Abstract
Introduction.
Maternal vaccination is critical for improving maternal and child health. Quality Improvement (QI) models1, such as the Centers for Disease Control and Prevention’s (CDC) Assessment, Feedback, Incentives, eXchange (AFIX)2 model, have not yet been adapted to maternal vaccinations. This study assesses the impact of AFIX-OB, an adapted version of AFIX for obstetric settings, on maternal vaccination rates.
Methods.
Between December 2016 and May 2018, state health departments and obstetric practices in Colorado and Georgia implemented the adapted AFIX-OB model. The model addressed unique patterns in patient encounters, practice flow, health records systems and competing clinical priorities in the obstetric setting through a menu of clearly-defined QI strategies, bi-weekly technical assistance meetings with designated immunization champions, incentives for champions/staff, and adapted tools to aid each practice during implementation. Vaccination rates were assessed by random chart reviews pre- and post-intervention.
Results.
The AFIX-OB model was evaluated in eleven obstetric practices in two states as part of a multi-level intervention to increase maternal vaccination. Post AFIX-OB implementation, documented influenza vaccination rates increased from 56% at baseline to 65% (p<0.01); and tetanus, diphtheria, and acellular pertussis (Tdap) vaccination rates increased from 77% at baseline to 84% (p<0.02) across all practices.
Conclusions.
The AFIX-OB model showed improvement in maternal vaccination rates for both influenza and Tdap vaccines. AFIX-OB may provide a useful framework for obstetric practices, as well as for other health care specialties. The focused goal should be on broader dissemination among those interested in adopting an evidence-based model for increasing vaccine uptake.
Introduction
Immunization delivery during pregnancy is becoming routine clinical practice.1 Vaccination became routinely recommended against influenza in any trimester in 2004 and against Bordetella pertussis during each pregnancy in 2012.2–6 According to a survey conducted by the Centers for Disease Control and Prevention (CDC), 36.8% of pregnant women reported receiving influenza vaccination during the 2017–2018 season and 54.4% reported receiving the Tetanus, diphtheria and acellular pertussis (Tdap) vaccine in 2018 during pregnancy,7 much lower than the current Healthy People 2020 goal of 80%.7–9 Barriers to maternal vaccine uptake exist at the patient, provider, and practice level. Patient level barriers are largely based on vaccine safety and efficacy concerns.10,11 Provider and practice level barriers include inadequate reimbursement for vaccination and administration, challenges stocking and storing vaccines, lack of provider recommendations to pregnant patients, and difficulties integrating vaccination activities into clinical workflows.12,13
While quality improvement (QI) strategies have been applied to increase maternal vaccination rates, success of such interventions in obstetric settings is limited.14 The CDC’s four-phase model - Assessment, Feedback, Incentives and eXchange (AFIX) - is a vaccine-related QI model that has demonstrated success in vaccine uptake in other clinical settings.15–18 First established among public pediatric clinics in Georgia in the 1990’s,16,19 the premise of AFIX is to improve providers’ motivation to vaccinate through assessment of current vaccination coverage coupled with feedback, goal setting, and incentives. In 1993, CDC adopted the model as a national requirement for pediatric clinics enrolled in the Vaccines for Children (VFC) program.20 Due to the success of AFIX, we adapted the model and sought to test its use in obstetrics settings. The objective of this paper is to describe the adapted AFIX-OB model and assess the effectiveness of AFIX-OB on improving maternal vaccination uptake.
Methods
This adaptation and evaluation of AFIX-OB was embedded within a larger study aimed at improving maternal and childhood vaccination uptake through a multi-level intervention at the practice, provider and patient levels.21 The adapted model was implemented and evaluated at the practice level in the intervention arm. The AFIX-OB intervention was carried out in eleven geographically and socio-demographically diverse obstetric practices in Colorado and Georgia. The study was reviewed and approved by the Emory University Institutional Review Board and a HIPAA waiver was granted for the collection of de-identified immunization data for assessment at the practice-level.
AFIX affiliates at the Colorado and Georgia health departments were engaged to align the adaptation with the traditional AFIX model.20 The components of the adapted AFIX-OB model were informed by: (1) identifying the strengths and challenges of the traditional AFIX model, (2) the unique barriers that obstetric providers face in implementing QI measures related to vaccination, and (3) the tools needed to carry out AFIX-OB (Supplemental Table 1). QI strategies were drawn directly from the CDC’s AFIX model and previous studies adapting these strategies to obstetric settings.22–27
Assessment.
The goal of the Assessment phase is to obtain an estimate of baseline maternal influenza and Tdap vaccination rates. Obstetric practices face multiple obstacles to evaluating vaccination rates including the lack of centralized immunization information systems9 and variability in how providers document vaccine administration.27 These barriers lead to varying and unreliable provider-specific rates. This can be complicated further because pregnant patients are often seen by different providers during their prenatal care. It was determined that the most feasible option for obtaining vaccination rates would be to conduct manual chart reviews at each practice. To streamline this process, a data collection instrument from a prior maternal vaccination study was adapted to collect data in REDCap.22,23,26,27 Details of these measures are described below.
Feedback.
Feedback entails presenting baseline immunization rates to providers and staff within each practice. To share Assessment data, we adapted a standardized pediatric “immunization report card” to maternal vaccinations (Supplemental Figure 1). The adapted “maternal immunization report card” compares baseline practice-specific maternal immunization rates to other obstetrics sites, current state and national rates, and Healthy People 2020 goals. As part of the overall parent study, we also utilized an immunization delivery scale (IDS) to collect data about vaccine delivery procedures already utilized in each practice.22,28 This IDS revealed three common shortfalls in maternal vaccination at participating clinics: (1) utilization of Vaccine Information Sheets (VIS) and/or educational materials addressing vaccine concerns, (2) routine and systematic Electronic Health Record (EHR)/paper-based vaccine tracking protocols, and (3) adoption of a formal standing order program to reduce missed opportunities to vaccinate. These identified gaps informed which of CDC’s evidence-based QI strategies found in the AFIX model would be most applicable for improving maternal vaccination.
Key decision-makers were encouraged to attend a one-hour Feedback session at each clinic. During these meetings, the “maternal immunization report card” and results of the IDS were shared. Based on prior research experiences in obstetric clinics,22,23,26 we limited the menu of QI strategies to those that directly addressed gaps in each practices’ current procedures and developed a two-point approach to the initial Feedback session. The first approach was vaccine promotion messaging as endorsed by CDC and the American College of Obstetricians and Gynecologists (ACOG) focused on educating pregnant patients about the risks of disease and benefits of vaccination using laminated VIS sheets, website improvements and branded vaccine promotion flyers. The second approach was to select one of the two following QI strategies to reduce missed opportunities: (1) establishing a standardized immunization tracking EHR protocol, or (2) formalizing a standing orders protocol.24 Each practice set a goal either to increase their rates by at least 5 percentage points if baseline rates were under 80% coverage, or to increase their rates by at least 1 percentage point if the baseline rates were at or above that goal. Each practice committed to at least one QI measure to implement for a six-month cycle.
Incentives.
Incentives identifies ways to motivate providers and staff to implement QI strategies. As vaccination becomes an expectation of obstetric providers, practices are still working to incorporate vaccinations into routine workflows and balance promoting vaccines with competing demands.24,27,29 Incentivizing providers and clinical staff was vital for developing buy-in around engaging in tasks and achieving goals. Prior experience and input from participating practices resulted in the following incentives: (1) Monetary incentives - Each immunization champion (the primary point of contact at each clinic) received a stipend of $500 for a two-year commitment to implement AFIX-OB.22,23,25 In four clinics, stipends could not be accepted due to institutional policies and the funds were reallocated towards general practice support. (2) Support - Immunization champions received bi-weekly technical assistance with study staff. Study staff also utilized an adapted meeting model26 that tracked processes, provided structure, and focused on reaching AFIX-OB QI goals. Once a QI strategy was successfully implemented, the strategy was moved to the sustainability phase and further assistance given only if needed. (3) Food incentives - Food was provided for staff at the initial Feedback meeting and follow-up eXchange meeting (described below) as an incentive to attend meetings. (4) Professional development - By participating in the initial meeting, completing an educational CME module30 (requisite for the provider-level intervention), and adhering to the six-month intervention commitment, providers were eligible to receive American Board of Obstetrics and Gynecology Maintenance of Certification (MOC) Part IV credit.
eXchange.
The eXchange phase involves reassessing practice vaccination rates and updating the report cards six months after the Feedback session. After six months, chart reviews used the same data collection methods and criteria used in the Assessment phase. An adapted follow-up report card highlighted results of each practice’s vaccination rate progression. Follow-up data were presented in fifteen to twenty minute in-person meetings with practice providers, staff, and immunization champions. A “sustainability package” detailed suggestions to encourage clinics’ successful progression toward unmet goals or maintenance of successfully implemented QI strategies. Hard copies of branded flyers, website content, standing orders and/or EHR protocols were given to each intervention practice to utilize as desired.
Measures.
In 2017, each practice carried out the six-month intervention with a start date dependent on each practice’s readiness to launch. Maternal influenza and Tdap vaccination rates were assessed for all eleven practices before baseline interventions were initiated (December 2016 to June 2017) and after the six-month interventions were completed (January 2018 to May 2018). A random sample of 40 charts were reviewed at each practice. Because this portion of the larger trial was designed to be a QI study, the number of charts chosen to be reviewed was consistent with a similar study that looked at the impact of EHR systems on maternal vaccination uptake27 and trended similarly to its parent randomized controlled trial across practices of different sizes.31 We calculated percent vaccinated, percent refusing one or both vaccines, and percent missing vaccine documentation. The sample included charts of women who had at least two prenatal care visits after September 1 of the assessment year and had delivered by the day of chart review. Consistency of gathering data for chart reviews was facilitated first by assessing what fields were utilized most often for data capture (e.g., patient demographics, vaccination history) and if record keeping seemed provider-dependent. Once all desired data fields were identified in each charting system, data was recorded in a password-protected, secured electronic database, Research Electronic Data Capture (REDCap).32
The demographic variables collected at both baseline and follow-up included race, ethnicity, insurance and mother’s age. Vaccination-related variables collected included: (1) if the patient was flagged for needing influenza and/or Tdap vaccinations; (2) if the patient was offered influenza and/or Tdap vaccines; (3) date and location vaccine(s) were administered; (4) date of refusal if patient refused either or both vaccines; (5) location of data in each field (eg: notes/comments field versus structured field) and; (6) notation of any missing data.
Statistical Analysis.
The AFIX-OB model was evaluated by assessing change in reported uptake of both the influenza and Tdap vaccines. Fisher’s exact tests and t-tests were used for pre-/post-intervention comparison of descriptive characteristics and immunization rates with p ≤ 0.05 were considered statistically significant. All data analyses were conducted using SAS 9.4 (Cary, NC).
Results
Eleven intervention practices, five in Georgia and six in Colorado, successfully followed AFIX-OB between Fall 2017 and Spring 2018. For the educational QI component of AFIX-OB, ten practices utilized laminated VIS sheets for Tdap and influenza, five practices incorporated content on their website, all eleven practices had providers (N=62 total) complete the CME module30 and all eleven practices utilized study branded vaccine promotion flyers. For the missed opportunities QI component, one practice instituted a standing orders protocol, eight practices instituted the EHR protocol and two practices implemented both.
We assessed 446 random charts during the 2016–2017 influenza season (pre-intervention/baseline) and 443 charts during the 2017–2018 influenza season (post-intervention/follow-up); approximately 40 charts per practice (see Table 1). Race/ethnicity data was not available for approximately 40% of charts during pre-assessment and 30% in post-assessment. The average number of visits per woman was higher pre-intervention than post (mean visits 12.7 pre vs. 11.4 post; p < 0.001). Approximately two-thirds of individuals whose charts were selected were covered by private insurance (pre = 69%, post = 67%). The remainder were primarily covered by Medicaid (pre = 25%, post = 28%). Mean age was 31 years.
Table 1.
Descriptive characteristics of study population
| Baseline Chart Review (N = 446) | 6-Month Follow-Up Chart Review (N =443) | ||||||
|---|---|---|---|---|---|---|---|
| Overall | Georgia | Colorado | Overall | Georgia | Colorado | p-value | |
| Race/Ethnicitya – N | <0.001* | ||||||
| (%) | |||||||
| White | 163 (36.6) | 54 (27.0) | 109 (44.3) | 174 (39.3) | 32 (15.8) | 142 (58.9) | |
| Black | 31 (7.0) | 19 (9.5) | 12 (4.9) | 73 (16.5) | 59 (29.2) | 14 (5.8) | |
| Hispanic | 49 (11.0) | 6 (3.0) | 43 (17.5) | 41 (9.3) | 1 (0.5) | 40 (16.6) | |
| Asian | 10 (2.2) | 5 (2.5) | 5 (2.0) | 11 (2.5) | 2 (1.0) | 9 (3.7) | |
| Native American / Alaskan Native | 1 (0.2) | 0 (0.0) | 1 (0.4) | 1 (0.2) | 0 (0.0) | 1 (0.4) | |
| Other | 8 (1.8) | 6 (3.0) | 2 (0.8) | 7 (1.6) | 6 (3.0) | 1 (0.4) | |
| Unknown | 184 (41.3) | 110 (55.0) | 74 (30.1) | 136 (30.7) | 102 (50.5) | 34 (14.1) | |
| Insurance Coverage – N (%) | 0.64 | ||||||
| Private | 307 (68.8) | 156 (78.0) | 151 (61.4) | 296 (66.8) | 145 (71.8) | 151 (62.7) | |
| Medicaid | 111 (24.9) | 33 (16.5) | 78 (31.7) | 126 (28.4) | 51 (15.3) | 75 (31.1) | |
| Medicare | 2 (0.5) | 0 (0.0) | 2 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other | 18 (4.0) | 7 (3.5) | 11 (4.5) | 14 (3.2) | 3 (1.5) | 11 (4.6) | |
| Uninsured | 3 (0.7) | 2 (1.0) | 1 (0.4) | 2 (0.5) | 2 91.0) | 0 (0.0) | |
| Unknown | 5 (1.1) | 2 (1.0) | 3 (1.2) | 5 (1.1) | 1 (0.5) | 4 (1.7) | |
| Number of office visits – Mean(SD) | 12.7 (3.1) | 12.7 (3.1) | 11.4 (3.8) | 11.4 (3.3) | 10.7 (3.6) | 12.0 (2.9) | <0.01** |
| Maternal Age – Mean (SD) | 32.0 (5.5) | 32.0 (5.5) | 30.9 (5.3) | 31.5 (5.0) | 31.4 (4.8) | 31.6 (5.1) | 0.82 |
Boldface indicates statistical significance of (*p<0.001, **p<0.01) comparing overall proportions between pre-intervention and post-intervention sample
All races are non-Hispanic; Hispanic can be of any race
Baseline documented influenza vaccine receipt was 56% and increased to 65% following the six-month intervention (p < 0.01) across both states (Figure 1a). Influenza vaccine receipt increased from baseline in Colorado (60% to 69%; p < 0.001) and in Georgia (51% to 59%; p = 0.08). Baseline Tdap vaccine receipt among all practices was 77% and increased to 84% following the six-month intervention (p<0.02) (Figure 1b). Similar to influenza vaccine, Tdap vaccine receipt increased from baseline in Colorado (81% to 90%; p < .01) and in Georgia (72% to 78%; p = 0.43). Baseline documented vaccine receipt varied widely by clinic; from 10% to 83% for influenza vaccine and from 12% to 98% for Tdap vaccine (Table 2). All eleven clinics saw improved uptake of Tdap vaccine and seven clinics saw increases in influenza vaccine uptake.
Figure 1a.
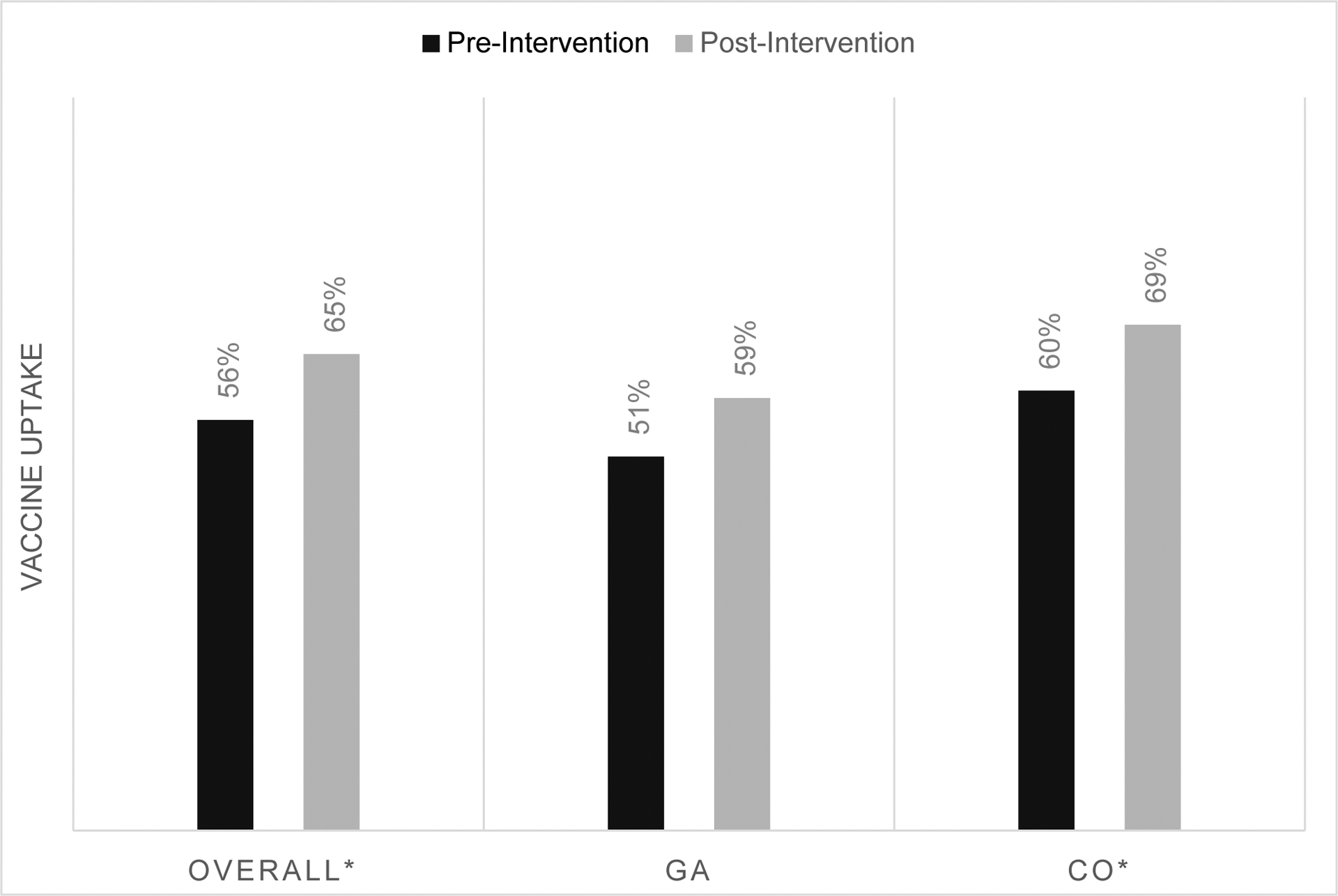
Influenza vaccine uptake pre- and post-intervention
*p < 0.05
Figure 1b.
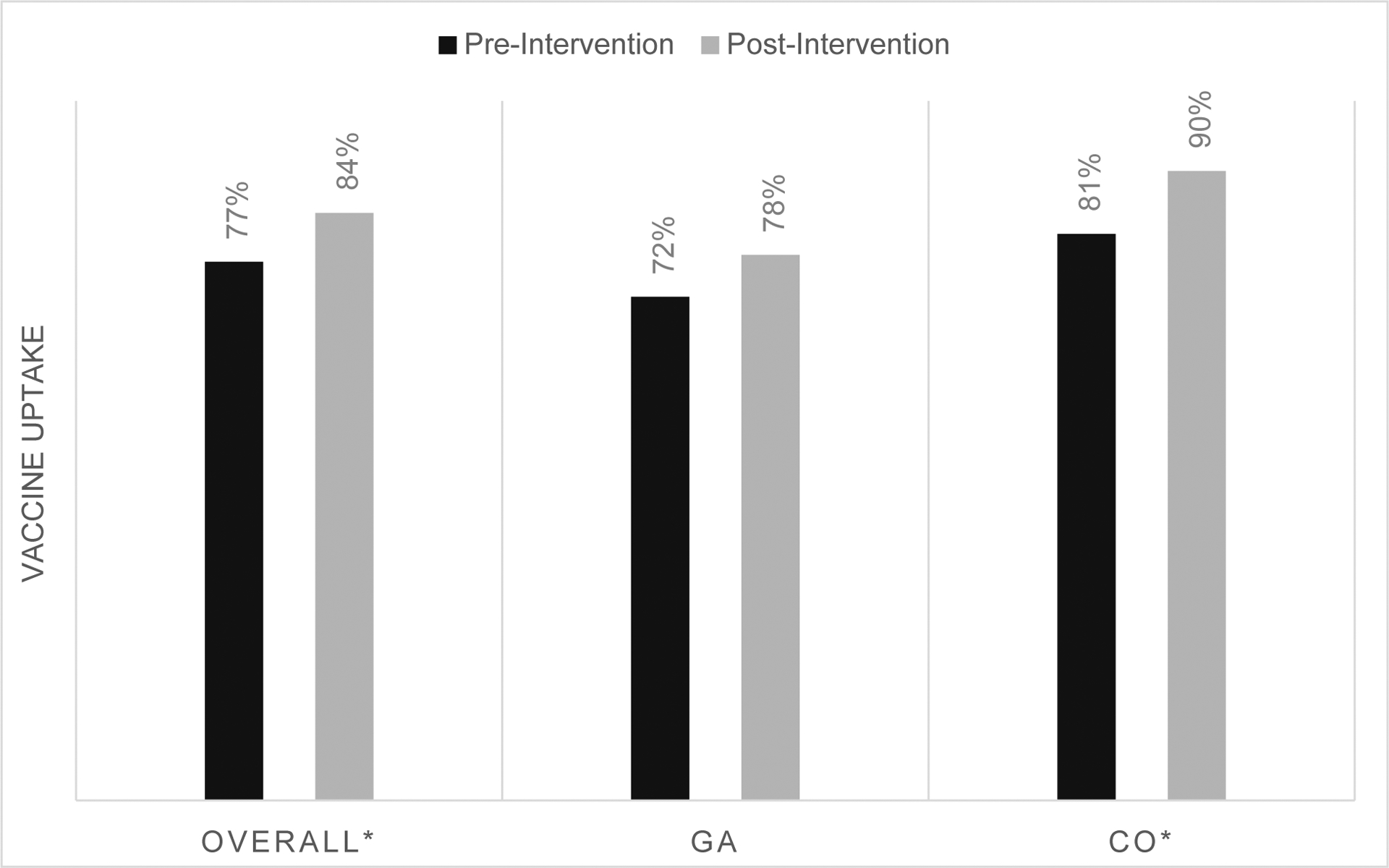
Tetanus, diphtheria and acellular pertussis (Tdap) vaccine uptake pre- and post-intervention
*p < 0.05
Table 2.
Pre- and Post-AFIX vaccination and documentation rates
| Influenza | Tdap | |||||||
|---|---|---|---|---|---|---|---|---|
| Vaccine Uptake | No Documentation | Vaccine Uptake | No Documentation | |||||
| Baseline (%) | 6-Month Follow-Up (%) | Baseline (%) | 6-Month Follow-Up (%) | Baseline (%) | 6-Month Follow-Up (%) | Baseline (%) | 6-Month Follow-Up (%) | |
| Colorado | ||||||||
| Practices | ||||||||
| Aa | 32.5 | 57.5 | 55.0 | 12.5 | 62.5 | 75.0 | 22.5 | 5.0 |
| Bb | 60.0 | 60.0 | 25.0 | 17.0 | 82.5 | 97.5 | 10.0 | 2.5 |
| Ca | 37.5 | 65.0 | 35.0 | 10.0 | 70.0 | 90.0 | 7.5 | 0.0 |
| Dc | 63.4 | 85.0 | 26.8 | 5.0 | 97.6 | 100.0 | 0.0 | 0.0 |
| Ec | 79.5 | 75.0 | 6.8 | 5.0 | 86.4 | 90.0 | 2.3 | 0.0 |
| Fb | 82.9 | 73.2 | 7.3 | 9.8 | 85.4 | 87.8 | 2.4 | 2.4 |
| Georgia | ||||||||
| Practices | ||||||||
| Ha | 37.5 | 62.5 | 57.5 | 38.0 | 80.0 | 90.0 | 15.0 | 10.0 |
| Ia | 55.0 | 62.5 | 25.0 | 22.5 | 90.0 | 95.0 | 10.0 | 0.0 |
| Ja | 75.6 | 67.5 | 2.4 | 10.0 | 82.9 | 92.5 | 0.0 | 0.0 |
| Ka | 10.0 | 32.5 | 87.5 | 40.0 | 12.5 | 25.0 | 85.0 | 67.5 |
| Lc | 75.0 | 78.4 | 5.0 | 3.0 | 90.0 | 91.9 | 0.0 | 0.0 |
Implemented EHR improvements
Implemented Standing Orders protocol
Implemented both
Documentation of vaccine receipt or refusal varied widely among EHRs/paper charts. Documentation of influenza vaccine activities in each clinic was largely inconsistent (median = 25% missing; range: 2% to 88% of charts with missing data) but generally better for Tdap at baseline (median = 8% missing; range: 0% to 85% of charts missing data). Only one clinic was missing Tdap immunization data for more than 25% of charts reviewed whereas for influenza, seven clinics were missing documentation of receipt or refusal for 25% or more. Improvement at six months in percent charts reviewed with missing documentation of influenza activities was noted in ten practices (median = 10% missing; range 3% to 40% for influenza at follow-up). There was 100% documentation of Tdap receipt or refusal in six practices at follow-up (median = 0% missing; range 0% to 68%).
Discussion
This study demonstrated that it is possible to adapt and implement the traditional AFIX model in obstetric settings to successfully improve documented receipt of recommended maternal immunizations. However, it should be acknowledged that oversight by study staff along with additional incentives may have augmented the level of successful fulfillment of all required QI activities. Eleven participating practices successfully adapted each phase of AFIX-OB and implemented both educational materials, as well as either a formalized EHR or standing orders protocol. Effectively adapting AFIX to obstetrics required a novel approach that addressed the unique factors in obstetrics that influence maternal immunization uptake including differences in EHR systems, the lack of interoperability in utilizing a centralized system, variability in provider dependent EHR tracking, differences in clinical workflow and the potential for patients to be seen by multiple providers during their prenatal care.
Overall, there was a significant increase in uptake of influenza vaccine by approximately 9 percentage points and Tdap by 7 percentage points across all clinics. These improvements are similar to another QI initiative for maternal Tdap vaccination, which achieved 7.6% absolute increase over 15 months.33 It is important to highlight that many of the practices in this study had baseline vaccination rates that exceeded state and national averages.7 Despite this, many of these clinics with high baseline rates still had improved coverage at the end of the intervention, suggesting that AFIX-OB could be a powerful QI approach in obstetrics.
Improvement in documentation for maternal vaccination in our study lines up with a similar study examining impacts of a multimodal intervention in obstetrics where EHR documentation of vaccination greatly increased (27% to 60% for influenza vaccine and 13% to 87% for Tdap vaccine) over a 3-year intervention. However, participating practices reported significant barriers to both implementing standing orders and documenting in EHRs.24,27 This AFIX-OB model was able to make significant gains in a shorter time frame. It is important to note that our study’s somewhat higher documentation rates from baseline to six months could be due to the limited set of practice-level interventions,20 allowing practices’ to focus exclusively on implementing consistent charting practices. It is also possible that observed improvement in documentation within our study illustrates the secular trend associated with improving maternal vaccine administration since the 2012 recommended changes in maternal vaccination; however, state and national surveillance data do not provide sufficient information on documentation to understand if this is the case. While the impacts of documentation on maternal vaccination uptake among pregnant women should be further explored, these outcomes suggest the importance of the U.S. Department of Health and Human Services National Vaccine Plan (NVP)’s recommendation to team up with state health departments to collect and track adult immunization data through their state’s Immunization Information System (IIS).34 Tracking maternal immunization data in a centralized system could decrease barriers such as the variability of documentation among providers and increase interoperability between different EHR systems. Adaptation of AFIX-OB required substantial support from outside immunization delivery experts, such as public health professionals and academic researchers. Furthermore, because there is no requirement in obstetric settings to incorporate AFIX into practice, as is the case in VFC enrolled primary care practices, obstetric practices required additional incentives. Once support and incentives are withdrawn, it will be important to ascertain sustainability of QI strategies. The AFIX-OB model should also be implemented and tested in additional obstetric settings to evaluate effectiveness in supporting improved vaccine uptake and how it can be more widely endorsed and adopted. This research is important as both the list of vaccines during pregnancy grows and CDC rolls out its latest iteration of its vaccination QI program called Immunization Quality Improvement for Providers (IQIP).35
The strength of AFIX-OB lies in its comprehensive and customizable nature. Systematic reviews of barriers to maternal vaccine uptake have noted that barriers exist at all levels of clinical care.9,13 Although we primarily focused on those QI interventions designed to improve delivery of vaccines at the practice-level, this was complemented by offering educational materials for both patients (flyers) and providers (educational module) to ensure that a wide variety of barriers could be addressed. However, these barriers are not constant across obstetric settings as three clinics had nearly perfect documentation of both influenza and Tdap vaccination and therefore did not need assistance in improving their EHRs. Practices with such high documentation rates may benefit from other QI strategies and evidence-based vaccination interventions.
The AFIX-OB process was implemented as part of a larger study (NCT#: 02898688) evaluating the impact of a set of patient-, provider- and practice-level interventions. Design of this larger study was such that we cannot completely separate the impact of the AFIX-OB quality improvement approach from that of the provider and patient interventions. Second, random chart reviews were an evaluation measure conducted to assess vaccination rates and was not compared to random chart reviews in control practices. Third, since many clinics were inconsistent in their documentation at baseline but improved throughout the course of the study, we cannot determine if increases were exclusively due to increased vaccine uptake or an artifact of improved documentation.
Conclusions
Obstetric practices were able to successfully adapt and implement the AFIX-OB model by following detailed QI methodology resulting in improvement in maternal immunization rates. AFIX-OB may provide a useful framework for adaptation and implementation of QI activities for other obstetric practices, as well as for other health care specialties, for increasing vaccine uptake.
Supplementary Material
Highlights:
Quality improvement model shows advancement in maternal vaccination rate increases
Tdap and influenza uptake increase in obstetrics with quality improvement model
Successful adaption of quality improvement model around maternal vaccination
Acknowledgements
Nikki Griffin (Georgia Department of Public Health), Lynn Trefren, RN, MSN (Colorado Department of Public Health and Environment) and Lori Stone Quick, RN, MSN (Colorado Department of Public Health and Environment) all consulted with study staff regarding the development and implementation of the AFIX-OB model.
Disclosure of Financial Support: This work was support in part by the National Institutes of Health [grant number R01AI110482]. The funder had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interest: CI Spina, SE Brewer, RJ Limaye, SB Omer, ST O’Leary, MK Ellingson, and WT Orenstein have no conflicts and report no financial disclosures. AT Chamberlain received paid consultancy with the American College of Obstetricians and Gynecologists regarding provider-to-patient communications. D Salmon received consulting and/or research support form Merck, Walgreens and Pfizer.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviation QI: Quality Improvement
Abbreviation AFIX: Assessment, Feedback, Incentives, eXchange
References
- 1.American College of Obstetricians and Gynecologists. Immunizations and Routine Obstetric-Gynecologic Care: A Guide for Providers and Patients. https://immunizationforwomen.org/downloads/Toolkits/Immunization/Imm-Routine-Care-Guide-Toolkit-FINAL-5-17-13.pdf Published 2013. Accessed November 5, 2019.
- 2.Liang JL,DVM, Tiwari T,MD, Moro P,MD, et al. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 2018;67(RR-2). doi: 10.15585/mmwr.rr6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women - Advisory Committee on Immunization Practices (ACIP), 2012. MMWR. 2013;62(07):131–135. [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Guidelines for Vaccinating Pregnant Women. https://www.cdc.gov/vaccines/pregnancy/hcp/guidelines.html. Published 2017. Accessed2018. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Flu Vaccine Safety and Pregnancy. https://www.cdc.gov/flu/highrisk/pregnant.htm. Published 2017. Accessed November 5, 2019. [Google Scholar]
- 6.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report: Recommendations and Reports. 2004;53(RR-6):1–39. [PubMed] [Google Scholar]
- 7.Kahn KE, Black CL, Ding H, et al. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2018. Morbidity and Mortality Weekly Report. 2018;67(38):1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health Interview Survey, Centers for Disease Control and Prevention, National Center for Health Statistics. Increase the percentage of pregnant women who are vaccinated against seasonal influenza. Office of Disease Prevention and Health Promotion. https://www.healthypeople.gov/2020/data-search/Search-the-Data#objid=6362;. Published 2018. Accessed2018. [Google Scholar]
- 9.O’Leary ST, Riley LE, Lindley MC, et al. Immunization Practices of US Obstetrician/Gynecologists for Pregnant Patients. American journal of preventive medicine. 2018;54(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moniz MH, Hasley S, Meyn LA, Beigi RH. Improving influenza vaccination rates in pregnancy through text messaging: a randomized controlled trial. Obstetrics and gynecology. 2013;121(4):734–740. [DOI] [PubMed] [Google Scholar]
- 11.Lutz CS, Carr W, Cohn A, Rodriguez L. Understanding barriers and predictors of maternal immunization: Identifying gaps through an exploratory literature review. Vaccine. 2018;36(49):7445–7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Vaccine Advisory Committee T. The National Vaccine Advisory Committee: Reducing Patient and Provider Barriers to Maternal Immunizations: Approved by the National Vaccine Advisory Committee on June 11, 2014. Public Health Reports. 2015;130(1):10–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuen CY, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women - a systematic review. Vaccine. 2014;32(36):4602–4613. [DOI] [PubMed] [Google Scholar]
- 14.MacDougall DM, Halperin SA. Improving rates of maternal immunization: Challenges and opportunities. Human Vaccines & Immunotherapeutics. 2016;12(4):857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeBaron CW, Chaney M, Baughman AL, Dini EF, Maes E, Dietz V, & Bernier R,. Impact of measurement and feedback on vaccination coverage in public clinics, 1988–1994. JAMA. 1997;277(8):631–635. [PubMed] [Google Scholar]
- 16.Dini EF, Chaney M, Moolenaar RL, LeBaron CW. Information as intervention: how Georgia used vaccination coverage data to double public sector vaccination coverage in seven years. Journal of public health management and practice: JPHMP. 1996;2(1):45–49. [DOI] [PubMed] [Google Scholar]
- 17.Silberman TA, Fernandez MI, Golden RE, Baranov SM, Whitfield D,. Implementation of an Enhanced-AFIX Intervention in Central and South Los Angeles. The 37th National Immunization Conference of CDC; March 17, 2003, 2003; California. [Google Scholar]
- 18.Washburn T, Devi Wold A, Raymond P, Duggan-Ball S, Marceau K, Beardsworth A,. Current initiatives to protect Rhode Island adolescents through increasing HPV vaccination. Human vaccines & immunotherapeutics. 2016;12(6):1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeBaron CW, Mercer JT, Massoudi MS, et al. Changes in clinic vaccination coverage after institution of measurement and feedback in 4 states and 2 cities. Archives of pediatrics & adolescent medicine. 1999;153(8):879–886. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Assessment, Feedback, Incentives, eXchange (AFIX) Program: Policies and Procedures Guide. https://www.cdc.gov/vaccines/programs/afix/downloads/standards-guide.pdf. Published 2013. Accessed February 1, 2019.
- 21.National Institutes of Health. P3+ Intervention Phase: Clinical Trial Registration NCT02898688. https://clinicaltrials.gov/ct2/show/NCT02898688. Published 2016. Updated August 5, 2020. Accessed October 12, 2020. [Google Scholar]
- 22.O’Leary ST, Pyrzanowski J, Brewer SE, Dickinson LM, Dempsey AF. Evidence-based vaccination strategies in obstetrics and gynecology settings: Current practices and methods for assessment. Human vaccines & immunotherapeutics. 2016;12(4):866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzoni S, Brewer SE, Durfee J, et al. Patient perspectives of obstetrician-gynecologists as primary care providers. Journal of Reproductive Medicine. 2017;62(1–2):3–8. [PubMed] [Google Scholar]
- 24.Barnard JG, Dempsey AF, Brewer SE, Pyrzanowski J, Mazzoni SE, O’Leary ST. Facilitators and barriers to the use of standing orders for vaccination in obstetrics and gynecology settings. Am J Obstet Gynecol. 2017;216(1):69.e61–69.e67. [DOI] [PubMed] [Google Scholar]
- 25.Chamberlain AT, Seib K, Ault KA, et al. Improving influenza and Tdap vaccination during pregnancy: A cluster-randomized trial of a multi-component antenatal vaccine promotion package in late influenza season. Vaccine. 2015;33(30):3571–3579. [DOI] [PubMed] [Google Scholar]
- 26.O’Leary S, Pyrzanowski J, Brewer S, et al. A Pragmatic Cluster-Randomized Trial to Increase Uptake of Vaccines During Pregnancy. Open Forum Infectious Diseases. 2016;3(suppl_1). [Google Scholar]
- 27.Brewer SE, Barnard J, Pyrzanowski J, O’Leary ST, Dempsey AF. Use of Electronic Health Records to Improve Maternal Vaccination. Women’s Health Issues. 2019. [DOI] [PubMed] [Google Scholar]
- 28.Ellingson MK, Dudley MZ, Limaye RJ, Salmon DA, O’Leary ST, Omer SB. Enhancing uptake of influenza maternal vaccine. Expert review of vaccines. 2019;18(2):191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellingson M, Chamberlain A,. Beyond the verbal: Pregnant women’s preferences for receiving influenza and Tdap vaccine information from their obstetric care providers. Human Vaccines & Immunotherapeutics. 2018;14(3):767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chamberlain AT, Limaye RJ, O’Leary ST, et al. Development and acceptability of a video-based vaccine promotion tutorial for obstetric care providers. Vaccine. 2019;37(19):2532–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Leary ST, Pyrzanowski J, Brewer SE, Sevick C, Dickinson LM, Dempsey AF. Effectiveness of a multimodal intervention to increase vaccination in obstetrics/gynecology settings. Vaccine. 2019;37(26):3409–3418. [DOI] [PubMed] [Google Scholar]
- 32.Colorado Clinical and Translational Sciences Institute (CCTSI). Translational Informatics. University of Colorado Denver. http://www.ucdenver.edu/research/CCTSI/programs-services/informatics/Pages/REDCap-Data-Storage.aspx. Published 2018. Accessed October 4, 2020. [Google Scholar]
- 33.Jina A, Wang TL, Seyferth ER, Cohen A, Bernstein HH. Increasing antepartum Tdap vaccine administration: A quality improvement initiative. Vaccine. 2019;37(28):3654–3659. [DOI] [PubMed] [Google Scholar]
- 34.The National Vaccine Program Office. National Adult Immunization Plan. U.S. Department of Health and Human Services. https://www.hhs.gov/sites/default/files/nvpo/national-adult-immunization-plan/naip.pdf. Published 2010. Accessed October 4, 2020. [Google Scholar]
- 35.Centers for Disease Control and Prevention. (IQIP) Immunization Quality Improvement for Providers. https://www.cdc.gov/vaccines/programs/iqip/index.html. Published 2019. Accessed October 18, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


