Abstract
Stem cell differentiation is of great interest in medical research; however, specifically and effectively regulating stem cell differentiation is still a challenge. In addition to chemical factors, physical signals are an important component of the stem cell ecotone. The mechanical microenvironment of stem cells has a huge role in stem cell differentiation. Herein, we describe the knowledge accumulated to date on the mechanical environment in which stem cells exist, which consists of various factors, including the extracellular matrix and topology, substrate stiffness, shear stress, hydrostatic pressure, tension, and microgravity. We then detail the currently known signalling pathways that stem cells use to perceive the mechanical environment, including those involving nuclear factor-kB, the nicotinic acetylcholine receptor, the piezoelectric mechanosensitive ion channel, and hypoxia-inducible factor 1α. Using this information in clinical settings to treat diseases is the goal of this research, and we describe the progress that has been made. In this review, we examined the effects of mechanical factors in the stem cell growth microenvironment on stem cell differentiation, how mechanical signals are transmitted to and function within the cell, and the influence of mechanical factors on the use of stem cells in clinical applications.
Keywords: Stem cell, Extracellular matrix, Shear stress, Hydrostatic pressure, Tension, Microgravity, NF-kB, nAChR, PIEZO, HIF-1α
Background
Stem cells have unquestionable importance in medicine and are receiving increasing attention due to their role in several diseases [1, 2]. Stem cell renewal [3], migration [4], adhesion [5], and differentiation [6] are integral to the proper functioning of living organisms, and their dysregulation can lead to multiple diseases. The majority of research on stem cell differentiation concerns how chemical stimulation affects differentiation [7]. However, there is growing evidence of the importance of physical signals in stem cell fate [8]. The application of specific physical factors can propel the differentiation of stem cells in a more clinically favourable and specialized direction [9–11].
The microenvironment in which stem cells grow contains not only a variety of biochemical molecules, but also a variety of mechanical factors [12]. The mechanical microenvironment of stem cells alters the fate of stem cells [13] and regulates multiple signalling cascade responses involved in stem cell differentiation [14]. And different types of stem cells will differentiate in different directions and show different fates when responding to the same microenvironmental signals. The effect of these mechanical signals on stem cell differentiation has an important role in clinical application [15] (Table 1).
Table 1.
Role and application of mechanical signals on stem cell differentiation
| Physical signal | Mechanical signal | Responsive cell | Effectiveness of mechanical signal | Application | References |
|---|---|---|---|---|---|
| ECM | dECM | BMSC | Enhancing osteogenic and angiogenic potential | Optimization of cell culture conditions | [20] |
| 3D Microenvironment | hESC | Promoting gene expression associated with differentiation to neural crest stem cells and osteoblasts | Optimizing artificial scaffolds as culture conditions | [23] | |
| ECM and artificial scaffolds | hASC | Corresponding cell-derived ECM promotes corresponding differentiation | Improving the regenerative capacity of unmodified scaffolds | [26] | |
| Substrate topology | Low pore size fibres | hMSC | Enhancing osteogenesis | Inducing stem cell-directed differentiation | [32] |
| Large pore size fibres | rAMSC | Promoting differentiation into islet-like clusters | [33] | ||
| Porous topology | NSPC | Promoting differentiation into astrocytes and neurons | [34] | ||
| Composite microstructure of nanofibres | rBMSC | Enhancing osteogenic differentiation | [35] | ||
| Substrate hardness | High hardness 3D-printed ECM | BMSC | Differentiating into sweat gland cells and hair follicle cells | [38] | |
| Hard alginate shells | hMSC | Promoting osteogenic differentiation | [39] | ||
| Soft hydrogel | VPC | Inducing differentiation towards endothelial cells | [40] | ||
| Shear stress | Oscillatory shear stress | rBMSC | Promoting osteogenic differentiation | Bone tissue engineering | [48] |
| Intermittent shear stress | rBMSC | Enhancing osteogenic differentiation | [49] | ||
| Perfusion culture | 3D MT-dASC | Changing in direction of osteogenic differentiation to lipogenic differentiation | [55] | ||
| Hydrostatic pressure | Circulating hydrostatic pressure | MSC | Enhancing osteogenic response | Changing the direction of stem cell differentiation | [59] |
| Circulating hydrostatic pressure and decalcified bone matrix scaffold | MSC | Reducing osteogenic properties and enhancing chondrogenic properties | [60] | ||
| Tension | Cyclic mechanical draft force | Human periodontal stem cells | Promoting osteogenic differentiation | Dental tissue engineering | [61–63] |
| Cyclic stretching | EPCs | Differentiating towards endothelium and angiogenesis | Vascular regeneration project | [64] | |
| Bone marrow-derived cells | Expressing smooth muscle cell markers | [65] | |||
| Microgravity | Microgravity | hBMSC | Inhibiting osteogenic differentiation and promoting adipogenic and chondrogenic differentiation | Treatment of diseases related to bone loss in space | [75, 76] |
| Nanostands and microgravity | hBMSC | Mitigating microgravity-induced osteoblast dysfunction | [77] | ||
| Simulation of microgravity | mESC | Differentiating towards the stereotyped endoderm | Contribution to the study of regeneration engineering | [79] |
ECM extracellular matrix, BMSCs bone marrow mesenchymal stem cells (MSCs), hESCs human embryonic stem cells, hASCs human adipose stem cells, hMSC human MSC, rAMSCs rat adipose MSCs, VPC vascular progenitor cell, rBMSC rat bone marrow MSC, 3D MT-dASC 3D microtissue-derived adult stem cell, mESC mouse embryonic stem cell, NSPC neural stem progenitor cell, EPCs endothelial progenitor cells
The cytoskeleton plays an indispensable role in the cell’s perception of the mechanical environment. Actin senses the stiffness of the environment and controls the persistence of the platelet foot through a specific cluster of actin/proto-myosin filaments, which in turn assembles a focal adhesion [16]. The focal adhesion then binds to extracellular matrix (ECM) ligands as well as intracellular proteins [17]. The mechanical signal is then transmitted to the nucleus via stress fibres [14]. Fibre stiffness in turn acts on ligand density at the cell surface and promotes the formation of the focal adhesion and associated signalling [18]. In addition to the transmission of perceived mechanical signals through the cytoskeleton to regulate stem cell differentiation, cells also transform mechanical signals into cell-recognizable chemical signals through various mechanosensors and transduction mechanisms [17], which in turn regulate stem cell differentiation.
This review aims to summarize the impact of mechanical factors in the stem cell growth microenvironment on stem cell differentiation, describe how stem cells sense and respond to mechanical signals to function, and further explore the clinical implications of the influence of mechanical factors on stem cells.
Mechanical microenvironment for stem cell growth
The stem cell microenvironment refers to all the environmental factors surrounding stem cells in tissues as they proliferate, self-renew, and differentiate into tissue cells at their residency sites, including soluble biomolecules, solid ECM with supporting cells, and the mechanical and physicochemical environment surrounding the stem cells. The mechanical microenvironment is an integral part of the stem cell microenvironment and includes the mechanical support of the ECM, the forces exerted on the cell by the environment, and the forces caused by the interaction of the cell with the surrounding support cells [19]. In vivo, the development, growth, proliferation, and differentiation of stem cells are inseparable from the mechanical environment in which they are embedded. In the usual physiological mechanical environment, stem cells can perform their functions normally; when the surrounding environment changes, the various functions of stem cells also change. Multiple aspects of the influence of the mechanical microenvironment of stem cell growth on their differentiation can be studied, including the extracellular matrix, substrate topology, substrate stiffness, shear stress, tension, hydrostatic pressure, and microgravity.
Extracellular matrix and topology
ECM is a three-dimensional network of various extracellular macromolecules, such as collagen, elastin, fibronectin, and laminin proteins, which provide a good environment for cells to survive. Cells cultured in vitro secrete ECM and the cell-derived ECM (dECM) produced after cell removal has great application in cell culture. For example, culturing bone marrow mesenchymal stem cell (MSC) (BMSC) with different combinations of distinct cell types of dECM showed that dECM enhanced the osteogenic and angiogenic potential of BMSC compared to tissue culture polystyrene; the different behaviour of the BMSC is related to the different proportions of cells that make up the dECM [20]. In addition, the natural living environment of cells is three-dimensional and 3D culture better simulates the realistic living environment of the cells. When cells are cultured in 3D, their biological behaviour and morphological size are altered, thus changing their surrounding mechanical microenvironment. Morphological changes and geometry of cells can modulate nanostructures and lipid assembly within cell membranes, thereby regulating stem cell signalling and differentiation fate [21]. The study of 3D materials will contribute to the development of tissue engineering and regenerative medicine [22]. Artificial scaffolds can be used to replace natural ECM as a cell culture medium. β-tricalcium phosphate (β-TCP) scaffolds with ECM-like properties provide a 3D microenvironment for human embryonic stem cell (hESC) and promote the expression of genes associated with neural crest stem cells and osteoblast differentiation [23]. Therefore, the continuous optimization of artificial scaffolds as culture conditions will help to further explore how ECM affects stem cell differentiation. Osteogenic differentiation of stem cells was significantly increased when 3D scaffolds were combined with heparin and bone morphogenetic protein 2 (BMP-2) [24]. Osteogenic differentiation was also significantly enhanced in MSCs growing on alginate/graphene oxide-printed 3D scaffolds [25]. Other groups have attempted to combine artificial stents with ECM, combining cell-derived ECM with 3D-printed polycaprolactone (PCL) scaffolds to culture human adipose stem cells (ASCs) (hASCs). Chondrocyte-derived ECM promoted cartilage differentiation and osteoblast-derived ECM was able to stimulate hASCs towards osteogenic differentiation [26]. Cell-derived ECM therefore has great potential to enhance the regenerative capacity of unmodified PCL stents and warrants further study, offering the possibility of determining the fate of stem cell differentiation. Interestingly, the effects of the 3D environment on stem cells are not static, but diverse. Induced pluripotent stem cell (iPSC) differentiates into a typical MSC-like phenotype on tissue culture plastic or on the surface of fibrin hydrogels. In contrast, iPSCs embedded in a 3D environment do not differentiate towards MSC and have reduced differentiation potential for osteogenic and lipogenic lineages [27].
The topology of the substrate is a physical characteristic of the microenvironment in which the cells are anchored and affects stem cell differentiation in many ways. Nanoscale materials have been widely used to model ECM and their topology has a significant impact on the fate of stem cells [28, 29]. For example, Jaswal et al. investigated electrospun nanofibre scaffolds for peripheral nerve regeneration [30] and found that precisely controlled concentrations of reduced graphene-encapsulated gold nanoparticles in PCL fibre scaffolds provided a microenvironment that mimicked natural ECM, and that their uniformly distributed topology might increase the stimulation of cell differentiation and could promote neuronal network formation. In addition, the nanotopography enhances the hydrophilicity of 3D-printed polylactic acid (PLA) scaffolds and significantly enhances osteogenic differentiation on the scaffold [31]. The study of how scaffold topology regulates cell behaviour can be considered from several perspectives, including pore size, porosity, fibre morphology, fibre diameter, and orientation. When human MSC (hMSC) were inoculated in 3D ECM-like fibrous structures, the smaller pore size exhibited higher overall stiffness and significantly enhanced hMSC collagen and mineral deposition, enhancing osteogenesis [32]. A 3D electrospun nanofibre scaffold with a large pore size supported the differentiation of rat adipose MSCs (rAMSCs) into islet-like clusters [33]. The addition of different copolymers to PCL produced micron fibres with a porous topology that allowed cultured rat neural stem progenitor cell (NSPC) to differentiate into astrocytes and neurons in the absence of any growth factors, demonstrating the role of the porous topology of the fibres [34]. Electrostatic spinning scaffolds of calcium phosphate nanoparticles with a composite microstructure of microbeads and nanofibres can enhance the osteogenic differentiation of rBMSCs by promoting scaffold biomineralization and protein adsorption through the exposure of bioactive components [35], which has potential in bone regeneration. The highest expression of insulin-differentiated cells was found on 300 nm-diameter fibres when mouse ESCs were cultured in a reticulated fibrous medium formed from polyamide (PA) fibres [36].
Substrate stiffness
Under physiological conditions, cells in vivo are anchored to tissue substrates of varying stiffness, and their specific stiffness influences the cells that grow on them. Substrate stiffness has a role in a wide range of stem cell differentiation profiles [37]. Cells perceive the stiffness of ECM through cytoskeletal contractility, and the relatively high stiffness of 3D-printed ECM facilitates the differentiation of BMSCs towards sweat cells and hair follicle cells [38]. The harder alginate shell promotes osteogenic differentiation of hMSCs [39], whereas the softer hydrogel will direct the differentiation of vascular progenitor cells (VPCs) towards endothelial cells (ECs) [40]. In addition, stiffness and topology have a synergistic effect on the maintenance of stem cell characteristics and the adipogenic or osteogenic differentiation of mouse MSCs (mMSCs) [41]. Matrix stiffness plays a dominant role in the maintenance of stemness on hard gels and hepatic differentiation on soft gels, whereas matrix morphology contributes to hepatocyte-like differentiation on soft gels [42]. MSC can interestingly no longer perceive the difference between soft and hard substrates after a period of incubation on a rigid substrate [43]. Therefore, in addition to the synergistic effect of substrate morphology and substrate hardness, incubation time also plays a role in regulating the differentiation of MSCs. The response of MSCs to substrate morphology varies depending on substrate stiffness and incubation time, and the effect of substrate stiffness and incubation time on MSCs also depends on the morphology of the substrate arrangement [44].
Shear stress
All types of tissue cells in the normal human body are continuously exposed to shear stresses caused by fluid flow in the tissue interstices under load. Shear force treatment of hESC simulated by the Microfluidic Dynamic Culture System promotes the expression of blood progenitors in the hESC lineage, reducing the proportion of mono-competent erythroid and megakaryocyte lineages and increasing the number of bone marrow and bipotent megakaryocyte–erythroid progenitors. Shear force treatment also promoted smooth muscle and cardiomyocyte production, suggesting a role for shear stress in both the haematopoietic spectrum and the arterial vascular system [45]. Human pluripotent stem cell-derived endothelial cells (hPSC-ECs) are more sensitive to low levels of shear stress and require prolonged exposure to shear stress to trigger stable phenotypic changes, exhibiting increased expression of arterial markers, suggesting that hPSC-ECs are transformed into an arterial phenotype [46]. Thus, shear stress influences the differentiation of stem cells.
Perfusion flow-induced shear stress in a fully automated bioreactor enhances osteogenic differentiation in hBMSC and modulates O2 concentration to improve osteogenic differentiation. This bioreactor is used to precisely control the fate of stem cells in terms of osteogenesis and has potential applications in the healthcare industry, for example in the prevention of osteoporosis [47]. However, although shear stress induced by perfusion flow was demonstrated to have a role in inducing stem cell differentiation, different patterns, sizes, and rates of fluid shear stimulation have different effects on stem cells. Osteogenic differentiation of rat bone marrow MSCs (rBMSCs) is more strongly promoted by 0.0225 Pa oscillatory shear stress [48]. Intermittent shear stresses of the order of 10 mPa can effectively enhance the osteogenic differentiation of rBMSCs [49]. The rate of fluid shear stress can also control the fate of rBMSCs towards osteogenic or chondrogenic cell differentiation [50]. The effect of osteogenic differentiation of rBMSCs under different shear stresses could be useful for bone tissue engineering applications. Therefore, the study of different stresses on the induction of osteogenic differentiation of stem cells has great importance.
However, a single application of shear stress is not sufficient. In practice, a combination of other culture conditions should be considered to improve the efficiency of targeted differentiation. Shear stress combined with polymeric biomaterials can enhance the osteogenic differentiation of MSCs [51]. This combination has driven improvements in the clinical approach to treating bone defects. High levels of cardiac-related gene expression were not observed in either of the 5-azacytidiner (5-Aza) or shear stress groups, whereas BMSCs cultured with 5-Aza in concert with shear stress showed significantly increased cardiac-related gene expression [52], which is expected to promote cardiac differentiation of stem cells. In addition to shear stress combining with biochemical conditions to regulate stem cell behaviour, shear stress can also work in concert with other physical conditions. Shear-stressed groove structures can promote the differentiation of BMSCs into myofibroblasts [53]. The hBMSCs embedded in the 3D scaffold are subjected to shear stress to produce a typical tendonogenic phenotype and promote the expression of tendon gene markers [54]. Osteogenic differentiation of 3D microtissue-derived human stem cells on bone bionic electrospun nanocomposites was evident, but shear stress led to lipogenic differentiation of 3D microtissue-derived human stem cells under perfusion culture [55]. This result not only links the 3D environment and composite materials to stem cell differentiation, but also contributes to changing the direction of stem cell differentiation.
Hydrostatic pressure
Hydrostatic pressure (HP) is a mechanical force that is widely present in the environment in which cells live. Using autologous platelet-rich fibrin (PRF) membranes as a growth factor-rich scaffold and culturing BMSCs pre-conditioned with HP prior to transplantation greatly enhanced the chondrogenic potential of the BMSC/PRF constructs. Further studies showed that the pressurized pretreated BMSC/PRF graft group showed a significant improvement in the integration of the regenerated cartilage with the host cartilage environment [56]. HP is therefore worth considering in the application of stem cell differentiation.
HP combined with the stromal microenvironment can induce directed differentiation of stem cells. Intermittent HP (IHP) and 3D microenvironments modified with ECM proteins, especially collagen, have a synergistic effect on the expression of chondrogenic genes by MSCs [57]. HP and piezoelectric scaffolds also have a synergistic effect on promoting chondrogenic differentiation in MSCs [58]. Circulating HP (CHP) increases the MSC osteogenic response through cytoskeletal reorganization [59]. However, when CHP was applied to hBMSCs in a decalcified bone matrix (DBM) scaffold, it reduced osteogenic properties and favoured chondrogenic properties [60], suggesting that HP combined with different induction conditions could alter the direction of stem cell differentiation.
Tension
Tension is the force exerted on an object by means of pulling in a certain direction, such as the pulling force produced on the cells in the body by the tissues. For example, muscle contraction is a movement produced by the cells being subjected to traction. Multiple studies confirm that cyclic mechanical tension can promote osteogenic differentiation of human periodontal stem cells [61–63]. Further studies have elucidated the contribution of tensile forces to stem cell differentiation. Circulating stretch not only promotes endothelial differentiation and angiogenesis of endothelial progenitor cells (EPCs) [64], but also enhances the expression of smooth muscle cell markers by bone marrow-derived cells [65], which has applications in vascular regeneration engineering. Appropriate tensile strain promotes osteogenic differentiation of BMSCs while inhibiting differentiation to adipocytes [66], and uniaxial cyclic stretch is even more significant in inducing MSC differentiation to osteoblasts in vitro [67]. This result also suggests that different types of tension act in different ways on stem cells.
In addition, the frequency and amplitude as well as the duration of stretching led to the differentiation of cells in different directions [68]. For example, the gene expression of type I collagen (Col I) and glycosaminoglycan (GAG) was significantly upregulated in the 10% and 15% stretch groups, whereas the gene expression of type II collagen (Col II) was downregulated, leading to differentiation towards fibrochondrocytes. However, a higher stretch stimulus (15%) simultaneously promoted the synthesis of α-smooth muscle actin. Therefore, 10% radial stretch stimulation is the optimal intensity to induce differentiation of BMSCs into fibrochondrocytes [69].
Microgravity
A microgravity environment is one in which the apparent weight of a system is much less than its actual weight in the presence of gravity. Microgravity is not common in daily life, but it has a major impact on astronauts conducting space operations. Microgravity can up- or downregulate differentiation-related genes [70, 71], which may lead to a range of related disorders in astronauts, such as bone loss [72] and cardiovascular disease [73]. In addition, simulated microgravity conditions may also disrupt the homeostasis of the immune system and lead to dynamic changes in hematopoietic stem cells (HSC) and lineage cells [74]. These results contribute to a better understanding of immune regulation and its changes during spaceflight, thus providing possible directions for the prevention or treatment of immune system disorders in astronauts.
Many studies have demonstrated that microgravity inhibits the osteogenic differentiation of hBMSCs while promoting adipogenesis and chondrogenic differentiation [75, 76]. However, growth on a nanocrystalline magnesium-doped hydroxyapatite/type I collagen composite scaffold (MHA/Coll) can attenuate microgravity-induced osteoblast dysfunction in hBMSCs and promote cell differentiation along the osteogenic lineage [77]. In addition, nanocomplexes loaded with BMP2 and BMP7 in simulated microgravity can also promote osteogenic differentiation of human adipose-derived stem cells (hADSCs) [78]. These results indicate the possibility of treating diseases associated with bone loss in space.
Microgravity has a positive effect in some ways. Mouse embryonic stem cell (mESC) cultured in a rotating bioreactor under simulated microgravity conditions can differentiate towards stereotyped endoderm, and these cells can further differentiate into cells from other related organs such as the pancreas, liver, and thyroid [79]. Simulated microgravity also promoted the proliferation and matrix production of tissue-engineered human chondrocyte-like cells [80]. MSCs cultured with a liver induction medium are more conducive to liver differentiation under long-term microgravity conditions [81]. Microgravity is therefore useful for regenerative engineering studies and has potential applications for disease prevention and treatment.
Mechanisms for perceiving the mechanical environment
As described in the previous section, mechanical stimuli, or the mechanical properties of the pericellular matrix material, play an important role in regulating the morphological development and function of stem cells. However, the exact mechanisms of how mechanical signals are sensed by and transmitted to and within stem cells, ultimately leading to a range of biological effects in stem cells, need to be further explored. There are two main mechanisms regarding the cellular perception of mechanical signals: transmission and transduction mechanisms. Signal transmission mechanisms occur when changes in the microenvironment are transmitted via sensors into the cell to cause rearrangement of the cytoskeleton, which in turn transmits signals to the cytoskeleton in the nucleus. Signal transduction mechanisms occur when changes in the microenvironment alter the permeability of ion channels or the activity of associated intracellular receptors, transducing mechanical signals into chemical signals to regulate the expression of associated genes. The two mechanisms work in synergy to transmit and convert mechanical signals. The cytoskeleton, integrins, and ion channels, among others, play important roles in perception [40]. In addition, various cellular sensory transduction pathways have been associated with mechanical stimulation, including signalling pathways such as NF-κB, nAChR, PIEZO, and HIF-1α.
NF-κB signalling pathway
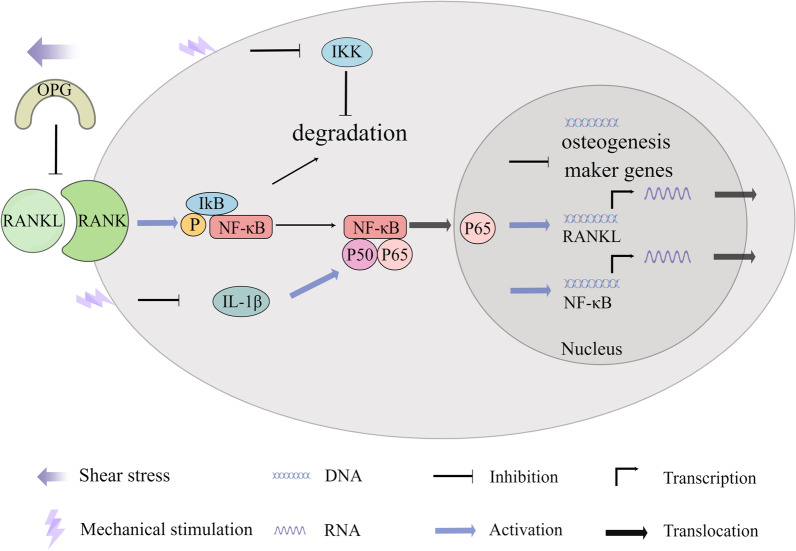
Nuclear factor κ-B (NF-κB) is a ubiquitous, inducible nuclear transcriptional activator that binds to enhancer elements in many different cell types and can also be activated by pathogenic stimuli. RANKL is a ligand for NF-κB receptor activator (RANK), which binds specifically to and activates RANK. RANKL-stimulated cells exhibit marked translocation of p65 from the cytoplasm to the nucleus, phosphorylating and activating NF-κB pathway-associated proteins. Activated NF-κB/p65 translocation to the nucleus reduces the expression of runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP), and osteoprotegerin (OPG). At the same time, OPG is a decoy receptor for RANKL, which competitively inhibits the binding of RANKL to RANK, inhibiting osteoclast production and its survival time. Therefore, the relative ratio of RANKL/OPG, which coordinates osteoblast/osteoclast production, is of great importance [82].
Binding of NF-κB dimers to NF-κB inhibitor (IκB) in the cytoplasm renders NF-κB dimers inactive. IκB proteins are phosphorylated and degraded following physical or chemical stimulation of cells. Subsequently, NF-κB dimers are transformed into an activated state, released, and transferred to the nucleus, leading to nuclear localization of p65 and increased expression of RANKL mRNA, resulting in an increased RANKL/OPG ratio, which in turn induces target gene transcription [83]. Mechanical stretching reduces phosphorylated IκB kinase and IκBα degradation is inhibited, resulting in increased IκBα, reduced phosphorylation and nuclear accumulation of P65, and downregulated activity, which in turn blocks NF-κB activity and promotes osteogenesis in hBMSCs [84]. The NF-κB signalling pathway provides a starting point for more precise regulation of stem cell differentiation at the molecular level.
NF-κB is expressed in rat growth plate chondrogenesis, stimulates chondrocyte proliferation and differentiation, prevents apoptosis, and promotes longitudinal bone growth [85], whereas in hyperchondrogenesis or arthritic cartilage, interleukin (IL)-1β is highly expressed, induces upregulation of miR-381, and promotes cartilage matrix resorption by inhibiting type II collagen and inducing metalloproteinase-13 (MMP-13) [86]. In addition, IL-1β significantly upregulates NF-κB, promotes p65 nuclear translocation, and activates Rac1 and reactive oxygen species (ROS), which in turn activate NF-κB translation in chondrocytes, thereby reshaping the microenvironment for the treatment of ROS and inflammatory factor-related chronic diseases such as osteoarthritis [87]. The increase in miR-320c inhibited cyclin-dependent kinase 6 (CDK6), attenuated IL-1β-induced chondrocyte inflammation, inhibited the activation of NF-kB pathway, and regulated the chondrogenesis of hBMSCs [88]. In contrast, mechanical loading can reduce the levels of oncogenes such as Rac1, MMP9, and IL-1β [89], which in turn reduces NF-κB expression and can modulate the bone microenvironment to reduce the growth and invasion of tumour cells (Fig. 1). Therefore, NF-κB signalling pathway-related proteins may be effective targets for cancer therapy.
Fig. 1.
Mechanical stimulation regulates the differentiation of stem cells into osteoblasts/osteoclasts and chondroblasts through the NF-κB pathway. Mechanical stretching can reduce phosphorylated IκB kinase, block NF-κB activity, and promote osteogenic differentiation of cells. Fluid shear stress also increases the expression of OPG, the decoy receptor for RANKL, upregulating the expression of osteoblast marker genes. Mechanical loading can reduce the levels of IL-1β, which in turn reduces NF-κB expression and regulates the chondrogenesis
Bone formation requires a balance between bone formation by osteoblasts and bone resorption by osteoclasts, the absence of either of which can cause specific corresponding diseases or abnormalities. Therefore, the effects of different mechanical forces on osteoblasts and osteoclasts were investigated. Fluid shear stress reduced RANKL expression and increased OPG expression in cells, which significantly reduced the RANKL/OPG ratio, upregulating the expression of osteoblast marker genes [90] (Fig. 1). Mechanical loading also inhibits osteoclastogenesis and promotes osteogenesis by downregulating NF-κB ligands and receptor activators of histone K, upregulating OPG, and downregulating peroxisome proliferators-activated receptor γ (PPARγ) [89]. However, circulating mechanical strain can stimulate more ALP and calcium deposition through activation of RANKL [91]. Certain circulating stresses can also induce osteoclast differentiation through upregulation of α 7 nAChR and activation of the classical Wnt pathway leading to increased RANKL expression and reduced expression of RUNX2, ALP, and OPG [92]. Exosomes are important mediators in maintaining the balance between bone formation and bone resorption. Exosomes from cyclic mechanical stretch (CMS)-treated BMSCs inhibit actin ring formation and suppress osteoclast differentiation by attenuating the NF-κB signalling pathway, which also provides new insights into intercellular communication between osteoblasts and osteoclasts under mechanical loading [93].
The nAChR signalling pathway
The nicotinic acetylcholine (ACh) receptor (nAChR) is a ligand-gated ion channel that signals through endogenous ACh and its agonists to drive organoid growth and differentiation [94]. Among these, the α7 nicotinic ACh receptor (α7nAChR) has been the main focus of research [95]. Although most of the research on α7nAChR has involved neural tissue and the inflammatory environment, and less on the stem cell and mechanistic environment, the cholinergic system also involves mammalian non-neuronal cells, such as stem cells. Cholinergic signalling plays a key role in controlling stem cells behaviour [96]. The α7nAChR has also been associated with mechanical signals [92]. In view of this, the α7 nAChR signalling pathway is promising in terms of its relationship to stem cell perception of mechanistic stimuli.
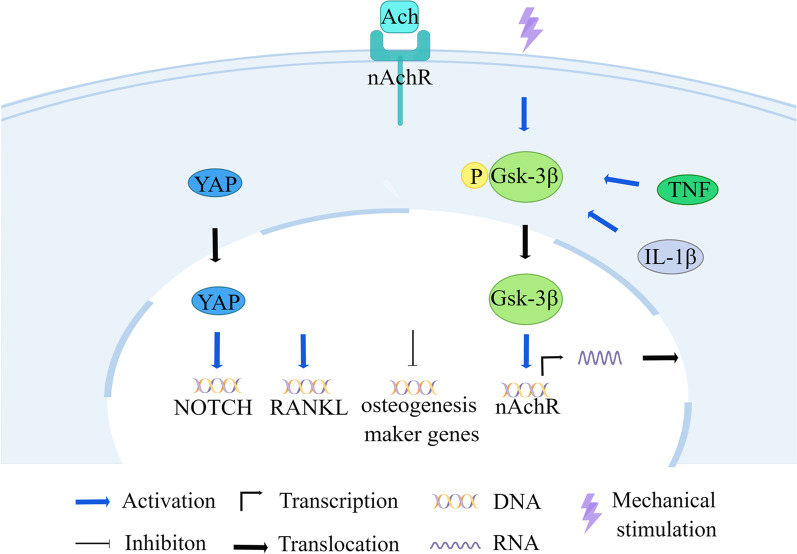
Tumour necrosis factor-α (TNF-α) and IL-1β significantly increased phosphorylated glycogen synthase kinase-3β (GSK-3β) levels in BMSCs and periodontal stem cells (PDLSCs), and increased expression of phosphorylated GSK-3β (p-GSK-3 β) upregulated α7 nAChR expression and promoted its function, which in turn upregulated RANKL, downregulated OPG, and decreased ALP, RUNX2, and OCN, leading to decreased osteogenic differentiation and increased osteoclast formation [97] (Fig. 2). This was also verified in another study in which PDLSCs upregulated the expression of p-GSK-3β, α7nAChR, and active β-catenin and decreased the expression of RUNX2, ALP, and OPG under hydrodynamic stress [92].
Fig. 2.
Mechanistic effects attenuate stem cell osteogenic differentiation via the nAchR signalling pathway. Under stress, TNF-α and IL-1β increase phosphorylated GSK-3β in stem cells, which then promotes the expression of α7 nAChR. nAChR is activated by the ligand Ach, which in turn upregulates RANKL and downregulates genes related to osteogenic differentiation
At the same time, the nAChR signalling pathway has synergistic effects with a variety of signalling pathways. Upregulation of α7nAChR activates the NF-κB signalling pathway. The receptor activator of RANKL is upregulated, which in turn induces osteoclast effects [92]. The nAChR signal not only coordinates with Wnt signalling to regulate intestinal stem cell (ISC) function [94], but also balances ISC differentiation by activating the Hippo and Notch signalling pathways [98]. The nAChR signalling activator nicotine blocks the osteogenic potential of hPDLC induced by cyclic tensile stress by binding to α7 nAChR and activating the classical Wnt pathway [99]. nAChR signalling activators can also upregulate the downstream effectors of the Hippo and Notch signalling pathways, YAP1/ TAZ and Notch1/Dll1, regulating the expression of target genes [98].
PIEZO signalling pathway
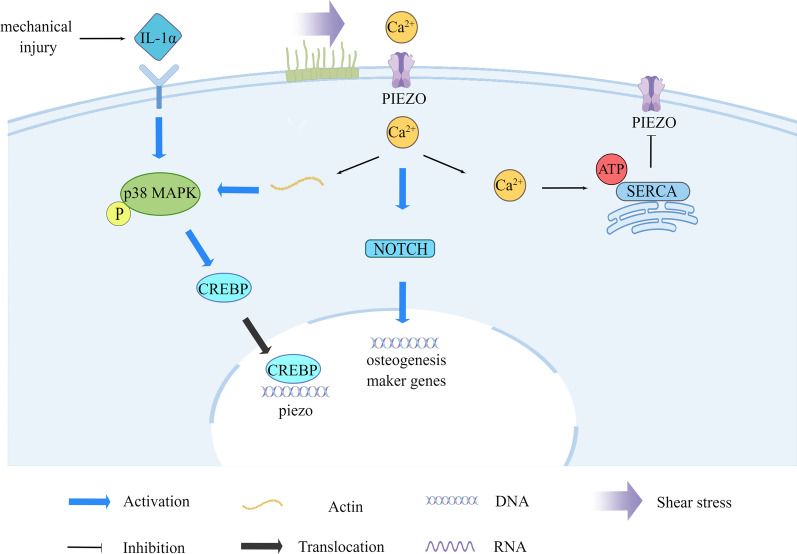
The piezoelectric mechanosensitive ion channel (PIEZO) acts as a mechanosensor and is a key receptor for sensing mechanical stimuli [100]. PIEZO strongly controls stem cell differentiation by coordinating WNT expression and ciliogenesis to link mechanical signals to intracellular signals [101]. Mechanical stretching can effectively stimulate osteogenic differentiation of stem cells by activating mechanosensitive ion channels [102] (Fig. 3). In human deciduous dentin stem cells, cyclic stress-induced ciliogenesis and the expression of WNT5b and WNT16, activating PIEZO1 and promoting nuclear translocation of RUNX2, which in turn promoted adult dentin cell differentiation [103]. In addition, stress loading increases PIEZO mRNA expression, which may be related to Ca2+ influx [104], which in turn is closely interrelated with cilia. The primary cilia are non-motile cilia and the influx of extracellular Ca2+ usually occurs first in the cilia, but the exact mechanism is not known [105]. Increased expression of the PIEZO 1 protein ion channel allows more Ca2+ influx into the cell, acting as a second messenger and activating the Notch1 signalling pathway, upregulating the expression of ALP, Runx2, and OCN, thereby promoting the osteogenic differentiation of human periodontal stem cells (hPDLSC) [106].
Fig. 3.
Mechanical stimulation induces osteogenic differentiation of stem cells via the PIEZO pathway. Mechanical stimulation induces cilia, which causes Ca2+ to enter the cell via PIEZO, activating the Notch signalling pathway and upregulating osteogenic differentiation genes. Mechanical damage also phosphorylates p38 MAPK via the IL-1α receptor, activating the transcription factor CREBP, which binds to the PIEZO gene promoter and can upregulate PIEZO
Shear stress associated with local blood flow is a key piezoelectric channel activator [107], and stimulation of fluid flow in vitro deflects primary cilia on osteocytes, resulting in an immediate rise in cytosolic Ca2+. PIEZO 1 responds to shear stress-induced stretching of the cell membrane after mechanical loading, leading to the release of ATP in the extracellular environment. Sarcoplasmic reticulum (SR) Ca2+ ATPase 2 (SERCA2) is an ATPase that interacts with PIEZO 1 in the membrane bilayer at the endoplasmic reticulum (ER)-plasma membrane junction and inhibits the mechanosensitivity of PIEZO 1. Specific mechanical forces can also transport cytosolic Ca2+ into the SR/ER for storage, maintain Ca2+ homeostasis, and regulate the PIEZO pathway [108].
Chondrocytes in articular cartilage are one of the terminal cells of MSC differentiation, and PIEZO channels exhibit a key signal transduction role in the fate of chondrocytes. During endochondral ossification, PIEZO 1 inactivation in chondrocytes impairs trabecular bone formation, resulting in reduced ossification [109]. PIEZO 1 and PIEZO 2 also confer mechanosensitivity to chondrocytes by synergistic action. Mechanical stress induces apoptosis through Ca2+ influx from PIEZO to chondrocytes [110] (Fig. 3). PIEZO 2 plays a central role in the apoptotic response to chondrocyte injury [111]. In addition, articular chondrocyte IL-1α receptors can sense mechanical damage and activate transcription factors cyclic AMP (cAMP) response element (CRE)-binding protein 1(CREBP1) by phosphorylating p38 MAPK. CREBP1 binds directly to the proximal PIEZO1 gene promoter and upregulates PIEZO1 expression. PIEZO 1 induces excess intracellular Ca2+, and elevated resting-state Ca2+ in turn alters the F-actin cytoskeleton and amplifies mechanically induced trauma [112].
HIF-1α signalling pathway
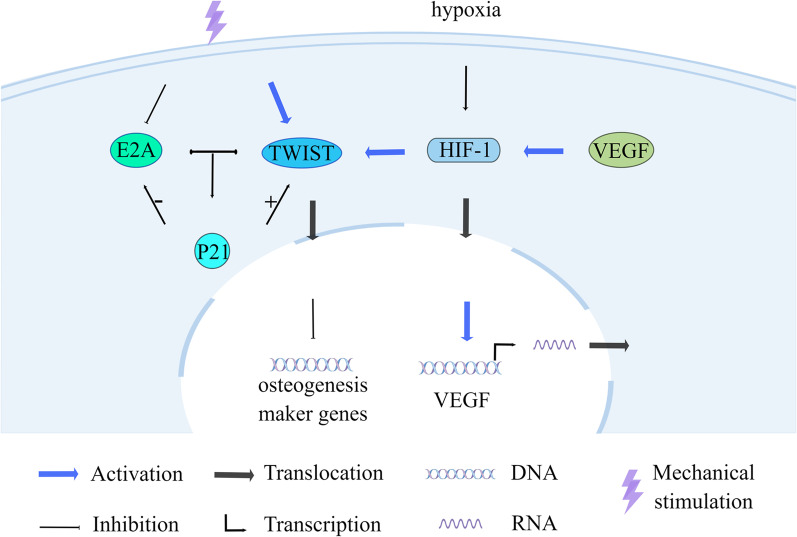
Hypoxia-inducible factor 1 (HIF-1) is a basic helix–loop–helix transcription factor that plays a role in apoptosis, and TWIST is also a helix–loop–helix transcription factor that controls gene expression during embryogenesis and the epithelial-mesenchymal transition. The HIF-1α/TWIST-mediated cellular response to oxygen affects stem cell differentiation and bone and cartilage histogenesis [113]. Natural cartilage formation requires hypoxic conditions, whereas bone formation is relatively normoxic [114]. Increased HIF-1α stability under hypoxic conditions stimulates prechondrogenic, anti-bone-forming, and anti-mast cell transcription. At higher oxygen concentrations, HIF-1α degradation promotes hypertrophy and osteoblast formation [115]. Hypoxia and HIF-1α also maintain the chondrogenic phenotype of cells by preventing cell hypertrophy or osteogenic differentiation [116] (Fig. 4). When HIF-1α is conditionally inactivated, the expression of the transcriptional regulator of chondrogenesis SOX9, and its downstream targets is reduced [117].
Fig. 4.
Maintenance of stem cell osteogenic factor homeostasis and maintenance of chondrocyte phenotype through the HIF-1 pathway. HIF-1α increases TWIST expression, which in turn regulates osteogenic differentiation. Mechanical stimulation also promotes TWIST and inhibits E2A; TWIST and E2A interact to activate p21. p21 has different regulatory effects on osteogenic factors. Also, p21 positively regulates the expression of TWIST and negatively regulates the expression of E2A. Hypoxia and HIF-1α maintain the chondrogenic phenotype of cells by preventing cell osteogenic differentiation
Under hypoxic conditions, HIF-1ɑ levels in bone marrow macrophages (BMM) are upregulated and can promote osteoclast formation [118]. HIF-1α increases TWIST expression, which in turn decreases RUNX2 and BMP2 expression [119] and regulates BMSC osteogenic differentiation [120]. However, the effect of cyclic tensile stress on Hif-1α expression varies across the stretch range and, in turn, affects different osteogenic differentiation capacities [121]. HIF-1α can also mediate the expression of RUNX2 in PDLSCs through the induction of vascular endothelial growth factor (VEGF) upregulation and promote early osteogenic differentiation [122]. YAP may also be involved in the mechanical stress-induced upregulation of HIF-1α [123].
Mechanical stimulation also induces osteogenic differentiation of BMSCs via the TWIST/E2A/p21 axis. Mechanical cyclic strain promotes TWIST and inhibits E2A, and TWIST and E2A interact to activate p21 expression. p21 has different regulatory effects on RUNX2 and BMP2, maintaining a relative balance between osteogenic factors. Meanwhile, p21 acts as a downstream gene of TWIST and E2A, regulating TWIST expression positively and E2A expression negatively [124] (Fig. 4). HIF-1 can also reduce the differentiation of peripheral blood MSCs (PBMSCs) into osteoblasts by increasing Notch1 expression [125].
Clinical applications of the mechanical environment of stem cells
Application of stem cells to treat diseases
Stem cells are receiving increasing attention in the fields of regenerative medicine and tissue engineering and are important for a wide range of diseases [1, 2]. Osteoarthritis, diabetes, osteoporosis, and blood-related diseases are specifically discussed below.
Human adipose mesenchymal progenitor cells (haMPCs) are stem cells with multiple differentiation potentials and immunomodulatory functions. Significant improvements in joint function, pain, quality of life, and cartilage regeneration were observed in patients with knee osteoarthritis after receiving intra-articular injections of ex vivo-expanded haMPCs from their own adipose tissue [126]. MSCs have also shown great potential for differentiation [51] and have applications in the clinic [127]. Injecting MSCs into the joint cavity provided significant relief of osteoarthritis symptoms and no serious adverse effects were observed [128]. Therefore, the application of stem cells in the field of osteoarthritis treatment is of interest. However, further integration and analysis of the efficacy and safety of stem cell therapy are still needed [129], and more research is needed. More types of stem cells are being studied which hold great promise for therapeutic use [130].
Diabetes mellitus (DM) is a serious metabolic disease characterized by hyperglycaemia and beta-cell dysfunction. Although medication is available to control the progression of the disease, it is difficult to cure it, so there is a real need for new and effective treatment modalities for DM. Stem cell therapy holds great promise for people with DM [131]. Stem cell-derived islets are a promising potential treatment for insulin-dependent diabetes [132]. Islet-like organs from progenitor cells are glucose-responsive and insulin-secreting and can reverse disease after transplantation in diabetic mice [133]. Tissue engineering of stem cells derived from adipose tissue can reduce hyperglycaemia and extend lifespan. There is also an opportunity for tissue-engineered islets in future stem cell therapy [33].
Osteoporosis is a widespread progressive bone disease that can pose a serious risk to people’s health and quality of life. Not only does it appear in older adults, but it also commonly afflicts space people who work in outer space. Researchers were inspired by the fact that exercise reduces the risk of osteoporosis in the population. Studies have shown that BMSCs can accelerate bone healing, ossification, and restoration of bone mechanical properties in osteoporotic fractures [134]. BMSCs have therefore become the subject of extensive research. Exosomes from cyclic mechanical stretch (CMS)-treated BMSCs inhibit osteoclastogenesis and ameliorate mechanical unloading-induced bone loss by attenuating NF-κB signalling pathway activity [135]. This result suggests that appropriate mechanical stimulation promotes osteogenic differentiation in BMSCs and provides a theoretical basis for why physical exercise prevents osteoporosis [136].
To overcome the limitations of the small amount of umbilical cord blood stem cells (UCB), intra-bone transfer of UCB (IB-UCB) is used. Intact haematopoietic stem cells were maintained by direct delivery of UCB into hypoxic HSC ecotopes, with rapid haematopoietic recovery and low incidence of graft-versus-host disease [137]. In addition, infusion of umbilical cord MSCs (UC-MSCs) may improve the efficacy of immunosuppressive therapy in children with severe aplastic anaemia and is safe [138]. Also, autologous stem cell transplantation therapy is safe and effective in newly diagnosed multiple myeloma [139]. Stem cells therefore have promising applications in a wide range of blood disorders.
In addition to these common diseases, stem cells have a wide range of applications, including periodontitis [140], acute liver injury [141], liver transplantation [142], and hereditary neonatal hyperammonemia [141], as well as those still waiting to be investigated.
Regulating the mechanical force of stem cells for disease control
Mechanical signals act as important influences on the fate of living organisms in a number of areas, including the circulatory system [143], neural tissue [144], tendon tissue engineering [145], periodontal tissue engineering [146], cartilage tissue engineering [15], and others. The effects of several mechanical forces on disease are specified below.
The external force generated using an in vitro mechanical device increases the stiffness of adipose tissue, thus affecting the migration and differentiation of ASCs. Different tissue stiffnesses have different effects in promoting the regeneration of adipose tissue. The use of mechanical devices to expand soft tissue holds promise for treating large soft tissue defects that are difficult to reconstruct through surgery [147]. Mechanical modulation of stiffness contributes to the use of MSCs in vascular tissue engineering [44].
Shear stress can cause a variety of clinical conditions. Platelet activation induced by shear stress is thought to be an important mechanism in acute coronary syndromes [148]. Shear stress is also associated with higher white matter lesion volume in migraine patients, which increases with lower endothelial shear stress [149]. Based on the effect of shear stress on disease, corresponding devices have also emerged that have good prospects for application in the medical industry. A fully automated bioreactor system (fABS) enhances the osteogenic differentiation of hBMSCs by generating shear stress on the one hand, and the hypoxia induced by fABS enhances the chondrogenic differentiation of hBMSCs on the other. Thus osteogenesis or chondrogenic differentiation can be balanced by regulating O2 concentration and controlling shear stress [47]. Also, a microfluidic dynamic culture system with shear treatment that promotes the expression of blood progenitors by mesenchymal cells and also differentiation towards smooth muscle and cardiomyocytes, acting in the haematopoietic spectrum and arterial vascular system, is promising for the simulation of human embryonic blood formation [45].
Intermittent shear flow has the potential to induce both circumferential stretch caused by HP of the fluid and shear stress caused by flow at the inner surface, while having a role in the simultaneous differentiation of MSC into epithelial and muscular lineages. Intermittent shear flow is more effective than steady shear flow for the development of oesophageal tissue engineering scaffolds [150]. The intermediate filament (IF) network under cyclic HP undergoes disruption and reorganization, translocating towards the perinuclear region, and is a potent mediator of cytoskeletal reorganization and increased osteogenic response in the MSC [59], which also demonstrates a potential new therapy for bone loss diseases such as osteoporosis.
The high HP in the periodontal ligament generated by the orthodontic force recruits the tooth cells and leaves a resorption pit on the root surface. Root resorption is more likely to occur when HP exceeds capillary blood pressure [151]. This finding offers new opportunities to combat orthodontically induced root resorption. In addition, low-intensity vibration therapy as a prophylactic strategy may have the potential as a non-pharmacological alternative to anti-resorptive and anabolic agents without adverse side effects [152], which also offers the possibility of non-pharmacological treatment of degenerative bone disease.
Microgravity has many well-known adverse effects on the human body. Prolonged exposure to microgravity during spaceflight can lead to severe osteoblast dysfunction, resulting in bone loss and causing conditions similar to osteoporosis [72] and disc herniation [153]. In addition, nearly half of the astronauts who landed after a long mission had reduced Hb and developed anaemia, and the magnitude of recovery depended on the duration of space exposure [73]. Astronauts in microgravity also suffer from immune system dysregulation [154] and elevated intracranial pressure (ICP) [155]. With so many adverse symptoms, there is an urgent need to understand the mechanisms by which microgravity causes disease in order to take preventive and remedial measures.
Conclusions
This paper summarises the impact of mechanical signals, including structural and force signals, in the microenvironment in which stem cells grow on stem cell differentiation and the mechanisms of how stem cells sense mechanical signals, and further discusses how mechanical factors affecting stem cells can be modified for clinical and disease applications. Existing studies on stem cell differentiation mostly ignore the role of mechanical cues in the environment. This review provides a timely summary of the impact of mechanical cues from the microenvironment in which stem cells reside. Mechanical signalling is an integral part of the study of stem cell differentiation that cannot be ignored and provides an important basis for future studies on the specialized differentiation of stem cells. However, the environmental components of stem cell growth are complex, and the mechanical signals generated by the interactions are also complex and diverse [150]. This article only summarises some of the clues, and more mechanistic factors will be discovered in the future. For example, differentiated daughter cells of stem cells are also a component of the stem cell ecotone [156] and daughter cells may also generate some sort of mechanical signal to participate in the composition of the mechanical microenvironment. Additionally, these mechanical cues do not have a single effect on stem cells [157, 158]. And the combination of different mechanical factors is more conducive to the differentiation of stem cells in a more favourable direction [159]. Therefore, how mechanical signals can better cooperate with each other to synergistically influence cell fate should also be considered. In addition, there are a variety of mechanosensing pathways [59, 160, 161], and in future both currently known (but not linked to mechanotransduction) pathways and undiscovered signalling pathways will increasingly be found to play a role in mechanosensing and deserve further investigation. Furthermore, cell membrane tension has an impact on cell fate [162]. Studies have shown that reduced cell membrane tension is a necessary but not sufficient condition for cell fate to shift from self-renewal to differentiation [160]. Therefore, whether cell membrane tension may act as a transmitter in the process of cells sensing the mechanical signals of the extracellular environment and mediate the effects of mechanical changes in the extracellular environment on cell fate deserves further study.
In addition, the performance of implanted stem cells depends not only on differentiation but also on migration, adhesion, proliferation, and paracrine secretion, which deserve to be explored in depth in the future. Different ECM stiffness affects cell-ECM adhesion, cell spreading and migration [163]. The directional migration of cells needs to be guided by certain signals, such as rigidity and topological chemotaxis. Cells have the ability to sense differences in base stiffness and migrate by migrating towards or away from areas of higher stiffness. In addition to this, cells can also sense topographical features of the surrounding environment, known as topological chemotaxis [164]. In addition, genetically reducing the stiffness of the basement membrane in the stratified epidermis increases membrane tension, leading to loss of membrane integrity and enhanced invasiveness of cancerous cells [165]. During tumorigenesis, the ECM undergoes remodelling, which is manifested by changes in molecular composition. The reconstituted ECM exhibits increased tension and stiffness, leading to hyperproliferation, poor differentiation, and invasion and metastasis of tumour cells. When tumour cells grow, the limited space generates compressive mechanical stress, limiting cell proliferation. During epithelial-mesenchymal transition, epithelial cells lose their polarity and cell-to-cell adhesion and acquire the ability to migrate. At the same time, epithelial–mesenchymal transition may promote cell morphological changes and promote proliferation [166]. In addition, different polymers were mixed in any ratio to make microstrip structures and cross-linked into 3D scaffolds to culture MSCs. These mixed microstrips could induce synergy through paracrine signalling to accelerate cartilage regeneration of MSCs [167].
Experiments in which stem cells respond to a mechanical environment sometimes show results that are contrary to previous studies [168], possibly because the cells have a memory of past mechanical cues and this memory remains useful for the behaviour of the stem cells over time [169]. By storing and removing proteins associated with mechanical memory [170], it is possible to alter cell fate, which also provides a direction for stem cell research. The role of stem cell differentiation in medicine is no longer in doubt. In the future, not only will stem cells play a role in the treatment of more diseases, but stem cell-based mechanical microenvironments are expected to be used in a variety of models [77, 171]. Mechanical microenvironments of stem cells will also become increasingly relevant to clinical applications.
In conclusion, this review summarizes the effects of the mechanical microenvironment of stem cell growth on stem cell differentiation and the corresponding mechanisms, which have important implications for clinical disease treatment.
Acknowledgements
We sincerely thank the free drawing support provided by the Figdraw platform (www. figdraw. com). Figures 1, 2, 3 and 4 in the paper were drawn using figdraw. We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- ECM
Extracellular matrix
- dECM
Cell-derived ECM
- BMSC
Bone marrow mesenchymal stem cell
- β-TCP
β-Tricalcium phosphate
- hESC
Human embryonic stem cell
- BMP2
Bone morphogenetic protein 2
- PCL
Polycaprolactone
- ASCs
Adipose stem cells
- hASCs
Human adipose stem cells
- iPSC
Induced pluripotent stem cell
- PLA
Polylactic acid
- hMSC
Human mesenchymal stem cell
- rAMSCs
Rat adipose mesenchymal stem cells
- NSPC
Neural stem progenitor cell
- PA
Polyamide
- VPCs
Vascular progenitor cells
- ECs
Endothelial cells
- mMSCs
Mouse mesenchymal stem cells
- MSC
Mesenchymal stem cell
- hPSC-ECs
Human pluripotent stem cell-derived endothelial cells
- rBMSCs
Rat bone marrow mesenchymal stem cells
- 5-Aza
5-Azacytidiner
- HP
Hydrostatic pressure
- PRF
Platelet-rich fibrin
- IHP
Intermittent hydrostatic pressure
- CHP
Circulating hydrostatic pressure
- DBM
Decalcified bone matrix
- EPCs
Endothelial progenitor cells
- Col I
Type I collagen
- GAG
Glycosaminoglycan
- Col II
Type II collagen
- HSC
Hematopoietic stem cell
- MHA/Coll
Magnesium-doped hydroxyapatite/type I collagen composite scaffold
- hADSCs
Human adipose-derived stem cells
- mESC
Mouse embryonic stem cell
- NF-κB
Nuclear factor κ-B
- RANK
NF-κB receptor activator
- RANKL
Ligand for RANK
- RUNX2
Runt-related transcription factor 2
- ALP
Alkaline phosphatase
- OPG
Osteoprotegerin
- IκB
NF-κB inhibitor
- IL
Interleukin
- ROS
Reactive oxygen species
- PPARγ
Peroxisome proliferators-activated receptor γ
- CMS
Cyclic mechanical stretch
- nAChR
Nicotinic acetylcholine receptor
- ACh
Acetylcholine
- α7nAChR
α7 Nicotinic acetylcholine receptor
- TNF-α
Tumour necrosis factor-α
- GSK-3β
Glycogen synthase kinase-3β
- PDLSCs
Periodontal stem cells
- p-GSK-3 β
Phosphorylated GSK-3β
- ISC
Intestinal stem cell
- PIEZO
Piezoelectric mechanosensitive ion channel
- hPDLSC
Human periodontal stem cell
- SR
Sarcoplasmic reticulum
- SERCA2
Sarcoplasmic reticulum Ca2+ ATPase 2
- ER
Endoplasmic reticulum
- CREBP1
Cyclic AMP response element-binding protein 1
- HIF-1
Hypoxia-inducible factor 1
- BMM
Bone marrow macrophage
- VEGF
Vascular endothelial growth factor
- PBMSCs
Peripheral blood mesenchymal stem cells
- haMPCs
Human adipose mesenchymal progenitor cells
- DM
Diabetes mellitus
- CMS
Cyclic mechanical stretch
- UCB
Umbilical cord blood stem cell
- IB-UCB
Intra-bone transfer of umbilical cord blood stem cell
- UC-MSCs
Umbilical cord mesenchymal stem cells
- fABS
Fully automated bioreactor system
- IF
Intermediate filament
- ICP
Intracranial pressure
- MMP-13
Metalloproteinase-13
- CDK6
Cyclin-dependent kinase 6
Author contributions
XZ contributed to the literature review, manuscript writing, and dissertation revision. SZ participated in its modification. TW conceived and designed the study. All authors read and approved the final manuscript.
Funding
This work is supported by the Liaoning Province Natural Science Foundation [2020-ZLLH-47, 2020-MS-065, 2021-YGJC-02], the Joint Fund of Liaoning Province Science and Technology Department and State Key Laboratory of Robotics [2019-KF-01-01], the Medical Engineering Cross Research Fund [LD202127], the Liaoning Province Science and Technology Plan Project [2017225054], and the Tumor Mass spectrometry Center Project [ZP202008].
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaofang Zhang and Sibo Zhang contributed equally to this work
References
- 1.Yamada Y, et al. Clinical potential and current progress of dental pulp stem cells for various systemic diseases in regenerative medicine: a concise review. Int J Mol Sci. 2019;20(5):1132. doi: 10.3390/ijms20051132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzola M, Di Pasquale E. Toward cardiac regeneration: combination of pluripotent stem cell-based therapies and bioengineering strategies. Front Bioeng Biotechnol. 2020;8:455. doi: 10.3389/fbioe.2020.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daadi MM. Isolation and purification of self-renewable human neural stem cells from iPSCs for cell therapy in experimental model of ischemic stroke. Methods Mol Biol. 2022;2389:165–175. doi: 10.1007/978-1-0716-1783-0_14. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo Y, et al. Isolation of adipose tissue-derived stem cells by direct membrane migration and expansion for clinical application. Hum Cell. 2021;34(3):819–824. doi: 10.1007/s13577-021-00505-3. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez SJ, et al. Cooperation of cell adhesion and autophagy in the brain: functional roles in development and neurodegenerative disease. Matrix Biol Plus. 2021;12:100089. doi: 10.1016/j.mbplus.2021.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazini L, et al. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs) Int J Mol Sci. 2019;20(10):2523. doi: 10.3390/ijms20102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atashi F, et al. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev. 2015;24(10):1150–1163. doi: 10.1089/scd.2014.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghaddam MM, et al. The effect of physical cues on the stem cell differentiation. Curr Stem Cell Res Ther. 2019;14(3):268–277. doi: 10.2174/1574888X14666181227120706. [DOI] [PubMed] [Google Scholar]
- 9.Helms F, et al. Complete myogenic differentiation of adipogenic stem cells requires both biochemical and mechanical stimulation. Ann Biomed Eng. 2020;48(3):913–926. doi: 10.1007/s10439-019-02234-z. [DOI] [PubMed] [Google Scholar]
- 10.Rinoldi C, et al. Tendon tissue engineering: effects of mechanical and biochemical stimulation on stem cell alignment on cell-laden hydrogel yarns. Adv Healthc Mater. 2019;8(7):e1801218. doi: 10.1002/adhm.201801218. [DOI] [PubMed] [Google Scholar]
- 11.Halim A, et al. Recent progress in engineering mesenchymal stem cell differentiation. Stem Cell Rev Rep. 2020;16(4):661–674. doi: 10.1007/s12015-020-09979-4. [DOI] [PubMed] [Google Scholar]
- 12.Wilems T, et al. The influence of microenvironment and extracellular matrix molecules in driving neural stem cell fate within biomaterials. Brain Res Bull. 2019;148:25–33. doi: 10.1016/j.brainresbull.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang P, et al. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res Ther. 2019;10(1):327. doi: 10.1186/s13287-019-1422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han S-B, et al. Mechanical properties of materials for stem cell differentiation. Adv Biosyst. 2020;4(11):2000247. doi: 10.1002/adbi.202000247. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Yao Y. The role of mechanical regulation in cartilage tissue engineering. Curr Stem Cell Res Ther. 2021;16(8):939–948. doi: 10.2174/1574888X16666210303151538. [DOI] [PubMed] [Google Scholar]
- 16.Cagigas ML, et al. Correlative cryo-ET identifies actin/tropomyosin filaments that mediate cell-substrate adhesion in cancer cells and mechanosensitivity of cell proliferation. Nat Mater. 2022;21(1):120–128. doi: 10.1038/s41563-021-01087-z. [DOI] [PubMed] [Google Scholar]
- 17.Naqvi SM, McNamara LM. Stem cell mechanobiology and the role of biomaterials in governing mechanotransduction and matrix production for tissue regeneration. Front Bioeng Biotechnol. 2020;8:597661. doi: 10.3389/fbioe.2020.597661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker BM, et al. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater. 2015;14(12):1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, et al. 3D spatiotemporal mechanical microenvironment: a hydrogel-based platform for guiding stem cell fate. Adv Mater. 2018;30(49):e1705911. doi: 10.1002/adma.201705911. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho MS, et al. Cultured cell-derived extracellular matrices to enhance the osteogenic differentiation and angiogenic properties of human mesenchymal stem/stromal cells. J Tissue Eng Regen Med. 2019;13(9):1544–1558. doi: 10.1002/term.2907. [DOI] [PubMed] [Google Scholar]
- 21.von Erlach TC, et al. Cell-geometry-dependent changes in plasma membrane order direct stem cell signalling and fate. Nat Mater. 2018;17(3):237–242. doi: 10.1038/s41563-017-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zonderland J, Moroni L. Steering cell behavior through mechanobiology in 3D: a regenerative medicine perspective. Biomaterials. 2021;268:120572. doi: 10.1016/j.biomaterials.2020.120572. [DOI] [PubMed] [Google Scholar]
- 23.Arpornmaeklong P, Pressler MJ. Effects of ss-TCP scaffolds on neurogenic and osteogenic differentiation of human embryonic stem cells. Ann Anat. 2018;215:52–62. doi: 10.1016/j.aanat.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Ji S, et al. Polyester-based ink platform with tunable bioactivity for 3D printing of tissue engineering scaffolds. Biomater Sci. 2019;7(2):560–570. doi: 10.1039/C8BM01269E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choe G, et al. Graphene oxide/alginate composites as novel bioinks for three-dimensional mesenchymal stem cell printing and bone regeneration applications. Nanoscale. 2019;11(48):23275–23285. doi: 10.1039/C9NR07643C. [DOI] [PubMed] [Google Scholar]
- 26.Blum JC, et al. Artificial decellularized extracellular matrix improves the regenerative capacity of adipose tissue derived stem cells on 3D printed polycaprolactone scaffolds. J Tissue Eng. 2021;12:20417314211022242. doi: 10.1177/20417314211022242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetzke R, et al. Differentiation of induced pluripotent stem cells towards mesenchymal stromal cells is hampered by culture in 3D hydrogels. Sci Rep. 2019;9(1):15578. doi: 10.1038/s41598-019-51911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamloo A, et al. Investigation into the interaction between quartz nanostructures and human cell lines for tissue engineering. Sci Iran. 2020;27(5):2343–2352. [Google Scholar]
- 29.Wu C, et al. Rapid nanomolding of nanotopography on flexible substrates to control muscle cell growth with enhanced maturation. Microsyst Nanoeng. 2021;7:89. doi: 10.1038/s41378-021-00316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaswal R, et al. Nanographene enfolded AuNPs sophisticatedly synchronized polycaprolactone based electrospun nanofibre scaffold for peripheral nerve regeneration. Mater Sci Eng C Mater Biol Appl. 2020;116:111213. doi: 10.1016/j.msec.2020.111213. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, et al. Simultaneously constructing nanotopographical and chemical cues in 3D-printed polylactic acid scaffolds to promote bone regeneration. Mater Sci Eng C Mater Biol Appl. 2021;118:111457. doi: 10.1016/j.msec.2020.111457. [DOI] [PubMed] [Google Scholar]
- 32.Brennan CM, et al. The effect of pore size within fibrous scaffolds fabricated using melt electrowriting on human bone marrow stem cell osteogenesis. Biomed Mater. 2019;14(6):065016. doi: 10.1088/1748-605X/ab49f2. [DOI] [PubMed] [Google Scholar]
- 33.Anitha R, et al. Tissue-engineered islet-like cell clusters generated from adipose tissue-derived stem cells on three-dimensional electrospun scaffolds can reverse diabetes in an experimental rat model and the role of porosity of scaffolds on cluster differentiation. J Biomed Mater Res A. 2020;108(3):749–759. doi: 10.1002/jbm.a.36854. [DOI] [PubMed] [Google Scholar]
- 34.Fernández D, et al. Fibrous materials made of poly(ε-caprolactone)/poly(ethylene oxide)-b-poly(ε-caprolactone) blends support neural stem cells differentiation. Polymers (Basel) 2019;11(10):1621. doi: 10.3390/polym11101621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, et al. β-Tricalcium phosphate contained beaded-fiber scaffolds characterized by high early osteoinductive activity for vascularized bone regeneration. Colloids Surf B Biointerfaces. 2021;201:111639. doi: 10.1016/j.colsurfb.2021.111639. [DOI] [PubMed] [Google Scholar]
- 36.Kaitsuka T, et al. A culture substratum with net-like polyamide fibers promotes the differentiation of mouse and human pluripotent stem cells to insulin-producing cells. Biomed Mater. 2019;14(4):045019. doi: 10.1088/1748-605X/ab261c. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, et al. Stiffness regulates the morphology, adhesion, proliferation, and osteogenic differentiation of maxillary Schneiderian sinus membrane-derived stem cells. Stem Cells Int. 2021;2021:8868004. doi: 10.1155/2021/8868004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enhejirigala, et al. Influence of the stiffness of three-dimensionally bioprinted extracellular matrix analogue on the differentiation of bone mesenchymal stem cells into skin appendage cells. Zhonghua Shao Shang Za Zhi. 2020;36(11):1013–1023. doi: 10.3760/cma.j.cn501120-20200811-00375. [DOI] [PubMed] [Google Scholar]
- 39.Wei D, et al. Dynamically modulated core-shell microfibers to study the effect of depth sensing of matrix stiffness on stem cell fate. ACS Appl Mater Interfaces. 2021;13(32):37997–38006. doi: 10.1021/acsami.1c06752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong L, et al. Substrate stiffness directs diverging vascular fates. Acta Biomater. 2019;96:321–329. doi: 10.1016/j.actbio.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 41.Ventre M, et al. Aligned fibrous decellularized cell derived matrices for mesenchymal stem cell amplification. J Biomed Mater Res A. 2019;107(11):2536–2546. doi: 10.1002/jbm.a.36759. [DOI] [PubMed] [Google Scholar]
- 42.Luo C, et al. Hepatic differentiation of human embryonic stem cells by coupling substrate stiffness and microtopography. Biomater Sci. 2021;9(10):3776–3790. doi: 10.1039/D1BM00174D. [DOI] [PubMed] [Google Scholar]
- 43.Lenzini S, et al. Leveraging biomaterial mechanics to improve pluripotent stem cell applications for tissue engineering. Front Bioeng Biotechnol. 2019;7:260. doi: 10.3389/fbioe.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parandakh A, et al. Substrate topography interacts with substrate stiffness and culture time to regulate mechanical properties and smooth muscle differentiation of mesenchymal stem cells. Colloids Surf B Biointerfaces. 2019;173:194–201. doi: 10.1016/j.colsurfb.2018.09.066. [DOI] [PubMed] [Google Scholar]
- 45.Li J, et al. the role of shear stress in the generation of definitive haematopoietic lineages and arterial vasculature from human pluripotent stem cells at the single-cell level. Exp Hematol. 2019;76:S73–S74. doi: 10.1016/j.exphem.2019.06.390. [DOI] [Google Scholar]
- 46.Arora S, et al. Determination of critical shear stress for maturation of human pluripotent stem cell-derived endothelial cells towards an arterial subtype. Biotechnol Bioeng. 2019;116(5):1164–1175. doi: 10.1002/bit.26910. [DOI] [PubMed] [Google Scholar]
- 47.Lim K-T, et al. A fully automated bioreactor system for precise control of stem cell proliferation and differentiation. Biochem Eng J. 2019;150:107258. doi: 10.1016/j.bej.2019.107258. [DOI] [Google Scholar]
- 48.Li Q, et al. Optimization of the theoretical model for growth rate of mesenchymal stem cells on three-dimensional scaffold under fluid shear stress. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2019;36(5):795–802. doi: 10.7507/1001-5515.201904025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dash SK, et al. Low intermittent flow promotes rat mesenchymal stem cell differentiation in logarithmic fluid shear device. Biomicrofluidics. 2020;14(5):054107. doi: 10.1063/5.0024437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yue D, et al. The rate of fluid shear stress is a potent regulator for the differentiation of mesenchymal stem cells. J Cell Physiol. 2019;234:16312–16319. doi: 10.1002/jcp.28296. [DOI] [PubMed] [Google Scholar]
- 51.Elashry MI, et al. Combined macromolecule biomaterials together with fluid shear stress promote the osteogenic differentiation capacity of equine adipose-derived mesenchymal stem cells. Stem Cell Res Ther. 2021;12(1):116. doi: 10.1186/s13287-021-02146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izadpanah P, et al. The effect of shear stress on cardiac differentiation of mesenchymal stem cells. Mol Biol Rep. 2022;49:3167–3175. doi: 10.1007/s11033-022-07149-y. [DOI] [PubMed] [Google Scholar]
- 53.Lei X, et al. The effect of fluid shear stress on fibroblasts and stem cells on plane and groove topographies. Cell Adhes Migr. 2020;14(1):12–23. doi: 10.1080/19336918.2020.1713532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciardulli MC, et al. Tendon and cytokine marker expression by human bone marrow mesenchymal stem cells in a hyaluronate/poly-lactic-co-glycolic acid (PLGA)/fibrin three-dimensional (3D) scaffold. Cells. 2020;9(5):1268. doi: 10.3390/cells9051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider I, et al. 3D microtissue-derived human stem cells seeded on electrospun nanocomposites under shear stress: modulation of gene expression. J Mech Behav Biomed Mater. 2020;102:103481. doi: 10.1016/j.jmbbm.2019.103481. [DOI] [PubMed] [Google Scholar]
- 56.Cheng B, et al. A novel construct with biomechanical flexibility for articular cartilage regeneration. Stem Cell Res Ther. 2019;10(1):1–16. doi: 10.1186/s13287-018-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jahanbakhsh A, et al. Evaluation of alginate modification effect on cell-matrix interaction, mechanotransduction and chondrogenesis of encapsulated MSCs. Cell Tissue Res. 2020;381(2):255–272. doi: 10.1007/s00441-020-03216-7. [DOI] [PubMed] [Google Scholar]
- 58.Khorshidi S, et al. Concurrent effects of piezoelectricity and hydrostatic pressure on chondrogenic differentiation of stem. Mater Lett. 2019;246:71–75. doi: 10.1016/j.matlet.2019.03.038. [DOI] [Google Scholar]
- 59.Stavenschi E, Hoey DA. Pressure-induced mesenchymal stem cell osteogenesis is dependent on intermediate filament remodeling. FASEB J. 2019;33(3):4178–4187. doi: 10.1096/fj.201801474RR. [DOI] [PubMed] [Google Scholar]
- 60.Shahmoradi SR, et al. Induction of chondrogenic differentiation in human mesenchymal stem cells cultured on human demineralized bone matrix scaffold under hydrostatic pressure. Tissue Eng Regen Med. 2019;16(1):69–80. doi: 10.1007/s13770-018-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xi X, et al. Nrf2 activation is involved in osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch. Exp Cell Res. 2021;403(2):112598. doi: 10.1016/j.yexcr.2021.112598. [DOI] [PubMed] [Google Scholar]
- 62.Wu X, et al. Mechanism of cyclic tensile stress in osteogenic differentiation of human periodontal ligament stem cells. Calcif Tissue Int. 2021;108(5):640–653. doi: 10.1007/s00223-020-00789-x. [DOI] [PubMed] [Google Scholar]
- 63.Tantilertanant Y, et al. Cyclic tensile force stimulates BMP9 synthesis and in vitro mineralization by human periodontal ligament cells. J Cell Physiol. 2019;234(4):4528–4539. doi: 10.1002/jcp.27257. [DOI] [PubMed] [Google Scholar]
- 64.Li N, et al. MicroRNA-129-1-3p regulates cyclic stretch-induced endothelial progenitor cell differentiation by targeting Runx2. J Cell Biochem. 2019;120(4):5256–5267. doi: 10.1002/jcb.27800. [DOI] [PubMed] [Google Scholar]
- 65.Maeda E, et al. Shape-dependent regulation of differentiation lineages of bone marrow-derived cells under cyclic stretch. J Biomech. 2019;96:109371. doi: 10.1016/j.jbiomech.2019.109371. [DOI] [PubMed] [Google Scholar]
- 66.Zhu G, et al. Tensile strain promotes osteogenic differentiation of bone marrow mesenchymal stem cells through upregulating lncRNA-MEG3. Histol Histopathol. 2021;36:18365–18365. doi: 10.14670/HH-18-365. [DOI] [PubMed] [Google Scholar]
- 67.Zhang D, et al. Uniaxial cyclic stretching promotes chromatin accessibility of gene loci associated with mesenchymal stem cells morphogenesis and osteogenesis. Front Cell Dev Biol. 2021;9:1613. doi: 10.3389/fcell.2021.664545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y-Q, et al. Advances on research of physical environment affecting stem cell differentiation in ligament tissue engineering. China J Orthop Traumatol. 2020;33(11):1080–1084. doi: 10.12200/j.issn.1003-0034.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 69.Su X, et al. Effects of dynamic radial tensile stress on fibrocartilage differentiation of bone marrow mesenchymal stem cells. Biomed Eng Online. 2020;19(1):8. doi: 10.1186/s12938-020-0751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han Y, et al. Molecular genetic analysis of neural stem cells after space flight and simulated microgravity on earth. Biotechnol Bioeng. 2021;118:3832–3846. doi: 10.1002/bit.27858. [DOI] [PubMed] [Google Scholar]
- 71.Wang P, et al. The maintaining and directed differentiation of hematopoietic stem cells under microgravity. In: Duan E, Long M, et al., editors. Life science in space: experiments on board the SJ-10 recoverable satellite. Singapore: Springer; 2019. pp. 205–233. [Google Scholar]
- 72.Li H, et al. Spaceflight promoted myocardial differentiation of induced pluripotent stem cells: results from Tianzhou-1 space mission. Stem Cells Dev. 2019;28(6):357–360. doi: 10.1089/scd.2018.0240. [DOI] [PubMed] [Google Scholar]
- 73.Trudel G, et al. Characterizing the effect of exposure to microgravity on anemia: more space is worse. Am J Hematol. 2020;95(3):267–273. doi: 10.1002/ajh.25699. [DOI] [PubMed] [Google Scholar]
- 74.Cao D, et al. Hematopoietic stem cells and lineage cells undergo dynamic alterations under microgravity and recovery conditions. FASEB J. 2019;33(6):6904–6918. doi: 10.1096/fj.201802421RR. [DOI] [PubMed] [Google Scholar]
- 75.Li L, et al. Effects of simulated microgravity on the expression profiles of RNA during osteogenic differentiation of human bone marrow mesenchymal stem cells. Cell Prolif. 2019;52(2):e12539. doi: 10.1111/cpr.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen L, et al. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells following transfection with Indian hedgehog and sonic hedgehog using a rotary cell culture system. Cell Mol Biol Lett. 2019;24:16. doi: 10.1186/s11658-019-0144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Avitabile E, et al. Bioinspired scaffold action under the extreme physiological conditions of simulated space flights: osteogenesis enhancing under microgravity. Front Bioeng Biotechnol. 2020;8:722. doi: 10.3389/fbioe.2020.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao XH, et al. Osteogenic differentiation system based on biopolymer nanoparticles for stem cells in simulated microgravity. Biomed Mater. 2021;16:044102. doi: 10.1088/1748-605X/abe9d1. [DOI] [PubMed] [Google Scholar]
- 79.Oss-Ronen L, et al. Enhanced induction of definitive endoderm differentiation of mouse embryonic stem cells in simulated microgravity. Stem Cells Dev. 2020;29(19):1275–1284. doi: 10.1089/scd.2020.0097. [DOI] [PubMed] [Google Scholar]
- 80.Ke C, et al. Effect of simulated microgravity on human chondrocyte-like cells. Int J Clin Exp Med. 2019;12(7):8718–8724. [Google Scholar]
- 81.Sun S, et al. Cell growth and differentiation under microgravity. In: Duan E, Long M, et al., editors. Life science in space: experiments on board the SJ-10 recoverable satellite. Berlin: Springer; 2019. pp. 167–188. [Google Scholar]
- 82.Nishida D, et al. RANKL/OPG ratio regulates odontoclastogenesis in damaged dental pulp. Sci Rep. 2021;11(1):4575. doi: 10.1038/s41598-021-84354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sakamoto M, et al. Vibration enhances osteoclastogenesis by inducing RANKL expression via NF-κB signaling in osteocytes. Bone. 2019;123:56–66. doi: 10.1016/j.bone.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 84.Chen X, et al. Mechanical stretch-induced osteogenic differentiation of human jaw bone marrow mesenchymal stem cells (hJBMMSCs) via inhibition of the NF-κB pathway. Cell Death Dis. 2018;9(2):207. doi: 10.1038/s41419-018-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Luca F. Regulatory role of NF-κB in growth plate chondrogenesis and its functional interaction with Growth Hormone. Mol Cell Endocrinol. 2020;514:110916. doi: 10.1016/j.mce.2020.110916. [DOI] [PubMed] [Google Scholar]
- 86.Hou C, et al. The role of MicroRNA-381 in chondrogenesis and interleukin-1-β induced chondrocyte responses. Cell Physiol Biochem. 2015;36(5):1753–1766. doi: 10.1159/000430148. [DOI] [PubMed] [Google Scholar]
- 87.Hou W, et al. Excavating bioactivities of nanozyme to remodel microenvironment for protecting chondrocytes and delaying osteoarthritis. Bioact Mater. 2021;6(8):2439–2451. doi: 10.1016/j.bioactmat.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun H, et al. CDK6 and miR-320c co-regulate chondrocyte catabolism through NF-κB signaling pathways. Cell Physiol Biochem. 2018;51(2):909–923. doi: 10.1159/000495392. [DOI] [PubMed] [Google Scholar]
- 89.Huang M, et al. Mechanical loading attenuates breast cancer-associated bone metastasis in obese mice by regulating the bone marrow microenvironment. J Cell Physiol. 2021;236(9):6391–6406. doi: 10.1002/jcp.30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, et al. Fluid shear stress increases osteocyte and inhibits osteoclasts via downregulating receptor-activator of nuclear factor kappaB (RANK)/osteoprotegerin expression in myeloma microenvironment. Med Sci Monit. 2019;25:5961–5968. doi: 10.12659/MSM.915986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang R, et al. Mechanical strain triggers differentiation of dental mesenchymal stem cells by activating osteogenesis-specific biomarkers expression. Am J Transl Res. 2019;11(1):233–244. [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Y, et al. Mechanical stress modulates the RANKL/OPG system of periodontal ligament stem cells via alpha7 nAChR in human deciduous teeth: an in vitro study. Stem Cells Int. 2019;2019:5326341. doi: 10.1155/2019/5326341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiao F, et al. Exosomes derived from cyclic mechanical stretch-exposed bone marrow mesenchymal stem cells inhibit RANKL-induced osteoclastogenesis through the NF-kappa B signaling pathway. Ann Transl Med. 2021;9(9):798. doi: 10.21037/atm-21-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi T. Roles of nAChR and Wnt signaling in intestinal stem cell function and inflammation. Int Immunopharmacol. 2020;81:106260. doi: 10.1016/j.intimp.2020.106260. [DOI] [PubMed] [Google Scholar]
- 95.Ihnatovych I, et al. iPSC model of CHRFAM7A effect on α7 nicotinic acetylcholine receptor function in the human context. Transl Psychiatry. 2019;9(1):59. doi: 10.1038/s41398-019-0375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi T. Multiple roles for cholinergic signaling from the perspective of stem cell function. Int J Mol Sci. 2021;22(2):666. doi: 10.3390/ijms22020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Z, et al. Inflammation has synergistic effect with nicotine in periodontitis by up-regulating the expression of α7 nAChR via phosphorylated GSK-3β. J Cell Mol Med. 2020;24(4):2663–2676. doi: 10.1111/jcmm.14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takahashi T, et al. Upregulated nicotinic ACh receptor signaling contributes to intestinal stem cell function through activation of Hippo and Notch signaling pathways. Int Immunopharmacol. 2020;88:106984. doi: 10.1016/j.intimp.2020.106984. [DOI] [PubMed] [Google Scholar]
- 99.Yu W, et al. Nicotine inhibits osteogenic differentiation of human periodontal ligament cells under cyclic tensile stress through canonical Wnt pathway and α7 nicotinic acetylcholine receptor. J Periodontal Res. 2018;53(4):555–564. doi: 10.1111/jre.12545. [DOI] [PubMed] [Google Scholar]
- 100.Hao L, et al. Synergistic osteogenesis promoted by magnetically actuated nano-mechanical stimuli. Nanoscale. 2019;11(48):23423–23437. doi: 10.1039/C9NR07170A. [DOI] [PubMed] [Google Scholar]
- 101.Tallapragada NP, et al. Inflation-collapse dynamics drive patterning and morphogenesis in intestinal organoids. Cell Stem Cell. 2021;28(9):1516–1532e14. doi: 10.1016/j.stem.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu T, et al. Involvement of mechanosensitive ion channels in the effects of mechanical stretch induces osteogenic differentiation in mouse bone marrow mesenchymal stem cells. J Cell Physiol. 2021;236(1):284–293. doi: 10.1002/jcp.29841. [DOI] [PubMed] [Google Scholar]
- 103.Miyazaki A, et al. Coordination of WNT signaling and ciliogenesis during odontogenesis by piezo type mechanosensitive ion channel component 1. Sci Rep. 2019;9:1–13. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morozumi W, et al. Piezo channel plays a part in retinal ganglion cell damage. Exp Eye Res. 2020;191:107900. doi: 10.1016/j.exer.2019.107900. [DOI] [PubMed] [Google Scholar]
- 105.Saternos H, et al. Primary cilia and calcium signaling interactions. Int J Mol Sci. 2020;21(19):7109. doi: 10.3390/ijms21197109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang L, et al. Mechanisms of the mechanically activated ion channel Piezo1 protein in mediating osteogenic differentiation of periodontal ligament stem cells via the Notch signaling pathway. West China J Stomatol. 2020;38(6):628–636. doi: 10.7518/hxkq.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shah V, et al. Emerging role of piezo ion channels in cardiovascular development. Dev Dyn. 2021;251:276–286. doi: 10.1002/dvdy.401. [DOI] [PubMed] [Google Scholar]
- 108.Bratengeier C, et al. High shear stress amplitude in combination with prolonged stimulus duration determine induction of osteoclast formation by hematopoietic progenitor cells. FASEB J. 2020;34(3):3755–3772. doi: 10.1096/fj.201901458R. [DOI] [PubMed] [Google Scholar]
- 109.Hendrickx G, et al. Piezo1 inactivation in chondrocytes impairs trabecular bone formation. J Bone Miner Res. 2021;36(2):369–384. doi: 10.1002/jbmr.4198. [DOI] [PubMed] [Google Scholar]
- 110.Lee W, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A. 2014;111(47):E5114–E5122. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Du G, et al. Roles of TRPV4 and piezo channels in stretch-evoked Ca(2+) response in chondrocytes. Exp Biol Med (Maywood, N.J.) 2020;245(3):180–189. doi: 10.1177/1535370219892601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee W, et al. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc Natl Acad Sci U S A. 2021;118(13):e2001611118. doi: 10.1073/pnas.2001611118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fu L, et al. Roles of oxygen level and hypoxia-inducible factor signaling pathway in cartilage, bone and osteochondral tissue engineering. Biomed Mater. 2021;16(2):022006. doi: 10.1088/1748-605X/abdb73. [DOI] [PubMed] [Google Scholar]
- 114.Taheem DK, et al. Hypoxia inducible factor-1α in osteochondral tissue engineering. Tissue Eng Part B Rev. 2020;26(2):105–115. doi: 10.1089/ten.teb.2019.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nilsson O, et al. Gradients in bone morphogenetic protein-related gene expression across the growth plate. J Endocrinol. 2007;193(1):75–84. doi: 10.1677/joe.1.07099. [DOI] [PubMed] [Google Scholar]
- 116.Amarilio R, et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134(21):3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 117.Provot S, et al. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007;177(3):451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu X, et al. High levels of HIF-1ɑ in hypoxic dental pulps associated with teeth with severe periodontitis. J Mol Histol. 2020;51(3):265–275. doi: 10.1007/s10735-020-09878-5. [DOI] [PubMed] [Google Scholar]
- 119.Liu Y, et al. HIF-1 alpha-TWIST pathway restrains cyclic mechanical stretch-induced osteogenic differentiation of bone marrow mesenchymal stem cells. Connect Tissue Res. 2019;60(6):544–554. doi: 10.1080/03008207.2019.1601185. [DOI] [PubMed] [Google Scholar]
- 120.Cai H, et al. BMP2 induces hMSC osteogenesis and matrix remodeling. Mol Med Rep. 2021;23(2):1. doi: 10.3892/mmr.2020.11764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu H, et al. Expression of HIF1alpha in cycling stretchinduced osteogenic differentiation of bone mesenchymal stem cells. Mol Med Rep. 2019;20(5):4489–4498. doi: 10.3892/mmr.2019.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu Q, et al. Hypoxia mediates runt-related transcription factor 2 expression via induction of vascular endothelial growth factor in periodontal ligament stem cells. Mol Cells. 2019;42(11):763–772. doi: 10.14348/molcells.2019.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jing X, et al. Mechanical loading induces HIF-1α expression in chondrocytes via YAP. Biotechnol Lett. 2020;42(9):1645–1654. doi: 10.1007/s10529-020-02910-4. [DOI] [PubMed] [Google Scholar]
- 124.Guo Q, et al. Mechanical stimulation induced osteogenic differentiation of BMSCs through TWIST/E2A/p21 axis. Biosci Rep. 2020;40:BSR20193876. doi: 10.1042/BSR20193876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang M, et al. Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression. Connect Tissue Res. 2019;60(6):583–596. doi: 10.1080/03008207.2019.1611792. [DOI] [PubMed] [Google Scholar]
- 126.Lu L, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 2019;10(1):143. doi: 10.1186/s13287-019-1248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mishra VK, et al. Identifying the therapeutic significance of mesenchymal stem cells. Cells. 2020;9(5):1145. doi: 10.3390/cells9051145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang P, et al. Efficacy and safety of mesenchymal stem cell injections for patients with osteoarthritis: a meta-analysis and review of RCTs. Arch Orthop Trauma Surg. 2021;141(7):1241–1251. doi: 10.1007/s00402-020-03703-0. [DOI] [PubMed] [Google Scholar]
- 129.Tani S, et al. The progress of stem cell technology for skeletal regeneration. Int J Mol Sci. 2021;22(3):1404. doi: 10.3390/ijms22031404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shariatzadeh M, et al. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019;378(3):399–410. doi: 10.1007/s00441-019-03069-9. [DOI] [PubMed] [Google Scholar]
- 131.Kassem DH, Kamal MM. Therapeutic efficacy of umbilical cord-derived stem cells for diabetes mellitus: a meta-analysis study. Stem Cell Res Ther. 2020;11(1):484. doi: 10.1186/s13287-020-01996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshihara E, et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature. 2020;586(7830):606–611. doi: 10.1038/s41586-020-2631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang D, et al. Long-term expansion of pancreatic islet organoids from resident procr(+) progenitors. Cell. 2020;180(6):1198–1211.e19. doi: 10.1016/j.cell.2020.02.048. [DOI] [PubMed] [Google Scholar]
- 134.Jin Z, et al. Bone mesenchymal stem cell therapy for ovariectomized osteoporotic rats: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2019;20(1):556. doi: 10.1186/s12891-019-2851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiao F, et al. Exosomes derived from cyclic mechanical stretch-exposed bone marrow mesenchymal stem cells inhibit RANKL-induced osteoclastogenesis through the NF-κB signaling pathway. Ann Transl Med. 2021;9(9):798. doi: 10.21037/atm-21-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu G, et al. Tensile strain promotes osteogenic differentiation of bone marrow mesenchymal stem cells through upregulating lncRNA-MEG3. Histol Histopathol. 2021;36(9):939–946. doi: 10.14670/HH-18-365. [DOI] [PubMed] [Google Scholar]
- 137.Bonifazi F, et al. Intrabone transplant provides full stemness of cord blood stem cells with fast hematopoietic recovery and low GVHD rate: results from a prospective study. Bone Marrow Transplant. 2019;54(5):717–725. doi: 10.1038/s41409-018-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lan Y, et al. Combination of umbilical cord mesenchymal stem cells and standard immunosuppressive regimen for pediatric patients with severe aplastic anemia. BMC Pediatr. 2021;21(1):102. doi: 10.1186/s12887-021-02562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moreau P, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29–38. doi: 10.1016/S0140-6736(19)31240-1. [DOI] [PubMed] [Google Scholar]
- 140.Li Q, et al. Stem cell therapies for periodontal tissue regeneration: a network meta-analysis of preclinical studies. Stem Cell Res Ther. 2020;11(1):427. doi: 10.1186/s13287-020-01938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spada M, et al. Intrahepatic administration of human liver stem cells in infants with inherited neonatal-onset hyperammonemia: a phase I study. Stem Cell Rev Rep. 2020;16(1):186–197. doi: 10.1007/s12015-019-09925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Takeishi K, et al. Assembly and function of a bioengineered human liver for transplantation generated solely from induced pluripotent stem cells. Cell Rep. 2020;31(9):107711. doi: 10.1016/j.celrep.2020.107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li H, et al. Biomechanical cues as master regulators of hematopoietic stem cell fate. Cell Mol Life Sci. 2021;78(16):5881–5902. doi: 10.1007/s00018-021-03882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luo Y, et al. Physical cues of matrices reeducate nerve cells. Front Cell Dev Biol. 2021;9:731170. doi: 10.3389/fcell.2021.731170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sheng R, et al. The application of mechanical stimulations in tendon tissue engineering. Stem Cells Int. 2020;2020:8824783. doi: 10.1155/2020/8824783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tomokiyo A, et al. Periodontal ligament stem cells: regenerative potency in periodontium. Stem Cells Dev. 2019;28(15):974–985. doi: 10.1089/scd.2019.0031. [DOI] [PubMed] [Google Scholar]
- 147.Li Y, et al. Application of external force regulates the migration and differentiation of adipose-derived stem/progenitor cells by altering tissue stiffness. Tissue Eng Part A. 2019;25(23–24):1614–1622. doi: 10.1089/ten.tea.2019.0046. [DOI] [PubMed] [Google Scholar]
- 148.Kim M, et al. Comparison of shear stress-induced thrombotic and thrombolytic effects among 3 different antithrombotic regimens in patients with acute coronary syndrome. Clin Appl Thromb Hemost Off J Int Acad Clin Appl Thromb Hemost. 2020;26:1076029620912814. doi: 10.1177/1076029620912814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hoogeveen ES, et al. MRI evaluation of the relationship between carotid artery endothelial shear stress and brain white matter lesions in migraine. J Cereb Blood Flow Metab. 2020;40(5):1040–1047. doi: 10.1177/0271678X19857810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu Y, et al. Mechanical stimuli enhance simultaneous differentiation into oesophageal cell lineages in a double-layered tubular scaffold. J Tissue Eng Regen Med. 2019;13(8):1394–1405. doi: 10.1002/term.2881. [DOI] [PubMed] [Google Scholar]
- 151.Zhong J, et al. In vivo effects of different orthodontic loading on root resorption and correlation with mechanobiological stimulus in periodontal ligament. J R Soc Interface. 2019;16(154):20190108. doi: 10.1098/rsif.2019.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rajapakse CS, et al. Effect of low-intensity vibration on bone strength, microstructure, and adiposity in pre-osteoporotic postmenopausal women: a randomized placebo-controlled trial. J Bone Miner Res. 2021;36(4):673–684. doi: 10.1002/jbmr.4229. [DOI] [PubMed] [Google Scholar]
- 153.Treffel L, et al. DI-5-cuffs: lumbar intervertebral disc proteoglycan and water content changes in humans after five days of dry immersion to simulate microgravity. Int J Mol Sci. 2020;21(11):3748. doi: 10.3390/ijms21113748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baba S, et al. Space flight diet-induced deficiency and response to gravity-free resistive exercise. Nutrients. 2020;12(8):2400. doi: 10.3390/nu12082400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Petersen LG, et al. Lower body negative pressure to safely reduce intracranial pressure. J Physiol. 2019;597(1):237–248. doi: 10.1113/JP276557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ning W, et al. Differentiated daughter cells regulate stem cell proliferation and fate through intra-tissue tension. Cell Stem Cell. 2021;28(3):436–452.e5. doi: 10.1016/j.stem.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu Y, et al. Combinational effects of mechanical forces and substrate surface characteristics on esophageal epithelial differentiation. J Biomed Mater Res A. 2019;107(3):552–560. doi: 10.1002/jbm.a.36571. [DOI] [PubMed] [Google Scholar]
- 158.Fang B, et al. The effects of mechanical stretch on the biological characteristics of human adipose-derived stem cells. J Cell Mol Med. 2019;23(6):4244–4255. doi: 10.1111/jcmm.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gungordu HI, et al. Effect of mechanical loading and substrate elasticity on the osteogenic and adipogenic differentiation of mesenchymal stem cells. J Tissue Eng Regen Med. 2019;13(12):2279–2290. doi: 10.1002/term.2956. [DOI] [PubMed] [Google Scholar]
- 160.De Belly H, et al. Membrane tension gates ERK-mediated regulation of pluripotent cell fate. Cell Stem Cell. 2021;28(2):273–284.e6. doi: 10.1016/j.stem.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fan T, et al. Spatial organization and crosstalk of vimentin and actin stress fibers regulate the osteogenic differentiation of human adipose-derived stem cells. FASEB J. 2021;35(2):e21175. doi: 10.1096/fj.202000378RR. [DOI] [PubMed] [Google Scholar]
- 162.Simunovic M, et al. Curving cells inside and out: roles of BAR domain proteins in membrane shaping and its cellular implications. Ann Rev Cell Dev Biol. 2019;35:111–129. doi: 10.1146/annurev-cellbio-100617-060558. [DOI] [PubMed] [Google Scholar]
- 163.Chaudhuri O, et al. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020;584(7822):535–546. doi: 10.1038/s41586-020-2612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.SenGupta S, et al. The principles of directed cell migration. Nat Rev Mol Cell Biol. 2021;22(8):529–547. doi: 10.1038/s41580-021-00366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fiore VF, et al. Publisher correction: mechanics of a multilayer epithelium instruct tumour architecture and function. Nature. 2020;586(7827):E9. doi: 10.1038/s41586-020-2751-5. [DOI] [PubMed] [Google Scholar]
- 166.Hosseini K, et al. EMT-induced cell-mechanical changes enhance mitotic rounding strength. Adv Sci (Weinh) 2020;7(19):2001276. doi: 10.1002/advs.202001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gegg C, et al. Mixed composition microribbon hydrogels induce rapid and synergistic cartilage regeneration by mesenchymal stem cells in 3D via paracrine signaling exchange. ACS Biomater Sci Eng. 2020;6(7):4166–4178. doi: 10.1021/acsbiomaterials.0c00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Berger AJ, et al. Mechanical memory impairs adipose-derived stem cell (ASC) adipogenic capacity after long-term in vitro expansion. Cell Mol Bioeng. 2021;14(5):397–408. doi: 10.1007/s12195-021-00705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wei D, et al. Mechanics-controlled dynamic cell niches guided osteogenic differentiation of stem cells via preserved cellular mechanical memory. ACS Appl Mater Interfaces. 2020;12(1):260–274. doi: 10.1021/acsami.9b18425. [DOI] [PubMed] [Google Scholar]
- 170.Zhang C, et al. Mechanics-driven nuclear localization of YAP can be reversed by N-cadherin ligation in mesenchymal stem cells. Nat Commun. 2021;12(1):6229. doi: 10.1038/s41467-021-26454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Feng Q, et al. Engineering the cellular mechanical microenvironment to regulate stem cell chondrogenesis: Insights from a microgel model. Acta Biomater. 2020;113:393–406. doi: 10.1016/j.actbio.2020.06.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.