Abstract
Islet autoimmunity may contribute to β-cell dysfunction in type 2 diabetes (T2D). Its prevalence and clinical significance have not been rigorously determined. In this ancillary study to the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE), we investigated the prevalence of cellular and humoral islet autoimmunity in patients with T2D duration of 4.0 ± 3.0 years (HbA1c 7.5 ± 0.5% on metformin alone). We measured T-cell autoreactivity against islet proteins, islet autoantibodies against 65-kDa GAD antigen, IA-2, and zinc transporter-8, and β-cell function. Cellular islet autoimmunity was present in 41.3%, humoral islet autoimmunity in 13.5%, and both in 5.3%. β-Cell function calculated as incremental area under the curve of glucose from 0–120 min (iAUC-CG) and ΔC-peptide(0–30)/Δglucose(0–30) from an oral glucose tolerance test was lower among T-cell–positive (T+) than T-cell–negative (T−) individuals using two different adjustments for insulin sensitivity (iAUC-CG: 13.2% [95% CI 0.3, 24.4] or 11.4% [95% CI 0.4, 21.2] lower; ΔC-peptide[0–30]/Δglucose[0–30]: 19% [95% CI 3.1, 32.3] or 17.7% [95% CI 2.6, 30.5%] lower). T+ patients had 17% higher HbA1c (95% CI 0.07, 0.28) and 7.7 mg/dL higher fasting plasma glucose levels (95% CI 0.2, 15.3) than T− patients. We conclude that islet autoimmunity is much more prevalent in patients with T2D than previously reported. T-cell–mediated autoimmunity is associated with diminished β-cell function and worse glycemic control.
Introduction
Deficient insulin secretion by β-cells of the islets of Langerhans is critical to the development of type 2 diabetes (T2D) (1). However, islet autoimmunity traditionally has not been considered a significant underlying defect in T2D, whereas this is accepted as the pathophysiological basis of type 1 diabetes (T1D). Increasing evidence suggests that islet autoimmunity might in fact contribute to β-cell dysfunction in patients with T2D (2–6). Among Pima Indians, an ethnic group with a high propensity to develop T2D, people with a clinical phenotype of T2D have been found to possess unique islet autoantibodies, an HLA haplotype associated with defective insulin secretion, and differences in T-cell receptor repertoires associated with diabetes (7–10). Some patients with T2D have islet-specific inflammation and autoimmune responses due to islet-reactive T cells (11–16). For example, the Th17 subset of CD4+ T cells, which is elevated in systemic inflammation associated with insulin resistance, is also an autoimmune destruction effector (13–15). Sarikonda et al. (17) have identified islet-reactive CD4+ T cells in patients with T2D, and Butcher et al. (11) demonstrated an association of proinflammatory cytokines and increased islet leukocyte content with β-cell dysfunction in patients with T2D.
Collectively, these data suggest that both humoral and cellular autoimmunity directed against islet antigens may contribute significantly to β-cell dysfunction in patients defined clinically as having T2D. We previously demonstrated immune recognition of islet proteins by T cells in small cohorts of patients with established T2D (3,6,16,18).
Islet-reactive T cells were present in the circulation of these patients with T2D both with and without classic T1D-associated islet autoantibodies (16). We also demonstrated that the presence of islet-reactive T cells was associated with accelerated β-cell functional decline in patients with T2D (3,6) and that attenuation of the islet-specific T-cell responses was associated with improved β-cell function (18). Confirmation of these findings in a large T2D cohort could revise our understanding of the pathophysiology of T2D, influence its clinical classification, and identify new targets for therapy.
This ancillary study to the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) was initiated to rigorously evaluate islet autoimmunity in patients carefully selected for having established T2D (19,20). We investigated the frequency of both humoral and cellular islet autoimmunity at baseline in a subset of GRADE patients with T2D and determined their relationships to β-cell function and glycemic control (19,20).
Research Design and Methods
GRADE Design Overview
GRADE is a 36-center randomized controlled trial evaluating the clinical effectiveness of the addition of four classes of glucose-lowering medications to metformin in patients with T2D (19,20). Prospective GRADE participants had a run-in period when the metformin dose was increased to 2 g/day or the maximal tolerated dose ≥1 g/day; 5,047 adults with HbA1c 6.6–8.5% at the end of run-in were enrolled and underwent baseline testing, including an oral glucose tolerance test (OGTT) (19). The current “β-cell Ancillary Study” was nested within GRADE to measure humoral and cellular islet autoimmunity in baseline blood samples and determine their relationship to β-cell function measurements derived from the OGTT samples. All GRADE clinical centers were invited to participate in this ancillary study. Nineteen centers obtained local Institutional Review Board approval for the ancillary study and contributed participants.
Subjects
The entry criteria were those of the parent GRADE study (ClinicalTrials.gov reg. no. NCT01794143) (19,20). Briefly, men or women diagnosed with T2D at ≥30 years of age (≥20 years for American Indians), duration of <10 years, on metformin ≥1,000 mg/day were included. Key exclusion criteria were: clinical suspicion of T1D, treatment with any glucose-lowering medication other than metformin in the previous 6 months, major cardiovascular events in the previous year, planning pregnancy during the course of the study, heart failure, pancreatitis, cancer, serum creatinine >1.4 mg/dL in women or >1.5 mg/dL in men, liver disease or ALT more than three times the upper limit of normal, alcoholism, glucocorticoid or antipsychotic use, and conditions rendering HbA1c results unreliable. There were no additional eligibility criteria for the β-cell Ancillary Study.
The 19 GRADE centers participating in this ancillary study recruited 419 participants with T2D (representing 8.3% of the overall GRADE study cohort), which exceeded the sample size estimated to power this study adequately.
Sample Collection
Blood samples were collected at the time of the GRADE participants’ baseline OGTT. At the fasting draw, blood was collected into heparin-coated tubes and shipped overnight from the clinical site to Seattle for processing. Plasma was separated from 5 cc blood and frozen at −80°C for autoantibody assays. The remaining blood sample was used for the islet-specific T-cell reactivity assay. A total of 1 cc blood was collected at each OGTT time point for measurements of glucose, insulin, and C-peptide by the GRADE central laboratory (University of Minnesota Advanced Research and Diagnostics Laboratory, Minneapolis, MN). Of the 419 GRADE participants who gave informed consent and provided baseline samples, autoantibodies could not be measured in 27 samples because of insufficient volume or severe hemolysis. Seventy samples could not be included in the T-cell reactivity assay because of delayed delivery, insufficient volume, nonviability of peripheral blood mononuclear cells (PBMCs), or severe hemolysis.
Cellular Immunoblotting (Islet-Specific T-Cell Reactivity Assay)
Cellular islet autoimmunity was measured by the well-established cellular immunoblotting T-cell assay, which is highly specific for islet proteins (83%) and sensitive (94%) for identifying patients with T1D among masked blood samples (21–23). Assay accuracy was validated in two National Institutes of Health–sponsored workshops (21,22). Blood samples shipped overnight from the clinical sites were processed immediately upon receipt. Each sample was evaluated for hemolysis after Ficoll separation of the PBMCs. If severe hemolysis was visible or the PBMCs were observed by microscopy to be of poor quality or insufficient numbers, the blood sample was discarded. Mitogen (concanavalin A; Sigma-Aldrich, St. Louis, MO) was used as a viability control and tetanus toxoid as a positive antigenic control to determine functional ability of cells. Stimulation indexes (SI = cpm experimental wells/cpm control wells) were calculated to determine proliferative responses of the PBMCs (23). If the PBMCs were unable to respond to concanavalin A with a value >20 SI, the data were excluded from the analysis.
Briefly, the cellular immunoblotting assay was performed as follows (23): human islet cell preparations (obtained from the National Institutes of Health–supported Integrated Islet Distribution Program, https://iidp.coh.org) were subjected to preparative one-dimensional SDS-PAGE, the gels electroblotted onto nitrocellulose, cut according to molecular weight regions, solubilized, and reprecipitated with DMSO and sodium carbonate/bicarbonate buffers. The PBMCs were incubated with nitrocellulose containing islet proteins separated by molecular weights (“blots,” i.e., bands of nitrocellulose defined by molecular weight) for 5 days. Tritiated thymidine (1 mCi/well) was added, the cells were harvested 18 h later, and radioactivity measured in a β-scintillation counter (LKB Pharmacia). Positive proliferative responses were taken as SI ≥2.1. Controls for the T-cell assay were patient PBMCs incubated with nitrocellulose blots without islet proteins and patient PBMCs only. In this assay, PBMCs from control subjects without diabetes respond to 0–3 blots (considered T-cell negative, or T−), whereas patients with diabetes associated with islet autoimmunity respond to 4–18 blots (considered T-cell positive, or T+). T-cell reactivity in this assay is specific for islets (and not other tissues) and stable using islets obtained from different donors (21–23). The specificity and sensitivity of the cellular immunoblotting assay compare favorably with other assays of cellular immune reactivity to islet antigens as reviewed by the Immunology of Diabetes Society’s T-Cell Workshop Committee (24). The threshold that determines a true-positive (“T+”) result in this assay (four or more blots) was established from several studies in a range of healthy control subjects (n = 237), aged 18–75 years, BMI 18–40 kg/m2, without T1D or T2D (21–23,25), and in people with autoimmune diseases (in the absence of diabetes) such as rheumatoid arthritis and Graves disease, all of whom have circulating T cells that react to three or fewer blots. Longitudinal responses of 10 of the healthy control subjects, sampled repeatedly over a period of 63 months, demonstrated no T-cell reactivity to islet proteins above the established threshold (three or fewer blots). T-cell reactivity to islet antigens was measured in 322 samples.
Islet Autoantibody Assays
We evaluated autoantibodies to 65-kDa GAD antigen (GAD65Ab), IA-2 autoantibody (IA-2Ab), and zinc transporter-8 autoantibody (ZnT8Ab). The islet autoantibody assays are well-established assays with a sensitivity of 70% and specificity of 98% for GAD65Ab, 66% sensitive and 98% specific for IA-2Ab, and 54% sensitive and 100% specific for ZnT8Ab (26). Autoantibody-positive (Ab+) and autoantibody-negative (Ab−) samples were included in every assay to correct for interassay variation and used to calculate an antibody index for GAD65Ab and IA-2Ab. For ZnT8Ab, pan-reactive serum from a patient with T1D was included as a standard and used to express Ig binding levels as a relative unit. Samples are considered ZnT8Ab-positive if binding to ZnT8-Arg, ZnT8Trp, or ZnT8-Glu is detected. Cutoffs are established based on the 98th percentile among 100 healthy human sera. The laboratory participates in the Diabetes Antibody Standardization Program (26). Autoantibodies were measured in 392 samples.
β-Cell Function and Insulin Sensitivity Measurements
Plasma glucose and C-peptide values were measured in samples collected at 0, 30, 60, 90, and 120 min of the OGTT, and two measures of β-cell function were calculated (after adjusting for insulin sensitivity): ratio of the incremental area under the curve (iAUC) of C-peptide to the iAUC of glucose from 0–120 min (iAUC-CG; in nanomoles per milligram), with iAUC calculated using the trapezoidal rule (27); and ratio of the increment of C-peptide to that of glucose over the first 30 min (C-peptide index; i.e., ΔC-peptide [0–30 min]/Δglucose [0–30 min]; in nanomoles per gram) (28). Supplementary Figure 1 shows the strong correlation between these two measures of β-cell response. Insulin sensitivity (to adjust the β-cell response) was estimated using C-peptide-based homeostasis model assessment of insulin sensitivity (HOMA2-S.cpep) or 1/fasting C-peptide.
Statistical Analysis
To assess the association between the measures of β-cell function and autoantibodies or T-cell reactivity, linear models with log-transformed measures of β-cell function as outcome and autoimmune status (autoantibodies and islet-reactive T cells) as exposure of interest were fit. Either C-peptide–based HOMA2-S or 1/fasting C-peptide was used to adjust the β-cell response for insulin sensitivity. Additional covariates, including age, sex, BMI, duration of diabetes, and medications other than metformin, were prespecified and used to adjust regression models for hypotheses involving autoantibodies or T-cell reactivity and β-cell function. (See Supplementary Material for additional details on the choice of models and covariates, including justification for the use of C-peptide–based HOMA2-S or 1/fasting C-peptide.)
Nine covariates had >10% missingness, and multiple imputation was used to handle the missingness (29) (see Supplementary Material for details). The original sample size for this study was determined to achieve 90% power for detecting differences in β-cell function among T+ and T− patients in the longitudinal GRADE study without adjustment for additional covariates.
When possible, robust statistical procedures for assessing associations were used, including using the Spearman correlation coefficient as a rank-based alternative to the Pearson correlation coefficient and exact tests for proportions to test the null hypotheses about the proportion of Ab+ or T+ patients. Robust (sandwich) SEs for multiply imputed data were used for inference in linear regressions involving autoantibody or T-cell reactivity and β-cell function. In all models, β-cell function measures used as the regression outcome were log-transformed. With this transformation, 100(eβ − 1) represents the percentage change in the outcome per unit change in the covariate. Alternatively, regression coefficients are interpreted as the change in β-cell function measures for a unit increase in covariates for a reference patient, defined as a male with average covariate values taking no medications other than metformin (referred to hereafter as “average male”). The Holm procedure for controlling the family-wise error rate was used to account for hypotheses corresponding to different choices of β-cell function and measure of insulin sensitivity (29) (see Supplementary Material for details).
Data and Resource Availability
The protocol is available by contacting A.B. at ashokb@bcm.edu. The statistical code is available by contacting A.S. at ashojaie@uw.edu. Deidentified data are available by contacting A.S. or A.B.
Results
Participant Characteristics
Participants were 33.9% female, aged 57.4 ± 10.1 years, with BMI 33.6 ± 6.2 kg/m2, diabetes duration 4.0 ± 3.0 years, and HbA1c 7.5 ± 0.5% (mean ± SD). These were similar to the characteristics of all participants in the GRADE study: 36.4% female, aged 57.2 ± 10.0 years, with BMI 34.3 ± 6.8 kg/m2, diabetes duration 4.2 ± 2.8 years, and HbA1c 7.5 ± 0.5% (19,20).
Prevalence of Islet-Specific T-Cell Reactivity
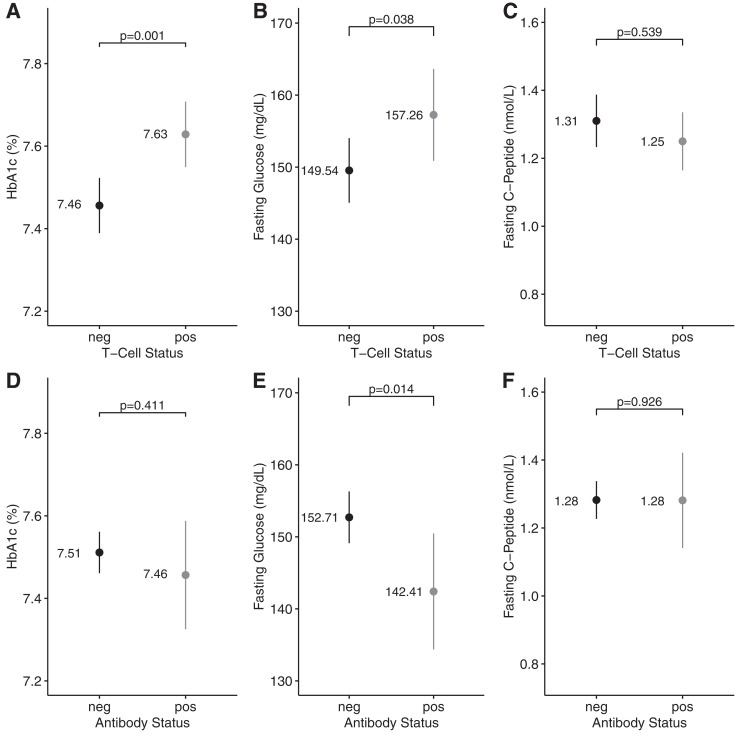
Of the 322 participants with available islet-specific T-cell reactivity data, 133 (41.3%) were T+. Supplementary Figure 2 displays the number of reactive blots in the T+ and T− groups. The proportions of T+ patients were similar between men and women and among different racial/ethnic groups (Table 1). Mean age, BMI, diabetes duration, systolic and diastolic blood pressure, and LDL- and HDL-cholesterol levels were similar between T+ and T− patients (Table 1). The mean (95% CI) for HbA1c was higher among those who were T+ than T− (7.63% [7.55, 7.71] vs. 7.46% [7.39, 7.52], mean [95% CI]), as was the mean fasting glucose level (157.26 mg/dL [150.87, 163.65] vs. 149.54 mg/dL [145.05, 154.03]) (Fig. 1A and B). However, fasting C-peptide levels were similar in T+ and T− patients (1.25 nmol/L [1.16, 1.34] vs. 1.31 nmol/L [1.23, 1.39]) (Fig. 1C).
Table 1.
Demographic/biochemical characteristics of the total study cohort, T+ vs. T− participants, and Ab+ vs. Ab− participants
| Total cohorta (N = 419) | T+b (N = 133) | T−b (N = 189) | Ab+c (N = 53) | Ab−c (N = 339) | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 277 | 89 (42) | 123 (58) | 31 (12) | 225 (88) |
| Female | 142 | 44 (40) | 66 (60) | 22 (16) | 114 (84) |
| Race | |||||
| African American | 70 | 16 (33) | 32 (67) | 12 (18) | 54 (82) |
| Asian | 19 | 6 (35) | 11 (65) | 4 (21) | 15 (79) |
| Hawaiian/Pacific Islander | 3 | 1 (33) | 2 (67) | 1 (33) | 2 (67) |
| White | 279 | 96 (44) | 121 (58) | 29 (11) | 230 (89) |
| American Indian/Alaska Native | 14 | 3 (30) | 7 (70) | 3 (23) | 10 (77) |
| Other/multiple | 3 | 0 (0) | 2 (100) | 0 (0) | 2 (100) |
| Unknown/not reported | 31 | 11 (44) | 14 (56) | 4 (13) | 26 (87) |
| Hispanic ethnicity | |||||
| Hispanic | 82 | 28 (44) | 35 (56) | 11 (15) | 64 (85) |
| Non-Hispanic | 334 | 103 (40) | 153 (60) | 42 (13) | 272 (87) |
| Unknown/not reported | 3 | 2 (66) | 1 (34) | 0 (0) | 3 (100) |
| Age (years), mean (SD) | 57.41 (10.13) | 56.8 (9.41) | 57.33 (9.84) | 56.26 (11.5) | 57.37 (9.9) |
| BMI (kg/m2), mean (SD) | 33.57 (6.2) | 33.78 (6.23) | 32.98 (5.76) | 34.26 (6.45) | 33.38 (6.15) |
| Diabetes duration (years), mean (SD) | 4 (2.95) | 4.06 (3) | 3.81 (2.88) | 3.64 (3.05) | 4.07 (2.95) |
| Systolic BP (mmHg), mean (SD) | 129.47 (15.32) | 129.05 (15.22) | 128.57 (14.91) | 130.79 (15.52) | 129.15 (15.34) |
| Diastolic BP (mmHg), mean (SD) | 77.95 (10.32) | 76.94 (9.84) | 78.53 (9.84) | 76.88 (9.66) | 78.18 (10.24) |
| LDL cholesterol (mg/dL), mean (SD) | 88.62 (31.04) | 89.43 (28.08) | 87.57 (32.21) | 88.65 (32.87) | 88.9 (31.02) |
| HDL cholesterol (mg/dL), mean (SD) | 43.68 (11.81) | 43.78 (11.1) | 43.41 (12.55) | 45.54 (12.35) | 43.66 (11.84) |
Data are n (%) unless otherwise indicated.
BP, blood pressure.
A total of 419 GRADE participants consented to participate in the β-cell Ancillary Study. Autoantibodies were measured in 392 samples and T-cell reactivity in 322. See text for details.
T+ indicates reactivity to ≥4 blots; T− indicates reactivity to ≤3 blots.
Ab+ is the presence of any one of the three islet autoantibodies; Ab− is the absence of all three islet autoantibodies.
Figure 1.
Comparison of glycemic parameters and fasting C-peptide in T+/T− and Ab+/Ab− patients. Unadjusted means and 95% CIs for HbA1c, fasting glucose, and fasting C-peptide levels in T− (n = 133) compared with T+ (n = 189) participants (A–C) and Ab+ (n = 53) compared with Ab− (n = 339) participants with T2D (D–F). A and D show group comparisons for HbA1c; B and E show comparisons for fasting glucose; and C and F show comparisons for fasting C-peptide. neg, negative; pos, positive.
Prevalence of Islet Autoantibodies
The prevalence of at least one islet autoantibody was 13.5%. Table 2 shows autoantibody frequencies in all 392 participants with complete autoantibody data and in the 322 participants who were T+ or T−. There was no significant association between T-cell positivity and autoantibody positivity (odds ratio 1.05 [95% CI 0.54, 2.06]; Fisher exact P = 0.87), and 5.3% of T+ participants were also positive for at least one autoantibody. Autoantibody frequencies in men and women and in different racial/ethnic groups were similar (Table 1). Mean age, BMI, diabetes duration, systolic and diastolic blood pressure, and LDL and HDL cholesterol were similar between participants who were Ab+ or Ab− (Table 1). Mean fasting glucose was lower in Ab+ than Ab− participants (142.41 mg/dL [134.36, 150.46] vs. 152.71 mg/dL [149.11, 156.30]); however, mean HbA1c (7.46% [7.33, 7.59] vs. 7.51% [7.46, 7.56]) and fasting C-peptide levels (1.28 nmol/L [1.14, 1.42] vs. 1.28 nmol/L [1.23, 1.34]) were similar between these two groups (Fig. 1D–F).
Table 2.
Islet autoantibody frequencies in T+ and T− patients with T2D
| Autoantibody | T+ (N = 133) | T− (N = 189) | All participants (N = 392) |
|---|---|---|---|
| GAD65Ab | 9 (4.8, 15.2) | 6.3 (3.3, 10.8) | 7.8 (5.4, 10.9) |
| IA2Ab | 4.5 (1.7, 9.6) | 7.4 (4.1, 12.1) | 6 (3.9, 8.9) |
| ZnT8Ab | 2.3 (0.5, 6.4) | 0.5 (0, 2.9) | 1.5 (0.6, 3.3) |
| One autoantibody | 12.8 (7.6, 19.7) | 12.2 (7.9, 17.7) | 13.5 (10.3, 17.3) |
| Two autoantibodies | 1.5 (0.2, 5.3) | 2.1 (0.6, 5.3) | 1.5 (0.6, 3.3) |
| Three autoantibodies | 1.5 (0.2, 5.3) | 0 (0, 1.9) | 0.5 (0.1, 1.8) |
Data are % (95% CI). T-cell assays (cellular immunoblotting tests) were not completed in 70 of 392 participants who had serum autoantibody tests.
T+ is reactivity to ≥4 blots; T− is reactivity to ≤3 blots.
Relationship of Islet-Specific T-Cell Autoimmunity to β-Cell Function
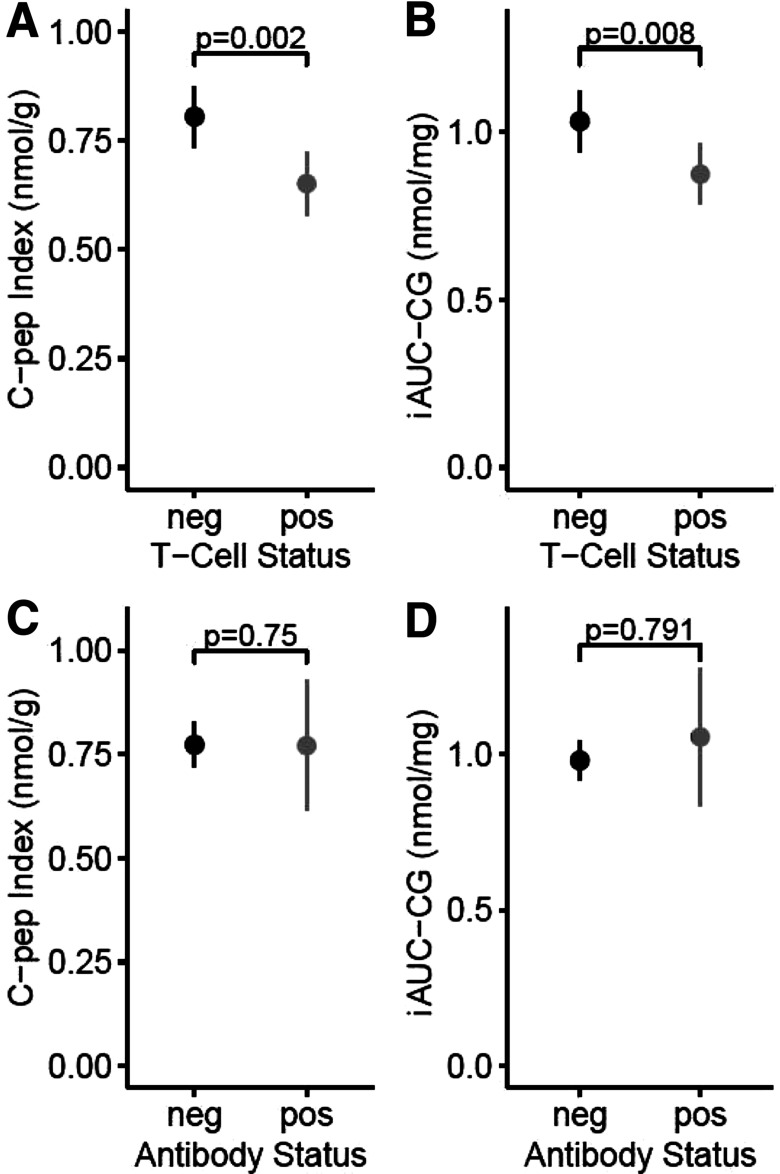
Patients who were T+ had significantly lower levels of β-cell function than T− patients, as measured by iAUC-CG after adjusting for age, sex, antibody positivity, BMI, duration of diabetes, medications other than metformin, and insulin sensitivity. Using 1/fasting C-peptide to adjust for insulin sensitivity, an average T+ male patient had 0.09 nmol/mg lower iAUC-CG compared with a similar T− participant (adjusted 95% CI −0.17, −0.01). This corresponds to 11.4% lower iAUC-CG in T+ patients compared with T− participants (adjusted 95% CI −21.2, −0.4). Using HOMA2-S.cpep to adjust for insulin sensitivity, the same T+ participant had 0.10 nmol/mg lower iAUC-CG than a T− participant (adjusted 95% CI −0.19, −0.02). This corresponds to 13.2% lower iAUC-CG in T+ participants compared with T− participants (adjusted 95% CI −24.4, −0.3).
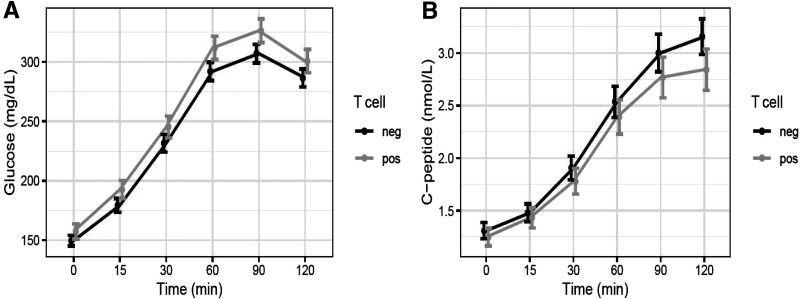
Participants who were T+ also had significantly lower C-peptide index values than T− participants after adjusting for insulin sensitivity, age, sex, antibody positivity, BMI, duration of diabetes, and medications other than metformin. Using 1/fasting C-peptide to adjust for insulin sensitivity, an average T+ male participant had 0.11 nmol/g lower C- peptide index compared with a similar T− participant (adjusted 95% CI −0.19, −0.04). This corresponds to 17.7% lower C-peptide in T+ compared with T− participants (adjusted 95% CI −30.5, −2.6). Using HOMA2-S.cpep to adjust for insulin sensitivity, the same T+ participant had 0.12 nmol/mg lower C-peptide index than a similar T− participant (adjusted 95% CI −0.20, −0.05). This corresponds to 19% lower C-peptide in T+ compared with T− participants (adjusted 95% CI −32.3, −3.1). Figure 2A and B show that both β-cell function measures were also significantly lower among T+ versus T− participants without adjusting for covariates. Figure 3 displays the curves of glucose and C-peptide response to oral glucose challenge in the two groups.
Figure 2.
Comparison of β-cell function in patients positive or negative for islet autoantibodies and T-cell reactivity to islet proteins. Unadjusted means and 95% CIs for two measures of β-cell function in T− (n = 133) compared with T+ (n = 189) patients with T2D (A and B) and in Ab+ (n = 53) compared with Ab− (n = 339) patients with T2D (C and D). A and C show group comparisons for C-peptide Index (C-pep Index). B and D show comparisons for iAUC-CG. See text for details. neg, negative; pos, positive.
Figure 3.
Curves of serum glucose (A) and C-peptide (B) response to oral glucose during the OGTT in T+ and T− participants. Values are ±95% CIs. neg, negative; pos, positive.
There was also a significant negative association between T-cell reactivity (number of blots as a continuous variable) and iAUC-CG after adjusting for the same covariates. Using 1/fasting C-peptide to adjust for insulin sensitivity, each additional blot for an average male participant decreased iAUC-CG by 0.01 nmol/mg (adjusted 95% CI −0.02, −0.002). This corresponds to 1.3% reduction in iAUC-CG for each additional blot (adjusted 95% CI −2.4, −0.2). Using HOMA2-S.cpep to adjust for insulin sensitivity for the same participant, each additional blot decreased iAUC-CG by 0.01 nmol/mg (adjusted 95% CI −0.02, −0.003). This corresponds to a 1.5% reduction in iAUC-CG for each additional blot (adjusted 95% CI −2.8, −0.2).
Similarly, there was a significant negative association between T-cell reactivity and C-peptide index after adjusting for the same covariates. Using 1/fasting C-peptide to adjust for insulin sensitivity, each additional blot for an average male participant decreased C-peptide index by 0.01 nmol/g (adjusted 95% CI −0.02, −0.004). This corresponds to a 1.9% reduction in C-peptide index for each additional blot (adjusted 95% CI −3.5, −0.3). Using HOMA2-S.cpep to adjust for insulin sensitivity for the same participant, each additional blot decreased C-peptide index by 0.01 nmol/g (adjusted 95% CI −0.02, −0.004). This corresponds to a 2% reduction in C-peptide index for each additional blot (adjusted 95% CI −3.7, −0.3).
Relationships Between Islet Autoantibodies and β-Cell Function
There was no significant association between iAUC-CG and autoantibody positivity using either 1/fasting C-peptide (difference 0.003 [adjusted 95% CI −0.15, 0.15]) or HOMA2-S.cpep (difference 0.01 [adjusted 95% CI −0.15, 0.16]) as the insulin sensitivity factor, while adjusting for the same covariates. Similarly, there was no significant association between C-peptide index and autoantibody positivity, using either 1/fasting C-peptide (difference −0.08 [adjusted 95% CI −0.2, 0.035]) or HOMA2-S.cpep (difference −0.08 [adjusted 95% CI −0.2, 0.04]) as the insulin sensitivity factor and adjusting for the same covariates. Figure 2C and D show that both β-cell function measures were similar in Ab+ versus Ab− participants without adjusting for covariates. The lack of statistical significance may be due to the small number of Ab+ patients (n = 53). Being both T+ and Ab+ did not confer significant association with either measure of β-cell function (P = 0.74 and 0.66 for iAUC-CG and C-peptide index, respectively), likely due to the small number of patients who were both T+ and Ab+ (n = 17). There were no noticeable associations between diabetes duration and prevalence of either T-cell positivity (difference 0.017 [95% CI −1.7, 1.7]) or autoantibody positivity (difference −0.77 [95% CI −2.3, 0.75]) using the same adjusting variables.
Discussion
Our most striking finding is the high prevalence of islet autoimmunity among a large cohort of patients with T2D. The T-cell assay, which measures cellular autoimmune responses against a range of islet autoantigens, revealed a high, 41.3% prevalence of cellular islet autoimmunity. The prevalence of humoral autoimmunity, defined as the presence of antibodies against three T1D-associated islet autoantigens, was 13.5%. These results suggest that islet autoimmunity is far more prevalent in patients with T2D than previously recognized. T-cell reactivity was inversely related to β-cell function; hence, T-cell–mediated autoimmunity could contribute to the β-cell defect in a substantial proportion of patients with T2D. This association is clinically significant because of the higher fasting glucose level and HbA1c in T+ compared with T− patients with T2D.
The frequency of humoral islet autoimmunity in this GRADE cohort is higher than reported in other large cohorts of adults with T2D (30–35). Variations in geography and ethnicity of the populations, sensitivity and cutoffs of the assays, and number of autoantibodies measured could account for the differences. Our study included measurements of all three current T1D-associated islet autoantibodies. Only a few other studies measured autoantibodies other than GAD65Ab in different laboratories (30–34). Comparing islet autoantibody frequencies across studies using different laboratories, methods, and cutoff values for positivity is problematic; we have demonstrated the importance of measuring GAD65Ab in the same laboratory using a uniform, biologically validated cutoff for comparative studies (35).
The lack of association between autoantibody positivity and β-cell function in the current study is at variance with previous reports of latent autoimmune diabetes in adults (LADA) (30–32). Those studies estimated β-cell function using fasting values for glucose and insulin or C-peptide. We used a more integrated assessment of β-cell function, the dynamic response of C-peptide to glucose over a 2-h OGTT. Recently, LADA has been shown to display endotypic heterogeneity, with varying degrees of β-cell dysfunction that could guide different treatment approaches (36). Ethnic differences may affect the relationship; a study in African American youth with T2D found no association between autoantibody status and β-cell function (37). Furthermore, β-cell dysfunction has been observed mainly in patients with LADA who have high autoantibody titers or multiple autoantibodies (30,32,38–42) at diagnosis and lose β-cell function faster than those with low titers (31,41). GAD65Ab positivity may be transient among those with low titers (42). Titers of GAD65Ab in our Ab+ patients were relatively low, with no significant difference between White and African American patients (Supplementary Fig. 3) (mean difference 0.02 [95% CI −0.66, 0.69]).
Finally, islet autoantibodies originally identified in patients with T1D or LADA may not reflect a broader form of autoimmunity in patients with T2D because pathways leading to islet autoimmunity could generate different immune-stimulating antigens in patients with T1D compared with T2D (4). In a study of Pima Indians with T2D, none had elevated classical T1D-associated autoantibodies, even though a proportion had novel autoantibodies associated with insulin secretion (8). Our T-cell assay is agnostic with respect to specific autoantigens; it assesses cellular immune response to a broad array of islet antigens.
Thus, the T-cell assay could define islet autoimmunity in patients with T2D more clearly than T1D-associated autoantibodies. In support of this concept, Frankl et al. (10) observed differences in T-cell receptor repertoires to be associated with and predictive of T2D development among Pima Indians. Genetic factors may also affect the autoimmune response, leading to subgroups of T2D with different markers of islet autoimmunity development. Of note, the racial and ethnic frequencies of the participants with T2D in our study are well representative of patients with T2D in both the overall GRADE study and the U.S. population, albeit with a slight overrepresentation of Hispanic patients (Table 1).
The overlap between Ab+ patients and T+ patients was small, only 5.4%, and the association between T-cell positivity and autoantibody positivity was not significant. We have previously noted discordance between the higher rates of T-cell positivity and the lower rates of T1D-associated autoantibody positivity in patients with T2D (16,43). This may be because islet autoantibodies associated with T2D are different from those associated with T1D and hence may not be recognized by GAD65, IA2, or ZnT8 autoantibody assays. Other research groups have identified antigenic differences in autoantibody specificities between T1D and T2D (44,45). Dissociation between the presence of autoantibodies and T-cell responses in groups of patients with T1D and T2D (5,16) supports the existence of a subpopulation of Ab−, T+ people with autoimmune diabetes. GAD65Ab+ patients with T2D are defined as LADA, whereas T+ patients are likely a novel subtype of T2D with islet dysfunction exacerbated by cellular immune responses to a range of islet antigens. Systemic inflammation contributes to the pathophysiology of T2D, and chronic elevations of inflammatory cytokines and chemokines in the islet milieu may play a role in β-cell dysfunction (12,46). Natural history studies and evidence of systemic and islet inflammation in patients with T2D (5–8) suggest that early, ongoing islet injury is a physiological feature of T2D. In susceptible patients, this could lead to breakdown of immune tolerance and development of islet-specific T-cell reactivity (4) or autoantibodies with disease-specific epitope patterns (47). It is difficult to determine to what extent increased T-cell reactivity in our assay reflects an active autoimmune process or serves as a marker for islet autoimmunity or inflammation. However, the clinical significance of these autoimmune pathways of β-cell dysfunction is supported by our preliminary data that attenuation of islet-reactive T cells may improve β-cell function in patients with T2D (18). The variations in islet autoimmunity noted in the current study also contribute to understanding the emerging heterogeneity of T2D. “Clustering” analyses have described distinct phenotypic subgroups of T2D, including some defined by β-cell dysfunction (48). Many among our T+ participants could fit the category denoted “severe insulin-deficient diabetes” by Ahlqvist et al. (48).
Metformin has been shown to have immune-regulating functions that could modulate cellular immune signaling in some autoimmune diseases (49). However, metformin does not appear to affect the development of islet cellular or autoantibody reactivity in patients with T2D in a manner that might affect the outcomes reported in this study. We previously assessed the longitudinal impact of development of islet autoimmunity over time on β-cell functional decline in patients with T2D taking a range of oral glucose-lowering medications (3). Of the patients with T2D who developed islet-reactive T cells, 86% had been taking metformin, and this was not associated with development or attenuation of any of the measures of islet autoimmunity. More recently, we investigated islet autoimmunity longitudinally in a large cohort of people with impaired glucose tolerance or recently diagnosed T2D placed on metformin alone or with liraglutide or insulin glargine (43). Again, we found no effect of metformin on the development of islet-reactive T cells or its association with diminished β-cell function and hyperglycemia. Since all participants in the GRADE study at baseline were taking metformin as the sole glucose-lowering medication, any effect of the drug would likely be distributed similarly among all the participants and their T-cell and autoantibody assays.
Could T-cell–mediated islet autoimmunity be suppressed by any interventions, and would its presence influence treatment of T2D? We have previously shown that attenuation of islet-reactive T cells in patients with T2D is associated with improved β-cell function (18,43). The present GRADE β-cell Ancillary Study has a longitudinal component, and assessment of the effects of the GRADE treatments on islet autoimmunity, progression of β-cell function, and glycemic control is pending. Data on progression of the GRADE participants await complete analysis of the primary outcome data of the parent GRADE study.
The islet-reactive immune responses in patients with T2D identified by the cellular immunoblotting test may not reflect a “primary,” early destructive process in the islets (as is presumed to occur in T1D) but rather result from the chronic, systemic inflammatory stress characteristic of obesity and T2D. The development of cellular islet autoimmunity in patients with T2D may be part of a secondary process that contributes to the decline in β-cell function (4). This concept is supported by the present data and our previous demonstrations of inverse correlations between T-cell reactivity and glycemic control (3) and between T-cell reactivity and both β-cell function and glycemic control (43). The collective evidence suggests that the differences in β-cell function and glycemic control between T+ and T− participants with T2D in the current study reflect pathophysiologically and clinically significant phenomena.
A potential limitation of this study is the sampling frame. Of those who consented, we were unable to measure autoantibodies in 6% and T-cell reactivity in 23%. However, our ancillary study did not use any additional inclusion or exclusion criteria, and there were no notable differences in demographic or relevant biochemical data between the parent GRADE cohort at baseline and those who completed the tests of humoral or cellular islet autoimmunity. Post hoc analysis indicated sufficient power for detecting the minimum effect size for the association of β-cell function with T-cell status.
In conclusion, cellular islet autoimmunity is prevalent in a large percentage of people with T2D and is a significant component in the pathophysiology of β-cell dysfunction. Of clinical importance is the negative association of islet-reactive T cells with β-cell function and glycemic control. This is possibly a characteristic of the natural history of T2D, given the recent finding that islet-reactive T cells were present in a large percentage of obese adults with prediabetes and recently diagnosed, treatment-naive T2D in the Restoring Insulin SEcretion (RISE) study (43). Presence of islet-reactive T cells among RISE participants was associated with increased fasting and 2-h glucose levels, and T+ patients with T2D also had lower steady-state C-peptide levels compared with T− patients (43). Our study reveals an immune-mediated mechanism that could contribute to β-cell dysfunction in T2D, define T2D subtypes, and uncover new therapeutic approaches to treat this heterogeneous condition.
Article Information
Acknowledgments. All authors affirm that authorship is merited based on the International Committee of Medical Journal Editors authorship criteria.
Funding. This ancillary study to the GRADE study was independently funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK104832 (to A.B. and J.P.P.). The GRADE study is supported by a grant from the NIDDK of the National Institutes of Health under award number U01DK098246. The planning of GRADE was supported by a U34 planning grant from the NIDDK (U34-DK-088043). The American Diabetes Association supported the initial planning meeting for the U34 proposal. The National Heart, Lung, and Blood Institute and the Centers for Disease Control and Prevention also provided funding support. The Department of Veterans Affairs provided resources and facilities. Additional support was provided by grants P30 DK017047, P30 DK020541-44, P30 DK020572, P30 DK072476, P30 DK079626, P30 DK092926, U54 GM104940, UL1 TR000439, UL1 TR000445, UL1 TR001108, UL1 TR001409, UL1 TR001449, UL1 TR002243, UL1 TR002345, UL1 TR002378, UL1 TR002489, UL1 TR002489, UL1 TR002529, UL1 TR002535, UL1 TR002537, and UL1 TR002548. Educational materials have been provided by the National Diabetes Education Program. Material support in the form of donated medications and supplies has been provided by Becton, Dickinson and Company, Bristol-Myers Squibb, Merck, Novo Nordisk, Roche Diagnostics, and Sanofi.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. S.E.K. has received fees from Bayer, Boehringer Ingelhein, Casma Therapeutics, Eli Lilly, Intarcia, Merck, Novo Nordisk, and Pfizer. C.D. has received fees from Novo Nordisk, Bayer, and AstraZeneca. K.J.M. has received a grant from Eli Lilly. No other potential conflicts of interest relevant to this work were reported.
Author Contributions. B.B.-W. and C.S.H. contributed to the conception and design of the study, acquisition of data, interpretation of data and results, supervision and management of research, and drafting of this manuscript. E.G.H. and B.P. contributed to acquisition of data and review of this manuscript. S.Z.Z. contributed to the statistical analysis and interpretation of data and results and the drafting and review of this manuscript. K.U., K.J.M., N.R., C.D., R.M.C., J.Y.P., H.J.F., and W.M.V. contributed to the acquisition of data, interpretation of data and results, and critical review of this manuscript. S.E.K. contributed to the supervision and management of the research, interpretation of data and results, and critical review of this manuscript. M.E.L. and M.L.J. contributed to the acquisition of data, supervision and management of the research, and critical review of this manuscript. N.Y. contributed to the statistical analysis of data and critical review of this manuscript. A.S. contributed to the conception and design of the research, statistical analysis and interpretation of data and results, acquisition of funding, supervision and management of research, and the drafting and review of this manuscript. All members of the GRADE β-cell Ancillary Study Network contributed to the acquisition of data. J.P.P. and A.B. contributed to the conception and design of the research, acquisition of data, interpretation of data and results, acquisition of funding, supervision and management of research, and drafting of the manuscript. A.B., J.P.P., and A.S. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
B.B.-W. and C.S.H. are joint first authors.
See accompanying article, p. 1167.
This article contains supplementary material online at https://doi.org/10.2337/figshare.18621602.
A complete list of the members of the GRADE Research Group can be found in the supplementary material online.
References
- 1. Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 2009;52:1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brooks-Worrell B, Palmer JP. Immunology in the Clinic Review Series; focus on metabolic diseases: development of islet autoimmune disease in type 2 diabetes patients: potential sequelae of chronic inflammation. Clin Exp Immunol 2012;167:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brooks-Worrell BM, Boyko EJ, Palmer JP. Impact of islet autoimmunity on the progressive β-cell functional decline in type 2 diabetes. Diabetes Care 2014;37:3286–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brooks-Worrell BM, Palmer JP. Setting the stage for islet autoimmunity in type 2 diabetes: obesity-associated chronic systemic inflammation and endoplasmic reticulum (ER) stress. Diabetes Care 2019;42:2338–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang H, Cheng Y, Tang W, et al. Clinical manifestation and islet β-cell function of a subtype of latent autoimmune diabetes in adults (LADA): positive for T cell responses in phenotypic type 2 diabetes. Acta Diabetol 2019;56:1225–1230 [DOI] [PubMed] [Google Scholar]
- 6. Goel A, Chiu H, Felton J, Palmer JP, Brooks-Worrell B. T-cell responses to islet antigens improves detection of autoimmune diabetes and identifies patients with more severe β-cell lesions in phenotypic type 2 diabetes. Diabetes 2007;56:2110–2115 [DOI] [PubMed] [Google Scholar]
- 7. Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima Indians: contributions of obesity and parental diabetes. Am J Epidemiol 1981;113:144–156 [DOI] [PubMed] [Google Scholar]
- 8. Chang DC, Piaggi P, Hanson RL, et al. Use of a high-density protein microarray to identify autoantibodies in subjects with type 2 diabetes mellitus and an HLA background associated with reduced insulin secretion. PLoS One 2015;10:e0143551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams RC, Muller YL, Hanson RL, et al. HLA-DRB1 reduces the risk of type 2 diabetes mellitus by increased insulin secretion. Diabetologia 2011;54:1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frankl JA, Thearle MS, Desmarais C, Bogardus C, Krakoff J. T-cell receptor repertoire variation may be associated with type 2 diabetes mellitus in humans. Diabetes Metab Res Rev 2016;32:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butcher MJ, Hallinger D, Garcia E, et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia 2014;57:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 13. Abdel-Moneim A, Bakery HH, Allam G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed Pharmacother 2018;101:287–292 [DOI] [PubMed] [Google Scholar]
- 14. Kamali AN, Noorbakhsh SM, Hamedifar H, et al. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol Immunol 2019;105:107–115 [DOI] [PubMed] [Google Scholar]
- 15. Tao L, Liu H, Gong Y. Role and mechanism of the Th17/Treg cell balance in the development and progression of insulin resistance. Mol Cell Biochem 2019;459:183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brooks-Worrell BM, Reichow JL, Goel A, Ismail H, Palmer JP. Identification of autoantibody-negative autoimmune type 2 diabetic patients. Diabetes Care 2011;34:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarikonda G, Pettus J, Phatak S, et al. CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J Autoimmun 2014;50:77–82 [DOI] [PubMed] [Google Scholar]
- 18. Brooks-Worrell BM, Palmer JP. Attenuation of islet-specific T cell responses is associated with C-peptide improvement in autoimmune type 2 diabetes patients. Clin Exp Immunol 2013;171:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nathan DM, Buse JB, Kahn SE, et al.; GRADE Study Research Group . Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013;36:2254–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wexler DJ, Krause-Steinrauf H, Crandall JP, et al.; GRADE Research Group . Baseline characteristics of randomized participants in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care 2019;42:2098–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seyfert-Margolis V, Gisler TD, Asare AL, et al. Analysis of T-cell assays to measure autoimmune responses in subjects with type 1 diabetes: results of a blinded controlled study. Diabetes 2006;55:2588–2594 [DOI] [PubMed] [Google Scholar]
- 22. Herold KC, Brooks-Worrell B, Palmer J, et al.; Type 1 Diabetes TrialNet Research Group . Validity and reproducibility of measurement of islet autoreactivity by T-cell assays in subjects with early type 1 diabetes. Diabetes 2009;58:2588–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brooks-Worrell BM, Starkebaum GA, Greenbaum C, Palmer JP. Peripheral blood mononuclear cells of insulin-dependent diabetic patients respond to multiple islet cell proteins. J Immunol 1996;157:5668–5674 [PubMed] [Google Scholar]
- 24. Mannering SI, Wong FS, Durinovic-Belló I, et al.; Immunology of Diabetes Society T-Cell Workshop Committee . Current approaches to measuring human islet-antigen specific T cell function in type 1 diabetes. Clin Exp Immunol 2010;162:197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brooks-Worrell B, Gersuk VH, Greenbaum C, Palmer JP. Intermolecular antigen spreading occurs during the preclinical period of human type 1 diabetes. J Immunol 2001;166:5265–5270 [DOI] [PubMed] [Google Scholar]
- 26. Williams AJK, Lampasona V, Schlosser M, et al.; Participating Laboratories . Detection of antibodies directed to the N-terminal region of GAD is dependent on assay format and contributes to differences in the specificity of GAD autoantibody assays for type 1 diabetes. Diabetes 2015;64:3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seltzer HS, Allen EW, Herron AL Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 1967;46:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Little R, Rubin D. Statistical Analysis With Missing Data. 3rd ed. Hoboken, NJ, John Wiley & Sons, Inc., 2019 [Google Scholar]
- 30. Turner R, Stratton I, Horton V, et al.; UK Prospective Diabetes Study Group . UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet 1997;350:1288–1293 [DOI] [PubMed] [Google Scholar]
- 31. Zinman B, Kahn SE, Haffner SM, O’Neill MC, Heise MA; ADOPT Study Group . Phenotypic characteristics of GAD antibody-positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes 2004;53:3193–3200 [DOI] [PubMed] [Google Scholar]
- 32. Monge L, Bruno G, Pinach S, et al. A clinically orientated approach increases the efficiency of screening for latent autoimmune diabetes in adults (LADA) in a large clinic-based cohort of patients with diabetes onset over 50 years. Diabet Med 2004;21:456–459 [DOI] [PubMed] [Google Scholar]
- 33. Xiang Y, Huang G, Shan Z, et al. Glutamic acid decarboxylase autoantibodies are dominant but insufficient to identify most Chinese with adult-onset non-insulin requiring autoimmune diabetes: LADA China study 5. Acta Diabetol 2015;52:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pilla SJ, Balasubramanyam A, Knowler WC, et al.; Look AHEAD Research Group . Islet autoantibody positivity in overweight and obese adults with type 2 diabetes. Autoimmunity 2018;51:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rolandsson O, Hampe CS, Wennberg P, Radtke J, Langenberg C; EPIC-InterAct Study Group . Prevalence and regional distribution of autoantibodies against GAD65Ab in a european population without diabetes: the EPIC-InterAct study. Diabetes Care 2015;38:e114–e115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buzzetti R, Tuomi T, Mauricio D, et al. Management of latent autoimmune diabetes in adults: a consensus statement from an international expert panel. Diabetes 2020;69:2037–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gumus P, Gomez R, Vargas A, Chalew S. The relationship of insulin secretion and GAD65 antibody levels at diagnosis on glycemic control in type 2 diabetes. J Pediatr Endocrinol Metab 2010;23:1025–1029 [DOI] [PubMed] [Google Scholar]
- 38. Buzzetti R, Di Pietro S, Giaccari A, et al.; Non Insulin Requiring Autoimmune Diabetes Study Group . High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care 2007;30:932–938 [DOI] [PubMed] [Google Scholar]
- 39. Lohmann T, Kellner K, Verlohren HJ, et al. Titre and combination of ICA and autoantibodies to glutamic acid decarboxylase discriminate two clinically distinct types of latent autoimmune diabetes in adults (LADA). Diabetologia 2001;44:1005–1010 [DOI] [PubMed] [Google Scholar]
- 40. Liu L, Li X, Xiang Y, et al.; LADA China Study Group . Latent autoimmune diabetes in adults with low-titer GAD antibodies: similar disease progression with type 2 diabetes: a nationwide, multicenter prospective study (LADA China Study 3). Diabetes Care 2015;38:16–21 [DOI] [PubMed] [Google Scholar]
- 41. Li X, Chen Y, Xie Y, et al. Decline pattern of beta- cell function in adult-onset latent autoimmune diabetes: an 8-year prospective study. J Clin Endocrinol Metab 2020;105:2331–2340 [DOI] [PubMed] [Google Scholar]
- 42. Sørgjerd EP, Thorsby PM, Torjesen PA, Skorpen F, Kvaløy K, Grill V. Presence of anti-GAD in a non-diabetic population of adults; time dynamics and clinical influence: results from the HUNT study. BMJ Open Diabetes Res Care 2015;3:e000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brooks-Worrell BM, Tjaden AH, Edelstein SL, et al.; RISE Consortium . Islet autoimmunity in adults with impaired glucose tolerance and recently diagnosed, treatment naïve type 2 diabetes in the Restoring Insulin SEcretion (RISE) Study. Front Immunol 2021;12:640251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li R, Huang J, Yu Y, Yang Y. Islet autoantibody patterns in patients with type 2 diabetes aged 60 and higher: a cross-sectional study in a Chinese hospital. Front Endocrinol (Lausanne) 2018;9:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gao X, Sun W, Wang Y, et al. Prevalence of positive islet autoantibody in type 2 diabetes patients: a cross-sectional study in a Chinese community. Endocr Connect 2019;8:1493–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Donath MY, Dinarello CA, Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat Rev Immunol 2019;19:734–746 [DOI] [PubMed] [Google Scholar]
- 47. Padoa CJ, Banga JP, Madec A-M, et al. Recombinant Fabs of human monoclonal antibodies specific to the middle epitope of GAD65 inhibit type 1 diabetes-specific GAD65Abs. Diabetes 2003;52:2689–2695 [DOI] [PubMed] [Google Scholar]
- 48. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–369 [DOI] [PubMed] [Google Scholar]
- 49. Ursini F, Russo E, Pellino G, et al. Metformin and autoimmunity: a “new deal” of an old drug. Front Immunol 2018;9:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]