Abstract
SecB is a cytosolic protein required for rapid and efficient export of particular periplasmic and outer membrane proteins in Escherichia coli. SecB promotes export by stabilizing newly synthesized precursor proteins in a nonnative conformation and by targeting the precursors to the inner membrane. Biochemical studies suggest that SecB facilitates precursor targeting by binding to the SecA protein, a component of the membrane-embedded translocation apparatus. To gain more insight into the functional interaction of SecB and SecA, in vivo, mutations in the secA locus that compensate for the export defect caused by the secB missense mutation secBL75Q were isolated. Two suppressors were isolated, both of which led to the overproduction of wild-type SecA protein. In vivo studies demonstrated that the SecBL75Q mutant protein releases precursor proteins at a lower rate than does wild-type SecB. Increasing the level of SecA protein in the cell was found to reverse this slow-release defect, indicating that overproduction of SecA stimulates the turnover of SecBL75Q-precursor complexes. These findings lend additional support to the proposed pathway for precursor targeting in which SecB promotes targeting to the translocation apparatus by binding to the SecA protein.
In the gram-negative bacterium Escherichia coli, proteins destined to be localized to the periplasmic space or outer membrane are transported out of the cytoplasm and through the inner membrane via the general export pathway (10, 16). Translocation of proteins across the inner membrane is catalyzed by the preprotein translocase, a multisubunit enzyme complex consisting of the SecA, SecY, SecE, SecG, SecD, SecF, and YajC proteins (17). The core of the translocase consists of an integral domain composed of the SecY, SecE, and SecG proteins and a peripheral domain composed of a dimer of SecA. SecA associates with the membrane through an affinity for acidic phospholipids and for the SecY subunit of the translocase (13, 60). SecA, SecY, and SecE are sufficient for translocation into proteoliposomes reconstituted with purified Sec components (3, 48, 51), although in the absence of the other components of the translocase, translocation is very inefficient (17, 49, 52).
SecA is an ATPase that is found both in the cytoplasm and associated with the inner membrane (4, 38). Cytosolic SecA functions as a repressor of its own translation (12, 57, 58). When SecA is bound to the SecYEG subunits of the translocase, acidic phospholipids and a precursor protein, SecA becomes fully active as an ATPase (39). SecA couples the energy from ATP binding and hydrolysis to protein translocation through repeated cycles of ATP-driven membrane insertion and deinsertion (18).
The initial step in the export process is delivery of the precursor protein to the inner membrane. A number of soluble cytosolic factors, including SecB, GroEL, GroES, DnaK, DnaJ, and the E. coli signal recognition particle, are involved in targeting precursors to the membrane (9, 29, 35, 62). Mutations affecting these components result in defective export of subsets of secreted proteins.
The SecB protein is required for efficient export of particular proteins to the periplasmic compartment and outer membrane of E. coli (29, 31). In vivo, SecB binds to nascent and fully elongated species of protein precursors (33) and stabilizes them in a nonnative conformation that is essential for translocation across the cytoplasmic membrane (6, 27). In the absence of SecB, export is much slower than in wild-type strains, and a significant amount of precursor protein folds into an export-incompetent conformation. In the case of the SecB ligand pre-maltose binding protein (preMBP), 25% of the protein fails to be exported (34). In addition, in the absence of SecB, export of MBP is completely posttranslational, indicating that SecB is required for cotranslational processing of preMBP (34). These data demonstrate that SecB plays a role in modulating the folding of precursor proteins and, in addition, is required for rapid targeting of precursors to the membrane.
Biochemical analyses suggest that SecB facilitates the targeting of precursor proteins to the translocation apparatus by binding to the SecA protein. Purified soluble SecA interacts with SecB with low affinity in vitro (11, 25). In contrast, SecB binds with high affinity to inner membrane vesicles in a SecA-dependent manner, and the high-affinity binding of SecB is promoted by precursor proteins (25).
Removal of the last 70 amino acids of SecA abolishes the ability of SecA to mediate high-affinity binding of SecB and SecB-PhoE precursor complexes to inner membrane vesicles, suggesting that SecB binds the carboxy terminus of SecA (2). More recently, direct binding of SecB to the C-terminal 22 amino acids of SecA has been demonstrated (20). Expression of a truncated SecA protein missing 66 amino acids of the C terminus reduces the export efficiency of SecB-dependent proteins in vivo (53). Interestingly, export of a SecB-independent protein was not affected by this truncation. Taken together, these results indicate that the C terminus of SecA is required for SecB binding and that efficient targeting of precursor proteins by SecB requires a functional SecB binding site on SecA.
Mutational studies have been used to identify specific residues important for SecB function (22, 28). Amino acid substitutions at Leu-75 or Glu-77 result in a strong defect in the rate of export in vivo but do not compromise complex formation between SecB and precursor proteins (28). SecBL75Q and SecBE77K are capable of binding unfolded MBP and blocking its refolding in vitro (22). SecBL75Q and SecBE77K are unable to support SecA-dependent membrane binding of the precursor protein proOmpA in vitro due to a defect in SecA binding (19). Thus, these residues may be involved in the formation of a SecA binding site on SecB.
To gain more insight into the SecB-SecA protein interaction, mutations in the secA gene that improve export of MBP in a strain containing the secB missense mutation secBL75Q were isolated. Two suppressor mutations were isolated, and both were found to lead to overproduction of the SecA protein. The effect of overproduction of SecA on the binding and release of precursor proteins from the SecBL75Q mutant protein was analyzed. Precursors were found to be released from SecBL75Q much more slowly than from wild-type SecB. Overproduction of SecA was found to reverse the slow-release defect caused by the secBL75Q mutation. These in vivo results lend additional support to the biochemical data, which indicate that the interaction between SecB and SecA is critical for efficient protein export.
MATERIALS AND METHODS
Bacterial strains, phages, and plasmids.
The E. coli strains used for these studies are listed in Table 1. Generalized transduction with phage P1vir was performed as described previously (46). The plasmids pMF8 and pT7-secA (58) were obtained from Don Oliver, pSR47 (45) and strain SR202 were obtained from Ralph Isberg, pHAsecEYG (13) was obtained from Bill Wickner, and pBAD22 (24) was obtained from Jon Beckwith.
TABLE 1.
Genotypes of E. coli strains
| Strain | Genotype | Source |
|---|---|---|
| MC4100 | F− Δlac-169 araD139 thiA rpsL relA motA | Lab collection |
| CK2163 | MC4100 secBL75Q malE+ | Lab collection |
| OF133 | MC4100 secBL75Q malE10-1 malT(Con) | Lab collection |
| HAC10 | MC4100 zab-1::Tn10 secA+ | This study |
| HAC12 | MC4100 secBL75Q zjb::Tn5 ΔmalB101 zab-1::Tn10 secA+ malT(Con) | This study |
| HAC13 | MC4100 secB+ malE10-1 malT(Con) zab-1::Tn10 secA+ | This study |
| HAC14 | MC4100 secBL75Q malE10-1 malT(Con) zab-1::Tn10 secA+ | This study |
| HAC15 | MC4100 secBL75Q zab-1::Tn10 secA+ | This study |
| HAC49 | MC4100 secBL75Q recA1 srl::Tn10 | This study |
| HAC50 | MC4100 secB+ recA1 srl::Tn10/pBR322 | This study |
| HAC52 | MC4100 secBL75Q recA1 srl::Tn10/pBR322 | This study |
| HAC53 | MC4100 secBL75Q recA1 srl::Tn10/pMF8 | This study |
| HAC82 | MC4100 secB+ ara+ recA1 srl::Tn10 | This study |
| HAC83 | MC4100 secBL75Q ara+ recA1 srl::Tn10 | This study |
| HAC97 | MC4100 secB+ ara+ recA1 srl::Tn10/pHAsecEYG | This study |
| HAC98 | MC4100 secBL75Q ara+ recA1 srl::Tn10/pHAsecEYG | This study |
| HAC151 | MC4100 secB+ ara+ recA1 srl::Tn10/pBAD22 | This study |
| HAC152 | MC4100 secBL75Q ara+ recA1 srl::Tn10/pBAD22 | This study |
| HAC214 | MC4100 secBL75Q malE10-1 malT(Con) zab-1::Tn10 secA1180 | This study |
| HAC215 | MC4100 secBL75Q malE10-1 malT(Con) zab-1::Tn10 secA4250 | This study |
| HAC216 | MC4100 secBL75Q malE+ zab-1::Tn10 secA1180 | This study |
| HAC217 | MC4100 secBL75Q malE+ zab-1::Tn10 secA4250 | This study |
Bacterial growth.
L broth, L agar, maltose tetrazolium agar, and M63 and M9 salts were prepared as previously described (46). Minimal media were supplemented with thiamine (5 μg/ml) and with 0.5% glycerol or with the combination of 0.2% glycerol and 0.4% maltose. Some cultures were supplemented with 18 amino acids (no methionine or cysteine) as described previously (8). When appropriate, antibiotics were added to the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; kanamycin, 10 μg/ml; and tetracycline, 20 μg/ml.
In experiments where the SecYEG complex was overexpressed from plasmid pHAsecEYG, cells were grown in M63 minimal medium containing 0.4% glucose, ampicillin (100 μg/ml), and 18 amino acids (1/100 of stock) at 37°C. When pHAsecEYG-containing strains reached a cell density of 2 × 108 to 3 × 108 cells/ml, the cells were pelleted, washed twice in M63 salts (at 37°C), resuspended in M63 minimal medium containing 0.4% arabinose and ampicillin (at 37°C), and grown for 4 h at 37°C.
Localized mutagenesis and isolation of Mal+ suppressor mutations.
Cells of strain HAC12 (secBL75Q zjb::Tn5 ΔmalB101 zab-1::Tn10 secA) were treated with nitrosoguanidine (62.5 μg/ml) as described previously (59). The mutagenized cells were split into pools. P1vir was prepared on each pool and used to transduce cells of strain OF133 [secB75Q malT(Con) malE10-1] as described previously (59). Tetracycline-resistant (Tcr) transductants were selected on minimal agar containing tetracycline at 37°C. Tcr transductants were pooled, grown overnight in liquid medium, and plated on minimal maltose agar plates containing tetracycline and sodium citrate. Mal+ colonies from individual plates were pooled, a P1vir lysate was prepared, and the phage was used to transduce OF133 cells. Tcr transductants were selected and analyzed as described above. Pools containing suppressor mutations showed approximately a 50-fold enrichment of Mal+ colonies. Individual Mal+ colonies were purified on minimal medium containing tetracycline, and linkage to the secA locus was analyzed by P1 transduction with the recipient OF133. Approximately 35,000 Tcr transductants were analyzed for growth on maltose by using the enrichment procedure described above.
Pulse-chase analysis of protein export.
Cells (2 × 108 to 3 × 108 cells/ml) were pulse-labeled for 15 s with Tran35S-label (ICN) (10 μCi/ml) at 37°C. The incorporation of label was terminated by the addition of nonradioactive methionine (100 μg/ml) and chloramphenicol (0.5 mg/ml). At various chase times, 1-ml samples were taken and precipitated with trichloroacetic acid (5% final concentration) on ice. MBP and OmpA were immunoprecipitated as previously described (34) with IgSORB (New England Enzyme Center, Inc., Boston, Mass.).
SDS-PAGE and fluorography.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done by the method of Laemmli (36). The gels were processed for fluorography with either sodium salicylate (5) or diphenyl oxazole (1).
Genetic mapping and DNA sequence analysis of suppressors.
DNA manipulations and bacterial transformations were as described previously (41). For mapping the suppressor mutations, DNA fragments encompassing the 5′ end of the geneX-secA operon from the end of the envA gene to the SalI restriction site in secA (5′secA) and from the SalI site to the end of the secA gene (3′secA) were amplified by PCR (47) from boiled colonies and cloned into pSR47, a suicide plasmid encoding kanamycin resistance (45). To determine whether the amplified fragments contained the mutations, the resulting plasmids were transformed into HAC14 (secBL75Q malE10-1 secA+) and HAC214 (secBL75Q malE10-1 secA1180) or HAC215 (secBL75Q malE10-1 secA4250) where appropriate. Transformants containing plasmid integrations at the secA locus were isolated and tested for growth on minimal maltose medium. The results from these experiments indicated that the lesion conferring Mal+ in secA4250 mutants was located in the region encompassed by the 5′ fragment. To map the mutation more finely, subclones of 5′secA plasmids containing DNA fragments from the end of envA to the EcoRI site in geneX or to the NcoI site in secA were generated. The subclones were tested as described above. Both subclones derived from secA4250 conferred Mal+ when introduced into strain HAC14 (secA+ Mal−), indicating that the secA4250 mutation was contained within the 463-bp fragment upstream of the secA gene. This fragment was sequenced, and a single-nucleotide substitution of adenosine for guanine was observed 3 nucleotides upstream of the translational start of geneX.
Attempts to map the secA1180 mutation were unsuccessful. Therefore, the entire geneX-secA region was amplified from the chromosome of HAC214 (secA1180) by PCR and sequenced by the Tufts University sequencing facility. No nucleotide changes were observed, suggesting that the lesion lay outside the region that was analyzed.
Antisera.
Anti-MBP, anti-OmpA, and anti-SecB antisera have been described previously (32). For preparation of anti-SecA antiserum, SecA protein was purified as described previously (7). Rabbits were immunized with denatured SecA as described previously (32), except that Hunter’s TiterMax (CytRx Corporation, Norcross, Ga.) was used as an adjuvant. Anti-SecE and anti-SecY antisera were the kind gifts of Jon Beckwith and Bill Wickner, respectively.
Preparation of cell lysates and immunoblotting.
For quantitation of total SecA, cells were grown in L broth to an A600 of 0.75 to 1.0. The cultures were poured over crushed ice, cells were harvested by centrifugation at 7,000 rpm for 10 min at 4°C in a Beckman JA-14 rotor, and the pellet was resuspended in 1/100th volume of lysis buffer (10 mM Tris-acetate [pH 7.6], 50 mM KCl, 10 mM Mg acetate, 1 mM dithiothreitol) plus 17.4 μg of phenylmethylsulfonyl fluoride per ml and 2 μg of DNase I per ml. Cells were lysed in a prechilled French pressure cell (two passes at 14,000 lb/in2), and the extracts were clarified by centrifugation at 10,000 rpm (12,000 × g) for 10 min. Protein concentrations were determined by the method of Lowry et al. (40) in the presence of SDS. Clarified extracts were subjected to SDS-PAGE, transferred to Immobilon polyvinylidene difluoride (PVDF) membranes (Millipore), and probed with polyclonal anti-SecA antiserum. The blot was developed with the Western-Light chemiluminescent detection system (Tropix, Bedford, Mass.) as recommended by the manufacturer.
Detection of SecB complexes.
Cells were grown in M63 minimal medium supplemented with 0.2% glycerol, 0.4% maltose, and 100 μg of ampicillin per ml at 37°C. Cultures were labeled with Tran35S-label (ICN), and the label was chased with nonradioactive methionine (2.2 × 10−5 M) as described previously (54). Cells were harvested over ice, converted to spheroplasts, and extracted as described previously (54). Material which was bound nonspecifically to the protein A-Sepharose matrix was removed, and cell membranes were pelleted as follows: 0.1 ml of protein A-Sepharose (1:1 slurry in phosphate-buffered saline [PBS]) was added to the extract, and the extract was centrifuged for 15 min at 83,000 rpm with a Beckman TLA 100.2 rotor at 4°C.
Chromatography was performed at 4°C. Anti-SecB antibodies were bound to a column of protein A-Sepharose as previously described (30). Precleared extracts were passed slowly through the anti-SecB column, and the column was washed once with 0.5 ml of PBS–0.1% Tween 20 and twice with 0.5 ml of PBS–0.5% Tween 20. Proteins bound to the column were eluted with 200 μl of boiling SDS buffer (250 mM Tris-HCl [pH 6.8], 4.0% SDS, 30% glycerol, 10% β-mercaptoethanol), and the column was washed twice with 100 μl of 10 mM Tris-HCl (pH 8)–0.5% Tween 20. The washes were pooled with the eluent.
Densitometry.
Densitometric analysis was performed with a Molecular Dynamics computing densitometer and ImageQuant 3.3 software. When the amount of exported MBP or exported OmpA was calculated, the obtained values were corrected for the loss of methionine residues in the mature form relative to the precursor form of the protein.
RESULTS
Isolation of suppressors.
MBP is required for the uptake and utilization of maltose as a carbon source. SecB facilitates the export of MBP to the periplasm, and mutations in the secB gene, including the missense mutation secBL75Q, result in a kinetic export defect for MBP. However, posttranslational export of MBP occurs in secB mutants, allowing these strains to grow on maltose. Mutations altering the signal sequence of the gene encoding MBP (malE) also compromise MBP export, but, as seen with secB mutants, malE signal sequence mutants are able to utilize maltose. The presence of both secB and malE signal sequence mutations abolishes MBP export, and the double-mutant cells are unable to grow on maltose (21). In this study, the Mal− phenotype of the secBL75Q malE10-1 double-mutant strain was used in a selection for secA suppressors of the secB missense mutation.
Localized nitrosoguanidine mutagenesis of the secA gene was used to isolate suppressors that enabled strain OF133 (secBL75Q malE10-1) to grow on minimal maltose medium (described in Materials and Methods). Linkage of the suppressor mutation to the secA locus was demonstrated by P1 transduction. Twelve Mal+ isolates which carried suppressor mutations linked to the secA gene were obtained.
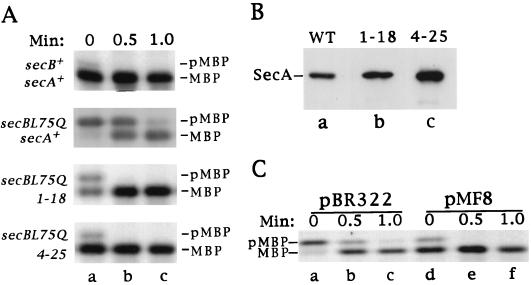
To determine whether any of the mutations affected the export defect caused by the secBL75Q mutation, MBP export in derivatives of strain CK2163 (secBL75Q malE+) was analyzed by pulse-chase labeling experiments. Cells were incubated with Tran35S-label (10 μCi/ml) for 15 s, and the chase was initiated by the addition of nonradioactive methionine and chloramphenicol. Samples were removed at various times and processed for immunoprecipitation with anti-MBP antiserum as described in Materials and Methods. The samples were analyzed by SDS-PAGE and fluorography (Fig. 1A). In wild-type (secB+) cells, export was very rapid, and the majority of MBP was exported during the 15-s pulse. By contrast, only 6% of the MBP was exported in the secBL75Q mutant strain during the pulse. Two strains, HAC216 (secBL75Q secA1180) and HAC217 (secBL75Q secA4250), containing suppressor mutations, showed a reversal of the secBL75Q defect. In both HAC216 (secBL75Q secA1180) and HAC217 (secBL75Q secA4250), the majority of MBP was exported during the pulse (72 and 93%, respectively), and MBP was completely exported by the 30-s chase point. The secA4250 mutation consistently appeared to have a stronger effect than secA1180. Radiolabeling experiments performed with strains carrying the malE10-1 signal sequence mutation and wild-type secB indicated that secA1180 and secA4250 did not suppress the signal sequence mutation (data not shown). Thus, these mutations enabled strain OF133 (secBL75Q malE10-1) to grow on maltose by suppressing the secBL75Q export defect. The remaining mutants contained suppressors of the malE signal sequence mutation.
FIG. 1.
(A) Suppression of secBL75Q by secA1180, secA4250, and SecA overproduction. Cells were grown in maltose-glycerol minimal medium and labeled for 15 s with Tran35S-label. Chase was initiated with nonradioactive methionine and chloramphenicol. Samples were taken at the time points indicated, immunoprecipitated with anti-MBP antiserum, and analyzed by SDS-PAGE (10% polyacrylamide) and fluorography as described in Materials and Methods. The positions of preMBP (pMBP) and mature MBP are indicated. (B) Total SecA levels in secA1180 and secA4250 mutant cells. Strains HAC15 (secA+), HAC216 (secA1180), and HAC217 (secA4250) were grown in L broth, and clarified cell lysates were made as described in Materials and Methods. Samples containing 10 μg of total protein were separated by SDS-PAGE, transferred to an Immobilon PVDF membrane, and probed with anti-SecA antiserum. WT, wild type. (C) Overproduction of wild-type SecA from plasmid pMF8 suppresses secBL75Q. Cells were grown in maltose minimal medium and subjected to pulse-chase analysis as described for panel A. The positions of preMBP and mature MBP are indicated.
Pulse-chase labeling experiments analyzing MBP export in secB::Tn5 strains indicated that both suppressors could improve export in the absence of SecB (data not shown). Although the suppression of the secB::Tn5 defect was very weak, this result indicated that both secA1180 and secA4250 were not allele-specific suppressors of the secBL75Q mutation.
To determine whether HAC214 (secA1180) and HAC215 (secA4250) contained lesions in the secA gene, the region of the secA locus from the end of the envA gene to the end of the secA gene was subjected to genetic mapping experiments and DNA sequence analyses (described in Materials and Methods). The results of these experiments indicated that both suppressors contained lesions outside the secA structural gene (summarized in Materials and Methods).
Overproduction of wild-type SecA protein suppresses secBL75Q.
Since the suppressor mutations mapped outside the secA gene, we hypothesized that these mutations might affect secA expression. To determine whether the secA1180 and secA4250 mutations affected the amount of SecA protein synthesized, the total amount of SecA in HAC216 (secA1180) and HAC217 (secA4250) mutant cells was determined. Cells of strains HAC15 (secA+), HAC216 (secA1180), and HAC217 (secA4250) were extracted as described in Materials and Methods. Ten micrograms of total cellular protein was resolved by SDS-PAGE and analyzed by immunoblotting with anti-SecA antiserum. Figure 1B shows that the extracts from the suppressor strains contained more SecA protein than the control extract. Quantitative immunoblotting of dilution series demonstrated that extracts from secA1180 and secA4250 strains contained 2- to 3-fold and 12-fold (n = 2) more SecA, respectively, than extracts from control cells. These data indicated that both mutants were overproducing SecA protein.
To test whether overproduction of wild-type SecA was responsible for the suppressor phenotype, MBP export in the SecA-overproducing strain HAC49 (secBL75Q/pMF8 [secA+]) and the control strain HAC49 (secBL75Q/pBR322 [secA]) was analyzed. pMF8 contains the geneX-secA-mutT operon under control of the operon’s natural promoter and results in approximately eightfold overproduction of SecA (data not shown) (58). Figure 1C shows that MBP was exported at a higher rate in the strain containing the SecA-overexpressing plasmid, pMF8, than in the secBL75Q strain with pBR322 (compare lanes a and d). Also, the kinetics of MBP export were comparable to those seen in HAC216 (secA1180) and HAC217 (secA4250) (Fig. 1A). Export of the SecB-dependent proteins preLamB, proOmpA, and precursor galactose binding protein was also improved in strains containing pMF8 (data not shown).
Pulse-chase experiments analyzing export in strains containing the plasmid pT7-secA, which contains only the secA gene under control of the φ10 promoter of phage T7, also demonstrated suppression of the export defect caused by secBL75Q (data not shown). Thus, overproduction of SecA alone is sufficient for suppression of secBL75Q.
Overexpression of the SecYEG complex fails to suppress secBL75Q.
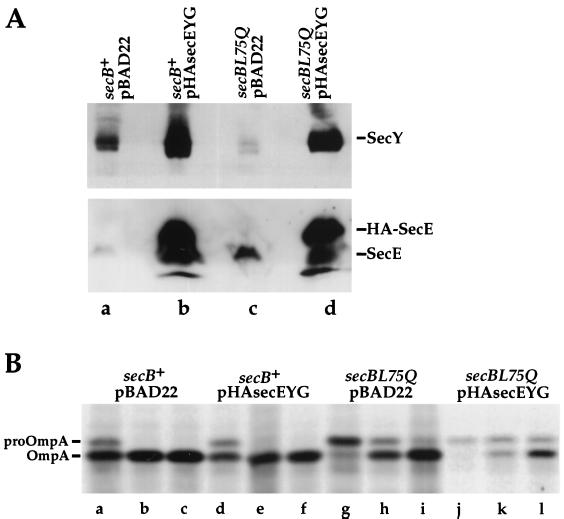
To determine whether overproduction of other components of the translocation apparatus would suppress the secBL75Q defect, the SecY-SecE-SecG complex (SecYEG) was overexpressed from plasmid pHAsecEYG. Overproduction of the SecYEG complex has been shown to increase the amount of functional translocation sites in the membrane and to enhance SecA-dependent translocation in vitro (13, 61). Cells of strains HAC82 (secB+ ara+) and HAC83 (secBL75Q ara+) were transformed with plasmid pHAsecEYG or vector pBAD22. pHAsecEYG contains the genes for an epitope-tagged SecE, SecY, and SecG under control of the PBAD promoter of the araBAD (arabinose) operon. For overexpression of SecYEG, cells were grown in M63 minimal medium containing 0.4% arabinose and supplemented with ampicillin and 18 amino acids (no cysteine or methionine) at 37°C. After 4 h of induction, samples were taken for analysis of total SecY and SecE by immunoblotting and for analysis of OmpA export (described in Materials and Methods). After 4 h of growth in the presence of arabinose, both SecE and SecY were overproduced at a very high level in pHAsecEYG-containing cells (Fig. 2A, lanes b and d). This high level of overexpression did not affect the growth of secB+ strains. However, it did dramatically slow the growth of the secBL75Q strain and interfered with the incorporation of label (Fig. 2B, lanes g to i). Nevertheless, as shown in Fig. 2B, overexpression of SecYEG did not interfere with the export of OmpA. OmpA was exported with similar kinetics in strains HAC152 (secBL75Q/pBAD22) (Fig. 2B, lanes g to i) and HAC98 (secBL75Q/pHAsecEYG) (Fig. 2B, lanes j to l). Thus, increasing the number of translocation sites did not suppress the secBL75Q export defect.
FIG. 2.
Overexpression of the SecYEG complex does not improve export in secBL75Q mutant strains. Cells were grown in minimal arabinose medium supplemented with ampicillin and a mix of 18 amino acids. (A) Immunoblots of total SecY and SecE protein. Samples (1 ml) were precipitated with trichloroacetic acid and analyzed by high-Tris SDS-PAGE (3). Proteins were transferred to a PVDF membrane and probed with anti-SecY and anti-SecE antisera. (B) Pulse-chase analysis of OmpA export. Cells were labeled with Tran35S-label for 15 s, and the label was chased with nonradioactive methionine and chloramphenicol. Samples were removed at the times indicated and treated as for Fig. 1, except that the samples were immunoprecipitated with anti-OmpA antiserum. Samples are as follows: lane a, HAC151 (secB+/pBAD22), 15-s pulse; lane b, HAC151, 0.5-min chase; lane c, HAC151, 1.0-min chase; lane d, HAC97 (secB+/pHAsecEYG), 15-s pulse; lane e, HAC97, 0.5-min chase; lane f, HAC97, 1.0-min chase; lane g, HAC152 (secBL75Q/pBAD22), 15-s pulse; lane h, HAC152, 0.5-min chase; lane i, HAC152, 1.0-min chase; lane j, HAC98 (secBL75Q/pHAsecEYG), 15-s pulse; lane k, HAC98, 0.5-min chase; lane l, HAC98, 1.0-min chase.
Overproduction of SecA improves release of precursor polypeptides from SecBL75Q.
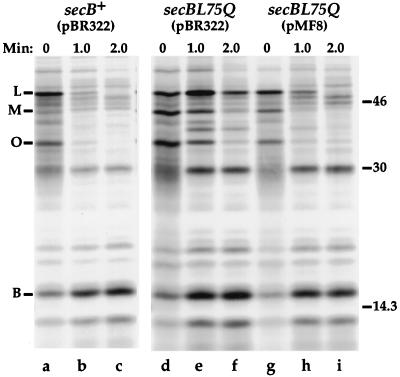
Previous studies have shown that substitutions at Leu-75 in SecB, which result in defective MBP export, do not disrupt SecB-preMBP complex formation in vivo (28). Therefore, the secBL75Q mutant is most likely defective in a step subsequent to SecB-precursor complex formation. One possibility is that the SecBL75Q protein may not release precursor proteins normally, resulting in slow turnover of SecB-precursor complexes. To test this hypothesis, polypeptide binding and release from SecB were analyzed. Cells of strains HAC50 (secB+/pBR322) and HAC52 (secBL75Q/pBR322) were incubated with Tran35S-label for 15 s, a cytoplasmic extract was prepared, and the extract was subjected to anti-SecB affinity chromatography as described in Materials and Methods. Proteins bound to the column were analyzed by SDS-PAGE and fluorography. After a 15-s pulse-labeling, SecB and proteins bound to SecB were observed in the anti-SecB-bound fraction from extracts of wild-type (secB+) cells (Fig. 3, lane a) and mutant (secBL75Q) cells (Fig. 3, lane d). Previous studies demonstrated that the SecB-bound proteins are nascent and fully elongated precursors of LamB, MBP, and the major outer membrane proteins OmpA and OmpF (33). Thus, SecBL75Q formed complexes with SecB-dependent proteins in vivo.
FIG. 3.
Defective release of precursors from SecBL75Q is suppressed by overproduction of SecA. Cells were grown in M63 minimal maltose-glycerol medium containing ampicillin. Cells were pulse-labeled with Tran35S-label for 15 s, and the label was chased with nonradioactive methionine. Samples were taken after the pulse and after 1 and 2 min of chase. Cells were extracted, and the extract was subjected to anti-SecB affinity chromatography as described in Materials and Methods. Proteins bound to the column were eluted and analyzed by SDS-PAGE (12.5% polyacrylamide) and fluorography. Samples were as follows: lane a, HAC50 (secB+/pBR322), 15-s pulse; lane b, HAC50, 1-min chase; lane c, HAC50, 2-min chase; lane d, HAC52 (secBL75Q/pBR322), 15-s pulse; lane e, HAC52, 1-min chase; lane f, HAC52, 2-min chase; lane g, HAC53 (secBL75Q/pMF8), 15-s pulse; lane h, HAC53, 1-min chase; lane i, HAC53, 2-min chase. The numbers at the right are molecular weight markers (in thousands). The mobilities of SecB (B), proOmpA (O), preMBP (M), and preLamB (L) are indicated on the left.
In wild-type cells, when the label was chased with nonradioactive methionine, radiolabeled SecB was observed in the anti-SecB-bound fractions, but SecB-bound precursors, such as preLamB, preMBP, and proOmpA, rapidly disappeared. After 1 min of chase, precursors were barely detectable in the bound fraction (Fig. 3, lane b), and after 2 min, radiolabeled preLamB, preMBP, and proOmpA were not observed in the bound fraction (Fig. 3, lane c), indicating that the precursors had been released from SecB. In contrast, when an extract of secBL75Q cells was analyzed, large amounts of radiolabeled precursors were observed in the SecB-bound fraction after 1 min of chase (Fig. 3, lane e). Even after 2 min of chase, a significant amount of preLamB and a small amount of radiolabeled proOmpA and preMBP were bound to SecBL75Q. Thus, the secBL75Q mutation results in slower release of precursors from the mutant SecB protein.
To determine whether overproduction of SecA would reverse the slow-release defect caused by secBL75Q, precursor binding and release were analyzed in strain HAC53 (secBL75Q/pMF8). Analysis of radiolabeled cells demonstrated that after a 15-s pulse-labeling, preLamB, preMBP, and proOmpA were bound to SecBL75Q in extracts from cells overproducing SecA (Fig. 3, lane g). This result indicated that precursor proteins were not bypassing SecBL75Q in cells with high levels of SecA protein. After 1 min of chase, small amounts of preLamB, preMBP, and proOmpA were observed in the bound fraction (Fig. 3, lane h), and after 2 min of chase, these precursors could barely be detected (Fig. 3, lane i). These data indicated that increasing SecA levels in the cell reversed the defective release of precursors from SecBL75Q.
DISCUSSION
The results of this study demonstrate that overproduction of wild-type SecA protein reverses the defect caused by the secB missense mutation secBL75Q. Overproduction of SecA did not lead to a bypass of the SecBL75Q protein. In cells with normal amounts of SecA, nascent precursor proteins were bound by SecBL75Q but were released at a significantly lower rate than from wild-type SecB. However, when SecA was overproduced eightfold, precursors dissociated from SecBL75Q at close-to-wild-type rates. These findings lend additional support to the biochemical studies which indicate that binding of SecB to SecA is critical for efficient protein export.
SecB promotes rapid export by maintaining precursor proteins in a translocation-competent conformation (6, 54) and by facilitating the delivery of precursors to the translocation machinery via the SecA protein (25). In general, substitutions at Leu-75 in the SecB protein result in a strong export defect, yet changes at this position do not disrupt complex formation between SecB and precursor proteins (28). Furthermore, the SecBL75Q mutant protein exhibits enhanced activity in blocking the folding of unfolded MBP in vitro (22). These results suggest that in vivo SecBL75Q is defective at a step in the export pathway that comes after precursor binding.
Biochemical studies suggest that efficient precursor targeting involves binding of SecB to membrane-bound SecA and that the SecBL75Q mutant is defective in binding SecA in vitro (19). Consistent with in vivo studies (28), Fekkes et al. found that purified SecBL75Q could bind the precursor proOmpA but was defective for in vitro translocation and had a lower affinity for membrane-bound SecA than wild-type SecB (19). These data suggest that the secBL75Q mutation disrupts the interaction between SecB and SecA, causing a defect in precursor targeting. The finding that precursors are bound by SecBL75Q but are released at a low rate in vivo indicates that in strains in which the interaction of SecB and SecA is defective, targeting of precursors to the membrane becomes rate-limiting, resulting in the accumulation of cytosolic SecB-precursor complexes (Fig. 3, lanes d to f). Increasing cellular SecA levels would be expected to promote complex formation between SecB and SecA. Therefore, overproduction of SecA most likely improves the rate at which precursors are released by SecBL75Q by improving the SecB-SecA interaction through mass action.
Consistent with the notion that secBL75Q disrupts the SecB-SecA interaction, overexpression of the SecY, SecE, and SecG proteins from a multicopy plasmid did not suppress the export defect of the secBL75Q mutant. Although inner membrane vesicles prepared from cells overexpressing SecYEG show enhanced translocation ATPase and protein translocation activities (13), increasing the number of functional translocation sites had no effect on the secBL75Q defect in vivo. Thus, enhancement of steps downstream of the SecB-SecA interaction does not lead to suppression of secBL75Q.
Overexpression of SecYEG would be expected to lead to an increase in the amount of SecA bound to SecYEG at the membrane, since there is approximately 10-fold more SecA than SecYEG complexes in cells (14). However, as just discussed, overproduction of SecYEG does not suppress the export defect of secBL75Q mutants. SecB-precursor complexes may be bound by SecA in the cytosol prior to targeting, as has been previously suggested (26), and not by SecA bound to SecYEG. Overproduction of SecA has been shown to lead to an increase in cytosolic SecA (reference 4 and unpublished results), and this population may be responsible for suppression.
Overproduction of SecA also improved the rate of export in strains lacking SecB (secB::Tn5). This observation is consistent with the previous findings of Oliver (50). Thus, overproduction of SecA is able to bypass the requirement for SecB altogether. This is in contrast to suppression of the secBL75Q defect, where overproduction of SecA was shown not to bypass SecBL75Q but to reverse the slow-release defect of the mutant protein. In cells with normal SecA levels, in the absence of SecB, export of precursor MBP is much slower than in wild-type cells and is completely posttranslational. Approximately 60% of the intracellular preMBP is exported, indicating that a significant quantity of preMBP is exported in strains lacking SecB. Thus, in secB::Tn5 strains, targeting of precursors to the membrane is most likely the rate-limiting step. It is possible that in the absence of SecB, precursors are bound directly by SecA. If this is the case, then overproduction of SecA could improve export in the absence of SecB by increasing the efficiency with which precursors are bound by SecA.
Since the secA4250 mutation is a G-to-A mutation 3 nucleotides upstream of the translational start site for geneX and strains carrying this mutation express high levels of SecA protein, it seems likely that translation of geneX is affected in secA4250 mutant strains. Initiation regions show a bias in favor of adenosine (A) at most positions, especially downstream of the Shine-Dalgarno sequence (15). Therefore, the secA4250 mutation could improve the efficiency of translation initiation by increasing the adenosine content of the region. This idea is supported by random-mutagenesis studies of the E. coli trp leader region, which demonstrated that A at position −3 from the initiator codon favored translation initiation over guanine (G) at this position.
Improving the efficiency of translation initiation of geneX could lead to overproduction of the SecA protein through a mechanism involving translational coupling. Translational coupling is a common form of regulation in E. coli operons and occurs when the translation of one cistron affects translation initiation of the downstream cistron(s). For some operons, translation of the upstream cistron helps to destabilize mRNA structures which sequester the Shine-Dalgarno sequence and/or the initiator codon (37, 56). Studies of secA regulation demonstrated that secA expression is translationally coupled to that of geneX (44). Translation of the distal region of geneX is thought to open up an RNA secondary structure located in the geneX-secA intergenic region which blocks access to the secA Shine-Dalgarno sequence. Therefore, increasing the amount of geneX translation could increase secA expression by melting the inhibitory RNA structure, enabling ribosomes to bind to the secA Shine-Dalgarno sequence.
In conclusion, the results of this study support the proposed role for SecB in targeting precursor proteins to SecA. Newly synthesized precursors bound by SecB are guided to the translocation site through the affinity of SecB for SecA. Upon docking at the translocation site, the precursor is transferred to SecA and SecB is released from the membrane, freeing it to bind a newly synthesized precursor.
ACKNOWLEDGMENTS
We thank Jon Beckwith for the generous gifts of plasmid pBAD22 and anti-SecE antisera; Ralph Isberg and Susanna Rankin for providing plasmid pSR47 and strain SR202; Don Oliver for plasmids pMF8 and pT7-secA; Bill Wickner for plasmid pHAsecEYG and anti-SecY antisera; Olivera Francetic, Harvey Kimsey, Lin Randall, Debu Raychaudhuri, and Perry Riggle for helpful discussions; Meckie Pohlschröder for technical assistance; and Arnold Driessen for valuable discussions and for communicating the method for immunodetection. We are grateful to Linc Sonenshein, Cathy Squires, and Andrew Wright for helpful discussions and for critical reading of the manuscript.
This work was supported by grant GM36415 from the National Institutes of Health (to C.A.K.). Part of the work was performed during the tenure of an American Heart Established Investigator Award (to C.A.K.).
REFERENCES
- 1.Bonner W M, Lasky R A. Detection method for tritium-labeled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974;46:83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- 2.Breukink E, Nouwen N, van Raalte A, Mizushima S, Tommassen J, de Kruijff B. The C terminus of SecA is involved in both lipid binding and SecB binding. J Biol Chem. 1995;270:7902–7907. doi: 10.1074/jbc.270.14.7902. [DOI] [PubMed] [Google Scholar]
- 3.Brundage L, Hendrick J P, Scheibel E, Driessen A J M, Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990;62:649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- 4.Cabelli R J, Dolan K M, Qian L, Oliver D B. Characterization of membrane-associated and soluble states of SecA protein from wild-type and secA52(Ts) mutant strains of Escherichia coli. J Biol Chem. 1991;266:24420–24427. [PubMed] [Google Scholar]
- 5.Chamberlain J B. Fluorographic detection of radioactivity in polyacrylamide gels with water soluble fluor, sodium salicylate. Anal Biochem. 1979;98:132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- 6.Collier D N, Bankaitis V A, Weiss J B, Bassford P J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988;53:273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham K, Lill R, Crooke E, Rice M, Moore K, Wickner W, Oliver D. SecA protein, a peripheral protein of the Escherichia coli plasma membrane, is essential for the functional binding and translocation of proOmpA. EMBO J. 1989;8:955–959. doi: 10.1002/j.1460-2075.1989.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 9.De Gier J-W L, Valent Q A, Von Heijne G, Luirink J. The E. coli SRP: preferences of a targeting factor. FEBS Lett. 1997;408:1–4. doi: 10.1016/s0014-5793(97)00402-x. [DOI] [PubMed] [Google Scholar]
- 10.Den Blaauwen T, Driessen A J M. Sec-dependent preprotein translocation in bacteria. Arch Microbiol. 1996;165:1–8. doi: 10.1007/s002030050289. [DOI] [PubMed] [Google Scholar]
- 11.Den Blaauwen T, Terpetschnig E, Lakowicz J R, Driessen A J M. Interaction of SecB with soluble SecA. FEBS Lett. 1997;416:35–38. doi: 10.1016/s0014-5793(97)01142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolan K M, Oliver D B. Characterization of Escherichia coli SecA protein binding to a site on its mRNA involved in autoregulation. J Biol Chem. 1991;266:23329–23333. [PubMed] [Google Scholar]
- 13.Douville K, Price A, Eichler J, Economou A, Wickner W. SecYEG and SecA are the stoichiometric components of the preprotein translocase. J Biol Chem. 1995;270:20106–20111. doi: 10.1074/jbc.270.34.20106. [DOI] [PubMed] [Google Scholar]
- 14.Driessen A, de Wit J G, Kuiper W, van der Wolk J P W, Fekkes P, van der Does C, van Wely K, Manting E, den Blaauwen T. SecA, a novel ATPase that converts chemical energy into a mechanical force to drive precursor protein translocation. Biochem Soc Trans. 1995;23:981–985. doi: 10.1042/bst0230981. [DOI] [PubMed] [Google Scholar]
- 15.Dreyfus M. What constitutes the signal for the initiation of protein synthesis on Escherichia coli mRNAs? J Mol Biol. 1988;204:79–94. doi: 10.1016/0022-2836(88)90601-8. [DOI] [PubMed] [Google Scholar]
- 16.Duong F, Eichler J, Price A, Leonard M R, Wickner W. Biogenesis of the gram-negative bacterial envelope. Cell. 1997;91:567–573. doi: 10.1016/s0092-8674(00)80444-4. [DOI] [PubMed] [Google Scholar]
- 17.Duong F, Wickner W. Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 1997;16:2756–2768. doi: 10.1093/emboj/16.10.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 19.Fekkes P, de Wit J G, van der Wolk J P W, Kimsey H H, Kumamoto C A, Driessen A J M. Preprotein transfer to the Escherichia coli translocase requires the cooperative binding of SecB and the signal sequence to SecA. Mol Microbiol. 1998;29:1179–1190. doi: 10.1046/j.1365-2958.1998.00997.x. [DOI] [PubMed] [Google Scholar]
- 20.Fekkes P, van der Does C, Driessen A K M. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 1997;16:6105–6113. doi: 10.1093/emboj/16.20.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francetic O, Hanson M P, Kumamoto C A. prlA suppression of defective export of maltose-binding protein mutants of Escherichia coli. J Bacteriol. 1993;175:4036–4044. doi: 10.1128/jb.175.13.4036-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gannon P M, Kumamoto C A. Mutations of the molecular chaperone protein SecB which alter the interaction between SecB and Maltose-binding Protein. J Biol Chem. 1993;268:1590–1595. [PubMed] [Google Scholar]
- 23.Gold L, Pribnow D, Schneider T, Shinedling S, Swebilius Singer B, Stormo G. Translation initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- 24.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartl F-U, Lecker S, Schiebel E, Hendrick J P, Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990;63:269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- 26.Hoffschulte H K, Drees B, Müller M. Identification of a soluble SecA/SecB complex by means of a subfractionation cell-free export system. J Biol Chem. 1994;269:12833–12839. [PubMed] [Google Scholar]
- 27.Khisty V J, Randall L L. Demonstration in vivo that interaction of maltose-binding protein with SecB is determined by a kinetic partitioning model. J Bacteriol. 1995;177:3277–3282. doi: 10.1128/jb.177.11.3277-3282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimsey H H, Dagarag M D, Kumamoto C A. Diverse effects of mutation on the activity of the Escherichia coli export chaperone SecB. J Biol Chem. 1995;270:22831–22835. doi: 10.1074/jbc.270.39.22831. [DOI] [PubMed] [Google Scholar]
- 29.Kumamoto C, Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985;154:267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumamoto C A. Escherichia coli SecB protein associates with exported proteins in vivo. Proc Natl Acad Sci USA. 1989;86:5320–5324. doi: 10.1073/pnas.86.14.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumamoto C A. SecB protein: a cytosolic export factor that associates with nascent exported proteins. J Bioenerg Biomembr. 1990;22:337–351. doi: 10.1007/BF00763171. [DOI] [PubMed] [Google Scholar]
- 32.Kumamoto C A, Chen L, Fandl J, Tai P C. Purification of the Escherichia coli secB gene product and demonstration of its activity in an in vitro protein translocation system. J Biol Chem. 1989;264:2242–2249. [PubMed] [Google Scholar]
- 33.Kumamoto C A, Francetic O. Highly selective binding of nascent polypeptides by an Escherichia coli chaperone protein in vivo. J Bacteriol. 1993;175:2184–2188. doi: 10.1128/jb.175.8.2184-2188.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumamoto C A, Gannon P M. Effects of Escherichia coli secB mutations on pre-maltose binding protein conformation and export kinetics. J Biol Chem. 1988;263:11554–11558. [PubMed] [Google Scholar]
- 35.Kusukawa N, Yura T, Ueguchi C, Akiyama Y, Ito K. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 1989;8:3517–3521. doi: 10.1002/j.1460-2075.1989.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Lesage P, Chiaruttini C, Graffe M, Doddon J, Milet M, Springer M. Messenger RNA secondary structure and translational coupling in the Escherichia coli operon encoding translation initiation factor IF3 and the ribosomal proteins, L35 and L20. J Mol Biol. 1992;228:366–386. doi: 10.1016/0022-2836(92)90827-7. [DOI] [PubMed] [Google Scholar]
- 38.Lill R, Cunningham R, Brundage L, Ito K, Oliver D, Wickner W. The SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of E. coli. EMBO J. 1989;8:961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 40.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 41.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 42.Matteucii M D, Heyneker H L. Targeted random mutagenesis: the use of ambiguously synthesized oligonucleotides to mutagenize sequences immediately 5′ of an ATG initiation codon. Nucleic Acids Res. 1983;11:3113–3121. doi: 10.1093/nar/11.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCabe P C. Production of single-stranded DNA by asymmetric PCR. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. pp. 76–83. [Google Scholar]
- 44.McNicholas P, Salavati R, Oliver D. Dual regulation of Escherichia coli secA translation by distinct upstream elements. J Mol Biol. 1997;265:128–141. doi: 10.1006/jmbi.1996.0723. [DOI] [PubMed] [Google Scholar]
- 45.Merriam J J, Mathur R, Maxfield-Boumil R, Isberg R R. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect Immun. 1997;65:2497–2501. doi: 10.1128/iai.65.6.2497-2501.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 47.Mullis K, Faloona F, Scharf S, Daiki R, Horn G, Erlich H. Specific enzyme amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp Quant Biol. 1986;51:263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 48.Murphy C K, Stewart E J, Beckwith J. A double counter-selection for the study of essential genes in Escherichia coli. Gene. 1995;155:1–7. doi: 10.1016/0378-1119(94)00920-n. [DOI] [PubMed] [Google Scholar]
- 49.Nishiyama K, Hanada M, Tokuda H. Disruption of the gene encoding p12 (SecG) reveals the direct involvement and important function of SecG in the protein translocation of Escherichia coli at low temperature. EMBO J. 1994;13:3272–3277. doi: 10.1002/j.1460-2075.1994.tb06628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliver, D. 1998. Personal communication.
- 51.Oliver D B, Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982;30:311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- 52.Pogliano J A, Beckwith J. SecD and SecF facilitate protein export in Escherichia coli. EMBO J. 1994;13:554–561. doi: 10.1002/j.1460-2075.1994.tb06293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajapandi T, Oliver D B. Carboxy-terminal region of Escherichia coli SecA ATPase is important to promote its protein translocation activity in vivo. Biochem Biophys Res Commun. 1994;200:1477–1483. doi: 10.1006/bbrc.1994.1617. [DOI] [PubMed] [Google Scholar]
- 54.Randall L L, Hardy S J S. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986;46:921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- 55.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito K, Nomura M. Post-transcriptional regulation of the str operon in Escherichia coli: structural and mutational analysis of the target site for translational repressor S7. J Mol Biol. 1994;235:125–139. doi: 10.1016/s0022-2836(05)80021-x. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt M G, Dolan K M, Oliver D B. Regulation of Escherichia coli SecA mRNA translation by a secretion-responsive element. J Bacteriol. 1991;173:6605–6611. doi: 10.1128/jb.173.20.6605-6611.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt M G, Oliver D B. SecA protein autogenously represses its own translation during normal protein secretion in Escherichia coli. J Bacteriol. 1989;171:643–649. doi: 10.1128/jb.171.2.643-649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silhavy T J, Berman M L, Enquist L E. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 60.Snyders S, Ramamurthy V, Oliver D. Identification of a region of interaction between Escherichia coli SecA and SecY proteins. J Biol Chem. 1997;272:11302–11306. doi: 10.1074/jbc.272.17.11302. [DOI] [PubMed] [Google Scholar]
- 61.Van der Does C, den Blaaudwen T, de Wit J G, Mantig E H, Groot N A, Fekkes P, Driessen A J M. SecA is an intrinsic subunit of the Escherichia coli preprotein translocase and exposes its carboxyl terminus to the periplasm. Mol Microbiol. 1996;22:619–629. doi: 10.1046/j.1365-2958.1996.d01-1712.x. [DOI] [PubMed] [Google Scholar]
- 62.Wild J, Altman E, Yura T, Gross T. DnaK and DnaJ heat shock proteins participate in protein export in Escherichia coli. Genes Dev. 1992;6:1165–1172. doi: 10.1101/gad.6.7.1165. [DOI] [PubMed] [Google Scholar]