Abstract
Purine, an abundant substrate in organisms, is a critical raw material for cell proliferation and an important factor for immune regulation. The purine de novo pathway and salvage pathway are tightly regulated by multiple enzymes, and dysfunction in these enzymes leads to excessive cell proliferation and immune imbalance that result in tumor progression. Maintaining the homeostasis of purine pools is an effective way to control cell growth and tumor evolution, and exploiting purine metabolism to suppress tumors suggests interesting directions for future research. In this review, we describe the process of purine metabolism and summarize the role and potential therapeutic effects of the major purine-metabolizing enzymes in ovarian cancer, including CD39, CD73, adenosine deaminase, adenylate kinase, hypoxanthine guanine phosphoribosyltransferase, inosine monophosphate dehydrogenase, purine nucleoside phosphorylase, dihydrofolate reductase and 5,10-methylenetetrahydrofolate reductase. Purinergic signaling is also described. We then provide an overview of the application of purine antimetabolites, comprising 6-thioguanine, 6-mercaptopurine, methotrexate, fludarabine and clopidogrel. Finally, we discuss the current challenges and future opportunities for targeting purine metabolism in the treatment-relevant cellular mechanisms of ovarian cancer.
Graphical Abstract
Keywords: Purine metabolism, Ovarian cancer, Metabolizing enzyme, Antimetabolites, Purinergic signaling
Introduction
Ovarian cancer (OC) is the seventh most common cancer and the fifth leading cause of cancer-related death among women worldwide, with a 5-year relative survival rate of 49% [1, 2]. Primary debulking surgery and adjuvant platinum-based chemotherapy are the first-line standard-of-care treatments [3]. More than 70% of patients experience will relapse after first-line treatment and acquire drug resistance, which highlights the need for novel treatment options.
As one of the most abundant components in organisms, purine, in addition to forming DNA and RNA, is involved in the stabilization of immune regulation and the formation of energy carriers and functions as an essential cofactor in biochemical reactions, thereby influencing the growth of both cancer and non-cancer cells [4, 5]. The metabolic enzymes implicated in purine metabolism cause imbalances in purine pools that interfere with cell proliferation, migration and death [6, 7]. Furthermore, various purine antimetabolites exert antitumor effects through multiple mechanisms, such as direct toxicity, interference with the tumor microenvironment (TME), inhibition of DNA synthesis and interference with DNA damage repair [8–12]. Disorders of extracellular ATP (eATP), extracellular adenosine (eADO) and subsequent purinergic signaling also delineate pro-oncogenic or anti-oncogenic outlines [13]. Overall, purine metabolism is closely related to tumor progression.
This review presents recent reports of major purine-metabolizing enzymes in purine synthetic pathways (Table 1), outlines the multiplicity of purinergic signaling in OC development, gives an overview of the application of purine antimetabolites in OC, and discusses potential therapeutic strategies to target purine metabolism in OC.
Table 1.
Summary of the main molecular features of purine-metabolizing enzymes associated with OC
| Purine-metabolising Enzyme | Enzyme Number | Size/ Molecular Mass | Coding Gene | Gene Location | Subcellular Location | Function | Reaction in Purine Metabolism | Expression or Activity in OC | Ref |
|---|---|---|---|---|---|---|---|---|---|
| CD39 | EC 3.6.1.5 | 510aa/58 kDa | ENTPD1 | 10q24.1 | Plasma membrane, extracellular | Purine metabolism, Purinergic neurotransmitter regulation, Blocking platelet aggregation, Immunomodulation | eATP → eAMP | High | [14, 15] |
| CD73 | EC 3.1.3.5 | 574aa/63 kDa | NT5E | 6q14.3 | Plasma membrane, extracellular, nucleus | Purine metabolism, Water-soluble vitamins and cofactor metabolism | eAMP → eAdo | High | [14–17] |
| ADA | EC 3.5.4.4 | ADA1: 363aa/41 kDa | ADA | 20q13.12 | Plasma membrane, cytosol, lysosome, extracellular | Purine metabolism, Adenosine homeostasis, Immunomodulation | Adenosine → Inosine, Deoxyadenosine → Deoxyinosine | High | [18–20] |
| ADA2: 511aa/59 kDa | ADA2 | 22q11.1 | Extracellular, lysosome | High | |||||
| ADAR | EC 3.5.4.37 | ADAR1:1226aa/136 kDa | ADAR | 1q21.3 | Cytoplasm, Nucleus | RNA editing | A-to-I RNA editing | High | [21–24] |
| ADAR2:741aa/81 kDa | ADARB1 | 21q22.3 | Cytoplasm, Nucleus | Unknown | |||||
| ADAR3:739aa/81 kDa | ADARB2 | 10p15.3 | Nucleus | Unknown | |||||
| AK | EC 2.7.4.3 | AK4:223aa/25 kDa | AK4 | 1p31.3 | Mitochondrion matrix | Purine nucleotide salvage, ATP level regulation | AMP + ATP ↔ ADP | High | [25–27] |
| AK7:723aa/83 kDa | AK7 | 14q32.2 | Cytoplasm, cytosol, Cell projection, cilium, flagellum | Adenosine → AMP | Low | ||||
| IMPDH | EC 1.1.1.205 | IMPDH1:514aa/55 kDa | IMPDH1 | 7q32.1 | Cytoplasm, Nucleus | Purine nucleotides de novo biosynthesis, Immunomodulation | IMP → XMP | Unknown | [28–31] |
| IMPDH2:514aa.56 kDa | IMPDH2 | 3p21.31 | Cytoplasm, Nucleus, cytosol | High | |||||
| PNP | EC 2.4.2.1 | 289aa/32 kDa | PNP | 14q11.2 | Cytoplasm | Pyrimidine metabolism, Purine salvage, Immunomodulation | Inosine → Hypoxanthine, Guanosine → Guanine, 2'-deoxyguanosine → Guanine, 2'-deoxyinosine → Hypoxanthine | Unknown | [32] |
| HPRT | EC 2.4.2.8 | 218aa/25 kDa | HPRT1 | Xq26.2-q26.3 | Cytoplasm | Purine salvage | Guanine → GMP, Hypoxanthine → IMP | Unknown | [33–35] |
| DHFR | EC 1.5.1.3 | 187aa/21 kDa | DHFR | 5q14.1 | Mitochondrion, Cytoplasm | Folate metabolism, Nitric oxide metabolism, Mitochondrial thymidylate de novo synthesis | Dihydrofolate → THF | High or Low | [36–39] |
| MTHFR | EC 1.5.1.20 | 656aa/75 kDa | MTHFR | 1p36.22 | Cytoplasm | Folate metabolism | Methylene-THF → Methyl-THF | Low | [40–43] |
Purine metabolism pathways
The stabilization of purine pools is determined by the balance between the synthesis and degradation of purine nucleotides (Fig. 1). The salvage pathway and de novo pathway are two different pathways for the synthesis of purine nucleotides in mammals. The salvage pathway recycles the degraded purine bases or nucleosides via 5-phosphoribosyl-1-pyrophosphate (PRPP) and catalysis by adenine phosphoribosyltransferase (APRT) and hypoxanthine guanine phosphoribosyltransferase (HPRT). There are other enzymes involved in the recovery of purines. Generally, this simple pathway meets most of the cellular requirements of a large percentage of normal cells with very low energy consumption [44, 45]. Rapidly dividing cancer cells rely more heavily on the de novo pathway, the basis for replenishing purine pools, to meet high energy demands [46]. The de novo pathway is triggered by a dynamic complex called purinosome [47]. Purinosome accumulates in the vicinity of mitochondria and microtubules to accelerate purine nucleotide synthesis by catalyzing the important step PRPP to inosine monophosphate (IMP) [48–50]. Two important rate-limiting enzymes, adenylosuccinate synthase (ADSS) and IMP dehydrogenase (IMPDH), catalyze the conversion of intracellular IMP to succinyl-AMP and xanthosine monophosphate (XMP) and then to AMP and GMP which are gradually degraded to xanthine and eventually hydroxylated to uric acid (UA) under the action of xanthine oxidase (XO) [51].
Fig. 1.
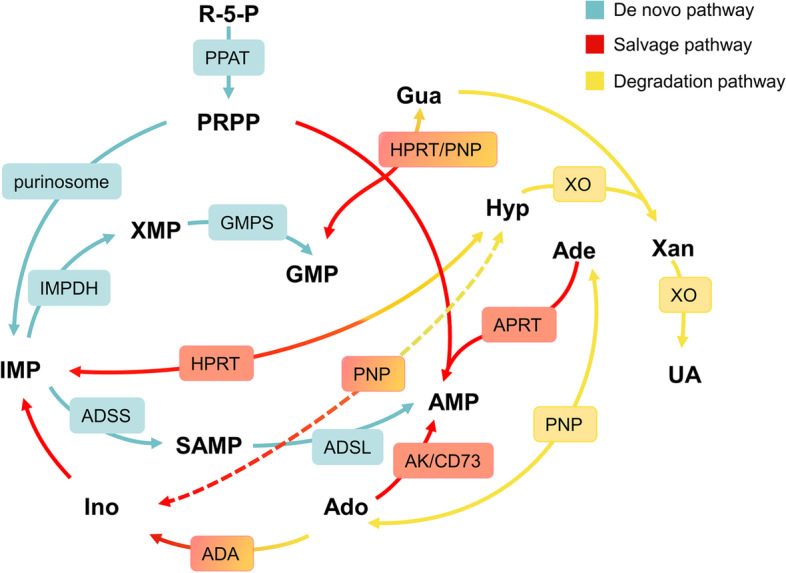
De novo, salvage and degradation pathways of purine nucleotides under the regulation of purine-metabolizing enzymes. The de novo pathway converts PRPP to IMP and, ultimately, GMP and AMP that further involve in nucleotide synthesis. The salvage pathway recovers purine bases and purine nucleosides to generate purine nucleotides. The degraded purine base becomes Xan with eventual conversion to UA. Cyan: de novo pathway; red: salvage pathway; yellow: degradation pathway; gradient color: involved in multiple metabolic pathways; arrows: purine metabolic pathways; squares: purine-metabolizing enzymes involved in related pathways. R-5-P: ribose 5-phosphate; PRPP: 5-phosphoribosyl-1-pyrophosphate; Gln: glutamine; THF: Tetrahydrofolate; Asp: aspartate; Hyp: hypoxanthine; Ino: Inosine; IMP: inosine monophosphate; Xan: xanthine; XMP: xanthosine monophosphate; Gua: guanine; GMP: guanosine monophosphate; Ade: adenine; Ado: adenosine; AMP: ado monophosphate; SAMP: succinyl-AMP; UA: uric acid; PPAT: phosphoribosyl pyrophosphate amidotransferase; IMPDH: IMP dehydrogenase; GMPS: GMP synthase; ADSS: adenylosuccinate synthase; ADSL: adenylosuccinate lyase; HPRT: Hyp Gua phosphoribosyltransferase; APRT: Ade phosphoribosyltransferase; ADA: Ado deaminase; AK: adenylate kinase; PNP: purine nucleoside phosphorylase; XO: xanthine oxidase
Purine-metabolizing enzymes and mechanisms in OC
Ectonucleoside triphosphate diphosphohydrolase and ectosolic-5′-nucleotidase
Ectonucleoside triphosphate diphosphohydrolase (CD39, EC 3.6.1.5) and ectosolic-5′-nucleotidase (CD73, EC 3.1.3.5) are two membrane-bound ectonucleotidases. CD39 is the rate-limiting enzyme for the continuous dephosphorylation of eATP to extracellular AMP (eAMP) [52]. The 5'-nucleotidase activity of CD73 limits the rate of hydrolysis from eAMP to membrane-permeable eADO (Fig. 2) [53]. The eADO creates a highly immunosuppressive microenvironment by inhibiting the cytotoxicity of CD8 + T cells and NK cells while increasing activation of Treg cells and M2 macrophages [54–57]. This implies the balance between eATP and adenosine, which is jointly maintained by CD39 and CD73, is closely related to the immune-suppressive tumor microenvironment.
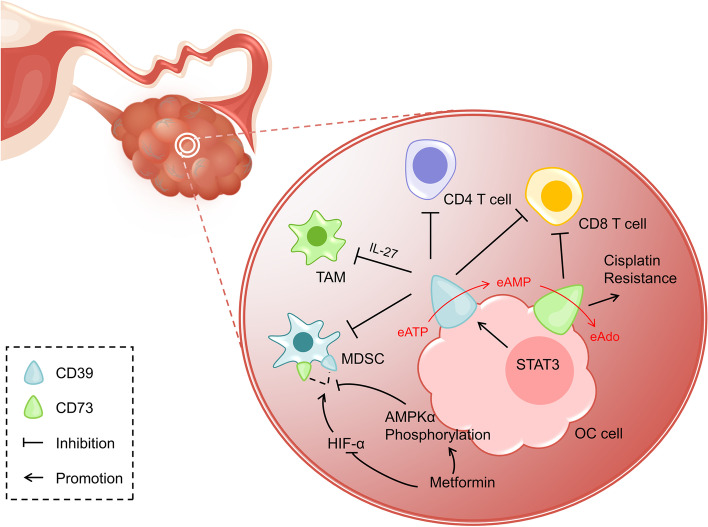
Fig. 2.
CD39 and CD73 in TME of OC. CD39 and CD73 localized on the surface of OC cells inhibit immune responses mediated by T cells, MDSC, and TAM in TME, and also induce cisplatin resistance. CD39 and CD73 dephosphorylate eATP to eAMP, ultimately converting it to eAdo. STAT3 induces cell surface acquiring CD39 in TME to promote immunosuppression. Metformin facilitates AMPKα phosphorylation and inhibits the HIF-α pathway to block the immunosuppression caused by high expression of CD39 and CD73 on MDSC. MDSC: myeloid-deriver suppressor cell; TAM: tumor-associated macrophage; TME: tumor microenvironment; eATP: extracellular ATP; eAMP: extracellular AMP; eAdo: extracellular Ado
Traditionally, CD39 is considered as a contactor between immune cells. Tumor-reactive T cells can be identified by the expression of CD39 alone or the co-expression with CD103 [58, 59]. In recent years, it has been shown that CD39 is widely expressed in multiple human cancers, and high-level CD39 is found in M2-polarized tumor-associated macrophages (TAMs) in OC tissues [10, 14, 15, 60, 61]. STAT3 induces the acquisition of CD39 on cell surface in TME to suppress T cell response [62]. Noteworthy, it is IL-27 that mediates the immunosuppression with CD39 involvement, whose polymorphisms is related to the susceptibility to OC [10, 63]. Furthermore, CD73 is expressed on the surface of OC, and high-level CD73 appears to be significantly associated with poor prognosis in patients with high-grade serous OC, which, as mentioned previously, is probably because that CD73 indirectly suppresses CD8 + T cells and promotes immune escape [16].
Surprisingly, the study by Hoon Kyu Oh et al. found that patients with CD73 overexpression present more frequently with low stage, moderate differentiation, no lymph node metastasis, and negative cytologic results, which means that more CD73 may lead to a better prognosis [17]. A study regarding ovarian tumor-initiating cells found that CD73 is essential for OC initiation and growth, and is able to regulate tumor-initiating cells at the transcriptional level to promote expression of epithelial–mesenchymal transition (EMT)-related genes [64]. Besides, blocking CD73 reverses drug resistance in cisplatin-resistant OC cells [65]. Studies on other human tumors have reported that AKT signaling plays a role when CD73 promotes tumor progression and metastasis [66–69]. However, more mechanistic studies are needed in OC.
Current researches have noted that the anticancer effect of CD39 or CD73 inhibitors depends overwhelmingly on relieving T cell targeted immunosuppression and additionally on myeloid-deriver suppressor cells (MDSCs), which suggest CD39 and CD73 may serve as potential therapeutic targets for OC treatment [70–73]. A study found the antitumor activity can be mediated by blocking CD39 via eATP-P2X7-ASC-NALP3-inflammasome-IL18 pathway [74]. Besides, CD39 inhibitor POM-1 partially relieves the immunosuppressive function of TAMs [10]. As we would expect, anti-CD73 antibody reverses the immunosuppressive environment developed by docetaxel in OC but the inhibition of CD73 alone rather than CD39 is less effective [75]. Thus, the protocol for co-suppressing CD39 and CD73 is expected. An important finding is that metformin, the first-line drug for type-2 diabetes, blocks CD39 and CD73 by increasing AMPKα phosphorylation and inhibiting HIF-α pathway to interrupt immunosuppression caused by MDSC as well as enhance the anti-tumor activity of CD8 + T cells [14]. In addition, a pivotal immunosuppressive factor programmed death-1 receptor (PD-1) encourages tumor spread through interfering protective immunity [76]. Anti-PD-1 combined with anti-CD39 or anti-CD73 demonstrates a more pronounced slowing of tumor growth [74, 77]. In any case, there is still a long way to go to clarify the specific roles and mechanisms of CD39 and CD73, and deep studies will provide new insights into tumor immune networks.
Adenosine deaminase
Adenosine deaminase (ADA, EC 3.5.4.4) catalyzes the irreversible hydrolytic deamination of adenosine and deoxyadenosine, the final products of which are inosine and deoxyinosine (Fig. 3) [18, 78, 79]. There are two isozymes of human ADA. ADA1 regulates adenosine concentration in the intracellular and interacts extracellularly with the adenosine receptor or dipeptidyl peptidase 4 (DDP4, EC 3.4.14.5) on the surface of immune cells [80, 81]. DDP4, expressed as a type II transmembrane protein, plays a pro-tumor role in a variety of human tumors and has the potential to act as a positive prognostic predictor [82]. It was reported that the interaction between ADA and DDP4 on the cell surface could regulate T cell activation and lymphocyte-epithelial cell adhesion [18, 83]. ADA2, with low abundance in humans, is mainly found in serum and its deficiency is associated with autoinflammatory diseases [84–87].
Fig. 3.
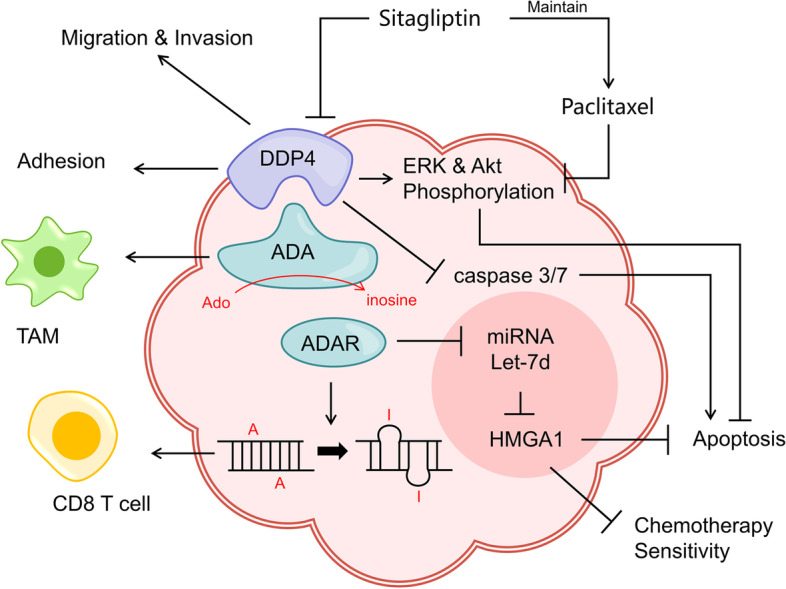
Role and mechanism of ADAR, ADA and its receptor DDP in OC. ADAR mediates A to I RNA editing to elicit CD8 T cell response and interferes with HMGA1 via miRNA Let-7d acting on OC apoptosis and chemotherapy sensitivity. ADA enhances the immune potency of TAM in TME with the capacity to convert Ado to inosine. DDP4, an important receptor for ADA, facilitates the migration, invasion and adhesion to mesothelial cells of OC. The DDP inhibitor Sitagliptin, increases caspase 3/7 activity to induce OC apoptosis on the one hand, and maintains the effect of paclitaxel on OC apoptosis via ERK and Akt pathways on the other hand
ADA level is significantly elevated in the serum and peritoneal fluid of OC patients and is positively correlated with pathological subtype and grade [19]. This may be a self-rescue measure in response to apoptosis caused by accumulation of toxic substrates adenosine and deoxyadenosine. D'Almeida SM et al. found that ADA, similar to CD39 inhibitors, partially blocked immunosuppression of TAMs [10]. At the same time, as a receptor of ADA, DDP4 is overexpressed strongly and the expression level is associated with FIGO stage or lymph node metastasis [88]. Besides, DDP4 increases the adhesion potency of OC to mesothelial cells with the participation of fibrin, and blocking DDP4 significantly inhibits cancer migration and invasiveness [89]. Sitagliptin, a selective DDP4 inhibitor, increases caspase 3/7 activity in OC by co-treatment with paclitaxel, and maintains apoptosis induction through the ERK and Akt signaling pathway [90, 91]. These studies imply that the application of ADA and DDP4 inhibitors seems to favor tumor-cell death. However, it is interesting to note that the addition of ADA reversed the decreased metastatic capacity of OC caused by adenosine [92]. Similarly, overexpression of DDP4 leads to enhanced chemosensitivity of OC cells to paclitaxel and reduced tumor cell invasiveness due to aberrant expression of E-cadherin, MMP-2 and TIMP [93–95]. These puzzling results suggest that ADA and DDP4 still need substantial and in-depth studies and their interaction could act as potential biomarkers or therapeutic targets in OC.
Adenosine deaminases acting on RNA(ADAR, EC 3.5.4.37)catalyzes the deamidation of adenosine on dsRNA to inosine, which means A-to-I RNA editing (Fig. 3) [96]. There are three members of the ADAR family: ADAR1 and ADAR2 are primarily used for RNA editing, and ADAR3 inhibits their editing activity by binding to substrates of the first two [97–99]. ADAR-triggered aberrant RNA editing regulation to promote tumor growth is widely observed in a variety of human solid tumors [100, 101]. The frequent over-editing of Cyclin I (CCNI) by ADAR1 in OC was found to produce peptide products that activate T-cell responses and specifically kill tumor cells [21]. Marek Cybulski et al. detected that the immune responses to CCNI in the nucleus and cytoplasm of epithelial OC are related to abnormal cell cycle but not to chemosensitivity [102]. Furthermore, ADAR1 is reported to induce tumor progression by impairing Let-7d in malignant tumors [103]. Studies have found that miRNA Let-7d plays a suppressive role in a variety of human tumors and its overexpression may inhibit HMGA1 by regulating the p53 signaling pathway to enhance chemosensitivity [104–107]. These studies provide us with inspiration for gene editing to regulate tumor progression. Overall, this is a promising therapeutic concept, but the specific mechanisms still need to be explored more deeply.
Adenylate kinase
Adenylate kinase (AK, EC 2.7.4.3) catalyzes the reversible transfer of phosphate group from ATP to AMP in purine synthesis pathway. AK-induced AMP signal changes affect adenosine pools status and energy information through metabolic sensors, which subsequently balance ATP level to regulate energy metabolism [108, 109]. AK family isozymes AK1-9 have been identified in human tissues [110], and existing studies have reported that AK1, 2, 4, 6 and 7 subtypes function in the regulation of tumor growth, metabolism, energy allocation and invasion [111–114]. So far, researches on AK in OC have been focused on AK4 and AK7.
AK4 is mainly expressed in kidney, heart and liver tissues, probably caused by the high-mitochondrial content. Significantly elevated level of AK4 is also found in some highly aggressive tumors [115–117]. Research has revealed that AK4 stabilizes the purine nucleotide pools and balances energy through the regulation of AMPK [118], and is able to interact with HIF-1 to regulate mitochondrial activity and enhance cellular hypoxia tolerance by promoting hypoxia [116, 119]. Cellular drug resistance is enhanced due to the progress in resistance to hypoxia. The interaction of hypoxia on AK4 level is different in diverse cell lines [120], however, it is clear that changes in AK4 level are able to protect cells from environmental stimuli, and blocking AK4 can resist the protective mechanism of cells against hypoxic stress [119]. AK4 can be found in oocytes, follicular epithelial cells and corpus luteum cells in normal ovary tissue [121]. AK4 is overexpressed in OC and the expression level is significantly correlated with tumor size and FIGO stage [26]. A meaningful finding is that upregulation of miRNA-3666 inhibits OC cell proliferation and migration by blocking the STAT3/AK4 axis and inducing apoptosis simultaneously [27]. Besides, AK4 favors tumor development and metastasis in an ATF3-dependent manner [122], which interestingly plays contradictory roles of either inducing apoptosis or promoting proliferation in different types of OC [123, 124].
AK7 is mainly expressed in cilia-rich sites such as respiratory tract and fallopian tube [109, 110]. Ciliary structural abnormalities caused by AK7 damage often led to primary male infertility or primary ciliary dyskinesia [125–127]. Abnormal activation or loss of primary cilia will affect the progression and prognosis of diverse tumors. Study reported that cilia-related genes are lowly expressed in glioblastoma, breast cancer, OC and colon adenocarcinoma; in contrast, they are overexpressed in clear cell renal cell carcinoma, rectal adenocarcinoma, lung adenocarcinoma and lung squamous cell carcinoma [128]. It is well known that cilia play a key role in assisting transport and picking in the female reproductive system. Furthermore, some studies support the ability of cilia as angiopoietin (Ang) sensory organ to maintain the morphological and motor homeostasis of ovarian tissue [129, 130]. Recent studies support the standpoint that the majority of OC originate from the cilia-rich fallopian tube [131] and the Ang/Tie signaling pathway associated with cilia formation may be relevant to OC development and formation [129, 132] and the expression of Ang2 is closely related to angiogenesis in OC [133]. Thus, it is reasonable to speculate that AK7 may partially influence the ability of cilia to intervene in OC progression. Zhang et al. found that AK7 level in OC is significantly reduced by analysis of the TCGA database, the degree of which is positively correlated with tumor stage [25]. And this phenomenon is mainly related to conduction pathways such as EMT, TGF-b signaling and UV response. More importantly, OC patients with lower AK7 expression have a worse prognosis. These studies not only suggest that AK7 exhibits potential as a prognostic indicator for OC but the possibility of future treatment by elevating AK7 expression level or interfering with cilia activity.
Hypoxanthine guanine phosphoribosyltransferase
HPRT (EC 2.4.2.8) is one of the classical enzymes of the purine salvage pathway, which utilizes the transfer of ribose phosphate from PRPP to form IMP and GMP for DNA synthesis and repair [134]. As a housekeeping gene, HPRT is maintained at a low level in all somatic cells except the central nervous system [135]. HPRT deficiency leads to failure of the salvage for hypoxanthine and guanine, increasing purine degradation and UA production and triggering a series of diseases: partial deficiency causes gout-like symptoms, while complete deficiency results in Lesch-Nyhan syndrome [136]. Although other enzymes have complementary roles, these symptoms are unique to HPRT deficiency [134].
HPRT is closely associated with tumor development and is overexpressed in various malignancies. However, it has been reported that HPRT level is fluctuating in OC but stable in borderline ovarian tumor and normal ovarian tissue, making it a suitable reference gene for biochemical processes [33, 137]. HPRT performs a potential auxiliary role in DNA mismatch-repair. The reason for mismatch is that most DNA polymerases preferentially pair 7,8-dihydro-8-oxoguanine with adenine rather than cytosine during DNA oxidation [138]. The MutY DNA glycosylase homologue recognizes and repairs mismatches, whose level changes lead to opposite changes in the mutation rate of HPRT in OC cell line A2780 [139]. It implies that high-level HPRT mutation is associated with DNA mismatch activity. Nevertheless, HPRT mutation rate is reduced in OC with defects in DNA mismatch repair gene hMSH2 [140]. Also, HPRT mutation may be in connection with tumor risk and chemotherapy resistance in OC [141–145]. These contradictory results suggest that more research is needed to elucidate the relationship between HPRT mutation and DNA mismatch repair and effects on tumors. In general, current research on HPRT is focused on report gene, while tumor-related research on HPRT is relatively stagnant. In-depth investigations are still needed to explore its specific effects on tumors and therapeutic strategies.
Inosine monophosphate dehydrogenase
The catalytic oxidation of IMP to XMP by inosine monophosphate dehydrogenase (IMPDH, EC 1.1.1.205) in cytoplasm is the NAD + -dependent rate-limiting step in de novo synthesis of GTP [146]. GTP, a classical signaling molecule that regulates various cellular activities as well as an energy supplier for protein synthesis, is elevated in a variety of tumor cells [147]. Although HPRT recovers guanines in salvage pathway, it is unable to meet the demand of malignant tumor cells. Elevated IMPDH activity enhances the ability of cancer cells to synthesize GTP, which provides raw material to supply rapid proliferation.
Abnormally high level of IMPDH is found in multiple malignancies, including OC [28, 148, 149]. Among the two isoforms of human IMPDH, IMPDH2 rather than IMPDH1 is upregulated in a variety of human tumors [29, 149–153]. Researches have reported that high expression of IMPDH2 in OC is associated with different tumor types, lower survival rates and higher stage, which implies a poorer prognosis [29]. IMPDH2 activates PI3K/Akt and Wnt/β-catenin pathways to promote progression or drive EMT processes in diverse cancers [28, 148]. The abnormally increased activity makes IMPDH, especially IMPDH2, a new target for anti-cancer drug development.
Indeed, the use of IMPDH inhibitor as an antineoplastic agent has a long history [154]. A few studies have linked IMPDH inhibitor to apoptosis: downregulation of MEK/ERK pathway leads to Bcl-2 inhibition [155]; downregulation of the Src/PI3K/Akt pathway with inhibition of mTOR to activate Bax and Bak; inhibition of mTORC1 or c-Myc signaling reduces ribosomal RNA synthesis [147, 156, 157]; drive of mitochondria-dependent mechanisms causes apoptosis [158]. Katherine Y et al. found that the addition of thiazole-4-carboxamide adenine dinucleotide, an intracellular active metabolite of tiazofurin, is effective in inhibiting IMPDH activity in OC [28]. Utilizing the IMPDH inhibitor benzamide riboside (BR) makes it possible to observe apoptosis induced by cell cycle arrest due to c-Myc and downstream Cdc25A inhibition [28, 30, 159]. Unfortunately, BR-induced apoptosis is only seen in partial OC cell lines, and the specific regulatory mechanisms between IMPDH inhibitor and apoptosis remain unknown. Furthermore, the development and application of IMPDH inhibitor has been limited by unstable effects, adverse effects at high doses, and discrepancies in IMPDH levels in different tumors [154]. For now, it is still of much value to explore IMPDH such as immunosuppression or biomarkers and this may be an opportunity to reactivate it as an antitumor agent.
Purine nucleoside phosphorylase
Purine nucleoside phosphorylase (PNP, EC 2.4.2.1), which catalyzes the reversible phosphorylation of adenosine, guanosine and inosine, is an important enzyme in salvage and degradation pathway [32]. Among them, homologous E. coli PNP (ePNP) rather than human PNP is able to enrich purine pools with adenosine as a substrate [160]. Gene directed enzyme prodrug therapy (GDEPT), also known as suicide gene therapy, relies on transgenic methods to encode enzymes that convert non-toxic drug precursors into active toxic metabolites to target tumor cells injury [161]. The anti-tumor effect of selective expression of the suicide gene PNP has been observed in a variety of malignancy researches (Fig. 4) [162, 163]. PNP-GDEPT exerts anti-tumor activity in a unique way compared to others. It cleaves 6-methylpurine-2'-deoxyriboside and fludarabine phosphate to toxic purine analogs 6-methylpurine and 2-Fluoroadenine, which inhibits nucleic acid and protein synthesis [164]. These toxins are then released into extracellular matrix and remain active [32]. That is, toxic metabolites can spread to surroundings to achieve significant bystander killing effect even if only few tumor cells express ePNP, which suggests a potential chemotherapeutic advantage [165, 166].
Fig. 4.
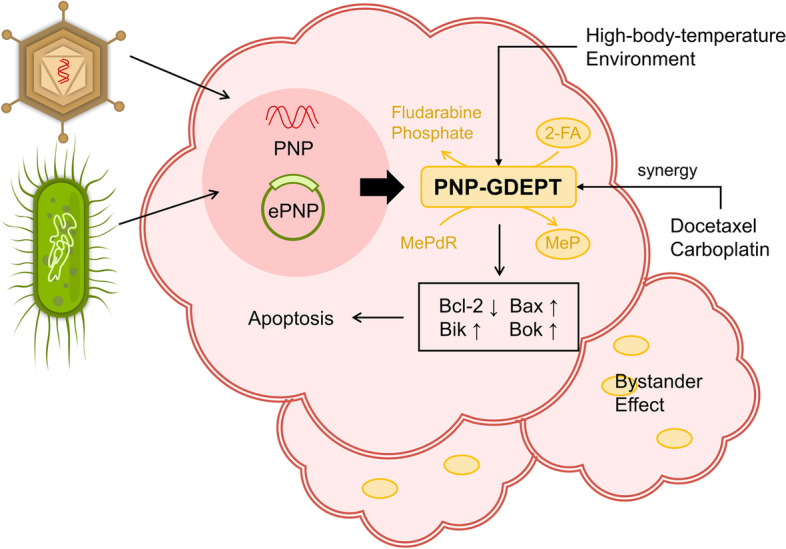
Application of PNP-GDEPT in OC. PNP cleaved MePdR and Fludarabine phosphate to the toxic products MeP and 2-FA. Implementation of GDEPT using ePNP or adenovirus-mediated PNP is able to induce apoptosis in OC cells and exert the bystander effect. PNP-GDEPT acts synergistically with docetaxel and cisplatin and high-body-temperature environment enhance the expression efficiency of ePNP. GDEPT: gene directed enzyme prodrug therapy; ePNP: E. coli PNP; MePdR: 6-methylpurine-2’-deoxyriboside; MeP: 6-methylpurine; 2-FA: 2-Fluoroadenine
A few attempts of this approach have been tested in OC cells. V K Gadi et al. have successfully modeled ePNP bystander killing which exerts significant antitumor effects of OC in vitro and in vivo [166]. Other researchers reported that adenovirus-mediated PNP-GDEPT caused upregulation of Bax, Bik and Bok and downregulation of Bcl-2 [167]. Meanwhile, there was synergy between PNP-GDEPT and docetaxel or carboplatin on platinum-resistant OC cells, implying the potential for reversing drug resistance. Unfortunately, this approach exhibited a low success rate. In this regard, researchers combined PNP-GDEPT with high-body-temperature environment created by human telomerase reverse transcriptase and heat shock elements to improve PNP expression efficiency [168]. Thus, while novel evidence is currently limited, these investigations may provide a new strategy for GDEPT in OC.
Other purine-metabolizing enzyme
Dihydrofolate reductase (DHFR, EC 1.5.1.3) and 5,10-methylenetetrahydrofolate reductase (MTHFR, EC 1.5.1.20) are not conventional purine metabolizing enzymes as they control the metabolism of purines and pyrimidines indirectly by regulating folate synthesis (Fig. 5). Folate acts as a raw material and coenzyme as well as a methyl donor in the biosynthesis of nucleotides [169]. DHFR reduces dihydrofolate to tetrahydrofolate (THF) which subsequently acquires one-carbon units from amino acids to install methyl groups [170]. MTHFR continues to convert methylene-THF to methyl-THF for further participation in nucleic acid biosynthesis. In fact, methyl-THF, methylene-THF and formyl-THF are interconverted to provide one-carbon units for methionine cycle pathway, thymidylate synthesis pathway and purine synthesis pathway in turn [171]. Studies have reported that folate-mediated one-carbon metabolism plays an important supporting role for rapidly proliferating cells, especially tumor cells [169] and DNA methylation is expected to be used as an early diagnostic marker for various malignancies [172–174]. Overall, folate metabolism, the upstream of purine metabolism, serves an important role in tumor development.
Fig. 5.
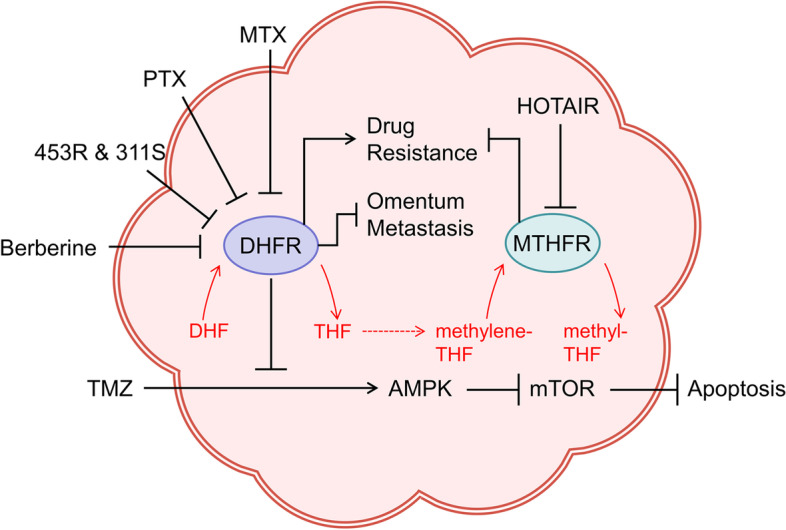
Role of DHFR and MTHFR in OC. DHFR and MTHFR are involved in the formation of important one-carbon units for purine metabolism. DHFR promotes drug resistance and inhibits omentum metastasis, while resisting apoptosis caused by TMZ through AMPK pathway activation and mTOR pathway inhibition. Berberine, PTX, MTX, and some quinoxalines (453R&311S) have been found to act as DHFR inhibitors. MTHFR inhibits FBP expression and enhances drug sensitivity, which is inhibited by HOTAIR. TMZ: temozolomide; PTX: pemetrexed; MTX: methotrexate; 453R: 3-methyl-7-trifluoromethyl-2(R)-[3,4,5-trimethoxyanilino]-quinoxaline; 311S: 3-piperazinilmethyl-2[4(oxymethyl)-phenoxy]-quinoxaline; FBP: folate binding protein
Multiple studies have shown that the overexpression of DHFR is associated with platinum resistance in OC [36–38]. Interestingly, Jia Chen et al. reported that DHFR expression is increased in benign ovarian tumor but decreased in malignant OC with a significant correlation with omentum metastasis [39]. In this case, however, DHFR expression is still significantly higher in platinum-resistant patients than platinum-sensitive patients, highlighting the potential diagnostic value of DHFR in chemoresistance. Actually, the commonly used chemotherapy drug methotrexate (MTX) targets DHFR to block THF production to inhibit rapidly proliferating tumor cells [175]; however, given its toxicity and resistance, there has been a search for new DHFR inhibitors. Pemetrexed, a DHFR and thymidylate synthase inhibitor, activates AMPK and inhibits mTOR signaling pathway by synergizing with temozolomide (TMZ) to obstruct tumor growth [176]. Berberine disturbs folate-metabolizing enzymes including DHFR to reduce the viability of OC cells, especially platinum-resistant cells. In addition, there is evidence that some compounds with quinoxaline structures are not cross-resistant with cisplatin and show remarkably inhibition on OC growth, demonstrating the potential as DHFR inhibitors [37, 177].
As a rate-limiting enzyme for folate metabolism, MTHFR with low viability slows down the synthesis and repair of nucleotides and disrupts the methylation of homocysteine [178, 179]. Defective MTHFR in OC combined with overexpression of high-affinity folate-binding proteins lead to increased folate uptake, implying that low-activity MTHFR is a potential risk factor for OC [40]. Current studies have found that MTHFR single nucleotide polymorphisms (SNPs), particularly C677T polymorphism which affects enzyme activity, are likely to increase tumor risk [41, 180]. Many studies on the association of C677T with susceptibility and risk of OC in Asians rather than whites have been performed [181–184]. However, no effect of C677T polymorphism in Asians was found in a 2012 meta-analysis, possibly because of the limitation of sample size [42]. A possible association between high folic acid intake and low patient survival was reported in research by S C Dixon et al. [43]. However, an interesting finding is that a reduced risk of OC in Chinese with high folate intake was more pronounced in MTHFR 677CC mutation, but no such protection was observed in Australians [185, 186]. This discrepancy could be attributed to the limitations of the sample and ethnicity, emphasizing the need for more research. Recently investigators have examined the effect of MTHFR on the efficacy of 5-fluorouracil (5-FU) chemotherapy in OC patients. Silencing HOX transcript antisense RNA elevates the sensitivity to 5-FU due to decreased MTHFR methylation [187]. Besides, MTHFR SNP has been found correlated with prognosis or hematologic toxicity in 5-FU-treated patients in other human tumors [188–190]. Yet research on this link in OC has remained an under-explored domain and more in-depth explorations are needed.
Purinergic signaling in OC
Key agonist, eATP and eADO
The extracellular purine, mainly eATP and eADO, has active traffic with intracellular signals. In normal physiological environments, high eATP and low eADO levels are maintained through exocytosis or vesicles [191]. Nevertheless, the overall level is subject to strict limitations [192]. The eATP concentration increases dramatically with intracellular ATP in disordered TME (even up to two times the intracellular concentration), which may be due to active release from tumor cells or immune cells, or to abnormal metabolites. As previously described, CD39 and CD73 contribute to the progressive dephosphorylation of eATP to eADO. Continuous stimulation of ATP causes active phospholipase D to promote ATP degradation [193]. Meanwhile, hypoxia effectively stimulates adenosine release [194–196], possibly due to HIF-1α-induced adenosine kinase inhibition that impedes the conversion of ADO to AMP and thus promotes its efflux [197], or possibly as a consequence of retaliatory metabolism [196]. The cascade response results in abnormal levels of both intra- and extracellular ATP and ADO to regulate tumor development via purinergic signaling as autocrine or paracrine messengers [13].
Generally, eATP and eADO are considered as important signals to regulate immunity with pro-inflammatory and anti-inflammatory effects, respectively [198, 199]. The sharp increase in eADO hinders immune cell activation to exert pro-cancer effects and reduces survival of OC patients [200–203]. This effect is closely related to purinergic receptors, which will be described later (Fig. 6). For other malignant features of ovarian cancer, eATP and eADO also play see-saw roles. The eATP activates corresponding purinergic receptors and its co-expressed KCa3.1 channel, triggering subsequent complex electrical membrane responses that contribute to cancer migration [204]. Conversely, eADO supplementation or its analogs have been reported to inhibit the ability of OC to invasion or angiogenesis, partially dependent on the promotion of RhoGDI2 subsequently related gene expression [205, 206]. The effect of adenosine analog supplementation on OC chemoresistance likewise depends on the type of receptor activated [207]. Notably, low doses of adenosine were associated with G0/G1 phase arrest, whereas high concentrations of adenosine induced both early and late apoptosis in a dose-dependent manner by activating the Bcl-2/Bax and caspase-3 pathway in OC cells [208]. These complex effects shift our thinking to future studies of eATP/eADP ratios rather than single substance levels.
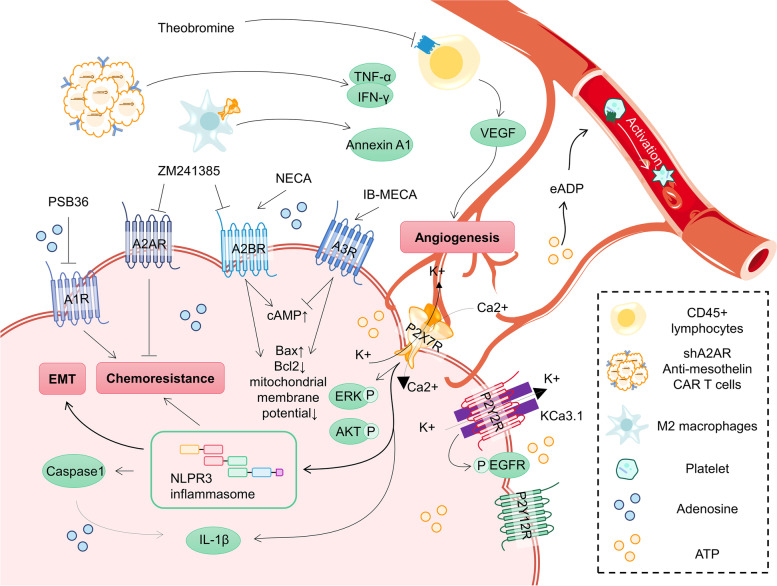
Fig. 6.
Purinergic signaling pathway in OC. Extra- and intracellular adenosine and ATP are key agonists. Purinergic receptors are expressed in a variety of cells in OC TME. Activation or antagonism of these receptors, as well as interaction with other signaling will ultimately affect the progression and malignant features of OC
Purinergic receptors and their therapeutic potential
Previous studies have shown that purinergic receptors are expressed in almost all immune cells, and an increasing number of studies have identified the presence of purinergic receptors in tumor cells [209]. The identified purinergic receptors include P1 receptors that mediate signals triggered by adenosine and P2 receptors that respond to adenine and uridine nucleotides. In general, P1 receptors and P2 receptors mediate pro-inflammatory and anti-inflammatory responses, respectively, which should actually be more dependent on specific receptor types and signaling transduction ratios [210].
P1 receptors
P1 receptors, also known as adenosine receptors, are divided into four subtypes, A1R, A2AR, A2BR and A3R, of which A2R is a key factor in protecting tissues from excessive immune responses and commonly expressed in a variety of human tumours [211–214]. A2AR is a high-affinity receptor involved in the regulation of T-cell function to promote immune evasion, and inhibits the secretion of defensive substances by neutrophils to attenuate the inflammatory response [54, 215]. A2BR appears to have low affinity and is more expressed in macrophages and dendritic cells. Another study found that activation of A2BR was able to interfere with TME via MDSCs to promote cancer growth [216]. Besides, the high-affinity receptors A1R and A3R are considered as immune-promoting adenosine receptors, possibly because of promoting IL-10 expression or inhibiting cAMP production [217, 218]. Therefore, adenosine receptors deserve to be another target of intervention in tumor immunotherapy and hold considerable promise.
A2BR and A3R are the predominantly expressed adenosine receptors in OC [219]. It has been reported that activation of A2BR significantly increases cAMP levels in OC, while activation of A3R exerts the opposite effect, and the other two receptors do not affect cAMP levels, implying that A2BR and A3R are major functional adenosine receptors in OC. In addition, A2BR activation significantly inhibits the migration ability of OC and maintains the epithelioid phenotype [220]. Kaplan–Meier survival analyses showed that OC patients with low A2BR expression levels, especially with early-stage OC, had shorter OS. Interestingly, however, Hajiahmadi et al. found that either the A2BR agonist NECA or the A3R agonist IB-MECA dose-dependently inhibited OC cell viability in a manner that related to the loss of mitochondrial membrane potential to activate apoptosis [221, 222]. Although these findings reflect the potential OC-inhibitory effect of activating A2R and A3R, changes in cAMP, which link essential malignant features such as invasion, metastasis, apoptosis resistance and chemoresistance, are not mentioned [223–225]. The non-dominantly expressed adenosine receptor, A2AR, plays a role in assisting chimeric antigen receptor (CAR) T cells from hindrance by the immunosuppressive microenvironment [226]. Liu G et al. found that anti-mesothelin CAR T cells released more TNF-α and IFN-γ after suppressing A2AR, and extremely enhanced anti-OC efficacy, which provided a new perspective for OC treatment [227]. It should be emphasized that the complex regulatory mechanisms and effects are still not completely clear because different cell status, receptor types, and environmental conditions may cause influences, and further studies to identify the pro- or anti-cancer roles played by adenosine receptors and the specific mechanisms are urgent and necessary.
In fact, targeting adenosine receptors has shown anticancer therapeutic potential in OC. Theobromine, a non-selective adenosine receptor antagonist, inhibits OC angiogenic activity by reducing vascular endothelial growth factor production [228]. Further studies revealed that the inhibition of angiogenesis was associated with A2BR interaction of CD45 lymphocytes [229, 230]. However, blind application of the adenosine analogue ZM241385, an A2R antagonist, may increase the resistance to cisplatin [207], and the A1R antagonist PSB36 brought a sensitizing effect. Overall, activation of adenosine receptors can be anti- and pro-cancer, and the opposing efficacy of their dual action may bring valuable insights for future clinical therapeutic interventions with further studies.
P2 receptors
P2 receptors consist of two subfamilies, P2XRs and P2YRs. P2XRs are ATP-gated non-selective cationic conductance channels with seven isoforms (P2X1-7R) that are expressed in a variety of tumor cells, immune cells, and stromal cells [209]. The eATP is a recognized agonist, and some non-nucleotide compounds have been reported to modulate it as well [231–233]. P2XRs, especially P2X7R, are the ones that have been focused on for their multiple roles in mediating tumor growth, metastasis, invasion, drug resistance, and death, in addition to pro- and anti-inflammatory effects [234–238]. Overexpression of P2X7R in OC contributes to cell proliferation and viability [239, 240]. Vázquez-Cuevas FG et al. found that activation of P2X7R caused an increase in intracellular Ca(2+) concentration and phosphorylation of ERK and AKT, but did not cause apoptosis [241]. Notably, eATP, or actually mainly the activation of P2X7R, promotes NLPR3 inflammasome activation and assembly, followed by activation of caspase 1, which is dependent on ATP induction and subsequently K(+) efflux [75]. Meanwhile, P2X7R drives IL-1β maturation in response to activation of the NLRP3 inflammasome [242]. It was shown that excessive NLRP3 in OC promotes EMT [243, 244] and mediates gemcitabine resistance [245] through the Wnt/β-catenin signaling pathway, and is associated with poorer OS [246]. Once NLRP3 is absent, the expression of P2X7R will be promoted, which contributes to cancer growth as a negative feedback loop [247]. Co-localization of P2X7R/NLRP3 in the adipocyte plasma membrane of omental tissue of OC patients implies its possible contribution to tumor metastasis [248]. However, it is noteworthy that antagonizing P2X7R inhibits pyroptosis via NLRP3/caspase1 and promotes apoptosis mediated by Bcl-2/caspase9/caspase3 pathway at the same time [249]. In addition, P2X7R stimulation in alternative activated M2 macrophages has been reported to release abundant anti-inflammatory proteins, suggesting a contribution to the inflammation resolution [238]. The P2X7R/NLRP3 complex lacks detailed studies in OC, and the pro- and anti-inflammatory and even pro- and anti-cancer responses it mediates are incompletely understood as well. The balance between mutually inhibited pyroptosis and apoptosis may bring a fresh perspective to precisely fight OC.
P2YRs are G protein-coupled and contain a total of eight isoforms (P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R and P2Y14R) in mammals. Compared to P2XRs, P2YRs are more sensitive to slight changes in local nucleotide or agonist concentrations [250]. These members are activated by different nucleotides, and only P2Y2R and P2Y11R are responsive to ATP [251]. The prominent contribution of P2YRs in tumor growth and metastasis cannot be ignored, which is associated with the regulation of intracellular Ca(2+) concentration and subsequently cAMP changes. In this regard, P2Y1R, P2Y2R, P2Y4R, P2Y6R bind to Gq-dominated protein subunits to activate PLCβ/IP3/DAG signaling pathway to increase intracellular Ca(2+) concentration, which may be affected by the acidic intracellular environment [251, 252]. P2Y12-14R couple to Gi/o to inhibit adenylate cyclase activity to reduce intracellular cAMP levels. As previously described, low levels of ATP stimulate P2Y2R, and activate co-localized KCa3.1 channels to promote migration of OC cells [204]. Martínez-Ramírez AS et al. suggested that the contribution of P2Y2R to OC cell migration may derive from an interaction with EGFR [253]. In addition, there is an increasing number of studies reporting the special status of platelets in OC. OC cells induce platelets activation, and in turn, platelets stimulate OC growth [254, 255]. Cho MS et al. revealed that activation of P2Y12R on platelets by ticagrelor contributes to OC growth, for which eADP secreted by OC cells is an important activator [256]. P2Y2R and P2Y12R may play as pivots in OC progression, however, more specific mechanisms need to be further investigated.
Altogether, the understanding of purinergic receptors in OC is currently incompletely clear. More in-depth studies need to be conducted to map a detailed purinergic signaling network in OC to provide novel insights for precise treatment.
Purine antimetabolites
Purine antimetabolites are one of the classical approaches to antitumor, which are chemical analogues of purine metabolism substrates and block purine metabolism by two mechanisms: mimic physiological substrates and compete for the same metabolic enzyme binding site to interfere with biochemical reaction rates resulting in reduced production of normal metabolites [257]; bind to the active site and generate inactive or even toxic metabolites which cause DNA damage and induce apoptosis [258]. Purine antimetabolites are mainly classified into thiopurines and purine deoxynucleoside analogues according to the structure and mechanism [257]. Current researches on OC focuses on 6-thioguanine (6-TG), 6-mercaptopurine (6-MP), MTX and fludarabine (Table 2). Besides, here we classify clopidogrel, which targets purinergic receptors, as an atypical purine antimetabolite.
Table 2.
The basic information and effects of 6-thioguanine, 6-mercaptopurine, methotrexate, fludarabine and clopidogrel in OC
| CAS number | Molecular Formula | Target | FDA-Approved Date | Effects in OC | Ref | |
|---|---|---|---|---|---|---|
| 6-Thioguanine | 154–42-7 | C5H5N5S | PRPP amidotransferase | 1966 | Not exactly | [259] |
| 6-Mercaptopurine | 50–44-2 | C5H4N4S | PRPP amidotransferase | 1953 | Antitumor | [260] |
| Methotrexate | 59–05-2 | C20H22N8O5 | DHFR | 1971 | Antitumor | [261, 262] |
| Fludarabine | 21,679–14-1 | C10H12FN5O4 | Nucleotide reductase; DNA polymerase; DNA ligase | 1991 | Antitumor | [263–266] |
| Clopidogrel | 113,665–84-2 | C16H16ClNO2S | P2Y12R (in platelets) | 1997 | Not exactly | [267–269] |
6-TG is a guanosine analogue that is converted by HPRT to toxic 6-thioguanine nucleotides, which exert pharmacological effects by binding to DNA [11]. It has been suggested that 6-TG may be an effective therapeutic agent for BRCA-deficient tumors, especially for platinum-resistant and PARP inhibitor-resistant tumors [259]. This is due to the fact that homologous recombination is reactivated in resistant cells without complete recovery from the damage caused by 6-TG. Unfortunately, long-term treatment is not recommended because of its hepatotoxicity [270]. 6-MP is a structural analogue of hypoxanthine with a similar mechanism as 6-TG and a lower toxicity [271]. After treatment with 6-MP and MTX for two months, 30% of OC patients with BRCA mutations showed stable disease status afterwards, and 14% showed longer-term clinical benefit [260]. Nevertheless, neutropenia and anemia are the most common adverse effects. A phase II clinical trial continues to evaluate the safety of 6-MP, which may provide an attack on BRCA-deficient OC [270]. The mechanism of MTX resistance to purine metabolism was described above. MTX was recently reported to induce apoptosis by increasing ROS, inducing DNA damage and modulating mitochondrial membrane potential [261]. Oral low-dose MTX and cyclophosphamide may serve as maintenance therapy after chemotherapy for patients with advanced OC [272]. Furthermore, Courtney A Penn et al. found that therapeutic combination of MTX and nanoparticle targeting TAM for OC exhibited superior activity over cisplatin alone [262]. At the same time, MTX ovarian toxicity cannot be ignored [273]. As for fludarabine, a fluorinated nucleotide analogue of vidarabine, is relatively resistant to ADA inactivation [274]. It is first converted to 9-β-D-arabino-furanosyl-2-fluoradenine (F-ara-A) that can be taken up by cells, and then to F-ara-A triphosphate which inhibits nucleotide reductase, DNA polymerase and DNA ligase, and ultimately causes impaired DNA synthesis and apoptosis [12]. Fludarabine was found to reduce OC migration and adhesion by inhibiting the FAK/STAT1 pathway [263] and was able to inhibit VEGF via Hif-1α and PI3K/AKT signaling pathways to arrest the progression of OC [264]. Moreover, it exerts synergistic effects with cisplatin, seemingly supporting its potential as a modulator of chemotherapeutic agents [265]. Two clinical trials utilize fludarabine as an immunosuppressant to deplete lymphocytes for allogeneic NK cell therapy in OC patients [275, 276]. There were also some important differences in other purine antimetabolites that contribute to the development of OC [277]. These conflicting roles indicate the need for more research and clinical trials on purine antimetabolites in OC. Additionally, interventions on purinergic signaling are the result of atypical purine antimetabolites, which lack sufficient evidence for therapeutic trials in OC. High-dose clopidogrel has been shown to cause high-level P2Y12 blockade [267]. A concern is that clopidogrel may increase the risk of toxicity when used with paclitaxel, as was found in a 60-year-old patient with OC [268]. A higher risk is also seen in the report by Park SH et al. [269]. It remains to be considered whether interventions on purinergic signaling pathways can provide benefits to OC patients.
Conclusions
Homeostasis of purine pools in vitro and in vivo is essential for the maintenance of healthy state and normal function of cells. The depletion of purine in normal cells can be saved by an increase in purine synthesis [278]. In some cases, excessive purine consumption is beyond the capacity of synthesis and is unable to be compensated. For example, excessive DNA synthesis in S1 phase leads to a dramatic increase in purine consumption, or a plethora of purine neurotransmitters are released during the active phase of certain neurons [279]. Imbalance of purine pools contributes to dysregulation of genomic stability and ultimately to metabolic disease or tumor development [280, 281]. Herein, we summarize the role of major purine-metabolizing enzymes, describe purinergic signaling pathways, outline the roles and mechanisms of partial purine antimetabolites and point out potential therapeutic strategies for targeting purine metabolism in OC.
In fact, purine-metabolizing enzymes also participate in other biological processes. For instance, CD39 and ADA are involved in immune regulation [18, 58, 59, 83]; IMPDH carries out the function of a transcription factor [282]; SAM Domain And HD Domain-Containing Protein-1 assists in regulating the cell cycle [283] and phosphoribosylaminoimidazole succinocarboxamide synthetase is correlated with DNA damage repair [284, 285]. It can help to identify new interventions in tumor process if we focus on purine metabolic processes and understand the mechanisms of action of the relevant enzymes as well as the interaction with other biological processes. Additionally, uncontrolled tumor proliferation due to excessive purine synthesis plays a role in chemoresistance, which may be sensitized by inhibitors of purine metabolism [286]. Purine antimetabolites and other anti-purine-metabolizing agents may provide an additional line of attack that would be a promising strategy to overcome tumor resistance in combination with conventional chemotherapy.
However, it cannot be denied that purine metabolism in OC has not been comprehensively studied and is still at a relatively superficial stage. So far, most studies have only briefly described purine-metabolizing enzyme changes and subsequent cell apoptosis, without exploring the relationship and mechanisms between the two in detail. Several studies even showed contradictory treatment results, probably caused by the lack of samples. Evidence above casts doubt on the credibility of the link between the two. Besides, it is difficult to determine whether adenosine disorders are caused by enzyme effect or an action of Adenosine itself, or are related to adenosine receptors. Likewise, it is a critical option to activate or antagonize purinergic receptors in OC. The interaction of purinergic signaling with hypoxia or NLRP3 inflammasomes is also a valuable reflection on how to intervene to obtain maximum benefit. Another limitation is that basic researches on OC treatment options targeting purine metabolizing enzymes or purinergic signaling pathways are currently stagnant and lack the support of clinical research results. Therefore, it is necessary to focus on the association of purine metabolism with other human tumors, which may open up new possibilities for OC research.
Recent studies have reported that multiple kinases interfere with tumor progression by affecting purine de novo pathway via regulation of important transcription factors or intervention with rate-limiting enzymes: DYRK3 regulates ATF4 transcriptional activity and inhibits PPAT to suppress hepatocellular carcinoma proliferation and metastasis [287]; UHMK1 modulates the NCOA3/ATF4 axis and may activates ATIC to promote gastric cancer development [288]; CLK3 stabilizes the USP13/Fbxl14/c-Myc axis to enhance cholangiocarcinoma aggressiveness [289]. These outcomes broaden the horizon, including targets beyond the enzymes that are directly involved in purine metabolism.
In any case, more researches are needed to understand the mechanisms of aberrant purine metabolism in OC. In-depth knowledge of purine metabolic processes may help to better understand cancer-related metabolic reprogramming and develop new inhibitors accordingly, which presents exciting opportunities for OC therapy. We will investigate this in more depth in the future and believe that this may become a promising novel strategy for OC therapy.
Acknowledgements
Not applicable.
Abbreviations
- ADA
Adenosine deaminase
- ADAR
Adenosine deaminases acting on RNA
- ADSS
Adenylosuccinate synthase
- AK
Adenylate kinase
- Ang
Angiopoietin
- APRT
Adenine phosphoribosyltransferase
- BR
Benzamide riboside
- CAR
Chimeric antigen receptor
- CCNI
Cyclin I
- CD39
Ectonucleoside triphosphate diphosphohydrolase
- CD73
Ectosolic-5′-nucleotidase
- DDP4
Dipeptidyl peptidase 4
- DHFR
Dihydrofolate reductase
- eADO
Extracellular adenosine
- eAMP
Extracellular AMP
- eATP
Extracellular ATP
- EMT
Epithelial–mesenchymal transition
- ePNP
E. coli PNP
- F-ara-A
9-β-D-arabino-furanosyl-2-fluoradenine
- GDEPT
Gene directed enzyme prodrug therapy
- HPRT
Hypoxanthine guanine phosphoribosyltransferase
- IMP
Inosine monophosphate
- IMPDH
Inosine monophosphate dehydrogenase
- MDSC
Myeloid-deriver suppressor cell
- MTHFR
5,10-Methylenetetrahydrofolate reductase
- MTX
Methotrexate
- OC
Ovarian cancer
- PD-1
Programmed death-1
- PNP
Purine nucleoside phosphorylase
- PRPP
5-Phosphoribosyl-1-pyrophosphate
- SNP
Single nucleotide polymorphisms
- TAM
Tumor-associated macrophage
- THF
Tetrahydrofolate
- TME
Tumor microenvironment
- TMZ
Temozolomide
- UA
Uric acid
- XMP
Xanthosine monophosphate
- XO
Xanthine oxidase
- 5-FU
5-Fluorouracil
- 6-MP
6-Mercaptopurine
- 6-TG
6-Thioguanine
Authors’ contributions
J Liu and S Hong provided ideas and wrote articles. J Yang, X Zhang and L Hong reviewed and proofread the article. Y Wang, H Wang and J Peng checked and modified the figures and tables. J Liu and S Hong contributed equally to this work. The author(s) read and approved the final manuscript.
Funding
Grant sponsor: Hubei Province's Outstanding Medical Academic Leader Programme and the Fundamental Research Funds for the Central Universities; Grant numbers: 2042021kf0125.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingchun Liu and Shasha Hong contributed equally to this work.
References
- 1.Doherty JA, Peres LC, Wang C, Way GP, Greene CS, Schildkraut JM. Challenges and opportunities in studying the epidemiology of ovarian cancer subtypes. Curr Epidemiol Rep. 2017;4(3):211–220. doi: 10.1007/s40471-017-0115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;10(4):211–224. doi: 10.1038/nrclinonc.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess JR, Greenberg NA. The role of nucleotides in the immune and gastrointestinal systems: potential clinical applications. Nutr Clin Pract. 2012;27(2):281–294. doi: 10.1177/0884533611434933. [DOI] [PubMed] [Google Scholar]
- 5.De Vitto H, Arachchige DB, Richardson BC, French JB. The intersection of purine and mitochondrial metabolism in cancer. Cells-Basel. 2021;10(10):2603. doi: 10.3390/cells10102603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camici M, Garcia-Gil M, Pesi R, Allegrini S, Tozzi MG. Purine-metabolising enzymes and apoptosis in cancer. Cancers (Basel) 2019;11(9):1354. doi: 10.3390/cancers11091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meuth M. The molecular basis of mutations induced by deoxyribonucleoside triphosphate pool imbalances in mammalian cells. Exp Cell Res. 1989;181(2):305–316. doi: 10.1016/0014-4827(89)90090-6. [DOI] [PubMed] [Google Scholar]
- 8.Reilly NM, Novara L, Di Nicolantonio F, Bardelli A. Exploiting DNA repair defects in colorectal cancer. Mol Oncol. 2019;13(4):681–700. doi: 10.1002/1878-0261.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br Med Bull. 2006;79–80:153–170. doi: 10.1093/bmb/ldl020. [DOI] [PubMed] [Google Scholar]
- 10.D’Almeida SM, Kauffenstein G, Roy C, Basset L, Papargyris L, Henrion D, Catros V, Ifrah N, Descamps P, Croue A, et al. The ecto-ATPDase CD39 is involved in the acquisition of the immunoregulatory phenotype by M-CSF-macrophages and ovarian cancer tumor-associated macrophages: Regulatory role of IL-27. Oncoimmunology. 2016;5(7):e1178025. [DOI] [PMC free article] [PubMed]
- 11.Coulthard S, Hogarth L. The thiopurines: an update. Invest New Drugs. 2005;23(6):523–532. doi: 10.1007/s10637-005-4020-8. [DOI] [PubMed] [Google Scholar]
- 12.Anderson VR, Perry CM. Fludarabine: a review of its use in non-Hodgkin’s lymphoma. Drugs. 2007;67(11):1633–55. [DOI] [PubMed]
- 13.Campos-Contreras A, Diaz-Munoz M, Vazquez-Cuevas FG. Purinergic signaling in the hallmarks of cancer. Cells-Basel. 2020;9(7):1612. doi: 10.3390/cells9071612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, Zhang C, Yue D, Qin G, Zhang T, et al. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 2018;78(7):1779–1791. doi: 10.1158/0008-5472.CAN-17-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montalban DBI, Penski C, Schlahsa L, Stein RG, Diessner J, Wockel A, Dietl J, Lutz MB, Mittelbronn M, Wischhusen J, et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages - a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer. 2016;4:49. doi: 10.1186/s40425-016-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, Chen GM, Gendoo DM, Haibe-Kains B, Karn T, et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015;75(21):4494–4503. doi: 10.1158/0008-5472.CAN-14-3569. [DOI] [PubMed] [Google Scholar]
- 17.Oh HK, Sin JI, Choi J, Park SH, Lee TS, Choi YS. Overexpression of CD73 in epithelial ovarian carcinoma is associated with better prognosis, lower stage, better differentiation and lower regulatory T cell infiltration. J Gynecol Oncol. 2012;23(4):274–281. doi: 10.3802/jgo.2012.23.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gines S, Marino M, Mallol J, Canela EI, Morimoto C, Callebaut C, Hovanessian A, Casado V, Lluis C, Franco R. Regulation of epithelial and lymphocyte cell adhesion by adenosine deaminase-CD26 interaction. Biochem J. 2002;361(Pt 2):203–209. doi: 10.1042/bj3610203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urunsak IF, Gulec UK, Paydas S, Seydaoglu G, Guzel AB, Vardar MA. Adenosine deaminase activity in patients with ovarian neoplasms. Arch Gynecol Obstet. 2012;286(1):155–159. doi: 10.1007/s00404-012-2279-5. [DOI] [PubMed] [Google Scholar]
- 20.Zavialov AV, Gracia E, Glaichenhaus N, Franco R, Zavialov AV, Lauvau G. Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J Leukoc Biol. 2010;88(2):279–290. doi: 10.1189/jlb.1109764. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Fritsche J, Roszik J, Williams LJ, Peng X, Chiu Y, Tsou CC, Hoffgaard F, Goldfinger V, Schoor O, et al. RNA editing derived epitopes function as cancer antigens to elicit immune responses. Nat Commun. 2018;9(1):3919. doi: 10.1038/s41467-018-06405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson JB, Samuel CE. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol Cell Biol. 1995;15(10):5376–5388. doi: 10.1128/MCB.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barraud P, Banerjee S, Mohamed WI, Jantsch MF, Allain FH. A bimodular nuclear localization signal assembled via an extended double-stranded RNA-binding domain acts as an RNA-sensing signal for transportin 1. Proc Natl Acad Sci U S A. 2014;111(18):E1852–E1861. doi: 10.1073/pnas.1323698111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan TY, Sedmik J, Fitzgerald MP, Halevy RS, Keegan LP, Helbig I, Basel-Salmon L, Cohen L, Straussberg R, Chung WK, et al. Bi-allelic ADARB1 variants associated with microcephaly, intellectual disability, and seizures. Am J Hum Genet. 2020;106(4):467–483. doi: 10.1016/j.ajhg.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XY, Zhou LL, Jiao Y, Li YQ, Guan YN, Zhao YC, Zheng LW. Adenylate kinase 7 is a prognostic indicator of overall survival in ovarian cancer. Medicine (Baltimore) 2021;100(1):e24134. doi: 10.1097/MD.0000000000024134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang M, Qin X, Wang Y, Mao F. Identification of AK4 as a novel therapeutic target for serous ovarian cancer. Oncol Lett. 2020;20(6):346. doi: 10.3892/ol.2020.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan H, Wu C, Huang B, Jin L, Jiang X. MiR-3666 serves as a tumor suppressor in ovarian carcinoma by down-regulating AK4 via targeting STAT3. Cancer Biomark. 2021;30(4):355–363. doi: 10.3233/CBM-190538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Look KY, Sutton GP, Natsumeda Y, Eble JN, Stehman FB, Ehrlich CE, Olah E, Prajda N, Bosze P, Eckhardt S, et al. Inhibition by tiazofurin of inosine 5’-phosphate dehydrogenase (IMP DH) activity in extracts of ovarian carcinomas. Gynecol Oncol. 1992;47(1):66–70. [DOI] [PubMed]
- 29.Tian Y, Zhang J, Chen L, Zhang X. The expression and prognostic role of IMPDH2 in ovarian cancer. Ann Diagn Pathol. 2020;46:151511. doi: 10.1016/j.anndiagpath.2020.151511. [DOI] [PubMed] [Google Scholar]
- 30.Hunakova L, Bies J, Sedlak J, Duraj J, Jakubikova J, Takacsova X, Novotny L, Chorvath B. Differential sensitivity of ovarian carcinoma cell lines to apoptosis induced by the IMPDH inhibitor benzamide riboside. Neoplasma. 2000;47(5):274–279. [PubMed] [Google Scholar]
- 31.Duong-Ly KC, Kuo YM, Johnson MC, Cote JM, Kollman JM, Soboloff J, Rall GF, Andrews AJ, Peterson JR. T cell activation triggers reversible inosine-5’-monophosphate dehydrogenase assembly. J Cell Sci. 2018;131(17):jcs223289. [DOI] [PMC free article] [PubMed]
- 32.Giuliani P, Zuccarini M, Buccella S, Pena-Altamira LE, Polazzi E, Virgili M, Monti B, Poli A, Rathbone MP, Di Iorio P, et al. Evidence for purine nucleoside phosphorylase (PNP) release from rat C6 glioma cells. J Neurochem. 2017;141(2):208–221. doi: 10.1111/jnc.14004. [DOI] [PubMed] [Google Scholar]
- 33.Ofinran O, Bose U, Hay D, Abdul S, Tufatelli C, Khan R. Selection of suitable reference genes for gene expression studies in normal human ovarian tissues, borderline ovarian tumours and ovarian cancer. Mol Med Rep. 2016;14(6):5725–5731. doi: 10.3892/mmr.2016.5933. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Wang Y, Han N, Wang X, Ruan M. HPRT promotes proliferation and metastasis in head and neck squamous cell carcinoma through direct interaction with STAT3. Exp Cell Res. 2021;399(1):112424. doi: 10.1016/j.yexcr.2020.112424. [DOI] [PubMed] [Google Scholar]
- 35.Keough DT, Hockova D, Holy A, Naesens LM, Skinner-Adams TS, Jersey J, Guddat LW. Inhibition of hypoxanthine-guanine phosphoribosyltransferase by acyclic nucleoside phosphonates: a new class of antimalarial therapeutics. J Med Chem. 2009;52(14):4391–4399. doi: 10.1021/jm900267n. [DOI] [PubMed] [Google Scholar]
- 36.Scanlon KJ, Kashani-Sabet M. Elevated expression of thymidylate synthase cycle genes in cisplatin-resistant human ovarian carcinoma A2780 cells. Proc Natl Acad Sci U S A. 1988;85(3):650–653. doi: 10.1073/pnas.85.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marverti G, Ligabue A, Paglietti G, Corona P, Piras S, Vitale G, Guerrieri D, Luciani R, Costi MP, Frassineti C, et al. Collateral sensitivity to novel thymidylate synthase inhibitors correlates with folate cycle enzymes impairment in cisplatin-resistant human ovarian cancer cells. Eur J Pharmacol. 2009;615(1–3):17–26. doi: 10.1016/j.ejphar.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 38.Trent JM, Buick RN, Olson S, Horns RJ, Schimke RT. Cytologic evidence for gene amplification in methotrexate-resistant cells obtained from a patient with ovarian adenocarcinoma. J Clin Oncol. 1984;2(1):8–15. doi: 10.1200/JCO.1984.2.1.8. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Li L. Aberrant Expression of Folate Metabolism Enzymes and Its Diagnosis and Survival Prediction in Ovarian Carcinoma. Anal Cell Pathol (Amst) 2019;2019:1438628. doi: 10.1155/2019/1438628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viel A, Dall’Agnese L, Simone F, Canzonieri V, Capozzi E, Visentin MC, Valle R, Boiocchi M. Loss of heterozygosity at the 5,10-methylenetetrahydrofolate reductase locus in human ovarian carcinomas. Br J Cancer. 1997;75(8):1105–10. [DOI] [PMC free article] [PubMed]
- 41.Gong JM, Shen Y, Shan WW, He YX. The association between MTHFR polymorphism and cervical cancer. Sci Rep. 2018;8(1):7244. doi: 10.1038/s41598-018-25726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Liao SG, Wang YJ. MTHFR polymorphisms and ovarian cancer risk: a meta-analysis. Mol Biol Rep. 2012;39(11):9863–9868. doi: 10.1007/s11033-012-1852-0. [DOI] [PubMed] [Google Scholar]
- 43.Dixon SC, Ibiebele TI, Protani MM, Beesley J, Defazio A, Crandon AJ, Gard GB, Rome RM, Webb PM, Nagle CM. Dietary folate and related micronutrients, folate-metabolising genes, and ovarian cancer survival. Gynecol Oncol. 2014;132(3):566–572. doi: 10.1016/j.ygyno.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 44.Natsumeda Y, Prajda N, Donohue JP, Glover JL, Weber G. Enzymic capacities of purine de Novo and salvage pathways for nucleotide synthesis in normal and neoplastic tissues. Cancer Res. 1984;44(6):2475–2479. [PubMed] [Google Scholar]
- 45.Yamaoka T, Kondo M, Honda S, Iwahana H, Moritani M, Ii S, Yoshimoto K, Itakura M. Amidophosphoribosyltransferase limits the rate of cell growth-linked de novo purine biosynthesis in the presence of constant capacity of salvage purine biosynthesis. J Biol Chem. 1997;272(28):17719–17725. doi: 10.1074/jbc.272.28.17719. [DOI] [PubMed] [Google Scholar]
- 46.Yin J, Ren W, Huang X, Deng J, Li T, Yin Y. Potential Mechanisms Connecting Purine Metabolism and Cancer Therapy. Front Immunol. 2018;9:1697. doi: 10.3389/fimmu.2018.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pareek V, Pedley AM, Benkovic SJ. Human de novo purine biosynthesis. Crit Rev Biochem Mol Biol. 2021;56(1):1–16. doi: 10.1080/10409238.2020.1832438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedley AM, Benkovic SJ. A New view into the regulation of purine metabolism: the purinosome. Trends Biochem Sci. 2017;42(2):141–154. doi: 10.1016/j.tibs.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H, Chiaro CR, Zhang L, Smith PB, Chan CY, Pedley AM, Pugh RJ, French JB, Patterson AD, Benkovic SJ. Quantitative analysis of purine nucleotides indicates that purinosomes increase de novo purine biosynthesis. J Biol Chem. 2015;290(11):6705–6713. doi: 10.1074/jbc.M114.628701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320(5872):103–106. doi: 10.1126/science.1152241. [DOI] [PubMed] [Google Scholar]
- 51.Miyazono KI, Ishino S, Makita N, Ito T, Ishino Y, Tanokura M. Crystal structure of the novel lesion-specific endonuclease PfuEndoQ from Pyrococcus furiosus. Nucleic Acids Res. 2018;46(9):4807–4818. doi: 10.1093/nar/gky261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moesta AK, Li XY, Smyth MJ. Targeting CD39 in cancer. Nat Rev Immunol. 2020;20(12):739–755. doi: 10.1038/s41577-020-0376-4. [DOI] [PubMed] [Google Scholar]
- 53.Regateiro FS, Cobbold SP, Waldmann H. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol. 2013;171(1):1–7. doi: 10.1111/j.1365-2249.2012.04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103(35):13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young A, Ngiow SF, Gao Y, Patch AM, Barkauskas DS, Messaoudene M, Lin G, Coudert JD, Stannard KA, Zitvogel L, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78(4):1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 56.Zheng X, Wang D. The adenosine A2A receptor agonist accelerates bone healing and adjusts Treg/Th17 cell balance through interleukin 6. Biomed Res Int. 2020;2020:2603873. doi: 10.1155/2020/2603873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SJ, Gause WC, Leibovich SJ, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. Faseb J. 2012;26(1):376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kortekaas KE, Santegoets SJ, Sturm G, Ehsan I, van Egmond SL, Finotello F, Trajanoski Z, Welters M, van Poelgeest M, van der Burg SH. CD39 identifies the CD4(+) tumor-specific T-cell population in human cancer. Cancer Immunol Res. 2020;8(10):1311–1321. doi: 10.1158/2326-6066.CIR-20-0270. [DOI] [PubMed] [Google Scholar]
- 59.Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, Mcdermott JE, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9(1):2724. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ni X, Wan W, Ma J, Liu X, Zheng B, He Z, Yang W, Huang L. A Novel Prognostic Biomarker of Luminal Breast Cancer: High CD39 Expression Is Related to Poor Survival. Front Genet. 2021;12:682503. doi: 10.3389/fgene.2021.682503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giatromanolaki A, Kouroupi M, Pouliliou S, Mitrakas A, Hasan F, Pappa A, Koukourakis MI. Ectonucleotidase CD73 and CD39 expression in non-small cell lung cancer relates to hypoxia and immunosuppressive pathways. Life Sci. 2020;259:118389. doi: 10.1016/j.lfs.2020.118389. [DOI] [PubMed] [Google Scholar]
- 62.Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14(10):1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z, Zhou B, Wu Y, Gao Q, Zhang K, Song Y, Zhang L, Xi M. Prognostic value of IL-27 polymorphisms and the susceptibility to epithelial ovarian cancer in a Chinese population. Immunogenetics. 2014;66(2):85–92. doi: 10.1007/s00251-013-0753-2. [DOI] [PubMed] [Google Scholar]
- 64.Lupia M, Angiolini F, Bertalot G, Freddi S, Sachsenmeier KF, Chisci E, Kutryb-Zajac B, Confalonieri S, Smolenski RT, Giovannoni R, et al. CD73 regulates stemness and epithelial-mesenchymal transition in ovarian cancer-initiating cells. Stem Cell Rep. 2018;10(4):1412–1425. doi: 10.1016/j.stemcr.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nevedomskaya E, Perryman R, Solanki S, Syed N, Mayboroda OA, Keun HC. A systems oncology approach identifies NT5E as a key metabolic regulator in tumor cells and modulator of platinum sensitivity. J Proteome Res. 2016;15(1):280–290. doi: 10.1021/acs.jproteome.5b00793. [DOI] [PubMed] [Google Scholar]
- 66.Ma XL, Shen MN, Hu B, Wang BL, Yang WJ, Lv LH, Wang H, Zhou Y, Jin AL, Sun YF, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110beta and predicts poor prognosis. J Hematol Oncol. 2019;12(1):37. doi: 10.1186/s13045-019-0724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma XL, Hu B, Tang WG, Xie SH, Ren N, Guo L, Lu RQ. CD73 sustained cancer-stem-cell traits by promoting SOX9 expression and stability in hepatocellular carcinoma. J Hematol Oncol. 2020;13(1):11. doi: 10.1186/s13045-020-0845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou L, Jia S, Chen Y, Wang W, Wu Z, Yu W, Zhang M, Ding G, Cao L. The distinct role of CD73 in the progression of pancreatic cancer. J Mol Med (Berl) 2019;97(6):803–815. doi: 10.1007/s00109-018-01742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu J, Wang X, Lu Q, Wang J, Li L, Liao X, Zhu W, Lv L, Zhi X, Yu J, et al. Extracellular 5’-nucleotidase (CD73) promotes human breast cancer cells growth through AKT/GSK-3beta/beta-catenin/cyclinD1 signaling pathway. Int J Cancer. 2018;142(5):959–67. [DOI] [PubMed]
- 70.Hausler SF, Montalban DBI, Strohschein J, Chandran PA, Engel JB, Honig A, Ossadnik M, Horn E, Fischer B, Krockenberger M, et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother. 2011;60(10):1405–1418. doi: 10.1007/s00262-011-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang F, Yu M, Cavanagh MM, Hutter SJ, Qi Q, Ye Z, Le Saux S, Sultan W, Turgano E, Dekker CL, et al. Expression of CD39 on activated t cells impairs their survival in older individuals. Cell Rep. 2016;14(5):1218–1231. doi: 10.1016/j.celrep.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, Wang L, Chen X, Li L, Li Y, Ping Y, Huang L, Yue D, Zhang Z, Wang F, et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-beta-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology. 2017;6(6):e1320011. doi: 10.1080/2162402X.2017.1320011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Limagne E, Euvrard R, Thibaudin M, Rebe C, Derangere V, Chevriaux A, Boidot R, Vegran F, Bonnefoy N, Vincent J, et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res. 2016;76(18):5241–5252. doi: 10.1158/0008-5472.CAN-15-3164. [DOI] [PubMed] [Google Scholar]
- 74.Li XY, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, Madore J, Lepletier A, Aguilera AR, Sundarrajan A, et al. Targeting CD39 in cancer reveals an extracellular ATP- and inflammasome-driven tumor immunity. Cancer Discov. 2019;9(12):1754–1773. doi: 10.1158/2159-8290.CD-19-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li H, Lv M, Qiao B, Li X. Blockade pf CD73/adenosine axis improves the therapeutic efficacy of docetaxel in epithelial ovarian cancer. Arch Gynecol Obstet. 2019;299(6):1737–1746. doi: 10.1007/s00404-019-05139-3. [DOI] [PubMed] [Google Scholar]
- 76.Salmaninejad A, Khoramshahi V, Azani A, Soltaninejad E, Aslani S, Zamani MR, Zal M, Nesaei A, Hosseini SM. PD-1 and cancer: molecular mechanisms and polymorphisms. Immunogenetics. 2018;70(2):73–86. doi: 10.1007/s00251-017-1015-5. [DOI] [PubMed] [Google Scholar]
- 77.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19(20):5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 78.Lindley ER, Pisoni RL. Demonstration of adenosine deaminase activity in human fibroblast lysosomes. Biochem J. 1993;290(Pt 2):457–462. doi: 10.1042/bj2900457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eltzschig HK, Faigle M, Knapp S, Karhausen J, Ibla J, Rosenberger P, Odegard KC, Laussen PC, Thompson LF, Colgan SP. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006;108(5):1602–1610. doi: 10.1182/blood-2006-02-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Franco R, Pacheco R, Gatell JM, Gallart T, Lluis C. Enzymatic and extraenzymatic role of adenosine deaminase 1 in T-cell-dendritic cell contacts and in alterations of the immune function. Crit Rev Immunol. 2007;27(6):495–509. doi: 10.1615/CritRevImmunol.v27.i6.10. [DOI] [PubMed] [Google Scholar]
- 81.Zhong J, Rao X, Deiuliis J, Braunstein Z, Narula V, Hazey J, Mikami D, Needleman B, Satoskar AR, Rajagopalan S. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes. 2013;62(1):149–157. doi: 10.2337/db12-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Enz N, Vliegen G, De Meester I, Jungraithmayr W. CD26/DPP4 - a potential biomarker and target for cancer therapy. Pharmacol Ther. 2019;198:135–159. doi: 10.1016/j.pharmthera.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 83.Martinez-Navio JM, Casanova V, Pacheco R, Naval-Macabuhay I, Climent N, Garcia F, Gatell JM, Mallol J, Gallart T, Lluis C, et al. Adenosine deaminase potentiates the generation of effector, memory, and regulatory CD4+ T cells. J Leukoc Biol. 2011;89(1):127–136. doi: 10.1189/jlb.1009696. [DOI] [PubMed] [Google Scholar]
- 84.Gakis C. Adenosine deaminase (ADA) isoenzymes ADA1 and ADA2: diagnostic and biological role. Eur Respir J. 1996;9(4):632–633. doi: 10.1183/09031936.96.09040632. [DOI] [PubMed] [Google Scholar]
- 85.Zavialov AV, Engstrom A. Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity. Biochem J. 2005;391(Pt 1):51–57. doi: 10.1042/BJ20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, Stone DL, Chae JJ, Rosenzweig SD, Bishop K, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370(10):911–920. doi: 10.1056/NEJMoa1307361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caorsi R, Penco F, Grossi A, Insalaco A, Omenetti A, Alessio M, Conti G, Marchetti F, Picco P, Tommasini A, et al. ADA2 deficiency (DADA2) as an unrecognised cause of early onset polyarteritis nodosa and stroke: a multicentre national study. Ann Rheum Dis. 2017;76(10):1648–1656. doi: 10.1136/annrheumdis-2016-210802. [DOI] [PubMed] [Google Scholar]
- 88.Zhang M, Xu L, Wang X, Sun B, Ding J. Expression levels of seprase/FAPalpha and DPPIV/CD26 in epithelial ovarian carcinoma. Oncol Lett. 2015;10(1):34–42. doi: 10.3892/ol.2015.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kikkawa F, Kajiyama H, Ino K, Shibata K, Mizutani S. Increased adhesion potency of ovarian carcinoma cells to mesothelial cells by overexpression of dipeptidyl peptidase IV. Int J Cancer. 2003;105(6):779–783. doi: 10.1002/ijc.11177. [DOI] [PubMed] [Google Scholar]
- 90.El-Kashef DH, Serrya MS. Sitagliptin ameliorates thioacetamide-induced acute liver injury via modulating TLR4/NF-KB signaling pathway in mice. Life Sci. 2019;228:266–273. doi: 10.1016/j.lfs.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 91.Kosowska A, Garczorz W, Klych-Ratuszny A, Aghdam M, Kimsa-Furdzik M, Simka-Lampa K, Francuz T. Sitagliptin Modulates the Response of Ovarian Cancer Cells to Chemotherapeutic Agents. Int J Mol Sci. 2020;21(23):8976. doi: 10.3390/ijms21238976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez-Ramirez AS, Diaz-Munoz M, Battastini AM, Campos-Contreras A, Olvera A, Bergamin L, Glaser T, Jacintho MC, Ulrich H, Vazquez-Cuevas FG. Cellular Migration Ability Is Modulated by Extracellular Purines in Ovarian Carcinoma SKOV-3 Cells. J Cell Biochem. 2017;118(12):4468–4478. doi: 10.1002/jcb.26104. [DOI] [PubMed] [Google Scholar]
- 93.Kajiyama H, Shibata K, Ino K, Mizutani S, Nawa A, Kikkawa F. The expression of dipeptidyl peptidase IV (DPPIV/CD26) is associated with enhanced chemosensitivity to paclitaxel in epithelial ovarian carcinoma cells. Cancer Sci. 2010;101(2):347–354. doi: 10.1111/j.1349-7006.2009.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of DPPIV/CD26 in epithelial morphology and suppressed invasive ability in ovarian carcinoma cells. Ann N Y Acad Sci. 2006;1086:233–240. doi: 10.1196/annals.1377.007. [DOI] [PubMed] [Google Scholar]
- 95.Kajiyama H, Kikkawa F, Khin E, Shibata K, Ino K, Mizutani S. Dipeptidyl peptidase IV overexpression induces up-regulation of E-cadherin and tissue inhibitors of matrix metalloproteinases, resulting in decreased invasive potential in ovarian carcinoma cells. Cancer Res. 2003;63(9):2278–2283. [PubMed] [Google Scholar]
- 96.Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. RNA. 2000;6(7):1004–18. [DOI] [PMC free article] [PubMed]
- 97.Wang C, Zou J, Ma X, Wang E, Peng G. Mechanisms and implications of ADAR-mediated RNA editing in cancer. Cancer Lett. 2017;411:27–34. doi: 10.1016/j.canlet.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 98.Nishikura K. Editor meets silencer: crosstalk between RNA editing and RNA interference. Nat Rev Mol Cell Biol. 2006;7(12):919–931. doi: 10.1038/nrm2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA. 2000;6(5):755–767. doi: 10.1017/S1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng X, Xu X, Wang Y, Hawke DH, Yu S, Han L, Zhou Z, Mojumdar K, Jeong KJ, Labrie M, et al. A-to-I RNA editing contributes to proteomic diversity in cancer. Cancer Cell. 2018;33(5):817–828. doi: 10.1016/j.ccell.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y, Qian H, Xu J, Gao W. ADAR, the carcinogenesis mechanisms of ADAR and related clinical applications. Ann Transl Med. 2019;7(22):686. doi: 10.21037/atm.2019.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cybulski M, Jarosz B, Nowakowski A, Jeleniewicz W, Seroczynski P, Mazurek-Kociubowska M. Cyclin I correlates with VEGFR-2 and cell proliferation in human epithelial ovarian cancer. Gynecol Oncol. 2012;127(1):217–222. doi: 10.1016/j.ygyno.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 103.Zipeto MA, Court AC, Sadarangani A, Delos SN, Balaian L, Chun HJ, Pineda G, Morris SR, Mason CN, Geron I, et al. ADAR1 activation drives leukemia stem cell self-renewal by impairing Let-7 biogenesis. Cell Stem Cell. 2016;19(2):177–191. doi: 10.1016/j.stem.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang ZY, Wang Y, Liu Q, Wu M. microRNA cluster MC-let-7a-1~let-7d promotes autophagy and apoptosis of glioma cells by down-regulating STAT3. Cns Neurosci Ther. 2020;26(3):319–331. doi: 10.1111/cns.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garcia-Vazquez R, Gallardo RD, Ruiz-Garcia E, Meneses GA, Hernandez DLCO, Astudillo-De LVH, Isla-Ortiz D, Marchat LA, Salinas-Vera YM, Carlos-Reyes A, et al. let-7d-3p is associated with apoptosis and response to neoadjuvant chemotherapy in ovarian cancer. Oncol Rep. 2018;39(6):3086–3094. doi: 10.3892/or.2018.6366. [DOI] [PubMed] [Google Scholar]
- 106.Jiang J, Liu HL, Tao L, Lin XY, Yang YD, Tan SW, Wu B. Let7d inhibits colorectal cancer cell proliferation through the CST1/p65 pathway. Int J Oncol. 2018;53(2):781–790. doi: 10.3892/ijo.2018.4419. [DOI] [PubMed] [Google Scholar]
- 107.Chen YN, Ren CC, Yang L, Nai MM, Xu YM, Zhang F, Liu Y. MicroRNA let7d5p rescues ovarian cancer cell apoptosis and restores chemosensitivity by regulating the p53 signaling pathway via HMGA1. Int J Oncol. 2019;54(5):1771–1784. doi: 10.3892/ijo.2019.4731. [DOI] [PubMed] [Google Scholar]
- 108.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, et al. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2001;98(13):7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dzeja P, Terzic A. Adenylate kinase and AMP signaling networks: metabolic monitoring, signal communication and body energy sensing. Int J Mol Sci. 2009;10(4):1729–1772. doi: 10.3390/ijms10041729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Panayiotou C, Solaroli N, Karlsson A. The many isoforms of human adenylate kinases. Int J Biochem Cell Biol. 2014;49:75–83. doi: 10.1016/j.biocel.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 111.Jan YH, Lai TC, Yang CJ, Huang MS, Hsiao M. A co-expressed gene status of adenylate kinase 1/4 reveals prognostic gene signature associated with prognosis and sensitivity to EGFR targeted therapy in lung adenocarcinoma. Sci Rep. 2019;9(1):12329. doi: 10.1038/s41598-019-48243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vasseur S, Malicet C, Calvo EL, Dagorn JC, Iovanna JL. Gene expression profiling of tumours derived from rasV12/E1A-transformed mouse embryonic fibroblasts to identify genes required for tumour development. Mol Cancer. 2005;4(1):4. doi: 10.1186/1476-4598-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lanning NJ, Looyenga BD, Kauffman AL, Niemi NM, Sudderth J, Deberardinis RJ, Mackeigan JP. A mitochondrial RNAi screen defines cellular bioenergetic determinants and identifies an adenylate kinase as a key regulator of ATP levels. Cell Rep. 2014;7(3):907–917. doi: 10.1016/j.celrep.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bai D, Zhang J, Li T, Hang R, Liu Y, Tian Y, Huang D, Qu L, Cao X, Ji J, et al. The ATPase hCINAP regulates 18S rRNA processing and is essential for embryogenesis and tumour growth. Nat Commun. 2016;7:12310. doi: 10.1038/ncomms12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J, Yin YT, Wu CH, Qiu RL, Jiang WJ, Deng XG, Li ZX. AK4 Promotes the Progression of HER2-Positive Breast Cancer by Facilitating Cell Proliferation and Invasion. Dis Markers. 2019;2019:8186091. doi: 10.1155/2019/8186091. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Jan YH, Lai TC, Yang CJ, Lin YF, Huang MS, Hsiao M. Adenylate kinase 4 modulates oxidative stress and stabilizes HIF-1alpha to drive lung adenocarcinoma metastasis. J Hematol Oncol. 2019;12(1):12. doi: 10.1186/s13045-019-0698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xin F, Yao DW, Fan L, Liu JH, Liu XD. Adenylate kinase 4 promotes bladder cancer cell proliferation and invasion. Clin Exp Med. 2019;19(4):525–534. doi: 10.1007/s10238-019-00576-5. [DOI] [PubMed] [Google Scholar]
- 118.Liu R, Strom AL, Zhai J, Gal J, Bao S, Gong W, Zhu H. Enzymatically inactive adenylate kinase 4 interacts with mitochondrial ADP/ATP translocase. Int J Biochem Cell Biol. 2009;41(6):1371–1380. doi: 10.1016/j.biocel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fujisawa K, Terai S, Takami T, Yamamoto N, Yamasaki T, Matsumoto T, Yamaguchi K, Owada Y, Nishina H, Noma T, et al. Modulation of anti-cancer drug sensitivity through the regulation of mitochondrial activity by adenylate kinase 4. J Exp Clin Cancer Res. 2016;35:48. doi: 10.1186/s13046-016-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kong F, Binas B, Moon JH, Kang SS, Kim HJ. Differential expression of adenylate kinase 4 in the context of disparate stress response strategies of HEK293 and HepG2 cells. Arch Biochem Biophys. 2013;533(1–2):11–17. doi: 10.1016/j.abb.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 121.Miyoshi K, Akazawa Y, Horiguchi T, Noma T. Localization of adenylate kinase 4 in mouse tissues. Acta Histochem Cytochem. 2009;42(2):55–64. doi: 10.1267/ahc.08012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jan YH, Tsai HY, Yang CJ, Huang MS, Yang YF, Lai TC, Lee CH, Jeng YM, Huang CY, Su JL, et al. Adenylate kinase-4 is a marker of poor clinical outcomes that promotes metastasis of lung cancer by downregulating the transcription factor ATF3. Cancer Res. 2012;72(19):5119–5129. doi: 10.1158/0008-5472.CAN-12-1842. [DOI] [PubMed] [Google Scholar]
- 123.Syed V, Mukherjee K, Lyons-Weiler J, Lau KM, Mashima T, Tsuruo T, Ho SM. Identification of ATF-3, caveolin-1, DLC-1, and NM23-H2 as putative antitumorigenic, progesterone-regulated genes for ovarian cancer cells by gene profiling. Oncogene. 2005;24(10):1774–1787. doi: 10.1038/sj.onc.1207991. [DOI] [PubMed] [Google Scholar]
- 124.Murph MM, Liu W, Yu S, Lu Y, Hall H, Hennessy BT, Lahad J, Schaner M, Helland A, Kristensen G, et al. Lysophosphatidic acid-induced transcriptional profile represents serous epithelial ovarian carcinoma and worsened prognosis. PLoS ONE. 2009;4(5):e5583. doi: 10.1371/journal.pone.0005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lores P, Coutton C, El KE, Stouvenel L, Givelet M, Thomas L, Rode B, Schmitt A, Louis B, Sakheli Z, et al. Homozygous missense mutation L673P in adenylate kinase 7 (AK7) leads to primary male infertility and multiple morphological anomalies of the flagella but not to primary ciliary dyskinesia. Hum Mol Genet. 2018;27(7):1196–1211. doi: 10.1093/hmg/ddy034. [DOI] [PubMed] [Google Scholar]
- 126.Mata M, Lluch-Estelles J, Armengot M, Sarrion I, Carda C, Cortijo J. New adenylate kinase 7 (AK7) mutation in primary ciliary dyskinesia. Am J Rhinol Allergy. 2012;26(4):260–264. doi: 10.2500/ajra.2012.26.3784. [DOI] [PubMed] [Google Scholar]
- 127.Milara J, Armengot M, Mata M, Morcillo EJ, Cortijo J. Role of adenylate kinase type 7 expression on cilia motility: possible link in primary ciliary dyskinesia. Am J Rhinol Allergy. 2010;24(3):181–185. doi: 10.2500/ajra.2010.24.3468. [DOI] [PubMed] [Google Scholar]
- 128.Shpak M, Goldberg MM, Cowperthwaite MC. Cilia gene expression patterns in cancer. Cancer Genomics Proteomics. 2014;11(1):13–24. [PubMed] [Google Scholar]
- 129.Teilmann SC, Christensen ST. Localization of the angiopoietin receptors Tie-1 and Tie-2 on the primary cilia in the female reproductive organs. Cell Biol Int. 2005;29(5):340–346. doi: 10.1016/j.cellbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 130.Dominska K. Involvement of ACE2/Ang-(1–7)/MAS1 axis in the regulation of ovarian function in mammals. Int J Mol Sci. 2020;21(13):4572. doi: 10.3390/ijms21134572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lohmussaar K, Kopper O, Korving J, Begthel H, Vreuls C, van Es JH, Clevers H. Assessing the origin of high-grade serous ovarian cancer using CRISPR-modification of mouse organoids. Nat Commun. 2020;11(1):2660. doi: 10.1038/s41467-020-16432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hata K, Udagawa J, Fujiwaki R, Nakayama K, Otani H, Miyazaki K. Expression of angiopoietin-1, angiopoietin-2, and Tie2 genes in normal ovary with corpus luteum and in ovarian cancer. Oncology. 2002;62(4):340–348. doi: 10.1159/000065066. [DOI] [PubMed] [Google Scholar]
- 133.Lin Z, Liu Y, Sun Y, He X. Expression of Ets-1, Ang-2 and maspin in ovarian cancer and their role in tumor angiogenesis. J Exp Clin Cancer Res. 2011;30:31. doi: 10.1186/1756-9966-30-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Townsend MH, Robison RA, O’Neill KL. A review of HPRT and its emerging role in cancer. Med Oncol. 2018;35(6):89. [DOI] [PubMed]
- 135.Stout JT, Caskey CT. HPRT: gene structure, expression, and mutation. Annu Rev Genet. 1985;19:127–148. doi: 10.1146/annurev.ge.19.120185.001015. [DOI] [PubMed] [Google Scholar]
- 136.Mateos EA, Puig JG. Purine metabolism in Lesch-Nyhan syndrome versus Kelley-Seegmiller syndrome. J Inherit Metab Dis. 1994;17(1):138–142. doi: 10.1007/BF00735419. [DOI] [PubMed] [Google Scholar]
- 137.Zoref-Shani E, Lavie R, Bromberg Y, Beery E, Sidi Y, Sperling O, Nordenberg J. Effects of differentiation-inducing agents on purine nucleotide metabolism in an ovarian cancer cell line. J Cancer Res Clin Oncol. 1994;120(12):717–722. doi: 10.1007/BF01194269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 139.Dorn J, Ferrari E, Imhof R, Ziegler N, Hubscher U. Regulation of human MutYH DNA glycosylase by the E3 ubiquitin ligase mule. J Biol Chem. 2014;289(10):7049–7058. doi: 10.1074/jbc.M113.536094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Richards B, Zhang H, Phear G, Meuth M. Conditional mutator phenotypes in hMSH2-deficient tumor cell lines. Science. 1997;277(5331):1523–1526. doi: 10.1126/science.277.5331.1523. [DOI] [PubMed] [Google Scholar]
- 141.Wang SS, O’Neill JP, Qian GS, Zhu YR, Wang JB, Armenian H, Zarba A, Wang JS, Kensler TW, Cariello NF, et al. Elevated HPRT mutation frequencies in aflatoxin-exposed residents of daxin, Qidong county, People’s Republic of China. Carcinogenesis. 1999;20(11):2181–4. [DOI] [PubMed]
- 142.Watanabe N, Okochi E, Mochizuki M, Sugimura T, Ushijima T. The presence of single nucleotide instability in human breast cancer cell lines. Cancer Res. 2001;61(21):7739–7742. [PubMed] [Google Scholar]
- 143.Chang YJ, Tseng CY, Lin PY, Chuang YC, Chao MW. Acute exposure to DEHP metabolite, MEHP cause genotoxicity, mutagenesis and carcinogenicity in mammalian Chinese hamster ovary cells. Carcinogenesis. 2017;38(3):336–345. doi: 10.1093/carcin/bgx009. [DOI] [PubMed] [Google Scholar]
- 144.Kamiyama H, Rauenzahn S, Shim JS, Karikari CA, Feldmann G, Hua L, Kamiyama M, Schuler FW, Lin MT, Beaty RM, et al. Personalized chemotherapy profiling using cancer cell lines from selectable mice. Clin Cancer Res. 2013;19(5):1139–1146. doi: 10.1158/1078-0432.CCR-12-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gercel-Taylor C, Scobee JJ, Taylor DD. Effect of chemotherapy on the mutation frequency of ovarian cancer cells at the HPRT locus. Anticancer Res. 2005;25(3B):2113–2117. [PubMed] [Google Scholar]
- 146.Isakovic AM, Dulovic M, Markovic I, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V, Isakovic A. Autophagy suppression sensitizes glioma cells to IMP dehydrogenase inhibition-induced apoptotic death. Exp Cell Res. 2017;350(1):32–40. doi: 10.1016/j.yexcr.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 147.Kofuji S, Sasaki AT. GTP metabolic reprogramming by IMPDH2: unlocking cancer cells’ fuelling mechanism. J Biochem. 2020;168(4):319–28. [DOI] [PMC free article] [PubMed]
- 148.Huang F, Ni M, Chalishazar MD, Huffman KE, Kim J, Cai L, Shi X, Cai F, Zacharias LG, Ireland AS, et al. Inosine Monophosphate Dehydrogenase Dependence in a Subset of Small Cell Lung Cancers. Cell Metab. 2018;28(3):369–382. doi: 10.1016/j.cmet.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Duan S, Huang W, Liu X, Liu X, Chen N, Xu Q, Hu Y, Song W, Zhou J. IMPDH2 promotes colorectal cancer progression through activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 signaling pathways. J Exp Clin Cancer Res. 2018;37(1):304. doi: 10.1186/s13046-018-0980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu H, Ma H, Zha L, Li Q, Yang G, Pan H, Fei X, Xu X, Xing C, Zhang L. IMPDH2 promotes cell proliferation and epithelial-mesenchymal transition of non-small cell lung cancer by activating the Wnt/beta-catenin signaling pathway. Oncol Lett. 2020;20(5):219. doi: 10.3892/ol.2020.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kofuji S, Hirayama A, Eberhardt AO, Kawaguchi R, Sugiura Y, Sampetrean O, Ikeda Y, Warren M, Sakamoto N, Kitahara S, et al. IMP dehydrogenase-2 drives aberrant nucleolar activity and promotes tumorigenesis in glioblastoma. Nat Cell Biol. 2019;21(8):1003–1014. doi: 10.1038/s41556-019-0363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.He Y, Zheng Z, Xu Y, Weng H, Gao Y, Qin K, Rong J, Chen C, Yun M, Zhang J, et al. Over-expression of IMPDH2 is associated with tumor progression and poor prognosis in hepatocellular carcinoma. Am J Cancer Res. 2018;8(8):1604–1614. [PMC free article] [PubMed] [Google Scholar]
- 153.Xu Y, Zheng Z, Gao Y, Duan S, Chen C, Rong J, Wang K, Yun M, Weng H, Ye S, et al. High expression of IMPDH2 is associated with aggressive features and poor prognosis of primary nasopharyngeal carcinoma. Sci Rep. 2017;7(1):745. doi: 10.1038/s41598-017-00887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Naffouje R, Grover P, Yu H, Sendilnathan A, Wolfe K, Majd N, Smith EP, Takeuchi K, Senda T, Kofuji S, et al. Anti-Tumor Potential of IMP Dehydrogenase Inhibitors: A Century-Long Story. Cancers (Basel) 2019;11(9):1346. doi: 10.3390/cancers11091346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Floryk D, Thompson TC. Antiproliferative effects of AVN944, a novel inosine 5-monophosphate dehydrogenase inhibitor, in prostate cancer cells. Int J Cancer. 2008;123(10):2294–2302. doi: 10.1002/ijc.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Valvezan AJ, Mcnamara MC, Miller SK, Torrence ME, Asara JM, Henske EP, Manning BD. IMPDH inhibitors for antitumor therapy in tuberous sclerosis complex. JCI Insight. 2020;5(7):e135071. doi: 10.1172/jci.insight.135071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang F, Huffman KE, Wang Z, Wang X, Li K, Cai F, Yang C, Cai L, Shih TS, Zacharias LG, et al. Guanosine triphosphate links MYC-dependent metabolic and ribosome programs in small-cell lung cancer. J Clin Invest. 2021;131(1):e139929. doi: 10.1172/JCI139929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meli M, Tolomeo M, Grifantini M, Franchetti P, Cappellacci L, Simoni D, Invidiata FP, Aiello S, Dusonchet L. The synergistic apoptotic effects of thiophenfurin, an inosine monophosphate dehydrogenase inhibitor, in combination with retinoids in HL60 cells. Oncol Rep. 2007;17(1):185–192. [PubMed] [Google Scholar]
- 159.Grusch M, Rosenberger G, Fuhrmann G, Braun K, Titscher B, Szekeres T, Fritzer-Skekeres M, Oberhuber G, Krohn K, Hengstschlaeger M, et al. Benzamide riboside induces apoptosis independent of Cdc25A expression in human ovarian carcinoma N.1 cells. Cell Death Differ. 1999;6(8):736–744. doi: 10.1038/sj.cdd.4400546. [DOI] [PubMed] [Google Scholar]
- 160.Bennett EM, Li C, Allan PW, Parker WB, Ealick SE. Structural basis for substrate specificity of Escherichia coli purine nucleoside phosphorylase. J Biol Chem. 2003;278(47):47110–47118. doi: 10.1074/jbc.M304622200. [DOI] [PubMed] [Google Scholar]
- 161.Zhang J, Kale V, Chen M. Gene-directed enzyme prodrug therapy. Aaps J. 2015;17(1):102–110. doi: 10.1208/s12248-014-9675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fu W, Lan H, Li S, Han X, Gao T, Ren D. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2’-deoxyriboside via Salmonella against murine tumors. Cancer Gene Ther. 2008;15(7):474–84. [DOI] [PubMed]
- 163.Deharvengt S, Wack S, Uhring M, Aprahamian M, Hajri A. Suicide gene/prodrug therapy for pancreatic adenocarcinoma by E. coli purine nucleoside phosphorylase and 6-methylpurine 2’-deoxyriboside. Pancreas. 2004;28(2):E54–64. [DOI] [PubMed]
- 164.Zhang Y, Parker WB, Sorscher EJ, Ealick SE. PNP anticancer gene therapy. Curr Top Med Chem. 2005;5(13):1259–1274. doi: 10.2174/156802605774463105. [DOI] [PubMed] [Google Scholar]
- 165.Sorscher EJ, Peng S, Bebok Z, Allan PW, Bennett LJ, Parker WB. Tumor cell bystander killing in colonic carcinoma utilizing the Escherichia coli DeoD gene to generate toxic purines. Gene Ther. 1994;1(4):233–238. [PubMed] [Google Scholar]
- 166.Gadi VK, Alexander SD, Kudlow JE, Allan P, Parker WB, Sorscher EJ. In vivo sensitization of ovarian tumors to chemotherapy by expression of E. coli purine nucleoside phosphorylase in a small fraction of cells. Gene Ther. 2000;7(20):1738–1743. doi: 10.1038/sj.gt.3301286. [DOI] [PubMed] [Google Scholar]
- 167.Singh PP, Joshi S, Russell PJ, Nair S, Khatri A. Purine Nucleoside Phosphorylase mediated molecular chemotherapy and conventional chemotherapy: a tangible union against chemoresistant cancer. BMC Cancer. 2011;11:368. doi: 10.1186/1471-2407-11-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang X, Sun L, Sun X, Yu J, Wang K, Wu Y, Gao Q, Zheng J. Antitumor effects of a dual-specific lentiviral vector carrying the Escherichia coli purine nucleoside phosphorylase gene. Int J Oncol. 2017;50(5):1612–1622. doi: 10.3892/ijo.2017.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang C, Zhang J, Liao M, Yang Y, Wang Y, Yuan Y, Ouyang L. Folate-mediated one-carbon metabolism: a targeting strategy in cancer therapy. Drug Discov Today. 2021;26(3):817–825. doi: 10.1016/j.drudis.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 170.Duff MJ, Desai N, Craig MA, Agarwal PK, Howell EE. Crowders steal dihydrofolate reductase ligands through quinary interactions. Biochemistry-Us. 2019;58(9):1198–1213. doi: 10.1021/acs.biochem.8b01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 172.Tang Q, Cheng J, Cao X, Surowy H, Burwinkel B. Blood-based DNA methylation as biomarker for breast cancer: a systematic review. Clin Epigenetics. 2016;8:115. doi: 10.1186/s13148-016-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tahara T, Arisawa T. DNA methylation as a molecular biomarker in gastric cancer. Epigenomics-Uk. 2015;7(3):475–486. doi: 10.2217/epi.15.4. [DOI] [PubMed] [Google Scholar]
- 174.Pan Y, Liu G, Zhou F, Su B, Li Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin Exp Med. 2018;18(1):1–14. doi: 10.1007/s10238-017-0467-0. [DOI] [PubMed] [Google Scholar]
- 175.Goldman ID, Matherly LH. The cellular pharmacology of methotrexate. Pharmacol Ther. 1985;28(1):77–102. doi: 10.1016/0163-7258(85)90083-X. [DOI] [PubMed] [Google Scholar]
- 176.Zhao M, Tan B, Dai X, Shao Y, He Q, Yang B, Wang J, Weng Q. DHFR/TYMS are positive regulators of glioma cell growth and modulate chemo-sensitivity to temozolomide. Eur J Pharmacol. 2019;863:172665. doi: 10.1016/j.ejphar.2019.172665. [DOI] [PubMed] [Google Scholar]
- 177.Marverti G, Ligabue A, Lombardi P, Ferrari S, Monti MG, Frassineti C, Costi MP. Modulation of the expression of folate cycle enzymes and polyamine metabolism by berberine in cisplatin-sensitive and -resistant human ovarian cancer cells. Int J Oncol. 2013;43(4):1269–1280. doi: 10.3892/ijo.2013.2045. [DOI] [PubMed] [Google Scholar]
- 178.Eroglu A, Akar N. Methylenetetrahydrofolate reductase C677T polymorphism in breast cancer risk. Breast Cancer Res Treat. 2010;122(3):897–898. doi: 10.1007/s10549-010-0938-4. [DOI] [PubMed] [Google Scholar]
- 179.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 180.Pepe C, Guidugli L, Sensi E, Aretini P, D’Andrea E, Montagna M, Manoukian S, Ottini L, Radice P, Viel A, et al. Methyl group metabolism gene polymorphisms as modifier of breast cancer risk in Italian BRCA1/2 carriers. Breast Cancer Res Treat. 2007;103(1):29–36. [DOI] [PubMed]
- 181.He L, Shen Y. MTHFR C677T polymorphism and breast, ovarian cancer risk: a meta-analysis of 19,260 patients and 26,364 controls. Onco Targets Ther. 2017;10:227–238. doi: 10.2147/OTT.S121472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pu D, Jiang SW, Wu J. Association between MTHFR gene polymorphism and the risk of ovarian cancer: a meta-analysis of the literature. Curr Pharm Des. 2014;20(11):1632–1638. doi: 10.2174/13816128113199990564. [DOI] [PubMed] [Google Scholar]
- 183.Terry KL, Tworoger SS, Goode EL, Gates MA, Titus-Ernstoff L, Kelemen LE, Sellers TA, Hankinson SE, Cramer DW. MTHFR polymorphisms in relation to ovarian cancer risk. Gynecol Oncol. 2010;119(2):319–324. doi: 10.1016/j.ygyno.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ma C, Liu Y, Zhang W, Liu P. The association between MTHFR C677T polymorphism and ovarian cancer risk: a meta-analysis of 18,628 individuals. Mol Biol Rep. 2013;40(3):2061–2068. doi: 10.1007/s11033-012-1970-8. [DOI] [PubMed] [Google Scholar]
- 185.Zhang L, Liu W, Hao Q, Bao L, Wang K. Folate intake and methylenetetrahydrofolate reductase gene polymorphisms as predictive and prognostic biomarkers for ovarian cancer risk. Int J Mol Sci. 2012;13(4):4009–4020. doi: 10.3390/ijms13044009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Webb PM, Ibiebele TI, Hughes MC, Beesley J, van der Pols JC, Chen X, Nagle CM, Bain CJ, Chenevix-Trench G. Folate and related micronutrients, folate-metabolising genes and risk of ovarian cancer. Eur J Clin Nutr. 2011;65(10):1133–1140. doi: 10.1038/ejcn.2011.99. [DOI] [PubMed] [Google Scholar]
- 187.Zhang S, Zheng F, Zhang L, Huang Z, Huang X, Pan Z, Chen S, Xu C, Jiang Y, Gu S, et al. LncRNA HOTAIR-mediated MTHFR methylation inhibits 5-fluorouracil sensitivity in esophageal cancer cells. J Exp Clin Cancer Res. 2020;39(1):131. doi: 10.1186/s13046-020-01610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yeh CC, Lai CY, Chang SN, Hsieh LL, Tang R, Sung FC, Lin YK. Polymorphisms of MTHFR C677T and A1298C associated with survival in patients with colorectal cancer treated with 5-fluorouracil-based chemotherapy. Int J Clin Oncol. 2017;22(3):484–493. doi: 10.1007/s10147-016-1080-z. [DOI] [PubMed] [Google Scholar]
- 189.Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y. Polymorphisms in ERCC1, GSTs, TS and MTHFR predict clinical outcomes of gastric cancer patients treated with platinum/5-Fu-based chemotherapy: a systematic review. Bmc Gastroenterol. 2012;12:137. doi: 10.1186/1471-230X-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tang C, Yu S, Jiang H, Li W, Xu X, Cheng X, Peng K, Chen E, Cui Y, Liu T. A Meta-Analysis: Methylenetetrahydrofolate Reductase C677T Polymorphism in Gastric Cancer Patients Treated with 5-Fu Based Chemotherapy Predicts Serious Hematologic Toxicity but Not Prognosis. J Cancer. 2018;9(6):1057–1066. doi: 10.7150/jca.23391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2017;36(3):293–303. doi: 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS ONE. 2008;3(7):e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schultze-Mosgau A, Katzur AC, Arora KK, Stojilkovic SS, Diedrich K, Ortmann O. Characterization of calcium-mobilizing, purinergic P2Y(2) receptors in human ovarian cancer cells. Mol Hum Reprod. 2000;6(5):435–442. doi: 10.1093/molehr/6.5.435. [DOI] [PubMed] [Google Scholar]
- 194.Saito H, Nishimura M, Shinano H, Makita H, Tsujino I, Shibuya E, Sato F, Miyamoto K, Kawakami Y. Plasma concentration of adenosine during normoxia and moderate hypoxia in humans. Am J Respir Crit Care Med. 1999;159(3):1014–1018. doi: 10.1164/ajrccm.159.3.9803100. [DOI] [PubMed] [Google Scholar]
- 195.Poth JM, Brodsky K, Ehrentraut H, Grenz A, Eltzschig HK. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. J Mol Med (Berl) 2013;91(2):183–193. doi: 10.1007/s00109-012-0988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kusek J, Yang Q, Witek M, Gruber CW, Nanoff C, Freissmuth M. Chaperoning of the A1-adenosine receptor by endogenous adenosine - an extension of the retaliatory metabolite concept. Mol Pharmacol. 2015;87(1):39–51. doi: 10.1124/mol.114.094045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111(12):5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 198.Allard B, Beavis PA, Darcy PK, Stagg J. Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol. 2016;29:7–16. doi: 10.1016/j.coph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 199.Linden J, Koch-Nolte F, Dahl G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu Rev Immunol. 2019;37:325–347. doi: 10.1146/annurev-immunol-051116-052406. [DOI] [PubMed] [Google Scholar]
- 200.Fredholm BB. Adenosine–a physiological or pathophysiological agent? J Mol Med (Berl) 2014;92(3):201–206. doi: 10.1007/s00109-013-1101-6. [DOI] [PubMed] [Google Scholar]
- 201.Vijayan D, Young A, Teng M, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17(12):709–724. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 202.Ohta A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front Immunol. 2016;7:109. doi: 10.3389/fimmu.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gaudreau PO, Allard B, Turcotte M, Stagg J. CD73-adenosine reduces immune responses and survival in ovarian cancer patients. Oncoimmunology. 2016;5(5):e1127496. doi: 10.1080/2162402X.2015.1127496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Robles-Martinez L, Garay E, Martel-Gallegos MG, Cisneros-Mejorado A, Perez-Montiel D, Lara A, Arellano RO. Kca3.1 activation via P2y2 purinergic receptors promotes human ovarian cancer cell (Skov-3) migration. Sci Rep. 2017;7(1):4340. doi: 10.1038/s41598-017-04292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xia B, Wang J. Adenosine inhibits ovarian cancer growth through regulating rhogdi2 protein expression. Drug Des Devel Ther. 2019;13:3837–3844. doi: 10.2147/DDDT.S219028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhu F, Liu XG, Liang NC. Inhibitory effect of adenosine analogues on invasion of human ovarian cancer cell line HO-8910PM] Ai Zheng. 2004;23(12):1646–1650. [PubMed] [Google Scholar]
- 207.Bednarska-Szczepaniak K, Krzyzanowski D, Klink M, Nowak M. Adenosine analogues as opposite modulators of the cisplatin resistance of ovarian cancer cells. Anticancer Agents Med Chem. 2019;19(4):473–486. doi: 10.2174/1871520619666190118113201. [DOI] [PubMed] [Google Scholar]
- 208.Shirali S, Aghaei M, Shabani M, Fathi M, Sohrabi M, Moeinifard M. Adenosine induces cell cycle arrest and apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian cancer cell line OVCAR-3. Tumour Biol. 2013;34(2):1085–1095. doi: 10.1007/s13277-013-0650-1. [DOI] [PubMed] [Google Scholar]
- 209.Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18(10):601–618. doi: 10.1038/s41568-018-0037-0. [DOI] [PubMed] [Google Scholar]
- 210.Woods LT, Forti KM, Shanbhag VC, Camden JM, Weisman GA. P2Y receptors for extracellular nucleotides: Contributions to cancer progression and therapeutic implications. Biochem Pharmacol. 2021;187:114406. doi: 10.1016/j.bcp.2021.114406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Braganhol E, Wink MR, Lenz G, Battastini A. Purinergic Signaling in Glioma Progression. Adv Exp Med Biol. 2020;1202:87–108. doi: 10.1007/978-3-030-30651-9_5. [DOI] [PubMed] [Google Scholar]
- 212.Kasama H, Sakamoto Y, Kasamatsu A, Okamoto A, Koyama T, Minakawa Y, Ogawara K, Yokoe H, Shiiba M, Tanzawa H, et al. Adenosine A2b receptor promotes progression of human oral cancer. BMC Cancer. 2015;15:563. doi: 10.1186/s12885-015-1577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lan J, Lu H, Samanta D, Salman S, Lu Y, Semenza GL. Hypoxia-inducible factor 1-dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment. Proc Natl Acad Sci U S A. 2018;115(41):E9640–E9648. doi: 10.1073/pnas.1809695115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhou Y, Chu X, Deng F, Tong L, Tong G, Yi Y, Liu J, Tang J, Tang Y, Xia Y, et al. The adenosine A2b receptor promotes tumor progression of bladder urothelial carcinoma by enhancing MAPK signaling pathway. Oncotarget. 2017;8(30):48755–48768. doi: 10.18632/oncotarget.17835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16(3):177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 216.Morello S, Miele L. Targeting the adenosine A2b receptor in the tumor microenvironment overcomes local immunosuppression by myeloid-derived suppressor cells. Oncoimmunology. 2014;3:e27989. doi: 10.4161/onci.27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Winerdal M, Winerdal ME, Wang YQ, Fredholm BB, Winqvist O, Aden U. Adenosine A1 receptors contribute to immune regulation after neonatal hypoxic ischemic brain injury. Purinergic Signal. 2016;12(1):89–101. doi: 10.1007/s11302-015-9482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Butler M, Sanmugalingam D, Burton VJ, Wilson T, Pearson R, Watson RP, Smith P, Parkinson SJ. Impairment of adenosine A3 receptor activity disrupts neutrophil migratory capacity and impacts innate immune function in vivo. Eur J Immunol. 2012;42(12):3358–3368. doi: 10.1002/eji.201242655. [DOI] [PubMed] [Google Scholar]
- 219.Hajiahmadi S, Panjehpour M, Aghaei M, Mousavi S. Molecular expression of adenosine receptors in OVCAR-3, Caov-4 and SKOV-3 human ovarian cancer cell lines. Res Pharm Sci. 2015;10(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- 220.Campos-Contreras A, Gonzalez-Gallardo A, Diaz-Munoz M, Vazquez-Cuevas FG. Adenosine Receptor A2B Negatively Regulates Cell Migration in Ovarian Carcinoma Cells. Int J Mol Sci. 2022;23(9):4585. doi: 10.3390/ijms23094585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hajiahmadi S, Panjehpour M, Aghaei M, Shabani M. Activation of A2b adenosine receptor regulates ovarian cancer cell growth: involvement of Bax/Bcl-2 and caspase-3. Biochem Cell Biol. 2015;93(4):321–329. doi: 10.1139/bcb-2014-0117. [DOI] [PubMed] [Google Scholar]
- 222.Abedi H, Aghaei M, Panjehpour M, Hajiahmadi S. Mitochondrial and caspase pathways are involved in the induction of apoptosis by IB-MECA in ovarian cancer cell lines. Tumour Biol. 2014;35(11):11027–11039. doi: 10.1007/s13277-014-2396-9. [DOI] [PubMed] [Google Scholar]
- 223.Jiang K, Yao G, Hu L, Yan Y, Liu J, Shi J, Chang Y, Zhang Y, Liang D, Shen D, et al. MOB2 suppresses GBM cell migration and invasion via regulation of FAK/Akt and cAMP/PKA signaling. Cell Death Dis. 2020;11(4):230. doi: 10.1038/s41419-020-2381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yue W, Ma J, Xiao Y, Wang P, Gu X, Xie B, Li M. The Apoptotic Resistance of BRCA1-Deficient Ovarian Cancer Cells is Mediated by cAMP. Front Cell Dev Biol. 2022;10:889656. doi: 10.3389/fcell.2022.889656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhu P, Wang L, Xu P, Tan Q, Wang Y, Feng G, Yuan L. GANT61 elevates chemosensitivity to cisplatin through regulating the Hedgehog, AMPK and cAMP pathways in ovarian cancer. Future Med Chem. 2022;14(7):479–500. doi: 10.4155/fmc-2021-0310. [DOI] [PubMed] [Google Scholar]
- 226.Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10:128. doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu G, Zhang Q, Liu G, Li D, Zhang L, Gu Z, Tian H, Zhang Y, Tian X. Disruption of adenosine 2A receptor improves the anti-tumor function of anti-mesothelin CAR T cells both in vitro and in vivo. Exp Cell Res. 2021;409(1):112886. doi: 10.1016/j.yexcr.2021.112886. [DOI] [PubMed] [Google Scholar]
- 228.Barcz E, Sommer E, Sokolnicka I, Gawrychowski K, Roszkowska-Purska K, Janik P, Skopinska-Rozewska E. The influence of theobromine on angiogenic activity and proangiogenic cytokines production of human ovarian cancer cells. Oncol Rep. 1998;5(2):517–520. doi: 10.3892/or.5.2.517. [DOI] [PubMed] [Google Scholar]
- 229.Barcz E, Sommer E, Janik P, Marianowski L, Skopinska-Rozewska E. Adenosine receptor antagonism causes inhibition of angiogenic activity of human ovarian cancer cells. Oncol Rep. 2000;7(6):1285–1291. doi: 10.3892/or.7.6.1285. [DOI] [PubMed] [Google Scholar]
- 230.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host A(2B) adenosine receptors promote carcinoma growth. Neoplasia. 2008;10(9):987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Di Virgilio F, Giuliani AL, Vultaggio-Poma V, Falzoni S, Sarti AC. Non-nucleotide Agonists Triggering P2X7 Receptor Activation and Pore Formation. Front Pharmacol. 2018;9:39. doi: 10.3389/fphar.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tomasinsig L, Pizzirani C, Skerlavaj B, Pellegatti P, Gulinelli S, Tossi A, Di Virgilio F, Zanetti M. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J Biol Chem. 2008;283(45):30471–30481. doi: 10.1074/jbc.M802185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Norenberg W, Hempel C, Urban N, Sobottka H, Illes P, Schaefer M. Clemastine potentiates the human P2X7 receptor by sensitizing it to lower ATP concentrations. J Biol Chem. 2011;286(13):11067–11081. doi: 10.1074/jbc.M110.198879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang Y, Cheng H, Li W, Wu H, Yang Y. Highly-expressed P2X7 receptor promotes growth and metastasis of human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3beta/beta-catenin and mTOR/HIF1alpha/VEGF signaling. Int J Cancer. 2019;145(4):1068–1082. doi: 10.1002/ijc.32207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Brisson L, Chadet S, Lopez-Charcas O, Jelassi B, Ternant D, Chamouton J, Lerondel S, Le Pape A, Couillin I, Gombault A, et al. P2X7 receptor promotes mouse mammary cancer cell invasiveness and tumour progression, and is a target for anticancer treatment. Cancers (Basel) 2020;12(9):2342. doi: 10.3390/cancers12092342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Arnaud-Sampaio VF, Rabelo I, Ulrich H, Lameu C. The P2X7 receptor in the maintenance of cancer stem cells, chemoresistance and metastasis. Stem Cell Rev Rep. 2020;16(2):288–300. doi: 10.1007/s12015-019-09936-w. [DOI] [PubMed] [Google Scholar]
- 237.Hofman P, Cherfils-Vicini J, Bazin M, Ilie M, Juhel T, Hebuterne X, Gilson E, Schmid-Alliana A, Boyer O, Adriouch S, et al. Genetic and pharmacological inactivation of the purinergic P2RX7 receptor dampens inflammation but increases tumor incidence in a mouse model of colitis-associated cancer. Cancer Res. 2015;75(5):835–845. doi: 10.1158/0008-5472.CAN-14-1778. [DOI] [PubMed] [Google Scholar]
- 238.de Torre-Minguela C, Barbera-Cremades M, Gomez AI, Martin-Sanchez F, Pelegrin P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci Rep. 2016;6:22586. doi: 10.1038/srep22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vazquez-Cuevas FG, Cruz-Rico A, Garay E, Garcia-Carranca A, Perez-Montiel D, Juarez B, Arellano RO. Differential expression of the P2X7 receptor in ovarian surface epithelium during the oestrous cycle in the mouse. Reprod Fertil Dev. 2013;25(7):971–984. doi: 10.1071/RD12196. [DOI] [PubMed] [Google Scholar]
- 240.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72(12):2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 241.Vazquez-Cuevas FG, Martinez-Ramirez AS, Robles-Martinez L, Garay E, Garcia-Carranca A, Perez-Montiel D, Castaneda-Garcia C, Arellano RO. Paracrine stimulation of P2X7 receptor by ATP activates a proliferative pathway in ovarian carcinoma cells. J Cell Biochem. 2014;115(11):1955–1966. doi: 10.1002/jcb.24867. [DOI] [PubMed] [Google Scholar]
- 242.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159(3):1451–1458. [PubMed] [Google Scholar]
- 243.Luborsky J, Barua A, Edassery S, Bahr JM, Edassery SL. Inflammasome expression is higher in ovarian tumors than in normal ovary. PLoS ONE. 2020;15(1):e227081. doi: 10.1371/journal.pone.0227081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wu H, Liu J, Zhang Y, Li Q, Wang Q, Gu Z. miR-22 suppresses cell viability and EMT of ovarian cancer cells via NLRP3 and inhibits PI3K/AKT signaling pathway. Clin Transl Oncol. 2021;23(2):257–264. doi: 10.1007/s12094-020-02413-8. [DOI] [PubMed] [Google Scholar]
- 245.Alrashed MM, Alharbi H, Alshehry AS, Ahmad M, Aloahd MS. MiR-624-5p enhances NLRP3 augmented gemcitabine resistance via EMT/IL-1beta/Wnt/beta-catenin signaling pathway in ovarian cancer. J Reprod Immunol. 2022;150:103488. doi: 10.1016/j.jri.2022.103488. [DOI] [PubMed] [Google Scholar]
- 246.Ding Y, Yan Y, Dong Y, Xu J, Su W, Shi W, Zou Q, Yang X. NLRP3 promotes immune escape by regulating immune checkpoints: A pan-cancer analysis. Int Immunopharmacol. 2022;104:108512. doi: 10.1016/j.intimp.2021.108512. [DOI] [PubMed] [Google Scholar]
- 247.Salaro E, Rambaldi A, Falzoni S, Amoroso FS, Franceschini A, Sarti AC, Bonora M, Cavazzini F, Rigolin GM, Ciccone M, et al. Involvement of the P2X7-NLRP3 axis in leukemic cell proliferation and death. Sci Rep. 2016;6:26280. doi: 10.1038/srep26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Solini A, Cobuccio L, Rossi C, Parolini F, Biancalana E, Cosio S, Chiarugi M, Gadducci A. Molecular characterization of peritoneal involvement in primary colon and ovary neoplasm: the possible clinical meaning of the p2x7 receptor-inflammasome complex. Eur Surg Res. 2021:1–9. [DOI] [PubMed]
- 249.Zhang Y, Li F, Wang L, Lou Y. A438079 affects colorectal cancer cell proliferation, migration, apoptosis, and pyroptosis by inhibiting the P2X7 receptor. Biochem Biophys Res Commun. 2021;558:147–153. doi: 10.1016/j.bbrc.2021.04.076. [DOI] [PubMed] [Google Scholar]
- 250.Jacobson KA, Muller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology. 2016;104:31–49. doi: 10.1016/j.neuropharm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Erb L, Weisman GA. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip Rev Membr Transp Signal. 2012;1(6):789–803. doi: 10.1002/wmts.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wei WC, Jacobs B, Becker EB, Glitsch MD. Reciprocal regulation of two G protein-coupled receptors sensing extracellular concentrations of Ca2+ and H. Proc Natl Acad Sci U S A. 2015;112(34):10738–10743. doi: 10.1073/pnas.1506085112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Martinez-Ramirez AS, Garay E, Garcia-Carranca A, Vazquez-Cuevas FG. The P2RY2 receptor induces carcinoma cell migration and EMT through cross-talk with epidermal growth factor receptor. J Cell Biochem. 2016;117(4):1016–1026. doi: 10.1002/jcb.25390. [DOI] [PubMed] [Google Scholar]
- 254.Egan K, Crowley D, Smyth P, O’Toole S, Spillane C, Martin C, Gallagher M, Canney A, Norris L, Conlon N, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS ONE. 2011;6(10):e26125. [DOI] [PMC free article] [PubMed]
- 255.Davis AN, Afshar-Kharghan V, Sood AK. Platelet effects on ovarian cancer. Semin Oncol. 2014;41(3):378–384. doi: 10.1053/j.seminoncol.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Cho MS, Noh K, Haemmerle M, Li D, Park H, Hu Q, Hisamatsu T, Mitamura T, Mak S, Kunapuli S, et al. Role of ADP receptors on platelets in the growth of ovarian cancer. Blood. 2017;130(10):1235–1242. doi: 10.1182/blood-2017-02-769893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Parker WB. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem Rev. 2009;109(7):2880–2893. doi: 10.1021/cr900028p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lee SA, Jung M. The nucleoside analog sangivamycin induces apoptotic cell death in breast carcinoma MCF7/adriamycin-resistant cells via protein kinase Cdelta and JNK activation. J Biol Chem. 2007;282(20):15271–15283. doi: 10.1074/jbc.M701362200. [DOI] [PubMed] [Google Scholar]
- 259.Issaeva N, Thomas HD, Djureinovic T, Jaspers JE, Stoimenov I, Kyle S, Pedley N, Gottipati P, Zur R, Sleeth K, et al. 6-thioguanine selectively kills BRCA2-defective tumors and overcomes PARP inhibitor resistance. Cancer Res. 2010;70(15):6268–6276. doi: 10.1158/0008-5472.CAN-09-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Roberts C, Strauss VY, Kopijasz S, Gourley C, Hall M, Montes A, Abraham J, Clamp A, Kennedy R, Banerjee S, et al. Results of a phase II clinical trial of 6-mercaptopurine (6MP) and methotrexate in patients with BRCA-defective tumours. Br J Cancer. 2020;122(4):483–490. doi: 10.1038/s41416-019-0674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Albasher G, Alkahtane AA, Alarifi S, Ali D, Alessia MS, Almeer RS, Abdel-Daim MM, Al-Sultan NK, Al-Qahtani AA, Ali H, et al. Methotrexate-induced apoptosis in human ovarian adenocarcinoma SKOV-3 cells via ROS-mediated bax/bcl-2-cyt-c release cascading. Onco Targets Ther. 2019;12:21–30. doi: 10.2147/OTT.S178510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Penn CA, Yang K, Zong H, Lim JY, Cole A, Yang D, Baker J, Goonewardena SN, Buckanovich RJ. Therapeutic impact of nanoparticle therapy targeting tumor-associated macrophages. Mol Cancer Ther. 2018;17(1):96–106. doi: 10.1158/1535-7163.MCT-17-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhang L, Zou W. Inhibition of integrin beta1 decreases the malignancy of ovarian cancer cells and potentiates anticancer therapy via the FAK/STAT1 signaling pathway. Mol Med Rep. 2015;12(6):7869–7876. doi: 10.3892/mmr.2015.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Fang J, Cao Z, Chen YC, Reed E, Jiang BH. 9-beta-D-arabinofuranosyl-2-fluoroadenine inhibits expression of vascular endothelial growth factor through hypoxia-inducible factor-1 in human ovarian cancer cells. Mol Pharmacol. 2004;66(1):178–186. doi: 10.1124/mol.66.1.178. [DOI] [PubMed] [Google Scholar]
- 265.Zaffaroni N, Orlandi L, Gornati D, De Marco C, Vaglini M, Silvestrini R. Fludarabine as a modulator of cisplatin activity in human tumour primary cultures and established cell lines. Eur J Cancer. 1996;32A(10):1766–1773. doi: 10.1016/0959-8049(96)00176-1. [DOI] [PubMed] [Google Scholar]
- 266.Xue J, Bi X, Wu G, Meng D, Fang J. Fludarabine reduces survivability of HepG2 cells through VEGF under hypoxia. Arch Biochem Biophys. 2007;468(1):100–106. doi: 10.1016/j.abb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 267.Frelinger AR, Michelson AD, Wiviott SD, Trenk D, Neumann FJ, Miller DL, Jakubowski JA, Costigan TM, Mccabe CH, Antman EM, et al. Intrinsic platelet reactivity before P2Y12 blockade contributes to residual platelet reactivity despite high-level P2Y12 blockade by prasugrel or high-dose clopidogrel. Results from PRINCIPLE-TIMI 44. Thromb Haemost. 2011;106(2):219–226. doi: 10.1160/TH11-03-0185. [DOI] [PubMed] [Google Scholar]
- 268.Bergmann TK, Filppula AM, Launiainen T, Nielsen F, Backman J, Brosen K. Neurotoxicity and low paclitaxel clearance associated with concomitant clopidogrel therapy in a 60-year-old Caucasian woman with ovarian carcinoma. Br J Clin Pharmacol. 2016;81(2):313–315. doi: 10.1111/bcp.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Park SH, Byon JS, Lee SW, Lee SJ, Jin DK, Shin WY. Coronary artery thrombosis associated with Paclitaxel in advanced ovarian cancer. Korean Circ J. 2009;39(3):124–127. doi: 10.4070/kcj.2009.39.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Nicum S, Roberts C, Boyle L, Kopijasz S, Gourley C, Hall M, Montes A, Poole C, Collins L, Schuh A, et al. A phase II clinical trial of 6-mercaptopurine (6MP) and methotrexate in patients with BRCA defective tumours: a study protocol. BMC Cancer. 2014;14:983. doi: 10.1186/1471-2407-14-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Estlin EJ. Continuing therapy for childhood acute lymphoblastic leukaemia: clinical and cellular pharmacology of methotrexate, 6-mercaptopurine and 6-thioguanine. Cancer Treat Rev. 2001;27(6):351–363. doi: 10.1053/ctrv.2002.0245. [DOI] [PubMed] [Google Scholar]
- 272.El-Husseiny K, Motawei H, Ali MS. Continuous low-dose oral cyclophosphamide and methotrexate as maintenance therapy in patients with advanced ovarian carcinoma after complete clinical response to platinum and paclitaxel chemotherapy. Int J Gynecol Cancer. 2016;26(3):437–442. doi: 10.1097/IGC.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 273.Hortu I, Ozceltik G, Ergenoglu AM, Yigitturk G, Atasoy O, Erbas O. Protective effect of oxytocin on a methotrexate-induced ovarian toxicity model. Arch Gynecol Obstet. 2020;301(5):1317–1324. doi: 10.1007/s00404-020-05534-1. [DOI] [PubMed] [Google Scholar]
- 274.O’Brien S, Kantarjian H, Keating MJ. Purine analogs in chronic lymphocytic leukemia and Waldenstrom’s macroglobulinemia. Ann Oncol. 1996;7(Suppl 6):S27–33. [DOI] [PubMed]
- 275.Hoogstad-Van EJ, Bekkers R, Ottevanger N, Schaap N, Hobo W, Jansen JH, Massuger L, Dolstra H. Intraperitoneal infusion of ex vivo-cultured allogeneic NK cells in recurrent ovarian carcinoma patients (a phase I study) Medicine (Baltimore) 2019;98(5):e14290. doi: 10.1097/MD.0000000000014290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, Mckenna D, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13(1):98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Teneriello M, Farley J, Parker M, O’Connor D, Shaver T, Park R, Barnhill D. Management of advanced ovarian epithelial cancer in the renal transplant patient. Gynecol Oncol. 1993;50(3):374–8. [DOI] [PubMed]
- 278.Fu R, Sutcliffe D, Zhao H, Huang X, Schretlen DJ, Benkovic S, Jinnah HA. Clinical severity in Lesch-Nyhan disease: the role of residual enzyme and compensatory pathways. Mol Genet Metab. 2015;114(1):55–61. doi: 10.1016/j.ymgme.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32(1):19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 280.Chatzidoukaki O, Goulielmaki E, Schumacher B, Garinis GA. DNA damage response and metabolic reprogramming in health and disease. Trends Genet. 2020;36(10):777–791. doi: 10.1016/j.tig.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 281.Mathews CK. DNA precursor metabolism and genomic stability. Faseb J. 2006;20(9):1300–1314. doi: 10.1096/fj.06-5730rev. [DOI] [PubMed] [Google Scholar]
- 282.Kozhevnikova EN, van der Knaap JA, Pindyurin AV, Ozgur Z, van Ijcken WF, Moshkin YM, Verrijzer CP. Metabolic enzyme IMPDH is also a transcription factor regulated by cellular state. Mol Cell. 2012;47(1):133–139. doi: 10.1016/j.molcel.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 283.Coquel F, Silva MJ, Techer H, Zadorozhny K, Sharma S, Nieminuszczy J, Mettling C, Dardillac E, Barthe A, Schmitz AL, et al. SAMHD1 acts at stalled replication forks to prevent interferon induction. Nature. 2018;557(7703):57–61. doi: 10.1038/s41586-018-0050-1. [DOI] [PubMed] [Google Scholar]
- 284.Du B, Zhang Z, Di W, Xu W, Yang L, Zhang S, He G, Yang R, Wang M. PAICS is related to glioma grade and can promote glioma growth and migration. J Cell Mol Med. 2021;25(16):7720–7733. doi: 10.1111/jcmm.16647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Huang N, Xu C, Deng L, Li X, Bian Z, Zhang Y, Long S, Chen Y, Zhen N, Li G, et al. PAICS contributes to gastric carcinogenesis and participates in DNA damage response by interacting with histone deacetylase 1/2. Cell Death Dis. 2020;11(7):507. doi: 10.1038/s41419-020-2708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Fulda S. Tumor resistance to apoptosis. Int J Cancer. 2009;124(3):511–515. doi: 10.1002/ijc.24064. [DOI] [PubMed] [Google Scholar]
- 287.Ma F, Zhu Y, Liu X, Zhou Q, Hong X, Qu C, Feng X, Zhang Y, Ding Q, Zhao J, et al. Dual-specificity tyrosine phosphorylation-regulated kinase 3 loss activates purine metabolism and promotes hepatocellular carcinoma progression. Hepatology. 2019;70(5):1785–1803. doi: 10.1002/hep.30703. [DOI] [PubMed] [Google Scholar]
- 288.Feng X, Ma D, Zhao J, Song Y, Zhu Y, Zhou Q, Ma F, Liu X, Zhong M, Liu Y, et al. UHMK1 promotes gastric cancer progression through reprogramming nucleotide metabolism. Embo J. 2020;39(5):e102541. doi: 10.15252/embj.2019102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhou Q, Lin M, Feng X, Ma F, Zhu Y, Liu X, Qu C, Sui H, Sun B, Zhu A, et al. Targeting CLK3 inhibits the progression of cholangiocarcinoma by reprogramming nucleotide metabolism. J Exp Med. 2020;217(8):e20191779. doi: 10.1084/jem.20191779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.