Abstract
Background and Aim:
Lepidium meyenii Walp (Maca) is an herbaceous plant that grows in the Peruvian Andes and it has been widely used as a nutritional supplement and fertility enhancer and has been used in the treatment of a variety of diseases, such as rheumatism, respiratory disorders, and anemia. The most notable feature of Maca is its potent antioxidant capacity, which helps in the scavenging of free radicals and protection of cells from oxidative stress. This study aimed to evaluate the in vitro effect of Maca extract on thawed sperm cells from bulls.
Materials and Methods:
Three dilutions of 1, 10, and 100 mg/mL of Maca extract were incubated with frozen–thawed bovine semen and analyzed at 1, 3, and 24 h of exposure time, evaluating the activity of the extract on the DNA, motility, morphology, viability, integrity of the membrane and acrosome of spermatozoa.
Results:
The Maca extract improved the studied sperm parameters of motility, acrosome integrity, vitality, and DNA integrity of sperm cells at a concentration of 10 mg/mL, and at 1 mg/mL, an improvement was observed in the morphology and integrity of the membrane. However, the best activity of the Maca extract was observed on the DNA integrity of the sperm, which was effective at the three concentrations evaluated after 24 h of incubation.
Conclusion:
The results indicate that L. meyenii can help in maintaining spermatozoa cellular integrity after the frozen–thaw process, especially in the protection against DNA fragmentation. Therefore, Maca would be a feasible supplementation to protect sperm to maintain their fertile ability after thawing.
Keywords: Aberdeenangus, antioxidant, bull, frozen-thawed semen, Lepidium meyenii (Maca), reproductive health
Introduction
Lepidium meyenii Walp, commonly known as Maca, belonging to the Brassicaceae family and growing exclusively between 3500 and 4500 m above sea level, is an herb known as a traditional nutritional supplement, widely used in Peru, North America, and Europe, and is considered a millenary food for the Andean cultures [1]. According to numerous studies, the hypocotyls of Maca have been widely used as a nutritional supplement [1–4]. Furthermore, in folk medicine, it is used to increase fertility and sexual function, so it is popularly known as the “Andean Viagra” [4]. Maca has elements of high nutritional value, such as proteins, carbohydrates, essential amino acids, lipids, free fatty acids, and a series of secondary metabolites, such as macamides, alkaloids, and glucosinolates [4], and the composition and several medicinal effects of Peruvian Maca were reported very recently [2, 3]. Several studies have demonstrated the effects of Maca on semen quality, spermatogenesis, sperm count, and sperm motility in different species [1, 5–7]. These effects were observed in both healthy [1, 4, 6, 8] and animals with induced subfertility problems [9, 10]. In addition, a recent study has shown that Maca has a beneficial effect on the amount of fresh semen and its quality after storage at a temperature of 5°C for 72 h [6]. A systematic review based on trials on infertile and healthy men showed positive results of L. meyenii on semen quality [11]. A review of the properties of L. meyenii on sperm quality, sexual behavior, and male genital tract pathologies was recently published [12].
These data are interesting for the preservation of semen quality in reproductive biotechnology, such as artificial insemination (AI) and in vitro fertilization (IVF). In livestock, the use of AI has increased considerably in recent decades. From an industrial point of view, AI has several advantages over natural service, such as higher pregnancy rates and lower costs of transporting animals for riding [13]. A fundamental part of AI is the use of frozen semen. Therefore, samples must be carefully managed to prevent damage that occurs due to oxidative stress (OS) [6]. A strategy to prevent oxidative damage to sperm can be found in the supplementation of food with antioxidants or in the direct use of these supplements in conjunction with semen samples. One of the main characteristics of Maca is the presence of compounds such as macamides and glucosinolates that help in eliminating free radicals and protect cells from OS [14]. During spermatogenesis and steroidogenesis, sperm accumulate reactive oxygen species (ROS) [15]. For proper fertilization to occur, it is important to keep ROS levels low. In addition, sperm capacitation, hyperactivation, acrosome reaction, and sperm fusion require low levels of ROS to be more efficient [16]. ROS activity is a major concern for sperm quality both in vivo and during in vitro incubation, as well as during cold storage [17]. Tafuri et al. [17] observed on stallions that Maca contains some effective antioxidants, a high percentage of glucosinolates, and other important components with high antioxidant capacity that can protect sperm and keep ROS levels low. Despite the significance of sperm freezability affecting bull fertility and its economic impact on cattle farming, the efficiency of cryopreservation is still limited to 40–50% of cellular survival [18]. Although physical damage to membranes and biochemical changes has been linked to cryo-induced OS [19–21], the causes of differing freezabilities remain unknown.
This study aimed to analyze, for the first time, the in vitro effect of L. meyenii on frozen–thawed bull sperm to determine the activity of the extract as a protective agent against sperm cell damage after thawing.
Materials and Methods
Ethical approval
Not applicable.
Study period and location
The study was carried out from March and December 2019 in the Assisted Reproduction Laboratory of the Center for the Development of Scientific Research (CEDIC), Asuncion, Paraguay.
Experimental design
To evaluate the effect of L. meyenii extract on frozen–thawed sperm cells, three dilutions of the extract were tested at different times. Tests were conducted by preparing plastic tubes containing 1 mL of Sperm Prep Medium (SPM) (IVF Bioscience, Manhattan, USA) at a concentration of 2×106 sperm/mL. After 1, 3, and 24 h of incubation at 38°C, control (U) and sperm cells with 1, 10, and 100 g/mL of Maca extract were evaluated (Figure-1). Samples were assessed for motility, morphology, membrane integrity, vitality, and DNA fragmentation at selected time points.
Figure-1.
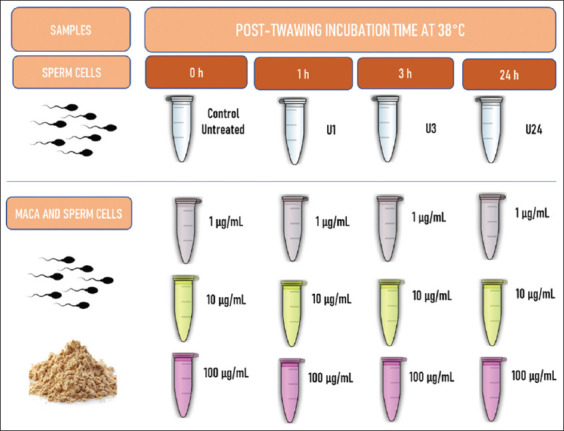
Design of the in vitro activity assays of Lepidium meyenii on frozen-thawed bull spermatozoa.
Preparation of L. meyenii extracts
Commercial powder of L. meyenii Walp, composed of dry and raw yellow hypocotyl phenotypes [22, 23] (Maca en pó - Herbalsave Ltda., São Paulo, Brazil), was purchased in February 2019 in São Paulo, Brazil. A stock was prepared by weighing 1 mg of the powder and adding it to 1 mL of dimethyl sulfoxide (DMSO) (Sigma–Aldrich, St. Louis, USA) just before use. Three successive dilutions were prepared from the 1 mg/mL stock to be used at final concentrations of 1, 10, and 100 μg/mL in a final volume of 3 μL (DMSO <0.003%).
Preparation of bull semen frozen–thawed samples
Two commercial straws of 0.25 mL of frozen bull semen (Aberdeen Angus, 18 months old) (Covepa group, Asunción, Paraguay) were thawed in a water bath at 37°C for 30 s. Both samples were pooled together and then washed twice with 2 mL of SPM medium by centrifugation at 1200 RPM for 5 min (Biosan LCM-3000, Riga, Latvia). The pellet was resuspended in 2 mL of SPM medium. Three independent experiments were performed.
Sperm concentration
The number of sperm cells was counted by adding 10 μL of sperm suspension to a pre-warmed Makler chamber under an inverted phase-contrast microscope with a thermal stage (38°C) (IX70; Olympus, Centre Valley, PA, USA) at 200×. The final concentration to perform the routine and functional tests was 2×106 sperm/mL in SPM medium.
Motility assessment
Sperm motility and vigor were evaluated twice by the same observer after each treatment using an inverted phase-contrast microscope with a thermal stage at 38°C at 200× (IX70). A droplet (10 μL) of sperm was placed on a prewarmed microscope slide and covered to be counted immediately. The motility test was classified into four types: rectilinear, circular, static, and immobile tests. A minimum of 200 sperm cells were counted in each sample [24].
Morphology test
For each group, 10 μL of sperm was placed on each slide for a thin smear and then air-dried for 5 min. Slides were stained with hematoxylin solution for 5 min, washed with distilled water, and air-dried for further processing. The presence of sperm cells were analyzed under a light microscope at 100× magnification (Axio LabA1, Zeiss, Germany) under oil immersion and classified as normal or abnormal conditions. Morphology of sperm cells refers to the structure of the individual sperm cells, and the minimum threshold for a bull to be classified as a satisfactory potential breeder is 70% normal, live cells [25]. Sperm defects (abnormalities) observed were grouped as follows: Head defects (e.g., detached head, defects in size and shape, nuclear vacuoles, and acrosomal defects), midpiece defects (e.g., distal midpiece reflex, bowed midpiece, and proximal droplet), and tail defects (e.g., bent tail and coiled tail) [26]. A total of 200 sperm cells were counted per slide from each group assayed.
Membrane integrity − hypo-osmotic swelling (HOS) test
The procedure used for the HOS test was similar to the one described by Correa et al. [27]. The HOS test was performed by combining 0.1 mL of semen with 0.1 mL of hypo-osmotic solution prepared by mixing 7.35 g of sodium citrate 2H2O and 13.51 g of fructose in 1 L of distilled water. The solutions (sperm mixture) were then incubated at 37°C for 2 h. A total of 200 spermatozoa were counted in at least five different fields of view. Sperm swelling was assessed following incubation by placing a drop of a well-mixed sample on a slide. The slide was covered with a cover glass and observed under a phase-contrast microscope (IX70) at 400× magnification. The total proportion and different swelling patterns of swollen spermatozoa were calculated by dividing the number of reacted cells (100×) by the total spermatozoa counted in the same area. The proportion of swollen spermatozoa from a control sample was subtracted from the calculations. Swelling patterns were recorded and expressed on a percentage basis of the total spermatozoa that reacted to the HOS test.
Acrosomal integrity and vitality test (AIT)
Trypan blue (Sigma–Aldrich, St. Louis, USA) and Giemsa dye (Merck, Darmstadt, Germany) stock solutions were prepared according to the manufacturer’s protocol. A total of 100 μL of each sample was placed in 100 μL of 2% Trypan blue and incubated for 10 min at 37°C. Samples were washed twice with 1 mL of SPM by centrifugation (6 min, 2000 RPM), and 10 μL of a smear was drawn and dried at 37°C. After that, slides were stained with 20% Giemsa for 40 min, rinsed underwater, and air-dried for further processing. The presence of sperm cells was analyzed under a light microscope (Axio LabA1, Zeiss, Germany) at 400× and classified into four groups: Dead with an acrosome, dead without an acrosome, alive with an acrosome, and alive without an acrosome. A minimum of 200 spermatozoa were counted in each group [28].
DNA fragmentation with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The amount of DNA fragmentation was evaluated by a TUNEL assay using a commercially available kit (In situ Cell Death Detection Kit, TMR red, Roche, Indianapolis, IN, USA), in which the free 3-OH ends of the DNA were labeled with fluorescein-conjugated dUTP by enzyme terminal deoxynucleotidyl transferase [29]. Briefly, fixed spermatozoa on the slides were washed three times for 5 min with phosphate-buffered saline (PBS) 1× and permeabilized with 0.1% (v/v) Triton X-100 containing 0.1% (w/v) sodium citrate for 2 min on ice. Samples were then incubated in 50 μL of the TUNEL reaction mixture for 1 h at 37°C in a dark and humidified atmosphere. For positive control, slides with spermatozoa were treated with RNase-free DNase I (400 U/mL, Qiagen, Valencia, CA, USA) at 23ºC for 10 min before incubation with the TUNEL reagent. For negative control, slides with spermatozoa were incubated with the TUNEL reagent in the absence of terminal deoxynucleotidyl transferase. Slides were washed three times with PBS, and mounted using a mounting medium (Vectasheld®, Vector Laboratories, Burlingame, CA, USA). Positive TUNEL staining was observed under a fluorescence microscope (Axio LabA1, Zeiss, Germany) using the filter set 14 Ex BP 510–560 nm excitation filter (Zeiss, Germany). The sperm TUNEL percentage was determined by counting the positive and negative stained spermatozoa. At least 200 cells were counted in each group.
Statistical analysis
Values were compared using a one-way analysis of variance, followed by Dunnett’s post hoc test using the GraphPad Prism version 9.0 software (GraphPad Software, San Diego CA, USA). Differences were considered statistically significant at p < 0.05.
Results
Table-1 details the effect of the treatment of the three concentrations of Maca dilutions at three exposure times on thawed and capacitated bovine sperm cells. The results of the motility, HOS, vitality, AIT, morphology, and TUNEL tests are expressed as the means of three experiments.
Table 1.
Sperm quality evaluation tests of Aberdeen Angus treated with three concentrations of Maca and at three different times. The results are expressed as a percentage.
| Test | Sperm cells status | Post-thawing incubation time and concentration of L. meyenii | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| 0 h | 1 h | 3 h | 24 h | |||||||||||
| Control | Control | 1 µg/mL | 10 µg/mL | 100 µg/mL | Control | 1 µg/mL | 10 µg/mL | 100 µg/mL | Control | 1 µg/mL | 10 µg/mL | 100 µg/mL | ||
| Motility (%) | Rectilinear | 42 | 50 | 46 | 63 | 57 | 54 | 54 | 55 | 53 | 49 | 60 | 53 | 48 |
| Circular | 9 | 9 | 7 | 5 | 8 | 5 | 4 | 4 | 7 | 3 | 5 | 3 | 3 | |
| Static | 5 | 2 | 8 | 5 | 5 | 0 | 0 | 5 | 3 | 8 | 11 | 15 | 12 | |
| Immotile | 45 | 39 | 45 | 28 | 31 | 42 | 43 | 37 | 38 | 42 | 24 | 30 | 39 | |
| HOS (%) | Normal | 44 | 46 | 44 | 59 | 47 | 44 | 52 | 47 | 47 | 44 | 42 | 46 | 41 |
| Abnormal | 56 | 54 | 57 | 41 | 53 | 56 | 49 | 54 | 54 | 56 | 58 | 54 | 59 | |
| Acrosomal | Damaged - Died | 35 | 19 | 26 | 27 | 27 | 19 | 37 | 21 | 29 | 30 | 37 | 34 | 43 |
| Integrity (%) | Intact - Died | 22 | 31 | 26 | 42 | 35 | 33 | 29 | 35 | 44 | 49 | 38 | 26 | 27 |
| Damaged - Live | 7 | 2 | 1 | 2 | 3 | 15 | 3 | 2 | 2 | 1 | 1 | 1 | 0 | |
| Intact - Live | 37 | 48 | 48 | 30 | 36 | 34 | 32 | 43 | 26 | 21 | 25 | 40 | 30 | |
| Vitality (%) | Died | 57 | 50 | 52 | 68 | 62 | 52 | 66 | 56 | 73 | 78 | 75 | 60 | 70 |
| Live | 44 | 50 | 49 | 32 | 39 | 49 | 35 | 45 | 28 | 22 | 25 | 41 | 30 | |
| Morphology (%) | Normal | 91 | 91 | 92 | 91 | 91 | 88 | 90 | 88 | 87 | 88 | 90 | 87 | 87 |
| Abnormal – Head | 3 | 2 | 2 | 3 | 3 | 5 | 3 | 2 | 3 | 3 | 3 | 2 | 4 | |
| Abnormal – Neck | 3 | 3 | 3 | 3 | 3 | 4 | 3 | 5 | 6 | 6 | 5 | 4 | 5 | |
| Abnormal - Tail | 3 | 5 | 3 | 3 | 3 | 3 | 4 | 4 | 5 | 3 | 3 | 5 | 4 | |
| TUNEL (%) | Normal DNA | 95.43 | 95.34 | 95.59 | 94.27 | 95.71 | 93.35 | 95.03 | 93.52 | 93.10 | 90.60 | 92.29 | 91.35 | 91.44 |
| Fragmented DNA | 4.57 | 4.66 | 4.41 | 5.73 | 4.29 | 6.65 | 4.97 | 6.48 | 6.90 | 9.40 | 7.71 | 8.65 | 8.56 | |
HOS=Hypo-osmotic swelling, TUNEL=DNA fragmentation with terminal deoxynucleotidyl transferase dUTP nick end labeling
The motility test determined an improvement with 10 μg/mL of L. meyenii at 1 h of incubation time but not in the other incubation times and concentrations assayed (Figure-2). Regarding the type of sperm motility, no differences in rectilinear, circular, or static movements were observed with treatments over time (p > 0.99) (Table-1). The HOS test showed a higher percentage of spermatozoa with greater plasma membrane integrity at 3 h of incubation when treated with 1 μg/mL of L. meyenii (Figure-3). No differences were observed in the other concentrations at 1, 3, and 24 h of incubation (p > 0.05).
Figure-2.
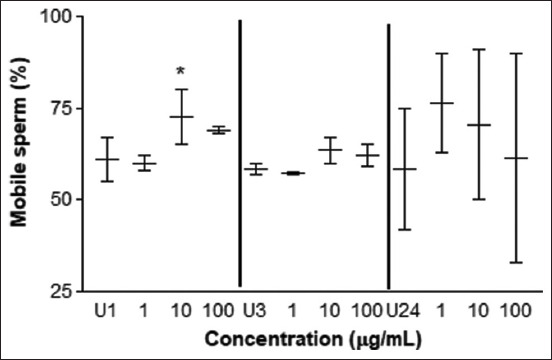
Motility test. Percentage of motile sperm cells treated with different concentrations of Lepidium meyenii versus control (U) at 1, 3, and 24 h of exposure. U1: untreated 1 h, U3: untreated 3h, U24: untreated 24 h of incubation time. The * means p < 0.05.
Figure-3.
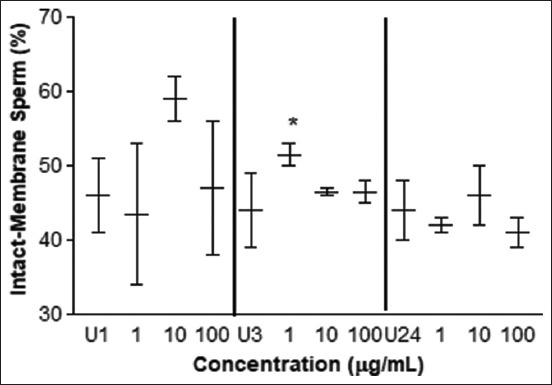
Hypo-osmotic swelling test. Percentage of sperm cells with intact plasma membrane treated with different concentrations of L. meyenii versus control (U) at 1, 3 and 24 h of exposure. U1: untreated 1 h, U3: untreated 3h, U24: untreated 24 h of incubation time. The *means p < 0.05.
In the AIT, no statistically significant differences were observed between the percentage of the integrity and damage of the acrosome in live and dead sperm cells (Table-1 and Figure-4). Nevertheless, an improvement in acrosome integrity was observed in live spermatozoa at 10 μg/mL after 3 h of exposure and at concentrations of 10 and 100 μg/mL after 24 h of incubation (Figure-5). Values below control were observed at concentrations of 10 μg/mL at 1 h of exposure (p < 0.01) and 100 μg/mL at 3 h of exposure (p < 0.01).
Figure-4.
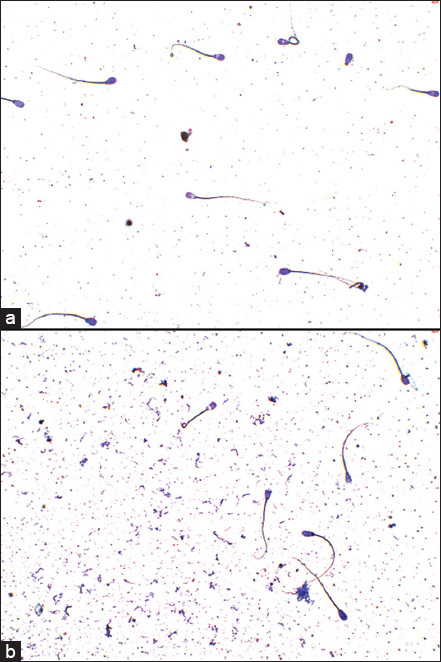
Acrosomal integrity and vitality test microscopic images at 3 h of incubation time under 400×. (a) Control sperm cells, (b) Sperm cells incubated with Maca at 100 μg/mL. Slightly stained acrosomes and/or cytoplasm indicates viable sperm, and strongly stained acrosomes and/or cytoplasm are damaged or dead sperm cells.
Figure-5.
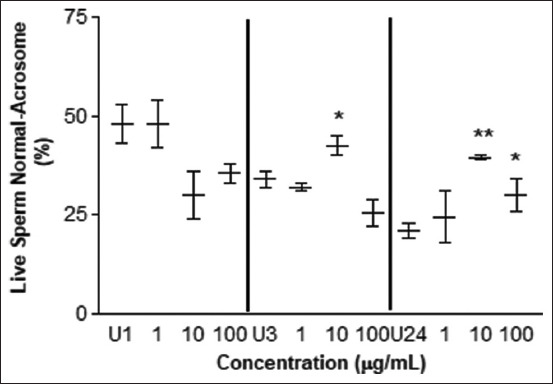
Acrosome intactness test. Percentage of intact acrosome in live sperm cells treated with different concentrations of L. meyenii at different times. U1: untreated 1 h, U3: untreated 3h, U24: untreated 24 h of incubation time. The * means p < 0.05 and ** p < 0.01.
The vitality test determined a greater amount of live spermatozoa treated with 10 μg/mL at 24 h of exposure (Figure-6), but values below the control were observed at concentrations of 10 and 100 μg/mL at 1 h of exposure (p < 0.05) and 100 μg/mL at 3 h of exposure (p < 0.05). The morphology test determined an improvement in sperm cells incubated with 1 μg/mL of L. meyenii after 1 and 3 h (p < 0.01) (Figure-7). No differences were observed at the other concentrations and incubation times (p > 0.05). The results obtained after 24 h of incubation were not statistically significant (p = 0.30). Values below control were observed at concentrations of 10 μg/mL after 3 h (p < 0.01) of exposure to 100 μg/mL (p < 0.05). By evaluating the morphological anomalies in the sperm head, neck, and tail, no differences were observed (Table-1) (p = 0.6, data not shown).
Figure-6.
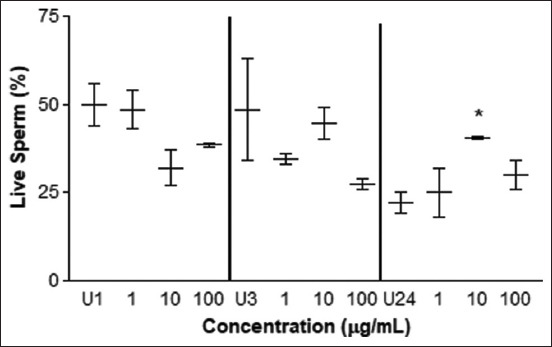
Vitality test. Percentage of live sperm cells treated with different concentrations of L. meyenii versus control (U). U1: untreated 1 h, U3: untreated 3h, U24: untreated 24 h of incubation time. The * means p<0.05.
Figure-7.
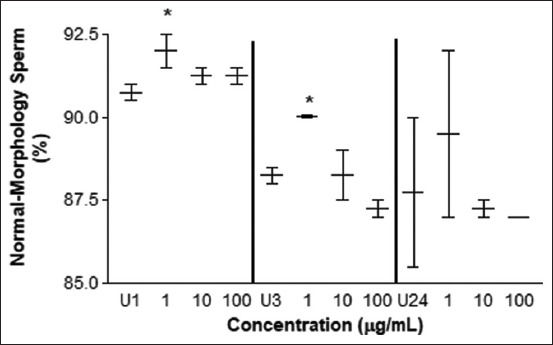
Morphology test. Percentage of sperm cells with normal morphology treated with different concentrations of L. meyenii versus control (U). U1: untreated 1 h, U3: untreated 3h, U24: untreated 24 h of incubation time. The ** means p<0.01.
In the TUNEL test, less sperm DNA damage was observed at concentrations of 100 μg/mL after 1 h of incubation, 1 μg/mL after 3 h, and 1, 10, and 100 μg/mL after 24 h of exposure (Figure-8). However, at concentrations of 10 μg/mL at 1 h of exposure (p < 0.01) and 100 μg/mL at 3 h of exposure (p < 0.05), values below the control were observed, indicating greater DNA damage.
Figure-8.
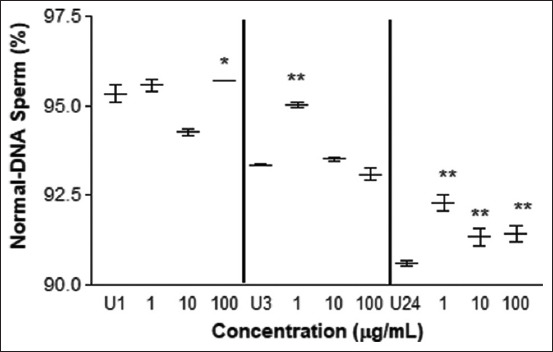
TUNEL test. Average percentage of sperm cells without DNA damage treated with different concentrations of L. meyenii versus control (U). U1: untreated 1 h, U3: untreated 3h, U24: untreated 24 h of incubation time. The * means p<0.05 and ** p<0.01.
Discussion
This is the first study on the in vitro effect of Maca on bovine spermatozoa. The only in vitro study on Maca was conducted by Aoki et al. [7], and it was on mouse and human spermatozoa. The effects of the components of L. meyenii have been extensively studied in recent years. However, there are few studies performed on bulls [30, 31]. The present study showed the in vitro effects of L. meyenii on the sperm parameters of capacitated frozen–thawed bull semen. Several studies have shown that Maca has an effect on seminal quality in humans [5, 12, 32], mice [1, 2, 8–10], and stallions [6, 17] that is related to its antioxidant capacity [4, 11, 33–36] because, during cryopreservation, sperm is damaged by OS [18–21]. In addition to the high concentration of antioxidants, L. meyenii contains several secondary metabolites [3, 14, 22, 23], which help in keeping ROS levels low [14, 17]. A study showed that the secondary metabolites of Maca act on the ferric reducing antioxidant potential, hydroxyl radical scavenging ability, and 2,2-diphenyl-1-picrylhydrazyl free-radical scavenging capacity and increase the levels of superoxide dismutase and glutathione [37]. All of these secondary metabolites or a distinct combination of them may explain the potential effects of Maca on male fertility.
Regarding the tests carried out in this study, one of them is the HOS test, which evaluates whether an intact membrane is biochemically active [27]. Thus, the assessment of sperm membrane function appears to be a significant marker for the fertilizing capacity of spermatozoa [27]. The results obtained in the present study showed that frozen–thawed bovine spermatozoa reacted to HOS and that minimal swelling occurred at the lowest concentration evaluated. By itself, the HOS test is a much better predictor of the outcome of fertilization in humans than the sperm number, motility, or morphology parameters by themselves, as shown by the outcome of the zona-free hamster oocyte penetration assay and pregnancy rates in humans [27].
Regarding the morphology and motility test results, they improved at concentrations of 1 and 10 μg/mL, respectively, but an adverse effect on morphology was also observed at the highest concentration of 100 μg/mL. A study presented by Rubio et al. [38] indicated that male rats with acetate-induced damage on spermatogenesis treated with Maca showed an improvement in spermatogenesis, as well as increased motile sperm count and a count of sperm with normal morphology. The adverse effect of the concentration of 100 μg/mL was also observed in the TUNEL and AIT, which would indicate a hormetic effect of the extract in these parameters. Hormesis is defined as a cellular adaptive response to stressors that results in a biphasic dose–response relationship, such that low-dose stimulation results in a beneficial adaptation whereas a high dose results in a toxic effect [39]. Furthermore, this deleterious effect could be followed by an excessive concentration of antioxidants that reduce too many oxidants that, in low concentration, are necessary for spermatozoa functions. Therefore, the in vitro use of L. meyenii extract at high concentrations would not be recommended.
The best results of Maca on the DNA of spermatozoa were observed at 24 h of incubation at all concentrations tested. Maintaining the DNA integrity of thawed semen is critical in assisted reproduction because, in the case of bulls, sperm DNA fragmentation values of 7–10% were associated with low AI success [40], and genetic and epigenetic alterations in sperm can result in early pregnancy loss [41].
Sperm viability was significantly lower after 1 and 3 h of incubation with the Maca dilutions, except at 24 h. A previous study indicated that sperm viability is related to bull fertility [42]. However, as these are not the only parameters of importance in determining fertility, reliance on only these parameters will not intuitively provide enough information regarding potential fertility to be a useful predictor [43].
On spermatozoa, the plasma membrane, mitochondrial membrane, and acrosomal membrane contain polyunsaturated fatty acids, and hence, they are very susceptible to OS, especially during freezing procedures [44]. OS is one of the factors that increase cell damage due to ROS. Excessive ROS production in sperm is dangerous because of its negative effects on functional sperm count [45]. Acrosome integrity is one of the determinant factors for the success of fertilization. Only acrosome-intact spermatozoa can penetrate the zona pellucida and can fuse with the oocyte plasma membrane [46]. In this assay, no damage was observed in the acrosomal membrane of spermatozoa incubated with Maca at concentrations of 1 and 10 μg/mL after 3 and 24 h. This result showed the protective effect of the Maca components on the integrity of the spermatozoa membrane, which indicates that they might be acting as a protector agent.
Conclusion
The in vitro results showed a general improvement in all sperm parameters analyzed (motility, vitality, acrosome integrity, morphology, and DNA fragmentation) in frozen–thawed bovine spermatozoa treated with L. meyenii extract, with the best results obtained at a concentration of 10 μg/mL. The observed improvement in these parameters plays an important role in predicting the sperm quality and thus the efficiency of embryo production. However, in vivo studies are required to elucidate the effects of L. meyenii in semen used to produce bovine embryos.
Authors’ Contributions
JL, MR, VN: conception and designed the study, methodology, analysis and interpretation of data, investigation, writing-original draft. JL, MR, AS, MLP, VN: Methodology, investigation, analysis and interpretation of data.MR, VN: Project administration, investigation, writing-original draft, writing-reviewing and editing. All authors read and approved the final manuscript.
Acknowledgments
We thank Oscar Romero and Jorge Alfonso for their collaboration and comments on the statistical analysis and Natalia Ramirez for the English grammar of the manuscript. This study was partially supported by CEDIC’s (Paraguay) own funds. MR and JLR would like to thank PRONII-CONACYT (National Incentive Program to Researchers of the National Science and Technological Council) (Paraguay) for financial support. MLP is grateful to Project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC).
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Gonzales G, Ruiz A, Gonzales C, Villegas L, Cordova A. Effect of Lepidium meyenii (Maca) roots on spermatogenesis of male rats. Asian J. Androl. 2001;3(3):231–233. [PubMed] [Google Scholar]
- 2.Wang S, Zhu F. Chemical composition and health effects of Maca (Lepidium meyenii) Food Chem. 2019;288:422–443. doi: 10.1016/j.foodchem.2019.02.071. [DOI] [PubMed] [Google Scholar]
- 3.da Silva Leitão Peres N, Cabrera Parra Bortoluzzi L, Medeiros Marques L.L, Formigoni M, Fuchs R.H.B, Droval A.A, Reitz Cardoso F.A. Medicinal effects of Peruvian Maca (Lepidium meyenii):A review. Food Funct. 2020;11(1):83–92. doi: 10.1039/c9fo02732g. [DOI] [PubMed] [Google Scholar]
- 4.Canales M, Aguilar J, Prada A, Marcelo A, Huamán C, Carbajal L. Nutritional evaluation of Lepidium meyenii (MACA) in albino mice and their descendants. Arch. Latinoam Nutr. 2000;50(2):126–133. [PubMed] [Google Scholar]
- 5.Gonzales G, Cordova A, Gonzales C, Chung A, Vega K, Villena A. Lepidium meyenii (Maca) improved semen parameters in adult men. Asian J. Androl. 2001;3(4):301–303. [PubMed] [Google Scholar]
- 6.Del Prete C, Tafuri S, Ciani F, Pasolini M.P, Ciotola F, Albarella S, Carotenuto D, Peretti V, Cocchia N. Influences of dietary supplementation with Lepidium meyenii (Maca) on stallion sperm production and on preservation of sperm quality during storage at 5°C. Andrology. 2018;6(2):351–361. doi: 10.1111/andr.12463. [DOI] [PubMed] [Google Scholar]
- 7.Aoki Y, Tsujimura A, Nagashima Y, Hiramatsu I, Uesaka Y, Nozaki T, Ogishima T, Shirai M, Shoyama Y, Tanaka H, Horie S. Effect of Lepidium meyenii on in vitro fertilization via improvement in acrosome reaction and motility of mouse and human sperm. Reprod. Med. Biol. 2019;18(1):57–64. doi: 10.1002/rmb2.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue N, Farfan C, Gonzales G.F. Effect of butanolic fraction of yellow and black Maca (Lepidium meyenii) on the sperm count of adult mice. Andrologia. 2016;48(8):915–921. doi: 10.1111/and.12679. [DOI] [PubMed] [Google Scholar]
- 9.Onaolapo A.Y, Oladipo B.P, Onaolapo O.J. Cyclophosphamide-induced male subfertility in mice:An assessment of the potential benefits of Maca supplement. Andrologia. 2018;50(3):1–10. doi: 10.1111/and.12911. [DOI] [PubMed] [Google Scholar]
- 10.Valdivia Cuya M, Yarasca De La Vega K, Lévano Sánchez G, Vásquez Cavero J, Temoche García H, Torres Torres L, Cruz Ornetta V. Effect of Lepidium meyenii (Maca) on testicular function of mice with chemically and physically induced subfertility. Andrologia. 2016;48(8):927–934. doi: 10.1111/and.12682. [DOI] [PubMed] [Google Scholar]
- 11.Lee M.S, Lee H.W, You S, Ha K.T. The use of Maca (Lepidium meyenii) to improve semen quality:A systematic review. Maturitas. 2016;92:64–69. doi: 10.1016/j.maturitas.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Tafuri S, Cocchia N, Vassetti A, Carotenuto D, Esposito L, Maruccio L, Avallone L, Ciani F. Lepidium meyenii (Maca) in male reproduction. Nat. Prod. Res. 2019;35(22):4550–4559. doi: 10.1080/14786419.2019.1698572. [DOI] [PubMed] [Google Scholar]
- 13.Hasler J.F. Forty years of embryo transfer in cattle:A review focusing on the journal Theriogenology, the growth of the industry in North America, and personal reminisces. Theriogenology. 2014;81(1):152–169. doi: 10.1016/j.theriogenology.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Long-Bo Z, Zhi-Lai Z, Qing-Xiu H, Min C, Li-Ping K. Research progress on chemical constituents and bioactivities of Lepidium meyenii. Zhongguo Zhong Yao Za Zhi. 2019;44(19):4142–4151. doi: 10.19540/j.cnki.cjcmm.20190419.202. [DOI] [PubMed] [Google Scholar]
- 15.Mathur P.P, D'Cruz S.C. The effect of environmental contaminants on testicular function. Asian J. Androl. 2011;13(4):585–591. doi: 10.1038/aja.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bardaweel S.K, Gul M, Alzweiri M, Ishaqat A, ALSalamat H.A, Bashatwah R.M. Reactive oxygen species:The dual role in physiological and pathological conditions of the human body. Eurasian J. Med. 2018;50(3):193–201. doi: 10.5152/eurasianjmed.2018.17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tafuri S, Cocchia N, Carotenuto D, Vassetti A, Staropoli A, Mastellone V, Peretti V, Ciotola F, Albarella S, Del Prete C, Palumbo V, Esposito L, Vinale F, Ciani F. Chemical analysis of Lepidium meyenii (Maca) and its effects on redox status and on reproductive biology in stallions. Molecules. 2019;24(10):1–12. doi: 10.3390/molecules24101981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hitit M, Ugur M.R, Dinh T.T.N, Sajeev D, Kaya A, Topper E, Tan W, Memili E. Cellular and functional physiopathology of bull sperm with altered sperm freezability. Front. Vet. Sci. 2020;7:581137. doi: 10.3389/fvets.2020.581137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro L.S, Hamilton T.R.S, Mendes C.M, Nichi M, Barnabe V.H, Visintin J.A, Assumpção M.E.O. Sperm cryodamage occurs after rapid freezing phase:Flow cytometry approach and antioxidant enzymes activity at different stages of cryopreservation. J. Anim. Sci. Biotechnol. 2016;7(1):17. doi: 10.1186/s40104-016-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gürler H, Malama E, Heppelmann M, Calisici O, Leiding C, Kastelic J.P, Bollwein H. Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm. Theriogenology. 2016;86(2):562–571. doi: 10.1016/j.theriogenology.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Peris-Frau P, Soler A.J, Iniesta-Cuerda M, Martín-Maestro A, Sánchez-Ajofrín I, Medina-Chávez D.A, Fernández-Santos M.R, García-Álvarez O, Maroto-Morales A, Montoro V, Garde J.J. Sperm cryodamage in ruminants:Understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int. J. Mol. Sci. 2020;21(8):2781. doi: 10.3390/ijms21082781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganzera M, Zhao J, Muhammad I, Khan I.A. Chemical profiling and standardization of Lepidium meyenii (Maca) by reversed-phase high-performance liquid chromatography. Chem. Pharm. Bull. 2002;50(7):988–991. doi: 10.1248/cpb.50.988. [DOI] [PubMed] [Google Scholar]
- 23.Dini A, Migliuolo G, Rastrelli L, Saturnino P, Schettino O. Chemical composition of Lepidium meyenii. Food Chem. 1994;49(4):347–349. [Google Scholar]
- 24.Ax R.L, Dally M, Didion B.A, Lenz R.W, Love C.C, Varner D.D, Hafez B, Bellin M.E. Reproduction in farm animals. Baltimore, Maryland, USA: Lippincott Williams &Wilkins; 2016. Semen evaluation; pp. 363–375. [Google Scholar]
- 25.Chenoweth P. Bull breeding soundness exams and beyond. Manhattan, KS: Proceedings of the applied reproductive strategies in beef cattle workshop; 2002. pp. 174–180. [Google Scholar]
- 26.Menon A, Thundathil J, Wilde R, Kastelic J, Barkema H. Validating the assessment of bull sperm morphology by veterinary practitioners. Can. Vet. J. 2011;52(4):407–408. [PMC free article] [PubMed] [Google Scholar]
- 27.Correa J.R, Zavos P.M. The hypoosmotic swelling test:Its employment as an assay to evaluate the functional integrity of the frozen-thawed bovine sperm membrane. Theriogenology. 1994;42(2):351–360. doi: 10.1016/0093-691x(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 28.Didion B.A, Dobrinsky J.R, Giles J.R, Graves C.N. Staining procedure to detect viability and the true acrosome reaction in spermatozoa of various species. Gamete Res. 1989;22(1):51–57. doi: 10.1002/mrd.1120220106. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Traganos F, Melamed M.R, Darzynkiewicz Z. Single-step procedure for labeling DNA strand breaks with fluorescein-or bodipy-conjugated deoxynucleotides:Detection of apoptosis and bromodeoxyuridine incorporation. Cytometry. 1995;20(2):172–180. doi: 10.1002/cyto.990200210. [DOI] [PubMed] [Google Scholar]
- 30.Clément C, Kneubühler J, Urwyler A, Witschi U.,, Kreuzer M. Effect of Maca supplementation on bovine sperm quantity and quality followed over two spermatogenic cycles. Theriogenology. 2010;74(2):173–183. doi: 10.1016/j.theriogenology.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Clément C. Relationship between colour type, environment and composition of the Andean plant Maca (Lepidium meyenii Walp.) and of its utility for enhancing fertility in breeding bulls. 2011 [Google Scholar]
- 32.Melnikovova I, Tomas F, Huml L, Kolarova M, Lapcik O, Cusimamani E. Effect of Lepidium meyenii on semen quality and reproductive hormones level in healthy adult men. Climacteric. 2014;17(1):87. [Google Scholar]
- 33.Rodríguez-Huamán Á, Casimiro-Gonzales S, Chávez-Pérez J.A, Gonzales-Arimborgo C, Cisneros-Fernández R, Aguilar-Mendoza L.Á, Gonzales G.F. Antioxidant and neuroprotector effect of Lepidium meyenii (Maca) methanol leaf extract against 6-hydroxy dopamine (6-OHDA)-induced toxicity in PC12 cells. Toxicol. Mech. Methods. 2017;27(4):279–285. doi: 10.1080/15376516.2016.1275908. [DOI] [PubMed] [Google Scholar]
- 34.Korkmaz S. Anti-oxidants in foods and its applications. India: InTech; 2018. Anti-oxidants in Maca (Lepidium meyenii) as a supplement in nutrition. [Google Scholar]
- 35.Lee Y.K, Chang Y.H. Physicochemical and anti-oxidant properties of methanol extract from Maca (Lepidium meyenii Walp.) leaves and roots. Food Sci. Technol. 2019;39(1):278–286. [Google Scholar]
- 36.Buyanbadrakh E, Hong H.S, Lee K.W, Huang W.Y, Oh J.H. Anti-oxidant activity, macamide B content and muscle cell protection of Maca (Lepidium meyenii) extracted using ultrasonication-assisted extraction. Microbiol. Biotechnol. Lett. 2020;48(2):129–137. [Google Scholar]
- 37.Gan J, Feng Y, He Z, Li X, Zhang H. Correlations between anti-oxidant activity and alkaloids and phenols of Maca (Lepidium meyenii) J. Food Qual. 2017;2017:3185945. [Google Scholar]
- 38.Rubio J, Riqueros M.I, Gasco M, Yucra S, Miranda S, Gonzales G.F. Lepidium meyenii (Maca) reversed the lead acetate induced-damage on reproductive function in male rats. Food Chem. Toxicol. 2006;44(7):1114–1122. doi: 10.1016/j.fct.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Calabrese E.J, Mattson M.P, Calabrese V. Resveratrol commonly displays hormesis:Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010;29(12):980–1015. doi: 10.1177/0960327110383625. [DOI] [PubMed] [Google Scholar]
- 40.Karoui S, Díaz C, González-Marín C, Amenabar M.E, Serrano M, Ugarte E, Gosálvez J, Roy R, López-Fernández C, Carabaño M.J. Is sperm DNA fragmentation a good marker for field AI bull fertility? J. Anim. Sci. 2012;90(8):2437–2449. doi: 10.2527/jas.2011-4492. [DOI] [PubMed] [Google Scholar]
- 41.Khadem N, Poorhoseyni A, Jalali M, Akbary A, Heydari S.T. Sperm DNA fragmentation in couples with unexplained recurrent spontaneous abortions. Andrologia. 2014;46(2):126–130. doi: 10.1111/and.12056. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Martínez H. Semen evaluation techniques and their relationship with fertility. Anim. Reprod. 2013;10(3):148–159. [Google Scholar]
- 43.Kumaresan A, Johannisson A, Al-Essawe E.M, Morrell J.M. Sperm viability, reactive oxygen species, and DNA fragmentation index combined can discriminate between above-and below-average fertility bulls. J. Dairy Sci. 2017;100(7):5824–5836. doi: 10.3168/jds.2016-12484. [DOI] [PubMed] [Google Scholar]
- 44.Chelucci S, Pasciu V, Succu S, Addis D, Leoni G.G, Manca M.E, Naitana S, Berlinguer F. Soybean lecithin-based extender preserves spermatozoa membrane integrity and fertilizing potential during goat semen cryopreservation. Theriogenology. 2015;83(6):1064–1074. doi: 10.1016/j.theriogenology.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Rath D, Bathgate R, Rodriguez-Martinez H, Roca J, Strzezek J, Waberski D. Recent advances in boar semen cryopreservation. Soc. Reprod. Fertil. 2009;66:51–66. [PubMed] [Google Scholar]
- 46.Celeghini E.C.C, Nascimento J, Raphael C.F, Andrade A.F.C, Arruda R.P. Simultaneous assessment of plasmatic, acrosomal, and mitochondrial membranes in ram sperm by fluorescent probes. Arq. Bras. Med. Vet. Zootec. 2010;62(3):536–543. [Google Scholar]


