Abstract
Exosomes are extracellular vesicles with a diameter ranging from 30 to 150 nm, which are an important medium for intercellular communication and are closely related to the progression of certain diseases. Therefore, exosomes are considered promising biomarkers for the diagnosis of specific diseases, and thereby, treatments based on exosomes are being widely examined. For exosome-related research, a rapid, simple, high-purity, and recovery isolation method is the primary prerequisite for exosomal large-scale application in medical practice. Although there are no standardized methods for exosome separation and analysis, various techniques have been established to explore their biochemical and physicochemical properties. In this review, we analyzed the progress in exosomal isolation strategies and proposed our views on the development prospects of various exosomal isolation techniques.
Graphical abstract
Keywords: Exosome, Extracellular vesicle, Microfluidic, Isolation, Separation
Introduction
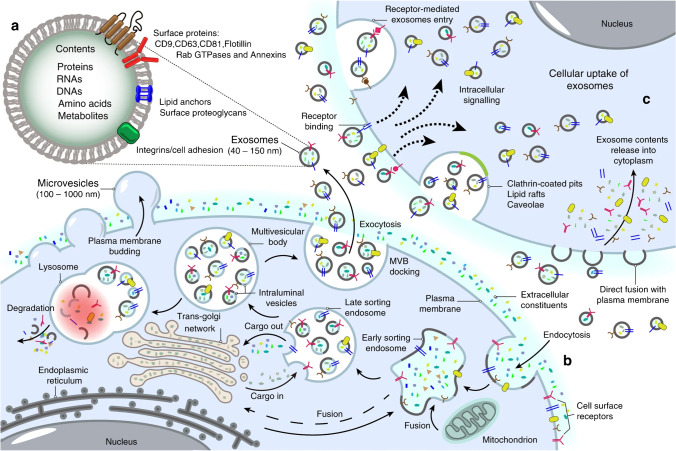
Exosome is one of the main subclasses of extracellular vesicles (EVs), and exosomes are secreted by almost all types of cells and are widely found in bodily fluids. They can carry specific signaling molecules to mediate intercellular communication (Fig. 1), regulate physiological and pathological status of receptor cells, and participate in the occurrence and development of a variety of diseases. Therefore, exosomes can be used as diagnostic biomarkers of certain diseases. Recently, exosome-based cell-free therapy has attracted considerable interest in the medical field, and the study of exosomes as drug delivery systems has also attracted significant attention among many researchers [1, 2].
Fig. 1.
Biogenesis of exosomes. Reproduced with permission [3]. Copyright 2020, Springer Nature
However, the following technical challenges have hindered the application of exosomes: simplifying the extraction process, improving the yield of exosomes, distinguishing exosomes from other EVs, and effectively analyzing and identifying exosomes. Hence, exosome therapy can demonstrate significant advantages in the future if the aforementioned problems are solved at the technical level. Therefore, it is imperative to explore an efficient and rapid exosomal isolation method. In this review, we conducted an analytical overview of the latest progress on exosomal isolation methods, established comparative analyses of various exosomal isolation techniques, and proposed our suggestions and opinions on the prospects of exosomal isolation techniques.
Main isolation techniques of exosomes
Different exosome isolation methods have been developed based on their size, shape, density, and surface proteins (Table 1). The large overlap in physical and chemical properties between exosome and non-exosome vesicles led to inclusion of a large number of non-exosome vesicles, such as microvesicles and apoptotic bodies, in “exosome samples” prepared via prior art. Therefore, unless otherwise specified, the term “exosome” used in this article refers to a mixture of small extracellular vesicles such as exosomes, apoptotic bodies, microparticles, and microvesicles.
Table 1.
Current exosome isolation methods and their advantages and disadvantages
| Method | Principle | Advantage | Disadvantage | Recovery | Purity | Time | Scalability | Sample volume | Refs |
|---|---|---|---|---|---|---|---|---|---|
| DUC | Different particles have different sedimentation coefficients |
• Suitable for large-volume samples • No other markers will be introduced • Low cost |
• High equipment • Labor-intensive • Potential destruction of exosomes |
High | Low | ≈4 h | Low | Large | [2, 4] |
| Density gradient UC | Different particles are concentrated in a specific position in the gradient medium |
• Higher purity vs. DUC • Separate exosomal subpopulation |
• Tedious operation • Limited treatment capacity • Low throughput |
Low | High | 10–18 h | Medium | Medium | [5] |
| UF | Use a membrane with a specified pore diameter or MWCO |
• Low cost • Time efficient • Simple |
• Rupture of exosome • Membrane blockage |
Medium | Medium | ≈2–4 h | High | Medium | [6] |
| SEC | Separates exosomes based on hydrodynamic radii |
• Preserve the integrity and natural biological activity • No additional preprocessing |
• Potential contamination • High equipment cost |
High | High | 15 min | High | Small | [7, 8] |
| Immunoaffinity capture | Based on antigen–antibody specific recognition and binding |
• High specificity • Simple |
• Destroy the integrity of the exosome • Expensive • Nonspecific binding |
Low | High | 2–6 h | High | Small | [9] |
| Polymer precipitation | Polymer can adhere and precipitate exosomes |
• Suitable for all types of samples • Simple and rapid • No deformation of the exosomes |
• Lead to the wrong quantification • Additional steps for higher purity |
High | Medium | 0.5–12 h | Medium | Small | [10] |
| AF4 | Laminar flow |
• Preserve the integrity • Reproducibility |
• Low resolution | Low | High | < 2 h | Low | Small | [11, 12] |
Ultracentrifugation (UC)—an active isolation method
Ultracentrifugation is the most commonly used method for isolating different biological components, and it has been considered as a classical method for exosomal isolation. It can be divided into density gradient ultracentrifugation and differential ultracentrifugation (DUC).
Differential ultracentrifugation
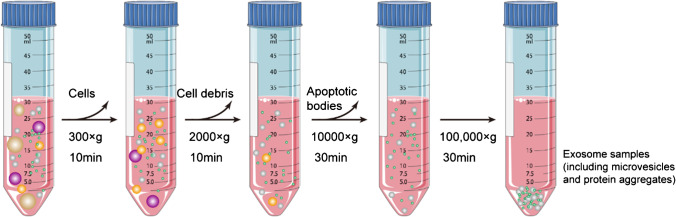
DUC is the earliest and the most frequently reported exosomal isolation strategy [13, 14]. The operation steps are shown in Fig. 2. This method involves a simple process and no other markers are introduced, and thereby, it is suitable for high-dose sample analysis. However, given that the extracellular fluid is highly heterogeneous, DUC can lead to non-ideal exosome aggregation [15]; therefore, it may not be an appropriate exosome purification method because different vesicles of similar size and protein aggregates can be co-formed at 100,000 × g. Therefore, exosomes prepared using this method should be purified further. Additionally, centrifugal force can lead to the destruction of the exosome structure, which in turn affects downstream experiments, particularly functional analysis of exosomes.
Fig. 2.
Schematic of differential ultracentrifugation–based exosome isolation. Differential ultracentrifugation is performed via multiple cycles of centrifugation with centrifugal forces in the range of 300 × g to 100,000 × g. Cells, cell debris, and apoptotic bodies are sequentially removed by controlling different centrifugal forces and time periods. After the final centrifugation (i.e., 100,000 × g), exosomes are collected by removing the supernatant. The centrifuge is operated at 4 °C
Density gradient ultracentrifugation
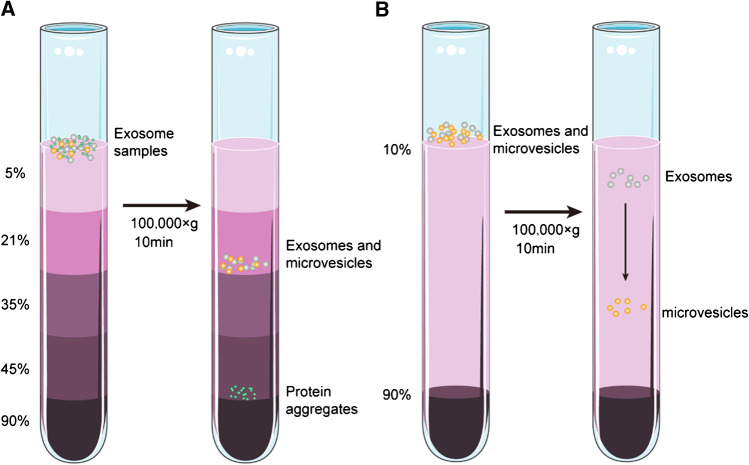
Density gradient ultracentrifugation is an improved technique based on DUC, and it involves the addition of sample particles to inert media with a density gradient (such as sucrose and cesium chloride), and thereby, it utilizes differences between the density of the particles and that of the medium. Different particles concentrate on their specific positions in the gradient medium under the action of a certain relative centrifugal force. Finally, different zones are obtained such that various high-purity particles can be collected from different zones (Fig. 3A). This method is suitable for the isolation of particles with slight difference in their sedimentation coefficient. Given the inherent density difference between different extracellular components, a more purified exosome can be obtained using this method [16]. The steps are shown in Fig. 3A.
Fig. 3.
Schematic of gradient density ultracentrifugation–based exosome isolation. A Isopycnic density gradient ultracentrifugation. B Moving-zone gradient ultracentrifugation
This technique can separate particles with poor sedimentation coefficients, such as DUC, and also separate particles with a certain density difference. The particles remain active and do not squeeze or deform.
However, this method requires the preparation of an inert gradient medium solution, long time, and relatively tedious operation. Additionally, given that this method is completely dependent on the density difference, exosomes cannot be isolated from EVs with buoyant densities (but different sizes) similar to those of exosomes [17, 18]. To effectively solve this technical issue, researchers have used moving-zone density gradient centrifugation, which separates particles based on density and size. It is used for the isolation of particles with different sizes and shapes but with similar densities. As shown in Fig. 3B, when the sample passes through the density gradient zone, with increasing density, particles with a higher density pass through the gradient layer and reach the bottom of the tube more quickly. Therefore, all solutes during centrifugation are sequentially separated based on density and mass/size. This in turn allows separation of vesicles with the same density but different sizes [17]. However, to obtain the optimum exosomal isolation, the centrifugation should be immediately stopped when the distance between the zones reaches the maximum, or the high-density medium should be installed as a buffer at the bottom of the tube. Otherwise, particles with similar density and largely distinct sedimentation coefficient can concentrate in the same zone.
Ultrafiltration (UF)—a passive isolation method
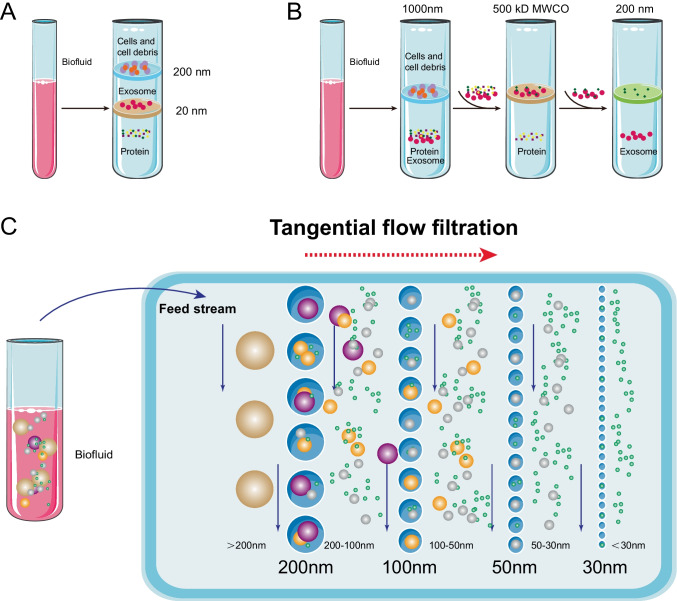
The main principle of UF involves the use of a membrane with a specified pore diameter or molecular weight cut-off (MWCO) for separating particles in a predetermined size range. Therefore, it is a size-based isolation technology [19]. Based on this principle, two types of ultrafiltration devices have been well developed: tandem-configured ultrafiltration and sequential ultrafiltration (Fig. 4A and B). Exosome recovery is dependent on the type of a filter. In a previous study, it was observed that a cellulose membrane with a pore size of 10 kDa exhibits the highest recovery efficiency [20]. UF can drastically shorten the processing time and does not require special equipment.
Fig. 4.
Schematic of ultrafiltration-based exosome isolation. A Tandem-configured ultrafiltration. When biofluids pass through the two membranes, large vesicles, including cell debris, apoptotic bodies, and cells, are trapped in the 200-nm membrane, while vesicles with diameter in the range of 20–200 nm are retained on the lower 20-nm filter. B Sequential ultrafiltration. Larger particles (e.g., cells or cell debris) are first removed via a 1000-nm filter; then the filtrate is passed through a second filter with 500-kD cut-off to remove free proteins; finally, exosomes with diameters in the range of 50–200 nm are collected from the filtrate via a 200-nm filter. C Tangential flow filtration
However, membrane blockage is a noticeable problem, which reduces the lifetime of expensive membranes and leads to low efficiency. Tangential flow filtration (TFF) techniques provide an ideal solution. TFF is in the form of cross-flow filtration (Fig. 4C). The tangential flow component destroys the formation of the concentration polarization layer, which can significantly reduce permeation flux [21]. Given that the membrane is always under a parallel flow force, potential clogging can be effectively minimized [22, 23]. Furthermore, TFF can improve the yield [24] and bioactivity of exosomes without any side effects derived from impurities [25]. Additionally, TFF exhibits greater batch-to-batch consistency than conventional isolation methods [26]. Apart from pore blockage, another limitation of UF corresponds to the co-presence of nanoparticles with sizes comparable to those of exosomes. A feasible approach to address this problem involves combining UF and other methods [2, 9].
Size exclusion chromatography (SEC)—a passive isolation method
SEC uses the initial biological fluid as the mobile phase and porous gel-filtered polymers as the stationary phase [27, 28]. The nature of the stationary phase allows different elutions (Fig. 5): The larger particles are eluted first. They are followed by the smaller vesicles and then the non-membrane-bound proteins (as larger particles can pass through fewer pores, they pass through a shorter path to the end of the column and elute faster). Therefore, eluents at different stages contain particles of different sizes.
Fig. 5.
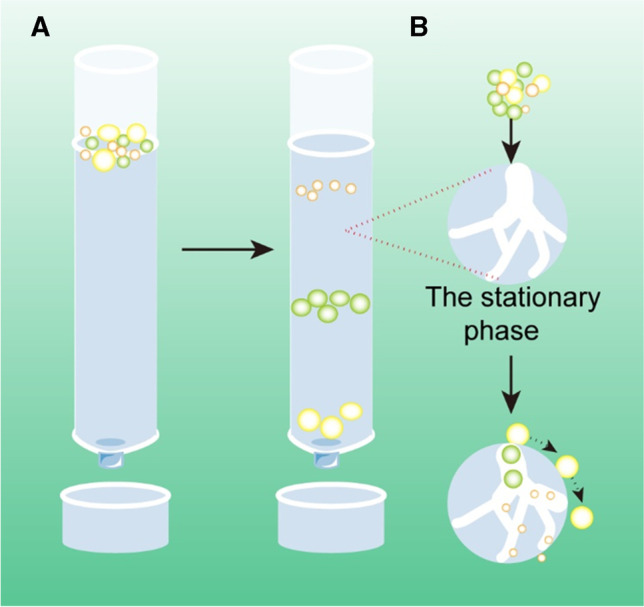
Schematic of size exclusion chromatography–based exosome isolation
When compared with DUC, SEC exhibits certain significant advantages. First, SEC has been shown superior to other techniques in terms of the purity of isolated exosomes, which is mainly realized by improving protein contamination [29]. Second, it can preserve the integrity and natural biological activity of isolated exosomes [30]. This is probably because SEC does not rely on centrifugal force, which is in sharp contrast to the DUC. Third, SEC is suitable for use with a small sample size, which is reported to be as low as 15 µL [31]. Fourth, the SEC is efficient in terms of time and effort, and the entire process can be completed in a short time (15 min) [31, 32]. Fifth, similar to the UF method, different pore sizes of the materials that are used can produce specific subsets of EVs. Finally, the SEC’s non-contact strategy (solute does not interact with the stationary phase) is more efficient than UF, and thereby, ensuring no or minimal sample loss [33, 34]. In view of these advantages, SEC has recently been considered as the optimum method for isolating morphologically and functionally intact exosomes from plasma [35]. Importantly, this method is easy to expand and automate, and it can be used for the preparation of high-throughput exosomes.
SEC has several shortcomings. First, SEC cannot avoid the existence of contaminants of similar size. To eliminate these pollutants, Gardiner proposed an exosomal isolation strategy that combines UF and SEC [36]. Later, Shu et al. confirmed the feasibility of this strategy [37]. Second, the total yield isolated from SEC is low. However, SEC isolation can be expanded. To avoid this problem, combining SEC with DUC can evidently reduce exosomal loss, ensure the yield, and effectively remove impurity proteins, which is more suitable for target proteomics and RNA analysis [30, 38].
Polymer precipitation method—an active isolation method
Polymers can create a hydrophobic microenvironment by hijacking water molecules that surround exosomes and, thereby, forces exosomes to leave the solution and sediment under low-speed centrifugation (Fig. 6) [39]. Currently, the commonly used polymers are polyethylene glycol (PEG), lectins, protamine, and sodium acetate.
Fig. 6.
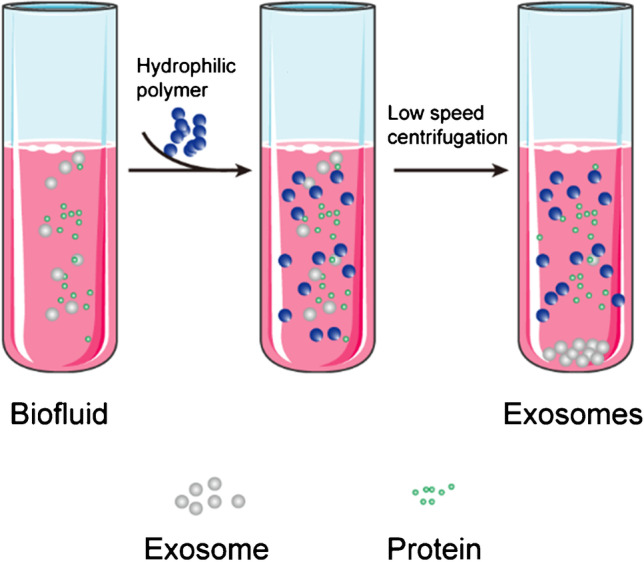
Schematic of polymer precipitation–based exosome isolation
When compared with ultracentrifugation or SEC, this method exhibits the advantages of simplicity, scalability, high yield, no deformation of exosomes, and can be used for a large number of samples without additional equipment [9]. Additionally, it can be used to quickly diagnose diseases by integrating various detection platforms for exosome (or protein/genetic material content) analysis [40]. These advantages show that this method has potential advantages for future clinical research.
However, the purity of exosomes obtained by polymer is lower, which is mainly due to the co-precipitation of soluble non-exosomal proteins, virions, immune complexes, and other pollutants [13, 41]. Currently, the quantification of exosomes is generally dependent on the total protein content of test samples. Therefore, given the existence of protein pollutants, this method can inevitably lead to incorrect quantification of exosomal preparations, and it can affect downstream analysis. Hence, it is not considered as an appropriate method for descriptive or functional analysis of exosomes [42].
Immunoaffinity capture—a label-based isolation method
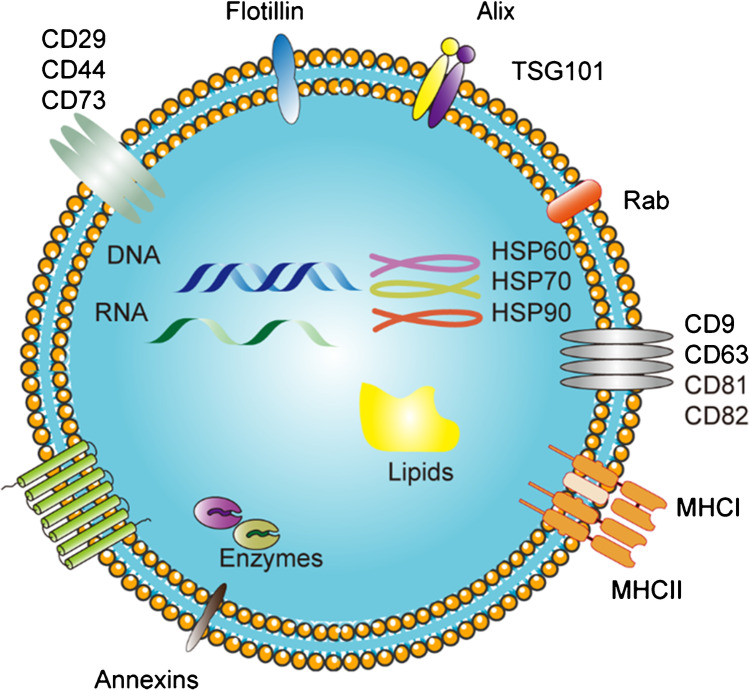
Some specific proteins, lipids, and polysaccharides exist on the surface of exosomes (Fig. 7), which are ubiquitous in all exosomes; therefore, exosomes can be separated based on the principle of antigen–antibody specific recognition and binding.
Fig. 7.
Typical representative composition of exosomal cargo
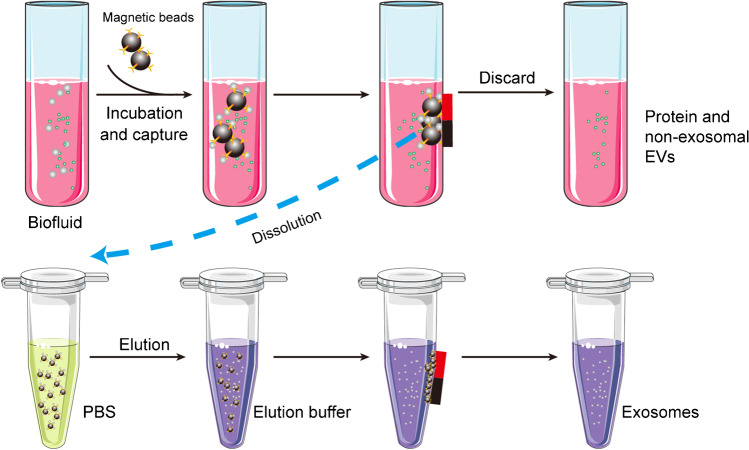
To effectively separate exosomes based on immunoaffinity, the antibody must be immobilized on a solid surface. Hence, magnetic beads are most commonly used (Fig. 8).
Fig. 8.
Schematic of the magnetic bead–based exosome isolation
When compared with ultracentrifugation, exosomes isolated via immunoaffinity techniques contain at least two times the number of exosome markers and proteins, indicating that exosomes isolated by this method exhibit higher specificity and yield [43, 44]. Conversely, this method usually leads to a “biased” isolation, for example, the isolation of CD9+exosome can also lead to the exclusion of CD9− exosome, and thereby, reducing the yield. Furthermore, isolated exosomes exhibit higher purity, and this “biased” isolation is conducive to the study of parental cells and can provide an important index for the diagnosis of certain specific diseases. Among them, the most typical method involves evaluating the existence of epithelial cell adhesion molecule (EpCAM)–related cancer by detecting EpCAM-positive exosomes [45].
Generally, the use of antibodies can shorten the time of isolation, improve the purity of exosomes, and enable the acquisition of specific exosomal components [45]. However, the isolation efficiency is related to the specificity and quality of antibodies because most of the commercially available antibodies for immunoprecipitation are non-specific. Thus, exosomes are non-specifically adsorbed on the solid phase. Additionally, the non-neutral pH and non-physiological elution buffers (to separate exosomes and antibodies) can irreversibly affect the biological function of the collected exosomes. Although these exosomes can typically be used for diagnostic purposes, they are not conducive to exosome-based functional research and various therapeutic applications [46, 47]. Moreover, it can only be suitable for small-sample studies because more expensive antibodies are required to address large samples, which limits its large-scale use to a certain extent. To solve these problems, a feasible method involves the use of immunoaffinity as an additional step in conjunction with DUC to improve the purity of isolated exosomes [48].
Aptamer-based method—a label-based isolation method
Aptamers are short single-stranded DNA or RNA sequences that can recognize and bind to their targets with high affinity and specificity in a manner similar to antibodies. In contrast to traditional antibodies, aptamers can be produced by chemical synthesis in vitro and exhibit several advantages such as small variation between batches, low or no immunogenicity, low cost, and easy chemical modification [49, 50]. Therefore, they can be used as substitutes for antibodies. In 2021, Zhang et al. presented an electrochemical micro-aptasensor that can realize sensitive, specific, and reliable detection of exosomes from cancer cells and enable quantitative evaluation of the exosome concentration by introducing anti-EpCAM aptamers [51]. In the past few years, several aptamer-mediated exosomal isolation platforms have been developed [52, 53]. Therefore, we believe that aptamer-mediated exosomal isolation exhibits significant potential for various applications.
Microfluidic technique based on fluid properties
Microfluidic technique is a high-throughput method, which is compatible with a variety of exosomal isolation methods. Importantly, microchannels can be connected together according to specific requirements [54]. A microfluidic device exhibits several advantages. First, it consumes less sample volume, reagents, and isolation time [55]. Second, when combined with other exosomal isolation methods, it can synergistically improve the yield and purity of exosomes. However, certain related unsolved problems still persist. First, the samples used for analysis can block the channel. Second, the output of exosomes is also low because of the small sample size. Additionally, this method requires advanced equipment, which limits its large-scale applications.
Size-based microfluidic isolation technique—a passive and label-free isolation method
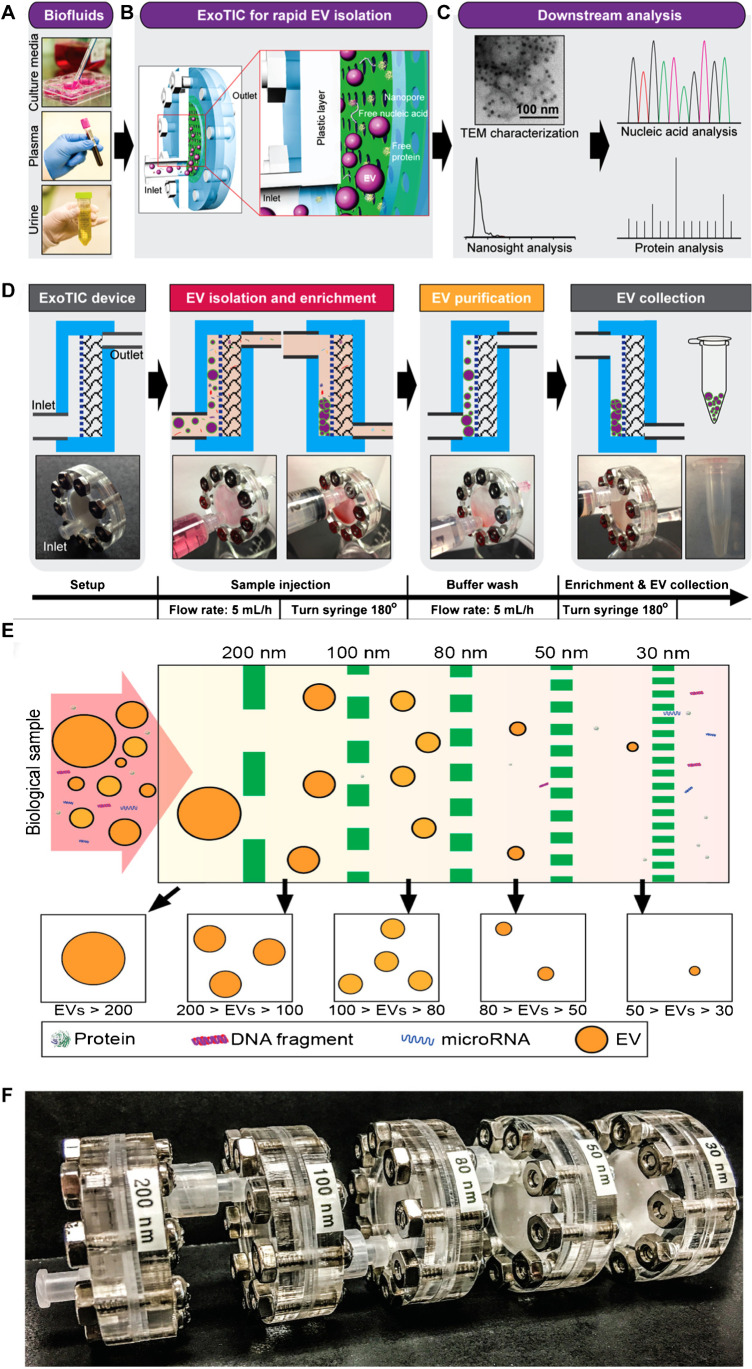
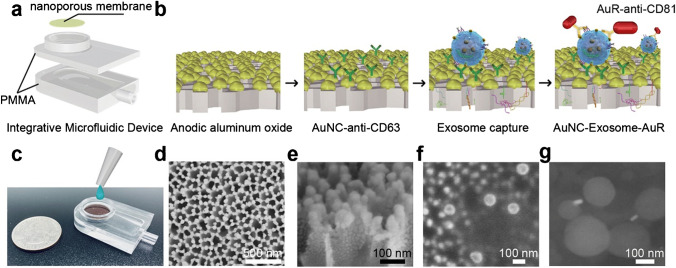
Several size-based microfluidic devices for exosomal isolation have been developed to date. The first device corresponds to the exosome total isolation chip (ExoTIC) [79]. It is a module unit composed of membranes with different pore sizes (Fig. 9). Multiple module units can be connected to isolate exosomes of a specific range of sizes. ExoTIC has a simple structure, easy to use, and has little effect on the natural exosomal structure. The system can realize high recovery and isolation purity with a small sample size (10–100 μL), making it suitable for clinical testing [56]. The second device corresponds to the nanowire-based exosome trip system. This device can separate exosomes with 60% recovery [80]. In 2020, Yang et al. presented an integrated microfluidic device with high sensitivity and specificity for isolating and detecting exosomes (Fig. 10). The principle of this device involves adjusting the membrane pore size by ion-sputtering gold layers of different exosomes [60]. Although size-based microfluidic isolation techniques (similar to SEC) can realize high throughput, the purity should be further improved.
Fig. 9.
Schematic illustration of the ExoTIC device for EV isolation. A Various biofluids can be processed. B Schematic illustration of the ExoTIC device. Intact EVs are enriched and purified at the filter, whereas the free proteins and nucleic acids are washed out. C Downstream analysis of isolated EVs. D Schematic of EV isolation from sample-in to EV-out. Total operation time for 5–10 mL of sample is under 3 h. E Design schematic of the ExoTIC device. F Image of an actual modular ExoTIC device. It is comprised of 5 modules, each module with a different membrane pore size, that connect in series for isolation of EVs. Reproduced with permission [56]. Copyright 2017, American Chemical Society
Fig. 10.
Integrative microfluidic device for exosome isolation and detection. a Design of the integrative microfluidic device. b Schematic illustration of in-situ detection of exosome. c A photographic image of the integrative microfluidic device. d SEM image of nanoporous gold (Au) nanoparticles deposited on AAO membrane with a thickness of 50 nm. e, f The side view of Au coating. g SEM image of the formed complex nanoporous gold nanocluster (AuNC)-Exosome-AuR. Reproduced with permission [60]. Copyright 2020, Elsevier
Deterministic lateral displacement (DLD) technique—a passive and label-free isolation method
DLD device is primarily composed of a cylindrical gradient array with a specific critical size for particle isolation [81]. The principle of DLD involves changing the flow path of particles larger than the critical size without affecting the paths of other particles. In 2014, Santana et al. developed a DLD microfluidic device that can be used to isolate microvesicles from different populations of cancer cell–derived EVs (including exosomes) [62]. In 2016, Wunsch et al. designed, for the first time, nanoscale DLD arrays. Importantly, exosomes were successfully separated using this device, demonstrating its potential for exosomal chip sorting and quantification [63]. Recently, DLD has been used as a label-free and easy-to-use technology to separate exosomes in microfluidic chips. However, current size-based technologies are still limited by exosomal saturation and low recovery [63]. Additionally, owing to the risk of blockage, it presents the challenge of low purity.
Immune microfluidic technique—a label-based isolation method
Similar to the general method of exosomal isolation based on immunoaffinity, exosomes are isolated via specific binding of antibodies fixed on microfluidic devices (also known as chips) to exosome markers. The advantages of this technique include efficiency, fast processing, simple operation, and small sample size. Therefore, it has attracted a considerable interest in the exosomal research.
However, the integrity and purity of exosomes obtained by this method should be improved. Non-specific binding is another problem in microfluidic immunoaffinity isolation because blocking and washing steps cannot be conducted in microfluidic devices. Nanotechnology has provided valuable opportunities for solving this problem. In 2014, Ramanathan et al. presented a microfluidic system with a fivefold increase in capture and detection performance when compared to that of hydrodynamic fluid flow, and this approach exhibits the potential of being applicable to essentially any biochemical assay based on immunocapture [82]. Dudani et al. used inertial manipulation of antagonist-coated magnetic beads to realize a high signal-to-noise exosomal isolation [65]. Another solution involves the use of monoclonal antibodies to reduce non-specific binding. Additionally, the exosomes bound to the antibodies should be eventually released by dissolving in an acidic solution, which can contaminate the collected exosomes, and thereby, affecting its downstream application. A possible solution involves allowing release of the exosomes in a neutral solution. In 2021, Suwatthanarak et al. used NaCl solution to release captured exosomes, which showed integrity in downstream miRNA analysis [67].
Dielectrophoretic (DEP) technique—an active and label-free method isolation
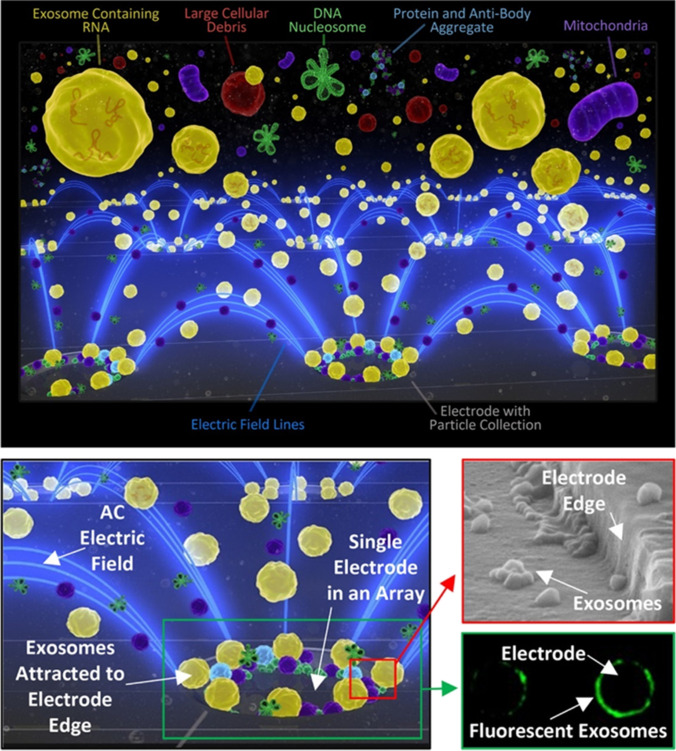
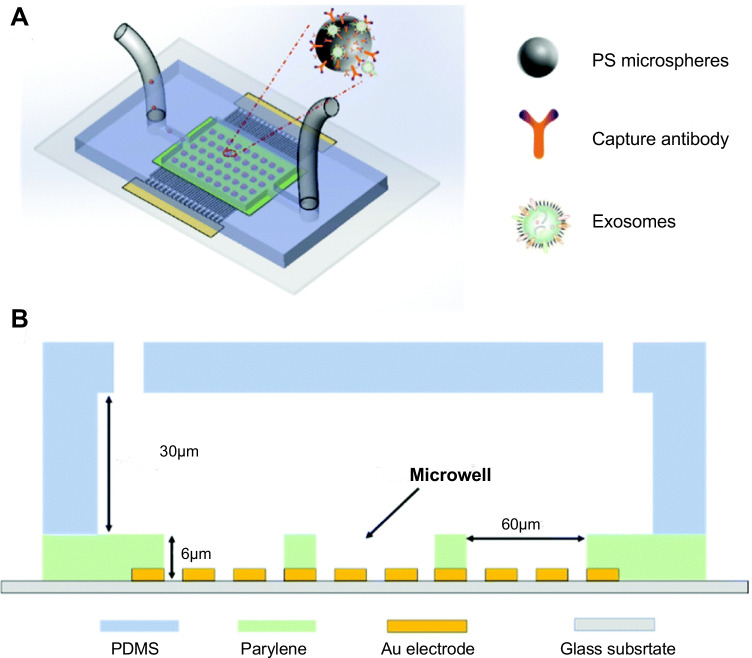
In a non-uniform electric field, particles are polarized and subjected to dielectric force related to their size (inversely proportional to its radius) and electrical properties. Therefore, under this electric field, smaller particles can be captured via a larger gradient of the square electric field (and vice versa), and size-dependent exosomal isolation can be realized [68, 83]. In 2017, Ibsen et al. used alternating current electrokinetic microarray chips to rapidly isolate glioblastoma exosomes from undiluted plasma (Fig. 11). The samples required by this method can be as low as 30 μL and exosomes can be separated within 30 min [68]. In 2019, Ayala-Mars et al. designed a direct current insulator-based dielectrophoretic approach that can simultaneously capture and separate exosomes based on size [70]. In 2020, Stanley et al. designed a unique microfluidic device based on the DEP force and electrohydrodynamic drag in low- and high-conductivity media, which can separate high-quality and high-purity nanoparticles, such as exosomes, from biological body fluids [84]. In 2021, Zhao et al. developed a microfluidic device (ExoDEP-chip) for exosome isolation and detection (Fig. 12). Exosomes were first isolated via binding to antibodies pre-immobilized on the polystyrene (PS) microsphere surface. Then, single microspheres were trapped into single microwells under the DEP force in the ExoDEP-chip, which realized microsphere mediated DEP isolation and immunoaffinity detection. The method exhibited a low limit of detection and a large detection range and also realized protein analysis of different cell lines [71]. However, given Joule heating (JH) and electrothermal heating effects, a good electrode design must be adopted to avoid their effect on the isolation performance [85]. Currently, a few reports examined the isolation of exosomes based on DEP technology, and this is mainly due to the low resolution and low purity. In addition, the complete integrity of exosomal function remains to be explored. Therefore, further in vitro research and downstream analyses, such as RNA sequencing and proteomic analyses, are necessary. However, a method based on DEP is unlabeled, non-contact, rapid, and provides a high throughput, suggesting good application prospects.
Fig. 11.
Schematic representation of exosome isolation on the ACE device (chip) microelectrodes. Electric field lines (blue) run between individual microelectrodes on the microarray and converge onto the edges of the microelectrodes, thereby forming the DEP high-field regions. The exosomes collect in the high-field regions while cells or larger particles in the sample are concentrated into the DEP low-field areas between microelectrodes, and lower molecular weight biomolecules are unaffected by DEP electric fields. A fluid wash removes any cells and the other plasma materials while the nanosize biomarkers (exosomes, etc.) remain concentrated in the DEP high-field regions. Reproduced with permission [68]. Copyright 2017, American Chemical Society
Fig. 12.
Schematic of exosome isolation on the ExoDEP-chip. A Schematic of the ExoDEP-chip. B Cross-section view of the ExoDEP-chip. Reproduced with permission [71]. Copyright 2021, The Royal Society of Chemistry
Acoustofluidic isolation—an active and label-free isolation method
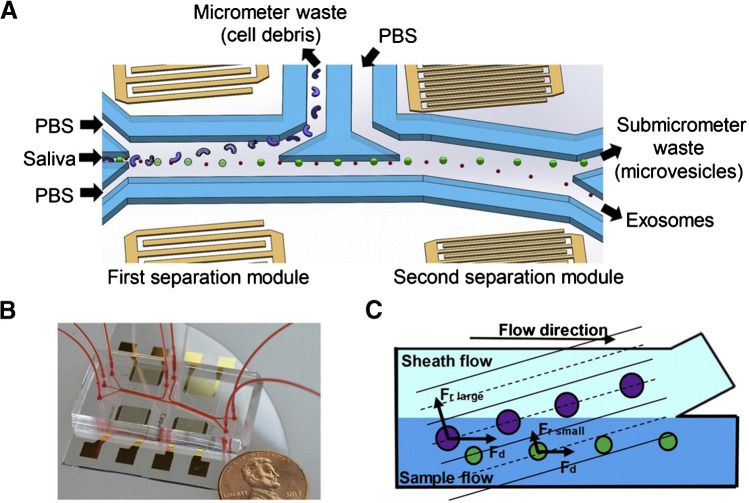
Particles with different mechanical properties (such as size and density) are subjected to different acoustic forces in the sound field, thereby realizing isolation of different particles. In 2017, Wu et al. developed an exosomal isolation chip based on acoustofluidic technology capable of directly isolating exosomes or other types of EVs from undiluted whole-blood samples in an automated fashion, and the purity of the exosomes isolated from a mixture of EVs was 98.4% [72]. In 2020, Wang et al. developed an acoustic flow control platform that separated exosomes, and the average yield of exosomal small RNA extracted from this platform was 15 times higher than that of the DUC (Fig. 13) [75].
Fig. 13.
Schematic and mechanism of the acoustofluidic device for exosome separation. A, B Schematic and optical image of the acoustofluidic device (penny shown for size comparison). C There is a size-based separation in each module. Given the acoustic radiation force (Fr) and a drag force induced by fluid (Fd), larger particles are separated into the sheath flow while small particles remain in the primary sample flow. Reproduced with permission [75]. Copyright 2020, Elsevier
However, acoustofluidic isolation is mainly used to isolate larger objects, such as cells and bacteria, and few breakthroughs were achieved in the isolation of exosomes because it is still challenging to manipulate objects below 100 nm. Currently, although few studies have reported exosome isolation based on acoustics, it is expected that with the unlabeled, rapid, and non-contact technology, a variety of acoustics-based microfluidic chips may garner more interest in the field of exosomal isolation (Table 2).
Table 2.
Studies of microfluidic techniques for exosome isolation
| Study | Principle | Year | Biofluids | Recovery | Purity | Time | Sample volume |
|---|---|---|---|---|---|---|---|
| Liu et al. [56] | Size-based | 2017 | Plasma, lavage, and urine | > 90% | High | < 3 h | 10 μL–120 mL |
| Sunkara et al. [57] | Size-based | 2019 | Blood, plasma | 75% | 107–108 particles/μg protein | 10–40 min | 30–600 μL |
| Han et al. [58] | Size-based (TFF) | 2021 | Cell culture supernatant, plasma | 80% | 97% | < 3 h | 500 μL |
| Liu et al. [59] | Size-based | 2020 | Cell culture medium and serum | 94.3% | 87.9% | 1 h | 100 μL |
| Yang et al. [60] | Size-based | 2020 | Urine | 5 × 109 particles/5nL | High | Long | 5 mL |
| Inci et al. [61] | Size-based | 2022 | Cell culture medium | 1.46 × 108 ± 2.82 × 107 particles/mL | NA | NA | 2 mL |
| Santana [62] | DLD | 2014 | Cell culture supernatant | < 40% | > 98% | < 1.5 h | 170 μL |
| Wunsch et al. [63] | DLD | 2016 | Urine | NA | NA | > 24 h | 10 μL |
| Smith et al. [64] | DLD | 2018 | Urine, serum | 50% | NA | 1 h | 900 μL |
| Dudani et al. [65] | Immunoaffinity | 2015 | Cell culture supernatant, blood | NA | NA | > 6 h | NA |
| Hisey et al. [66] | Immunoaffinity | 2018 | Serum | ~ 60% | NA | < 1 h | 100 μL |
| Suwatthanarak et al. [67] | Immunoaffinity | 2021 | Cell culture medium | ~ 70% | fourfold high than UC | < 2 h | 1 mL |
| Ibsen et al. [68] | DEP | 2017 | Plasma | NA | NA | 30 min | 30–50 μL |
| Lewis et al. [69] | DEP | 2018 | Blood | NA | NA | 30 min | < 25 μL |
| Ayala-Mars et al. [70] | DEP | 2019 | Cell culture supernatant | High | NA | < 30 min | 100 |
| Zhao et al. [71] | DEP | 2021 | Exosomes suspended in PBS | 83.5% | NA | 1 µL/min | NA |
| Wu et al. [72] | Acoustic force | 2017 | Blood | 82.4% | 98.4% | < 30 min | 100 μL |
| Evander et al. [73] and Ku et al. [74] | Acoustic force | 2018 | Cell culture supernatant, blood, urine | 9.3% | NA | 30 min | 0.3–0.5 mL |
| Wang et al. [75] | Acoustic force | 2020 | Saliva | 15.18-fold higher than DUC | High | 10–20 min | NA |
| Tayebi et al. [76] | Acoustic and electric forces | 2021 | Cell culture supernatant | 81% | > 95% | NA | NA |
| Tay et al. [77] | Spiral inertia | 2021 | Blood | threefold increase compared to UC | High (details unknown) | 1 h | 5 mL |
| Chen et al. [78] | A chitosan-modified shuttle flow | 2021 | Serum | > 84% | > 90% | 15 min | 10 μL |
Asymmetric flow field-flow fractionation (AF4)—a passive isolation method
The AF4 is a size-based isolation technique. In contrast to SEC, AF4 exhibits programmable cross-flow intensity, which can be optimized in exosomal isolation to improve efficiency [86]. Thus, AF4 is very attractive to isolate of EV subsets. Zhang et al. successfully classified the subclasses of EVs based on the technique [11, 12]. The significant potential of AF4 in separating exosomes with high reproducibility and purity is demonstrated in extant studies [87, 88]. Therefore, AF4 technique can serve as a powerful platform for the isolation/purification of exosomes, and the technology can promote the development of exosomes including proteomics, biomarker discovery, and functions.
Prospects and challenges
Currently, research on exosomes is mainly focused on the following aspects: (1) the role of exosome in various diseases, (2) exploration of the therapeutic effect of exosome in diseases, (3) use of exosome as a drug delivery system to deliver drugs to specific areas of the body. All of the aforementioned aspects are based on obtaining high-quality exosomes. Therefore, exploring an efficient isolation method is the first prerequisite for exosomal application. In the past few decades, exosomal isolation technique has also undergone rapid development. However, a reality for most researchers is that they are often unable to immediately find an exosome isolation method that is completely appropriate for their research. Additionally, due to financial constraints, not all researchers have the ability to attempt different methods. Hence, a reference is of great significance for the progress of the entire research and cost savings.
The ideal exosomal isolation method is relatively simple, fast, does not require complex or expensive equipment, and allows exosome isolation from a large number of samples with high throughput, purity, and recovery rate. Unfortunately, no specific technique (even the ultracentrifugation, which is the current gold standard (Table 1)) does not satisfy these requirements. Existing techniques should be fully evaluated in large-scale studies to determine their stability and repeatability. In addition, it is also a challenge to ensure that the exosomes exhibit high biological stability and activity without compromising the subsequent analysis of exosomes.
Thus, the problem of obtaining an efficient exosomal isolation method is not resolved, which is largely due to the complexity of biological body fluids, significant overlap between physical, chemical, and biochemical properties of exosomes, lipoproteins, viruses and other EVs, and heterogeneity of exosomes themselves [18]. These factors further limit the downstream analysis of exosomes. Thus, the combined application of two or more isolation techniques provides a feasible strategy for effective isolation. In 2021, Tayebi et al. demonstrated an approach based on electrical and acoustic forces to manipulate exosomes for deterministic sorting. The acoustic/electric approach and specifically exosomes (< 200 nm) and microvesicles (> 300 nm) can be used to sort subpopulations of EVs. More importantly, the purity of the exosomes obtained via this approach exceeded 95%, and the recovery rate was 81%, which significantly exceeded those of comparable approaches [76].
However, although combined different isolation techniques can improve the purity of exosomes, they typically increase the difficulty and cost of operation and can lead to decreases in overall yield and unreliable downstream analysis. Therefore, prior to selecting an isolation strategy, it is necessary to carefully consider the nature of the sample and the purpose of the study to choose an appropriate combination of techniques. For example, when using immunoaffinity-based methods, pretreatment of polymer precipitation can improve the efficiency of exosomal isolation and avoid the use of several expensive antibodies [89].
The advantages of UF include simple operation, convenient analysis of large quantities of biological samples, and the ability to separate exosomes of specific sizes. However, it also displays evident limitations, particularly membrane blockage, which shortens the life of the expensive membrane and reduces isolation efficiency, thereby leading to the misinterpretation of test results. Hence, TFF technology can effectively solve this problem. Although a few limitations continue to exist in current TFF technology, we believe that by considering the continuous progress of fluid mechanics and material science, the use of the TFF technique will significantly increase in the field of exosomal isolation. Similarly, SEC offers high-quality isolation of exosomes, good repeatability, and considerable potential for high-throughput industrial applications. A significant advantage of SEC is that it preserves the integrity and natural biological activity of the isolated exosomes. Therefore, isolation by SEC can maximize exosomal functions. In addition, SEC exhibits technical advantages such as removing most excess soluble plasma proteins to allow for the recovery of purer exosome preparations. Specifically, SEC is more user-friendly and less time-consuming than other isolation methods and maintains characteristics of exosomes to ensure upcoming applications. Based on the aforementioned advantages, we expect that SEC can be easily adapted in most laboratories. Although SEC is a feasible candidate for exosomal isolation, additional direct comparative studies are needed to support this conclusion. Hence, we also expect that a combination of UF and SEC can facilitate the preparation of clinical-grade exosome products in the future.
Moreover, for any isolation method, it is essential to standardize protocols used by all laboratories. Even with the same exosomal isolation methods, the diversity of protocols interferes with the verification, comparison, and analysis of data obtained via different research teams. Therefore, it is very important to comprehensively examine exosomal isolation schemes and standardize characterization of exosomes. Several methods (transmission electron microscopy, nanoparticle tracking analysis, dynamic light scattering, flow cytometry, and immunohistochemical analysis) can be combined to characterize morphology, biochemical components, and receptors of exosomes [90]. In addition, when using a specific method to isolate exosomes, it is necessary to consider the characteristics of the sample to be analyzed because the specific scheme should be adapted to the specific characteristics of the sample such as the presence of specific proteins and viscosity. Different methods can produce different EV subsets. Each exosome isolation method displays its own advantages and disadvantages (Table 1), and thus it may be impossible to develop a general exosomal isolation method. Therefore, a specific method or several specific combinations of different techniques can be selected based on the type of the initial sample to be processed and the purpose of the study. We believe that it is currently important to develop one or more isolation methods that are suitable for various scientific and clinical studies.
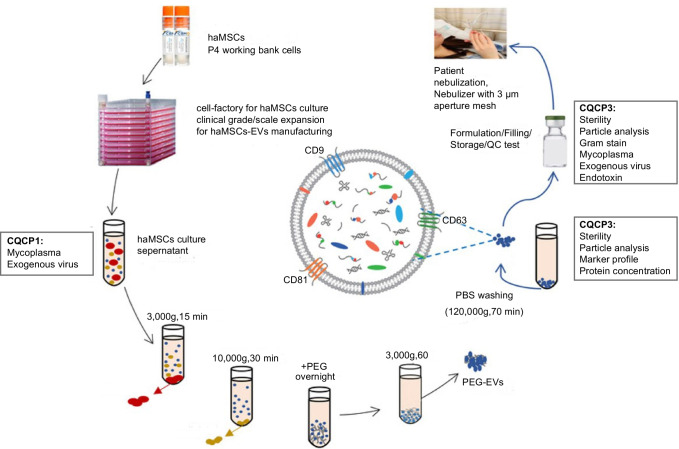
Currently, scientific research on exosomes mainly focuses on basic research, where only a small quantity of exosomes is sufficient for research purposes. In contrast to basic research, clinical research focuses on convenience, stability, and accuracy of isolation techniques. In addition, the yield of exosomes should also be considered. Requirements for exosomal yield are not extremely strict for diagnosis purposes while large-scale exosome production with high quality must be guaranteed for treatment purposes. However, current techniques are limited in their ability to produce clinical-grade exosomes for scaled-up manufacturing processes [91]. In a preclinical study in 2021, Shi et al. successfully prepared clinical-grade extracellular vesicles via a technical combination of ultracentrifugation and PEG (Fig. 14) [92], and they used this method in a subsequent clinical study (MEXCOVID, NCT04276987) to obtain clinical-grade exosomes and explore the safety and efficacy of aerosol inhalation of the exosomes in patients with COVID-19 and indicated a good therapeutic effect [93].
Fig. 14.
Manufacture of haMSC-EVs. haMSC-EVs, human adipose–derived MSC-extracellular vesicles; NTA, nanoparticle tracking analysis; CQCP, critical quality control points. Reproduced with permission [92]. Copyright 2021, The authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles
In summary, despite significant progress in microfluidic technology in recent years, it necessitates considerable development prior to clinical use. The relatively low efficiency of microfluidic techniques can adversely affect downstream assessment (genome and proteome analysis) and lead to inaccurate diagnostic results. Therefore, it is imperative to develop an unlabeled passive microfluidic technique for exosomal isolation because it is simpler and does not lead to outfield or surface markers. In 2021, Tay et al. developed a continuous-flow and scalable microfluidic device to isolate exosomes from whole blood. This device is a passive (hydrodynamic) isolation technique with high efficiency (threefold increase in yield compared to UC) [77]. Liu et al. reported a novel microfluidic device that can separate exosomes from microliters of cell culture media and human serum in a label-free, continuous-flow, and size-dependent manner and achieved a high recovery rate (94.3%) and high purity (87.9%) [59]. In 2021, Chen et al. proposed a simple, fast, and label-free chitosan-modified shuttle flow microchip to isolate exosomes from trace serum of patients. This chip could flexibly capture and release exosomes with a purity of over 90% and an RNA recovery ratio over 84% within 15 min, which is not possible for ultracentrifugation methods [78]. Therefore, it is expected that improvements in microfluidic devices will certainly bring more opportunities to the medical field in the future, and simple, continuous, and rapid exosomal isolation can be achieved via this method. However, further exploration is needed to overcome the Brownian motion of exosomes in microchannels to improve efficiency.
In addition, given that exosomes are a new class of drugs and specific guidelines on the exosomal manufacture and quality assessment have not been published, it is critical for developers to closely communicate with regulators to proactively address problems that may arise in biotherapeutic exosomes [91].
Conclusion
In this study, we reviewed the latest advances in exosomal isolation techniques including conventional and microfluidic techniques. Despite significant progress and the increasing maturity of exosomal isolation techniques, none of the existing techniques is perfect and considerable explorations are necessary prior to clinical application. We suggest that combining different isolation techniques will isolate specific exosome subsets with high purity. Currently, sufficient clinical samples are required to test the stability, accuracy, and convenience of each technique. Continuous efforts by researchers are believed to realize the development of an ideal technique that can be easily used in clinical applications in the future.
Abbreviations
- EVs
Extracellular vesicles
- DUC
Differential ultracentrifugation
- UF
Ultrafiltration
- MWCO
Molecular weight cut-off
- TFF
Tangential flow filtration
- SEC
Size exclusion chromatography
- PEG
Polyethylene glycol
- EpCAM
Epithelial cell adhesion molecule
- DLD
Deterministic lateral displacement
- DEP
Dielectrophoretic
- PS
Polystyrene
- JH
Joule heating
- AF4
Asymmetric flow field-flow fractionation
- FCM
Flow cytometry
Funding
This work was supported by the Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2021-QN-A19), A joint project of TCM and western medicine to tackle major diseases, Robot-assisted precise treatment solution for scoliosis (KXW-2021-J), and Cuiying Scientific Training Program for Undergraduates of Lanzhou University Second Hospital (CYXZ2020-02).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wen-zhao Liu and Zhan-jun Ma contributed equally to this study and share first authorship.
References
- 1.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32(3–4):623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ni Z, Zhou S, Li S, Kuang L, Chen H, Luo X, et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig AK, De Miroschedji K, Doeppner TR, Börger V, Ruesing J, Rebmann V, et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J Extracell Vesicles. 2018;7(1):1528109. doi: 10.1080/20013078.2018.1528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding L, Yang X, Gao Z, Effah CY, Zhang X, Wu Y, et al. A holistic review of the state-of-the-art microfluidics for exosome separation: an overview of the current status, existing obstacles, and future outlook. Small. 2021;17(29):e2007174. doi: 10.1002/smll.202007174. [DOI] [PubMed] [Google Scholar]
- 7.Takov K, Yellon DM, Davidson SM. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: yield, purity and functional potential. J Extracell Vesicles. 2019;8(1):1560809. doi: 10.1080/20013078.2018.1560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lozano-Ramos I, Bancu I, Oliveira-Tercero A, Armengol MP, Menezes-Neto A, Del Portillo HA, et al. Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J Extracell Vesicles. 2015;4:27369. doi: 10.3402/jev.v4.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel GK, Khan MA, Zubair H, Srivastava SK, Khushman M, Singh S, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep. 2019;9(1):5335. doi: 10.1038/s41598-019-41800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M, et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2020;9(1):1697028. doi: 10.1080/20013078.2019.1697028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc. 2019;14(4):1027–1053. doi: 10.1038/s41596-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347. doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9(26):8206–8220. doi: 10.7150/thno.37455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 16.Street JM, Koritzinsky EH, Glispie DM, Yuen PST. Urine exosome isolation and characterization. Methods Mol Biol. 2017;1641:413–423. doi: 10.1007/978-1-4939-7172-5_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim YJ, Lee SJ. Are exosomes the vehicle for protein aggregate propagation in neurodegenerative diseases? Acta Neuropathol Commun. 2017;5(1):64. doi: 10.1186/s40478-017-0467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen BY, Sung CW, Chen C, Cheng CM, Lin DP, Huang CT, et al. Advances in exosomes technology. Clin Chim Acta. 2019;493:14–19. doi: 10.1016/j.cca.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Xu R, Simpson RJ, Greening DW. A protocol for isolation and proteomic characterization of distinct extracellular vesicle subtypes by sequential centrifugal ultrafiltration. Methods Mol Biol. 2017;1545:91–116. doi: 10.1007/978-1-4939-6728-5_7. [DOI] [PubMed] [Google Scholar]
- 20.Vergauwen G, Dhondt B, Van Deun J, De Smedt E, Berx G, Timmerman E, et al. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci Rep. 2017;7(1):2704. doi: 10.1038/s41598-017-02599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh R. Rapid antibody screening by membrane chromatographic immunoassay technique. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844(1):163–167. doi: 10.1016/j.jchromb.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Lebreton B, Brown A, van Reis R. Application of high-performance tangential flow filtration (HPTFF) to the purification of a human pharmaceutical antibody fragment expressed in Escherichia coli. Biotechnol Bioeng. 2008;100(5):964–974. doi: 10.1002/bit.21842. [DOI] [PubMed] [Google Scholar]
- 23.Heinemann ML, Ilmer M, Silva LP, Hawke DH, Recio A, Vorontsova MA, et al. Benchtop isolation and characterization of functional exosomes by sequential filtration. J Chromatogr A. 2014;1371:125–135. doi: 10.1016/j.chroma.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 24.You DG, Lim GT, Kwon S, Um W, Oh BH, Song SH, et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021;7(23):eabe0083. [DOI] [PMC free article] [PubMed]
- 25.Kim JY, Rhim WK, Yoo YI, Kim DS, Ko KW, Heo Y, et al. Defined MSC exosome with high yield and purity to improve regenerative activity. J Tissue Eng. 2021;12:20417314211008626. doi: 10.1177/20417314211008626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busatto S, Vilanilam G, Ticer T, Lin WL, Dickson DW, Shapiro S, et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells. 2018;7(12):273. [DOI] [PMC free article] [PubMed]
- 27.Böing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3:23430. [DOI] [PMC free article] [PubMed]
- 28.Gandham S, Su X, Wood J, Nocera AL, Alli SC, Milane L, et al. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020;38(10):1066–1098. doi: 10.1016/j.tibtech.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baranyai T, Herczeg K, Onódi Z, Voszka I, Módos K, Marton N, et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS ONE. 2015;10(12):e0145686. doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An M, Wu J, Zhu J, Lubman DM. Comparison of an optimized ultracentrifugation method versus size-exclusion chromatography for isolation of exosomes from human serum. J Proteome Res. 2018;17(10):3599–3605. doi: 10.1021/acs.jproteome.8b00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stranska R, Gysbrechts L, Wouters J, Vermeersch P, Bloch K, Dierickx D, et al. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J Transl Med. 2018;16(1):1. doi: 10.1186/s12967-017-1374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navajas R, Corrales FJ, Paradela A. Serum exosome isolation by size-exclusion chromatography for the discovery and validation of preeclampsia-associated biomarkers. Methods Mol Biol. 2019;1959:39–50. doi: 10.1007/978-1-4939-9164-8_3. [DOI] [PubMed] [Google Scholar]
- 33.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Abramowicz A, Widlak P, Pietrowska M. Proteomic analysis of exosomal cargo: the challenge of high purity vesicle isolation. Mol Biosyst. 2016;12(5):1407–1419. doi: 10.1039/C6MB00082G. [DOI] [PubMed] [Google Scholar]
- 35.Hong CS, Funk S, Whiteside TL. Isolation of biologically active exosomes from plasma of patients with cancer. Methods Mol Biol. 2017;1633:257–265. doi: 10.1007/978-1-4939-7142-8_16. [DOI] [PubMed] [Google Scholar]
- 36.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. [DOI] [PMC free article] [PubMed]
- 37.Shu S, Yang Y, Allen CL, Hurley E, Tung KH, Minderman H, et al. Purity and yield of melanoma exosomes are dependent on isolation method. J Extracell Vesicles. 2020;9(1):1692401. doi: 10.1080/20013078.2019.1692401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gheinani AH, Vögeli M, Baumgartner U, Vassella E, Draeger A, Burkhard FC, et al. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci Rep. 2018;8(1):3945. doi: 10.1038/s41598-018-22142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015(4):319–323. doi: 10.1101/pdb.top074476. [DOI] [PubMed] [Google Scholar]
- 40.Yang L, Jia J, Li S. Advances in the application of exosomes identification using surface-enhanced Raman spectroscopy for the early detection of cancers. Front Bioeng Biotechnol. 2021;9:808933. doi: 10.3389/fbioe.2021.808933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, Franquesa M, Beyer K, Borràs FE. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci Rep. 2016;6:33641. doi: 10.1038/srep33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarovni N, Corrado A, Guazzi P, Zocco D, Lari E, Radano G, et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46–58. doi: 10.1016/j.ymeth.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 44.Kooijmans SA, Aleza CG, Roffler SR, van Solinge WW, Vader P, Schiffelers RM. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;5:31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rupp AK, Rupp C, Keller S, Brase JC, Ehehalt R, Fogel M, et al. Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage. Gynecol Oncol. 2011;122(2):437–446. doi: 10.1016/j.ygyno.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9(4):1015–1028. doi: 10.7150/thno.30853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xian P, Hei Y, Wang R, Wang T, Yang J, Li J, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9(20):5956–5975. doi: 10.7150/thno.33872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang T, Yin W, AlShamaileh H, Zhang Y, Tran PH, Nguyen TN, et al. A detailed protein-SELEX protocol allowing visual assessments of individual steps for a high success rate. Hum Gene Ther Methods. 2019;30(1):1–16. doi: 10.1089/hgtb.2018.237. [DOI] [PubMed] [Google Scholar]
- 50.Wang T, Philippovich S, Mao J, Veedu RN. Efficient epidermal growth factor receptor targeting oligonucleotide as a potential molecule for targeted cancer therapy. Int J Mol Sci. 2019;20(19):4700. [DOI] [PMC free article] [PubMed]
- 51.Zhang W, Tian Z, Yang S, Rich J, Zhao S, Klingeborn M, et al. Electrochemical micro-aptasensors for exosome detection based on hybridization chain reaction amplification. Microsyst Nanoeng. 2021;7:63. doi: 10.1038/s41378-021-00293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang K, Yue Y, Wu S, Liu W, Shi J, Zhang Z. Rapid capture and nondestructive release of extracellular vesicles using aptamer-based magnetic isolation. ACS Sens. 2019;4(5):1245–1251. doi: 10.1021/acssensors.9b00060. [DOI] [PubMed] [Google Scholar]
- 53.Yu X, He L, Pentok M, Yang H, Yang Y, Li Z, et al. An aptamer-based new method for competitive fluorescence detection of exosomes. Nanoscale. 2019;11(33):15589–15595. doi: 10.1039/C9NR04050A. [DOI] [PubMed] [Google Scholar]
- 54.Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 55.Iorember FM, Vehaskari VM. Uromodulin: old friend with new roles in health and disease. Pediatr Nephrol. 2014;29(7):1151–1158. doi: 10.1007/s00467-013-2563-z. [DOI] [PubMed] [Google Scholar]
- 56.Liu F, Vermesh O, Mani V, Ge TJ, Madsen SJ, Sabour A, et al. The exosome total isolation chip. ACS Nano. 2017;11(11):10712–10723. doi: 10.1021/acsnano.7b04878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sunkara V, Kim CJ, Park J, Woo HK, Kim D, Ha HK, et al. Fully automated, label-free isolation of extracellular vesicles from whole blood for cancer diagnosis and monitoring. Theranostics. 2019;9(7):1851–1863. doi: 10.7150/thno.32438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han Z, Peng C, Yi J, Zhang D, Xiang X, Peng X, et al. Highly efficient exosome purification from human plasma by tangential flow filtration based microfluidic chip. Sens Actuators, B Chem. 2021;333:129563. doi: 10.1016/j.snb.2021.129563. [DOI] [Google Scholar]
- 59.Liu Y, Zhao W, Cheng R, Logun M, Zayas-Viera MDM, Karumbaiah L, et al. Label-free ferrohydrodynamic separation of exosome-like nanoparticles. Lab Chip. 2020;20(17):3187–3201. doi: 10.1039/D0LC00609B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Q, Cheng L, Hu L, Lou D, Zhang T, Li J, et al. An integrative microfluidic device for isolation and ultrasensitive detection of lung cancer-specific exosomes from patient urine. Biosens Bioelectron. 2020;163:112290. doi: 10.1016/j.bios.2020.112290. [DOI] [PubMed] [Google Scholar]
- 61.Inci F. Benchmarking a microfluidic-based filtration for isolating biological particles. Langmuir. 2022;38(5):1897–1909. doi: 10.1021/acs.langmuir.1c03119. [DOI] [PubMed] [Google Scholar]
- 62.Santana SM, Antonyak MA, Cerione RA, Kirby BJ. Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations. Biomed Microdevices. 2014;16(6):869–877. doi: 10.1007/s10544-014-9891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wunsch BH, Smith JT, Gifford SM, Wang C, Brink M, Bruce RL, et al. Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat Nanotechnol. 2016;11(11):936–940. doi: 10.1038/nnano.2016.134. [DOI] [PubMed] [Google Scholar]
- 64.Smith JT, Wunsch BH, Dogra N, Ahsen ME, Lee K, Yadav KK, et al. Integrated nanoscale deterministic lateral displacement arrays for separation of extracellular vesicles from clinically-relevant volumes of biological samples. Lab Chip. 2018;18(24):3913–3925. doi: 10.1039/C8LC01017J. [DOI] [PubMed] [Google Scholar]
- 65.Dudani JS, Gossett DR, Tse HT, Lamm RJ, Kulkarni RP, Carlo DD. Rapid inertial solution exchange for enrichment and flow cytometric detection of microvesicles. Biomicrofluidics. 2015;9(1):014112. doi: 10.1063/1.4907807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hisey CL, Dorayappan KDP, Cohn DE, Selvendiran K, Hansford DJ. Microfluidic affinity separation chip for selective capture and release of label-free ovarian cancer exosomes. Lab Chip. 2018;18(20):3144–3153. doi: 10.1039/C8LC00834E. [DOI] [PubMed] [Google Scholar]
- 67.Suwatthanarak T, Thiodorus IA, Tanaka M, Shimada T, Takeshita D, Yasui T, et al. Microfluidic-based capture and release of cancer-derived exosomes via peptide-nanowire hybrid interface. Lab Chip. 2021;21(3):597–607. doi: 10.1039/D0LC00899K. [DOI] [PubMed] [Google Scholar]
- 68.Ibsen SD, Wright J, Lewis JM, Kim S, Ko SY, Ong J, et al. Rapid isolation and detection of exosomes and associated biomarkers from plasma. ACS Nano. 2017;11(7):6641–6651. doi: 10.1021/acsnano.7b00549. [DOI] [PubMed] [Google Scholar]
- 69.Lewis JM, Vyas AD, Qiu Y, Messer KS, White R, Heller MJ. Integrated analysis of exosomal protein biomarkers on alternating current electrokinetic chips enables rapid detection of pancreatic cancer in patient blood. ACS Nano. 2018;12(4):3311–3320. doi: 10.1021/acsnano.7b08199. [DOI] [PubMed] [Google Scholar]
- 70.Ayala-Mar S, Perez-Gonzalez VH, Mata-Gómez MA, Gallo-Villanueva RC, González-Valdez J. Electrokinetically driven exosome separation and concentration using dielectrophoretic-enhanced PDMS-based microfluidics. Anal Chem. 2019;91(23):14975–14982. doi: 10.1021/acs.analchem.9b03448. [DOI] [PubMed] [Google Scholar]
- 71.Zhao W, Zhang L, Ye Y, Li Y, Luan X, Liu J, et al. Microsphere mediated exosome isolation and ultra-sensitive detection on a dielectrophoresis integrated microfluidic device. Analyst. 2021;146(19):5962–5972. doi: 10.1039/D1AN01061A. [DOI] [PubMed] [Google Scholar]
- 72.Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017;114(40):10584–10589. doi: 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Evander M, Gidlöf O, Olde B, Erlinge D, Laurell T. Non-contact acoustic capture of microparticles from small plasma volumes. Lab Chip. 2015;15(12):2588–2596. doi: 10.1039/C5LC00290G. [DOI] [PubMed] [Google Scholar]
- 74.Ku A, Lim HC, Evander M, Lilja H, Laurell T, Scheding S, et al. Acoustic enrichment of extracellular vesicles from biological fluids. Anal Chem. 2018;90(13):8011–8019. doi: 10.1021/acs.analchem.8b00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Z, Li F, Rufo J, Chen C, Yang S, Li L, et al. Acoustofluidic salivary exosome isolation: a liquid biopsy compatible approach for human papillomavirus-associated oropharyngeal cancer detection. J Mol Diagn. 2020;22(1):50–59. doi: 10.1016/j.jmoldx.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tayebi M, Yang D, Collins DJ, Ai Y. Deterministic sorting of submicrometer particles and extracellular vesicles using a combined electric and acoustic field. Nano Lett. 2021;21(16):6835–6842. doi: 10.1021/acs.nanolett.1c01827. [DOI] [PubMed] [Google Scholar]
- 77.Tay HM, Leong SY, Xu X, Kong F, Upadya M, Dalan R, et al. Direct isolation of circulating extracellular vesicles from blood for vascular risk profiling in type 2 diabetes mellitus. Lab Chip. 2021;21(13):2511–2523. doi: 10.1039/D1LC00333J. [DOI] [PubMed] [Google Scholar]
- 78.Chen W, Cao R, Su W, Zhang X, Xu Y, Wang P, et al. Simple and fast isolation of circulating exosomes with a chitosan modified shuttle flow microchip for breast cancer diagnosis. Lab Chip. 2021;21(9):1759–1770. doi: 10.1039/D0LC01311K. [DOI] [PubMed] [Google Scholar]
- 79.Pang B, Zhu Y, Ni J, Thompson J, Malouf D, Bucci J, et al. Extracellular vesicles: the next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis. Theranostics. 2020;10(5):2309–2326. doi: 10.7150/thno.39486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z, Wu HJ, Fine D, Schmulen J, Hu Y, Godin B, et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13(15):2879–2882. doi: 10.1039/c3lc41343h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ranjan S, Zeming KK, Jureen R, Fisher D, Zhang Y. DLD pillar shape design for efficient separation of spherical and non-spherical bioparticles. Lab Chip. 2014;14(21):4250–4262. doi: 10.1039/C4LC00578C. [DOI] [PubMed] [Google Scholar]
- 82.Vaidyanathan R, Naghibosadat M, Rauf S, Korbie D, Carrascosa LG, Shiddiky MJ, et al. Detecting exosomes specifically: a multiplexed device based on alternating current electrohydrodynamic induced nanoshearing. Anal Chem. 2014;86(22):11125–11132. doi: 10.1021/ac502082b. [DOI] [PubMed] [Google Scholar]
- 83.Zhu H, Lin X, Su Y, Dong H, Wu J. Screen-printed microfluidic dielectrophoresis chip for cell separation. Biosens Bioelectron. 2015;63:371–378. doi: 10.1016/j.bios.2014.07.072. [DOI] [PubMed] [Google Scholar]
- 84.Kushigbor SDE, Tang Z, Yobas L. Railing nanoparticles along activated tracks towards continuous-flow electrokinetic enrichment from blood plasma() Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:2249–2252. doi: 10.1109/EMBC44109.2020.9176283. [DOI] [PubMed] [Google Scholar]
- 85.Aghilinejad A, Aghaamoo M, Chen X, Xu J. Effects of electrothermal vortices on insulator-based dielectrophoresis for circulating tumor cell separation. Electrophoresis. 2018;39(5–6):869–877. doi: 10.1002/elps.201700264. [DOI] [PubMed] [Google Scholar]
- 86.Fraunhofer W, Winter G. The use of asymmetrical flow field-flow fractionation in pharmaceutics and biopharmaceutics. Eur J Pharm Biopharm. 2004;58(2):369–383. doi: 10.1016/j.ejpb.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 87.Wu B, Chen X, Wang J, Qing X, Wang Z, Ding X, et al. Separation and characterization of extracellular vesicles from human plasma by asymmetrical flow field-flow fractionation. Anal Chim Acta. 2020;1127:234–245. doi: 10.1016/j.aca.2020.06.071. [DOI] [PubMed] [Google Scholar]
- 88.Kim YB, Yang JS, Lee GB, Moon MH. Evaluation of exosome separation from human serum by frit-inlet asymmetrical flow field-flow fractionation and multiangle light scattering. Anal Chim Acta. 2020;1124:137–145. doi: 10.1016/j.aca.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 89.Kornilov R, Puhka M, Mannerström B, Hiidenmaa H, Peltoniemi H, Siljander P, et al. Efficient ultrafiltration-based protocol to deplete extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2018;7(1):1422674. doi: 10.1080/20013078.2017.1422674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 91.Ahn SH, Ryu SW, Choi H, You S, Park J, Choi C. Manufacturing therapeutic exosomes: from bench to industry. Mol Cells. 2022;45(5):284–290. doi: 10.14348/molcells.2022.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi MM, Yang QY, Monsel A, Yan JY, Dai CX, Zhao JY, et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J Extracell Vesicles. 2021;10(10):e12134. doi: 10.1002/jev2.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu YG, Shi MM, Monsel A, Dai CX, Dong X, Shen H, et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: a pilot study. Stem Cell Res Ther. 2022;13(1):220. doi: 10.1186/s13287-022-02900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]