94. 10.1097/00008571-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez LA, Gatch MB, Taylor CM, Bell-Horner CL, Forster MJ, Dillon GH. Carisoprodol-mediated modulation of GABAA receptors: In vitro and in vivo studies. J Pharmacol Exp Ther. 2009;329(2):827–37. 10.1124/jpet.109.151142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DuBois DW, Damborsky JC, Fincher AS, Frye GD, Winzer-Serhan UH. Varenicline and nicotine enhance GABAergic synaptic transmission in rat CA1 hippocampal and medial septum/diagonal band neurons. Life Sci. 2013;92(6–7):337–44. 10.1016/j.lfs.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye JH, Ponnudurai R, Schaefer R. Ondansetron: a selective 5-HT(3) receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev. 2001;7(2):199–213. 10.1111/j.1527-3458.2001.tb00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel TK, Patel PB, Tripathi CB. Effect of pantoprazole and its interactions with vecuronium on the neuromuscular junction. Indian J Pharmacol. 2010;42(1):36–9. 10.4103/0253-7613.62410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witenko C, Moorman-Li R, Motycka C, et al. Considerations for the appropriate use of skeletal muscle relaxants for the management of acute low back pain. P T. 2014;39(6):427–35. [PMC free article] [PubMed] [Google Scholar]
- 43.Kaddar N, Vigneault P, Pilote S, Patoine D, Simard C, Drolet B. Tizanidine (Zanaflex): a muscle relaxant that may prolong the QT interval by blocking IKr. J Cardiovasc Pharmacol Ther. 2012;17(1):102–9. 10.1177/1074248410395020. [DOI] [PubMed] [Google Scholar]
- 44.McDonald MG, Au NT, Rettie AE. P450-based drug-drug interactions of amiodarone and its metabolites: diversity of inhibitory mechanisms. Drug Metab Dispos. 2015;43(11):1661–9. 10.1124/dmd.115.065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vries M, Seppala LJ, Daams JG, van de Glind EMM, Masud T, van der Velde N. Fall-risk-increasing drugs: a systematic review and meta-analysis: I cardiovascular drugs. J Am Med Dir Assoc. 2018;19(4):371.e1–371.e9. 10.1016/j.jamda.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Sill JC, Schumacher K, Southorn PA, Reuter J, Yaksh TL. Bradycardia and hypotension associated with baclofen used during general anesthesia. Anesthesiology. 1986;64(2):255–8. 10.1097/00000542-198602000-00022. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa S, Ishiki M, Nako K, et al. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, decreases systolic blood pressure in Japanese hypertensive patients with type 2 diabetes. Tohoku J Exp Med. 2011;223(2):133–5. 10.1620/tjem.223.133. [DOI] [PubMed] [Google Scholar]
- 48.Moga DC, Carnahan RM, Lund BC, et al. Risks and benefits of bladder antimuscarinics among elderly residents of veterans affairs community living centers. J Am Med Dir Assoc. 2013;14(10):749–60. 10.1016/j.jamda.2013.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


