Abstract
Objectives:
Glass ionomer cements (GICs) are among the most popular dental restorative materials, but their use is limited due to their clinical disadvantages. Many efforts have been made to improve the properties of these materials by adding various fillers. Incorporation of hydroxyapatite (HA) into the GICs is considered to improve the physical properties of restorations, and may prevent treatment failure. This study aimed to evaluate the surface roughness (Ra) of a conventional glass ionomer cement (CGIC), a resin-modified glass ionomer (RMGI) and a Zirconomer with and without micro-hydroxyapatite (μHA).
Materials and Methods:
This experimental study was conducted on 6 groups (n=10) including CGIC, CGIC + μHA, RMGI, RMGI + μHA, Zirconomer, and Zirconomer + μHA. A total of 60 disc-shaped samples (6 mm × 2 mm) were prepared in plastic molds and were stored in distilled water for 24 h. After polishing of the specimens, their Ra was measured by a profilometer in micrometers (μm). The data were analyzed using two and one-way ANOVA, Tukey’s HSD test, and independent t-test.
Results:
Incorporation of μHA resulted in statistically significant differences in Ra between the study groups (P<0.05). Following the incorporation of μHA, the Ra significantly decreased in CGIC (P=0.013) and Zirconomer (P=0.003). However, addition of μHA to RMGI resulted in a significant increase in its Ra (P<0.001).
Conclusion:
Addition of μHA decreased the Ra of Zirconomer and CGIC, and increased the surface roughness of RMGI samples.
Keywords: Glass Ionomer Cements, Hydroxyapatites, Surface Properties, Biocompatible Materials
INTRODUCTION
Surface roughness highly affects the erosion and clinical longevity of restorations. A rough surface facilitates plaque accumulation. It also provides a suitable environment for colonization of microorganisms, consequently leading to gingival and periodontal disease and increasing the risk of dental caries [1]. A smooth surface decreases the surface area and surface energy; therefore, accumulation of particles is lower on smooth surfaces [2]. Furthermore, the esthetic success of a restoration is directly correlated with its optical properties. Surface roughness is considered as one of the most important factors affecting the appearance of a tooth-colored restoration [3]. Glass ionomer cements (GICs) are among the popular dental materials commonly used for direct esthetic restorations in non-load-bearing areas [2]. The original GICs are composed of an aqueous solution of polyacrylic acid, which reacts with a powder consisting of calcium fluoroaluminosilicate glass. These glass fillers are a modification of the former dental silicate cements with an aim to enhance their physical, mechanical and biological properties [4]. The favorable properties of GICs include their optimal biocompatibility, adhesion to the tooth structure, and fluoride release potential. Beside their favorable properties, GICs have some clinical limitations such as long setting time, rough surface structure, and sensitivity to moisture and dehydration during their primary setting [5]. Attempts to overcome these limitations resulted in production of resin-modified glass ionomers (RMGIs) [6,7]. These materials were developed by adding resin monomers to GICs, resulting in much better esthetics, higher mechanical and handling properties, and higher bond strength to enamel and dentin. Another shortcoming of the conventional glass ionomer cements (CGICs) is their low mechanical strength [8]. The main reasons for the application of zirconia as a filler are its good chemical and dimensional stability, high toughness and mechanical strength, and a Young’s modulus similar to that of stainless steel alloys. Zirconia has been extensively used for strengthening and toughening of brittle hydroxyapatite, bioglass, and composite materials [9]. A high-strength GIC that is strengthened with zirconia fillers known as Zirconomer, could overcome the disadvantages of the previously used GICs [10]. Hydroxyapatite [Ca10(PO4)6(OH)2] (HA) is considered as the main component of the enamel and dentin [11,12]. Owing to its chemical composition and crystallographic structure, it could be used in bone tissue engineering, restoration of periodontal defects, endodontic treatments like pulp capping, apical barrier formation, treatment of primary caries, and also as a filler to reinforce the structure of restorative GICs and composite resins [11,13].
It has been reported that incorporation of HA into GICs can improve some of their mechanical features such as diametral and flexural strength, fracture resistance, compressive strength, and bond strength. It can also remineralize the demineralized dentin and increase its flexural strength [11]. In another study, HA was found to have antibacterial activity against Streptococcus mutans [14]. Due to the favorable biocompatibility of HA and high mechanical properties of Zirconomer, incorporation of HA as filler into Zirconomer has gained interest [9]. A recent study by sharafedin et al. [15] showed that addition of 5 and 15wt% micro-hydroxyapatite (μHA) to Zirconomer and RMGI increased their surface microhardness. Although the surface roughness of GICs and the factors affecting it have been previously evaluated in many studies [16–20], and efforts have been made to improve this property of GICs [2], limited studies have evaluated the effect of incorporation of μHA on the surface roughness of GICs. Thus, this study was designed to investigate the effect of incorporation of μHA on the surface roughness of three GICs namely CGIC, RMGI, and Zirconomer.
MATERIALS AND METHODS
The study protocol was approved by the Ethics Committee for Research of Shiraz University of Medical Sciences (IR.SUMS.REC.1394.S360). In this experimental study, 60 samples were fabricated in six groups (n=10). Disc-shaped samples were made by using cylindrical plastic molds measuring 6 mm × 2 mm. Table 1 presents the characteristics of the materials used in this study. Sample preparation in the six groups was as follows:
Table 1.
Composition of materials used in this study
| Materials (batch number) | Manufacturer | Composition |
|---|---|---|
| Fuji II GC (1407141) | GC Corp., Tokyo, Japan |
Powder: fluoroaluminosilicate glass Liquid: polyacrylic acid, itaconic acid, tartaric acid, maleic acid, water |
| Fuji II LC (1407021) | GC Corp., Tokyo, Japan |
Powder: aluminofluorosilicate glass, urethane dimethacrylate, camphorquinone Liquid: polymer acrylic acid, distill water, 2-hydroxyethylmethacrylate |
| Zirconomer (04140781) | Shofu INC, Kyoto, Japan |
Powder: aluminofluorosilicate glass, zirconium oxide, tartaric acid Liquid: polyacrylic acid, deionized water |
| Hydroxyapatite (5465141Q788) | Sigma-Aldrich, USA | Calcium hydroxyphosphate |
| Varnish (01071020) | Kimia, Iran | Copal resin, ethanol |
Group 1 (CGIC):
To prepare the CGIC samples based on the manufacturer’s instructions, one scoop of CGIC powder and one drop of the liquid (Fuji II GC, GC Corp., Tokyo, Japan) were mixed on a cold clean glass slab using a plastic spatula for 25 s. The mixture was applied into the mold; the upper and lower surfaces were covered with transparent Mylar strips (Fintrec Transparent Matrix, Pulpdent Corp., Water-town, MA, USA), and were then placed between two glass slabs for complete setting.
The material set within 5.5 min after mixing. A thin layer of copal varnish (Kimia, Tehran, Iran) was applied over the surface.
Group 2 (CGIC + μHA):
Each sample in this group contained 85wt% CGIC powder and 15wt% μHA (Sigma-Aldrich, St. Louis, USA), which were separately weighed by using a scale with 0.0001 g accuracy (GR+360; A&D, Tokyo, Japan).
The powders were blended on a glass slab using a plastic spatula. The mixture was transferred to clean amalgam capsules and mixed again in an amalgamator (FD-4300; Faghihi, Tehran, Iran) for 20 s to obtain uniform and equally homogenized powder in all samples. The obtained powder was mixed with CGIC liquid similar to group 1. The mixture was then applied into the molds and allowed to set for 5.5 min, and was finally coated with varnish.
Group 3 (RMGI):
The samples in this group were made of one scoop of RMGI (Fuji II LC, GC Corp., Tokyo, Japan) and 2 drops of liquid that were mixed for 25 s according to the manufacturer’s instructions. The mixture was inserted into the molds and covered with Mylar strips as in group 1. It was placed between two glass slabs, and cured with a LED curing unit (Bluelex GT 1200; Monitex, San-Chong, Taiwan) with 1200 mW/cm2 light intensity for 20 s. The tip of the device was in contact with the slab to achieve better cure. Finally, a thin layer of varnish was applied over the cured samples.
Group 4 (RMGI + μHA):
The powder containing 85wt% RMGI and 15wt% μHA was prepared as in group 2.
Group 5 (Zirconomer):
The same as group 1, two scoops of Zirconomer (Zirconomer, Shofu Inc., Kyoto, Japan) powder were mixed with one drop of liquid for 30 s. The mixture set within 3.5 min, and coated with varnish.
Group 6 (Zirconomer + μHA):
As in group 2, the powder containing 85wt% Zirconomer and 15wt% μHA was mixed with Zirconomer liquid. Figure 1 displays the study groups based on the materials.
Fig. 1.

Study groups based on the materials
All the fabricated samples were removed from the molds and stored in distilled water at room temperature for 24 h.
Then, the surface of the samples was polished using multistep polishing paper discs (Super Snap Rainbow Technique kit; Shofu Corp., San Marcos, USA) and low-speed handpiece under water coolant. Each specimen was polished for 30 s at each step. Polishing was performed with a rotational planar motion around the normal vector of the surface [21] To eliminate the debris, the samples were rinsed with distilled water for 1 min in an ultrasonic bath (Renfert GmbH, Hilzingen, Germany).
The surface roughness (Ra) was measured at three points of each sample, using a surface roughness tester (Rugosurf 20; Tesa Tec, Renens, Switzerland). The mean of the three values was recorded as the final surface roughness of each sample. The mean value of each group was used for statistical analysis. The study groups were analyzed by two and one-way ANOVA, Tukey’s HSD test, and independent t-test using SPSS version 24 (SPSS Inc., Chicago, IL, USA) (P<0.05).
RESULTS
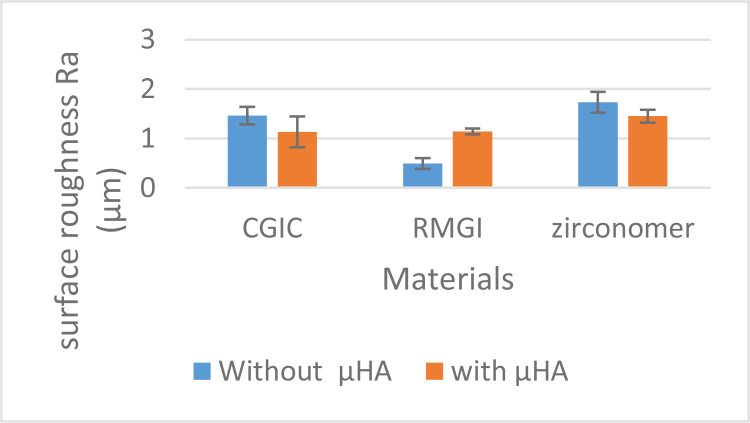
Two-way ANOVA revealed significant interaction effect of the type of GIC and μHA application on Ra (P<0.001). As shown in Figure 2, addition of μHA had different effects on the mean Ra values in different types of GICs. Therefore, subgroup analyses were performed to compare different groups.
Fig. 2.
Mean and standard deviations of the surface roughness of materials with and without μHA. two-way ANOVA revealed a significant interaction effect between the study groups
One-way ANOVA showed significant differences in Ra between the groups (P<0.001). The Tukey’s HSD test revealed that the mean Ra value in the RMGI+μHA group was slightly higher than that in the CGIC+μHA group; however, this difference was not statistically significant (P=0.992). The Ra in Zirconomer+μHA group was significantly higher than that in RMGI+μHA (P=0.016) and insignificantly higher than that in CGIC+μHA (P=0.12) groups. Without adding μHA, the mean Ra value of Zirconomer was significantly higher than that of RMGI (P<0.001) and CGIC (P=0.05). Furthermore, the mean Ra value of CGIC was significantly higher than that of RMGI without μHA (P<0.001). Independent t-test showed that while addition of μHA to the testing materials resulted in a significant decrease in the Ra values of Zirconomer (P=0.003) and CGIC (P=0.013), a significant increase was observed in RMGI following μHA addition (P<0.001). It should be noted that the lowest and the highest Ra values were recorded for RMGI (0.49 μm) and Zirconomer (1.73 μm), respectively. Table 2 summarizes the mean±standard deviation of Ra in each group.
Table 2.
Mean and standard deviation (SD) of surface roughness values (Ra in μm) of the study groups
| Groups | Mean±SD | P* | |
|---|---|---|---|
| − μHA | + μHA | ||
| CGI | 1.46±0.18A | 1.13±0.31A | 0.013 |
| RMGI | 0.49±0.11B | 1.14±0.06A | <0.001 |
| Zirconomer | 1.73±0.21C | 1.45±0.13B | 0.003 |
| P** | <0.001 | 0.006 | --- |
In each column, the mean Ra values with different uppercase letters are significantly different (Tukey’s HSD test).
P: independent t-test,
P: one-way ANOVA F test
CGIC: conventional glass ionomer cement; RMGI: resin-modified glass ionomer, μHA: micro-hydroxyapatite
DISCUSSION
It has been reported that incorporation of HA could improve some physical characteristics of GICs [15].
Surface roughness is considered as one of the most important parameters of dental restorations. In the present study, we investigated the effect of adding HA on surface roughness of GICs. Micro-HA was chosen in this study because it reinforces the GICs and could easily mix with resin (either Bis-GMA+HEMA or Bis-GMA+TEGDEMA). Although nano-HA has a more similar crystal size to the mineral phase of the tooth structure, nano-HA considerably increases the setting time of GICs [15]. It has also been reported that the surface mechanical properties of nano-GICs are not superior to those of GICs without nanofillers [22].
Surface roughness can be evaluated through different methods such as visual evaluation under an optical microscope, scanning electron microscopy, atomic force microscopy, and profilometric analysis [16]. The current study benefited from profilometric analysis to measure Ra due to the optimal accuracy, applicability, and simplicity of this method. Ra is the algebraic average height of the roughness component irregularities from the mean line measured within the sampling length. It should be noted that higher Ra values indicate higher surface roughness [2] Three profilometric measurements were made on each sample, and the mean value was recorded as the Ra of the respective sample in this study. The current study measured the mean Ra values to be 0.49–1.73 μm for specimens made of GICs without μHA and 1.13–1.45 μm for GICs mixed with μHA. Apparently, the material composition caused changes in the Ra values. Among all the tested GICs, RMGI had the lowest Ra value, which was in accord with other studies [2,18,23].
It has been reported that materials with larger particle size have a rougher surface [24,25]. Thus, particles size is a determining factor in the surface roughness of GICs [26]. The particle size of RMGI is smaller than that of Zirconomer [27] and CGIC [25]; therefore, it can justify the lower Ra of RMGI compared with Zirconomer and CGIC. In addition, presence of resin component contributes to a reduction in RMGI surface roughness. Resin improves the surface properties of GICs; thus, in presence of resin in GICs, smaller particles are detached from the material during the polishing process, and a smoother surface is achieved [18]; therefore, it probably causes smoother surface of RMGI after polishing.
According to the results, Zirconomer had the highest Ra value compared with other groups (regardless of the presence of μHA), which was in agreement with previous studies [28,29]. Higher Ra values in Zirconomer may be attributed to its larger particle size [25]. The mean Ra value in the CGIC group was between those of Zirconomer and RMGI samples. In line with the study by Gladys et al, [25] the mean particle size in CGIC was higher than that in RMGI and lower than that in Zirconomer.
The interaction between GIC and HA occurs through Ca ions in HA and the carboxylate group in acrylate polymer. The bond between GIC and HA is also affected by the adsorption between carboxylic acid and HA, as well as the interaction of the functional groups of polymer and HA. Due to their contribution to the reaction between the powder and liquid in GIC, HA particles probably participate in acid-base reactions through ion release [11]. Seemingly, in the present study, chemical curing increased the setting time of Zirconomer (3.5 min) and CGIC (5.5 min) compared with RMGI, which was light-cured. In other words, HA particles and glass-ionomer have more time to interact in CGIC and Zirconomer groups, probably resulting in a more homogenized mixture than RMGI + μHA leading to lower surface roughness. However, based on some reports, HA particles are likely to act as a barrier, preventing the bonding of components in light-cure RMGI [11,30], which can be the cause of increased Ra in μHA-containing RMGI.
The ability to interact with HA led to smoother surface of HA-incorporated CGIC and Zirconomer compared with groups devoid of HA, which could be due to the small particle size of μHA. Polishing, its technique, and polishing tools are among other factors affecting the surface roughness. Although use of a Mylar strip results in the smoothest surface possible compared with any finishing treatment, some degrees of finishing and polishing are often required clinically [19]. Previous studies indicated that multi-step polishing would yield the best results [20,31], and also lower Ra values could be obtained in polishing with planar motions for any type of material composition and abrasive disc [21]. It has also been reported that polishing with aluminum-oxide discs results in a polished surface without dislodging of glass particles [31,32].
In the present study, according to the clinical conditions, multiple polishing instruments were used, and polishing was carried out with aluminum-oxide discs in a planar motion to achieve a better polished surface. Each specimen was polished for 120 s in four consecutive steps. Polishing was accomplished by four flexible discs with different grits to make uniform abrasion in the matrix and filler of samples. GIC with incorporated μHA is suggested as a potential dental restorative material. More investigations are recommended on the effects of HA with different amounts and particle sizes on surface roughness by simulating the oral conditions.
CONCLUSION
Considering the limitations of the present study, it can be concluded that addition of μHA had a significant effect on the surface roughness of CGIC, RMGI, and Zirconomer. Hence, the material composition can be a determining factor in the Ra value. It was found that incorporation of μHA may reduce the surface roughness of Zirconomer and CGIC, but it can increase the Ra of RMGI. RMGI yielded the smoothest surface in comparison with other groups. Moreover, Zirconomer showed the highest surface roughness among the tested materials.
ACKNOWLEDGMENTS
The authors thank the Vice-Chancellory of Research, Shiraz University of Medical Sciences for supporting this study (Grant# 9711). The authors would like to thank the Biomaterial Research Center and Mrs. Baghery for testing the specimens and Dr. Vosooghi for conducting the statistical analyses.
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Sharafeddin F, Jamalipour G. Effects of 35% carbamide peroxide gel on surface roughness and hardness of composite resins. J Dent (Tehran). 2010;7(1):6–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Mallya PL, Acharya S, Ballal V, Ginjupalli K, Kundabala M, Thomas M. Profilometric study to compare the effectiveness of various finishing and polishing techniques on different restorative glass ionomer cements. J Interdiscip Dent. 2013. May;3(2):86–91. [Google Scholar]
- 3.Hosoya Y, Shiraishi T, Ando S, Miyazaki M, Garcia-Godoy F. Effects of polishing on surface roughness and gloss of S-PRG filled flowable resin composite. Am J Dent. 2012. Aug;25(4):227–30. [PubMed] [Google Scholar]
- 4.Nicholson JW. Chemistry of glass-ionomer cements: a review. Biomaterials. 1998. Apr;19(6):485–94. [DOI] [PubMed] [Google Scholar]
- 5.Sharafeddin F, Choobineh MM. Assessment of the shear bond strength between nanofilled composite bonded to glass-ionomer cement using self-etch adhesive with different pHs and total-etch adhesive. J Dent (Shiraz). 2016. Mar;17(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Sharafeddin F, Tondari A, Alavi A. The effect of adding glass and polyethylene fibers on flexural strength of three types of glass-ionomer cements. Res J Biol Scien. 2013;8:66–70. [Google Scholar]
- 7.Sharafeddin F, Ghaboos SA, Jowkar Z. The effect of short polyethylene fiber with different weight percentages on diametral tensile strength of conventional and resin modified glass ionomer cements. J Clin Exp Dent. 2017. Mar;9(3):e466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharafeddin F, Bakhtiarvand NA, Jowkar Z. Evaluation of the effect of home bleaching gel on microleakage of different glass ionomers reinforced with micro-hydroxyapatite. J Conserv Dent. 2019. Jan;22(1):64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu YW, Yap AU, Cheang P, Khor KA. Effects of incorporation of HA/ZrO(2) into glass ionomer cement (GIC). Biomaterials. 2005. Mar;26(7):713–20. [DOI] [PubMed] [Google Scholar]
- 10.Abdulsamee N, Elkhadem AH. Zirconomer and Zirconomer improved (white amalgams): restorative materials for the future. Review. EC Dent Sci. 2017. Nov;15:134–50. [Google Scholar]
- 11.Sharafeddin F, Feizi N. Evaluation of the effect of adding micro-hydroxyapatite and nano-hydroxyapatite on the microleakage of conventional and resin-modified Glass-ionomer Cl V restorations. J Clin Exp Dent. 2017. Feb;9(2):e242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharafeddin F, Kowkabi M, Shoale S. Evaluation of the effect of home bleaching agents on surface microhardness of different glass-ionomer cements containing hydroxyapatite. J Clin Exp Dent. 2017. Sep;9(9):e1075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moheet IA, Luddin N, Ab Rahman I, Kannan TP, Abd Ghani NR. Evaluation of mechanical properties and bond strength of nano-hydroxyapatite-silica added glass ionomer cement. Ceram Int. 2018. Jun;44(8):9899–906. [Google Scholar]
- 14.Hilal MK. Antibacterial property of hydroxyapatite compared to Glass Ionomer Cement and Amalgam. Int J Sci Res. 2014. May;3(5):402–4. [Google Scholar]
- 15.Sharafeddin F, Shoale S, Kowkabi M. Effects of different percentages of microhydroxyapatite on microhardness of resin-modified glass-ionomer and Zirconomer. J Clin Exp Dent. 2017. Jun;9(6):e805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da Silva RC, Zuanon AC. Surface roughness of glass ionomer cements indicated for atraumatic restorative treatment (ART). Braz Dent J. 2006;17(2):106–9. [DOI] [PubMed] [Google Scholar]
- 17.Ozdemir-Ozenen D, Sungurtekin E, Issever H, Sandalli N. Surface roughness of fluoride-releasing restorative materials after topical fluoride application. Eur J Paediatr Dent. 2013. Mar;14(1):68–72. [PubMed] [Google Scholar]
- 18.Bala O, Arisu HD, Yikilgan I, Arslan S, Gullu A. Evaluation of surface roughness and hardness of different glass ionomer cements. Eur J Dent. 2012. Jan;6(1):79–86. [PMC free article] [PubMed] [Google Scholar]
- 19.Wu SS, Yap AU, Chelvan S, Tan ES. Effect of prophylaxis regimens on surface roughness of glass ionomer cements. Oper Dent. 2005. Mar;30(2):180–4. [PubMed] [Google Scholar]
- 20.Singh AK, Shivanna V, Shivamurthy GB, Kedia NB, Yadav AB, Yadav SK. Comparative surface roughness evaluation of A novel aesthetic restorative material using profilometer - an in vitro study. Int J Enhanc Res Med Dent Care. 2014;1(3):9–17. [Google Scholar]
- 21.Fruits TJ, Miranda FJ, Coury TL. Effects of equivalent abrasive grit sizes utilizing differing polishing motions on selected restorative materials. Quintessence Int. 1996. Apr;27(4):279–85. [PubMed] [Google Scholar]
- 22.Najeeb S, Khurshid Z, Zafar MS, Khan AS, Zohaib S, Marti JM, et al. Modifications in glass ionomer cements: Nano-sized fillers and bioactive nanoceramics. Int J Mol Sci. 2016. Jul; 17(7):1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolford MJ. Finishing glass polyalkenoate (glass-ionomer) cements. Br Dent J. 1988. Dec;165(11):395–9. [DOI] [PubMed] [Google Scholar]
- 24.Reis AF, Giannini M, Lovadino JR, Ambrosano GM. Effects of various finishing systems on the surface roughness and staining susceptibility of packable composite resins. Dent Mater. 2003. Jan;19(1):12–8. [DOI] [PubMed] [Google Scholar]
- 25.Gladys S, Van Meerbeek B, Braem M, Lambrechts P, Vanherle G. Comparative physico-mechanical characterization of new hybrid restorative materials with conventional glass-ionomer and resin composite restorative materials. J Dent Res. 1997. Apr;76(4):883–94. [DOI] [PubMed] [Google Scholar]
- 26.Yap AU, Tan WS, Yeo JC, Yap WY, Ong SB. Surface texture of resin-modified glass ionomer cements: effects of finishing/polishing systems. Oper Dent. 2002;27(4):381–6. [PubMed] [Google Scholar]
- 27.Prabhakar A, Kalimireddy P, Yavagal C, Sugandhan S. Assessment of the clinical performance of zirconia infused glass ionomer cement: An in vivo study. Int J Oral Heal Sci. 2015. Jul;5(2):74–9. [Google Scholar]
- 28.Pitkethy MJ. Nanoparticles as building blocks? Mater Today. 2003. Dec;6(12):36–42. [Google Scholar]
- 29.Asafarlal S. Comparative evaluation of microleakage, surface roughness and hardness of three glass ionomer cements – Zirconomer, Fujii IX Extra GC and Ketac Molar: An in vitro study. Dent J. 2017. Apr;7(5):1–5. [Google Scholar]
- 30.Moshaverinia A, Ansari S, Moshaverinia M, Roohpour N, Darr JA, Rehman I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008. Mar;4(2):432–40. [DOI] [PubMed] [Google Scholar]
- 31.Weitman RT, Eames WB. Plaque accumulation on composite surfaces after various finishing procedures. J Am Dent Assoc. 1975. Jul;91(1):101–6. [DOI] [PubMed] [Google Scholar]
- 32.Setcos JC, Tarim B, Suzuki S. Surface finish produced on resin composites by new polishing systems. Quintessence Int. 1999. Mar;30(3):169–73. [PubMed] [Google Scholar]