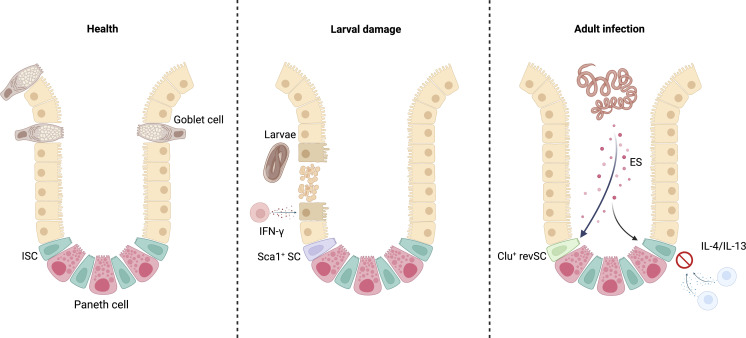
Karo-Atar et al. demonstrate that products released by a gut-dwelling helminth modify intestinal stem cells, which is associated with a loss of secretory cell types that support helminth expulsion.
Abstract
Helminth parasites are well known to have co-evolved a diverse arsenal of immunomodulatory factors to aid their persistence following infection. In this issue, Karo-Atar et al. (2022. J. Exp. Med. https://doi.org/10.1084/jem.20212311) demonstrate that products released by the gut-dwelling helminth Heligmosomoides polygyrus barkeri modify intestinal stem cells into a “revival” state, which is associated with a loss of helminth-expelling secretory cell types from the epithelium.
Parasitic helminth infections are prevalent worldwide, and high helminth burden, particularly from soil-transmitted helminths, is a major health concern for humans and livestock (Jourdan et al., 2018; Gilleard et al., 2021). Administration of therapeutics can be challenging, with re-infection common and vaccine responsiveness poor; moreover, drug resistance is increasing (Gilleard et al., 2021). As a counterpoint to this, helminths can also be beneficial for the immune system. For example, helminth infections are associated with suppression of allergies and immune-mediated inflammatory diseases (Maizels, 2020).

Insights from John R. Grainger and Rufus H. Daw.
Heligmosomoides polygyrus bakeri (Hpb) is a small-intestinal helminth of rodents that has been used experimentally to define immunomodulatory strategies used by helminth parasites (Osbourn et al., 2017; Maizels, 2020; Johnston et al., 2017). While in its larval stage, Hpb invades and then dwells in the submucosal intestinal tissue; as an adult it inhabits the lumen of the gut in intimate association with the epithelium (Nusse et al., 2018). Broadly speaking, type 2 immune responses, promoted by cytokines like IL-4, IL-5, and IL-13, are important for reducing helminth fecundity and supporting expulsion. One key mechanism of type 2–mediated immunity is “weep and sweep” that involves alterations to the fluid and mucus in the intestine as well as modulation of peristalsis. The weep element of weep and sweep is dependent on epithelial lineages, such as goblet cells and Tuft cells (Coakley and Harris, 2020).
Here Karo-Atar et al. (2022) found in vivo during the luminal adult stage of Hpb infection that the intestinal epithelium began expressing genes important in fetal development, such as Clu (encoding for clusterin). One strategy that helminths employ to target host immunity is by releasing excretory-secretory (ES) immunomodulatory factors (Maizels, 2020). Utilizing single-cell RNA sequencing (scRNA-seq) on small-intestinal organoids, the authors unexpectedly found that Hpb-conditioned medium (containing Hpb-ES) could support the emergence of Clu-expressing intestinal stem cells (ISCs) without the need for host-derived cytokines. Emergence of Clu+ stem cells was associated with reduced differentiation of secretory epithelial cell populations important in weep and sweep, such as goblet cells. Thus, Hpb has evolved to release factors that directly hijack tissue restorative pathways observed in fetal development to favor its own survival.
Until recently, much of our understanding of helminth immunomodulation has focused on downstream effects on hematopoietic populations (Maizels, 2020; Johnston et al., 2017). A number of key studies, including this new study from Karo-Atar et al. (2022), have now begun to explore the regulatory relationships between intestinal helminths and the epithelium (Drurey et al., 2022; Nusse et al., 2018; Coakley and Harris, 2020). Of note, at early stages of Hpb infection, while larvae are growing in the submucosa, fetal-like reversion of ISCs has already been described (Nusse et al., 2018). At this larval stage of infection, isolated fetal-like stem cells produced spheroids (observed when culturing fetal stem cells) rather than typical organoids. These fetal-like stem cells were Sca-1+ and had transcriptional features linked to IFN-γ exposure. Karo-Atar et al. (2022) also found that Hpb-conditioned medium (Hpb-CM) generated spheroids associated with the Clu+ ISC; however, Clu+ ISC did not express Sca-1 and were induced independently of IFN-γ. This implies that more than one mechanism is likely at play in supporting fetal ISC programs during Hpb infection that is tailored to helminth lifecycle stages. Further defining the relationship between these different types of ISCs will be crucial to better understanding epithelial replenishment processes.
Direct effects of Hpb-ES products on epithelial cell lineages were reported by Drurey et al. (2022). They showed that in vitro Hpb-ES was able to disfavor the accumulation of Tuft cells in response to IL-4 and IL-13. Interestingly, there was also evidence of fetal-like reversion in this context, and small-intestinal organoids took on spheroid growth characteristics (Drurey et al., 2022). In the Karo-Atar study, the authors utilized scRNA-seq of the organoids to profile specific stem cell populations following exposure to Hpb-CM. Analyzing this scRNA-seq dataset, Karo-Atar et al. (2022) were able to identify the Clu+ ISCs as revival stem cells (revSCs) that they had already described in the gut following irradiation and in the dextran sodium sulfate (DSS) model of colitis (Ayyaz et al., 2019). While rare in health, following DSS-mediated tissue damage Clu+ revSCs expand and are crucial to restore intestinal function. Dovetailing with the Drurey et al. (2022) study, development of the Clu+ revSC in Hpb-CM–treated organoids was associated with reduction in goblet and Paneth cells, and when organoids were co-exposed to Hpb-CM and type 2 cytokines, augmentation of Tuft and goblet cells by the type 2 cytokines was inhibited. Based on these findings, it will be interesting in the future to explore the precise cellular mechanisms that underlie the emergence of Clu+ revSCs and tandem suppression of IL-4 and IL-13 driven secretory cell generation. Is Hpb targeting a single pathway, or has it developed strategies to impact at multiple points in development of the epithelium?
The commonality of fetal-like reversion in helminth infection and other forms of intestinal injury highlights the importance of co-option of this state following damage of the epithelium (Ayyaz et al., 2019; Nusse et al., 2018). Whilst work by Nusse et al. (2018) and Ayyaz et al. (2019) depicts these stem cell reversion mechanisms as a response to generic epithelial damage, Karo-Atar et al. were able to draw a causal link between Hpb-ES products and modulation of the crypt niche. Indeed, a number of studies have now highlighted the importance of fetal-like reversion to tissue healing in inflammatory settings, including other external barrier surfaces and other cell populations, such as macrophages and endothelial cells in the skin (Reynolds et al., 2021). Although some of the mechanisms by which the epithelial barrier is replenished following injury are likely similar across mucosal surfaces, whether the same helminth products could drive fetal-like reversion in other organs and hence be used more broadly to therapeutically support healing pathways is unknown.
Terminal differentiation of ISCs drives epithelial cell and secretory lineage repopulation of the intestinal villus at homeostasis. Following intestinal damage, for example during Hpb larval infiltration, IFN-γ–dependent fetal-like reversion of Sca-1+ ISCs occurs. Adult-stage Hpb infection in the intestinal lumen co-opts this type of response to intestinal damage via its ES products. The ES favors the differentiation of activated fetal-like Clu+ revSCs and reduces development of IL-4/IL-13 responsive immunologically involved epithelial cell lineages, such as goblet cells, important in worm expulsion. Created with BioRender.com.
As discussed, the precise molecular mechanisms by which Hpb-CM favors development of revSC remain unclear. One pathway studied in the manuscript is the Hippo tumour-suppressor pathway that is linked to regulation of organ size during development and stem cell self-renewal (Hong et al., 2016). The authors found that during Hpb infection, nuclear translocation of one of the transcriptional effectors negatively regulated by Hippo, YAP, was evident in the intestinal epithelium. Interestingly, Clu is a target gene of YAP, but the authors found that Hpb-CM induced fetal-like reprogramming of organoids as well as induction of Clu during Hpb infection was only partially Yap-dependent. Moreover, treatment of organoids with recombinant Clusterin was not sufficient to drive organoid reprogramming. Taken together, this suggests that while YAP may play some role, a network involving more than just YAP-dependent factors is key to fetal reversion of ISCs.
In order to understand the mechanisms by which Hpb-CM favors fetal-like reversion, it will be crucial to identify specific molecules in Hpb-ES responsible for the observed effects. In this regard, a plethora of specific helminth molecules are known to modify the host immune system. One molecule that already exemplifies the epithelial targeting and immunomodulatory nature of Hpb-ES products is HpARI, which binds to IL-33 to attenuate T helper type 2 cell responses following intestinal epithelial cell damage (Osbourn et al., 2017). Other molecules include VAL proteins that bind sterols with potential immunomodulatory benefit (Asojo et al., 2018) and TGF-β mimics (Johnston et al., 2017). Immunomodulation of type 2 immunity is not restricted to Hpb; for example, the Trichuris muris ES product, p43, binds to IL-13, inhibiting its activity (Bancroft et al., 2019).
Establishing the mechanisms by which helminths modify the host immune system is of importance not only to understand basic biology of tissue remodeling, but also for identifying mechanisms to control helminth infection or develop therapeutics (Coakley and Harris, 2020; Maizels, 2020). Vaccines targeted against defined ES products could provide new approaches for helminth control. Moreover, given the inverse association between helminths and inflammatory diseases, a major area of focus in pre-clinical models has been to use helminths and their ES products therapeutically to suppress inflammation (Johnston et al., 2017; Maizels, 2020; Osbourn et al., 2017). Whether some of the positive outcomes in these settings were due to direct effects of ES on epithelial stem cells rather than impacting the hematopoietic compartment will need to be explored. While studies in humans are limited, mining of available human data to investigate the emergence of fetal-like signatures following therapeutic administration of helminths could shed light on whether similar mechanisms may be clinically relevant (Broadhurst et al., 2010). Another strategy to understand effects in humans is to employ human organoid cultures. These have recently been used to explore early interactions between the large intestinal human helminth Trichuris trichiura and the epithelium (Duque-Correa et al., 2022).
Overall, this important work by Karo-Atar et al. (2022) pushes forward our understanding of how helminths can modify their environment to favor their persistence. Whether such mechanisms are common across intestinal helminth species or indeed other types of infection is an area for future research. Finally, while this study largely focuses on their involvement in helminth persistence, revSC likely develop to concurrently prevent uncontrolled life-threatening epithelial damage to the host. Thus, learning to harness revSC development, as parasitic worms already have, holds much promise for the treatment of diseases associated with epithelial barrier disruption, from inflammatory bowel diseases to certain types of cancer.
Acknowledgments
J.R. Grainger is a Senior Fellow funded by the Kennedy Trust for Rheumatology Research.
References
- Asojo, O.A., et al. 2018. Int. J. Parasitol. 10.1016/j.ijpara.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyaz, A., et al. 2019. Nature. 10.1038/s41586-019-1154-y [DOI] [Google Scholar]
- Bancroft, A.J., et al. 2019. Nat. Commun. 10.1038/s41467-019-09996-z [DOI] [Google Scholar]
- Broadhurst, M.J., et al. 2010. Sci. Transl. Med. 10.1126/scitranslmed.3001500 [DOI] [PubMed] [Google Scholar]
- Coakley, G., and Harris N.L.. 2020. Trends Parasitol. 10.1016/j.pt.2020.07.002 [DOI] [PubMed] [Google Scholar]
- Drurey, C., et al. 2022. J. Exp. Med. 10.1084/jem.20211140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Correa, M.A., et al. 2022. Nat. Commun. 10.1038/s41467-022-29334-0 [DOI] [Google Scholar]
- Gilleard, J.S., et al. 2021. Int. J. Parasitol. 10.1016/j.ijpara.2021.10.007 [DOI] [Google Scholar]
- Hong, A.W., et al. 2016. Nat. Rev. Gastroenterol. Hepatol. 10.1038/nrgastro.2016.59 [DOI] [Google Scholar]
- Johnston, C.J.C, et al. 2017. Nat. Commun. 10.1038/s41467-017-01886-6 [DOI] [Google Scholar]
- Jourdan, P.M., et al. 2018. Lancet. 10.1016/S0140-6736(17)31930-X [DOI] [Google Scholar]
- Karo-Atar, D., et al. 2022. J. Exp. Med. 10.1084/jem.20212311 [DOI] [Google Scholar]
- Maizels, R.M. 2020. Allergy. 10.1111/all.13944 [DOI] [Google Scholar]
- Nusse, Y.M., et al. 2018. Nature. 10.1038/s41586-018-0257-1 [DOI] [Google Scholar]
- Osbourn, M., et al. 2017. Immunity. 10.1016/j.immuni.2017.09.015 [DOI] [Google Scholar]
- Reynolds, G., et al. 2021. Science. 10.1126/science.aba6500 [DOI] [Google Scholar]