Abstract
OBJECTIVE
High-deductible health plans (HDHPs) are increasingly more common but can be challenging for patients to navigate and may negatively affect care engagement for chronic conditions such as type 2 diabetes. We sought to understand how higher out-of-pocket costs affect participation in provider visits, medication adherence, and routine monitoring by patients with type 2 diabetes with an HDHP.
RESEARCH DESIGN AND METHODS
In a retrospective cohort of 19,379 Kaiser Permanente Northern California patients with type 2 diabetes (age 18–64 years), 6,801 patients with an HDHP were compared with those with a no-deductible plan using propensity score matching. We evaluated the number of telephone and office visits with primary care, oral diabetic medication adherence, and rates of HbA1c testing, blood pressure monitoring, and retinopathy screening.
RESULTS
Patients with an HDHP had fewer primary care office visits compared with patients with no deductible (4.25 vs. 4.85 visits per person; P < 0.001), less retinopathy screening (49.9% vs. 53.3%; P < 0.001), and fewer A1c and blood pressure measurements (46.7% vs. 51.4%; P < 0.001 and 93.2% vs. 94.4%; P = 0.004, respectively) compared with the control group. Medication adherence was not significantly different between patients with an HDHP and those with no deductible (57.4% vs. 58.6%; P = 0.234).
CONCLUSIONS
HDHPs seem to be a barrier for patients with type 2 diabetes and reduce care participation in both visits with out-of-pocket costs and preventive care without out-of-pocket costs, possibly because of the increased complexity of cost sharing under an HDHP, potentially leading to decreased monitoring of important clinical measurements.
Introduction
As U.S. health care spending is projected to reach 20% of the gross domestic product by 2025 (1), high-deductible health plans (HDHPs) are rising in prevalence. They accounted for one in three employer-sponsored plans as of 2020 (2), and almost half of privately insured adults had an HDHP in 2018 (3). HDHPs aim to control health care spending by encouraging patients to price shop and participate in their health care decisions (4–6). However, previous studies have shown that these plans can lead to unintentional decreases in overall use and preventive care (7,8). This can have harmful impacts on vulnerable patients with chronic conditions (9).
Type 2 diabetes continues to rise in prevalence, and in 2020, almost one in 10 adults were diagnosed with type 2 diabetes in the U.S. (10). The burden of disease is disproportionately higher in those with lower socioeconomic status (11). Prior evidence has shown that patients with type 2 diabetes with high-deductible plans experience increased out-of-pocket costs and increased rates of diabetic complications (12). Several studies have shown that higher out-of-pocket costs are associated with lower medication adherence in patients with chronic conditions, such as type 2 diabetes (13–16). The effect of high-deductible plans on other aspects of type 2 diabetes care is less well studied, but overall adherence to diabetes disease monitoring, such as HbA1c testing, blood pressure (BP) control, and retinopathy screening, remains poor throughout the U.S., with testing rates for biannual HbA1c monitoring as low as 7% in some populations (17) and for retinopathy screening as low as 31% (18). Patient participation in routine diabetes care is a key component of successful glycemic control (19,20), but how does an HDHP affect patient participation in type 2 diabetes?
While previous studies have evaluated the financial and health impacts of out-of-pocket costs and routine care in type 2 diabetes, such as medication adherence and complications, participation in routine medical care by patients with type 2 diabetes with a high-deductible plan is not well understood (12,17,20). For patients with diabetes, regular visits in their care with providers and adherence to multiple preventative measures are key to preventing long-term complications (21). To understand the association between HDHPs and patients’ health care behavior, we compared patients with an HDHP with those in a plan without any deductible using a propensity score model. Because there are likely significant differences among patients who choose a high-deductible plan, a propensity score model allows for matching on known patient factors that may influence this decision (22,23). We examined three aspects of patient behavior (i.e., use of scheduled visits with primary care providers, medication adherence, and routine diabetes monitoring) to better understand the difference in health care use and intermediate outcomes in patients with type 2 diabetes in an HDHP. We hypothesized that patients with an HDHP may have lower rates of primary health care visits, medication adherence, and routine diabetes monitoring.
Research Design and Methods
Study Setting
Kaiser Permanente Northern California (KPNC) is a large integrated health system that provides both primary and specialty care for ∼4 million members across northern California. Enrollment in an HDHP can be through an employer-sponsored health plan or individual purchase, including through Covered California (the California state health care exchange under the Affordable Care Act). An HDHP within KPNC requires a patient to pay the full cost for most medical services until the medical deductible is met. For HDHPs in this setting, inpatient and emergency visits apply to the deductible, although we excluded plans with only inpatient deductibles. Until the deductible is met, HDHP patients pay the full cost for services, except for Internal Revenue Service–defined preventive visits and procedures. For a no-deductible plan, the patient pays a copayment (variable depending on the plan) for the type of service rendered. Pharmacy benefits vary by plan, although common generic medications for chronic conditions, including diabetes oral medications and insulin prescriptions, are priced at consistently low costs for all health plan members. Pharmacy deductibles do not count toward the total medical deductible.
Within KPNC, all diabetes health care services are provided without differentiation of insurance plan. Diabetes care management is predominantly provided through primary care physicians and case managers aided by established protocols to encourage compliance and adherence. Regardless of insurance plan, each patient with type 2 diabetes has a patient-centered care team with regular patient outreach for routine monitoring. A once-per-year routine checkup examination with the primary care provider is free. Retinopathy testing, HbA1c testing, and BP measurement do not require referral and can be performed without a scheduled appointment. Within KPNC, retinopathy screening is free, because it is performed by digital retinal photography. In this setting, telephone visits are also free of cost sharing and occur as scheduled visits. At each visit in primary care, clinicians will review care gaps and medications, and patients can complete screening, such as HbA1c testing, BP measurement, and retinopathy screening, in the same trip.
Cohort
We identified members at KPNC who had continuous coverage in the same type of health plan (HDHP or no deductible) for 24 months starting anytime between 1 January 2014 and 1 January 2016 using electronic health records and other administrative databases. The first 6 months after study enrollment were used to define a baseline period, with the following 18 months as our study period. The last date of possible follow-up was 31 December 2017. We defined patients with type 2 diabetes as those who had at least one visit coded with an ICD-10 code of type 2 diabetes (code E11.xx) during the baseline period and either a second visit with an ICD-10 code of type 2 diabetes or A1c ≥6.5% (47 mmol/mol) to ensure patients in our cohort met the definition of type 2 diabetes. We also compared our cohort against our internal diabetes registry. We used the Internal Revenue Service definition of a Health Savings Account–eligible high-deductible plan between 2014 and 2016, with an annual minimum deductible between $1,205 and $1,300 for single coverage and $6,530 and $6,550 for family coverage and annual maximum deductible between $2,500 and $2,600 for single and $12,700 and $13,100 for family (24). Health Savings Account funding status during the study period was not available with our study data.
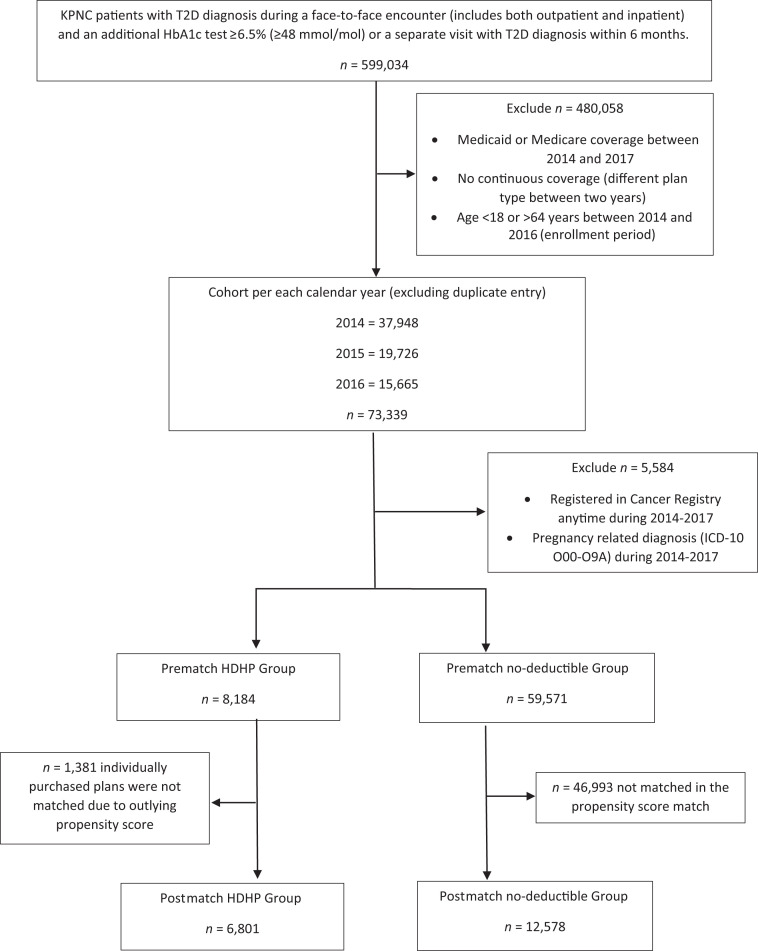
We excluded all members age ≥65 years because of the lack of deductible plans in Medicare as well as those who were enrolled through Medicaid. Other exclusion criteria included patients age <18 years, those pregnant during the study period, and those enrolled in the cancer registry during the study period, because these conditions would require a different diabetes management approach. The total study period was 24 months for each patient (6-month baseline period and 18-month study period). If a patient had multiple eligible entries into the study, we used their first eligible study period. The comparison group was extracted using the same exclusion criteria for patients enrolled in a KPNC health plan with no deductible and then further selected via propensity score matching with HDHP group in baseline characteristics (described below in Statistical Analysis) (Fig. 1).
Figure 1.
Flowchart of cohort selection and propensity score matching to final cohort of HDHP and no-deductible groups.
Outcome Variables
We examined three types of outcomes, including visits with provider, medication adherence, and routine diabetes monitoring adherence. We evaluated patient use of visits with providers through the number of office encounters and telephone encounters with primary care physicians (any visit with adult internal medicine or family medicine physician or midlevel provider). To measure medication adherence, we defined oral diabetes medication adherence as having at least 80% of days covered with any oral diabetes medication (allowing for carryovers from previous refills) over the 18-month period. Insulin adherence was excluded from the outcome measurement and included as a covariate instead. Routine diabetes monitoring was measured by A1c testing, retinopathy screening, and BP measurement. A1c testing was measured by having at least three HbA1c measurements within an 18-month period, each spaced at least 30 days apart. We defined adherence to diabetic retinopathy screening as at least one dilated eye photo with reading within the 18 months. These are part of routine type 2 diabetes management as recommended by the American Diabetes Association, with one HbA1c measurement every 6 months and diabetic retinopathy screening every 2 years (21). We also included BP measurement adherence by having at least one systolic BP (SBP) reading during the 18-month study period. We also considered whether patients met glycemic control and blood pressure control in the following 18 months with the value of the last HbA1c reading and last measured SBP.
Covariates
In our observational study, we identified a list of potential confounders that may affect both plan type selection and outcome, including patient demographics, insurance characteristics, and baseline clinical measures.
Baseline patient characteristics included sex, age, race, neighborhood socioeconomic status (SES), and English language proficiency. One key influential factor in choosing a health insurance plan and paying out-of-pocket costs was the socioeconomic status of the individual. We used neighborhood SES as a proxy measure, because individual-level SES was not available. Low SES neighborhoods were defined as ≥20% of the residents having household incomes below the federal poverty level or ≥25% of the residents age >25 years having a high school education or less in the census block group, based on the 2010 U.S. census (25). Language proficiency may influence participation in medical treatment and be a barrier in navigating insurance plans as well as medical care (26).
Insurance characteristics included whether the health plan was purchased through an employer or individually purchased. Employer-sponsored purchases typically constrain health insurance options. Direct insurance purchase still accounted for 16% of U.S. insurance coverage (27). We also included a variable indicating whether the patient was new to KPNC, because there may be differences in medical care participation in a new medical system.
Baseline clinical measures included the patient’s total number of prescription medications, as a proxy measure of overall health complexity, whether the patient was on insulin, and diabetes control status based on the last A1c measured during the 6-month baseline period.
Statistical Analysis
To reduce the selection bias in patients self-selecting their plan type, we used a propensity score–matching method to select a no-deductible comparison group matched to HDHP enrollees (28). The propensity score model estimated the likelihood of a patient selecting a high-deductible plan based on measurable variables as defined in the covariate section above. By matching patients on their propensity score, we sought to reduce the influence of confounding bias in a patient’s decision in selecting a high-deductible plan versus no-deductible plan. We used a logistic regression model with HDHP as a dependent variable and all the potential confounders described above as independent variables to calculate the propensity score. Then we used greedy matching (1:2 ratio) with exact match for race/ethnicity, new member status, and insurance type to obtain the propensity score–matched cohort. The 1:2 ratio was chosen after comparing common support between different values of m in 1:m matching and found that the 1:2 ratio provided the best overlap. Finally, we compared the outcome measures between the two matched patient groups. Proportions were calculated and compared using the χ2 test for categorical variables; means were calculated and compared using the t test for continuous variables. We considered significance with a two-tailed P value of <0.05. All analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC).
Because we excluded patients who were not matched in baseline characteristics from both HDHP and no-deductible groups in the propensity score–matched cohort, we conducted a sensitivity analysis among the same original cohort of patients with type 2 diabetes before propensity score matching. We used a logistic regression model for binary outcomes (e.g, A1c measurement, retinopathy screening) and negative binomial regression model for counts (office and telephone visits) adjusted for the potential confounders that we included in the propensity score calculation. We then calculated covariate-adjusted mean or percentage by applying the coefficients from models to all study participants as if they all had an HDHP plan and as if they all had a no-deductible plan, respectively.
Results
Cohort Characteristics
We identified 118,976 KPNC members who had a diagnosis of type 2 diabetes during the initial 6-month baseline period between 1 January 2014 and 30 June 2016. After applying our exclusion criteria and validating the diagnosis of type 2 diabetes, 8,184 (12%) had an HDHP and 59,571 (88%) had a no-deductible plan. The propensity-matched cohort consisted of 6,801 patients in the HDHP group and 12,578 patients in the no-deductible (copayments only) comparison group (Fig. 1).
Prior to matching, the HDHP group had a higher percentage of Hispanic and Asian patients, and the no-deductible group was more likely to be Black. HDHPs were more likely to be individually purchased, and patients were more likely to live in a lower SES neighborhood. The average number of prescription medications and the proportion of patients taking insulin were higher among the patients with no deductible. Approximately 29% of patients in the HDHP group did not have any prescriptions for oral diabetic medications, compared with 31% in the no-deductible group (Supplementary Table 1). After propensity score matching, baseline characteristics matched closely between the two study groups, as shown in Table 1. Of note, most patients with an individually purchased HDHP were unable to be matched to the no-deductible group in our propensity score–matched cohort.
Table 1.
Baseline patient characteristics
| HDHP | No deductible | P | |
|---|---|---|---|
| N of patients | 6,801 | 12,578 | |
| Age, years | 0.803 | ||
| 18 to <45 | 20.39 | 19.86 | |
| 45 to <50 | 15.54 | 15.69 | |
| 50 to <55 | 23.07 | 22.97 | |
| 55 to <60 | 25.44 | 26.25 | |
| 60 to <65 | 15.56 | 15.24 | |
| Sex | 0.773 | ||
| Male | 60.09 | 60.34 | |
| Female | 39.91 | 39.66 | |
| Neighborhood SES* | 0.260 | ||
| Nonlow | 67.18 | 68.42 | |
| Low | 30.10 | 29.10 | |
| Unknown | 2.72 | 2.48 | |
| Race (self-reported) | 1.000 | ||
| White | 36.98 | 36.98 | |
| Black | 4.82 | 4.82 | |
| Hispanic | 30.80 | 30.80 | |
| Asian | 25.39 | 25.39 | |
| Other | 2.00 | 2.00 | |
| New member† | 1.000 | ||
| No | 97.50 | 97.50 | |
| Yes | 2.50 | 2.50 | |
| Insurance type | 1.000 | ||
| Employer sponsored | 85.06 | 85.06 | |
| Individually purchased | 14.94 | 14.94 | |
| English as primary language (self-reported) | 0.663 | ||
| No | 20.61 | 20.31 | |
| Yes | 79.39 | 79.69 | |
| Baseline insulin prescription | 0.774 | ||
| No | 81.56 | 81.37 | |
| Yes | 18.44 | 18.63 | |
| Baseline A1c‡ | 0.329 | ||
| No measure | 5.60 | 5.05 | |
| <7 | 30.05 | 30.91 | |
| 7 to <8 | 26.91 | 26.25 | |
| 8+ | 37.44 | 37.79 | |
| Baseline medication prescriptions | 0.901 | ||
| <5 | 72.68 | 72.58 | |
| ≥5 | 27.32 | 27.42 |
Data are % unless otherwise indicated. Table depicts baseline properties of propensity score–matched cohort. Matched with greedy matching at 1:2 ratio and exact match of race, member status, and insurance type, weighted by match weight.
SES calculated based on zip code, with low SES defined as ≥20% of the residents having household incomes below the federal poverty level or ≥25% of the residents age >25 years having a high school education or less in the census block group, based on the 2010 U.S. census.
New member defined by no prior KPNC insurance plan before the start of the 2-year observation period.
Baseline A1c as last HbA1c obtained during 6-month baseline period.
Use Measures
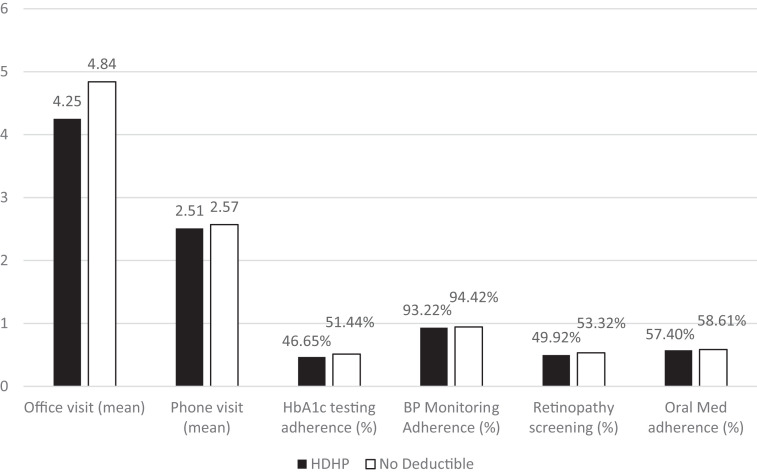
We compared the two matched groups in the three areas of interest: use of visits with primary care providers, medication adherence, and routine diabetes monitoring adherence (Fig. 2). On average, during the 18-month follow-up period, the HDHP group had 4.25 office visits per patient, compared with 4.84 office visits in the no-deductible group (P < 0.001). The average number of telephone visits was comparable at 2.51 per patient in the HDHP group and 2.57 per patient in the no-deductible group (P = 0.359). Patients in the HDHP group had statistically significantly fewer office visits with any provider in the primary care setting, although there was no statistically significant difference in telephone visits, which were free of out-of-pocket costs regardless of plan type.
Figure 2.
Participation in visits with providers, routine diabetes monitoring adherence, and medication adherence between propensity score–matched HDHP and no-deductible groups. Significant differences are noted between office visits (P < 0.001), HbA1c testing adherence (P < 0.001), BP monitoring adherence (P = 0.004), and retinopathy screening (P < 0.001). No significant difference was noted between telephone visits (P = 0.359) and oral medication adherence (P = 0.234). Office visits and telephone visits were measured as average visits per patient over 18 months.
The percentage of patients who were adherent to their oral diabetes medications was not statistically significantly different in either group among patients who had at least one oral diabetic medication prescription. The adherence rate during the 18-month follow-up period was 57.4% in the HDHP group and 58.6% in the no-deductible group (P = 0.234).
The HDHP group was less likely to have retinopathy screening, A1c measurement, or BP measurement. Retinopathy screening was lower in the HDHP group, with 49.9% of the HDHP patients completing retinopathy screening in the 18-month follow-up period, whereas 53.3% of the no-deductible group completed retinopathy screening (P < 0.001). Adherence to at least three HbA1c tests during the follow-up period of 18 months was 46.7% in the HDHP group compared with 51.4% in the no-deductible group (P < 0.001). During the study period of 18 months, 4.9% of patients in HDHP group and 3.8% of patients in the no-deductible group did not have any HbA1c measurements. For BP measurement, 6.8% in the HDHP group did not have any outpatient BP readings in the 18 months, whereas in the no-deductible group, 5.6% of patients did not have any outpatient BP readings (P = 0.004).
Intermediate Clinical Outcomes
Among those with at least one measurement during the follow-up period, HbA1c control (HbA1c value ≤7.0% [53 mmol/mol]) and BP control (SBP <140 mmHg) were not statistically different between the two groups (Table 2).
Table 2.
Comparisons of HbA1c and SBP control between propensity score–matched HDHP and no-deductible groups among those with at least one measurement in 18 months
| HDHP | No deductible | P | |
|---|---|---|---|
| N of patients | 6,801 | 12,578 | |
| HbA1c outcome | |||
| At least 1 HbA1c test in 18 months* | 6,465 (95.1) | 12,095 (96.2) | 0.002 |
| At least 3 HbA1c tests within 18 months | 3,173 (46.7) | 6,459 (51.4) | <0.001 |
| 1 or 2 HbA1c tests within 18 months | 3,292 (48.4) | 5,636 (44.8) | <0.001 |
| No HbA1c test within 18 months | 336 (4.9) | 483 (3.8) | <0.001 |
| Met HbA1c control (in those who were tested)† | 3,197 (47.0) | 6,000 (47.7) | 0.546 |
| BP measurement | |||
| At least 1 BP measurement in 18 months | 6,340 (93.2) | 11,873 (94.4) | 0.007 |
| No BP measurement during 18 months | 461 (6.8) | 700 (5.6) | |
| Last SBP <140 mmHg during 18 months‡ | 6,088 (89.5) | 11,305 (89.9) | 0.496 |
Data are n (%).
P value calculated by comparing cohort with at least 1 HbA1c in 18 months vs. cohort with no HbA1c test within 18 months.
A1c control as measured from last HbA1c value during the study period. Meeting HbA1c control defined as HbA1c value ≤7.0% (53 mmol/mol).
SBP ≤135 mmHg is recommended by the American Diabetes Association guidelines. We used <140 mmHg to account for measurement errors.
The results from the sensitivity analysis among all participants before propensity score matching, with adjustment for covariates, were comparable to those from our propensity score–matched cohort (Supplementary Table 2).
Conclusions
Among patients with type 2 diabetes in an integrated delivery system with strong population management support, we found that patients enrolled in an HDHP had lower rates of use of provider visits and diabetes monitoring. While those who had testing seemed to have similar rates of control, the lower frequency of monitoring may have led to a delay in identifying patients who were not in control and delay in treatment intensification.
In the study setting, KPNC has a strong population management strategy to reduce barriers to diabetes monitoring in HbA1c testing, retinopathy screening, and BP measurement regardless of insurance plan type. While the findings of similar rates of control between the two groups suggest similar health outcomes, the different rate of screening is concerning for delay in identifying patients who are not at goal. A previous study within KPNC highlighted significant complexity in navigating HDHP designs (8). Patients with a high-deductible plan may reduce care use more as a result of concerns of cost and perception of good health when the last reading was at goal, despite automated and personal reminders to continue to monitor. This could explain the difference in rates of use and monitoring adherence while medication adherence remained the same. While nonpreventive office visits involved cost sharing, and telephone visits did not have out-of-pocket expenses, we found lower rates of office visits among the HDHP group and similar rates of telephone visits across both plan types. However, diabetic retinopathy screening in the study setting was free of cost, but this screening was lower in the HDHP group. HbA1c testing had out-of-pocket costs, and BP screening was free in the study setting and could be captured as part of routine office visits or as free drop-in nurse BP checks; however, both measurement rates were lower in the HDHP group. Provider guidance and reminders through office visits and telephone visits help to center the patient to the treatment plan and allow patients to gain further insight and promote adherence (29,30). However, if patients do not engage with their providers in office visits because of concerns about cost, this becomes a missed opportunity to promote the importance of monitoring adherence, because the on-site office visit allows for BP checks, A1c testing, and retinopathy screening at the same visit (31).
Previous studies have shown that patients with type 2 diabetes with a high-deductible plan tend to delay their care, although there has not been a consistent difference in clinical outcomes such as emergency department or hospital visits (12,32). While differences between our study groups did not reach significance in measured A1c control or BP control, there were significant differences in both A1c and BP measurement rates. Patients in an HDHP had fewer HbA1c and BP measurements, although only HbA1c had cost sharing; BP could be checked free outside of a visit. Several studies within KPNC have shown that the population management programs for diabetes and hypertension are effective, and patients who engage in the programs have similar results despite insurance differences (30,33). However, if a patient chooses to delay care because of concerns about high out-of-pocket costs, this can limit the monitoring of clinical markers and result in both groups seeming to have similar short-term clinical outcomes.
Previous studies have noted that only one in four patients talk to their providers about cost under an HDHP plan, and only 10% of patients in an HDHP understand the cost-sharing design between preventive and nonpreventive tests and procedures (8,34). A previous study within the same KPNC population showed that 34% of patients with an HDHP were more likely to report avoiding office visits because of concerns about cost (35). Even in an integrated health care system such as KPNC, multiple efforts are made to reduce the barriers of out-of-pocket costs for patients with type 2 diabetes, such as medical assistant BP screenings, population manager telephone calls, and health education classes, but given the potential complexity of cost sharing in HDHPs and the sensitivity of patients in these plans to out-of-pocket costs, our study shows that barriers still exist in high-deductible plans that limit participation in treatment plans for patients with type 2 diabetes.
There are several limitations to our study. Our study took place in an integrated health care setting, which may be different from other practices in the U.S. Within KPNC, care protocols have been developed for the multifaceted care of diabetes using an integrated approach between primary care, case managers, and specialty groups. These may not be generalizable to other care systems, although they may be of interest to other systems. Also, our study does not represent some individually insured patients who were not able to be adequately propensity score matched because of the lack of common support in their propensity scores. While there was no significant difference in outcomes between the patients with employer-sponsored and individually purchased plans, the individually insured patients may have been from a different demographic, such as self-employed or unemployed patients or those whose employer does not offer health coverage (36). Because we were unable to further characterize or study this group, this may pose a limitation to our study. Further study is recommended to evaluate the difference in cost sensitivity between these individual plan purchasers. Another limitation in our methods is that we did not specifically examine medication adherence with insulin. Individuals with insulin-dependent diabetes require more complex management, and we could not capture medication adherence for insulin. We included this as a variable in our propensity score to account for the lack of insulin adherence information. Also, we were unable to report on actual out-of-pocket costs incurred between the two groups and could not account for seasonality with regard to high-deductible plans and actual costs accrued by the patients. Because most patients were enrolled in the study during the January to June 6-month baseline period in 2014, bias may exist in that the control group would have been more familiar with the medical plan compared with the high-deductible group. Lastly, because this is an observational study, even though we included a wide range of covariates in our analysis, we were unable to account for unmeasured factors that influence patient behavior, such as future medical needs and the patient’s perception of diabetes control.
In conclusion, in patients with type 2 diabetes, high-deductible plans can be a deterring factor in participating in diabetes care, including through lower rates of provider visits and lower rates of diabetes-related monitoring. HDHPs can have cost-free exemptions for some health care services, but this can be confusing for patients, and in our study, we identified decreased use of preventive services that were free. Because of the complexities of a high-deductible plan, patients in such a plan may have fewer points of clinical contact as a result of overall reduction of care, and this could lead to unintended bias and fewer points of intervention. With the rise in popularity of HDHPs and recent changes in the regulation of these plans, the complexities of the cost of care should be systematically addressed for patients with chronic medical conditions such as type 2 diabetes.
Article Information
Acknowledgments. The authors thank Andrea Millman for helping with the institutional review board submission process.
Funding. This study was funded through the Kaiser Permanente Division of Research Community Benefit Grant.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.M.W. contributed to the design of the study, data interpretation, and drafting of the manuscript. J.H. contributed to the design of the study, data extraction and statistical analysis, and review of the manuscript. M.E.R. contributed to the design of the study, data interpretation, and review of the manuscript. Y.M.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.19196546.
References
- 1. Keehan SP, Poisal JA, Cuckler GA, et al. National health expenditure projections, 2015-25: economy, prices, and aging expected to shape spending and enrollment. Health Aff (Millwood) 2016;35:1522–1531 [DOI] [PubMed] [Google Scholar]
- 2. Section 8: high-deductible health plans with savings option. In Claxton G, Rae M, Long M, Damico A, Foster G, Whitmore H. Kaiser Family Foundation and Health Research & Educational Trust Employer Health Benefits Survey. Accessed 5 August 2020. Available from https://www.kff.org/report-section/ehbs-2017-section-8-high-deductible-health-plans-with-savings-option/
- 3. Cohen RA, Martinez ME, Zammitti EP. Health Insurance Coverage: Early Release of Estimates From the National Health Interview Survey, January–March 2018. Accessed 5 August 2020. Available from https://www.cdc.gov/nchs/data/nhis/earlyrelease/Insur201808.pdf
- 4. Haviland AM, Eisenberg MD, Mehrotra A, Huckfeldt PJ, Sood N. Do “consumer-directed” health plans bend the cost curve over time? J Health Econ 2016;46:33–51 [DOI] [PubMed] [Google Scholar]
- 5. Robinson JC. Health savings accounts—the ownership society in health care. N Engl J Med 2005;353:1199–1202 [DOI] [PubMed] [Google Scholar]
- 6. Lee TH, Zapert K. Do high-deductible health plans threaten quality of care? N Engl J Med 2005;353:1202–1204 [DOI] [PubMed] [Google Scholar]
- 7. Brot-Goldberg ZC, Chandra A, Handel BR, Kolstad JT. What does a deductible do? The impact of cost-sharing on health care prices, quantities, and spending dynamics. Q J Econ 2017;132:1261–1318 [Google Scholar]
- 8. Reed ME, Graetz I, Fung V, Newhouse JP, Hsu J. In consumer-directed health plans, a majority of patients were unaware of free or low-cost preventive care. Health Aff (Millwood) 2012;31:2641–2648 [DOI] [PubMed] [Google Scholar]
- 9. Wharam JF, Ross-Degnan D, Rosenthal MB. The ACA and high-deductible insurance—strategies for sharpening a blunt instrument. N Engl J Med 2013;369:1481–1484 [DOI] [PubMed] [Google Scholar]
- 10. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health 2020;10:107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention . National Diabetes Statistics Report 2020. Estimates of Diabetes and Its Burden in the United States. Accessed 8 August 2020. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national- diabetes-statistics-report.pdf
- 12. Wharam JF, Zhang F, Eggleston EM, Lu CY, Soumerai S, Ross-Degnan D. Diabetes outpatient care and acute complications before and after high-deductible insurance enrollment: a Natural Experiment for Translation in Diabetes (NEXT-D) study. JAMA Intern Med 2017;177:358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karter AJ, Parker MM, Solomon MD, et al. Effect of out-of-pocket cost on medication initiation, adherence, and persistence among patients with type 2 diabetes: the Diabetes Study of Northern California (DISTANCE). Health Serv Res 2018;53:1227–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reed M. Reducing out-of-pocket costs to coordinate prescription medication benefit design with chronic disease outreach and clinical care. J Gen Intern Med 2017;32:495–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reed ME, Warton EM, Kim E, Solomon MD, Karter AJ. Value-based insurance design benefit offsets reductions in medication adherence associated with switch to deductible plan. Health Aff (Millwood) 2017;36:516–523 [DOI] [PubMed] [Google Scholar]
- 16. Fendrick AM, Buxbaum JD, Tang Y, et al. Association between switching to a high-deductible health plan and discontinuation of type 2 diabetes treatment. JAMA Netw Open 2019;2:e1914372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lian J, Liang Y. Diabetes management in the real world and the impact of adherence to guideline recommendations. Curr Med Res Opin 2014;30:2233–2240 [DOI] [PubMed] [Google Scholar]
- 18. Lee DJ, Kumar N, Feuer WJ, et al. Dilated eye examination screening guideline compliance among patients with diabetes without a diabetic retinopathy diagnosis: the role of geographic access. BMJ Open Diabetes Res Care 2014;2:e000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holman H, Lorig K. Patients as partners in managing chronic disease. Partnership is a prerequisite for effective and efficient health care. BMJ 2000;320:526–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imai C, Li L, Hardie RA, Georgiou A. Adherence to guideline-recommended HbA1c testing frequency and better outcomes in patients with type 2 diabetes: a 5-year retrospective cohort study in Australian general practice. BMJ Qual Saf 2021;30:706–714. DOI: https://doi.org/10.1136/bmjqs-2020-012026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes Association . 3. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in Diabetes-2018. Diabetes Care 2018;41(Suppl. 1):S28–S37 [DOI] [PubMed] [Google Scholar]
- 22. Austin PC. A tutorial and case study in propensity score analysis: an application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivariate Behav Res 2011;46:119–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haukoos JS, Lewis RJ. The propensity score. JAMA 2015;314:1637–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Internal Revenue Service . Internal Revenue Bulletin: 2004-15. Accessed 16 July 2017. Available from https://www.irs.gov/irb/2004-15_IRB
- 25. Krieger N, Chen JT, Waterman PD, Soobader M-J, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol 2002;156:471–482 [DOI] [PubMed] [Google Scholar]
- 26. Lu T, Myerson R. Disparities in health insurance coverage and access to care by English language proficiency in the USA, 2006–2016. J Gen Intern Med 2020;35:1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berchick ER, Hood E, Barnett JC. Health Insurance Coverage in the United States: 2017. Accessed 4 March 2019. Available from https://www.census.gov/content/dam/Census/library/publications/2018/demo/p60-264.pdf
- 28. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39:33–38 [Google Scholar]
- 29. Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med 2002;17:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rana JS, Karter AJ, Liu JY, Moffet HH, Jaffe MG. Improved cardiovascular risk factors control associated with a large-scale population management program among diabetes patients. Am J Med 2018;131:661–668 [DOI] [PubMed] [Google Scholar]
- 31. Karter AJ, Parker MM, Moffet HH, et al. Missed appointments and poor glycemic control: an opportunity to identify high-risk diabetic patients. Med Care 2004;42:110–115 [DOI] [PubMed] [Google Scholar]
- 32. Rabin DL, Jetty A, Petterson S, Saqr Z, Froehlich A. Among low-income respondents with diabetes, high-deductible versus no-deductible insurance sharply reduces medical service use. Diabetes Care 2017;40:239–245 [DOI] [PubMed] [Google Scholar]
- 33. Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA 2013;310:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kullgren JT, Cliff BQ, Krenz CD, et al. A survey of Americans with high-deductible health plans identifies opportunities to enhance consumer behaviors. Health Aff (Millwood) 2019;38:416–424 [DOI] [PubMed] [Google Scholar]
- 35. Reed M, Fung V, Price M, et al. High-deductible health insurance plans: efforts to sharpen a blunt instrument. Health Aff (Millwood) 2009;28:1145–1154 [DOI] [PubMed] [Google Scholar]
- 36. Hamel L, Firth J, Levitt L, Claxton G, Brodie M. Survey of Non-Group Health Insurance Enrollees, Wave 3. Accessed 6 August 2020. Available from https://www.kff.org/health-reform/poll-finding/survey-of-non-group-health-insurance-enrollees-wave-3/