Abstract
Background
Models predicting atrial fibrillation (AF) risk, such as Cohorts for Heart and Aging Research in Genomic Epidemiology AF (CHARGE‐AF), have not performed as well in electronic health records. Natural language processing (NLP) may improve models by using narrative electronic health record text.
Methods and Results
From a primary care network, we included patients aged ≥65 years with visits between 2003 and 2013 in development (n=32 960) and internal validation cohorts (n=13 992). An external validation cohort from a separate network from 2015 to 2020 included 39 051 patients. Model features were defined using electronic health record codified data and narrative data with NLP. We developed 2 models to predict 5‐year AF incidence using (1) codified+NLP data and (2) codified data only and evaluated model performance. The analysis included 2839 incident AF cases in the development cohort and 1057 and 2226 cases in internal and external validation cohorts, respectively. The C‐statistic was greater (P<0.001) in codified+NLP model (0.744 [95% CI, 0.735–0.753]) compared with codified‐only (0.730 [95% CI, 0.720–0.739]) in the development cohort. In internal validation, the C‐statistic of codified+NLP was modestly higher (0.735 [95% CI, 0.720–0.749]) compared with codified‐only (0.729 [95% CI, 0.715–0.744]; P=0.06) and CHARGE‐AF (0.717 [95% CI, 0.703–0.731]; P=0.002). Codified+NLP and codified‐only were well calibrated, whereas CHARGE‐AF underestimated AF risk. In external validation, the C‐statistic of codified+NLP (0.750 [95% CI, 0.740–0.760]) remained higher (P<0.001) than codified‐only (0.738 [95% CI, 0.727–0.748]) and CHARGE‐AF (0.735 [95% CI, 0.725–0.746]).
Conclusions
Estimation of 5‐year risk of AF can be modestly improved using NLP to incorporate narrative electronic health record data.
Keywords: atrial fibrillation, natural language processing, predicted risk
Subject Categories: Atrial Fibrillation, Risk Factors
Nonstandard Abbreviations and Acronyms
- CHARGE‐AF
Cohorts for Heart and Aging Research in Genomic Epidemiology Atrial Fibrillation
- NRI
net reclassification improvement
Clinical Perspective.
What Is New?
We derived and validated 2 new models to predict the incidence of atrial fibrillation: one model that used only codified data and a second model that added information from narrative data using natural language processing.
In internal and external validation, we found that estimation of the 5‐year risk of atrial fibrillation in a primary care population can be modestly improved by using natural language processing to incorporate narrative electronic health record data.
Both newly developed models (codified data only and codified plus natural language processing) demonstrated improved predictive utility compared with an established atrial fibrillation prediction model (Cohorts for Heart and Aging Research in Genomic Epidemiology Atrial Fibrillation).
What Are the Clinical Implications?
The amount of data available in unstructured form within electronic health records is immense, and optimizing the use of natural language processing to meaningfully process this data offers an opportunity to improve prognostic models used within clinical care.
Atrial fibrillation (AF) is a common arrhythmia in aging populations, 1 , 2 is a potent risk factor for ischemic stroke, 3 , 4 , 5 and is often first identified at the time of stroke. 6 , 7 Oral anticoagulation therapy is highly efficacious in preventing a large proportion of AF‐related strokes. 8 , 9 , 10 , 11 , 12 Screening for undiagnosed AF is a growing priority as it may enable earlier diagnosis of AF and implementation of oral anticoagulation therapy to prevent strokes.
Novel point‐of‐care technologies used in health care settings or at home, including wearable technology and mobile single‐lead ECGs, make mass screening for undiagnosed AF feasible. Prior randomized studies assessing the impact of AF screening interventions in subjects aged ≥65 years have been mixed 13 , 14 , 15 , 16 ; however, the effectiveness of these studies may have been limited by screening solely based on age, which may include many subjects with low short‐term risk of developing AF. 16 , 17 Using individual patient risk of AF can effectively allocate screening resources to those most likely to benefit. 18
A widely used model to predict AF, the Cohorts for Heart and Aging Research in Genomic Epidemiology AF (CHARGE‐AF) score, 19 was developed and validated in research cohorts with rigorously collected and uniformly formatted data and has demonstrated weaker performance in health care–related data sets. 20 , 21 AF risk stratification may be most often used in clinical settings that include subjects with more comorbidities than those included in research cohorts. Developing and validating an AF risk prediction model within a clinical setting using features available in electronic health records (EHRs) may improve model performance in these settings. Much of the data in EHRs exist as free‐form typed narrative text within provider notes or reports. Natural language processing (NLP) represents a range of computational techniques for processing text that can be used to extract medical concepts for analyses. Incorporating NLP in determination of risk factors within EHRs has several advantages and has resulted in improved risk prediction. 22 , 23 NLP provides data that may be missing from codified data (ie, entered in a structured format, such as International Classification of Diseases [ICD], Tenth Revision diagnosis codes), for example, information extracted from cardiology reports about left ventricular hypertrophy. 24 , 25 , 26
In this study, we developed and evaluated 2 models to identify patients at increased risk for AF in primary care patients at Massachusetts General Hospital. One model used codified and NLP data (codified+NLP), whereas one used codified data only (codified‐only). It is not known if incorporating NLP data to ascertain risk factors within EHRs will result in an improved AF risk prediction model. As such, we compared performance of newly developed models with and without NLP and compared each to an existing and widely used AF risk model (CHARGE‐AF) in both internal and external validation populations. 27
Methods
Mass General Brigham data contain protected health information and cannot be shared publicly. The data processing scripts used to perform analyses will be made available to interested researchers on reasonable request to the corresponding author.
Study Sample
Model Development and Internal Validation
The study cohort for model development and internal validation consisted of patients from the Primary Care Practice‐Based Research Network at Massachusetts General Hospital, identified using a validated attribution algorithm. 28 , 29 All 18 practices in the network use EHRs and share the same data systems. Individuals included were aged ≥65 years, with primary care visits between 2003 and 2013. Individuals were excluded if they had diagnosed prevalent AF with no study follow‐up before the AF diagnosis. Individuals aged <65 years were excluded because AF prevalence is strongly associated with age, and these individuals are less likely to benefit from oral anticoagulation or be targeted in an AF screening program. The eligible cohort was randomly split into 2 subsets: two thirds for model development and one third for internal validation.
External Validation
The external validation cohort consisted of patients aged ≥65 years from Brigham and Women's Hospital with primary care visits between 2015 and 2020 and without prevalent AF. Brigham and Women's 15 practices use EHRs, and data are accessed from the Mass General Brigham Research Patient Data Registry, a data warehouse containing data from 7 affiliated hospitals, including Massachusetts General Hospital. 30 This medical records–based study was approved with a waiver of informed consent by the local Mass General Brigham Institutional Review Board.
Ascertainment of Potential Features/Variables for the Model
Data extracted from the EHR included the following: (1) patient demographics, (2) diagnostic codes (International Classification of Diseases, Ninth Revision [ICD‐9], and International Classification of Diseases, Tenth Revision [ICD‐10]), (3) procedure codes (Current Procedural Terminology), (4) medications, (5) cardiology test reports, (6) progress notes, (7) visit notes, (8) history and physical notes, (9) laboratory notes, (10) discharge summaries, and (11) vital status. We assembled a list of potential predictors of AF incidence based on a review of prior studies. The full list of potential model features is available in Table 1, as well as the source of information (eg, codified EHR data versus data extracted using NLP). Although height, weight, and blood pressure are included in an existing AF prediction model, these were not considered as features for new models because of missing data.
Table 1.
List of Clinical Features Considered in Codified‐Only and Codified+NLP models to Predict Incident AF
| Variable | Codified | NLP |
|---|---|---|
| Age | X | |
| Sex | X | |
| Race and ethnicity | X | |
| Insurance | X | |
| Obesity | X | X |
| Current smoker | X | |
| Left ventricular hypertrophy | X | |
| Left atrial enlargement | X | |
| Mitral valve disease | X | X |
| Mitral valve prolapse | X | X |
| Mitral insufficiency | X | X |
| Mitral stenosis | X | X |
| Supraventricular tachycardia | X | X |
| Premature atrial contractions | X | X |
| Myocardial infarction | X | X |
| Chronic kidney disease | X | X |
| Chronic kidney disease: severe | X | X |
| Hyperlipidemia | X | X |
| Valvular disease | X | X |
| Prior stroke/transient ischemic attack | X | X |
| Systemic atherosclerosis | X | X |
| Cerebral atherosclerosis | X | X |
| Thyrotoxicosis | X | X |
| Hypothyroidism | X | X |
| Pulmonary disease | X | X |
| Chronic obstructive pulmonary disease | X | |
| Congenital heart disease | X | X |
| Cardiomegaly | X | X |
| Alcohol disorder | X | X |
| Pericarditis | X | X |
| Myocarditis | X | X |
| Sleep apnea | X | X |
| Prior cardiac surgery | X | |
| Hypertrophic cardiomyopathy | X | X |
| Other cardiomyopathy | X | X |
| Chronic liver disease | X | X |
| Cirrhosis | X | X |
| Liver complications | X | X |
| Diabetes | X | X |
| Hypertension | X | X |
| Congestive heart failure | X | X |
| Coronary artery disease | X | X |
| Peripheral vascular disease | X | X |
| Cerebrovascular disease | X | X |
| Long PR interval | X | |
| Shortened PR interval | X | |
| CRP | X | |
| NT‐proBNP | X |
AF indicates atrial fibrillation; CRP, C‐reactive protein; NLP, natural language processing; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Clinical Characteristics
Patient characteristics and comorbidities were ascertained using EHR data from the 3 years before each patient's first visit during the study period. Age, sex, and race and ethnicity were ascertained at the time of cohort entry. Height and weight recorded closest to cohort entry were obtained. Medication use was assessed on the basis of any medications listed in the EHR in the 3 years before cohort entry. Laboratory values were assessed within the 1 year before cohort entry. Current smoking status was assessed using the most recent smoking status update in the EHR before cohort entry. PR interval was extracted from ECGs and classified as shortened (<120 milliseconds), normal (120–200 milliseconds), or prolonged (>200 milliseconds). 31 We used patient home zip code to link to the National Neighborhood Data Archive and ascertain the percentage of the population within a zip code tabulation area with household income of <$50 000. 32
Features/Variables Defined Using Codified EHR Data
We used validated EHR algorithms to define the following variables: obesity, diabetes, hypertension, congestive heart failure, coronary artery disease, peripheral vascular disease, and cerebrovascular disease (Table S1). 33 , 34 , 35 , 36 Comorbidities without a validated algorithm were identified by a single ICD‐9/ICD‐10 code before and within 3 years of cohort entry. ICD‐9/ICD‐10 codes used are available in Table S2. We defined all components of the CHARGE‐AF model using codified data (Table S3).
Features/Variables From the Narrative EHR Data Extracted Using NLP
All potential features were also ascertained using NLP, unless fully populated within structured fields (eg, demographics) or if the positive predictive value of the NLP‐derived variable was low (Table 1). Health care provider progress notes, visit notes, history and physical notes, discharge summaries, laboratory notes, and cardiology test reports were processed to extract information from narrative data using a published approach 24 using Narrative Information Linear Extraction, an NLP package for EHR analysis (Figure 1). 37 Briefly, we created a dictionary of terms corresponding to potential model features (Table S4). The list of terms was mapped to concepts in the Unified Medical Language System. 38 For example, the terms “atrial fibrillation” and “auricular fibrillation” are different ways of expressing the same concept and are assigned a concept unique identifier (C0004238). We then processed free‐text clinical notes using NLP to count the number of positive mentions of each concept unique identifier, while disregarding negative mentions, such as “no evidence of ….” Patients with at least 1 positive concept unique identifier match before cohort entry were considered to have the feature at baseline. Medical record review of 100 randomly selected patients was performed by author J.M.A. for NLP‐defined variables with >10% absolute increase compared with expected published prevalences (current smoking, myocardial infarction, mitral valve disease, mitral insufficiency, mitral valve prolapse, and mitral stenosis). 39 , 40 , 41 , 42 , 43 Following this review, specific acronyms to be excluded and negation terms to be added were identified. We did not consider the NLP‐defined variable for model inclusion if the positive predictive value on medical record review was <60%. For all features identified via NLP, we randomly selected 20 patients per feature with positive concept unique identifier mentions for medical record review performed by J.M.A. No additional systematic problems were identified following this review.
Figure 1. Overview of the process of extracting and processing codified and narrative electronic health record (EHR) data to determine patient‐level predictors of atrial fibrillation.
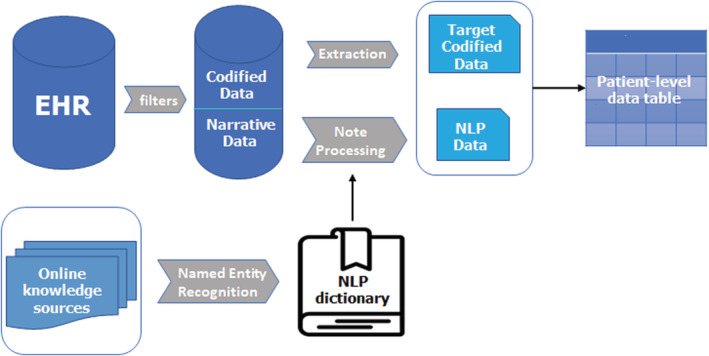
NLP indicates natural language processing.
Outcomes
The primary outcome was incident AF within 5 years of entry into the study cohort. Incident AF status was ascertained using a previously validated EHR algorithm, which used problem list entries and inpatient or outpatient ICD‐9/ICD‐10 codes (positive predictive value, 96.3%). 44 Cases included in analyses occurred between 2003 and 2018 for the development and internal validation cohorts, and between 2015 and 2020 for the external validation cohort.
Model Derivation
In the development cohort, we developed 2 Cox proportional hazards models to predict AF incidence within 5 years using the following: (1) codified+NLP data (codified+NLP) and (2) codified data only (codified‐only). Censoring occurred at the time of death, last primary care visit if leaving the primary care cohort, or after 5 years of follow‐up. For variable selection in each model, we considered all potential model features in Table 1 and ran 10‐fold cross‐validated Cox proportional hazards models using a least absolute shrinkage and selection operator penalty. 45 The largest tuning parameter (λ) was selected such that error was within 1 SD of the minimum cross‐validated error. 46 As shown in Table 1, most features were defined by both codified and NLP data. For the codified+NLP model, the least absolute shrinkage and selection operator selection process was allowed to select the codified version of a feature, select the NLP version of a feature, or select both the codified and NLP version of the feature. If both the codified and NLP data version of a feature were selected, we recoded it into a single 4‐level variable (0 indicates neither codified or NLP [feature not present]; 1, feature defined by codified data only; 2, feature defined by NLP data only; and 3, feature defined by both codified and NLP data). For example, both the codified and NLP versions of mitral valve disorder were selected in the codified+NLP model. In this model, there is a single variable representing both variables (0 indicates no mitral valve disorder by either codified version or NLP; 1, mitral valve disorder present in codified data, but not NLP; 2, mitral valve disorder present in NLP data, but not codified; and 3, mitral valve disorder present in both codified and NLP data). The proportional hazards assumption was verified graphically by examining the log of the minus log of the survival curve for each predictor. 47 Within each model, individual 5‐year risk of AF was calculated.
Statistical Analysis
In validation cohorts, we used the coefficients from the models established in the development cohort to estimate predicted 5‐year risks and used the product‐limit method, which accounts for censoring, to calculate observed 5‐year risks. In the external validation cohort, administrative censoring occurred at the end of follow‐up in 2020. Because many participants in the external validation cohort were not followed up for 5 years, we also evaluated models over a 3‐year period in supplementary analyses. For descriptive data, we calculated mean and SDs of numbers and percentages. In the validation data sets, we compared performance of the codified+NLP, codified‐only, and CHARGE‐AF models. To calculate the CHARGE‐AF score, we used the published components and coefficients (Table S3). 19 We compared hazard ratios (HRs) across groups, determined by the Cox method (based on the 16th, 50th, and 84th percentiles of the linear predictor values for each model). 48 In a normal distribution, the 84th percentile corresponds to the mean of the linear predictor +1 SD, whereas the 16th percentile corresponds to the mean of the linear predictor −1 SD. 48 We calculated the Harrell C‐statistic to assess the model's ability to separate who developed AF from those who did not. We also plotted cumulative incidence curves for risk groups. We compared the Harrell C‐statistic between models with bias correction using 200 bootstrap samples. In addition to our main models, which included race as a predictor, we also assessed performance of models that included insurance and zip code–defined income instead of race in supplemental analyses. We assessed calibration by comparing the predicted and observed 5‐year AF risks from each model with patients divided into risk groups based on deciles. To provide quantified summary measures of model calibration, we calculated the Integrated Calibration Index, which represents the weighted average absolute difference between observed and predicted probabilities. 49 For CHARGE‐AF, we also assessed calibration after recalibration. To recalibrate, instead of using the published CHARGE‐AF baseline survival when calculating predicted risk (published survival, 0.9718412736), we generated an updated baseline survival by calculating the average 5‐year AF‐free survival in the validation sample (updated baseline survival, 0.9331399). 50 To quantify how well each model reclassified subjects (codified+NLP compared with codified‐only, and each compared with CHARGE‐AF), we evaluated net reclassification improvement (NRI) for time‐to‐event data with censoring 51 , 52 using percentile‐based cut points, which allows for generalizability of the NRI when standard risk cut points are not available. 53 Percentile‐based cut points were based on the 16th, 50th, and 84th percentiles for each model in the validation data. 48 Because the NRI may be sensitive to the cut points chosen, we also evaluated the NRI with 4 groups based on quartiles (25th, 50th, and 75th percentiles). We considered a 2‐sided P<0.05 to indicate statistical significance.
Results
The development cohort included 32 960 patients aged ≥65 years without a diagnosis of AF at baseline. The mean age was 70.3 (SD, 6.9) years, 57.4% were women, and 85.3% were non‐Hispanic White race and ethnicity. The internal validation cohort included 16 233 patients meeting eligibility criteria, with 2311 patients missing data to calculate CHARGE‐AF, resulting in a population of 13 992 patients. The mean age was 69.5 (SD, 6.2) years, 57.5% were women, and 86.0% were non‐Hispanic White race and ethnicity (Table 2). The external validation cohort included 42 234 patients meeting eligibility criteria, with 3183 patients missing data to calculate CHARGE‐AF, resulting in a population of 39 051 patients (mean age, 71.5 years). Additional baseline patient characteristics for this cohort are included in Table S5.
Table 2.
Baseline Patient Characteristics for Development and Internal Validation Cohorts
| Characteristic | Development (n=32 960) | Internal validation (n=13 992) | External validation (n=39 051)* |
|---|---|---|---|
| Age, mean (SD), y | 70.3 (6.9) | 69.5 (6.2) | 71.5 (6.8) |
| Aged 65–75 | 25 233 (76.6) | 11 325 (80.9) | 29 153 (74.7) |
| Aged 75–85 y | 6200 (18.8) | 2252 (16.1) | 7680 (19.7) |
| Aged ≥85 | 1527 (4.6) | 415 (3.0) | 2218 (5.7) |
| Female sex | 18 910 (57.4) | 8041 (47.5) | 24 391 (62.5) |
| Race and ethnicity | |||
| Non‐Hispanic White | 28 111 (85.3) | 12 031 (86.0) | 30 043 (76.9) |
| Black | 1419 (4.3) | 572 (4.1) | 3280 (8.4) |
| Hispanic | 1339 (4.1) | 569 (4.1) | 1911 (4.9) |
| Asian | 1242 (3.8) | 488 (3.5) | 997 (2.6) |
| Unknown | 447 (1.4) | 177 (1.3) | 1279 (3.3) |
| Other† | 402 (1.2) | 155 (1.1) | 1541 (4.0) |
| Insurance | |||
| Commercial | 6980 (21.2) | 3009 (21.5) | 11 557 (29.6) |
| Medicare | 23 566 (71.5) | 9984 (71.4) | 26 383 (67.6) |
| Medicaid | 993 (3.0) | 401 (2.9) | 908 (2.3) |
| Self‐pay | 1421 (4.3) | 598 (4.3) | 203 (0.5) |
| % With income <$50 000 by zip code, mean (SD)‡ | 27.2 (14.6) | 27.0 (14.5) | 21.3 (16.4) |
| Characteristic | Codified data | NLP data | Codified data | NLP data | Codified data | NLP data |
|---|---|---|---|---|---|---|
| Obesity | 7707 (23.4) | 8107 (24.6) | 3532 (25.2) | 3668 (26.2) | 17 398 (44.6) | 10 785 (27.6) |
| Current smoker | 997 (3.0) | … | 443 (3.2) | … | … | … |
| Mitral valve disorder | 2988 (9.1) | 6266 (19.0) | 1290 (9.2) | 2704 (19.3) | 1137 (2.9) | 7776 (19.9) |
| Mitral valve prolapse | 2781 (8.4) | 923 (2.8) | 1196 (8.6) | 398 (2.8) | 758 (1.9) | … |
| Mitral valve insufficiency | 326 (1.0) | 5772 (17.5) | 137 (1.0) | 2504 (17.9) | 328 (0.8) | 7141 (18.3) |
| Mitral valve stenosis | 73 (0.2) | 95 (0.3) | 25 (0.2) | 44 (0.3) | 32 (0.1) | 265 (0.7) |
| Supraventricular tachycardia | 297 (0.9) | 1789 (5.4) | 133 (1.0) | 757 (5.4) | 427 (1.1) | 1276 (3.3) |
| Premature atrial contractions | 106 (0.3) | 5668 (17.2) | 41 (0.3) | 2357 (16.9) | 91 (0.2) | 6858 (17.6) |
| Left ventricular hypertrophy | … | 3803 (11.5) | … | 1623 (11.6) | … | 6241 (16.0) |
| Left atrial enlargement | … | 5543 (16.8) | … | 2387 (17.1) | … | … |
| Myocardial infarction | 1953 (5.9) | 4683 (14.2) | 782 (5.6) | 2018 (14.4) | 1565 (4.0) | 4847 (12.4) |
| Chronic kidney disease | 1033 (3.1) | 915 (2.8) | 475 (3.4) | 385 (2.8) | 3408 (8.7) | 3036 (7.8) |
| Chronic kidney disease: severe | 108 (0.3) | 383 (1.2) | 48 (0.3) | 182 (1.3) | 647 (1.7) | 802 (2.1) |
| Hyperlipidemia | 20 243 (61.4) | 13 356 (40.5) | 8605 (61.5) | 5927 (42.4) | … | … |
| Valvular disease | 941 (2.9) | 491 (1.5) | 391 (2.8) | 213 (1.5) | 1402 (3.6) | … |
| Prior stroke/transient ischemic attack | 2182 (6.6) | 7064 (21.4) | 827 (5.9) | 2980 (21.3) | 2332 (6.0) | … |
| Systemic atherosclerosis | 941 (2.9) | 327 (1.0) | 401 (2.9) | 135 (1.0) | 751 (1.9) | … |
| Cerebral atherosclerosis (codified) | 1062 (3.2) | … | 404 (2.9) | … | … | … |
| Thyrotoxicosis | 1115 (3.4) | 1190 (3.6) | 484 (3.5) | 508 (3.6) | … | … |
| Hypothyroidism | 5219 (15.8) | 4246 (12.9) | 2197 (15.7) | 1849 (13.2) | … | … |
| Pulmonary disease | 6632 (20.1) | 1926 (5.8) | 2855 (20.4) | 879 (6.3) | … | … |
| Chronic obstructive pulmonary disease | 3211 (9.7) | … | 1338 (9.6) | … | 2328 (6.0) | … |
| Congenital heart disease | 468 (1.4) | 32 (0.1) | 176 (1.3) | 20 (0.1) | 316 (0.8) | … |
| Cardiomegaly | 2353 (7.1) | 788 (2.4) | 955 (6.8) | 332 (2.4) | 300 (0.8) | … |
| Alcohol disorder | 554 (1.7) | 1944 (5.9) | 260 (1.9) | 819 (5.9) | … | … |
| Pericarditis | 315 (1.0) | 548 (1.7) | 150 (1.1) | 225 (1.6) | … | … |
| Myocarditis | 37 (0.1) | 125 (0.4) | 11 (0.1) | 57 (0.4) | … | … |
| Sleep apnea | 1093 (3.3) | 1278 (3.9) | 491 (3.5) | 584 (4.2) | … | … |
| Prior cardiac surgery | 848 (2.6) | … | 364 (2.6) | … | … | … |
| Hypertrophic cardiomyopathy | 83 (0.3) | 132 (0.4) | 24 (0.2) | 52 (0.4) | … | … |
| Other cardiomyopathy | 1296 (3.9) | 873 (2.7) | 489 (3.5) | 358 (2.6) | 630 (1.6) | 2186 (5.6) |
| Chronic liver disease | 2521 (7.7) | 176 (0.5) | 1160 (8.3) | 90 (0.6) | … | … |
| Cirrhosis | 260 (0.8) | 524 (1.6) | 135 (1.0) | 278 (2.0) | … | … |
| Liver complications | 358 (1.1) | 1294 (3.9) | 183 (1.3) | 670 (4.8) | … | … |
| Diabetes | 4063 (12.3) | 11 999 (36.4) | 1741 (12.4) | 5280 (37.7) | … | … |
| Hypertension | 16 295 (49.4) | 21 339 (64.7) | 6914 (49.4) | 9093 (65.0) | … | 26 678 (68.3) |
| Congestive heart failure | 663 (2.0) | 2757 (8.4) | 245 (1.8) | 1134 (8.1) | 3013 (7.7) | 3344 (8.6) |
| Coronary artery disease | 2973 (9.0) | 8983 (27.3) | 1163 (8.3) | 3752 (26.8) | 1196 (3.1) | 13 330 (34.1) |
| Peripheral vascular disease | 777 (2.4) | 1951 (5.9) | 328 (2.3) | 855 (6.1) | 172 (0.4) | 1576 (4.0) |
| Cerebrovascular disease | 954 (2.9) | 781 (2.4) | 407 (2.9) | 339 (2.4) | … | … |
| Long PR interval | … | 156 (0.5) | … | 68 (0.5) | … | … |
| Shortened PR interval | … | 57 (0.2) | … | 32 (0.2) | … | … |
Data are given as number (percentage), unless otherwise indicated. NLP indicates natural language processing.
Only final model parameters were ascertained in external validation cohort.
“Other” Race represents American Indian/Alaskan Native, Indian, Middle Eastern, Multiracial, and those with “Other” listed in registration data.
Ascertained from National Neighborhood Data Archive. 32 Missing income data in development population: n=1498; missing income data in internal validation population: n=507; missing income data in external validation population: n=54.
Development Cohort
In the development cohort, there were 2839 incident AF diagnoses (5‐year Kaplan‐Meier cumulative incidence, 9.5% [95% CI, 9.2%–9.9%]) and 8517 death events that occurred before an AF diagnosis or at the end of 5 years of follow‐up (25.8%). The mean duration of follow‐up among the entire development sample was 4.29 years; and among censored patients, it was 4.48 years. The estimated β coefficients and HRs for variables included in the codified+NLP model (22 features) and the codified‐only model (23 features) are shown in Table 3. The codified+NLP model included 3 features defined from structured demographics fields, 5 features defined from codified data only, 7 features defined from NLP data only, and 7 features where both the codified and NLP versions were selected. For the 7 features where both the codified and NLP versions were selected, the prevalence identified by codified data only, NLP data only, and by both codified and NLP data is shown in Figure S1.
Table 3.
Estimated β Coefficients and HRs for Features Included in the Codified+NLP Data Model and the Codified‐Only Data Model in the Development Cohort
| Variable | Codified+NLP model | Codified‐only model | ||
|---|---|---|---|---|
| Estimated β (SE) | HR (95% CI) | Estimated β (SE) | HR (95% CI) | |
| Age (per 5 y) | 0.067 (0.002) | 1.40 (1.37–1.43) | 0.067 (0.002) | 1.40 (1.37–1.43) |
| Sex (female) | −0.504 (0.039) | 0.60 (0.56–0.65) | −0.505 (0.040) | 0.60 (0.56–0.65) |
| Race and ethnicity | ||||
| Black | −0.221 (0.167) | 0.80 (0.58–1.11) | −0.089 (0.166) | 0.92 (0.66–1.27) |
| Hispanic | −0.103 (0.172) | 0.90 (0.64–1.26) | 0.014 (0.171) | 1.01 (0.73–1.42) |
| Other* | −0.516 (0.303) | 0.60 (0.33–1.08) | −0.464 (0.303) | 0.63 (0.35–1.14) |
| Unknown | 0.454 (0.195) | 1.58 (1.08–2.31) | 0.469 (0.195) | 1.60 (1.09–2.34) |
| Non‐Hispanic White | 0.273 (0.124) | 1.31 (1.03–1.68) | 0.347 (0.124) | 1.41 (1.11–1.80) |
| Obesity | ||||
| Codified data | … | … | 0.245 (0.044) | 1.28 (1.17–1.39) |
| NLP data | 0.222 (0.044) | 1.25 (1.14–1.36) | … | … |
| Mitral valve disorder | ||||
| Codified data | −0.101 (0.199) | 0.90 (0.61–1.33) | −0.110 (0.193) | 0.90 (0.61–1.31) |
| NLP data | 0.219 (0.165) | 1.25 (0.90–1.72) | … | … |
| NLP+codified data | 0.159 (0.246) | 1.17 (0.72–1.90) | … | … |
| Mitral valve prolapse (codified) | 0.312 (0.182) | 1.37 (0.96–1.95) | 0.455 (0.187) | 1.58 (1.09–2.27) |
| Mitral valve insufficiency | ||||
| Codified data | … | … | 0.208 (0.144) | 1.23 (0.93–1.63) |
| NLP data | −0.152 (0.168) | 0.86 (0.62–1.19) | … | … |
| Mitral valve stenosis | ||||
| Codified data | −0.085 (0.343) | 0.92 (0.47–1.80) | 0.641 (0.198) | 1.90 (1.29–2.80) |
| NLP data | 0.521 (0.262) | 1.68 (1.01–2.82) | … | … |
| NLP+codified data | 1.112 (0.233) | 3.04 (1.93–4.79) | … | … |
| Supraventricular tachycardia | ||||
| Codified data | 0.659 (0.212) | 1.93 (1.28–2.93) | 0.668 (0.121) | 1.95 (1.54–2.47) |
| NLP data | 0.405 (0.068) | 1.50 (1.31–1.71) | … | … |
| NLP+codified data | 0.634 (0.146) | 1.89 (1.42–2.51) | … | … |
| Premature atrial contractions | ||||
| Codified data | … | … | 0.543 (0.190) | 1.72 (1.19–2.50) |
| NLP data | 0.350 (0.046) | 1.42 (1.30–1.55) | … | … |
| Left ventricular hypertrophy (NLP) | 0.159 (0.053) | 1.17 (1.06–1.30) | … | … |
| Myocardial infarction | ||||
| Codified data | … | … | 0.136 (0.066) | 1.15 (1.01–1.30) |
| NLP data | 0.131 (0.052) | 1.14 (1.03–1.26) | … | … |
| Chronic kidney disease | ||||
| Codified data | … | … | 0.097 (0.073) | 1.10 (0.96–1.27) |
| NLP data | 0.041 (0.104) | 1.04 (0.85–1.28) | … | … |
| Chronic kidney disease: severe | ||||
| Codified data | … | … | 0.378 (0.138) | 1.46 (1.11–1.91) |
| NLP data | 0.367 (0.145) | 1.44 (1.09–1.92) | … | … |
| Valvular disease (codified) | 0.216 (0.087) | 1.24 (1.05–1.47) | 0.194 (0.097) | 1.21 (1.01–1.47) |
| Stroke/transient ischemic attack (codified) | … | … | 0.165 (0.061) | 1.18 (1.05–1.33) |
| Systemic atherosclerosis (codified) | 0.120 (0.081) | 1.13 (0.96–1.32) | 0.166 (0.081) | 1.18 (1.01–1.38) |
| Chronic obstructive pulmonary disease (codified) | 0.150 (0.053) | 1.16 (1.05–1.29) | 0.221 (0.053) | 1.25 (1.13–1.38) |
| Congenital heart disease (codified) | … | … | 0.236 (0.107) | 1.27 (1.03–1.56) |
| Cardiomegaly (codified) | 0.340 (0.061) | 1.41 (1.25–1.58) | 0.473 (0.059) | 1.60 (1.43–1.80) |
| Other cardiomyopathy | ||||
| Codified data | 0.162 (0.082) | 1.18 (1.00–1.38) | 0.176 (0.075) | 1.19 (1.03–1.38) |
| NLP data | 0.377 (0.121) | 1.46 (1.15–1.85) | … | … |
| NLP+codified data | 0.059 (0.117) | 1.06 (0.84–1.34) | … | … |
| Hypertension (NLP) | 0.212 (0.048) | 1.24 (1.13–1.36) | … | … |
| Congestive heart failure | ||||
| Codified data | 0.788 (0.203) | 2.20 (1.48–3.27) | 0.453 (0.087) | 1.57 (1.33–1.87) |
| NLP data | 0.137 (0.064) | 1.15 (1.01–3.30) | … | … |
| NLP+codified data | 0.397 (0.103) | 1.49 (1.22–1.82) | … | … |
| Coronary artery disease | ||||
| Codified data | 0.298 (0.207) | 1.35 (0.90–2.02) | 0.220 (0.057) | 1.25 (1.12–1.39) |
| NLP data | 0.030 (0.051) | 1.03 (0.93–1.14) | … | … |
| NLP+codified data | 0.024 (0.065) | 1.02 (0.90–1.16) | … | … |
| Peripheral vascular disease | ||||
| Codified data | 0.195 (0.145) | 1.22 (0.91–1.61) | 0.242 (0.087) | 1.27 (1.07–1.51) |
| NLP data | 0.184 (0.072) | 1.20 (1.05–1.38) | … | … |
| NLP+codified data | 0.242 (0.104) | 1.27 (1.04–1.56) | … | … |
All risk factors are classified at the start of follow‐up. The presence of an estimated β and HR indicates the variable was included in the corresponding model. For the codified+NLP model, features may be defined by only codified data (only “codified data” row has a β), defined by only NLP (only “NLP data” row has a β), or defined by both codified and NLP (“codified data,” “NLP data,” and “NLP+codified data” rows have a β). An ellipses indicates the variable was not selected for inclusion in the model. HR indicates hazard ratio; and NLP, natural language processing.
“Other” Race represents American Indian/Alaskan Native, Indian, Middle Eastern, Multiracial, and those with “Other” listed in registration data.
For the codified+NLP model, risk groups were defined on the basis of the ≥84th percentile of 5‐year predicted risk (≥14.6%), the 50th to <84th percentile (6.5%–<14.6%), the 16th to <50th percentile (3.6%–<6.5%), and the <16th percentile (<3.6%). For the codified‐only model, the risk groups were defined as ≥84th percentile (≥14.4%), the 50th to <84th percentile (6.8%–<14.4%), the 16th to <50th percentile (3.8%–<6.8%), and the <16th percentile (<3.8%). HRs for incidence of AF within 5 years by risk group for each model are shown in Table 4. The C‐statistic for the codified+NLP model was 0.744 (95% CI, 0.735–0.753), which was significantly greater (P<0.001) than the C‐statistic for the codified‐only model (0.730 [95% CI, 0.720–0.739]). Cumulative incidence plots stratified by groups of predicted risk for both models are shown in Figure 2. C‐statistics were similar, although slightly reduced, in models excluding race and ethnicity and adding insurance and neighborhood‐based income (Table S5). Calibration plots of both models are presented in Figure 3, with both models demonstrating good calibration (codified+NLP: Integrated Calibration Index=0.014 [95% CI, 0.003–0.024]; codified‐only: Integrated Calibration Index=0.011 [95% CI, 0.001–0.021]).
Table 4.
HRs and 95% CIs for Incidence of AF by Risk Groups Defined by the 16th, 50th, and 84th Percentiles for Each Model in the Development Cohort
| Variable | Codified+NLP HR (95% CI) | Codified‐only HR (95% CI) |
|---|---|---|
| ≥84th Percentile | 15.66 (12.68–19.34) | 12.73 (10.50–15.42) |
| 50th–<84th Percentile | 5.82 (4.71–7.20) | 4.53 (3.73–5.49) |
| 16th–<50th Percentile | 2.19 (1.75–2.74) | 2.03 (1.65–2.48) |
| <16th Percentile | … | … |
The 5‐year risk of AF was calculated for each model as where is the average AF‐free survival probability at 5 years in the sample, is an individual's risk score calculated using the regression coefficients from the development model (β) and the level for each risk factor (X), and is the average score of the sample. For the codified+NLP model, risk was calculated as ; and for codified‐only model, as . AF indicates atrial fibrillation; HR, hazard ratio; and NLP, natural language processing.
Figure 2. Cumulative incidence plots stratified by groups of predicted risk in development cohort.
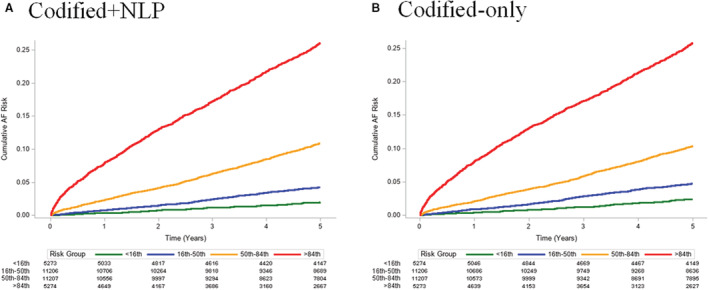
A, Depicts the cumulative risk of atrial fibrillation (AF) by groups defined by <16th percentile (green, <3.6%), 16th to <50th percentile (blue, 3.6%–<6.5%), 50th to <84th percentile (orange, 6.5%–<14.6%), and ≥84th percentile (red, ≥14.6%) of predicted AF risk for the codified+natural language processing (NLP) model. B, Depicts the cumulative risk of AF by groups defined by <16th percentile (green, <3.8%), 16th to <50th percentile (blue, 3.8%–<6.8%), 50th to <84th percentile (orange, 6.8%–<14.4%), and ≥84th percentile (red, ≥14.4%) of predicted AF risk for the codified‐only model.
Figure 3. Calibration plots of observed 5‐year atrial fibrillation (AF) risk vs predicted 5‐year AF risk with patients divided into risk groups based on deciles in the development cohort.
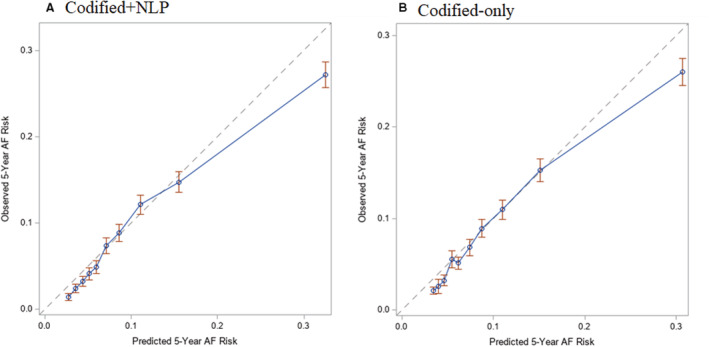
A, Depicts the plot of observed 5‐year AF risk (y axis) vs predicted 5‐year AF risk (x axis) for the codified+natural language processing (NLP) model in blue, whereas the optimal calibration is shown in gray. B, Depicts the plot of observed 5‐year AF risk (y axis) vs predicted 5‐year AF risk (x axis) for the codified‐only model in blue, whereas the optimal calibration is shown in gray.
Internal Validation Cohort
In the internal validation cohort, there were 1057 incident AF diagnoses (Kaplan‐Meier cumulative incidence, 8.1% [95% CI, 7.7%–8.6%]) and 3201 death events (22.9%). The mean duration of follow‐up among the entire internal validation sample was 4.44 years; and among censored patients, it was 4.61 years. Both the codified+NLP data model (mean, 8.4%; median, 5.8%) and codified‐only model (mean, 9.0%; median, 6.4%) predicted higher AF risk than CHARGE‐AF (mean, 5.6%; median, 5.6%). Distributions of risk predictions for all 3 models are displayed in Figure 4.
Figure 4. Distributions of predicted risk for codified+natural language processing (NLP), codified‐only, and Cohorts for Heart and Aging Research in Genomic Epidemiology Atrial Fibrillation (CHARGE‐AF) models in the internal validation cohort.
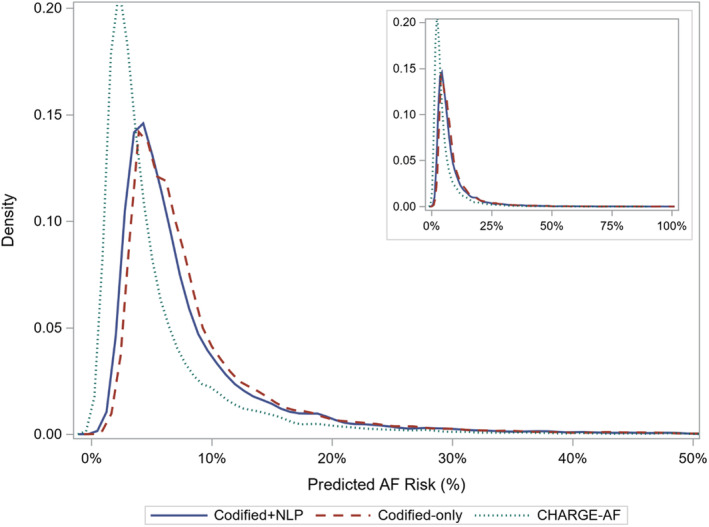
AF indicates atrial fibrillation.
In the internal validation cohort, the C‐statistic was modestly higher in the codified+NLP data model (0.735 [95% CI, 0.720–0.749]) compared with the codified‐only model (0.729 [95% CI, 0.715–0.744]; P=0.06) and the CHARGE‐AF model (0.717 [95% CI, 0.703–0.731]; P=0.002). The C‐statistic for the codified‐only model was also significantly higher than for CHARGE‐AF (P=0.01). HRs for AF by risk groups defined by the 16th, 50th, and 84th percentiles for each model are shown in Table 5. Like the development cohort, the C‐statistic was similar, but slightly reduced, when excluding race and ethnicity and adding insurance and neighborhood‐based income to the model (Table S5). Cumulative incidence plots stratified by risk groups of predicted risk are shown in Figure 5. Each model demonstrates separation in the cumulative incidence curves by risk group, with the codified+NLP data model (Kaplan‐Meier estimate for ≥84th percentile, 21.9%; and 50th–<84th percentile, 9.1%) and codified‐only model (Kaplan‐Meier estimate for ≥84th percentile, 21.4%; and 50th–<84th percentile, 9.1%) having greater separation between the highest and next highest risk group compared with CHARGE‐AF (Kaplan‐Meier estimate for ≥84th percentile, 19.3%; and 50th–<84th percentile, 9.9%). Table 6 summarizes percentile‐based NRI results. Codified+NLP and codified‐only models demonstrate significantly improved reclassification compared with CHARGE‐AF according to the overall NRI, with both positive event and nonevent NRI. The overall NRI comparing codified+NLP and the codified‐only model was small, and the CI crosses 0. NRI results were similar when establishing groups based on quartiles.
Table 5.
HRs and 95% CIs for Incidence of AF by Risk Groups Defined by the 16th, 50th, and 84th Percentiles for Each Model in Internal Validation Cohort
| Variable | Codified+NLP HR (95% CI) | Codified‐only HR (95% CI) | CHARGE‐AF HR (95% CI) |
|---|---|---|---|
| ≥84th Percentile | 16.28 (11.31–23.43) | 14.71 (10.47–20.66) | 13.05 (9.20–18.53) |
| 50th–<84th Percentile | 6.19 (4.30–8.92) | 5.67 (4.03–7.98) | 6.35 (4.48–9.00) |
| 16th–<50th Percentile | 2.59 (1.77–3.80) | 2.54 (1.77–3.63) | 2.60 (1.81–3.75) |
| <16th Percentile | … | … | … |
The 5‐year risk of AF was calculated for each model as where is the average AF‐free survival probability at 5 years in the sample. For codified+NLP and codified‐only, is an individual's risk score calculated using the regression coefficients from the development model (β) and the level for each risk factor (X), and is the average score of the sample. For CHARGE‐AF, is an individual's CHARGE‐AF score calculated using the regression coefficients from the original CHARGE‐AF publication (β) and the level for each risk factor (X), and is a published constant (12.5815600). 19 For the codified+NLP model, risk was calculated as ; for codified‐only, as ; and for CHARGE‐AF, as . AF indicates atrial fibrillation; CHARGE‐AF, Cohorts for Heart and Aging Research in Genomic Epidemiology AF; HR, hazard ratio; and NLP, natural language processing.
Figure 5. Cumulative incidence plots stratified by groups of predicted risk in the internal validation cohort.
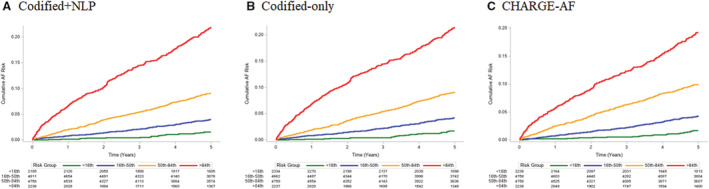
A, Depicts the cumulative risk of atrial fibrillation (AF) by groups defined by <16th percentile (green, <3.4%), 16th to <50th percentile (blue, 3.4%–<5.8%), 50th to <84th percentile (orange, 5.8%–<12.5%), and ≥84th percentile (red, ≥12.5%) of predicted AF risk for the codified+natural language processing (NLP) model. B, Depicts the cumulative risk of AF by groups defined by <16th percentile (green, <3.7%), 16th to <50th percentile (blue, 3.7%–<6.4%), 50th to <84th percentile (orange, 6.4%–<13.2%), and ≥84th percentile (red, ≥13.2%) of predicted AF risk for the codified‐only model. C, Depicts the cumulative risk of AF by groups defined by <16th percentile (green, <1.8%), 16th to <50th percentile (blue, 1.8%–<3.5%), 50th to <84th percentile (orange, 3.5%–<8.8%), and ≥84th percentile (red, ≥8.8%) of predicted AF risk for the Cohorts for Heart and Aging Research in Genomic Epidemiology AF (CHARGE‐AF) model.
Table 6.
Percentile‐Based NRI With Groups Determined by 16th, 50th, and 84th Percentiles of Each Model in Internal Validation Cohort
| Variable | Overall NRI | Event NRI | Nonevent NRI |
|---|---|---|---|
| Codified+NLP vs CHARGE‐AF | 0.070 (0.033–0.113) | 0.052 (0.017–0.093) | 0.018 (0.006–0.029) |
| Codified‐only vs CHARGE‐AF | 0.054 (0.015–0.091) | 0.036 (−0.002–0.069) | 0.019 (0.007–0.030) |
| Codified+NLP vs codified‐only | 0.016 (−0.012–0.044) | 0.020 (−0.007–0.046) | −0.004 (−0.013 to −0.005) |
CHARGE‐AF indicates Cohorts for Heart and Aging Research in Genomic Epidemiology Atrial Fibrillation; NLP, natural language processing; and NRI, net reclassification improvement.
The codified+NLP data model and the codified‐only model both appeared well calibrated in the internal validation cohort (Figure 6). In contrast, calibration of CHARGE‐AF was poor, with the plot of observed 5‐year AF risk versus predicted 5‐year AF risk demonstrating underestimation of AF risk (Figure 6). Calibration remained poor, even after recalibrating CHARGE‐AF with the baseline survival of the internal validation sample (Figure S2). The Integrated Calibration Index estimate was smallest for the codified+NLP model (0.011 [95% CI, 0.002–0.020]), compared with codified‐only (0.016 [95% CI, 0.006–0.027]) and CHARGE‐AF (0.023 [95% CI, 0.009–0.036]).
Figure 6. Calibration plots of observed 5‐year atrial fibrillation (AF) risk vs predicted 5‐year AF risk with patients divided into risk groups based on deciles in the internal validation cohort.
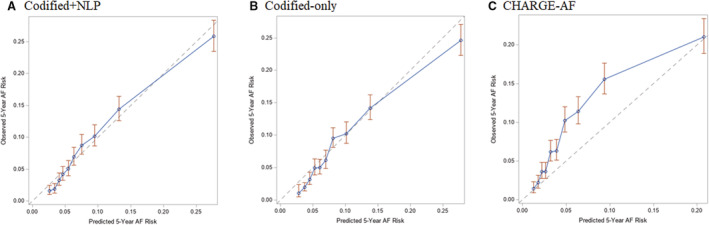
A, Depicts the plot of observed 5‐year AF risk (y axis) vs predicted 5‐year AF risk (x axis) for the codified+natural language processing (NLP) model in blue, whereas the optimal calibration is shown in gray. B, Depicts the plot of observed 5‐year AF risk (y axis) vs predicted 5‐year AF risk (x axis) for the codified‐only model in blue, whereas the optimal calibration is shown in gray. C, Depicts the plot of observed 5‐year AF risk (y axis) vs predicted 5‐year AF risk (x axis) for the Cohorts for Heart and Aging Research in Genomic Epidemiology AF (CHARGE‐AF) model in blue, whereas the optimal calibration is shown in gray.
External Validation Cohort
In the external validation cohort, there were 2226 incident AF diagnoses (Kaplan‐Meier cumulative incidence, 7.3% [95% CI, 7.0%–7.6%]). The mean duration of follow‐up among the entire external validation sample was 3.64 years; and among censored patients, it was 3.75 years. The C‐statistic was higher in the codified+NLP data model (0.750 [95% CI, 0.740–0.760]) compared with the codified‐only model (0.738 [95% CI, 0.727–0.748]; P<0.001) and the CHARGE‐AF model (0.735 [95% CI, 0.725–0.746]; P<0.001). HRs for AF by risk groups defined by the 16th, 50th, and 84th percentiles for each model are shown in Table S6. Cumulative incidence plots stratified by risk groups of predicted risk are shown in Figure S3. Table S7 summarizes the NRI results. In external validation, the codified+NLP model demonstrated significantly improved reclassification compared with CHARGE‐AF and codified‐only by overall NRI, with most of the improvement attributable to correctly up‐classifying events. The codified+NLP and codified‐only models were not as well calibrated in the external validation cohort, with both models overestimating AF risk (Figure S4). Evaluation of model performance was similar when limiting to 3 years of follow‐up (Table S8 and Figures S5 and S6).
Discussion
In >86 000 older primary care patients, we derived and validated models to predict the incidence of AF. In one model, we used only codified data. In the second model, we added information from narrative data using NLP to codified data. We observed that incorporating codified and NLP‐derived data modestly improved model performance compared with using only codified data. Furthermore, both newly developed models were superior to an established AF prediction model, CHARGE‐AF. In external validation, performance remained modestly greater for the model that incorporated NLP‐derived data. Our findings suggest that incorporating narrative EHR data using NLP may improve identification of clinical predictors of AF and yield a better prediction model.
Our results demonstrate that clinical and demographic features routinely ascertained from the EHR can predict 5‐year risk of AF, and that incorporating narrative data from the EHR using NLP can modestly improve model performance compared with using codified data only. We developed 2 models to predict incident AF in this study, 1 using only codified EHR data to identify clinical features and 1 that added narrative data using NLP. Prior studies have demonstrated the utility of NLP in increasing the sensitivity and positive predictive value of clinical features compared with using codified data only. 22 , 23 , 24 , 25 , 26 , 54 NLP may reduce misclassification of clinical features by extracting information from narrative text that would otherwise not be considered for an algorithm. Using least absolute shrinkage and selection operator as a variable selection strategy, we found the NLP version of several features to be selected in the model over the codified version (eg, obesity). In other situations, both the NLP and codified versions were selected in the models (eg, supraventricular tachycardia). Among 22 model features included in the codified+NLP model, the NLP version was selected instead of the codified version for 6 features (eg, obesity), whereas both the NLP and codified versions were selected for 7 features (eg, supraventricular tachycardia) (Table 3). In the development and validation populations, the C‐statistic and NRI were better in the model incorporating NLP compared with the model using codified data only. However, the magnitude of the improvement was modest. Accurately and efficiently assessing individual risk estimates for AF in clinical settings may enable targeted risk‐based screening or prevention interventions and may be implemented within the EHR to guide clinical decision making. For example, assessing risk of AF could serve as a guide for use of longer‐term cardiac monitor in survivors of acute stroke. 17 , 35
The CHARGE‐AF risk model was developed in community‐based research cohorts, which may represent subjects at lower underlying risk of developing AF and include more accurate assessment of covariates. External validation of CHARGE‐AF in EHR‐based cohorts has demonstrated poor calibration. 20 , 21 , 35 Within both our internal and external validation samples, CHARGE‐AF underestimated risk of developing AF, even after recalibrating to the baseline risk of each sample. In contrast, our newly developed models using codified data only or NLP and codified data demonstrated modestly improved C‐statistics compared with CHARGE‐AF and were well calibrated in an internal validation population, although calibration was not as good in external validation. Our findings support prior work suggesting that prediction models developed within a clinical setting using EHR data perform better in real‐world clinical settings than models derived from community‐based research cohort data. 21 This may be the result of differences in data quality and/or differences in populations between research cohorts and clinical settings.
NLP provides an opportunity to access the vast amount of data in narrative notes within EHRs. The use of NLP as part of clinical operations will be dependent on scaling the rapid processing of large amounts of data and optimizing the ability of NLP tools to efficiently and accurately identify clinical factors. 55 , 56 , 57 Quality of narrative data in the EHR may differ over time and by provider and institution. Thus, porting our algorithm to another institution will require at minimum validation, and potential refitting before implementation if there are large differences in the population. In our samples, the addition of NLP provided a statistically significant improvement in predictive performance, but the magnitude of improvement was modest. Most components of the codified+NLP model, except for those extracted from cardiology reports, were available in a codified format. However, we did observe for most variables that either the NLP version was more predictive or the combination of codified and NLP was more predictive. If easily implemented, using NLP may be worthwhile to achieve the best possible model. The added benefit of NLP may differ at different institutions, depending on the quality of codified and narrative data. For institutions unable to implement NLP, our model using only codified data performed well and was an improvement over CHARGE‐AF.
This study has several potential limitations. Our models were developed within a single‐center tertiary academic primary care practice network with patients who were largely of European ancestry, so generalizability may be limited. Like CHARGE‐AF, we included race and ethnicity in our models. 19 Although Black individuals have consistently had a lower prevalence of clinically detected AF compared with White individuals, this may represent differential detection rather than a biological mechanism. 58 Race and ethnicity may represent, and potentially poorly represent, a proxy for social determinants of health. As such, we presented models and evaluated the C‐statistic adding insurance and insurance plus zip code–defined income as predictors instead of race and ethnicity and the performance of the models did not materially deteriorate. Ascertainment of clinical features and incidence of AF were based on retrospective assessment of EHR documentation, which may be associated with misclassification when classifying features using codified or NLP data. Data on clinical features and ascertainment of incident AF are limited to what is available within the Mass General Brigham EHR. We do not have the ability to fully ascertain information on clinical features or incident diagnoses of AF for patients seen outside of our network.
In conclusion, estimation of the 5‐year risk of AF in a primary care population can be modestly improved by using NLP to incorporate narrative EHR data. The amount of data available in unstructured form within EHRs is immense. Optimizing the use of NLP tools to meaningfully process these data offers an opportunity to improve prognostic models used within clinical care.
Sources of Funding
Drs Ashburner, Lubitz, and Anderson are supported by American Heart Association (AHA) 18SFRN34250007 and 18SFRN34150007. Dr Ashburner is supported by National Institutes of Health (NIH) grant K01HL148506. Dr Lubitz is supported by NIH grants R01HL139731 and R01HL157635. Dr Anderson is supported by NIH grants R01NS103924 and U01NS069763, AHA‐Bugher Foundation Centers for Excellence in Hemorrhagic Stroke, the Massachusetts General Hospital Center for Neuroscience, and Henry and Allison McCance Center for Brain Health. Dr Liao is supported by the NIH grant P30AR072577 (VERITY Bioinformatics Core).
Disclosures
Dr Lubitz receives sponsored research support from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Fitbit, Medtronic, Premier, and IBM; and has consulted for Bristol Myers Squibb, Pfizer, Blackstone Life Sciences, and Invitae. Dr Anderson receives sponsored research support from Bayer AG; and has consulted for ApoPharma, Inc. Dr Singer receives research support from Bristol‐Myers Squibb; and has consulted for Boehringer Ingelheim, Bristol‐Myers Squibb, Fitbit, Johnson and Johnson, Merck, and Pfizer. Dr Atlas receives sponsored research support from Bristol Myers Squibb/Pfizer; and has consulted for Bristol Myers Squibb/Pfizer and Fitbit. Dr Murphy receives sponsored research support from AstraZeneca Pharmaceuticals LP, Analysis Group, Inc, Radius Health Inc, and Amgen, Inc. The remainder of the authors report no disclosures.
Supporting information
Tables S1–S8
Figures S1–S6
Acknowledgments
We thank Meghan L. Rieu‐Werden, BS, for help with data acquisition and cohort generation.
For Sources of Funding and Disclosures, see page 14.
References
- 1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370 [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147:1561–1564. [PubMed] [Google Scholar]
- 5. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063 [DOI] [PubMed] [Google Scholar]
- 6. Borowsky LH, Regan S, Chang Y, Ayres A, Greenberg SM, Singer DE. First diagnosis of atrial fibrillation at the time of stroke. Cerebrovasc Dis. 2017;43:192–199. doi: 10.1159/000457809 [DOI] [PubMed] [Google Scholar]
- 7. Lubitz SA, Yin X, McManus DD, Weng LC, Aparicio HJ, Walkey AJ, Rafael Romero J, Kase CS, Ellinor PT, Wolf PA, et al. Stroke as the initial manifestation of atrial fibrillation: the Framingham Heart Study. Stroke. 2017;48:490–492. doi: 10.1161/STROKEAHA.116.015071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bjorck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population‐based study. Stroke. 2013;44:3103–3108. doi: 10.1161/STROKEAHA.113.002329 [DOI] [PubMed] [Google Scholar]
- 9. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 10. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 11. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 12. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 13. Fitzmaurice DA, Hobbs FD, Jowett S, Mant J, Murray ET, Holder R, Raftery JP, Bryan S, Davies M, Lip GY, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335:383. doi: 10.1136/bmj.39280.660567.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaasenbrood F, Hollander M, de Bruijn SH, Dolmans CP, Tieleman RG, Hoes AW, Rutten FH. Opportunistic screening versus usual care for diagnosing atrial fibrillation in general practice: a cluster randomised controlled trial. Br J Gen Pract. 2020;70:e427–e433. doi: 10.3399/bjgp20X708161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uittenbogaart SB, Verbiest‐van Gurp N, Lucassen WAM, Winkens B, Nielen M, Erkens PMG, Knottnerus JA, van Weert HCPM, Stoffers HEJH. Opportunistic screening versus usual care for detection of atrial fibrillation in primary care: cluster randomised controlled trial. BMJ. 2020;370:m3208. doi: 10.1136/bmj.m3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lubitz SA, Atlas SJ, Ashburner JM, Lipsanopoulos ATT, Borowsky LH, Guan W, Khurshid S, Ellinor PT, Chang Y, McManus DD, et al. Screening for atrial fibrillation in older adults at primary care visits: VITAL‐AF randomized controlled trial. Circulation. 2022;145:946–954. doi: 10.1161/CIRCULATIONAHA.121.057014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ashburner JM, Khurshid S, Atlas SJ, Singer DE, Lubitz SA. Point‐of‐care screening for atrial fibrillation: where are we, and where do we go next? Cardiovasc Digit Health J. 2021;2:294–297. doi: 10.1016/j.cvdhj.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khurshid S, Mars N, Haggerty CM, Huang Q, Weng L‐C, Hartzel DN, Center RG, Lunetta KL, Ashburner JM, Anderson CD, et al. Predictive accuracy of a clinical and genetic risk model for atrial fibrillation. Circ Genomic Precis Med. 2021;14:e003355. doi: 10.1161/CIRCGEN.121.003355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kolek MJ, Graves AJ, Xu M, Bian A, Teixeira PL, Shoemaker MB, Parvez B, Xu H, Heckbert SR, Ellinor PT, et al. Evaluation of a prediction model for the development of atrial fibrillation in a repository of electronic medical records. JAMA Cardiol. 2016;1:1007–1013. doi: 10.1001/jamacardio.2016.3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hulme OL, Khurshid S, Weng L‐C, Anderson CD, Wang EY, Ashburner JM, Ko D, McManus DD, Benjamin EJ, Ellinor PT, et al. Development and validation of a prediction model for atrial fibrillation using electronic health records. JACC Clin Electrophysiol. 2019;5:1331–1341. doi: 10.1016/j.jacep.2019.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Himes BE, Dai Y, Kohane IS, Weiss ST, Ramoni MF. Prediction of chronic obstructive pulmonary disease (COPD) in asthma patients using electronic medical records. J Am Med Inform Assoc. 2009;16:371–379. doi: 10.1197/jamia.M2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wasfy JH, Singal G, O'Brien C, Blumenthal DM, Kennedy KF, Strom JB, Spertus JA, Mauri L, Normand S‐LT, Yeh RW. Enhancing the prediction of 30‐day readmission after percutaneous coronary intervention using data extracted by querying of the electronic health record. Circ Cardiovasc Qual Outcomes. 2015;8:477–485. doi: 10.1161/CIRCOUTCOMES.115.001855 [DOI] [PubMed] [Google Scholar]
- 24. Liao KP, Cai T, Savova GK, Murphy SN, Karlson EW, Ananthakrishnan AN, Gainer VS, Shaw SY, Xia Z, Szolovits P, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi: 10.1136/bmj.h1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liao KP, Ananthakrishnan AN, Kumar V, Xia Z, Cagan A, Gainer VS, Goryachev S, Chen P, Savova GK, Agniel D, et al. Methods to develop an electronic medical record phenotype algorithm to compare the risk of coronary artery disease across 3 chronic disease cohorts. PLoS One. 2015;10:e0136651. doi: 10.1371/journal.pone.0136651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li L, Chase HS, Patel CO, Friedman C, Weng C. Comparing ICD9‐encoded diagnoses and NLP‐processed discharge summaries for clinical trials pre‐screening: a case study. AMIA Annu Symp Proc. 2008;2008:404–408. [PMC free article] [PubMed] [Google Scholar]
- 27. Steyerberg EW, Harrell FE. Prediction models need appropriate internal, internal‐external, and external validation. J Clin Epidemiol. 2016;69:245–247. doi: 10.1016/j.jclinepi.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Atlas SJ, Chang Y, Lasko TA, Chueh HC, Grant RW, Barry MJ. Is this “my” patient? Development and validation of a predictive model to link patients to primary care providers. J Gen Intern Med. 2006;21:973–978. doi: 10.1111/j.1525-1497.2006.00509.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Atlas SJ, Grant RW, Ferris TG, Chang Y, Barry MJ. Patient‐physician connectedness and quality of primary care. Ann Intern Med. 2009;150:325–335. doi: 10.7326/0003-4819-150-5-200903030-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murphy SN, Morgan MM, Barnett GO, Chueh HC. Optimizing healthcare research data warehouse design through past COSTAR query analysis. Proc AMIA Symp. 1999;892–896. [PMC free article] [PubMed] [Google Scholar]
- 31. Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79 743 ambulatory subjects. J Electrocardiol. 2007;40:228–234. doi: 10.1016/j.jelectrocard.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 32. Robert Melendez U of MI for SR, Philippa Clarke U of MI for SR, Anam Khan U of MI for SR, Iris Gomez‐Lopez U of MI for SR, Mao Li U of MI for SR, Megan Chenoweth U of MI for SR . National Neighborhood Data Archive (NaNDA): Socioeconomic Status and Demographic Characteristics of ZIP Code Tabulation Areas, United States, 2008–2017 [Internet]. 2020. Available at: https://www.openicpsr.org/openicpsr/project/120462/version/V1/view. Accessed Jan 25, 2022.
- 33. Grant RW, Cagliero E, Sullivan CM, Dubey AK, Estey GA, Weil EM, Gesmundo J, Nathan DM, Singer DE, Chueh HC, et al. A controlled trial of population management: diabetes mellitus: putting evidence into practice (DM‐PEP). Diabetes Care. 2004;27:2299–2305. doi: 10.2337/diacare.27.10.2299 [DOI] [PubMed] [Google Scholar]
- 34. Leong A, Berkowitz SA, Triant VA, Porneala B, He W, Atlas SJ, Wexler DJ, Meigs JB. Hypoglycemia in diabetes mellitus as a coronary artery disease risk factor in patients at elevated vascular risk. J Clin Endocrinol Metab. 2016;101:659–668. doi: 10.1210/jc.2015-3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashburner JM, Wang X, Li X, Khurshid S, Ko D, Trisini Lipsanopoulos A, Lee PR, Carmichael T, Turner AC, Jackson C, et al. Re‐CHARGE‐AF: recalibration of the CHARGE‐AF model for atrial fibrillation risk prediction in patients with acute stroke. J Am Heart Assoc. 2021;10:e022363. doi: 10.1161/JAHA.121.022363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berkowitz SA, Atlas SJ, Grant RW, Wexler DJ. Individualizing HbA1c targets for patients with diabetes: impact of an automated algorithm within a primary care network. Diabet Med J Br Diabet Assoc. 2014;31:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu S, Cai T. NILE: Fast Natural Language Processing for Electronic Health Records. ArXiv13116063 Cs [Internet]. 2019; Available at: http://arxiv.org/abs/1311.6063. Accessed Feb 7, 2022.
- 38. Bodenreider O. The Unified Medical Language System (UMLS): integrating biomedical terminology. Nucleic Acids Res. 2004;32:D267–D270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cornelius ME, Loretan CG, Wang TW, Jamal A, Homa DM. Tobacco product use among adults ‐ United States, 2020. MMWR Morb Mortal Wkly Rep. 2022;71:397–405. doi: 10.15585/mmwr.mm7111a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu B, Akushevich I, Yashkin AP, Kravchenko J. Epidemiology of geographic disparities of myocardial infarction among older adults in the United States: analysis of 2000–2017 medicare data. Front Cardiovasc Med. 2021;8:707102. doi: 10.3389/fcvm.2021.707102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse. Circulation. 2014;129:2158–2170. doi: 10.1161/CIRCULATIONAHA.113.006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 43. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 44. Ashburner JM, Singer DE, Lubitz SA, Borowsky LH, Atlas SJ. Changes in use of anticoagulation in patients with atrial fibrillation within a primary care network associated with the introduction of direct oral anticoagulants. Am J Cardiol. 2017;120:786–791. doi: 10.1016/j.amjcard.2017.05.055 [DOI] [PubMed] [Google Scholar]
- 45. Tibshirani R. The lasso method for variable selection in the Cox Model. Stat Med. 1997;16:385–395. doi: 10.1002/(sici)1097-0258(19970228)16:4 [DOI] [PubMed] [Google Scholar]
- 46. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Springer Series in Statistics. 2nd ed. Berlin/Heidelberg, Germany: Springer; 2009. [Google Scholar]
- 47. Kuitunen I, Ponkilainen VT, Uimonen MM, Eskelinen A, Reito A. Testing the proportional hazards assumption in cox regression and dealing with possible non‐proportionality in total joint arthroplasty research: methodological perspectives and review. BMC Musculoskelet Disord. 2021;22:489. doi: 10.1186/s12891-021-04379-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cox D. Note on grouping. J Am Stat Assoc. 1957;52:543–547. [Google Scholar]
- 49. Austin PC, Harrell FE, van Klaveren D. Graphical calibration curves and the integrated calibration index (ICI) for survival models. Stat Med. 2020;39:2714–2742. doi: 10.1002/sim.8570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Calster B, McLernon DJ, van Smeden M, Wynants L, Steyerberg EW. Topic Group ‘Evaluating diagnostic tests and prediction models’ of the STRATOS initiative. Calibration: the Achilles heel of predictive analytics. BMC Med. 2019;17:230. doi: 10.1186/s12916-019-1466-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pencina MJ, D'Agostino RB, D'Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. discussion 207–212 [DOI] [PubMed] [Google Scholar]
- 52. Pencina MJ, D'Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McKearnan SB, Wolfson J, Vock DM, Vazquez‐Benitez G, O'Connor PJ. Performance of the net reclassification improvement for nonnested models and a novel percentile‐based alternative. Am J Epidemiol. 2018;187:1327–1335. doi: 10.1093/aje/kwx374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao SS, Hong C, Cai T, Xu C, Huang J, Ermann J, Goodson NJ, Solomon DH, Cai T, Liao KP. Incorporating natural language processing to improve classification of axial spondyloarthritis using electronic health records. Rheumatol Oxf Engl. 2020;59:1059–1065. doi: 10.1093/rheumatology/kez375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maddox TM, Matheny MA. Natural language processing and the promise of big data: small step forward, but many miles to go. Circ Cardiovasc Qual Outcomes. 2015;8:463–465. doi: 10.1161/CIRCOUTCOMES.115.002125 [DOI] [PubMed] [Google Scholar]
- 56. Liao KP, Sun J, Cai TA, Link N, Hong C, Huang J, Huffman JE, Gronsbell J, Zhang Y, Ho Y‐L, et al. High‐throughput multimodal automated phenotyping (MAP) with application to PheWAS. J Am Med Inform Assoc. 2019;26:1255–1262. doi: 10.1093/jamia/ocz066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yu S, Liao KP, Shaw SY, Gainer VS, Churchill SE, Szolovits P, Murphy SN, Kohane IS, Cai T. Toward high‐throughput phenotyping: unbiased automated feature extraction and selection from knowledge sources. J Am Med Inform Assoc. 2015;22:993–1000. doi: 10.1093/jamia/ocv034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heckbert SR, Austin TR, Jensen PN, Chen LY, Post WS, Floyd JS, Soliman EZ, Kronmal RA, Psaty BM. Differences by race/ethnicity in the prevalence of clinically detected and monitor‐detected atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e007698. doi: 10.1161/CIRCEP.119.007698 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8
Figures S1–S6


