Abstract
Background
Allergic reactions to the coronavirus disease 2019 (COVID-19) vaccines have raised concerns, particularly as repeated doses are required. Skin tests with the vaccines excipient were found to be of low value, whereas the utility of skin tests with the whole vaccine is yet to be determined.
Objective
To evaluate a panel of skin tests and the outcomes of subsequent doses of immunization among subjects who suffered an immediate allergic reaction to the BioNTech (BNT162b2) COVID-19 vaccine.
Methods
Between March and December 2021, patients who experienced symptoms consistent with immediate allergic reactions to the BNT162b2 vaccine and were referred to the Sheba Medical Center underwent skin testing with polyethylene glyol (PEG)-containing medicines, Pfizer-BNT162b2, and Oxford–AstraZeneca vaccine (AZD1222). Further immunization was performed accordingly and under medical observation.
Results
A total of 51 patients underwent skin testing for suspected allergy to the COVID vaccines, of which 38 of 51 (74.5%) were nonreactive, 7 of 51(13.7%) had no skin sensitization but suffered a clinical reaction during skin testing (mainly cough), and 6 of 51 (11.7%) exhibited immediate skin sensitization. Both skin sensitization and cough during testing were related to a higher use of adrenaline following immunization (P = .08 and P = .024, respectively). Further immunization with the BNT162b2 vaccine was recommended unless sensitization or severe reaction to previous immunization was evident. The latter were referred to be tested/receive the alternative AZD1222 vaccine. Ten patients underwent skin testing with AZD1222: 2 of 10 (20%) demonstrated skin sensitization to both vaccines; thus, 8 of 10 were immunized with the AZD1222, of which 2 of 8 (25%) had allergic reactions.
Conclusions
Immediate allergic reactions to COVID-19 vaccines are rare but can be severe and reoccur. Intradermal testing with the whole vaccine may discriminate sensitized subjects, detect cross-sensitization between vaccines, and enable estimation of patients at higher risk.
Key words: COVID-19, Allergy, Vaccine, BNT162b2, AZD1222, Skin test
Abbreviations used: AZD1222, Oxford–AstraZeneca chimpanzee adenovirus vectored vaccine; BNT162b2, Pfizer-BioNTech mRNA COVID-19 vaccine; COVID-19, coronavirus disease 2019; ID, intradermal; PEG, polyethylene glyol; PEG-med, PEG-containing medication
What is already known about this topic? Allergic reactions to coronavirus disease 2019 vaccines are rare but warrant evaluation when they occur. Skin tests with coronavirus disease 2019 vaccines excipient for evaluation of allergy are of low value. The utility of skin tests with the whole vaccine is yet to be determined.
What does this article add to our knowledge? Intradermal testing with the whole vaccine may discriminate sensitized subjects, detect cross-sensitization between vaccines, and enable estimation of patients at high risk.
How does this study impact current management guidelines? We recommend assessment of allergy to coronavirus disease 2019 vaccines using intradermal testing with the whole vaccine and offer tailored immunization protocols (eg, alternative vaccine, premedication, and desensitization) for patients at high risk.
Introduction
Since the declaration of coronavirus disease 2019 (COVID-19) as a global pandemic on March 11, 2020, the pandemic, caused by severe acute respiratory syndrome coronavirus 2, has overtaken the world in a never-before-seen pace and has taken a toll on the world both medically and economically.1 The invention of the COVID-19 vaccines Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273), and later the Oxford–AstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 (AZD1222) came with a sense of promise and hope to combat the spread of the disease. The reemergence of novel, more transmissible variants (eg, Alpha, Beta, Gamma, Delta, Omicron) resulted in further spikes of disease and a greater ensuing public health burden, maintaining the goal of vaccination highly relevant worldwide. Although efficacy and safety of these vaccines was proven,2 with mostly mild adverse effects as pain at the injection site, fatigue, and headache,3 allergic reactions ranging from mild responses to anaphylaxis have been reported.4 , 5 The latter was observed in up to 4.7, 2.5, and 7.4 cases per million doses of the Pfizer, Moderna, and AstraZeneca vaccines, respectively.6 , 7 This prompted the Centers for Disease Control and Prevention to state that patients with a history of allergy to vaccine components or a history of an immediate allergic reaction to the first inoculation dose should not receive further doses,8 , 9 thus creating an obstacle on the path to global immunization.
Although the causes of allergic reactions to the COVID-19 vaccine are yet to be determined, polyethylene glycol (PEG)-2000, a component of the mRNA COVID-19 vaccines,10 , 11 and polysorbate 80, which has structural similarities to PEG and is a component of the AZD1222 vaccine,12 were suggested as plausible causes. Other components of the vaccines as well as other mechanisms related to such allergic reactions were also proposed.12
Methods for skin testing with nonirritating concentrations of PEG, polysorbate,13, 14, 15 and the Pfizer-BioNTech COVID-19 vaccine have been published.16 Although the accuracy of skin testing with PEG, PEG-containing compounds, and polysorbate 80 is considered of high positive predictive value for assessment of sensitization to these compounds,17 there is limited supportive evidence to the usage of these tests in evaluating immediate reactions to the COVID vaccines. Notably, most studies relied on skin tests with PEG-3500–containing medication rather than PEG-2000 of the BNT162b2 vaccine with inconclusive results.17 , 18 Hence, there is a rational for assessing sensitivity to a COVID vaccine, via skin tests to the vaccine itself and/or alternative ones. The objective of this study was to evaluate subjects who experienced symptoms consistent with immediate allergic reactions to the COVID-19 BNT162b2 vaccine using intradermal (ID) tests with the BNT162b2 and/or AZD1222 whole vaccines, and their response to subsequent immunization doses.
Methods
In this study, patients with symptoms compatible with immediate allergic reactions to the BNT162b2 vaccine were included. Patients referred to our Clinical Immunology and Allergy Institute, in the Sheba Medical Center from March 2021 to December 2021, underwent clinical assessment, allergic risk assessment (eg, allergy history, prior anaphylactic reactions, adrenaline autoinjector carriage, use of other drugs, and characterization of reaction to the BNT162b2 vaccine), and skin testing with PEG-containing medication (PEG-med) and the whole vaccine. Thereafter, patients were referred for further immunization with the BNT162b2 vaccine or an alternative non-mRNA vaccine (AZD1222). The latter was available in Israel from November 2021.
Symptoms consistent with immediate allergic reactions to the BNT162b2 vaccine were defined by occurring within minutes and up to 2 hours following immunization and resolving in less than 12 hours. These reactions included urticarial rash, angioedema, shortness of breath, wheezing, cough, and hypotension. Patients who suffered immediate nonallergic reactions (eg, loss of sensation, headache, and presyncope) and/or late hypersensitivity reactions (eg, rash lasting more than 24 hours) were excluded from this cohort. Medical and allergy history was gathered from patients’ medical records, and during intake at a visit to our clinic. Patients were defined with multiple allergies if they had a history of 2 or more of the following: (1) drug allergy, (2) insect sting allergy, (3) food allergy, and (4) allergic rhinitis and/or asthma. Sequential reactions to second and third doses of anti-COVID immunizations were followed, and documented by our team, as all subsequent vaccinations were performed under observation in the Sheba Medical Center.
Allergic assessment included skin prick and ID testing using drugs containing PEG-3350, namely, Normalax- PEG 3350 100% w/w, a laxative, (prick) and methylprednisolone acetate 40 mg/mL, which contains 29.1 mg/mL of PEG 3350 (prick and ID with 1:100 and 1:10 dilutions). Thereafter, skin testing was done with the BNT162b2 and/or AZD1222 vaccines using the original concentration for epicutaneous (prick) followed by ID testing with a dilution of 1:100 and 1:10 of the relevant vaccine. All tests were performed by allergy and immunology nurses and results interpreted 20 minutes after each step. Sensitivity (skin response) was defined as the presence of wheal 3 mm larger than that with the negative control (saline). Notably, during testing with the vaccine, several patients suffered clinical reactions (prolonged cough, wheezing, and/or rash) that appeared within 30 minutes of ID injection of the diluted BNT162b2 vaccine and lasted up to 2 hours, regardless of skin sensitivity.
Patients who did not exhibit sensitization (nonreactive skin test) to either PEG-med and/or the BNT162b2 vaccine, nor clinical reactions during testing, were referred to further immunization with the BNT162b2 vaccine, with premedication (eg, antihistamine and/or bronchodilators). All subsequent immunizations were done under observation of our team.5 Exception was made for patients who suffered a severe reaction (eg, adrenaline treated) during prior full immunization or a reaction during skin testing, which were referred to immunization with the alternative vaccine the AZD1222. Alike, patients with evidence of sensitization (reactive skin tests) to BNT162b2 were declined from additional BNT162b2 inoculation and were recommended to receive the AZD1222. All patients referred to receive the AZD1222 vaccine underwent additional skin tests with this vaccine before inoculation.
Statistical methods
Study data were collected and managed using Microsoft Excel 2019 software (Microsoft, Redmond, Washington). Statistical analysis was performed using IBM SPSS statistics software, version 27 (IBM, Armonk, NY). The Fisher exact test and/or Student t tests were used as appropriate. All P values were from 2-sided tests, and results were deemed statistically significant at P less than .05.
This study was performed in accordance with the Declaration of Helsinki and in agreement with the institutional regulations and approval of the Sheba Medical Center institutional review board.
Results
Patients’ characteristics
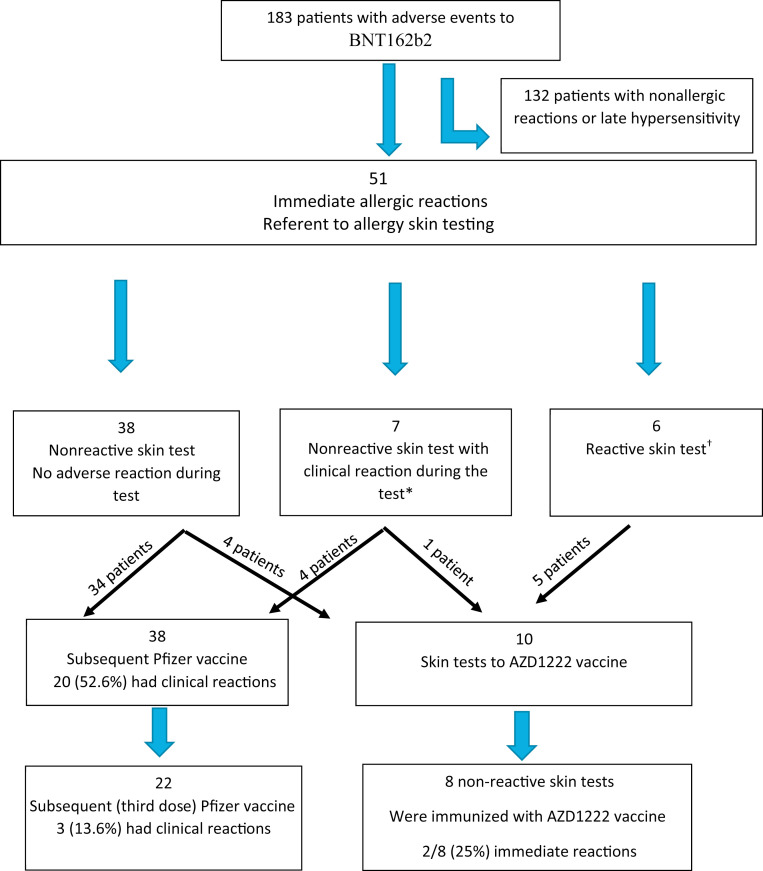
During the study period, 183 patients with adverse reactions to BNT162b2 vaccine were evaluated in our Clinical Immunology and Allergy Institute, of which 132 patients with nonallergic or late hypersensitivity reactions were excluded, whereas 51 patients who experienced symptoms consistent with immediate allergic reactions to BNT162b2 vaccine were included (Figure 1 ). Most patients were female (92.1%), with a mean age of 45 ± 15 years. More than 80% had a medical history of prior allergies, 52.4% had multiple allergies (ie, 2 or more of the following: drug allergy, insect sting allergy, food allergy, and allergic rhinitis and/or asthma), and 27.4% had prior anaphylactic reaction (Table I ). Of note, none fulfilled criteria for the diagnosis of multiple drug intolerance syndrome (ie, adverse drug reactions to at least 3 chemically, pharmacologically, and immunologically unrelated drugs, manifested on 3 different occasions, and with negative immunologic [allergic] test(s) results).19
Figure 1.
The diagram represents the assessment and subsequent immunization of patients who applied to the Clinical Immunology and Allergy Institute in the Sheba Medical Center from March 2021 to December 2021. ∗Two of 7 patients declined subsequent immunization/skin test. †One of 6 patients declined AZD skin tests.
Table I.
Demographic characteristics and allergy history
| Characteristic | Participants, n (%) (N = 51) |
|---|---|
| Age (y), mean ± SD | 45 ± 15 |
| Sex: female | 47 (92.1) |
| Prior allergic reaction, total∗ | 41 (80.3) |
| Drug allergy | 28 (54.9) |
| Insect bite allergy | 3 (5.8) |
| Food allergy | 10 (19.6) |
| Allergic rhinitis | 8 (15.6) |
| Prior anaphylaxis | 14 (27.4) |
| Asthma | 6 (11.7) |
| Multiple allergies† | 27 (52.9) |
| Adrenaline syringe carriers | 6 (11.7) |
Data represent the demographic characteristics and allergy history of the cohort of 51 patients with immediate allergic reaction to BNT162b2 vaccine who underwent skin tests.
Prior allergic reaction was defined if patient had allergy to drugs, insect bites, or food.
Multiple allergies were defined as fulfilling 2 or more of the following: (1) drug allergy (2) insect sting allergy, (3) food allergy, and (4) allergic rhinitis and/or asthma.
The primary immediate reaction to the BNT162b2 vaccine
Immediate reactions following immunization with the BNT162b2 vaccine were characterized by onset of symptoms within 25 minutes (range, 10-30 minutes). Most common symptoms were cutaneous (n = 27 [52.9%]), angioedema (n = 19 [19.6%]), shortness of breath (n = 19 [37.2%]), and cough (n = 14 [27.4%]). One patient (1.9%) had a severe reaction that included hypotension. Reactions were treated with antihistamines (31 [60.7%]), glucocorticoids (14 [27.4%]), inhaled bronchodilators (12 [23.5%]), and Adrenaline (10 [19.6%]) (Table II ). Symptoms resolved within 0.5 to 6 hours, and none required hospitalization.
Table II.
Immediate allergic reactions to Pfizer-BioNTech COVID-19 vaccine
| Reaction and treatment | First dose (N = 51) |
Second dose (N = 38) |
Third dose (N = 22) |
|---|---|---|---|
| Immediate allergic reaction | 51 (100) | 20 (52.6) | 3 (13.6) |
| Cutaneous | 27 (52.9) | 7 (18.4) | 1 (4.5) |
| Angioedema | 10 (19.6) | 6 (15.7) | 1 (4.5) |
| Dyspnea | 19 (37.2) | 10 (26.3) | 2 (9) |
| Cough | 14 (27.4) | 8 (21) | 1 (4.5) |
| Hypotension | 1 (1.9) | 0 (0) | 0 (0) |
| Anaphylaxis∗ | 14 (27.4) | 3 (7.8) | 1 (4.5) |
| Treatment | |||
| Antihistamines | 31 (60.7) | 12 (31.5) | 3 (13.6) |
| Oral corticosteroids | 14 (27.4) | 7 (18.4) | 3 (13.6) |
| Inhaled bronchodilator | 12 (23.5) | 6 (15.7) | 3 (13.6) |
| Adrenaline injection | 10 (19.6) | 4 (10.5) | 1 (4.5) |
Values represent n (%).
Data represent the immediate allergic reactions to the 3 doses of Pfizer-BioNTech COVID-19 vaccine, and the treatment that was given.
Anaphylaxis was defined according to Brighton’s anaphylaxis criteria.20
Skin testing
All patients underwent skin tests with PEG-meds and the BNT162b2 vaccine, of which 38 of 51 (74.5%) showed no sensitization (nonreactive skin test results) to any of the compounds. An additional 7 of 51 (13.7%) patients had nonreactive skin test results but suffered a clinical reaction following ID injection of the diluted vaccine, of which 6 exhibited persistent cough ± wheezing (1 of them also had a skin rash), while the seventh patient had flushing and skin rash (Table III ). Test-related reactions were regarded as mild-moderate though required treatments with antihistamines and/or inhaled bronchodilator and 1 patient was treated with inhaled adrenaline for persisted cough. All test-related reactions resolved within 30 to 60 minutes, and none required hospitalization. Interestingly, the presence of cough following initial immunization was linked to cough during ID testing because 4 of 6 (66.6%) were on both occasions versus 10 of 45 (22.2%) present only during immunization (P = .04). Furthermore, patients with cough following ID testing required more treatments with adrenaline during the 38 subsequent doses of immunization with the BNT162b2 vaccine compared with patients with no reactions during ID testing (2 of 3 [66.6%] vs 2 of 35 [5.7 %], respectively; P = .024).
Table III.
Skin testing with BNT162b2 vaccines: (A) Skin sensitivity and (B) systemic reactions to skin tests
| S. no. | Age (y) | Sex | Histamine | Saline | Normalax SPT | Depo SPT (mm) | Depo ID 1:100 (mm) | Depo ID 1:10 (mm) | BNT162b2 SPT (mm) | BNT162b2 ID 1:100 (mm) | BNT162b2 ID 1:10 (mm) | BNT162b2 systemic reaction to skin test | BNT162b2 systemic reaction to first dose |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. | |||||||||||||
| 1 | 16 | F | 5/10 | Neg | Neg | Neg | 5/10 | NA | Neg | 8/14 | Neg | No | Rash and dyspnea treated with adrenaline |
| 2 | 48 | F | 5/5 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 5/10 | Rash and dyspnea | Dyspnea rash and angioedma |
| 3 | 45 | F | 5/5 | Neg | Neg | Neg | Neg | 5/10 | NA | NA | NA | NA | Rash |
| 4 | 50 | F | 3/5 | Neg | Neg | Neg | Neg | Neg | Neg | 5/10 | Neg | Rash | Rash |
| 5 | 49 | F | 5/10 | Neg | 10/25 | Neg | Neg | Neg | Neg | 5/10 | NA | Neg | Dyspnea, rash, and angioedema treated with adrenaline X2 |
| 6 | 28 | F | 5/10 | Neg | Neg | Neg | 5/10 | NA | Neg | 5/10 | NA | No | Rash, cough, dyspnea, and angioedema treated with adrenaline |
| B. | |||||||||||||
| 1 | 37 | F | 5/5 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Cough | Dyspnea and rash treated with adrenaline |
| 2 | 39 | F | 5/10 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Rash | Angioedema of uvula and tongue |
| 3 | 56 | F | 5/10 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Cough | Rash and cough |
| 4 | 30 | F | 5/5 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Cough | Cough |
| 5 | 39 | F | 5/5 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Cough and rash | Rash and cough and dyspnea treated with adrenaline |
| 6 | 51 | F | 5/10 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Cough | No |
| 7 | 56 | F | 5/5 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Cough | Cough |
F, Female; Depo, depomedrol acetate; M, male; NA, not available; Neg, negative (in regard to saline control); SPT, skin prick test.
Data represent demographic characteristics, skin test results (wheel/flare [mm]), and the reactions to either skin test/vaccine of patients with positive skin test result to BNT162B2 vaccine.
Sensitization (skin reactivity) to PEG-med and/or to the vaccine was documented in 6 of 51 (11.7%) patients in our cohort, of which 3 of 6 (50%) were sensitized to both PEG and BNT162b2 vaccine, 2 of 6 (33%) only to the BNT162b2 vaccine, and 1 of 6 (17%) to PEG-med and refused further testing with the vaccine. Intriguingly, 6 of 6 (100%) patients with skin sensitization suffered cutaneous reactions during the initial immunization versus 21 of 45 (46.6%) patients with no sensitization (P = .023). During the initial reaction to the vaccine, 4 of 6 (66.6%) and 3 of 6 (50%) patients with sensitization fulfilled Brighton’s anaphylaxis criteria20 and required therapy with adrenaline versus 10 of 45 (22.2%) and 7 of 45 (15.5%) of those with nonreactive skin test results, respectively (P = .08 and P = .0413).
Reaction to subsequent doses of BNT162b2 vaccine
Of the 51 patients who reported an immediate reaction, those with no skin sensitization and mild-moderate clinical initial reaction to the vaccine were recommended to receive additional doses of the BNT162b2 vaccine with premedication and under observation. Thereafter, 38 of 51 (74.5%) received a second dose of the BNT162b2 vaccine and 22 of 38 (58%) received the booster (third dose) according to the Israeli Ministry of Health instructions. Among the 60 subsequent immunization doses, 23 of 60 (38%) were related to another allergic response (ie, 20 of 38 and 3 of 22 during second and third doses, respectively). Most of those additional reactions were milder than the primary event, although 4 of 38 (10.5%) had persistent cough that was not resolved with antihistamine and steroids and were treated with intramuscular adrenaline. Two of them were treated with intramuscular adrenaline due to similar reaction following the first dose. Only 1 of 22 (4.5%) patients required treatment with intramuscular adrenaline after the third dose, due to urticarial rash and persisted cough. This patient was also treated with adrenaline in the former 2 doses due to persistent cough that was not resolved with antihistamine and steroids. None of the patients required hospitalization (Table II).
AZD1222 skin testing and vaccination
In our cohort, 10 patients who suffered an immediate reaction to the BNT162b2 vaccine were referred to skin testing and immunization with the alternative AZD1222 vaccine. These patients either had evidence of sensitivity to BNT162b2 or a severe systemic reaction to the former dose of the BNT162b2 vaccine regardless of sensitization. Skin testing with the AZD1222 vaccine revealed 2 of 10 (20%) patients with sensitization to the AZD1222 vaccine (ID in dilution of 1:10; flare/wheal of 5/10 and 8/10 mm, respectively). Interestingly, these 2 patients also showed sensitization to the BNT162b2 vaccine, and were declined to receive both vaccines. The other 8 of 10 patients with nonreactive skin test results to AZD1222 underwent immunization with this vaccine, of which 2 of 8 (25%) had clinical reactions to the full dose of the vaccine: a 62-year-old woman who despite nonreactive skin test results to PEG-med and both vaccines required 3 shots of adrenaline following inoculation with the BNT162b2 vaccine, and 1 shot of adrenaline 10 minutes after injection of the AZD1222 vaccine due to vomiting, shortness of breath, and extensive rash. A milder reaction was documented in a 45-year-old woman who presented with angioedema after the BNT162b2 vaccine, and had evidence of sensitization to this vaccine. Similar reaction with angioedema appeared shortly after inoculation with the AZD1222, which was responded to with an additional dose of antihistamines and resolved within 2 hours.
Discussion
Immediate allergic reactions to the BNT162b2 vaccine are quite rare but can be severe.4, 5, 6, 7 Because additional boosters are required in times of pandemic such as the COVID-19, evaluating these allergic reactions and designing protocols for further immunization of allergic patients are of importance.
In this study, we assessed 51 patients who experienced symptoms consistent with immediate allergic reactions to the BNT162b2 vaccine. Our cohort consists of adults at a mean age of 45 years, which is characteristic of allergy to drugs.21 Other typical features of this cohort were female predominance (92.1%), and allergic background (80%) with a relatively high rate of prior anaphylactic reactions (27.4%), which stands in agreement with former studies of allergic reaction to the COVID-19 vaccines in which a female bias4 , 5 , 17 , 22 , 23 as well as prior allergy and particularly drug allergy4, 5, 6 , 24 were reported. Notably, the vast majority of patients in our cohort (46 of 51) received subsequent doses of immunization with either the BNT162b2 vaccine or the AZD1222 vaccine according to the severity of their initial clinical response and/or evidence of sensitization to these vaccines. Altogether 68 doses of subsequent immunization were given, and additional allergic reactions were evident in 38% of them. Although most subsequent allergic reactions were milder, some did require the use of adrenaline. This strengthens the need for medical observation by a specialized team during further immunization of patients at high risk.5 Our protocol included premedication during subsequent immunization. One may speculate that this approach reduced or ameliorated recurrent reactions though further studies are needed.
We assessed sensitivity to the COVID vaccine both directly using skin testing with the vaccines themselves and indirectly using PEG-med. Direct assessment included skin prick and ID tests with diluted BNT162b2 or AZD1222 vaccine. As far as we know, this is the largest report of assessment of sensitivity to these vaccines using vaccine ID testing. Herein, we found that 6 of 51 (12%) patients were sensitized to BNT162b2 via ID testing (though most skin test results were minimally positive—5/10 mm—Table III), of which 2 were also sensitized to the AZD1222 vaccine and 4 of 5 patients were also sensitized to PEG-med. This stands in agreement with several reports in which only a minority of patients who suffered an immediate reaction to the COVID vaccines demonstrated sensitivity to vaccine ingredients.17 , 18 , 25 Correspondingly, it may support the concept that in this minority of allergic patients, an IgE-mediated reaction to the vaccine and/or its excipients (eg, PEG in the BNT162b2) may play a role, and that cross-reactivity (eg, with polysorbate included in the AZD1222) between vaccines is plausible.15 In our study, sensitization was associated with cutaneous manifestation during the initial immunization (P = .023) and numerically with the need of adrenaline (50% of sensitized patients vs 15.5% of nonsensitized; P = .08). Lastly, during skin testing, 2 of 6 sensitized patients experienced systemic reactions following exposure to the minimal amount of BNT162b2 used for ID tests (ie, 1/10-100 dilution). Systemic reactions during skin testing were previously reported for PEG-containing compounds.13 , 26 , 27 Interestingly, in our cohort, systemic reactions (mainly cough) during ID testing with the diluted vaccine was observed also among 7 of 51 (13.7%) patients with nonreactive skin test results. Although anxiety as the reason for cough during skin testing cannot be excluded, the occurrence of these reactions on different dates (using different batches of the vaccine) and the similarity of responses among different patients as well as the prompt response to therapy support the idea that these are clinical reactions to the ID injection. This further strengthens the concept that some immediate reactions to the vaccine and/or its excipients are non–IgE-mediated (eg, complement mediated) and the exact mechanisms of these reactions is yet to be revealed.
Previous studies that assessed sensitization to the COVID vaccines mostly used an indirect method, relying on skin tests with PEG and/or polysorbate 80 only that were less conclusive.13 , 17 , 18 For instance, Wolfson et al17 demonstrated, via vaccine excipient skin testing, sensitivity in 4 of 65 subjects, 2 of whom underwent and tolerated a subsequent dose of vaccine. Recently, Pitlick el al25 assessed sensitization to COVID vaccines via skin tests with vaccines excipients and vaccines themselves, with the vaccines used mainly for prick tests. In that study, 4 of 55 patients, with allergic reaction to the first dose of vaccine, demonstrated sensitization only to polysorbate-containing products, and 3 of them underwent subsequent immunization, of which 1 developed allergic symptoms. Notably, among the 55 patients in Pitlick el al25 study, only 11 underwent skin prick testing with mRNA vaccine, all of which were nonreactive, whereas ID test was performed only in 1 patient. Similarly, in our study, all skin prick tests with the BNT162b2 and AZD1222 vaccines were not reactive and sensitization was documented only via ID injection (Table III). Therefore, we speculate that ID test with the whole vaccine is of higher utility for detection of IgE-mediated allergy
Taking it all together, one may suggest that the utility of skin testing particularly using ID testing with the whole vaccine may improve our ability to estimate sensitization to these vaccines. This may indicate the mechanisms of reaction and may serve as a tool for decision making regarding subsequent immunization. However, because of lack of large studies to assess the utility of skin testing with the whole vaccine, one should take into account the possibility of false-positive and false-negative results and until there will be more studies, these results should be interpreted cautiously and be used only as an aiding tool and not as a sole parameter for decision regarding subsequent vaccinations. In our protocol, evidence of sensitization precludes from further immunization with the relevant vaccine, as recommended by international bodies and the Israeli Ministry of Health.28
A broader consensus exists regarding most patients with immediate allergic reaction to COVID vaccines, in which no evidence of sensitization (ie, nonreactive skin test results) was documented as reported herein and by others.17 , 25 This may indicate a role for non–IgE-mediated mechanisms in the pathogenesis of such reactions.18 In our cohort, 45 of 51 patients were not sensitized to any reagent used for skin tests. However, 7 of them exhibited clinical reactions during testing that were linked with clinical symptoms during the initial immunization (ie, cough) and potentially with more severe reactions (patients with cough following ID testing required significantly more treatments with adrenaline during the 38 subsequent doses of immunization with the BNT162b2 vaccine [2 of 3 (66.6%) vs 2 of 35 (5.7 %); P = .024]). Taking into consideration the possibility of such reactions during skin testing, it is recommended that such evaluation be performed only under the observation of a specialized allergy team.
Reoccurrence of allergic reaction on subsequent inoculations was not rare and reached 38% in our cohort. Similarly, in the study of Wolfson et al,17 among patients who reported a history of an immediate allergic reaction and nonreactive skin test to PEG, 25% had an additional allergic reaction following another dose of vaccine. A higher incidence of adrenaline usage during subsequent immunizations was documented in our cohort compared with Wolfson et al’s17 cohort (4 of 38 [10.5%] vs 2 of 65 [3%], respectively). This may be explained by the different populations evaluated in these studies and/or the higher use of adrenaline in our cohort during the initial inoculation compared with Wolfson et al’s17 cohort (10 of 51 [19.6%] vs 6 of 80 [8%], respectively).
Although risk factors for further allergic reactions are yet to be revealed, in our cohort it seems that sensitization via ID testing or systemic reactions during skin testing regardless of sensitization, and potentially the severity of the initial reaction may be of prognostic value. Hence, in our study, patients estimated to be at high risk were offered, once they was eligible, immunization with an alternative non-mRNA vaccine (AZD1222). This was a solution only for some, because 2 patients exhibited sensitization to both vaccines and thus were not immunized, and 2 of 8 had allergic reactions also to the AZD1222, 1 of which was severe. This emphasizes the need to perform skin testing with each vaccine especially if cross-reactivity is possible, and to immunize high-risk patients under observation regardless of the vaccine used. Alternatively, there is a possibility of administering subsequent doses of the same vaccine by graded dosing as reported by Mustafa et al.29
Our study has some limitation derived from its designing, because the decision to undergo skin testing as well as further immunization was dependent on patient preferences, the Israeli Ministry of Health instructions, and vaccine availability. This is a single-center study, although patients were referred to our center following the primary event from various centers, and represent only Israeli population. Because previous medical and allergic history was in part gathered from patients’ medical history, the prevalence of anxiety (particularly in 4 patients who presented with shortness of breath as the only symptom) cannot be entirely excluded. Evaluation of PEG-med used in this study included PEG 3350 instead of PEG-2000 (used in the BNT162b2 vaccine) because the latter was not available for assessment, and we did not use polysorbate for skin tests (excipient of AZD1222 vaccine). Another limitation is the lack of knowledge regarding nonirritant doses of AZD1222 vaccine use for skin tests, because the number of tests done was limited by availability of this vaccine and controls were not studied. In this study, we used the same dilutions for both vaccines, and 2 of 10 patients tested with the AZD1222 vaccine were regarded as sensitized. Moreover, in our study, 2 of 8 (25%) patients developed symptoms suggestive of allergic reactions despite negative skin test results. More studies are needed to assess the utility, sensitivity, and specificity of skin tests with the AZD1222 vaccine. In addition, although both shortness of breath and cough may be induced by anxiety, they were considered in this cohort as immediate reactions. Notably, in our cohort, among 19 patients who reported shortness of breath, only 4 patients had shortness of breath as their only symptom. Lastly, we were unable to assess skin sensitivity and/or allergic reactions to other COVID-19 vaccines (eg, Spikevax [developed by Moderna] and COVID-19 Janssen Vaccine [developed by Johnson & Johnson])30 because they were not available in our country.
Conclusions
Immediate allergic reactions to COVID vaccines are rare but can be severe, and regardless of vaccine type and/or the mechanisms of reactions (ie, IgE- or non–IgE- mediated), a risk or recurrent event is substantial. This supports further immunization of patients at risk under medical observation of specialized teams.
Vaccine ID testing with either BNT162b2 or AZD1222 vaccine may enable identification of sensitized patients as well as potential cross-sensitization between vaccines. Moreover, regardless of skin sensitization, systemic reaction during testing may signify patients at a higher risk of further reactions. In addition, alike other drugs, female sex and previous allergy/drug hypersensitivity are related to COVID vaccines allergy.
Tailored immunization protocols (eg, alternative vaccine, premedication, and desensitization) are needed for patients at high risk of such immediate allergic reactions.
Footnotes
Conflicts of interest: The authors have no conflict of interest to disclose.
References
- 1.Kolahchi Z., De Domenico M., Uddin L.Q., Cauda V., Grossmann I., Lacasa L., et al. COVID-19 and its global economic impact. Adv Exp Med Biol. 2021;1318:825–837. doi: 10.1007/978-3-030-63761-3_46. [DOI] [PubMed] [Google Scholar]
- 2.Anand P., Stahel V.P. The safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 2021;15:20. doi: 10.1186/s13037-021-00291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur R.J., Dutta S., Bhardwaj P., Charan J., Dhingra S., Mitra P., et al. Adverse events reported from COVID-19 vaccine trials: a systematic review. Indian J Clin Biochem IJCB. 2021;36:427–439. doi: 10.1007/s12291-021-00968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325:780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shavit R., Maoz-Segal R., Iancovici-Kidon M., Offengenden I., Haj Yahia S., Machnes Maayan D., et al. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim M.A., Lee Y.W., Kim S.R., Kim J.H., Min T.K., Park H.S., et al. COVID-19 vaccine-associated anaphylaxis and allergic reactions: consensus statements of the KAAACI Urticaria/Angioedema/Anaphylaxis Working Group. Allergy Asthma Immunol Res. 2021;13:526–544. doi: 10.4168/aair.2021.13.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention COVID-19 vaccination. 2020. 2020. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/specific-groups/allergies.html [PubMed]
- 9.Centers for Disease Control and Prevention COVID-19 vaccines and severe allergic reactions. 2021. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html
- 10.Cabanillas B., Akdis C.A., Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy. 2021;76:1617–1618. doi: 10.1111/all.14711. [DOI] [PubMed] [Google Scholar]
- 11.Garvey L.H., Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021;126:e106–e108. doi: 10.1016/j.bja.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabanillas B., Novak N. Allergy to COVID-19 vaccines: a current update. Allergol Int. 2021;70:313–318. doi: 10.1016/j.alit.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerji A., Wickner P.G., Saff R., Stone C.A., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broyles A.D., Banerji A., Barmettler S., Biggs C.M., Blumenthal K., Brennan P.J., et al. Practical guidance for the evaluation and management of drug hypersensitivity: specific drugs. J Allergy Clin Immunol Pract. 2020;8:S16–S116. doi: 10.1016/j.jaip.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Stone C.A., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A., et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcelino J., Farinha S., Silva R., Didenko I., Proença M., Tomáz E. Nonirritant concentrations for skin testing with SARS-CoV-2 mRNA vaccine. J Allergy Clin Immunol Pract. 2021;9:2476–2477. doi: 10.1016/j.jaip.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfson A.R., Robinson L.B., Li L., McMahon A.E., Cogan A.S., Fu X., et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9:3308–3320.e3. doi: 10.1016/j.jaip.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren C.M., Snow T.T., Lee A.S., Shah M.M., Heider A., Blomkalns A., et al. Assessment of allergic and anaphylactic reactions to mRNA COVID-19 vaccines with confirmatory testing in a US regional health system. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behera S.K., Das S., Chengappa K.G., Xavier A.S., Selvarajan S. Multiple drug intolerance syndrome: an underreported distinct clinical entity. Curr Clin Pharmacol. 2019;14:84–90. doi: 10.2174/1574884713666181112125714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brighton Collaboration Anaphylaxis: case definition companion guide. 2021. 2021. https://brightoncollaboration.us/anaphylaxis-case-definition-companion-guide/
- 21.Thong B.Y.H., Tan T.C. Epidemiology and risk factors for drug allergy. Br J Clin Pharmacol. 2011;71:684–700. doi: 10.1111/j.1365-2125.2010.03774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumenthal K.G., Robinson L.B., Camargo C.A., Shenoy E.S., Banerji A., Landman A.B., et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iguchi T., Umeda H., Kojima M., Kanno Y., Tanaka Y., Kinoshita N., et al. Cumulative adverse event reporting of anaphylaxis after mRNA COVID-19 vaccine (Pfizer-BioNTech) injections in Japan: the first-month report. Drug Saf. 2021;44:1209–1214. doi: 10.1007/s40264-021-01104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitlick M.M., Sitek A.N., D’Netto M.E., Dages K.N., Chiarella S.E., Gonzalez-Estrada A., et al. Utility and futility of skin testing to address concerns surrounding messenger RNA coronavirus disease 2019 vaccine reactions. Ann Allergy Asthma Immunol. 2022;128:153–160. doi: 10.1016/j.anai.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sellaturay P., Nasser S., Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9:670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Wylon K., Dölle S., Worm M. Polyethylene glycol as a cause of anaphylaxis. Allergy Asthma Clin Immunol. 2016;12:67. doi: 10.1186/s13223-016-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Israel Ministry of Health Coronavirus (COVID-19) vaccines [In Hebrew] https://www.health.gov.il/UnitsOffice/HD/PH/epidemiology/td/docs/365_Corona.pdf
- 29.Mustafa S.S., Ramsey A., Staicu M.L. Administration of a second dose of the Moderna COVID-19 vaccine after an immediate hypersensitivity reaction with the first dose: two case reports. Ann Intern Med. 2021;174:1177–1178. doi: 10.7326/L21-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Medicines Agency . 2021. COVID-19 vaccines. 2021.https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines [Google Scholar]