Abstract
Background
Systemic lupus erythematosus (SLE) is rarely diagnosed before 5-years-old. Those with disease onset at a very young age are predicted by a higher genetic risk and a more severe phenotype. We performed whole-exome sequencing to survey the genetic etiologies and clinical manifestations in patients fulfilling 2012 SLICC SLE classification criteria before the age of 5.
Case presentation
Among the 184 childhood-onset SLE patients regularly followed in a tertiary medical center in Taiwan, 7 cases (3.8%) of which onset ≦ 5 years of age were identified for characteristic review and genetic analysis. Compared to those onset at elder age, cases onset before the age of 5 are more likely to suffer from proliferative glomerulonephritis, renal thrombotic microangiopathy, neuropsychiatric disorder and failure to thrive. Causative genetic etiologies were identified in 3. In addition to the abundance of autoantibodies, patient with homozygous TREX1 (c.292_293 ins A) mutation presented with chilblain-like skin lesions, peripheral spasticity, endocrinopathy and experienced multiple invasive infections. Patient with SLC7A7 (c.625 + 1 G > A) mutation suffered from profound glomerulonephritis with full-house glomerular deposits as well as hyperammonemia, metabolic acidosis and episodic conscious disturbance. Two other cases harbored variants in lupus associating genes C1s, C2, DNASE1 and DNASE1L3 and another with CFHR4. Despite fulfilling the classification criteria for lupus, many of the patients required treatments beyond conventional therapy.
Conclusions
Genetic etiologies and lupus mimickers were found among a substantial proportion of patients suspected with early-onset SLE. Detail clinical evaluation and genetic testing are important for tailored care and personalized treatment.
Keywords: Systemic lupus erythematous, Childhood lupus, Lupus mimics, Genetic study, TREX1, SLC7A7
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with heterogenous clinical manifestations. Characterized by immune dysregulation and production of autoantibodies against self-antigens, the disease can affect nearly any tissue or organ systems. To date, no diagnostic criteria for SLE is available [1]. To facilitate the suspicion of lupus and to compare between other autoimmune disorders, SLE classification criteria were established since 1972 and subsequently revised in 1982, 1997, 2012 and 2019 for refinement [2]. As the diagnosis of SLE mostly depend on clinical and serological clues, diagnosis of lupus can be challenging, especially among those with atypical manifestations and extreme phenotypes [1, 3].
Women of childbearing age are typically predisposed to developing SLE. Disease development before the age of 5 is relatively uncommon [4]. According to a nationwide study in Taiwan, the prevalence of SLE under the age of 5 was lower than 5/100,000 [5]. As genetic, hormonal and environmental factors are all known to contribute to disease development, through a large-scale multiracial SLE cohort study, Webb et al. found that the onset of lupus during childhood is predicted by a higher genetic risk and is associated with a more severe phenotype [6]. Moreover, considering the early-onset nature of “monogenic lupus” and some “SLE mimickers”, special attention and additional workup may be needed to assist the diagnosis of lupus in children before school age [7]. For decades, whole-exome sequencing (WES) and whole-genome sequencing have assisted in the identification of lupus-mimickers and rare monogenic variants associated with SLE with high penetrance [8]. Recognition of causative mutations in patients with early-onset lupus or lupus-mimics may provide crucial insights into the pathogenesis and improve personalized treatment [9].
To explore the clinical manifestations and causative mutations in patients suspected with early-onset SLE (age ≦ 5 years old) and lupus mimics in Taiwan, WES was performed with special attention on genes potentially responsible for lupus [10, 11]. Patient’s clinical features and treatment responses were also carefully reviewed.
Patients and methods
Study subjects
Between Jan. 2012 to Dec. 2019, one hundred and eighty-four childhood-onset SLE (cSLE) patients were regularly followed in the Pediatric Allergy, Asthma, and Rheumatology department in Chang-Gung Memorial Hospital, a tertiary medical center in Taiwan. The average age of disease onset was 12.9 ± 2.8 years old and 164 of them are female (89.1%). Among them, 7 cases (3.8%) of which onset ≦ 5 years of age were identified for detail characteristic review and genetic analysis. All cases fulfilled the 2012 Systemic Lupus International Collaborating Clinics (SLICC) criteria for the diagnosis of SLE [12].
Genetic analysis
Genomic deoxyribonucleic acid (DNA) was isolated from peripheral venous blood samples. WES was performed at Biotools (New Taipei city, Taiwan) using the Agilent SureSelect Human All Exon Kit 58 m (v6) (Agilent Technologies, Inc. Santa Clara, United States) for exome capture and the NovaSeq 6000 platform (Illumina, San Diego, CA) for massively parallel sequencing. Raw image analyses and base calling were performed using Illumina’s Pipeline with default parameters. Sequence data were aligned to the reference human genome (hg38) using the Burrows-Wheeler Aligner, and duplicate reads were removed using Picard tools. Results revealed a mean depth of 62.34 times, and 98.38% of the targets were covered with at least 10 times of depths. We used the Genome Analysis Toolkit to perform realignment and variation (SNP and InDel) detection. Annovar was utilized to catalog the detected variations. Variations were filtered with a homo-polymer length > 6 (and synonymous substitutions) or that were common (> 1%) in the Exome Aggregation Consortium database and the Genome Aggregation Database. Pathogenicity score was calculated using PolyPhen2, SIFT, DANN and CADD. Special attention was placed on the panel of reported genes associating lupus to identify possible causal mutations [10, 11]. Sanger sequencing was performed to confirm the genetic variants from patient DNA and the parental DNA when the samples were available.
Case presentation
Seven cases including 4 female (57.1%) and 3 male (42.9%) were suspected with early-onset SLE (age of onset ≦ 5 years old). None of the patients were of consanguineous marriage and the age of disease onset ranged from 20 to 60 months. Compared to the cSLE patients onset at elder age, cases fulfilling the SLE classification criteria before the age of 5 are less female dominate (57.1% vs 90.4%), and more likely to suffer from proliferative glomerulonephritis (71.4% vs 56.5%), renal thrombotic microangiopathy (TMA) (28.6% vs 4.5%), neuropsychiatric disorder (57.1% vs 11.3%), and failure to thrive (FTT) (42.9% vs 4.0%). Clinical manifestations and initial laboratorial findings of those suspected with early-onset SLE were summarized in Table 1. Patients with probable genetic etiologies were further discussed.
Table 1.
Clinical characteristics and laboratory data of patients suspected with early-onset systemic lupus erythematosus
| Cases | # 1 | # 2 | # 3 | # 4 | # 5 | # 6 | # 7 |
|---|---|---|---|---|---|---|---|
| Gender | F | M | M | F | F | F | M |
| Family history of SLE | no | yes | yes | no | no | no | no |
| Age when cSLE suspected (mon) | 20 | 24 | 38 | 48 | 51 | 60 | 60 |
| Age when WES performed (mon) | 21 | 27 | 46 | 50 | 101 | 96 | 103 |
| Identified genetic variants | homozygous TREX1 (c.292_293 ins A) | SLC7A7 (c.625 + 1 G > A) | SLC7A7 (c.625 + 1 G > A) | DNASE1 (c.G370A),C1s (c.G1241), C2 (c.C1558T) | CFHR4 (c.T103C) | DNASE1L3 (c.G764A) | negative finding |
| SLICC at diagnosis | |||||||
| Acute cutaneous lupus | - | - | - | - | - | + | - |
| Chronic cutaneous lupus | + | - | - | - | + | - | - |
| Oral or nasal ulcers | - | - | - | + | - | + | + |
| Nonscarring alopecia | - | - | - | - | - | - | - |
| Synovitis | - | - | - | - | + | + | + |
| Serositis | - | - | - | + | - | - | - |
| Renal | - | - |
+ LN IV |
+ LN IV |
+ LN IV |
+ LN IV |
+ LN IV |
| Neurologic | + | - | - | - | - | - | - |
| Hemolytic anemia | + | - | - | + | + | - | - |
| Leukopenia/ lymphopenia | - | + | - | - | - | - | - |
| Thrombocytopenia | + | + | - | + | - | - | - |
| Positive ANA | + | - | + | + | + | + | + |
| Positive anti-dsDNA | - | + | + | - | + | + | + |
| Positive anti-Sm | + | NA | + | + | - | + | NA |
| Positive APL | + | NA | NA | + | - | - | - |
| Low complement level | - | + | + | + | + | + | + |
| Positive direct Coombs test | - | NA | - | - | - | + | + |
| Initial Laboratory data | |||||||
| White blood cells (/μl) [RR: 4000–11000] | 7600 | 4400 | 4300 | 4500 | 4400 | 7200 | 6000 |
| Lymphocytes (/μl) [RR: 1000—4000] | 1800 | 900 | 1500 | 2000 | 2200 | 2100 | 1500 |
| Hemoglobin (g/dl) [RR: 12—18] | 8.5 | 12.2 | 11.2 | 9.4 | 7.2 | 13 | 15.3 |
| Platelets (K/μl) [RR: 150—400] | 94 | 95 | 200 | 34 | 239 | 430 | 248 |
| anti-dsDNA Ab (unit/ml) [RR: < 139] | 104 | 140.5 | 176.6 | 49.5 | 714 | 615.5 | > 2000 |
| C3 (mg/dL) [RR: 90—180] | 102 | 127 | 44.8 | 72 | 30.9 | 26.7 | 21.2 |
| C4 (mg/dL) [RR: 10—40] | 13.4 | 8.3 | 5.32 | 15.7 | 6.16 | 3.33 | 5.43 |
| ANA [RR: 1:80 negative] | 1:320 | - | 1:80 | 1:160 | 1:320 | 1:160 | 1:1280 |
| Proteinuria [RR: 0] | 0 | 1 + | 4 + | 4 + | 2 + | 4 + | 2 + |
| Hematuria [RR: 0] | 0 | 0 | 3 + | 3 + | 3 + | 3 + | Trace |
| Symptoms and comorbidities | |||||||
| Growth and development | FTT, developmental delay | - | FTT | - | FTT | - | - |
| CNS | conscious disturbance, encephalopathy with leukodystrophy | episodic conscious disturbance | episodic conscious disturbance | seizure with posterior reversible encephalopathy syndrome | - | - | - |
| Cardiovascular | patent foramen ovale, trivial tricuspid regurgitation | - | - | hypertension | hypertension | hypertension | hypertension |
| Gastrointestinal | gastroesophageal reflux, raise of hepatic enzyme | Hepatosplenomegaly, emesis | Hepatosplenomegaly, emesis | ascites | necrotizing pancreatitis with pancreatic pseudocysts | - | moderate hepatomegaly |
| Renal | - | - | GN | GN, TMA | GN, TMA | GN | GN |
| Metabolic | autoimmune thyroiditis with subclinical hypothyroidism, insulin-dependent diabetes mellitus | metabolic acidosis, hyperammonemia | metabolic acidosis, hyperammonemia | - | dyslipidemia | - | dyslipidemia |
| Mucocutaneous | chilblain-like skin rash | - | - | oral ulcer | discoid rash | oral ulcer, malar rash | oral ulcer |
| Musculoskeletal | Spastic quadriplegic cerebral palsy | - | delayed bone age | - | arthritis over bilateral knees | arthritis over bilateral ankles and knees | arthritis over bilateral knees |
| Serology | ANA, anti-Sm, anti-RNP, anti-Ro, anti-La, ANCA and APL Ab | anti-dsDNA, anti-Ro, low complement | ANA, anti-dsDNA, anti-Sm, and low complement | ANA, anti-Sm, APL and low complement | ANA, anti-dsDNA, anti-Ro and low complement | ANA, anti-dsDNA, anti-Sm, anti-Ro and low complement | ANA, anti-dsDNA, anti-Ro, APL and low complement |
| Invasive infections | recurrent aspiration pneumonia, P. aeruginosa pneumonia, Salmonella sepsis and K. pneumonia pyelonephritis | S. aureus bacteremia, M. catarrhalis pneumonia | - | - | Salmonella sepsis, C. albicans bacteremia, bacterial peritonitis | - | - |
| Treatment | |||||||
| Prednisolone | tapering dose from 3.5 mg/day × 1 mon | tapering dose from 2.5 mg/day × 3 mons | tapering dose from 20 mg/day × 15 mons | tapering dose from 60 mg/day × 5 yrs | steroid pulse + tapering dose from 60 mg/day until now | steroid pulse + tapering dose from 60 mg/day until now | steroid pulse + tapering dose from 60 mg/day until now |
| Cyclophosphamide | - | - | - | - | 0.5-1 g/m2 monthly × 5 doses | 0.5-1 g/m2 monthly × 12 doses | 0.5-1 g/m2 monthly × 12 doses |
| Azathioprine | - | - | - | - | - | - | 50 mg/day × 2 mons |
| Cyclosporin | - | - | - | 25–50 mg/day | 100–150 mg/day | 100–200 mg/day | - |
| MMF/MPA | - | - | - | MMF 500- 750 mg/day | MPA 360–1440 mg/day | MMF 500- 1500 mg/day | MPA 540–1440 mg/day |
| Hydroxychloroquine | - | - | - | - | 100–200 mg/day | 100–200 mg/day | 100–200 mg/day |
| Other treatments | thyroxine, insulin | neomycin sulfate, sodium benzoate, multivitamins, lactulose | neomycin sulfate, sodium benzoate, multivitamins, lactulose | plasma exchange, captopril, amlodipine, clonidine | plasma exchange, captopril, losartan, atorvastatin | amlodipine | amlodipine, losartan |
Abbreviations: SLE Systemic lupus erythematosus, F Female, M Male, SLICC Systemic Lupus International Collaborating Clinics, LN IV Lupus nephritis class 4, ANA Antinuclear antibody, anti-dsDNA Anti-double-stranded DNA antibody, anti-Sm Anti-Smith antibody, anti-Ro Anti-Ro antibody, anti-La Anti-La antibody, ANCA Anti-neutrophil cytoplasmic antibody, anti-RNP Anti-ribonucleoprotein antibody, APL Anti-phospholipid antibody, CNS Central nervous system, TMA Thrombotic microangiopathy, GN Glomerulonephritis, MMF Mycophenolate mofetil, MPA Mycophenolic acid, NA Not available, RR reference range
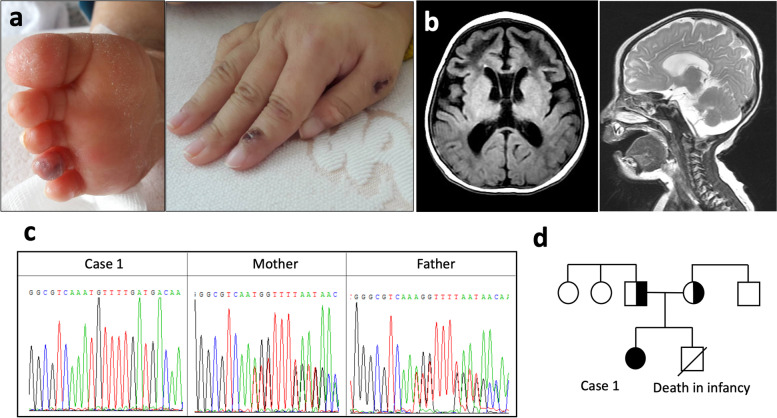
Case 1
Case 1 is a 20-month-old girl from a Taiwanese and Indonesian joint family presented with acute onset of drowsiness. On arrival, she was found with FTT, chilblain-like skin lesions and dystonic posturing with peripheral spasticity (Fig. 1A). Brain computed tomography imaging demonstrated encephalopathy with leukodystrophy (Fig. 1B). Serial laboratory workup revealed thrombocytopenia, hemolytic anemia, positive anti-nuclear antibody (ANA) and high titer of anti-extractable nuclear antibodies (anti-ENA), including anti-Smith antibody (anti-Sm), anti-ribonucleoprotein antibody (anti-RNP), anti-Ro antibody, anti-La antibody, anti-neutrophil cytoplasmic antibody (ANCA) and anti-phospholipid antibody (APL) (Table 1). The patient was initially treated with low dose corticosteroids (~ 0.5 mg/kg/day) for progressing thrombocytopenia and hemolytic anemia, but quickly tapered off in a month due to limited response and aspiration pneumonia.
Fig. 1.
Clinical features and genetic analysis of Case 1. a Chilblain lupus erythematosus lesions over the ventral aspect of 4th toe and dorsal aspect of the index finger and thumb. b The spectrum of brain changes, including encephalopathy with leukodystrophy on brain computed tomography. c Sanger sequencing of identified alterations with whole exome sequencing of a patient and her parents. d The family pedigree of case 1 with the TREX1 mutation
Because of her profound neurologic defects, chilblain-like skin rash and young age, WES and plasma interferon-α (IFN-α) was examined for type-1 interferonopathy survey. Her genetic analysis revealed homozygous TREX1 c.292_293 ins A; p.Cys99Met fs mutation. The reported allelic frequency (AF) in gnomAD is 0.007% and the pathogenicity scores were unavailable. Sanger sequencing of the patient and her parental DNA confirmed that the TREX1 variants were inherited from both of her parents. Level of her plasma IFN-α was significantly higher than both of her parents and health control (53.56 v.s. 38.91, 37.17 and 35.74 pg/ml, respectively). Under the diagnosis of Aicardi-Goutières syndrome (AGS), Janus kinase (JAK) inhibitor was suggested as an alternative choice but rapidly discontinued due to high expenses and recurrent infectious episodes. Autoimmune thyroiditis with subclinical hypothyroidism, glaucoma and insulin-dependent diabetes mellitus were noted during sequential follow-ups without steroid or immunosuppressants treatment. Despite careful care, the patient died at the age of 6 following multiple invasive infectious episodes, including recurrent aspiration pneumonia, Pseudomonas aeruginosa pneumonia, Salmonella sepsis and Klebsiella pneumonia pyelonephritis.
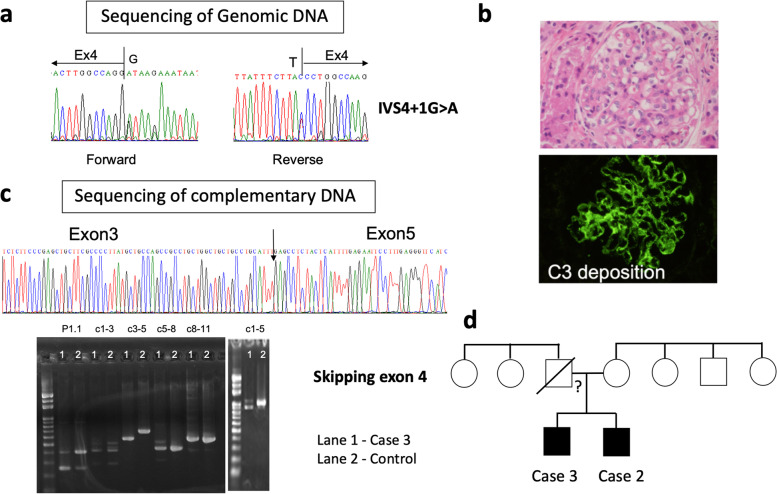
Case 2 and 3
In a nonconsanguineous family without documented family history of autoimmune diseases, 2 brothers sequentially fulfilled the 2012 SLICC SLE classification criteria at the ages of 24 and 38 months. Upon initial evaluation, the elder brother (case 3) suffered from FTT and glomerulonephritis presenting with profound proteinuria (> 1,000 mg/m2/day, urine protein/creatinine ratio: 98,198 mg/gm) and hematuria. Histopathological examination of his renal biopsy revealed membranoproliferative glomerulonephritis with strong IgG, IgA, IgM, C3 and C1q staining in diffuse pattern, compatible with lupus nephritis (LN) (Fig. 2B). Although his hemogram and clinical manifestations were unremarkable, positive ANA, anti-double stranded DNA antibody (anti-dsDNA), anti-Sm, low complements and the renal histopathological findings raised the suspicion of cSLE. Steroid (~ 2 mg/kg/day) was initially given for treatment and gradually tapered off in 15 months. His younger brother had a much milder symptoms with transient proteinuria, lymphopenia, thrombocytopenia, low C4 and positive autoantibody profile (Table 1). Episodic hyperammonemia, metabolic acidosis and conscious disturbance were noted during follow ups.
Fig. 2.
Renal histology and genetic/complementary DNA analysis of Case 2 & 3. a In the genomic level, the G before the last nucleotide of the intron is mutated to A, causing the splicing site to shift. b Histopathological examination of a renal biopsy showed membranoproliferative glomerulonephritis. An immunofluorescence micrograph illustrating diffuse glomerular C3 deposition. c Complementary DNA (cDNA) analysis revealed a skipping of exon 4. The band on gel electrophoresis confirmed a shorter cDNA product from the patient. d The family pedigree of Case 2 and 3 harboring heterozygous SLC7A7 mutations. The father of Case 2 and 3 deceased and the mother is negative for SLC7A7 mutation
Due to young age, family cluster, metabolic disruption and conscious disturbance, genetic study was arranged. Share splicing variant c.625 + 1 G > A was found in the SLC7A7 gene in both patients associating lysinuric protein intolerance (LPI), a rare metabolic disorder. The reported AF in gnomAD is 0.004% and the DANN and CADD pathogenicity score were 0.995 and 28, respectively. Although only one variant was identified by exome sequencing, sequencing of patient cDNA revealed a skipping of exon 4 compared to healthy controls for all transcripts (Fig. 2C). Sanger sequencing was not performed on the deceased father and the mother was negative for SLC7A7 mutation (Fig. 2D). Instead of using immunosuppressants, these brothers were recommended to consume a hypoproteinemic diet adapted for their age and receive citrulline as well as vitamin supplementation. Case 2 experienced scattered episodes of hyperammonemia without serious conscious disturbance and the 2 brothers grew up smoothly without further immunomodulatory medications.
Case 4
Case 4 is a 4-year-old girl with painless oral ulceration and swelling legs. Seizure with posterior reversible encephalopathy syndrome was also noted. Renal biopsy following progressing edema and elevation of serum creatinine revealed LN class IV and renal TMA. Prednisone, mycophenolate mofetil (MMF) and plasma exchange were prescribed for rapid progressive glomerulonephritis. WES was arranged for young age and TMA. Her genetic analysis revealed heterozygous mutations in 3 different genes: C1s (c.G1241A; p.R414H; AF: 0.00478%; PolyPhen2: 0.096; DANN: 0.078), C2 (c.C1558T; p.R520C; AF: 0.18%; PolyPhen2: 0.994; DANN: 0.999), and DNASE1 (c.G370A; p.E124K; AF: 0.00521%; PolyPhen2: 0.687; DANN: 0.998). The patient is under relative stable condition under MMF and cyclosporin treatment.
Case 5
Case 5 is a 4-year-old girl with a record of FTT. Fever, arthritis, discoid rashes and high titer of autoantibodies were also noted (Table 1). Renal biopsy following heavy proteinuria, hematologic changes and hypertension revealed LN class IV with TMA. She underwent a course of plasma exchange due to persistent proteinuria despite cyclophosphamide, steroid and cyclosporine treatment. WES was arranged for young age and TMA. While no genetic variants among the SLE associating genes were found, a heterozygous mutation in the CFHR4 gene (c.T103C; AF: 0.14%; PolyPhen2: 0.999; DANN: 0.952), was discovered. Deletions in the CFHR4 gene have been found in association with atypical hemolytic uremic syndrome (aHUS), a form of TMA. Our patient suffered from recurrent aHUS and required plasma exchange, steroid, MMF and cyclosporine for treatment. Three episodes of necrotizing pancreatitis with pancreatic pseudocysts were noted 5 years after her diagnosis of SLE.
Case 6
Case 6 is a 5-year-old girl presented with nephrotic-range proteinuria (urine protein/creatinine ratio: 4,218 mg/gm), malar rash, oral ulceration and arthritis. Her renal biopsy suggested class IV LN. A heterozygous mutation in DNASE1L3 (c.G764A; p.R255K; AF: 0.01%; PolyPhen2: 0.001; DANN: 0.705) was evidenced in her targeted WES screening. Treatment with cyclophosphamide pulse therapy, prednisolone, hydroxychloroquine and azathioprine lead to a complete remission of LN with improving proteinuria (urine protein/creatinine ratio: 38 mg/gm).
Discussion
Clinical variation of SLE across different age groups existed in different populations [4, 13–17]. Utilizing the nationwide, population-based retrospective cohort, Chen et al. revealed that juvenile-onset SLE patients (onset < 18 years of age) were at greatest risk of mortality likely due to higher disease severity in Taiwan [16]. Systemic review and meta-analysis study performed by Bundhun et al. showed that renal inflammation, hematological manifestations, seizure and ocular involvement were significantly higher among young SLE patients [18]. In America, Gomes found no differences in gender discrepancy, nephritis, neuropsychiatric involvement and disease activity, but a higher frequency of fever, hepatomegaly, splenomegaly and discoid lupus among the early-onset SLE patients (onset < 6 years of age) [15]. Data collected from Europe suggested that young children with SLE have higher frequency of autoimmune family history, neuropsychiatric manifestations, nephritis, hematological disorders and an increased risk of organ damages potentially contributed by the cumulative duration and dose of prednisone and immunosuppressive medications [14, 17]. Similar but not limited to the reported findings, cases fulfilling the SLE classification criteria before the age of 5 in our cohort are less female dominate and more likely to suffer from proliferative glomerulonephritis, neuropsychiatric disorder as well as TMA and FTT. FTT is used to describe infant and child with weight below the fifth percentile for sex and corrected age [19]. While inadequate caloric intake was its leading etiology, inflammatory conditions, inborn errors of metabolism and genetic defects can all attributed its presence. The hallmarks of TMAs are vascular thromboses, which lead to clinical signs of microangiopathic hemolysis, a decrease in platelet count and organ damage involving renal or neurological manifestations [20]. It is a form of endothelial injury that can occur in the kidneys of 1–4% LN patients and is associated with severe clinical manifestations and a high mortality rate [21, 21]. Plasma exchange and Eculizumab, a monoclonal antibody capable of inhibiting C5 activation was recommended for treatment of TMA secondary to SLE [20, 21, 23]. Considering the necessity of further evaluation and treatment adjustment in the presence of FTT and TMA, it is worthwhile to look for these manifestations in cases suspected with early-onset SLE.
Two children with early-onset SLE, fulfilling the American College of Rheumatology (ACR) criteria for SLE was reported by Hedrich et al. with atypical manifestations including severe liver dysfunction, coagulopathy and protein loss enteropathy [4]. Through a large-scale multiracial SLE cohort study covering 1317 patients, Webb et al. discovered that the age of disease onset during childhood is predicted by a higher genetic risk for lupus and is associated with a more severe phenotype [6]. Recently, Massias et al. hypothesized that the variations of clinical manifestation across different age groups may resulted from different mechanisms underlining SLE pathophysiology at different age [13]. The availability of next generation sequencing and the emerging evidence of genetic susceptibility in lupus over the last decade largely expanded our knowledge on the genetic basis of lupus [8]. Mutations in genes governing the pathways of complement cascade, immune tolerance, apoptosis, antigen clearance, type I interferonopathy, metabolism and more have been identified as genetic etiologies underling lupus [10, 11, 24, 25]. Recently, Tirosh et al. reported that causative monogenic mutations were identified in 4 out of 15 newly diagnosed cSLE patients in 5 different genes: C1QC, SLC7A7, MAN2B1, PTEN and STAT1 [11]. Analyzed 39 children with lupus manifestations associated with primary immunodeficiency diseases (PIDs), Al-Mayouf et al. discovered that complement deficiency was the most frequent PIDs associating lupus-like manifestations. Genetic defects in PNP, PIK3CD, STAT1, ISG15, IL2RB, GS3, DNASE2 and genes associating chronic granulomatous disease were found among 7 of the 25 patients who underwent genetical testing [25]. Screening through 117 cSLE patients fulfilling the ACR criteria for SLE, Belot et al. reported that the mendelian genotypes involving variants in C1QA, C1QC, C2, DNASE1L3, and IKZF1 were confirmed in 8 patients, while 7 additional cases harbored heterozygous variants in complement or type I interferon-associated autosomal recessive genes. Rare variants which were predicted to be damaging were significantly enriched in the cSLE cohort compared with controls [10]. In the present study, causative genetic etiologies TREX1 and SLC7A7 were identified in 3 out of 7 patients (42.9%). Rare and potentially damaging variants C1s, C2, DNASE, DNASE1L3 and CFHR4 were found in the other 3 (42.9%). Although monogenic lupus and lupus mimics were not exclusive for cases with early-onset disease, considering the high prevalence of causative genetic etiologies among the childhood population, detail clinical evaluation and genetic testing is recommended to help clarify the underling pathogenesis and predict disease course.
Abnormalities in the intracellular nucleic acid sensing machinery TREX1 and other critical players including RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1 and IFIH1 causes AGS, a monogenic interferonopathy [26, 27]. Due to the importance of type 1 interferon (IFN) in systemic autoimmune diseases, many overlapping clinical and laboratorial presentation were noted between patients with AGS and SLE. Genetic variant in TREX1 gene is the causative mutation for Case 1. It encodes a 3’ repair exonuclease that guards DNA synthesis, and loss of function can lead to an accumulation of endogenous DNA and increased expression of IFN [28, 29]. Individuals with microcephaly or TREX1-related AGS, such as Case 1, were the most severely affected and less likely to achieve normal developmental milestone [26]. Although less discussed, besides the profound neurologic defects and presence of various autoantibodies, manifestations of congenital glaucoma [30], hepatic inflammation [31], endocrinopathies [32] and susceptible to infections [33] of Case 1 are also likely attributed to TREX1 mutation. Worthwhile to mention, while the clinical features of heterozygous TREX1 mutations have been described in patients with lupus [34, 35], the identification of homozygous TREX1 (c.292_293 ins A) in SLE was reported for the first time. Comparing the age of disease onset in SLE mimics with monoallelic TREX1 variants, the lupus phenotype appeared much earlier in Case 1 (20 month vs. 14 ~ 50 years old) [35]. Recently, JAK inhibitors including Ruxolitinib and Baricitinib have been reported to not only control AGS related skin lesions but neurologic function even in cases with severe and long-standing disease [36–38]. Other developing regimens, including interferon-α/β receptor blockade, IFN-α targeting, reverse-transcriptase inhibitors and stimulator of interferon genes (STING) antagonist also provide various degree of benefits as these medications suppress IFN signaling [39–44]. As targeted therapy became available, clinical suspicion and genetic testing for AGS is especially important for patients presenting with early-onset lupus mimic disease.
SLC7A7 gene encoded a subunit of the cationic amino acid transporter found in epithelial cell membranes. Mutations in this gene causes LPI, a rare recessive disorder characterized by FTT, growth retardation, muscle hypotonia and hepatosplenomegaly [45]. Mostly appeared after weaning of breastmilk, clinical manifestations of LPI can be widely variable resembling the findings in urea cycle disorders such as hyperammonemia [46]. Overlapping manifestation of LPI and SLE has been reported in several case series [11, 46]. Renal involvement is a frequent and progressive complication in LPI. In a cohort of 39 LPI patients, 74% of the patients had proteinuria and 38% had hematuria [47]. Heterogeneous renal histological findings ranging from tubulointestinal disorder to distinct glomerulonephritis with polyclonal immunoglobulin deposition has been reported [47, 48]. Carefully reviewed by Contreras et al., the incidence of other LPI associating manifestations including FTT, metabolic disorder, neurologic symptoms and hepatosplenomegaly in Case 2 and 3 corresponded to 52%, 52%, 25% and 43% of all cases with LPI, respectively [46]. While recessive disorder requires homozygous SLC7A7 defects to become phenotypic, the clinical phenotypes, presence of hyperammonemia, segregation analysis and the complete skipping of exon 4 revealed by complementary DNA analysis of Case 2 and 3 suggested a diagnosis of LPI. It is hypothesized that an undetected large deletion or compound heterozygous mutation existed in the corresponding allele, leading to the LPI and lupus phenotype. Instead of aggressive immunosuppressant treatment, administration of arginine and nitrogen scavenger drugs such as sodium benzoate and sodium phenylpyruvate to lower the level of ammonia and a low-protein diet with oral supplementation of citrulline and carnitine kept the 2 brothers under a relative stable disease status. In fact, dietary adjustment and nitrogen scavenger drugs are recommended as the mainstays of long-term therapy [49]. Identification of LPI, a lupus mimicker, from classical lupus thoroughly explained the atypical metabolic and neurological presentations among Case 2 and 3 and limited the use of unnecessary immunosuppressants. Moreover, the genetic data provided physicians with a better understanding on how the disease would progress. Potential complications including renal, hematological, skeletal, and gastrointestinal features will be closely monitored [49].
Rare and potentially pathogenic variants in the lupus associating genes C1s, C2, DNASE1 and DNASE1L3 as well as CFHR4 were discovered in Cases 4–6 diagnosed with early-onset SLE in the present series. Homozygous deficiencies of early components within the complement cascades are among the strongest genetic risk factors for SLE in human [50]. SLE patient with C1s mutations has been reported with discoid rash, generalized seizure, autoimmune thyroiditis, autoimmune hepatitis and diffuse proliferative glomerulonephritis with full house deposition of glomerular immunofluorescence in their kidney biopsy consistent with LN [50–52]. Arthritis, mucocutaneous lesions, hematologic disorder and renal manifestations were documented among patients with C2 mutations [53–55]. DNASE1 mutation is associated with high titer of ANA, anti-dsDNA, anti-histones, anti-Ro and immune mediated glomerulonephritis [56]. Interestingly, although the direct contribution of these mutations in Case 4 presenting with various autoantibodies, proliferative LN and seizure associating posterior reversible encephalopathy syndrome remain unknown, reduced plasma DNase1 activity have recently been shown to cause the persistence of pro-thrombotic neutrophil extracellular traps, promote microvascular thrombosis and contribute the development of TMA [57]. Patients with DNASE1L3 mutations are prone for LN, high titer of ANA, APL, ANCA and low complement similar to Case 6 [58]. CFHR4 encodes one of the 5 complement factor H-related proteins and is linked to aHUS, a life threatening TMA characterized by dysregulation of the alternative pathway of complement [59]. While aHUS rarely causes acute pancreatitis and the association between these two diseases remain unclear, several reports revealed an episode of acute pancreatitis preceding TMA in cases with or without TMA related mutations [60–62]. Together, although the direct impact of these variants in disease manifestation requires further clarification, the accumulation of rare variants predicted-damaging variants in SLE-associated genes may contribute to disease expression and clinical heterogeneity [10].
Considering the prevalence and the severity of proliferative glomerulonephritis, TMA and neuropsychiatric disorder in cases suspected with early-onset SLE, identification of genetic variants associating these phenotypes may be as important as surveying for lupus associating mutations in young children with lupus-like manifestations. CNS manifestations of SLE expanded widely from nonspecific symptoms including headache, cognitive impairment to devastating features such as memory loss, seizures and stroke [63]. As previously reviewed, only handful of genetic variations were associated with neuropsychiatric symptoms in lupus [63, 64]. The HLA-DRB1Ã04 genotype and STAT4 rs10181656 were associated with stroke in SLE independent of the status of APL [65, 66]. Rare single-nucleotide polymorphisms (SNPs) and mutations in TREX1, have been reported in SLE cases with neurological manifestations, especially seizures and neuropsychiatric lupus [35, 66, 67]. The cumulative effect of having 10 or more SNPs in the HLADRB1, IRF5, STAT4, BLK, TNFAIP3, TNIP1, FCGR2B and TNFSF13 genes have also been shown to increase the risk of developing neurological manifestations by twofold [68]. Changes in mental status were noted in 3 of the 7 patients in the present series. While delirium, depression, dementia, and coma can all result in mental status alteration, TREX1 associated encephalopathy affecting the ascending reticular activating system and LPI related hyperammonemia and metabolic acidosis possibly attributed to their neuropsychiatric presentation. Proliferative glomerulonephritis can lead to end-stage kidney disease and usually requires aggressive treatment with immunosuppressants [69]. Recently, around 60 different disease susceptibility genes associated with LN were classified according to the pathways they’re involved [70]. Take BLK mutation for example, being a src family non-receptor tyrosine kinase mainly expressed by B-cells, BLK mutations in LN not only interrupt one’s adaptive immune signaling, but provide a rational for the application of B cell targeting regimen in the control of LN [70]. DNASE1 mutation in Case 4 alters program cell death and TREX1 mutation in Case 1 mainly affects the innate immunity. Despite the notion of its potential functionality, no pathway-specific therapeutic strategies, however, were recommended for LN related to these mutations. Finally, genetic mutations including CFH, CFI, CFB, C3, THBD, PLG, MCP, ADAMTS13, MMACHC and DGKE have recently been reported to result in TMA [20, 23, 71]. Spotting a genetic variant in the TMA associating genes by physician should raise the awareness of TMA in patients suspected with early-onset lupus.
During the past decades, treatment for SLE has moved from corticosteroids alone to a combination of disease-modifying antirheumatic drugs, immunosuppressants and biologics. A treat-to-target strategy was recently proposed for lupus, leading to individualized, patient-tailored regimens with multitargeted therapies [9, 72]. With rapid expansion of treatment options, early identification of patients with lupus mimics and those with causative mutations from sporadic lupus is necessary for precise and tailored treatment. Recently, it is proposed that genetic testing are recommended for those with disease onset at a young age; severe, life-threatening or organ-threatening presentation; aggressive disease course, rapid deterioration and/or accumulation of organ damage; and poor response to standard treatment [11]. Due to the rarity of early-onset SLE, we are unable to enroll large enough case number in the study to reflect the clinical significance of individualized treatment. International and multi-central collaboration is needed to better address the issue. In the coming era of precision medicine, patients with SLE will likely be stratified by their immunophenotypes or their genetics as technology advances [9, 72]. Revealing the molecular genetic diagnosis of SLE, especially among those early-onset cases, can promote personalized medical care with targeted therapies and monitoring.
Conclusions
Genetic etiologies and lupus mimickers were found among a substantial proportion of patients suspected with early-onset SLE. Detail clinical evaluation and genetic testing are important to predict disease course, organ damages and refine the therapeutic options for pathology-based precision medicine.
Acknowledgements
We thank the staff and participants in the study, without whom this work would not be possible.
Abbreviations
- ACR
American College of Rheumatology
- AF
Allelic frequency
- AGS
Aicardi-Goutières syndrome
- aHUS
Atypical hemolytic uremic syndrome
- Anti-dsDNA
Anti-double stranded DNA antibody
- anti-ENA
Anti-extractable nuclear antibody
- Anti-La
anti-La antibody
- ANA
Anti-nuclear antibody
- ANCA
Anti-neutrophil cytoplasmic antibody
- APL
Anti-phospholipid antibody
- anti-RNP
Anti-ribonucleoprotein antibody
- Anti-Ro
anti-Ro antibody
- anti-Sm
Anti-Smith antibody
- cSLE
Childhood-onset SLE
- CNS
Central nervous system
- DNA
Deoxyribonucleic acid
- FTT
Failure to thrive
- gnomAD
Genome Aggregation Database
- IFN
Interferon
- JAK
Janus kinase
- LPI
Lysinuric protein intolerance
- LN
Lupus nephritis
- MMF
Mycophenolate mofetil
- MPA
Mycophenolic acid
- PID
Primary immunodeficiency
- SLE
Systemic lupus erythematosus
- SLICC
Systemic Lupus International Collaborating Clinics
- SNP
Single nucleotide polymorphism
- TMA
Thrombotic microangiopathy
- WES
Whole-exome sequencing
Authors’ contributions
W-FL and C-YW carried out the case analysis and drafted the manuscript, W-LF participated in the sequence alignment, M-HT and H-YY critically reviewed the manuscript, CY-W and JL-H conceived of the study and participated in the design. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the research fund of Chang Gung Memorial Hospital, Linkou (CMRPG3H1201-3, NMRPG3G6241-3, CMRPG3J0501-02) and Ministry of Science and Technology, Taiwan (MOST106-2314-B-182A-139-MY3).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This research was in compliance with the Declaration of Helsinki and was approved by the CGMH Institutional Review Board (IRB No. 201800989A3).
Consent for publication
Informed consent forms were signed by the patients’ guardians / parents before study entrance.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wan-FangLee, Wen-LangFan, Jing-LongHuang and Chao-YiWu contributed equally to this work.
Contributor Information
Jing-Long Huang, Email: long@adm.cgmh.org.tw.
Chao-Yi Wu, Email: joywucgu@hotmail.com.
References
- 1.Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update omicronn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis. 2021;80(1):14–25. doi: 10.1136/annrheumdis-2020-218272. [DOI] [PubMed] [Google Scholar]
- 2.Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European league against rheumatism/american college of rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151–1159. doi: 10.1136/annrheumdis-2018-214819. [DOI] [PubMed] [Google Scholar]
- 3.Chasset F, Richez C, Martin T, Belot A, Korganow AS, Arnaud L. Rare diseases that mimic systemic lupus erythematosus (lupus mimickers) Joint Bone Spine. 2019;86(2):165–171. doi: 10.1016/j.jbspin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Hedrich CM, Zappel H, Straub S, Laass MW, Wieczorek K, Hahn G, et al. Early onset systemic lupus erythematosus: differential diagnoses, clinical presentation, and treatment options. Clin Rheumatol. 2011;30(2):275–283. doi: 10.1007/s10067-010-1576-2. [DOI] [PubMed] [Google Scholar]
- 5.Huang JL, Yao TC, See LC. Prevalence of pediatric systemic lupus erythematosus and juvenile chronic arthritis in a Chinese population- a nation-wide prospective population-based study in Taiwan. Clin Exp Rheumatol. 2004;22(6):776–780. [PubMed] [Google Scholar]
- 6.Webb R, Kelly JA, Somers EC, Hughes T, Kaufman KM, Sanchez E, et al. Early disease onset is predicted by a higher genetic risk for lupus and is associated with a more severe phenotype in lupus patients. Ann Rheum Dis. 2011;70(1):151–156. doi: 10.1136/ard.2010.141697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alperin JM, Ortiz-Fernandez L, Sawalha AH. Monogenic lupus: a developing paradigm of disease. Front Immunol. 2018;9:2496. doi: 10.3389/fimmu.2018.02496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung SA, Shum AK. Rare variants, autoimmune disease, and arthritis. Curr Opin Rheumatol. 2016;28(4):346–351. doi: 10.1097/BOR.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demirkaya E, Sahin S, Romano M, Zhou Q, Aksentijevich I. New horizons in the genetic etiology of systemic lupus erythematosus and lupus-like disease: monogenic lupus and beyond. J Clin Med. 2020;9(3):712. doi: 10.3390/jcm9030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belot A, Rice GI, Omarjee SO, Rouchon Q, Smith EMD, Moreews M, et al. Contribution of rare and predicted pathogenic gene variants to childhood-onset lupus: a large, genetic panel analysis of British and French cohorts. The Lancet Rheumatology. 2020;2(2):e99–e109. doi: 10.1016/S2665-9913(19)30142-0. [DOI] [PubMed] [Google Scholar]
- 11.Tirosh I, Spielman S, Barel O, Ram R, Stauber T, Paret G, et al. Whole exome sequencing in childhood-onset lupus frequently detects single gene etiologies. Pediatr Rheumatol Online J. 2019;17(1):52. doi: 10.1186/s12969-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massias JS, Smith EMD, Al-Abadi E, Armon K, Bailey K, Ciurtin C, et al. Clinical and laboratory characteristics in juvenile-onset systemic lupus erythematosus across age groups. Lupus. 2020;29(5):474–481. doi: 10.1177/0961203320909156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca R, Aguiar F, Rodrigues M, Brito I. Clinical phenotype and outcome in lupus according to age: a comparison between juvenile and adult onset. Reumatol Clin (Engl Ed) 2018;14(3):160–163. doi: 10.1016/j.reuma.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Gomes RC, Silva MF, Kozu K, Bonfa E, Pereira RM, Terreri MT, et al. Features of 847 childhood-onset systemic lupus erythematosus patients in three age groups at diagnosis: a Brazilian multicenter study. Arthritis Care Res (Hoboken) 2016;68(11):1736–1741. doi: 10.1002/acr.22881. [DOI] [PubMed] [Google Scholar]
- 16.Chen YM, Lin CH, Chen HH, Chang SN, Hsieh TY, Hung WT, et al. Onset age affects mortality and renal outcome of female systemic lupus erythematosus patients: a nationwide population-based study in Taiwan. Rheumatology (Oxford) 2014;53(1):180–185. doi: 10.1093/rheumatology/ket330. [DOI] [PubMed] [Google Scholar]
- 17.Descloux E, Durieu I, Cochat P, Vital-Durand D, Ninet J, Fabien N, et al. Influence of age at disease onset in the outcome of paediatric systemic lupus erythematosus. Rheumatology (Oxford) 2009;48(7):779–784. doi: 10.1093/rheumatology/kep067. [DOI] [PubMed] [Google Scholar]
- 18.Bundhun PK, Kumari A, Huang F. Differences in clinical features observed between childhood-onset versus adult-onset systemic lupus erythematosus: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96(37):e8086. doi: 10.1097/MD.0000000000008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo SD, Hwang EH, Lee YJ, Park JH. Clinical Characteristics of Failure to Thrive in Infant and Toddler: Organic vs. Nonorganic Pediatr Gastroenterol Hepatol Nutr. 2013;16(4):261–268. doi: 10.5223/pghn.2013.16.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aigner C, Schmidt A, Gaggl M, Sunder-Plassmann G. An updated classification of thrombotic microangiopathies and treatment of complement gene variant-mediated thrombotic microangiopathy. Clin Kidney J. 2019;12(3):333–337. doi: 10.1093/ckj/sfz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright RD, Bannerman F, Beresford MW, Oni L. A systematic review of the role of eculizumab in systemic lupus erythematosus-associated thrombotic microangiopathy. BMC Nephrol. 2020;21(1):245. doi: 10.1186/s12882-020-01888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lansigan F, Isufi I, Tagoe CE. Microangiopathic haemolytic anaemia resembling thrombotic thrombocytopenic purpura in systemic lupus erythematosus: the role of ADAMTS13. Rheumatology. 2010;50(5):824–829. doi: 10.1093/rheumatology/keq395. [DOI] [PubMed] [Google Scholar]
- 23.Kello N, Khoury LE, Marder G, Furie R, Zapantis E, Horowitz DL. Secondary thrombotic microangiopathy in systemic lupus erythematosus and antiphospholipid syndrome, the role of complement and use of eculizumab: Case series and review of literature. Semin Arthritis Rheum. 2019;49(1):74–83. doi: 10.1016/j.semarthrit.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Costa-Reis P, Sullivan KE. Monogenic lupus: it’s all new! Curr Opin Immunol. 2017;49:87–95. doi: 10.1016/j.coi.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Al-Mayouf SM, Alreefi HA, Alsinan TA, AlSalmi G, AlRowais A, Al-Herz W, et al. Lupus manifestations in children with primary immunodeficiency diseases: comprehensive phenotypic and genetic features and outcome. Mod Rheumatol. 2021;31(6):1171–1178. doi: 10.1080/14397595.2021.1886627. [DOI] [PubMed] [Google Scholar]
- 26.Adang L, Gavazzi F, De Simone M, Fazzi E, Galli J, Koh J, et al. Developmental outcomes of aicardi goutieres syndrome. J Child Neurol. 2020;35(1):7–16. doi: 10.1177/0883073819870944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crow YJ, Stetson DB. The type I interferonopathies: 10 years on. Nat Rev Immunol. 2022;22(8):471-83. [DOI] [PMC free article] [PubMed]
- 28.Grieves JL, Fye JM, Harvey S, Grayson JM, Hollis T, Perrino FW. Exonuclease TREX1 degrades double-stranded DNA to prevent spontaneous lupus-like inflammatory disease. Proc Natl Acad Sci U S A. 2015;112(16):5117–5122. doi: 10.1073/pnas.1423804112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice GI, Rodero MP, Crow YJ. Human disease phenotypes associated with mutations in TREX1. J Clin Immunol. 2015;35(3):235–243. doi: 10.1007/s10875-015-0147-3. [DOI] [PubMed] [Google Scholar]
- 30.Gowda VK, Vegda H, Shivappa SK, Benakappa N. Aicardi-goutieres syndrome presenting with congenital glaucoma. Indian J Pediatr. 2020;87(8):652. doi: 10.1007/s12098-020-03196-0. [DOI] [PubMed] [Google Scholar]
- 31.Gavazzi F, Cross ZM, Woidill S, McMann JM, Rand EB, Takanohashi A, et al. Hepatic involvement in aicardi-goutieres syndrome. Neuropediatrics. 2021;52(6):441–447. doi: 10.1055/s-0040-1722673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worth C, Briggs TA, Padidela R, Balmer E, Skae M. Endocrinopathies in aicardi goutieres syndrome-a descriptive case series. Clin Case Rep. 2020;8(11):2181–2185. doi: 10.1002/ccr3.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P, Du J, Goodier JL, Hou J, Kang J, Kazazian HH, Jr, et al. Aicardi-Goutieres syndrome protein TREX1 suppresses L1 and maintains genome integrity through exonuclease-independent ORF1p depletion. Nucleic Acids Res. 2017;45(8):4619–4631. doi: 10.1093/nar/gkx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee-Kirsch MA, Gong M, Chowdhury D, Senenko L, Engel K, Lee YA, et al. Mutations in the gene encoding the 3’-5’ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet. 2007;39(9):1065–1067. doi: 10.1038/ng2091. [DOI] [PubMed] [Google Scholar]
- 35.Namjou B, Kothari PH, Kelly JA, Glenn SB, Ojwang JO, Adler A, et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 2011;12(4):270–279. doi: 10.1038/gene.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chyuan IT, Tzeng H-T, Chen J-Y. Signaling Pathways of Type I and Type III Interferons and Targeted Therapies in Systemic Lupus Erythematosus. Cells. 2019;8(9):963. doi: 10.3390/cells8090963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briand C, Fremond ML, Bessis D, Carbasse A, Rice GI, Bondet V, et al. Efficacy of JAK1/2 inhibition in the treatment of chilblain lupus due to TREX1 deficiency. Ann Rheum Dis. 2019;78(3):431–433. doi: 10.1136/annrheumdis-2018-214037. [DOI] [PubMed] [Google Scholar]
- 38.Vanderver A, Adang L, Gavazzi F, McDonald K, Helman G, Frank DB, et al. Janus kinase inhibition in the aicardi-goutieres syndrome. N Engl J Med. 2020;383(10):986–989. doi: 10.1056/NEJMc2001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ronnblom L, Leonard D. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med. 2019;6(1):e000270. doi: 10.1136/lupus-2018-000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong Z, Mei J, Li C, Bai G, Maimaiti M, Hu H, et al. STING inhibitors target the cyclic dinucleotide binding pocket. Proc Natl Acad Sci U S A. 2021;118(24):e2105465118. doi: 10.1073/pnas.2105465118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice GI. Reverse-transcriptase inhibitors in the aicardi-goutières syndrome. N Engl J Med. 2018;379(23):2275–7. doi: 10.1056/NEJMc1810983. [DOI] [PubMed] [Google Scholar]
- 42.Crow YJ, Shetty J, Livingston JH. Treatments in Aicardi-Goutières syndrome. Dev Med Child Neurol. 2019;62(1):42–47. doi: 10.1111/dmcn.14268. [DOI] [PubMed] [Google Scholar]
- 43.Rice GI, Meyzer C, Bouazza N, Hully M, Boddaert N, Semeraro M, et al. Reverse-transcriptase inhibitors in the aicardi-goutieres syndrome. N Engl J Med. 2018;379(23):2275–2277. doi: 10.1056/NEJMc1810983. [DOI] [PubMed] [Google Scholar]
- 44.Mura E, Masnada S, Antonello C, Parazzini C, Izzo G, Garau J, et al. Ruxolitinib in aicardi-goutieres syndrome. Metab Brain Dis. 2021;36(5):859–863. doi: 10.1007/s11011-021-00716-5. [DOI] [PubMed] [Google Scholar]
- 45.Torrents D, Mykkänen J, Pineda M, Feliubadaló L, Estévez R, Cid RD, et al. Identification of SLC7A7, encoding y+LAT-1, as the lysinuric protein intolerance gene. Nature Genetics. 1999;21(3):293–6. doi: 10.1038/6809. [DOI] [PubMed] [Google Scholar]
- 46.Contreras JL, Ladino MA, Aranguiz K, Mendez GP, Coban-Akdemir Z, Yuan B, et al. Immune dysregulation mimicking systemic lupus erythematosus in a patient with lysinuric protein intolerance: case report and review of the literature. Front Pediatr. 2021;9:673957. doi: 10.3389/fped.2021.673957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicolas C, Bednarek N, Vuiblet V, Boyer O, Brassier A, De Lonlay P, et al. Renal involvement in a french paediatric cohort of patients with lysinuric protein intolerance. JIMD Rep. 2016;29:11–17. doi: 10.1007/8904_2015_509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esteve E, Krug P, Hummel A, Arnoux JB, Boyer O, Brassier A, et al. Renal involvement in lysinuric protein intolerance: contribution of pathology to assessment of heterogeneity of renal lesions. Hum Pathol. 2017;62:160–169. doi: 10.1016/j.humpath.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 49.Noguchi A, Takahashi T. Overview of symptoms and treatment for lysinuric protein intolerance. J Hum Genet. 2019;64(9):849–858. doi: 10.1038/s10038-019-0620-6. [DOI] [PubMed] [Google Scholar]
- 50.Wu YL, Brookshire BP, Verani RR, Arnett FC, Yu CY. Clinical presentations and molecular basis of complement C1r deficiency in a male African-American patient with systemic lupus erythematosus. Lupus. 2011;20(11):1126–1134. doi: 10.1177/0961203311404914. [DOI] [PubMed] [Google Scholar]
- 51.Amano MT, Ferriani VPL, Florido MPC, Reis ES, Delcolli MIMV, Azzolini AECS, et al. Genetic analysis of complement C1s deficiency associated with systemic lupus erythematosus highlights alternative splicing of normal C1s gene. Mol Immunol. 2008;45(6):1693–1702. doi: 10.1016/j.molimm.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 52.Batu ED, Koşukcu C, Taşkıran E, Sahin S, Akman S, Sözeri B, et al. Whole exome sequencing in early-onset systemic lupus erythematosus. J Rheumatol. 2018;45(12):1671–1679. doi: 10.3899/jrheum.171358. [DOI] [PubMed] [Google Scholar]
- 53.Liphaus BL, Umetsu N, Jesus AA, Bando SY, Silva CA, Carneiro-Sampaio M. Molecular characterization of the complement C1q, C2 and C4 genes in Brazilian patients with juvenile systemic lupus erythematosus. Clinics. 2015;70(3):220–227. doi: 10.6061/clinics/2015(03)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jönsson G, Sjöholm AG, Truedsson L, Bengtsson AA, Braconier JH, Sturfelt G. Rheumatological manifestations, organ damage and autoimmunity in hereditary C2 deficiency. Rheumatology. 2007;46(7):1133–1139. doi: 10.1093/rheumatology/kem023. [DOI] [PubMed] [Google Scholar]
- 55.Jönsson G, Truedsson L, Sturfelt G, Oxelius V-A, Braconier JH, Sjöholm AG. Hereditary C2 deficiency in Sweden. Medicine. 2005;84(1):23–34. doi: 10.1097/01.md.0000152371.22747.1e. [DOI] [PubMed] [Google Scholar]
- 56.Felux J, Erbacher A, Breckler M, Herve R, Lemeiter D, Mannherz HG, et al. Deoxyribonuclease 1-mediated clearance of circulating chromatin prevents from immune cell activation and pro-inflammatory cytokine production, a phenomenon amplified by low Trap1 activity: consequences for systemic lupus erythematosus. Front Immunol. 2021;12:613597. doi: 10.3389/fimmu.2021.613597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jimenez-Alcazar M, Napirei M, Panda R, Kohler EC, Kremer Hovinga JA, Mannherz HG, et al. Impaired DNase1-mediated degradation of neutrophil extracellular traps is associated with acute thrombotic microangiopathies. J Thromb Haemost. 2015;13(5):732–742. doi: 10.1111/jth.12796. [DOI] [PubMed] [Google Scholar]
- 58.Al-Mayouf SM, Sunker A, Abdwani R, Abrawi SA, Almurshedi F, Alhashmi N, et al. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat Genet. 2011;43(12):1186–1188. doi: 10.1038/ng.975. [DOI] [PubMed] [Google Scholar]
- 59.Martin Merinero H, Zhang Y, Arjona E, Del Angel G, Goodfellow R, Gomez-Rubio E, et al. Functional characterization of 105 factor H variants associated with aHUS: lessons for variant classification. Blood. 2021;138(22):2185–2201. doi: 10.1182/blood.2021012037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beck BB, van Spronsen F, Diepstra A, Berger RM, Komhoff M. Renal thrombotic microangiopathy in patients with cblC defect: review of an under-recognized entity. Pediatr Nephrol. 2017;32(5):733–741. doi: 10.1007/s00467-016-3399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jean-Marie EM, Cho JJ, Trevino JG. A case report of recurrent acute pancreatitis associated with life threatening atypical hemolytic uremic syndrome. Medicine (Baltimore) 2020;99(22):e19731. doi: 10.1097/MD.0000000000019731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandino-Perez J, Gutierrez E, Caravaca-Fontan F, Morales E, Aubert-Girbal L, Delgado-Lillo R, et al. Haemolytic uraemic syndrome associated with pancreatitis: report of four cases and review of the literature. Clin Kidney J. 2021;14(8):1946–1952. doi: 10.1093/ckj/sfaa245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartz N, Stock AD, Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol. 2019;15(3):137–152. doi: 10.1038/s41584-018-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fanouriakis A, Boumpas DT, Bertsias GK. Pathogenesis and treatment of CNS lupus. Curr Opin Rheumatol. 2013;25(5):577–583. doi: 10.1097/BOR.0b013e328363eaf1. [DOI] [PubMed] [Google Scholar]
- 65.Lundstrom E, Gustafsson JT, Jonsen A, Leonard D, Zickert A, Elvin K, et al. HLA-DRB1*04/*13 alleles are associated with vascular disease and antiphospholipid antibodies in systemic lupus erythematosus. Ann Rheum Dis. 2013;72(6):1018–1025. doi: 10.1136/annrheumdis-2012-201760. [DOI] [PubMed] [Google Scholar]
- 66.Rullo OJ, Tsao BP. Recent insights into the genetic basis of systemic lupus erythematosus. Ann Rheum Dis. 2013;72 Suppl(0 2):ii56–61. doi: 10.1136/annrheumdis-2012-202351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ceccarelli F, Perricone C, Borgiani P, Ciccacci C, Rufini S, Cipriano E, et al. genetic factors in systemic lupus erythematosus: contribution to disease phenotype. J Immunol Res. 2015;2015:745647. doi: 10.1155/2015/745647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koga M, Kawasaki A, Ito I, Furuya T, Ohashi J, Kyogoku C, et al. Cumulative association of eight susceptibility genes with systemic lupus erythematosus in a Japanese female population. J Hum Genet. 2011;56(7):503–507. doi: 10.1038/jhg.2011.49. [DOI] [PubMed] [Google Scholar]
- 69.Wu JY, Yeh KW, Huang JL. Early predictors of outcomes in pediatric lupus nephritis: focus on proliferative lesions. Semin Arthritis Rheum. 2014;43(4):513–520. doi: 10.1016/j.semarthrit.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Munroe ME, James JA. Genetics of lupus nephritis: clinical implications. Semin Nephrol. 2015;35(5):396–409. doi: 10.1016/j.semnephrol.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vieira-Martins P, El Sissy C, Bordereau P, Gruber A, Rosain J, Fremeaux-Bacchi V. Defining the genetics of thrombotic microangiopathies. Transfus Apheres Sci. 2016;54(2):212–219. doi: 10.1016/j.transci.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 72.Nagafuchi Y, Shoda H, Fujio K. Immune Profiling and Precision Medicine in Systemic Lupus Erythematosus. Cells. 2019;8(2):140. doi: 10.3390/cells8020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.