Abstract
A better understanding of COVID-19 in people with primary immunodeficiency (PI), rare inherited defects in the immune system, is important for protecting this population, especially as population-wide approaches to mitigation change. COVID-19 outcomes in the PI population could have broader public health implications because some people with PI might be more likely to have extended illnesses, which could lead to increased transmission and emergence of variants. We performed a systematic review on COVID-19-associated morbidity and mortality in people with PI. Of the 1114 articles identified through the literature search, we included 68 articles in the review after removing 1046 articles because they were duplicates, did not involve COVID-19, did not involve PI, were not in English, were commentaries, were gene association or gene discovery studies, or could not be accessed. The 68 articles included outcomes for 459 people with PI and COVID-19. Using data from these 459 people, we calculated a case fatality rate of 9%, hospitalization rate of 49%, and oxygen supplementation rate of 29%. Studies have indicated that a number of people with PI showed at least some immune response to COVID-19 vaccination, with responses varying by type of PI and other factors, although vaccine effectiveness against hospitalization was lower in the PI population than in the general population. In addition to being up-to-date on vaccinations, current strategies for optimizing protection for people with PI can include pre-exposure prophylaxis for those eligible and use of therapeutics. Overall, people with PI, when infected, tested positive and showed symptoms for similar lengths of time as the general population. However, a number of people with X-linked agammaglobulinemia (XLA) or other B-cell pathway defects were reported to have prolonged infections, measured by time from first positive SARS-CoV-2 test to first negative test. As prolonged infections might increase the likelihood of genetic variants emerging, SARS-CoV2 isolates from people with PI and extended illness would be good candidates to prioritize for whole genome sequencing.
Keywords: COVID-19, Primary immunodeficiency, SARS-CoV-2, Public health, Inborn errors of immunity
Abbreviations: PI, primary immunodeficiency; ICU, intensive care unit; COVID-19, coronavirus disease 2019; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; CVID, common variable immunodeficiency; ECMO, extracorporeal membrane oxygenation; XLA, X-linked agammaglobulinemia; RBD, receptor binding domain; JMF, Jeffery Modell Foundation; CID, combined immunodeficiency; SCID, severe combined immunodeficiency; APECED, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy; ALPS-like, autoimmune lymphoproliferative syndrome-like; VE, vaccine effectiveness; CDC, Centers for Disease Control and Prevention; NIH, National Institutes of Health; PCR, polymerase chain reaction
1. Introduction
Knowing whether certain underlying conditions increase a person's risk for severe COVID-19 is important for clinical and public health practice. People with primary immunodeficiency (PI), rare inherited defects in the immune system, are more susceptible to infectious diseases [1]. There are over 400 different types of PI with varying symptoms and severity depending partly on which components of the immune system are affected [2]. Understanding COVID-19-related health outcomes, such as mortality, hospitalization, and oxygen requirement, for people with PI is essential to better protect and treat this patient population. As the COVID-19 pandemic evolves, significant advances have been made with regard to diagnostic tests, vaccinations, and therapeutics to decrease the impact of COVID-19 on the general population. As mask mandates, community-wide use of masks, and adherence to other mitigation measures decrease, an accurate assessment of risk is essential for patients with PI to help guide them, their families, and their health care providers in deciding which mitigation measures to take. In addition, a focus on the PI population is of broader interest as patients with PI might be less likely to clear the virus or more likely to have extended illnesses, thus increasing transmission risk and creating the potential for the emergence of variants [3]. Furthermore, understanding the pathology of COVID-19 in people with PI can provide insights into how SAR-CoV-2 interacts with the immune system and which components are involved in severe disease, viral clearance, and other factors. While studies have established an increased risk for people with secondary immunodeficiencies, due to factors such as HIV infection [4], organ transplant [5], or chemotherapy [6], these findings do not necessarily extrapolate to PI [7].
We conducted a systematic review of the literature to evaluate health outcomes in people with PI and COVID-19, including cohort, cross-sectional, and case studies. We did not address the effects of the COVID-19 pandemic on mental health, healthcare delivery, or related issues. We aimed to determine whether any classes of PI showed increased susceptibility to severe COVID-19 and to identify gaps in the literature as areas for future study.
2. Methods
2.1. Literature search
We performed a systematic review of the literature following the PRISMA guidelines [8] to examine health-related outcomes of COVID-19 in people with PI. One author (M.K.) searched the WHO COVID-19 Database, Medline (Ovid), Embase (Ovid), CAB Abstracts (Ovid), Global Health (Ovid), PsycInfo (Ovid), the Cochrane Library, Scopus, Academic Search Complete (Ebsco), CINAHL (Ebsco), and ProQuest Central from January 2020–August 2021, limiting results to English language studies only. After using Endnote to identify duplicates, a total of 1114 articles were identified for manual article selection. Complete search strategies are included in Table S1. We based search terms on the Jeffery Modell Foundation (JMF) 2020–2021 Global Survey (F. and V. Modell, personal communication) and the IUIS classification of human inborn errors of immunity in table 1 of Tangye S, et al. [2] (Table S2). Inclusion criteria for articles were: 1) case reports or case series involving patients with pre-existing PI who had confirmed or suspected COVID-19, 2) cohort studies that looked at COVID-19 health outcomes among people with pre-existing PI, and 3) COVID-19 vaccination studies of people with pre-existing PI. We excluded articles that were: 1) not about both PI and COVID-19, 2) non-human studies, 3) commentaries, 4) genomic association studies, 5) plans for a currently unfinished study, 6) a duplicate of an already included study, and 7) gene discovery studies on novel or rare variants that increase risk for severe COVID-19 in previously undiagnosed individuals, as our focus was on people with known PI. One author (E.D.) screened titles and abstracts to select articles for full text review and checked the full text of selected articles to determine which ones to include based on the inclusion criteria.
Two authors (E.D. and R.F.G.) used a tool proposed by Murad et al. [9] to assess risk of selection and measurement bias for each included article and categorized each article as having a low, moderate, serious, or critical risk of bias (Table S3). This tool uses questions to evaluate the selection, ascertainment, follow-up, and level of detail in case reports and case series. The same reviewers used the GRADE assessment [10] to determine the certainty of evidence in each article, categorized as high, moderate, low, or very low (Table S3). We selected these methods because they allowed us to assess both case and cohort studies. We did not control for bias or certainty in the data analysis.
2.2. Data collection
For cohort studies that reported data in aggregate, we collected infection rate, fatality rate, hospitalization rate, and oxygen supplementation requirement.
For case reports and case series, one author (E.D.) extracted data, listed in Table S4, and merged this information to form a single dataset. Information extracted included patient demographics (age, sex), comorbidities, PI diagnosis, PI treatments, hospitalization, length of hospitalization (days), re-admissions/hospitalization, ICU admission, oxygen supplementation requirement, COVID-19 treatments, complications during SARS-CoV-2 infection, length of COVID-19 symptoms (days), length of SARS-CoV-2 infection (days), presence of anti-SARS-CoV-2 antibodies (including IgG, IgM, and not specified), persistent symptoms after COVID-19 diagnosis, and coinfections during COVID-19 illness. We used data from the 459 cases in the dataset to calculate percentages for each variable of interest after excluding cases with missing data for that variable, except for length of COVID-19 symptoms, length of SARS-CoV-2 infection, and presence of anti-SARS-CoV-2 antibodies. We used the 407 recovered cases to calculate the percentages for these three variables, excluding those who died or had not recovered. We performed analyses for all PI combined and for each of the 10 primary immunodeficiency classifications used by JMF, i.e., immunodeficiencies affecting cellular and humoral immunity, combined immunodeficiencies with associated or syndromic features, predominantly antibody deficiencies, diseases of immune dysregulation, autoinflammatory disorders, congenital defects of phagocyte number or function, defects in intrinsic and innate immunity, complement deficiencies, phenocopies of inborn errors of immunity, and unspecified.
3. Results
3.1. Article selection
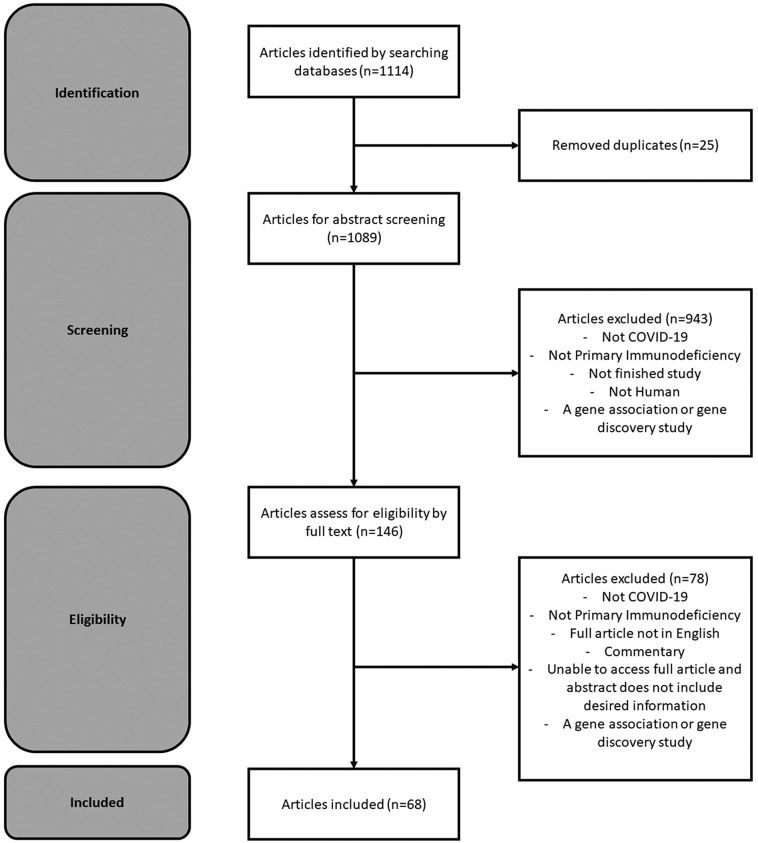
Of the 1114 articles identified through the literature search, we included 68 articles in this review after removing 25 articles for duplication that had not been identified through Endnote and removing 1021 articles for not including COVID-19, not including PI, not being a human study, being a commentary, being a gene association or gene discovery study, the full article not being in English, and being unable to access the article (Fig. 1 ). We assessed bias for all included publications and found 37 publications had a low risk of bias, 29 publications had a moderate risk of bias, and 2 publications had a high risk of bias (Table S3). Of the 68 included articles, 47 were case reports or case series, and 21 were cohort or cross-sectional studies. The cohort and cross-sectional studies covered a variety of COVID-19-related health outcomes including mortality, hospitalization, and oxygen requirement [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]] (Table 1 ). Some cohort and cross-sectional studies had COVID-19-related health outcomes at the individual level, and all of these studies had small numbers (n of 11 to 169) of people with PI and COVID-19. Three studies with small sample sizes assessed COVID-19 rates in PI populations. Using polymerase chain reaction (PCR) testing for COVID-19 diagnosis, Milito and colleagues found a similar cumulative incidence of COVID-19 by February 2021 between the PI population and general population of about 4% in the Italian PI population and 5% in the general population. Delvari and colleagues also found a similar incidence between the PI and general populations in Iran (0.7% in PI population in mid-2020 vs. 0.6% in general population in October 2020) [11,12]. Deya-Martinez and colleagues found a COVID-19 prevalence, using PCR and serology testing and SARS-CoV-2 ELISpot, of 7.7% in a pediatric cohort with moderate to severe PI in Spain [13].
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for primary immunodeficiency and COVID-19 literature curation.
Table 1.
Summary of cohort and cross-sectional study results included in this systematic review.
| Title | First author | Country | Number of participants | Findings |
|---|---|---|---|---|
| Adverse COVID-19 outcomes in immune deficiencies: Inequality exists between subclasses [16] | Karakoc Aydiner E. | Turkey | 34 | Overall, 67.6% of the patients required hospitalization and 23.5% required the ICU. The overall case fatality rate was 23.5%. The inpatient mortality rate was 34%. None of the admitted patients with predominantly antibody deficiencies died. Admitted patients with combined immunodeficiencies or immune dysregulation had mortality rates of 33% and 50% respectively. |
| CD19 + IgD + CD27- naïve B Cells as predictors of humoral response to COVID-19 mRNA vaccination in immunocompromised patients [17] | Schulz E. | Austria | 25 | After mRNA COVID-19 vaccination, patients with primary immunodeficiency had lower antibody production compared to healthy patients. |
| The clinical course and outcome of SARS-COVID 19 in patients with inborn errors of immunity, does it relate to the type of immune defects [18] | Sherkat R. | Iran | 14 | Not all patients with inborn errors of immunity are predisposed to severe COVID-19. Out of the 14 patients, 3 (21.4%) were hospitalized and 1 (7.1%) was admitted to the ICU. |
| Clinical outcome, incidence, and SARS-CoV-2 infection-fatality rates in Italian patients with inborn errors of immunity [11] | Milito C. | Italy | 131 | The cumulative incidence per 100,000 was 4.01 for patients with inborn errors of immunity and 5.22 for the general population. The infection fatality rate was 3.81% for patients with inborn errors of immunity and 3.28% for the general population. One third of the patients with inborn errors of immunity were positive for SARS-CoV-2 for over three weeks. |
| Clinical outcomes and features of COVID-19 in patients with primary immunodeficiencies in New York City [19] | Ho H. | United States | 16 | The median symptom duration was 29 days. Out of the 16 patients, 12 (75%) required hospitalization and 5 (31%) required the ICU. For oxygen supplementation, 5 (31%) required a standard nasal cannula and 5 (31%) required mechanical ventilation. Out of the 16 patients, 4 (25%) died. |
| Confirmed SARS-COV-2 Infection In A Cohort Of Children And Young Adults With Moderate Or Severe Primary Immunodeficiencies [13] | Deya-Martinez A. | Spain | 65 | The COVID-19 prevalence was 7.7% (5/65). Only one patient, with Jacobsen Syndrome and CVID-like phenotype, was symptomatic. |
| Coronavirus disease 2019 in patients with inborn errors of immunity: An international study [20] | Meyts I. | International | 94 | Out of the 94 patients with inborn errors of immunity and COVID-19, 59 (63%) required hospitalization, 13 (14%) required non-invasive ventilation, 15 (16%) required admission to the ICU, 15 (16%) required invasive ventilation, 3 (3%) required ECMO, and 9 (9.6%) patients died. |
| COVID-19 affecting hereditary angioedema patients with and without C1 inhibitor deficiency [21] | Grumach A. | Brazil | 13 | Out of the 13 patients with hereditary angioedema and COVID-19, 5 (38%) had a hereditary angioedema attack. One patient required hospitalization. |
| COVID-19 AND PRIMARY IMMUNODEFICIENCY: ONE-YEAR EXPERIENCE [22] | Al Yazidi L. | Oman | 140 | None of the children with primary immunodeficiencies were admitted to the hospital. A patient with XLA shed SARS-CoV-2 for 3 months. |
| COVID-19 in patients with primary and secondary immunodeficiency: The United Kingdom experience [23] | Shields A. | United Kingdom | 67 | Of the 67 patients with inborn errors of immunity, 34 (50.7%) required hospitalization, and 12 (17.9%) died. The inpatient mortality was 35.3% and the case fatality ratio was 28.5. |
| COVID-19 in Patients with Primary Immunodeficiency [24] | Esenboga S. | Turkey | 26 | Of the 26 patients, 8 (31%) were hospitalized, 2 (7.7%) were admitted to the ICU, and 2 (7.7%) died. The median recovery time was 8 days while one patient had a 60 day recovery time. |
| COVID-19 prevalence and outcomes in patients receiving biologic therapies at an infusion center in New York City [25] | Harada K. | United States | 11 | Of the 11 patients with primary immunodeficiency, 10 (91%) were hospitalized, 5 (45%) required a nasal canula, 4 (36%) required ventilation, 4 (36%) required the ICU, and 4 (36%) died. |
| Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity [14] | Hagin D. | Israel | 26 | In response to the Pfizer-BioNTech COVID-19 vaccine, 18 out of 26 individuals with PI developed a specific antibody response and 19 had a S-peptide-specific T-cell response. |
| Impact of SARS-CoV-2 Pandemic on Patients with Primary Immunodeficiency [12] | Delavari S. | Iran | 19 | Compared to the general population, patients with primary immunodeficiencies had a 1.23 higher risk of COVID-19 infection. The general population had an incidence of 1:178 and patients with primary immunodeficiency had an incidence of 1:144. |
| Low morbidity in Danish patients with common variable immunodeficiency disorder infected with severe acute respiratory syndrome coronavirus 2 [26] | Drabe C. | Denmark | 11 | All 11 patients had CVID and recovered. Three (27%) of patients were admitted to the hospital and 1 (9%) patient required oxygen supplementation. The median symptom duration was 12 days. |
| Minor Clinical Impact of COVID-19 Pandemic on Patients With Primary Immunodeficiency in Israel [27] | Marcus N. | Israel | 20 | Out of 1679 primary immunodeficiency patients followed, 20 (1.2%) tested positive for COVID-19. None of the patients required hospitalization and symptom duration ranged from 1 to 14 days. |
| Risk factors for hospitalization, disease severity and mortality in children and adolescents with COVID-19: Results from a nationwide German registry [28] | Armann J. | Germany | 169 | Children with primary immunodeficiency had a 2.7-fold risk for ICU admission compared with children without primary immunodeficiency. |
| SARS-CoV-2 vaccine induced atypical immune responses in antibody defects: everybody does their best [15] | Salinas A. | Italy | 75 | Compared to healthy controls, patients with CVID had lower post BNT162b2 vaccination antibodies for Spike and RBD. Patients with XLA did not generate antibodies for Spike or RBD after vaccination. However, 5 out of 6 XLA patients developed Spike-specific T-cells. |
3.2. Cases in the literature
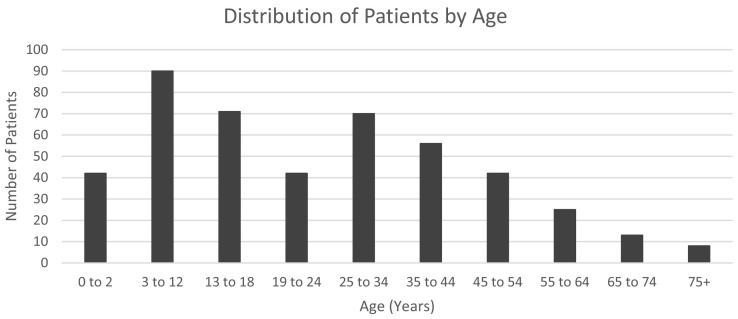
A total of 459 people with PI and COVID-19 are described from 54 of the 68 studies [12,13,16,[18], [19], [20], [21],24,26,27,[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71]] (Table 2 and Table S4). These cases came from case reports as well as cohort studies with information available for individual patients. These articles include information from 19 countries and primary immunodeficiencies from every class. The patients ranged from ages 0 years to 75 + years with most of the patients being between ages 3–12 years (Fig. 2 ). Among those PI reported in case reports, case series, and cohort studies, antibody deficiencies were most common, with 208 patients (45%) (Table 2 ), and phenocopies of inborn errors of immunity were the least common (0.4%, n = 2). The percentage of cases in each PI class generally correlates with the global prevalence of each PI class, which was calculated based on data from a 2018 JMF survey (F. and V. Modell, personal communication). Based on this survey, predominantly antibody deficiencies, such as X-linked agammaglobulinemia (XLA), were the most common PI class (46%, compared with 45% of COVID-19 PI cases), while phenocopies of inborn errors of immunity, such as Good Syndrome, was the rarest PI class (0.1%, compared with 0.4% of COVID-19 PI cases). The information available for the cases, including health outcomes and treatments, varied considerably among studies. For example, among 208 PI cases classified as predominantly antibody deficiency, only 123 included information on supplemental oxygen requirements.
Table 2.
COVID-19 outcomes for primary immunodeficiency cases included in this systematic review.
| Patient Characteristics | All Primary Immunodeficiencies | Immunodeficiencies Affecting Cellular and Humoral Immunity | Combined Immunodeficiencies with Associated or Syndromic Features | Predominantly Antibody Deficiencies | Diseases of Immune Dysregulation | Congenital Defects of Phagocyte Number or Function | Defects in Intrinsic and Innate Immunity | Autoinflammatory Disorders | Complement Deficiencies | Phenocopies of Inborn Errors of Immunity | Unspecified | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Number of Patients | 459 | 32 | 35 | 208 | 38 | 28 | 19 | 15 | 25 | 2 | 57 | |
| Supplemental Oxygen | Overall | 29% (76/264) | 17% (3/18) | 23% (6/26) | 33% (41/123) | 36% (10/28) | 12% (2/17) | 38% (5/13) | 14% (1/7) | Unknown | Unknown | 25% (8/32) |
| Nasal Cannula | 14% (30/218) | 0% (0/15) | 9% (2/22) | 20% (20/102) | 14% (3/21) | 0% (0/15) | 11% (1/9) | 14% (1/7) | Unknown | Unknown | 11% (3/27) | |
| Non-Invasive Ventilation | 4% (8/225) | 11% (2/18) | 8% (2/24) | 2% (2/103) | 5% (1/22) | 6% (1/16) | 0% (0/9) | 0% (0/7) | Unknown | Unknown | 0% (0/27) | |
| Invasive ventilation | 12% (32/257) | 0% (0/17) | 8% (2/26) | 15% (18/121) | 21% (6/28) | 0% (0/16) | 25% (3/12) | 0% (0/7) | Unknown | Unknown | 10% (3/30) | |
| ECMO | 2% (4/264) | 0% (0/18) | 0% (0/26) | 0% (0/123) | 0% (0/28) | 6% (1/17) | 8% (1/13) | 0% (0/7) | Unknown | Unknown | 6% (2/32) | |
| Hospitalization | Overall | 49% (212/433) | 44% (14/32) | 38% (11/29) | 50% (103/204) | 64% (23/36) | 46% (13/28) | 63% (12/19) | 47% (7/15) | 0% (0/12) | 100% (2/2) | 48% (27/56) |
| ICU | 16% (66/424) | 16% (5/32) | 14% (4/28) | 16% (31/200) | 21% (7/33) | 11% (3/28) | 26% (5/19) | 7% (1/15) | 0% (0/12) | 100% (2/2) | 15% (8/55) | |
| Death | 9% (42/448) | 19% (6/32) | 9% (3/34) | 8% (17/202) | 18% (7/38) | 8% (2/26) | 0% (0/19) | 7% (1/15) | 0% (0/25) | 100% (2/2) | 7% (4/55) | |
Fig. 2.
Distribution of primary immunodeficiency patients with COVID-19 in the literature by age.
3.3. Case fatality
We used data from the 459 people with PI and COVID-19 included in case reports, case series, and cohort studies to calculate the overall case fatality rate and case fatality rate by type of PI among people with PI and COVID-19 (Table 2). One caveat is that rates calculated using data from case reports and case series are likely elevated, as these publications are generally limited to those who are hospitalized and potentially biased toward including those with more severe health outcomes [72]. We calculated an overall case fatality rate of 9% (Table 2). In comparison, the case fatality rate reported in the cohort studies varied from 3.8% to 36%. Out of these cohorts, the two cohorts with the highest number of participants, 131 and 94 participants, had case fatality rates of 3.8% and 9.6%, respectively. Among types of PI from the 459 cases with COVID-19, complement deficiencies (n = 25) and defects in intrinsic and innate immunity, such as MyD88 deficiency, (n = 19) had the lowest case fatality rate of 0% (Table 2). Consistent with this, one cohort study also reported that none of the patients with complement deficiencies, such as hereditary angioedema, died. For other types of PI from the 459 cases with COVID-19, we calculated case fatality rates of 19% for immunodeficiencies affecting cellular and humoral immunity, such as Omenn Syndrome, and 18% for diseases of immune dysregulation, such as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (Table 2). The remaining primary immunodeficiency classes had case fatality rates between 6% and 9%. Of the published PI cases with COVID-19, those in the youngest (0–2 years) and oldest (75 years or older) age groups had the highest case fatality rates, 17% and 63%, respectively.
3.4. Hospitalization
We calculated hospitalization, ICU, and respiratory support rates among the 459 people with PI and COVID-19 from published reports (Table 2). The overall hospitalization rate was 49% and the ICU admission rate was 16%. Types of PI with the highest hospitalization rates were diseases of immune dysregulation, such as ALPS-like syndrome, (64%); defects in intrinsic and innate immunity, such as MyD88 deficiency, (63%); and predominantly antibody deficiencies, such as XLA, (50%). Complement deficiencies, such as hereditary angioedema, had the lowest hospitalization rates (0%, n = 12). Among all case reports, only 5 (1 chronic granulomatous disease, 4 XLA) reported readmission to the hospital and all 5 recovered (Table S4). The age groups with the highest percentage of hospitalized patients were those 0 to 2 years old (69% of 42 cases age 0 to 2 years) and those 75 years old or older (75% of 8 cases ages 75 and older). Overall, 29% of patients required oxygen supplementation or intubation: 14% with nasal cannula, 4% with non-invasive ventilation, 12% with invasive ventilation, and 2% with extra corporeal membrane oxygenation (ECMO) (Table 2). Patients with predominantly antibody deficiencies had the highest rates of required oxygen supplementation or intubation, required nasal canula, and required invasive ventilation. Information about required oxygen supplementation was not available for case reports or series on patients with complement deficiencies or phenocopies of inborn error of immunity. The only reports of ECMO use were among patients with congenital defects of phagocyte number or function, defects in intrinsic and innate immunity, and unspecified PI.
3.5. COVID-19 duration, symptoms, and antibodies
Of the 407 patients who recovered, 78 (19%) had information on their SARS-CoV-2 antibody status after infection, 102 (25%) had information on the duration of COVID-19 symptoms, and 61 (15%) had information on how long they continued to test positive for SARS-CoV-2 (Table S4). Of the 78 cases with information available, 52 (67%) developed antibodies against SARS-CoV-2, including patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) and those with PI that affect the B-cell pathway, including 19 patients with common variable immunodeficiency (CVID) and 1 patient with XLA. Of the 84 patients with information available, the median length of symptoms for the symptomatic patients was 8 days (range: 1 to 60 days), with a longer length of symptoms (range: 30 to 67 days) seen for patients with agammaglobulinemia, combined immunodeficiency (CID), CVID, severe combined immunodeficiency (SCID), Wiskott-Aldrich Syndrome, and XLA. Of the 61 patients with information available, the median time from first positive test for SARS-CoV-2 to first negative test was 15 days (range: 2 to 67 days). Of note, 4 patients with XLA showed longer durations of testing positive for SARS-CoV-2 (30 to 64 days), and 4 additional cases had reports of rehospitalization or of testing positive, then negative, then positive again. As most people with XLA have no or very few B cells and thus produce no or few antibodies, this finding supports the importance of antibodies in clearing SARS-CoV-2 [20,24,33,35,49,55,58]. No case reports included information on whole viral genome sequencing for SARS-CoV-2 to allow for evaluation of the impact of PI on the emergence of variants of concern.
3.6. COVID-19 vaccination
Only two studies focused on COVID-19 vaccination in people with PI in the time period covered by this review; patients received Pfizer-BioNTech COVID-19 vaccine in both studies [14,15]. The first study, by Hagin and colleagues, included 26 patients with primary immunodeficiencies, including XLA, CVID, NFkB1 haploinsufficiency, hypogammaglobulinemia, selective IgG2 deficiency, CID, autoimmune lymphoproliferative syndrome-like (ALPS-like), STAT1 GOF, and STAT3 LOF [14]. None of the patients had major adverse reactions to the vaccine. Out of 26 patients, 18 tested positive for antibodies against the SARS-CoV-2 spike protein following 2-dose vaccination, indicating a B-cell response to the vaccine [14]. As expected, none of the four XLA patients had evidence of antibody response [14]. The other patients who did not have evidence of an antibody response to vaccination had ALPS-like disease (1 out of 1), CID (1 out of 1), and CVID (2 out of 12). Compared with healthy vaccinated controls, patients with CVID who responded to vaccination had lower anti-SARS-CoV2 antibody responses, especially those who were older [14]. Excluding XLA patients, 18 out of 21 patients with PI produced antibodies that were able to block binding of the SARS-CoV-2 spike protein to the ACE2 receptor in vitro indicating that antibodies produced could block SARS-CoV-2 Infection. Out of 26 patients, 19 had a T-cell response to the vaccine, measured by IL-2 and IFN-ɣ production, including the patients with XLA. Those lacking a T-cell response were 1 patient with ALPS-like disease (1 out of 1), 2 with NFKB1-H1 (2 out of 2), and 4 with CVID (4 out of 12), with no difference in response by age.
The second article about COVID-19 vaccination and PI patients, by Salinas et al., studied patients with CVID and XLA [15]. Seven of the 41 patients with CVID already had COVID-19 prior to vaccination, and one had COVID-19 following vaccination with Pfizer-BioNTech COVID-19 vaccine. Most CVID patients had abnormal B- and T-cell responses to the vaccine that the authors suggested might not be sufficient to offer protection [15]. However, 5 of the 7 patients previously infected with SARS-CoV-2 had detectable IgG antibodies, indicating that they had mounted a B-cell response to infection, and vaccination boosted the IgG response in 6 out of 7. While the XLA patients did not have a B-cell response, most had a significant T-cell response to vaccination that the authors indicated might offer protection [15].
Two other studies, published outside of the time period of the 68 articles in this study, were identified during the preparation of this manuscript and are discussed due to their significance and the lack of publications in this area. One study provided information on COVID-19 vaccine effectiveness against severe disease requiring hospitalization for patients with intrinsic immune conditions or immunodeficiencies who had been vaccinated 14 days or more before hospitalization, from January to September 2021 [73]. The vaccine effectiveness (VE) for two doses of either the Moderna or Pfizer-BioNTech mRNA vaccine was 73% for PI patients and 87% for immunocompetent patients, for two doses of the Moderna vaccine the VE was 81% for PI patients and 93% for immunocompetent patients, and for two doses of the Pfizer-BioNTech vaccine VE was 64% for PI patients and 88% for immunocompetent patients [73]. Another study had 11 PI patients that received either the Pfizer-BioNTech or Moderna mRNA COVID-19 vaccine. All of the patients, except for the only patient with XLA, developed a positive antibody response [74].
4. Discussion
This systematic review provides insight into the information available in the literature regarding COVID-19 health outcomes in PI patients as well as gaps in knowledge. Of all the PI classes, predominantly antibody deficiencies had the most information available on health outcomes associated with COVID-19. The literature provides information on COVID-19 outcomes in PI patients, including mortality, hospitalization, and required oxygen supplementation. However, the varying number and level of detail for reported COVID-19 cases for the different PI classes limit our ability to compare the severity of COVID-19 between different PI classes. During the preparation of this manuscript, Bucciol and colleagues published a review on PI and COVID-19 outcomes [75]. Their review, which shared 38 articles with our review, looked at ICU admission and case fatality rates in different types of PI and had similar results to our study (Table S5). Both reviews found 16% ICU admission rates and 9% case fatality rates overall for people hospitalized with PI. Our review expands on their findings by providing information on factors beyond ICU admission and case fatality rates, such as oxygen requirements, treatments, comorbidities, complications, and length of COVID-19 infection.
Because of their increased risk for infection in general, most people with PI, even before the pandemic, routinely take proactive measures to avoid infection, including wearing masks, physical distancing, and avoiding crowds, and may take further steps to avoid infection compared to the general population [11,12,20,22,72,76]. Strict adherence to these mitigation practices would be expected to decrease the likelihood of exposure to SARS-CoV-2 resulting in the PI population having a lower level of exposure than the general population. Thus, finding a similar cumulative incidence of COVID-19 in the PI population compared with the general population might indicate that patients with PI are more likely to be infected with SARS-CoV-2 following exposure. However, this could also be explained by a higher likelihood of testing patients with PI for COVID-19. Whether mitigation measures provide the same level of protection for people with PI as for the general population cannot be determined from the data available.
Some studies have reported similar mortality rates between the general population and people with PI [11,12]. However, many people with PI have a decreased life expectancy due to their increased risk for infection, cancer, and other complications. Thus, the mean and median age of people with PI would be expected to be lower than that of the general population: based on the JMF 2020–2021 Global Survey, the largest age group among those with PI is ages 5–19 years (F. and V. Modell, personal communication). As younger age is a protective factor, finding similar overall case fatality rates between the PI and general population could indicate an increased risk of COVID-19-related mortality in the PI population. If this is the case, stratifying by age might be expected to show higher rates in each age category for people with PI compared with the general population. Our findings on mortality in children with PI support this hypothesis. Based on data from 201 children in the case and cohort studies, the case fatality rate in children with PI hospitalized for COVID-19 was 23%, substantially higher than the 0.18% rate reported for children in the general population.
Furthermore, PI patients are followed more carefully and frequently, so that asymptomatic and mild cases would be more likely to be detected than for people in the general population, potentially leading to lower rates of morbidity and mortality in this population when compared with the general population [12]. Our review found a higher COVID-19 case fatality rate for PI patients 56 years or older (20.5%) compared with those 55 years or younger (8.2%). The difference by age in the case-fatality rate is also found in the general population, 19.0% for patients 51 years or older compared with 3.0% for patients 50 years or younger [77]. Overall, people with PI die from COVID-19 at significantly younger ages than the general population and more often have comorbidities including chronic lung or liver disease [23].
Newborn screening for SCID allows for the early identification of children with this condition so that they can be treated with hematopoietic stem cell transplantation prior to 6 months of age, increasing the likelihood of successful transplantation, which can greatly improve their health outcomes. Of note, 10 people with SCID who had been treated with hematopoietic stem cell transplantation showed mild courses of COVID-19 [12,27]. This highlights the importance of newborn screening for SCID in the United States in preventing morbidity and mortality of these children during the pandemic, as they likely would have died of COVID-19 in the absence of successful transplantation, made possible through early identification by newborn screening.
An emerging area of interest for all PI is information about COVID-19 vaccination and its ability to prevent infection and limit transmission within this population. Current recommendations are that people with PI receive three primary doses of mRNA COVID-19 vaccine and one booster dose, with the possibility of a second booster dose at least 4 months after the first booster dose [78]. The studies by Hagin et al. and Salinas et al. showed that vaccination with two doses of mRNA vaccine produces an immune response in some people with PI, such as CVID. However, some people with PI, such as XLA, were found to have little to no antibody production following vaccination. Even among those who have evidence of mounting an immune response to vaccination the concentration of neutralizing antibodies may be suboptimal [14,15,73]. A better understanding of the effectiveness of vaccination, including boosters, in protecting patients with PI will be important for optimizing the vaccine recommendations for this population [14]. This will require determining the immune correlates of protection that provide the most accurate measure of vaccine effectiveness and immunity in people with PI. Of note, current Interim Guidelines for COVID-19 Antibody Testing from the U.S. Center of Disease Control and Prevention do not recommend antibody testing to assess for immunity to SARS-CoV-2 following COVID-19 [79].
While vaccinations remain the first line of defense, further study is needed to optimize prevention and treatments for people with PI who become infected with SARS-CoV-2. The National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel recommends pre-exposure prophylaxis using anti-SARS-CoV-2 monoclonal antibodies (tixagevimab 300 mg plus cilgavimab 300 mg (Evusheld) administered as 2 consecutive 3 mL intramuscular injections) for people ages 12 years and older who have a moderate or severe primary immunodeficiency [80]. IVIG, a standard treatment for many people with PI, and COVID-19 convalescent plasma can both provide protection to people with PI. Studies have shown that both IVIG and COVID-19 convalescent plasma now have increased anti-SARS-CoV2 antibody titers compared with early trials because of vaccine coverage, which generated higher antibody titers than infection in the general population, including plasma donors [81]. Some studies suggest that convalescent plasma can be especially beneficial for people with PI, helping avoid relapses and prolonged infection [42,58]. However, about 3% of convalescent plasma obtained from patients previously hospitalized for COVID-19 were found to contain neutralizing autoantibodies to type I interferons which have been associated with severe COVID-19. Whether convalescent plasma containing these autoantibodies is harmful for people with PI and COVID-19 is not yet known [82]. For people with moderate or severe PI who test positive for COVID-19, the NIH Treatment Guidelines Panel recommends treatment with the antivirals Paxlovid and Remdesivir [83].
Our review identified important areas for further study, including length of SARS-CoV-2 infection, length of symptoms in PI patients, and whole viral genome sequencing in people with PI. Serial whole viral genome sequencing in people with PI and prolonged infection at different time points is important to determine whether prolonged infection increases the likelihood of developing novel genetic variants of concern of SARS-CoV-2. While the length of SARS-CoV-2 infection and length of symptoms in PI patients does not appear to be longer overall than the general population, certain subsets of patients, such as those with XLA or other B cell pathway defects, may be more likely to have prolonged or repeat infections [11,22,24,33,42,55,58,84]. This is consistent with the current consensus that neutralizing antibodies are the primary immune correlate of protection [85]. In studies on people with PI, the presence of neutralizing antibodies was associated with less frequent infections and less severe outcomes, delayed seroconversion is associated with compromised viral control, and neutralizing antibodies prior to 14 days after disease onset can be important for recovery [58]. However, many with XLA and other B cell defects have a mild disease course, still mount an inflammatory response to infection, and mount an immune response to vaccination. Some have speculated that dysregulation of the B cell pathway might cause the inflammation pathology observed in otherwise healthy people from the general population [22]. Supporting this, some patients with PI with autoimmune or inflammatory complications had poorer outcomes [19], and some studies have found that severe COVID-19 cases are more likely to have a greater IgG response than mild cases [41]. In contrast, the more severe outcomes seen in people with defects in the T cell and innate pathways point to the importance of these pathways in preventing severe disease [11,12,47,86]. These findings support the development of vaccines that better target the T cells to better protect both people with PI and the general population. These types of vaccines would be less vulnerable to loss of effectiveness due to SARS-CoV2 variants, as T-cells target larger regions of the spike protein [87,88]. However, some with XLA and other B cell pathway defects have developed severe disease despite seemingly functional T cell and innate pathways, possibly indicating the importance of the role of B cells in the setting of intact cellular immunity in some people [58]. Of note, those with PI that result in absent B cell function, such as XLA, tend to fare better than those with a PI that leads to B-cell dysfunction, such as CVID [23]. Despite these trends, patients with PI can have complications from COVID-19, and severity cannot be predicted for all people with PI [57]. This might be partly due to the complex interplay and built-in redundancy of the B and T cell pathways, as T cells are required to make antibodies, just as B cells assist with T cell function.
Studies found a higher risk of severe disease in some people with immune dysregulation or autoimmune disease, possibly due to a higher likelihood of unregulated inflammatory response, which could provide clues to the mechanism of disease in some previously healthy people who develop severe COVID-19 and could suggest that treatments for these PI may have utility in treating people who develop severe COVID-19 [12]. Whether COVID-19 could trigger auto-immunity in predisposed people remains unknown [57]. For example, a recent study showed an association between COVID-19 infection and development of type 1 diabetes, with a higher risk in younger (age 0–1) and older (51–65) people [89]. Whether this risk is higher in people with pro-inflammatory PI remains unknown.
Strengths of this review include the broad nature of the search used to find relevant literature and the inclusion of case studies, cohort studies, and cross-sectional studies. One major limitation is publication bias. For case studies, the more severe cases with worse outcomes may be more likely to be published. For example, people with PI who were infected with SARS-CoV-2 and remained asymptomatic or had mild disease courses that did not require hospitalization were less likely to be included in the published literature. As a result, rates of outcomes such as hospitalization, ICU admission, oxygen requirement, and death are likely overestimated. The number of studies and included patients, particularly those focused on COVID-19 vaccine response, was small. These low numbers precluded a more sophisticated meta-analysis that controlled for publication bias, time period of infection, and other factors. The heterogeneity across the large number of PI conditions makes it challenging to draw overall conclusions about COVID-19 and PI. Furthermore, the low number of cases in most PI classes could mean that the cases included in this review are not representative of those classes. Another potential limitation is the exclusion of non-English articles. As a result, important findings for COVID-19 outcomes in PI patients may have been missed.
5. Conclusion
Evidence-based guidelines for managing emerging high-consequence pathogens such as SARS-CoV-2 are important for PI patients, their families, and their providers. Better understanding of COVID-19 in people with PI is important both for better protecting these people and potentially for limiting transmission and the development of SARS-CoV-2 variants of concern. SARS-CoV-2 isolates from people with PI and prolonged infection would be good candidates to prioritize for whole genome sequencing. Findings from studies on people with PI can provide important clues to the mechanisms of SARS-CoV-2 infection and protection offered by vaccination. Mitigation such as masking and physical distancing, while decreasing in use in the general population, remain important for many people with PI due to their increased risk for many different types of infections. Adherence to current guidance, including vaccination, use of pre-exposure prophylaxis, and early treatment if infected, is especially important for people with PI, who might be at increased risk for secondary infections if hospitalized with COVID-19.
Acknowledgments
Acknowledgments
We thank Tara Anderson, Carolyn Bridges, Jennifer Giovanni, Brendan Jackson, Harold Jaffe, Jefferson Jones, Pamela Logan, Sarah Meyer, Sara Elizabeth Oliver, LaVonne Ortega, Augustine Rajakumar, Jerome Tokars, and Bao-Ping Zhu for their careful reviews of the manuscript.
Disclaimer
The opinions expressed in this paper are those of the authors and do not reflect the official position of the Centers for Disease Control and Prevention.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2022.109097.
Appendix A. Supplementary data
Tables S1, S2 and S5
Tables S3: Bias and GRADE assessment
Table S4: Health outcomes for individual cases with PI and COVID-19 from case reports, case series, and cohort studies
References
- 1.McCusker C., Upton J., Warrington R. Primary immunodeficiency. Allergy asthma. Clin. Immunol. 2018;14(Suppl. 2):61. doi: 10.1186/s13223-018-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangye S.G., Al-Herz W., Bousfiha A., Chatila T., Cunningham-Rundles C., Etzioni A., Franco J.L., Holland S.M., Klein C., Morio T., Ochs H.D., Oksenhendler E., Picard C., Puck J., Torgerson T.R., Casanova J.L., Sullivan K.E. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2020;40(1):24–64. doi: 10.1007/s10875-019-00737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egeren D., Novokhodko A., Stoddard M., Tran U., Zetter B., Rogers M., Joseph-McCarthy D., Chakravarty A. Controlling long-term SARS-CoV-2 infections can slow viral evolution and reduce the risk of treatment failure. Sci. Rep. 2021;11(1):22630. doi: 10.1038/s41598-021-02148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown L., Spinelli M., Gandhi M. The interplay between HIV and COVID-19: summary of the data and responses to date. Curr. Opin. HIV AIDS. 2021;16(1):63–73. doi: 10.1097/COH.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzi Y., Bartash R., Scalea J., Loarte-Campos P., Akalin E. COVID-19 and solid organ transplantation: A review article. Transplantation. 2021;105(1):37–55. doi: 10.1097/TP.0000000000003523. [DOI] [PubMed] [Google Scholar]
- 6.Jee J., Foote M.B., Lumish M., Stonestrom A.J., Wills B., Narendra V., Avutu V., Murciano-Goroff Y.R., Chan J.E., Derkach A., Philip J., Belenkaya R., Kerpelev M., Maloy M., Watson A., Fong C., Janjigian Y., Diaz L.A., Jr., Bolton K.L., Pessin M.S. Chemotherapy and COVID-19 outcomes in patients with Cancer. J. Clin. Oncol. 2020;38(30):3538–3546. doi: 10.1200/JCO.20.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duraisingham S., Buckland M., Dempster J., Lorenzo L., Grigoriadou S., Longhurst H. Primary vs. secondary antibody deficiency: clinical features and infection outcomes of immunoglobulin replacement. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., PRISMA-P Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murad M., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., Jaeschke R., Rind D., Meerpohl J., Dahm P., Schünemann H.J. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Milito C., Lougaris V., Giardino G., Punziano A., Vultaggio A., Carrabba M., Cinetto F., Scarpa R., Delle Piane R.M., Baselli L., Ricci S., Rivalta B., Conti F., Marasco C., Marzollo A., Firinu D., Pulvirenti F., Lagnese G., Vivarelli E., Cancrini C., Martire B., Danieli M.G., Pession A., Vacca A., Azzari C., Fabio G., Matucci A., Soresina A.R., Agostini C., Spadaro G., Badolato R., Cicalese M.P., Aiuti A., Plebani A., Pignata C., Quinti I. Clinical outcome, incidence, and SARS-CoV-2 infection-fatality rates in Italian patients with inborn errors of immunity. J Allergy Clin Immunol Pract. 2021;9(7):2904–2906.e2. doi: 10.1016/j.jaip.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delavari S., Abolhassani H., Abolnezhadian F., Babaha F., Iranparast S., Ahanchian H., Moazzen N., Nabavi M., Arshi S., Fallahpour M., Bemanian M.H., Shokri S., Momen T., Sadeghi-Shabestari M., Molatefi R., Shirkani A., Vosughimotlagh A., Safarirad M., Sharifzadeh M., Pashangzadeh S., Salami F., Shirmast P., Rezaei A., Moeini Shad T., Mohraz M., Rezaei N., Hammarström L., Yazdani R., Aghamohamamdi A. Impact of SARS-CoV-2 pandemic on patients with primary immunodeficiency. J. Clin. Immunol. 2021;41(2):345–355. doi: 10.1007/s10875-020-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deya-Martinez A., Garcia-Garcia A.P., Vlagea A., Jordan Y., Fumado V., Fortuny C., Espanol M., Gonzalez A., Esteve-Sole A., Juan M., Pascal M., Alsina L. Confirmed SARS-COV-2 infection in A cohort of children and young adults with moderate or severe primary Immunodeficiencies. J. Clin. Immunol. 2021;41(Suppl. 1):S107. doi: 10.1007/s10875-021-01001-x. [DOI] [Google Scholar]
- 14.Hagin D., Freund T., Navon M., Halperin T., Adir D., Marom R., Levi I., Benor S., Alcalay Y., Freund N.T. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J. Allergy Clin. Immunol. 2021;148(3):739–749. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salinas A.F., Mortari E.P., Terreri S., Quintarelli C., Pulvirenti F., Di Cecca S., Guercio M., Milito C., Bonanni L., Auria S., Romaggioli L., Cusano G., Albano C., Zaffina S., Perno C.F., Spadaro G., Locatelli F., Carsetti R., Quinti I. SARS-CoV-2 vaccine induced atypical immune responses in antibody defects: everybody does their best. J. Clin. Immunol. 2021;41(8):1709–1722. doi: 10.1007/s10875-021-01133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karakoc Aydiner E., Bilgic Eltan S., Babayeva R., Aydiner O., Kepenekli E., Kolukisa B., et al. Adverse COVID-19 outcomes in immune deficiencies: Inequality exists between subclasses. Allergy. 2021;77(1):282–295. doi: 10.1111/all.15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz E., Hodl I., Forstner P., Hatzl S., Sareban N., Moritz M., Fessler J., Dreo B., Uhl B., Url C., Grisold A.J., Khalil M., Kleinhappl B., Enzinger C., Stradner M.H., Greinix H.T., Schlenke P., Steinmetz I. CD19+IgD+CD27- Naïve B cells as predictors of humoral response to COVID 19 mRNA vaccination in immunocompromised patients. Front. Immunol. 2021;12:5245. doi: 10.3389/fimmu.2021.803742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherkat R., Bolourinejad P., Salemi N., Rahmati F., Aria H., Azizi M., Jafari M., Najafi S. The clinical coarse and outcome of SARS-COVID 19 in patients with inborn error of immunity, does it relate to the type of the immune defects. J. Clin. Immunol. 2021;41(Suppl. 1):S39–S40. doi: 10.1007/s10875-021-01001-x. [DOI] [Google Scholar]
- 19.Ho H.-E., Mathew S., Peluso M.J., Cunningham-Rundles C. Clinical outcomes and features of COVID-19 in patients with primary immunodeficiencies in new York City. J Allergy Clin Immunol Pract. 2021;9(1):490–493.e2. doi: 10.1016/j.jaip.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., Lopez-Granados E., Gianelli C., Robles-Marhuenda A., Jeandel P.-Y., Paillard C., Sankaran V.G., Demirdag Y.Y., Lougaris V., Aiuti A., Plebani A., Milito C., Dalm V.A., Guevara-Hoyer K., Sánchez-Ramón S., Bezrodnik L., Barzaghi F., Gonzalez-Granado L.I., Hayman G.R., Uzel G., Mendonça L.O., Agostini C., Spadaro G., Badolato R., Soresina A., Vermeulen F., Bosteels C., Lambrecht B.N., Keller M., Mustillo P.J., Abraham R.S., Gupta S., Ozen A., Karakoc-Aydiner E., Baris S., Freeman A.F., Yamazaki-Nakashimada M., Scheffler-Mendoza S., Espinosa-Padilla S., Gennery A.R., Jolles S., Espinosa Y., Poli M.C., Fieschi C., Hauck F. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J. Allergy Clin. Immunol. 2021;147(2):520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grumach A.S., Goudouris E., Dortas Junior S., Marcelino F.C., Alonso M.L.O., Martins RdO, Arpon M.A., Valle S.O.R. COVID-19 affecting hereditary angioedema patients with and without C1 inhibitor deficiency. J Allergy Clin Immunol Pract. 2021;9(1):508–510. doi: 10.1016/j.jaip.2020.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Yazidi L.S., Al Rawahi H., Al Busaidi I., Al Tamemi S. COVID-19 and primary immunodeficiency: one-year experience. J. Paediatr. Child Health. 2021;57(4):594. doi: 10.1111/jpc.15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shields A.M., Burns S.O., Savic S., Richter A.G. COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience. J. Allergy Clin. Immunol. 2021;147(3):870–875.e1. doi: 10.1016/j.jaci.2020.12.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esenboga S., Ocak M., Akarsu A., Bildik H.N., Cagdas D., Iskit A.T., Tezcan I. COVID-19 in patients with primary immunodeficiency. J. Clin. Immunol. 2021;06:06. doi: 10.1007/s10875-021-01065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harada K., Ho H.E., Cunningham-Rundles C. COVID-19 prevalence and outcomes in patients receiving biologic therapies at an infusion center in new York City. Clin. Immunol. 2021;230 doi: 10.1016/j.clim.2021.108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drabe C.H., Hansen A.B.E., Rasmussen L.D., Larsen O.D., Møller A., Mogensen T.H., Helweg-Larsen J., Katzenstein T.L. Low morbidity in Danish patients with common variable immunodeficiency disorder infected with severe acute respiratory syndrome coronavirus 2. Infect Dis. 2021;53(12):953–958. doi: 10.1080/23744235.2021.1957144. [DOI] [PubMed] [Google Scholar]
- 27.Marcus N., Frizinsky S., Hagin D., Ovadia A., Hanna S., Farkash M., Maoz-Segal R., Agmon-Levin N., Broides A., Nahum A., Rosenberg E., Kuperman A.A., Dinur-Schejter Y., Berkun Y., Toker O., Goldberg S., Confino-Cohen R., Scheuerman O., Badarneh B., Epstein-Rigbi N., Ama Etzioni A., Dalal I., Somech R. Minor clinical impact of COVID-19 pandemic on patients with primary immunodeficiency in Israel. Front. Immunol. 2020;11:614086. doi: 10.3389/fimmu.2020.614086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armann J., et al. Risk factors for hospitalization, disease severity and mortality in children and adolescents with COVID-19: Results from a nationwide German registry. medRxiv. 2021 https://www.medrxiv.org/content/10.1101/2021.06.07.21258488v1 Available at: [Google Scholar]
- 29.Abraham R.S., Marshall J.M., Kuehn H.S., Rueda C.M., Gibbs A., Guider W., Stewart C., Rosenzweig S.D., Wang H., Jean S., Peeples M., King T., Hunt W.G., Honegger J.R., Ramilo O., Mustillo P.J., Mejias A., Ardura M.I., Shimamura M. Severe SARS-CoV-2 disease in the context of a NF-κB2 loss-of-function pathogenic variant. J. Allergy Clin. Immunol. 2021;147(2):532–544.e1. doi: 10.1016/j.jaci.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahanchian H., Moazzen N., Sezavar M., Khalighi N., Khoshkhui M., Aelami M.H., Motevalli Haghi N.S., Rezaei N. COVID-19 in a child with primary antibody deficiency. Clin Case Rep. 2021;9(2):755–758. doi: 10.1002/ccr3.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alicea Marrero M.M., Silio M., McQueen-Amaker K., Español M., Velez M., LeBlanc Z. Posthematopoietic stem cell transplant COVID-19 infection in a pediatric patient with IPEX syndrome. Pediatr. Blood Cancer. 2021;68(1):e28578. doi: 10.1002/pbc.28578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelino G., Cifaldi C., Zangari P., Di Cesare S., Di Matteo G., Chiriaco M., Francalanci P., Faraci S., Rea F., Romeo E.F., Amodio D., Ursu G.M., Bertocchini A., Accinni A., Crocoli A., Inserra A., Cozza R., Romano C., Licciardello M., Rinelli M., Dall Oglio L., Cancrini C., De Angelis P., Finocchi A. Gastric cancer, inflammatory bowel disease and polyautoimmunity in a 17-year-old boy: CTLA-4 deficiency successfully treated with Abatacept. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e1051–e1056. doi: 10.1097/MEG.0000000000002185. [DOI] [PubMed] [Google Scholar]
- 33.Ayaz Ahmed M., Verghese D., Sun C., Mohan A., Djondo D. The ‘x’ factor: exploring COVID-19 viral shedding in x-linked agammaglobulinemia. Can PCR cell cycle threshold play a role? Am. J. Respir. Crit. Care Med. 2021;203:A2007. doi: 10.1164/ajrccm-conference.2021.203.1. [DOI] [Google Scholar]
- 34.Beccuti G., Ghizzoni L., Cambria V., Codullo V., Sacchi P., Lovati E., Mongodi S., Iotti G.A., Mojoli F. A COVID-19 pneumonia case report of autoimmune polyendocrine syndrome type 1 in Lombardy, Italy: letter to the editor. J. Endocrinol. Investig. 2020;43(8):1175–1177. doi: 10.1007/s40618-020-01323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckland M.S., Galloway J.B., Fhogartaigh C.N., Meredith L., Provine N.M., Bloor S., Ogbe A., Zelek W.M., Smielewska A., Yakovleva A., Mann T., Bergamaschi L., Turner L., Mescia F., Toonen E.J.M., Hackstein C.-P., Akther H.D., Vieira V.A., Ceron-Gutierrez L., Periselneris J., Kiani-Alikhan S., Grigoriadou S., Vaghela D., Lear S.E., Török M.E., Hamilton W.L., Stockton J., Quick J., Nelson P., Hunter M., Coulter T.I., Devlin L., Bradley J.R., Smith K.G.C., Ouwehand W.H., Estcourt L., Harvala H., Roberts D.J., Wilkinson I.B., Screaton N., Loman N., Doffinger R., Lyons P.A., Morgan B.P., Goodfellow I.G., Klenerman P., Lehner P.J., Matheson N.J., Thaventhiran J.E.D. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat. Commun. 2020;11(1):6385. doi: 10.1038/s41467-020-19761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpino A., Buganza R., Matarazzo P., Tuli G., Pinon M., Calvo P.L., Montin D., Licciardi F., De Sanctis L. Autoimmune Polyendocrinopathy-candidiasis-ectodermal dystrophy in two siblings: same mutations but very different phenotypes. Genes (Basel) 2021;12(2):169. doi: 10.3390/genes12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castano-Jaramillo L.M., Yamazaki-Nakashimada M.A., Scheffler Mendoza S.C., Bustamante-Ogando J.C., Espinosa-Padilla S.E., Lugo Reyes S.O. A male infant with COVID-19 in the context of ARPC1B deficiency. Pediatr. Allergy Immunol. 2021;32(1):199–201. doi: 10.1111/pai.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhamija Y., Xie S., Zhang S., Wilson B., Spearman P., Paulsen G., Danziger-Isakov L., Assa’ad A. Treatment of COVID-19 with convalescent plasma in a patient with X-linked Agammaglobulinemia. J. Clin. Immunol. 2021;41(Suppl. 1):S54. doi: 10.1007/s10875-021-01001-x. [DOI] [Google Scholar]
- 39.Gabryszewski S., Sun D., Jyonouchi S., Sullivan K., Henrickson S. Self-limited Covid-19 infection in Artemis Hypomorphic Scid: are B cells dispensable? Annals of allergy. Asthma and Immunology. 2020;125(5 Supplement):S105. doi: 10.1016/j.anai.2020.08.353. [DOI] [Google Scholar]
- 40.Gupta S., Su H., Narsai T., Agrawal S. SARS-CoV-2-associated T-cell responses in the presence of humoral immunodeficiency. Int. Arch. Allergy Immunol. 2021;182(3):195–209. doi: 10.1159/000514193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hovey J.G., Tolbert D., Howell D. Burton’s Agammaglobulinemia and COVID-19. Cureus. 2020;12(11):3. doi: 10.7759/cureus.11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iaboni A., Betschel S. A patient with X-linked agammaglobulinemia and COVID-19 infection treated with remdesivir and convalescent plasma. J. Clin. Immunol. 2021;41(Suppl. 1):S106–S107. doi: 10.1007/s10875-021-01001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaminski M., Yoshimoto G., Silva L., Garcia A., Ferraroni N. COVID-19 and common variable immunodeficiency. J. Allergy Clin. Immunol. 2021;147(2 Supplement):AB65. doi: 10.1016/j.jaci.2020.12.259. [DOI] [Google Scholar]
- 44.Khakwani Z., Kumar S. Covid-19 in a patient with hyper-eosinophilic syndrome treated with Mepolizumab. Ann. Allergy Asthma Immunol. 2020;125(5 Supplement):S104. doi: 10.1016/j.anai.2020.08.350. [DOI] [Google Scholar]
- 45.Lee P.Y., Platt C.D., Weeks S., Grace R.F., Maher G., Gauthier K., Devana S., Vitali S., Randolph A.G., McDonald D.R., Geha R.S., Chou J. Immune dysregulation and multisystem inflammatory syndrome in children (MIS-C) in individuals with haploinsufficiency of SOCS1. J. Allergy Clin. Immunol. 2020;146(5):1194–1200.e1. doi: 10.1016/j.jaci.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemarquis A., Campbell T., Aranda-Guillén M., Hennings V., Brodin P., Kämpe O., Blennow K., Zetterberg H., Wennerås C., Eriksson K., Landegren N., Bryceson Y., Berg S., Ekwall O. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. J. Allergy Clin. Immunol. 2021;148(1):96–98. doi: 10.1016/j.jaci.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mantravadi V., Nguyen S.T., Morley S.C., Bednarski J.J., Kitcharoensakkul M., Cooper M.A. Recovery from COVID-19 in a child with chronic granulomatous disease and T cell lymphopenia. J. Clin. Immunol. 2021;41(1):23–25. doi: 10.1007/s10875-020-00896-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazer M.B., Turnbull I.R., Miles S., Blood T.M., Sadler B., Hess A., Botney M.D., Martin R.S., Bosanquet J.P., Striker D.A., Anand N.S., Morre M., Caldwell C.C., Brakenridge S.C., Moldawer L.L., Di Paola J.A., Hotchkiss R.S., Remy K.E. Interleukin-7 reverses lymphopenia and improves T-cell function in coronavirus disease 2019 patient with inborn error of toll-like receptor 3: A case report. Critical Care Explorations. 2021;3(7):e0500. doi: 10.1097/CCE.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palomba E., Carrabba M., Zuglian G., Alagna L., Saltini P., Fortina V., Hu C., Bandera A., Fabio G., Gori A., Muscatello A. Treatment of SARS-CoV-2 relapse with Remdesivir and neutralizing antibodies cocktail in a patient with X-linked Agammaglobulinemia. Int. J. Infect. Dis. 2021;110:338–340. doi: 10.1016/j.ijid.2021.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riberio L.C., Benites B.D., Ulaf R.G., Nunes T.A., Costa-Lima C., Addas-Carvalho M., Proenca-Modena J.L., Granja F., da Costa V.A., Duarte AdSS, Zangirolami A.B., Amaro E.C., Mansour E., Zollner R.L., Velloso L.A. Rapid clinical recovery of a SARS-CoV-2 infected common variable immunodeficiency patient following the infusion of COVID-19 convalescent plasma. Allergy asthma. Clin. Immunol. 2021;17(1):14. doi: 10.1186/s13223-021-00518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt A., Krawitz P.M., Engels H.N.M.M., Bagci S. TBK1 and TNFRSF13B mutations and an autoinflammatory disease in a child with lethal COVID-19. NPJ Genomic Medicine. 2021;6(1):55. doi: 10.1038/s41525-021-00220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stockdale C., Rice L., Carter C., Berry I., Poulter J., O’Riordan S., Pollard S., Anwar R., Tooze R., Savic S. Novel case of Tripeptidyl peptidase 2 deficiency associated with mild clinical phenotype. J. Clin. Immunol. 2021;41(5):1123–1127. doi: 10.1007/s10875-021-01006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szafron V., Pereira M., Vogel T., Leung D., Forbes-Satter L. A balancing act: treatment of COVID-19 and cytomegalovirus in A patient with primary immunodeficiency. Ann. Allergy Asthma Immunol. 2020;125(5):S106–S107. doi: 10.1016/j.anai.2020.08.359. [DOI] [Google Scholar]
- 54.van Oers N.S.C., Hanners N.W., Sue P.K., Aquino V., Li Q.-Z., Schoggins J.W., Wysocki C.A. SARS-CoV-2 infection associated with hepatitis in an infant with X-linked severe combined immunodeficiency. Clin. Immunol. 2021;224:108662. doi: 10.1016/j.clim.2020.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westreich A. Persistently positive SARS-CoV-2 RT-PCR testing in a pediatric patient with X-linked Agammaglobulinemia. J. Clin. Immunol. 2021;41(Suppl. 1):S95–S96. doi: 10.1007/s10875-021-01001-x. [DOI] [Google Scholar]
- 56.Zamperlini-Netto G., Fernandes J.F., Garcia J.L., Ribeiro A.A.F., Camargo L.F.A., de Moraes Terra C., Hamerschlak N. COVID-19 after hematopoietic stem cell transplantation: report of two children. Bone Marrow Transplant. 2021;56(3):713–715. doi: 10.1038/s41409-020-01041-8. [DOI] [PubMed] [Google Scholar]
- 57.Castano-Jaramillo L.M., Yamazaki-Nakashimada M.A., Farrill-Romanillos P.M., Muzquiz Zermeño D., Scheffler Mendoza S.C., Venegas Montoya E., García Campos J.A., Sánchez-Sánchez L.M., Gámez González L.B., Ramírez López J.M., Bustamante Ogando J.C., Vásquez-Echeverri E., Medina Torres E.A., Lopez-Herrera G., Blancas Galicia L., Berrón Ruiz L., Staines-Boone A.T., Espinosa-Padilla S.E., Segura Mendez N.H., Lugo Reyes S.O. COVID-19 in the context of inborn errors of immunity: a case series of 31 patients from Mexico. J. Clin. Immunol. 2021;41(7):1463–1478. doi: 10.1007/s10875-021-01077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delgado-Fernández M., García-Gemar G.M., Fuentes-López A., Muñoz-Pérez M.I., Oyonarte-Gómez S., Ruíz-García I., Martín-Carmona J., Sanz-Cánovas J., Castaño-Carracedo M.Á., Reguera-Iglesias J.M., Ruíz-Mesa J.D. Treatment of COVID-19 with convalescent plasma in patients with humoral immunodeficiency - three consecutive cases and review of the literature. Enferm Infecc Microbiol Clin (Engl Ed) 2021 doi: 10.1016/j.eimc.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fallatah E., Chang Y., Calderon J., Trujillo V.H. DiGeorge syndrome and COVID-19 in two pediatric patients. J. Allergy Clin. Immunol. 2021;147(2 Supplement):AB66. doi: 10.1016/j.jaci.2020.12.261. [DOI] [Google Scholar]
- 60.James A., Schmitt M., Ochoa S., Ferre E., Dimaggio T., Lionakis M. A compare and contrast of COVID-19 disease progression in two siblings with APECED in relation to the timing of treatment initiation. J. Clin. Immunol. 2021;41(Suppl. 1):S87–S88. doi: 10.1007/s10875-021-01001-x. [DOI] [Google Scholar]
- 61.Khalid M., Urban A., Darnell D., Freeman A. Clinical outcomes of SARS-CoV2 infection in STAT3 deficiency. J. Clin. Immunol. 2021;41(Suppl. 1):S46–S47. doi: 10.1007/s10875-021-01001-x. [DOI] [Google Scholar]
- 62.Khalid M., Urban A., Darnell D., Freeman A. Outcomes of SARS-CoV2 infection in STAT3 and PGM3 deficiency. J. Allergy Clin. Immunol. 2021;147(2):AB152. doi: 10.1016/j.jaci.2020.12.547. [DOI] [Google Scholar]
- 63.Kinoshita H., Durkee-Shock J., Jensen-Wachspress M., Kankate V.V., Lang H., Lazarski C.A., Keswani A., Webber K.C., Montgomery-Recht K., Walkiewicz M., Notarangelo L.D., Burbelo P.D., Fuss I., Cohen J.I., Bollard C.M., Keller M.D. Robust antibody and T cell responses to SARS-CoV-2 in patients with antibody deficiency. J. Clin. Immunol. 2021;41(6):1146–1153. doi: 10.1007/s10875-021-01046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lévy R., Bastard P., Lanternier F., Lecuit M., Zhang S.-Y., Casanova J.-L. IFN-α2a therapy in two patients with inborn errors of TLR3 and IRF3 infected with SARS-CoV-2. J. Clin. Immunol. 2021;41(1):26–27. doi: 10.1007/s10875-020-00933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahmood H.Z., Madhavarapu S., Almuqamam M. Varying illness severity in patients with myd88 deficiencyinfected with coronavirus SARS-CoV-2. Pediatrics. 2021;147(3):453–454. doi: 10.1542/peds.147.3MA5.453. [DOI] [Google Scholar]
- 66.Meisel C., Akbil B., Meyer T., Lankes E., Corman V.M., Staudacher O., Unterwalder N., Kölsch U., Drosten C., Mall M.A., Kallinich T., Schnabel D., Goffinet C., von Bernuth H. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J. Clin. Invest. 2021;131(14):e150867. doi: 10.1172/JCI150867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ochoa S., Rosen L., Lionakis M., Uzel G., Suez D. COVID-19 in 3 patients with CLTA4 haploinsufficiency and absence of autoantibodies to type 1 interferons. J. Clin. Immunol. 2021;41(Suppl. 1):S8–S9. doi: 10.1007/s10875-021-01001-x. [DOI] [Google Scholar]
- 68.Simioli F., Martino M., Annunziata A., Carannante N., Fiorentino G. Therapeutic approach for severe COVID-19 and immunocompromised patients. A case series. Respir Med Case Rep. 2021;33:101397. doi: 10.1016/j.rmcr.2021.101397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soresina A., Moratto D., Chiarini M., Paolillo C., Baresi G., Focà E., Bezzi M., Baronio B., Giacomelli M., Badolato R. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 2020;31(5):565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corse T., Dayan L., Kersten S., Battaglia F., Terlecky S.R., Han Z. Clinical outcomes of COVID-19 patients with pre-existing, compromised immune systems: A review of case reports. Int. J. Med. Sci. 2020;17(18):2974–2986. doi: 10.7150/ijms.50537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goudouris E.S., Pinto-Mariz F., Mendonça L.O., Aranda C.S., Guimarães R.R., Kokron C., Barros M.T., Anísio F., Alonso M.L.O., Marcelino F., Valle S.O.R., Junior S.D., Barreto I.D.P., Ferreira J.F.S., Roxo-Junior P., do Rego Silva A.M., Campinhos F.L., Bonfim C., Loth G., Fernandes J.F., Garcia J.L., Capelo A., Takano O.A., MIV Nadaf, Toledo E.C., LAO Cunha, RSW Di Gesu, Schidlowski L., Fillipo P., Bichuetti-Silva D.C., Soldateli G., Ferraroni N.R., de Oliveira Dantas E., Pestana S., Mansour E., Ulaf R.G., Prando C., Condino-Neto A., Grumach A.S. Outcome of SARS-CoV-2 infection in 121 patients with inborn errors of immunity: A cross-sectional study. J. Clin. Immunol. 2021;41(7):1479–1489. doi: 10.1007/s10875-021-01066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Immune Deficiency Foundation. Doctors provide updates on COVID-19 and PI 2021. https://primaryimmune.org/news/doctors-provide-updates-covid-19-and-pi (accessed 03 June 2022)
- 73.Embi P.J., Levy M.E., Naleway A.L., Patel P., Gaglani M., Natarajan K., Dascomb K., Ong T.C., Klein N.P., Liao I.C., Grannis S.J., Han J., Stenehjem E., Dunne M.M., Lewis N., Irving S.A., Rao S., McEvoy C., Bozio C.H., Murthy K., Dixon B.E., Grisel N., Yang D.H., Goddard K., Kharbanda A.B., Reynolds S., Raiyani C., Fadel W.F., Arndorfer J., Rowley E.A., Fireman B., Ferdinands J., Valvi N.R., Ball S.W., Zerbo O., Griggs E.P., Mitchell P.K., Porter R.M., Kiduko S.A., Blanton L., Zhuang Y., Steffens A., Reese S.E., Olson N., Williams J., Dickerson M., McMorrow M., Schrag S.J., Verani J.R., Fry A.M., Azziz-Baumgartner E., Barron M.A., Thompson M.G., DeSilva M.B. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults – nine states, January-September 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70(44):1553–1559. doi: 10.15585/mmwr.mm7044e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Squire J., Joshi A. Seroconversion after coronavirus disease 2019 vaccination in patients with immune deficiency. Ann. Allergy Asthma Immunol. 2021;127(3):383–384. doi: 10.1016/j.anai.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bucciol G., Tangye S., Meyts I. Coronavirus disease 2019 in patients with inborn errors of immunity: lessons learned. Curr. Opin. Pediatr. 2021;33(6):648–656. doi: 10.1097/MOP.0000000000001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Babaha F., Rezaei N. Primary immunodeficiency diseases in COVID-19 pandemic: A predisposing or protective factor? Am J Med Sci. 2020;360(6):740–741. doi: 10.1016/j.amjms.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alimohamadi Y., Tola H., Abbasi-ghahramanloo A., Janani M., Sepandi M. Case fatality rate of COVID-19: a systematic review and meta-analysis. J Prev Med Hyg. 2021;62(2):E311–E320. doi: 10.15167/2421-4248/jpmh2021.62.2.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.COVID-19 vaccination guidance for people who are moderately or severely immunocompromised 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#immunocompromised
- 79.Interim Guidelines for COVID-19 Antibody Testing 2022. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html
- 80.Prevention of SARS-CoV-2 Infection. 2022. https://www.covid19treatmentguidelines.nih.gov/overview/prevention-of-sars-cov-2/
- 81.Germanio C., Simmons G., Thorbrogger C., Martinelli R., Stone M., Gniadek T., Busch M. Vaccination of COVID-19 convalescent plasma donors increases binding and neutralizing antibodies against SARS-CoV-2 variants. Transfusion. 2022;131(14):e150867. doi: 10.1111/trf.16823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vazquez S., Bastard P., Kelly K., Gervais A., Norris P., Dumont L., Casanova J., Anderson M., DeRisi J. Neutralizing autoantibodies to type I interferons in COVID-19 convalescent donor plasma. J. Clin. Immunol. 2021;41(6):1169–1171. doi: 10.1007/s10875-021-01060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Therapeutic Management of Nonhospitalized Adults With COVID-19. 2022. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults--therapeutic-management/
- 84.Zhou X., Zhou J., Zhao J. Recurrent pneumonia in a patient with new coronavirus infection after discharge from hospital for insufficient antibody production: a case report. BMC Infect. Dis. 2020;20(1):500. doi: 10.1186/s12879-020-05231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khoury D., Cromer D., Reynaldi A., Schlub T., Wheatley A., Juno J., Subbarao K., Kent S., Triccas J., Davenport M. Neutralizing antibody levels are highly predicitive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 86.van der Made C.I., Simons A., Schuurs-Hoeijmakers J., van den Heuvel G., Mantere T., Kersten S., van Deuren R.C., Steehouwer M., van Reijmersdal S.V., Jaeger M., Hofste T., Astuti G., Corominas Galbany J., van der Schoot V., van der Hoeven H., Hagmolen Of Ten Have W, Klijn E., Van Den Meer C., Fiddelaers J., De Mast Q., Bleeker-Rovers C.P., LAB Joosten, Yntema H.G., Gilissen C., Nelen M., JWM Van Der Meer, Brunner H.G., Netea M.G., Van De Veerdonk F.L., Hoischen A. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324(7):663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naranbhai V., Nathan A., Kaseke C., Berrios C., Khatri A., Choi S., Getz M.A., Tano-Menka R., Ofoman O., Gayton A., Senjobe F., Denis K.J.S., Lam E.C., Garcia-Beltran W.F., Balazs A.B., Walker B.D., Iafrate A.J., Gaiha G.D. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all prior infected and vaccinated individuals. medRxiv. 2022 https://www.medrxiv.org/content/10.1101/2022.01.04.21268586v1 Available at: [Google Scholar]
- 88.Collier A.Y., Yu J., McMahan K., Liu J., Chandrashekar A., Maron J.S., Atyeo C., Martinez D.R., Ansel J.L., Aguayo R., Rowe M., Jacob-Dolan C., Sellers D., Barrett J., Ahmad K., Anioke T., VanWyk H., Gardner S., Powers O., Bondzie E.A., Wan H., Baric R.S., Alter G., Hacker M.R., Barouch D.H. Differential kinetics of immune responses elicited by Covid-19 vaccines. N. Engl. J. Med. 2021;385(21):2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qeadan F., Tingey B., Egbert J., Pezzolesi M., Burge M., Peterson K., Honda T. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: A nationwide cohort from the US using the Cerner real-world data. PLoS One. 2022;17(4):e0266809. doi: 10.1371/journal.pone.0266809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1, S2 and S5
Tables S3: Bias and GRADE assessment
Table S4: Health outcomes for individual cases with PI and COVID-19 from case reports, case series, and cohort studies