Abstract
Background
B‐type natriuretic peptide (BNP) is a well‐known biomarker for prognosis in heart failure with patients with preserved ejection fraction. However, the clinical predictive ability of BNP for the risk of stroke in HFpEF is not clear.
Methods and Results
A total of 799 patients with HFpEF from the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial were included. Association of baseline BNP with risk of stroke was assessed using the Cox proportional hazard model. The discriminatory ability of BNP was expressed using the C index. The improvement in 5‐year stroke prediction was assessed by C statistic, categorical net reclassification improvement index, and relative integrated discrimination improvement. A total of 34 (4.3%) patients among the 799 patients with HFpEF experienced stroke events over a median of 2.85 years of follow‐up. The stroke group showed a higher BNP level than the nonstroke group (375 pg/mL versus 241 pg/mL, respectively; P=0.006). Higher BNP levels were associated with increased risk of stroke after multivariable adjustment (hazard ratio, 3.29 [95% CI, 1.51–7.16]) and had a moderate performance for stroke prediction (C index, 0.67). Adding BNP to CHADS2/CHA2DS2‐VASc/R2CHADS2 scores improved their predictive value for stroke (CHADS2: C index, 0.67; BNP+CHADS2: C index, 0.77; net reclassification improvement, 40.9%; integrated discrimination improvement, 3.0%; CHA2DS2‐VASc: C index, 0.64; BNP+CHA2DS2‐VASc: C index, 0.74; net reclassification improvement, 41.4%; integrated discrimination improvement, 2.2%; R2CHADS2: C index, 0.70; BNP+R2CHADS2: C index, 0.78; net reclassification improvement, 40.9%; integrated discrimination improvement, 3.2%).
Conclusions
BNP is associated with an increased risk of stroke in patients with HFpEF and may be a valuable biomarker for stroke prediction in HFpEF.
Keywords: B‐type natriuretic peptide, heart failure, risk prediction, stroke
Subject Categories: Ischemic Stroke, Heart Failure
Nonstandard Abbreviations and Acronyms
- HFpEF
heart failure with preserved ejection fraction
- IDI
integrated discrimination improvement
- NRI
net reclassification improvement index
- NYHA
New York Heart Association
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist
Clinical Perspective.
What Is New?
We first evaluated the predictive value of B‐type natriuretic peptide for risk of stroke in patients with heart failure with preserved ejection fraction.
Higher B‐type natriuretic peptide levels are associated with increased risk of stroke and have a moderate performance for stroke prediction in heart failure with preserved ejection fraction.
Addition of B‐type natriuretic peptide to CHA2DS2/CHA2DS2‐VASc/R2CHADS2 scores significantly improved their stroke prediction in heart failure with preserved ejection fraction.
What Are the Clinical Implications?
The current findings suggest that B‐type natriuretic peptide might be a potential valuable biomarker for stroke prediction in heart failure with preserved ejection fraction.
Heart failure (HF) with preserved ejection fraction (HFpEF) is a highly complex clinical syndrome with a high prevalence with increasing age. 1 Stroke is considered a devastating outcome, with high levels of mortality and morbidity. 2 Epidemiological studies from several cohorts have shown that thrombus formation and stroke incidence in patients with HFpEF are similar or somewhat higher than those in patients with reduced ejection fraction. 1 , 3 , 4 , 5 Thus, the prediction of stroke in HFpEF remains an urgent clinical issue.
Several stroke risk scores, such as the CHADS2 and CHA2DS2‐VASc, have been developed to predict stroke in patients with atrial fibrillation (AF). 6 AF and HF, the vicious twins, often coexist and independently contribute to poor outcomes. 7 , 8 However, as we previously reported, the established stroke risk scores including CHA2DS2‐VASc and anticoagulation and risk factors in atrial fibrillation did not work well. 9 Moreover, although HFpEF is associated with the development of AF, concomitant AF cannot directly explain the risk of systemic thromboembolic events in patients with HFpEF. These observations supported the opinion raised by some authors that HFpEF itself is the substrate for stroke. 10
B‐type natriuretic peptide (BNP) is a well‐known biomarker for the presence and severity of HF and prognosis of patients with HF. 11 , 12 In regard to HFpEF, BNP has been statistically significantly associated with adverse outcomes, such as all‐cause mortality and hospitalization for HF. 13 , 14 Elevated plasma BNP levels were shown to predict the risk of stroke in the general population in several community‐based longitudinal cohorts. 15 , 16 More recently, in patients with chronic HF, the use of BNP to predict the occurrence of stroke has also been reported. 17 Given this background, by using the data set from the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial, a large, randomized controlled trial that investigated the effect of spironolactone in HFpEF, we performed a secondary analysis to (1) assess the association between BNP and the risk of stroke and (2) determine whether the addition of BNP to existing stroke risk scores (CHADS2/CHA2DS2‐VASc/R2CHADS2) could provide a better prediction of the risk of stroke in patients with HFpEF.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We used the TOPCAT trial data set, a multicenter, international, randomized, double‐blind, placebo‐controlled phase III trial of the spironolactone at 233 sites in 6 countries. Briefly, this trial enrolled 3445 patients with HFpEF from the Americas and Russia/Georgia, with (1) an age of 50 years or older, (2) left ventricular ejection fraction ≥45% and at least one sign and symptom of HF, (3) controlled systolic blood pressure, a serum potassium level <5.0 mmoL/L, and an estimated glomerular filtration rate (eGFR) of ≥30 mL/min per 1.73 m2 of body surface area, and (4) a history of HF hospitalization within 12 months before enrollment or an elevated level of natriuretic peptide within 60 days before randomization (a BNP of ≥100 pg/mL or NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) of ≥360 pg/mL). BNP or NT‐proBNP was locally collected and processed as previously described. 18 , 19 This trial duration was ≈6 years (August 10, 2006–January 31, 2012), with an average subject follow‐up of 3.3 years. TOPCAT complied with the Declaration of Helsinki and received ethical clearance. The study was approved by the institutional review board at each participating site. All patients signed informed consent before randomization. The primary results of the TOPCAT trial are published elsewhere. 18 , 19
Follow‐Up and End Point Definition
Patients were followed up for outcomes every 4 months during the study randomized in the first year and every 6 months thereafter. Stroke was defined as: (1) a focal neurologic deficit (resulting from a vascular cause involving the central nervous system) of sudden onset that is not reversible within 24 hours (including death) and not because of other readily identifiable causes (eg, brain tumor, trauma), or (2) a focal neurologic deficit (resulting from a vascular cause involving the central nervous system) of sudden onset that is reversible within 24 hours and brain imaging documenting a new infarction or hemorrhage (eg, magnetic resonance imaging with diffusion‐weighted imaging). All stroke events were adjudicated by a clinical end point committee at Brigham and Women's Hospital, Boston, Massachusetts, according to prespecified criteria. 18
Statistical Analysis
SPSS Statistics version 25.0 (IBM, Armonk, NY) and R version 4.0.3 software for Windows (R Foundation for Statistical Computing, Vienna, Austria) were used. The continuous variables are expressed as the mean with SD for the normally distributed data or median with interquartile range for the nonnormally distributed data. The normality of the data was analyzed using the Shapiro‐Wilk test. The differences between the groups in the continuous variables were compared using unpaired Student t tests (normal distribution) or Mann‐Whitney U tests (nonnormal distributions). The categorical variables, which are reported as count and percentage, were compared using χ2 tests. The best cutoff value of BNP for predicting a stroke event during follow‐up was explored by using regression tree analysis. Kaplan‐Meier analysis with log‐rank test was used for initial comparisons of stroke event among BNP groups. Proportionality assumption was assessed by plotting log minus log survival function. The Cox proportional hazard model calculated the adjusted hazard ratio (HR), and 95% CI of BNP associated with stroke risk was calculated. The adjusted variables were selected by stepwise methods (P<0.1) from all baseline factors and considering meaningful clinical variables. Furthermore, a Cox proportional hazard analysis with propensity score was performed using 1:1 nearest‐neighbor matching without replacement to match significant baselines characteristics among BNP groups. The propensity score was derived using a logistic regression model that included BNP>278 pg/mL as the outcome variable and confounders with significant difference BNP groups, including age, sex, body mass index, ejection fraction, eGFR, history of AF, administration of diuretics, and β‐blockers as explanatory variables. Standardized differences of <0.10 between propensity score–matched patients were considered negligible.
C index at 5 years in a crude Cox model was used to evaluate improvements of prognostic value by adding BNP to existing stroke risk models (CHADS2, CHA2DS2‐Vasc, R2CHADS2 scores). The calculation of these risk scores has been described previously. 20 The C index of a BNP‐added risk‐predicting model versus existing risk‐predicting model was compared by using the method of Delong et al. 21 The continuous net reclassification improvement (NRI) index and integrated discrimination improvement (IDI) index examined the improvement. CIs of C index, NRI scores, and IDI scores were computed by bootstrap resampling. P<0.05 was considered statistically significant in all analyses.
RESULTS
Characteristics of HFpEF Patients With or Without Baseline BNP Value
In total, 3445 subjects were recruited for the TOPCAT study; a total of 802 patients had baseline BNP values available, 799 of whom were included in our study after excluding individuals with missing covariates. There was a significant difference across the key characteristics of patients with HFpEF with or without baseline BNP value including age, race, and history of smoking (Table S1). In patients with available BNP levels, median (interquartile range) values were 71.0 years (64.0–79.0 years) for age, 31.2 kg/m2 (26.7–38.3 kg/m2) for body mass index, 63.1 mL/min per 1.73 m2 (50.2–76.7 L/min per 1.73 m2) for eGFR, and 61.8% had a history of AF. BNP ranged from 4 to 4943 pg/mL (median, 247 pg/mL; interquartile range, 143–443 pg/mL).
Characteristics of HFpEF Patients With or Without a Stroke Event After Randomization
Among 799 included subjects, there were 34 stroke events (1.52 per 100 patient‐years) after a mean of 2.85 years of follow‐up among 2238.51 patient‐years. As shown in Table 1, the stroke group had a higher prevalence of AF, higher BNP levels, and more warfarin administration than the nonstroke group. There were no significant differences among age, sex, country, body mass index, New York Heart Association functional class, or other comorbidities between the 2 groups. Only a small proportion of the enrolled patients had baseline echocardiography data, which are summarized in Table S2. The patients who experienced stroke events had higher left ventricular relative wall thickness, higher posterior wall thickness, and higher right ventricular outflow tract velocity time integral.
Table 1.
Basic Characteristics of Stroke and Nonstroke Group
| Variables | Nonstroke, n=765 | Stroke, n=34 | P value |
|---|---|---|---|
| Random to spironolactone | 390 (48.8) | 17 (50.0) | 0.911 |
| Demographics | |||
| Age, y | 71.0 (63.0, 79.0) | 74.0 (67.8, 81.3) | 0.067 |
| Sex, men, n (%) | 387 (48.4) | 23 (67.6) | 0.052 |
| Race, White, n (%) | 607 (76.0) | 28 (82.4) | 0.671 |
| Country, United States, n (%) | 565 (70.7) | 27 (79.4) | 0.469 |
| BMI, kg/m2 | 32.4 (27.8, 37.8) | 31.2 (26.7, 38.3) | 0.436 |
| Smoking history | 411 (51.4) | 23 (67.6) | 0.111 |
| Alcohol drinking, n (%) | 0.327 | ||
| 0 | 573 (71.7) | 28 (82.4) | |
| 1–5 drinks per wk | 132 (16.5) | 4 (11.8) | |
| 6–10 drinks per wk | 43 (5.4) | 2 (5.9) | |
| 10+ drinks per wk | 17 (2.1) | 0 (0) | |
| Physical examination | |||
| EF, % | 58.0 (53.0, 63.0) | 59.5 (55.0, 62.3) | 0.817 |
| NYHA, class III–IV, n (%) | 288 (36.0) | 13 (38.2) | 0.945 |
| Heart rate, bpm | 68.0 (60.0, 76.0) | 69.5 (60.0, 78.0) | 0.760 |
| SBP, mm Hg | 129.0 (118.0, 138.0) | 130.0 (122.0, 145.0) | 0.067 |
| DBP, mm Hg | 70.0 (62.0, 80.0) | 74.0 (65.5, 83.3) | 0.110 |
| eGFR, mL/min per 1.73 m2 | 63.3 (50.2, 77.1) | 61.3 (48.3, 72.1) | 0.253 |
| BNP, pg/mL | 241.0 (142.0, 439.5) | 375.0 (213.8, 610.3) | 0.006 |
| Comorbidities, n (%) | |||
| Previous hospitalization for CHF | 344 (43.1) | 17 (50) | 0.564 |
| Previous MI | 178 (22.3) | 12 (35.3) | 0.107 |
| Previous stroke | 81 (10.1) | 3 (8.8) | 0.966 |
| Previous CABG | 142 (17.8) | 8 (23.5) | 0.468 |
| Previous PCI | 169 (21.2) | 11 (32.4) | 0.161 |
| COPD | 123 (15.4) | 6 (17.6) | 0.808 |
| Hypertension | 693 (86.7) | 33 (97.1) | 0.328 |
| Peripheral artery disease | 88 (11.0) | 6 (17.6) | 0.415 |
| Dyslipidemia | 561 (70.2) | 28 (82.4) | 0.242 |
| AF | 309 (38.7) | 21 (61.8) | 0.013 |
| Diabetes | 320 (40.1) | 16 (47.1) | 0.546 |
| Medications, n (%) | |||
| Diuretic | 651 (81.5) | 29 (85.3) | 0.975 |
| ACEI | 379 (47.4) | 20 (58.8) | 0.290 |
| ARB | 217 (27.2) | 8 (23.5) | 0.540 |
| β‐blocker | 619 (77.5) | 28 (82.4) | 0.834 |
| Calcium channel blocker | 306 (38.3) | 13 (38.2) | 0.837 |
| Aspirin | 475 (59.4) | 24 (70.6) | 0.317 |
| Statin | 491 (61.5) | 26 (76.5) | 0.142 |
| Warfarin | 245 (30.7) | 17 (50.0) | 0.029 |
| Scores | |||
| CHADS2 | 3 (2, 3) | 3 (3, 3) | 0.087 |
| CHA2DS2‐VASc | 4 (4, 5) | 5 (2, 5) | 0.176 |
| R2CHADS2 | 4 (3, 5) | 3.5 (3, 5) | 0.348 |
The continuous variables are expressed as the mean with SD for the normally distributed data or median with interquartile range for the nonnormally distributed data. ACEI indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; CABG, coronary artery bypass grafting; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; EF, ejection fraction; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; and SBP, systolic blood pressure.
Baseline BNP Levels and Associated Risk of Stroke
Regression tree analysis revealed the cutoff point of BNP at 278 pg/mL in predicting stroke. This cutoff point was evaluated by Kaplan‐Meier analysis, which showed that patients with BNP>278 pg/mL experienced a higher risk of stroke (P<0.001) (Figure 1). The basic characteristics of subjects stratified by baseline BNP level are presented in Table S3. The patients in the lower BNP group (BNP≤278 pg/mL) were younger, more likely to be women, had higher body mass index, ejection fraction, and eGFR, and had lower prevalence of AF and diuretic administration, and lower β‐blocker administration than the higher BNP group (BNP>278 pg/mL). Baseline echocardiography with a small proportion of the enrolled patients is presented in Table S4.
Figure 1. Kaplan‐Meier analysis for stroke in high BNP>278 pg/mL and low BNP (BNP≤278 pg/mL) groups.
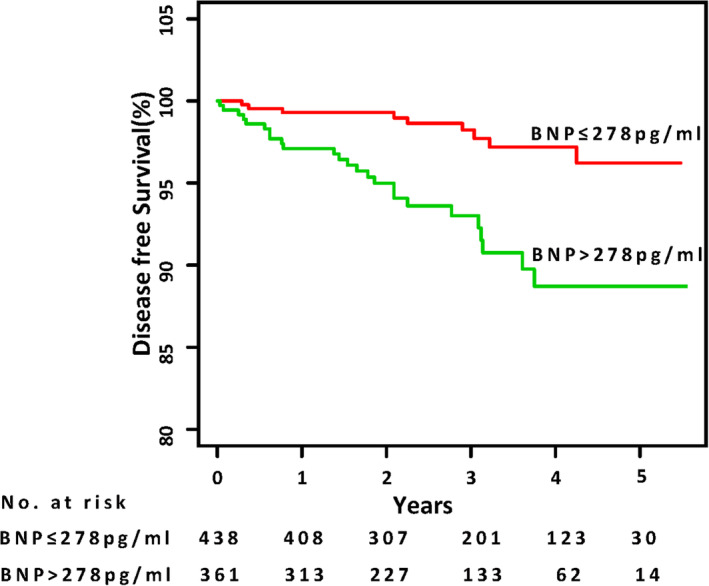
Stroke‐free survival was analyzed by a log‐rank test (P<0.001). BNP indicates B‐type natriuretic peptide.
The results of Cox regression showed that BNP levels >278 pg/mL were associated with an increased risk of stroke after adjusting for age, sex, previous stroke, diabetes, previous myocardial infarction, AF, smoking, spironolactone use, New York Heart Association class, warfarin, aspirin, and eGFR (hazard ratio [HR], 3.24 [95% CI, 1.49–7.06]) (Table 2).
Table 2.
Univariate and Multivariate Cox Regression Analysis of BNP Groups With Outcome of Stroke
| Sensitivity analyses | Cases (%) | Person‐years | Incidence rate, per 100 person‐years | Unadjusted HR (95% CI), P value | Adjusted HR (95% CI), P value |
|---|---|---|---|---|---|
| All included patients,* n=799 | 34 (4.26) | 2238.51 | 1.52 | ||
| BNP≤278 pg/mL, n=438 | 9 (2.05) | 1304.94 | 0.69 | Ref | Ref |
| BNP>278 pg/mL, n=361 | 25 (6.93) | 933.57 | 2.68 | 3.88 (1.81–8.31), 0.001¶ | 3.24 (1.49–7.06), 0.003¶ |
| Excluding patients with AF,† n=469 | 13 (2.77) | 1333.52 | 0.97 | ||
| BNP≤278 pg/mL, n=278 | 4 (1.44) | 829.49 | 0.48 | Ref | Ref |
| BNP>278 pg/mL, n=191 | 9 (4.71) | 441.03 | 2.04 | 3.64 (1.12–11.82), 0.032¶ | 3.42 (1.02–11.48), 0.047¶ |
| Excluding patients from Russia/Georgia,‡ n=695 | 31 (4.46) | 1987.47 | 1.56 | ||
| BNP≤278 pg/mL, n=373 | 8 | 1144.51 | 0.70 | Ref | Ref |
| BNP>278 pg/mL, n=322 | 23 | 842.96 | 2.73 | 3.93 (1.76–8.79), 0.001¶ | 3.28 (1.44–7.47), 0.005¶ |
| Excluding patients treated with warfarin,§ n=537 | 17 (3.17) | 1489.30 | 1.14 | ||
| BNP≤ 278 pg/mL, n=305 | 5 | 908.05 | 0.55 | Ref | Ref |
| BNP>278 pg/mL, n=232 | 12 | 581.25 | 2.06 | 3.67 (1.29–10.44), 0.015¶ | 3.42 (1.16–10.13), 0.026¶ |
| Propensity score matched‖ | |||||
| BNP≤278 pg/mL, n=305 | 6 | 929.21 | 0.65 | Ref | Ref |
| BNP>278 pg/mL, n=232 | 22 | 808.03 | 2.72 | 4.17 (1.69–10.29), 0.002¶ | 4.12 (1.66–10.24), 0.002¶ |
P≤0.05 is statistically significant. AF indicates atrial fibrillation; BNP, B‐type natriuretic peptide; HR, hazard ratio; and Ref, reference.
HR adjusted for age, sex, previous stroke, diabetes, previous myocardial infarction, AF, smoking, spironolactone using, New York Heart Association function class, warfarin, aspirin, estimated glomerular filtration rate.
HR adjusted for previous myocardial infarction, age, sex, previous stroke, diabetes, smoking, spironolactone using, New York Heart Association function class, warfarin, aspirin, estimated glomerular filtration rate.
HR adjusted for age, sex, previous stroke, diabetes, previous myocardial infarction, AF, smoking, spironolactone using, New York Heart Association function class, warfarin, aspirin, estimated glomerular filtration rate.
HR adjusted for age, sex, previous stroke, diabetes, previous myocardial infarction, AF, smoking, spironolactone using, New York Heart Association function class, aspirin, estimated glomerular filtration rate.
HR variables including age, sex, body mass index, ejection fraction, estimated glomerular filtration rate, history of AF; administration of diuretic and β‐blocker was included in the propensity score. Adjusted for previous stroke, diabetes, previous myocardial infarction, smoking, spironolactone, New York Heart Association function class, warfarin, aspirin.
P<0.05.
Discriminatory Capacity of Adding BNP to Existing Stroke Risk Scores
The discriminatory performances of BNP for predicting stroke in patients with HFpEF were expressed as the C index. A BNP level of >278 pg/mL showed a moderate performance for 5‐year stroke prediction (C index, 0.67 [95% CI, 0.60–0.74]), with a sensitivity of 0.81 and specificity of 0.56 (Table 3).
Table 3.
C Index, NRI, and IDI for Evaluating Improvement to Predict Stroke After Adding BNP to Scores of CHADS2, CHA2DS2‐VASc, and R2CHADS2 in Patients With Heart Failure With Preserved Ejection Fraction
| Clinical model | Clinical model+BNP | Clinical model vs clinical model+BNP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C index (95% CI) | Sensitivity, specificity | C index (95% CI) | Sensitivity, specificity | P value for C index | NRI % (95% CI) | P value for NRI | IDI % (95% CI) | P value for IDI | |
| BNP | 0.67 (0.60–0.74) | 0.81, 0.56 | |||||||
| CHADS2 | 0.67 (0.61–0.73) | 0.77, 0.51 | 0.77 (0.70–0.84) | 0.73, 0.73 | 0.004 | 40.9 (19.0–53.8) | <0.0001 | 3.0 (0.8–6.8) | <0.0001 |
| CHA2DS2‐VASc | 0.64 (0.55–0.72) | 0.79, 0.43 | 0.74 (0.67–0.81) | 0.75, 0.67 | 0.050 | 41.4 (22.7–56.0) | <0.0001 | 2.2 (0.7–7.0) | <0.0001 |
| R2CHADS2 | 0.70 (0.63–0.78) | 0.85, 0.41 | 0.78 (0.71–0.86) | 0.80, 0.65 | 0.029 | 40.9 (22.2–55.4) | <0.0001 | 3.20 (0.9–8.3) | <0.0001 |
BNP indicates B‐type natriuretic peptide; IDI, integrated discrimination improvement; and NRI, net reclassification improvement.
We assessed the predictive ability of BNP by adding a BNP level of >278 pg/mL as an additional parameter to existing stroke risk scores, including CHADS2, CHA2DS2‐VASc, and R2CHADS2 scores. In regard to 5‐year stroke risk, the addition of BNP to existing stroke risk scores significantly improved the discrimination assessed by C index, NRI, and IDI compared with that of the risk scores alone (CHADS2: C index, 0.67; BNP+CHADS2: C index, 0.77, P=0.004; NRI, 40.9%; IDI, 3.0%; CHA2DS2‐VASc: C index, 0.64; BNP+CHA2DS2‐VASc: C index, 0.74, P=0.050; NRI, 41.4%; IDI, 2.2%; R2CHADS2: C index, 0.70; BNP+ R2CHADS2: C index, 0.78, P=0.029; NRI, 40.9%; IDI, 3.2%) (Table 3, Figure 2).
Figure 2. Receiver operating characteristic curves of CHADS2/CHA2DS2‐VASc/R2CHADS2 score and BNP‐added CHADS2/CHA2DS2‐VASc/R2CHADS2 score for predicting stroke.
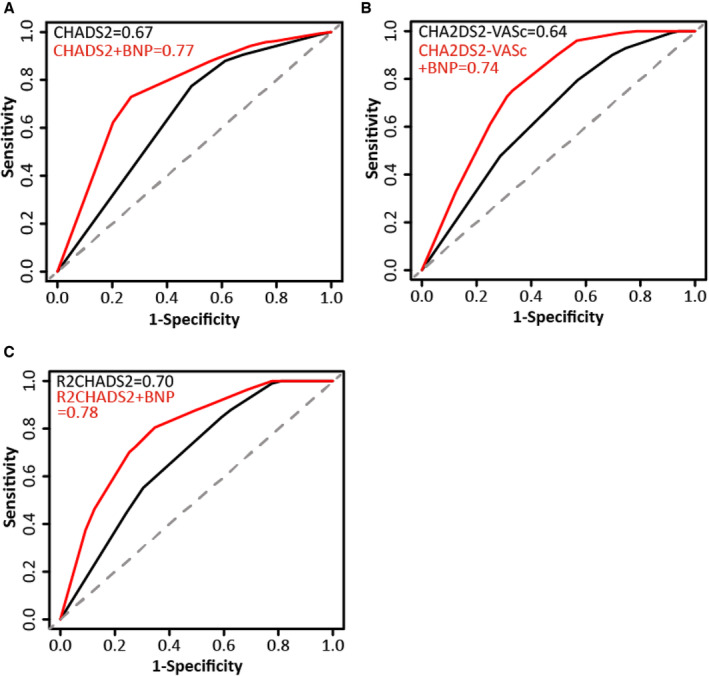
The BNP‐added CHADS2/CHA2DS2‐VASc/R2CHADS2 score was compared with CHADS2/CHA2DS2‐VASc/R2CHADS2 score for predicting future stroke by using the C index. The CHADS2,CHA2DS2‐VASc,R2CHADS2scores are simple clinical scores which were employed to stratify the risk of systemic thromboembolic complications in patients with atrial fibrillation. Sensitivity indicates the ability of a test to correctly identify patients with a disease. Specificity indicates the ability of a test to correctly identify people without the disease. BNP indicates B‐type natriuretic peptide.
Sensitivity Analysis
We performed a sensitivity analysis after excluding individuals from Russia/Georgia because of the regional variation in patients and outcomes in the TOPCAT trial. The results confirmed better discrimination of existing BNP‐added risk scores assessed by C index and the corresponding NRI and IDI values (Table S5). Sensitivity analysis performed by excluding patients treated with warfarin or those with AF showed similar results (Table S5). Sensitivity analyses by competing Cox generated confirmed results, with elevated BNP levels for stroke (HR, 3.77 [95% CI, 1.72–8.29]; P=0.001).
An additional sensitivity analysis using propensity score matching was further developed to verify the association between BNP and the risk of stroke in patients with HFpEF. A total of 626 individuals from 799 patients were identified, of which 313 were in each BNP group. The basic characteristics did not significantly differ among BNP groups after matching (Table S6). The risk of stroke was significantly higher in propensity score–matched patients with BNP levels >278 pg/mL (HR, 4.12 [95% CI, 1.66–10.24]) (Table 2).
DISCUSSION
Based on a secondary analysis from the TOPCAT trial, we showed that (1) high BNP was associated with an increased risk of stroke in patients with HFpEF; (2) addition of BNP to CHADS2/CHA2DS2‐VASc/R2CHADS2 scores significantly improved the discrimination for stroke prediction in HFpEF; and (3) sensitivity analyses by excluding patients with AF at baseline, those in Russia/Georgia, and those being treated with warfarin generated confirmatory results. Overall, our findings first suggest that BNP could predict the risk of stroke in patients with HFpEF.
Stroke is one of the devastating adverse events in HF and contributes to high mortality and morbidity. As the common cause of stroke among patients with HF, AF has been studied for several decades. 22 However, pooled analysis of Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM)‐Preserved and Irbesartan in Heart Failure With Preserved Systolic Function trials showed that in patients with HFpEF without AF, the stroke incidence (1.0% per year) was similar to the rate in HF with reduced ejection fraction without AF (1.2% per year). 4 Moreover, Cogswell et al found a high prevalence of subclinical cerebral infarcts in the HFpEF population with no prior AF diagnosis compared with those with AF (29.3% versus 23.5%, respectively). 23 Catheter ablation of AF in a randomized controlled clinical trial also failed to reduce the risk of stroke. 10 Overall, AF cannot directly explain stroke risk in HFpEF, and HFpEF itself might be a substrate for stroke incidents. 10 Recent studies have suggested the development of left atrial (LA) myopathy (as reflected by LA geometry, LA mechanical function, or LA fibrosis) is central to the pathogenesis of HFpEF. 10 Furthermore, accumulating evidence showed LA myopathy was a direct causal mechanism contributing to thromboembolic stroke independent of the presence or control of AF. 24 , 25 Our results supported this hypothesis. A prospective study by Patel et al 26 showed LA myopathy, indicated by LA reservoir strain abnormality, was associated with decreased stroke volume and poor left ventricle diastolic function, independently of AF status, and eventually contributed to elevated BNP. Therefore, BNP levels might be an indicator for LA myopathy, even in HFpEF without AF, and thus might be an early biomarker that can predict stroke, regardless of AF.
Several risk tools were tested for their predictive ability for stroke in patients with AF. We previously showed that CHA2DS2‐VASc (C statistic, 0.62) scores did not achieve an excellent predictive ability for stroke, regardless of AF status. 9 , 20 Abdul‐Rahim and coworkers developed a complicated model for stroke in patients with HFpEF without AF, with a higher performance from the CHARM‐Preserved (C statistic, 0.71) and I‐Preserve (C statistic, 0.73) trials; furthermore, the model worked exceptionally well with an external validation cohort of TOPCAT (C statistic, 0.73). 4 These results suggested the generality of risk scores across different baseline characteristics across the population. Notably, none of their models included the BNP level. 4 Our results showed that the predictive ability of BNP significantly improved the predictive power of established stroke risk scores, even in the absence of AF. However, our results should be considered exploratory and should be validated in more populations with HFpEF, especially in patients without AF.
In patients with AF, NT‐proBNP is independently associated with an increased risk of stroke. Our sensitivity analysis showed that among those with HFpEF without AF history, the addition of BNP to existing stroke risk scores still significantly improved the C statistic, which suggested BNP's predictive ability for stroke in HFpEF independent of AF history. Nevertheless, we should also point out that the comorbidities analyzed in our study were restricted to baseline characteristics, and the occurrence of AF during the follow‐up might have also largely contributed to the incidence of stroke; therefore, whether BNP predicts stroke independently of AF needs to be further validated. On the other hand, circulating BNP levels are largely influenced by renal function. 27 , 28 Therefore, we adjusted the Cox regression for eGFR, and the result is still significant. Although our result showed a statistically significant association between BNP and the risk of stroke, the predictive ability of BNP in patients with HFpEF with and without kidney dysfunction should also be evaluated in future research.
Besides cardiomyocytes, BNP also could be released from the hypothalamus in response to acute cerebral ischemia. 29 , 30 However, the TOPCAT trial excluded patients with stroke in the past 90 days 18 ; thus, the increased BNP level secreted from the hypothalamus was limited in our present study.
The most recent HF guidelines stated that anticoagulation should be considered for patients with HF and AF, if eligible, as assessed by the CHA2DS2‐VASc score. However, they did specify types of HF. Therefore, although CHA2DS2‐VASc is mainly based on HF with reduced ejection fraction, and criteria for anticoagulation for AF and HFpEF are in reality lacking, we fully agree with the opinion raised by Mulder et al that anticoagulation must be seriously considered in many patients with AF and HFpEF. 31 The benefit of anticoagulation therapy based on BNP's addition to CHA2DS2‐VASc in HFpEF and AF may be further studied. For HF without AF, a recent Cochrane systematic review by Lip et al does not support the routine use of oral anticoagulation therapy in patients with chronic HF with no AF because of the benefit‐to‐risk imbalance. 32 Therefore, it is still uncertain whether patients with HFpEF in sinus rhythm are at a particularly high risk of stroke benefit from the use of anticoagulants. New risk scores, such as combined BNP and other clinical risk factors, are needed to guide the use of anticoagulation therapy in HF without AF.
Interestingly, NT‐proBNP was one of the components of the ABC (age, biomarkers, clinical history) stroke risk score, a biomarker‐based risk score for predicting stroke in AF, which somewhat supports our finding. In addition to BNP, other biomarkers, such as inflammation and fibrotic myopathy, have also been considered effective predictors of stroke. 33 For example, plasma high‐sensitivity cardiac troponin levels, another component of the ABC risk score, has also been validated as a component for predicting stroke. Plasma high‐sensitivity cardiac troponin levels are a well‐reported prognostic marker in patients with cardiovascular diseases, including HFpEF. 34 , 35 However, there are limited studies examining the predictive ability of plasma high‐sensitivity cardiac troponin levels and stroke in patients with HFpEF, which might be studied in subsequent research.
Strengths and Limitations
Our study has several strengths. To our knowledge, this was the first study to evaluate the performance of BNP to predict the occurrence of stroke in patients with HFpEF.
Nevertheless, several limitations should be acknowledged. First, this is a post hoc analysis based on the TOPCAT data set, and measured and unmeasured covariates might have potentially influenced the results. Second, the specified subtypes of stroke, such as ischemic stroke and embolic stroke, were not analyzed in the study because of the limited number of stroke events. Third, a history of transient ischemic attack and thromboembolic events was not available in the TOPCAT; thus, whether the predictive ability of BNP is independent of a history of transient ischemic attack and thromboembolic events remains unclear. Fourth, because of availability of BNP values, our analysis was restricted to a minority of TOPCAT participants, and there was a significant difference between some characteristics of included and excluded participants. However, the average incidence rate of stroke was 1.5 per 100 patient‐years in the present study, which is similar to the patients with HFpEF in the overall TOPCAT trial and the CHARM‐Preserved and I‐Preserve trials. Thus, our cohort may be representative of general HFpEF in the association between BNP and stroke. Fifth, NT‐proBNP was not examined because of data restrictions (limited stroke cases). A previous meta‐analysis showed a comparable diagnostic performance between BNP and NT‐proBNP for cardioembolic stroke 36 ; however, the predictive ability of NT‐proBNP for stroke should be studied in future studies.
CONCLUSIONS
BNP is associated with an increased risk of stroke in patients with HFpEF and may be a valuable biomarker for stroke prediction in patients with HFpEF. However, our findings need to be further confirmed, and the specific threshold should be prospectively reexamined.
Sources of Funding
This work was supported in part by the National Natural Science Foundation of China (J.W., 82070237; Y.Z., 81870170; Y.C., 81970200; Y.C., 81770229; X.L., 82100347), Natural Science Foundation of Guangdong Province (Y.Z., 2019A1515011682; X.L., 2022A1515010582), China Postdoctoral Science Foundation (X.L., 2021M703724), and National High Technology Research and Development Program of Guangzhou (J.W., 20180304001; J.W., 2019GZR110406004; Y.Z., 201704020044). None of the funding institutions had a role in design, methods, subject recruitment, data collections, analysis, and preparation of the article.
Disclosures
None.
Supporting information
Tables S1–S6
Presented in part at the Great Wall International Congress of Cardiology 2021 and the Asian Heart Society Congress 2021 in Beijing, China from October 29–31, 2021, and published in abstract form [Cardiovascular Innovations and Applications. 6;C85 or https://doi.org/10.15212/CVIA.2021.0030].
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024302
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Yuling Zhang, Email: zhyul@mail.sysu.edu.cn.
Jingfeng Wang, Email: wjingf@mail.sysu.edu.cn.
References
- 1. Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, Deswal A, Dickson VV, Kosiborod MN, Lekavich CL. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140:e294–e324. doi: 10.1161/CIR.0000000000000691 [DOI] [PubMed] [Google Scholar]
- 2. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 3. Uhm JS, Kim J, Yu HT, Kim TH, Lee SR, Cha MJ, Choi EK, Lee JM, Kim JB, Park J, et al. Stroke and systemic embolism in patients with atrial fibrillation and heart failure according to heart failure type. ESC Heart Fail. 2021;8:1582–1589. doi: 10.1002/ehf2.13264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdul‐Rahim AH, Perez A‐C, MacIsaac RL, Jhund PS, Claggett BL, Carson PE, Komajda M, McKelvie RS, Zile MR, Swedberg K. Risk of stroke in chronic heart failure patients with preserved ejection fraction, but without atrial fibrillation: analysis of the CHARM‐Preserved and I‐Preserve trials. Eur Heart J. 2017;38:742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kotecha D, Chudasama R, Lane DA, Kirchhof P, Lip GY. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: a systematic review and meta‐analysis of death and adverse outcomes. Int J Cardiol. 2016;203:660–666. doi: 10.1016/j.ijcard.2015.10.220 [DOI] [PubMed] [Google Scholar]
- 6. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 7. Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community‐based study. Circulation. 2013;128:1085–1093. doi: 10.1161/CIRCULATIONAHA.113.001475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy YN, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. 2020;76:1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu W, Wu Y, Zhou Y, Liang W, Xue R, Wu Z, Dong Y, Liu C. CHA2DS2‐VASc and ATRIA Scores and clinical outcomes in patients with Heart failure with preserved ejection fraction. Cardiovasc Drugs Ther. 2020;34:763–772. doi: 10.1007/s10557-020-07011-y [DOI] [PubMed] [Google Scholar]
- 10. Packer M. HFpEF is the substrate for stroke in obesity and diabetes independent of atrial fibrillation. JACC Heart Fail. 2020;8:35–42. [DOI] [PubMed] [Google Scholar]
- 11. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, et al. Heart Failure association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21:715–731. doi: 10.1002/ejhf.1494 [DOI] [PubMed] [Google Scholar]
- 12. McCullough PA, Nowak RM, McCord J, Hollander JE, Herrmann HC, Steg PG, Duc P, Westheim A, Omland T, Knudsen CW. B‐type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106:416–422. doi: 10.1161/01.CIR.0000025242.79963.4C [DOI] [PubMed] [Google Scholar]
- 13. Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, Desai AS, O'Meara E, Fleg JL, Pfeffer MA, et al. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Fail. 2017;5:241–252. doi: 10.1016/j.jchf.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 14. van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B‐type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044 [DOI] [PubMed] [Google Scholar]
- 15. Everett BM, Berger JS, Manson JE, Ridker PM, Cook NR. B‐type natriuretic peptides improve cardiovascular disease risk prediction in a cohort of women. J Am Coll Cardiol. 2014;64:1789–1797. doi: 10.1016/j.jacc.2014.04.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, Huxley RR, Ballantyne CM. Troponin T, N‐terminal pro–B‐type natriuretic peptide, and incidence of stroke: the atherosclerosis risk in communities study. Stroke. 2013;44:961–967. doi: 10.1161/STROKEAHA.111.000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hotsuki Y, Sato Y, Yoshihisa A, Watanabe K, Kimishima Y, Kiko T, Yokokawa T, Abe S, Misaka T, Sato T, et al. B‐type natriuretic peptide is associated with post‐discharge stroke in hospitalized patients with heart failure. ESC Heart Fail. 2020;7:2508–2515. doi: 10.1002/ehf2.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162(966–972):e10. doi: 10.1016/j.ahj.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 19. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 20. Wu Y, Xie Z, Liang W, Xue R, Wu Z, Wu D, He J, Zhu W, Liu C. Usefulness of CHADS2, R2CHADS2, and CHA2DS2‐VASc scores for predicting incident atrial fibrillation in heart failure with preserved ejection fraction patients. ESC Heart Fail. 2021;8:1369–1377. doi: 10.1002/ehf2.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 22. Haeusler KG, Laufs U, Endres M. Chronic heart failure and ischemic stroke. Stroke. 2011;42:2977–2982. doi: 10.1161/STROKEAHA.111.628479 [DOI] [PubMed] [Google Scholar]
- 23. Cogswell RJ, Norby FL, Gottesman RF, Chen LY, Solomon S, Shah A, Alonso A. High prevalence of subclinical cerebral infarction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2017;19:1303–1309. doi: 10.1002/ejhf.812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, Miller C, Qi D, Ziegler PD. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ: Arrhythm Electrophysiol. 2009;2:474–480. [DOI] [PubMed] [Google Scholar]
- 25. Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJ, Morton JB, Sanders P, Kalman JM. Long‐term effects of catheter ablation for lone atrial fibrillation: progressive atrial electroanatomic substrate remodeling despite successful ablation. Heart Rhythm. 2012;9:473–480. doi: 10.1016/j.hrthm.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 26. Patel RB, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink‐Nelson L, Tromp J, Sanchez C, Njoroge J, et al. Disproportionate left atrial myopathy in heart failure with preserved ejection fraction among participants of the PROMIS‐HFpEF study. Sci Rep. 2021;11:4885. doi: 10.1038/s41598-021-84133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okamoto R, Ali Y, Hashizume R, Suzuki N, Ito M. BNP as a major player in the heart‐kidney connection. Int J Mol Sci. 2019;20:3581. doi: 10.3390/ijms20143581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takase H, Dohi Y. Kidney function crucially affects B‐type natriuretic peptide (BNP), N‐terminal proBNP and their relationship. Eur J Clin Invest. 2014;44:303–308. doi: 10.1111/eci.12234 [DOI] [PubMed] [Google Scholar]
- 29. Menon B, Ramalingam K, Conjeevaram J, Munisusmitha K. Role of brain natriuretic peptide as a novel prognostic biomarker in acute ischemic stroke. Anna Indian Acad Neurol. 2016;19:462. doi: 10.4103/0972-2327.194422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi K, Totsune K, Sone M, Ohneda M, Murakami O, Itoi K, Mouri T. Human brain natriuretic peptide‐like immunoreactivity in human brain. Peptides. 1992;13:121–123. doi: 10.1016/0196-9781(92)90149-W [DOI] [PubMed] [Google Scholar]
- 31. Mulder BA, van Veldhuisen DJ, Rienstra M. What should the C (‘congestive heart failure’) represent in the CHA2DS2‐VASc score? Eur J Heart Fail. 2020;22:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lip GY, Wrigley BJ, Pisters R. Anticoagulation versus placebo for heart failure in sinus rhythm. Cochrane Database Syst Revs. 2012;(6):Cd003336. [DOI] [PubMed] [Google Scholar]
- 33. Mesquita T, Lin YN, Ibrahim A. Chronic low‐grade inflammation in heart failure with preserved ejection fraction. Aging Cell. 2021;20:e13453. doi: 10.1111/acel.13453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki S, Motoki H, Minamisawa M, Okuma Y, Shoin W, Okano T, Kimura K, Ebisawa S, Okada A, Kuwahara K. Prognostic significance of high‐sensitivity cardiac troponin in patients with heart failure with preserved ejection fraction. Heart and vessels. 2019;34:1650–1656. doi: 10.1111/acel.13453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Myhre PL, O'Meara E, Claggett BL, de Denus S, Jarolim P, Anand IS, Beldhuis IE, Fleg JL, Lewis E, Pitt B. Cardiac troponin I and risk of cardiac events in patients with heart failure and preserved ejection fraction. Circ: Heart Fail. 2018;11:e005312. doi: 10.1161/CIRCHEARTFAILURE.118.005312 [DOI] [PubMed] [Google Scholar]
- 36. Bai J, Sun H, Xie L, Zhu Y, Feng Y. Detection of cardioembolic stroke with B‐type natriuretic peptide or N‐terminal pro‐BNP: a comparative diagnostic meta‐analysis. Int J Neurosci. 2018;128:1100–1108. doi: 10.1080/00207454.2017.1408612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6


