Abstract
Background
Intracoronary physiologic indexes such as coronary flow reserve (CFR) and left ventricular ejection fraction (LVEF) have been regarded as prognostic indicators in patients with coronary artery disease. The current study evaluated the association between intracoronary physiologic indexes and LVEF and their differential prognostic implications in patients with coronary artery disease.
Methods and Results
A total of 1889 patients with 2492 vessels with available CFR and LVEF were selected from an international multicenter prospective registry. Baseline physiologic indexes were measured by thermodilution or Doppler methods and LVEF was recorded at the index procedure. The primary outcome was target vessel failure, which was a composite of cardiac death, target vessel myocardial infarction, or clinically driven target vessel revascularization over 5 years of follow‐up. Patients with reduced LVEF <50% (162 patients [8.6%], 202 vessels [8.1%]) showed a similar degree of epicardial coronary artery disease but lower CFR values than those with preserved LVEF (2.4±1.2 versus 2.7±1.2, P<0.001), mainly driven by the increased resting coronary flow. Conversely, hyperemic coronary flow, fractional flow reserve, and the degree of microvascular dysfunction were similar between the 2 groups. Reduced CFR (≤2.0) was seen in 613 patients (32.5%) with 771 vessels (30.9%). Reduced CFR was an independent predictor for target vessel failure (hazard ratio, 2.081 [95% CI, 1.385–3.126], P<0.001), regardless of LVEF.
Conclusions
CFR was lower in patients with reduced LVEF because of increased resting coronary flow. Patients with reduced CFR showed a significantly higher risk of target vessel failure than did those with preserved CFR, regardless of LVEF.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04485234.
Keywords: coronary flow reserve, coronary physiology, left ventricular ejection fraction, prognosis
Subject Categories: Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- CFR
coronary flow reserve
- FFR
fractional flow reserve
- TVF
target vessel failure
Clinical Perspective.
What Is New?
Compared with patients with preserved left ventricular ejection fraction (LVEF), patients with reduced LVEF had increased resting coronary flow, which led to lower coronary flow reserve values, despite a similar degree of epicardial stenosis.
Conversely, hyperemic physiologic indexes including fractional flow reserve had no significant correlation with LVEF and were not significantly different between the 2 groups.
Depressed coronary flow reserve (≤2.0) was independently associated with an increased risk of target vessel failure, regardless of different patterns of resting and hyperemic coronary flow or LVEF.
What Are the Clinical Implications?
These results suggest that maximally achievable hyperemic flow may not necessarily be affected by the presence of LV dysfunction, and that fractional flow reserve–guided strategy would still be valuable in patients with reduced LVEF, consistent with prior studies showing clinical effectiveness of fractional flow reserve–guided decision making in this patient population.
Furthermore, the prognostic relevance of depressed coronary flow reserve are consistent despite heterogeneous underlying mechanism such as disturbed autoregulatory processes in coronary circulation, intraindividual variability in resting condition, uncontrolled blood pressure or heart rate, or coronary microcirculatory dysfunction.
Therefore, measurement of coronary flow reserve in patients with reduced LVEF would provide a significant benefit in risk stratification and enable an individualized approach.
Coronary flow reserve (CFR) is a coronary physiologic index defined as a ratio of maximal hyperemic coronary flow to resting coronary flow. Low CFR represents flow limitation of target vessel encompassing the entire coronary circulatory system, from epicardial coronary artery to coronary microvasculature. 1 , 2 In previous studies, CFR was found to be a prognostic indicator in patients with coronary artery disease (CAD), regardless of the significance of epicardial coronary stenosis or clinical presentation. 3 , 4 , 5 , 6 , 7
However, coronary physiologic assessments were mostly confined to patients with preserved left ventricular (LV) systolic function, 3 , 4 , 5 , 6 , 7 and the clinical implications of coronary physiologic assessment have not been sufficiently validated in patients with reduced LV ejection fraction (LVEF). Theoretically, epicardial coronary stenosis affects LV systolic function by diminished coronary flow and impairment of myocardial perfusion. Conversely, LV systolic function cannot alter the degree of epicardial coronary stenosis or maximal hyperemic flow in an epicardial coronary artery. 8 , 9 Furthermore, in contrast to hyperemic pressure–derived indexes such as fractional flow reserve (FFR), flow‐derived indexes including CFR are known to be affected by various clinical and hemodynamic factors, and there are limited data on the relationship between flow‐derived indexes and LVEF and the prognostic role of CFR in patients with reduced LVEF. 10
Therefore, the current study sought to evaluate (1) the association between intracoronary physiologic indexes and LVEF; and (2) the differential prognostic implications according to LVEF and CFR using 5‐year follow‐up data from the ILIAS, international multicenter vessel‐level pooled registry of intracoronary pressure and flow assessment.
METHODS
Anonymized patient‐level data will be made available by the executive and publication committee for reasonable requests. Consent was not obtained for data sharing but the presented data are anonymized and risk of identification is minimal.
Study Design of ILIAS Registry
The ILIAS (Inclusive Invasive Physiological Assessment in Angina Syndromes) registry is an international, multicenter, vessel‐level pooled registry of intracoronary pressure and flow assessment. The registry is gathered from 20 institutes located in Korea, the Netherlands, Japan, Spain, Italy, Denmark, and the United States. All data were prospectively recorded according to the protocols of each study. Patients who underwent clinically indicated coronary angiography and comprehensive intracoronary physiologic assessment of at least 1 native coronary artery were included. Patients with hemodynamic instability, significant valvular heart disease, prior coronary artery bypass graft surgery, or culprit vessels of acute coronary syndromes were excluded. Individual patient data were recorded using standardized and anonymized spreadsheets by a fully compliant cloud‐based clinical data platform (Castor EDC, Amsterdam, the Netherlands). Standardized definitions were used for all variables including clinical outcomes. The study protocol was approved by the Institutional Review Board or Ethics Committee at each participating center and written informed consent was obtained from all participants. The study protocol was in accordance with the Declaration of Helsinki. The ILIAS Registry is registered at Clinicaltrials.gov (NCT04485234).
Study Population
A total of 2322 patients with 3046 vessels were enrolled in the ILIAS registry. Among the total population, patients with unavailable baseline LVEF or CFR data and those with no follow‐up data were excluded for the current study, leaving 1889 patients with 2492 vessels with clinical outcomes during 5 years of follow‐up. According to CFR, the study population was classified into preserved CFR (>2.0) (1276 patients with 1721 vessels) or depressed CFR (≤2.0) (613 patients with 771 vessels) cohorts. The patients were further stratified by LVEF using a cut‐off value of 50% into 4 groups: preserved CFR and LVEF group (1185 patients with 1602 vessels); preserved CFR and reduced LVEF group (91 patients with 119 vessels); depressed CFR and preserved LVEF group (542 patients with 688 vessels); and depressed CFR and reduced LVEF group (71 patients with 83 vessels) (Figure 1).
Figure 1. Study flow.
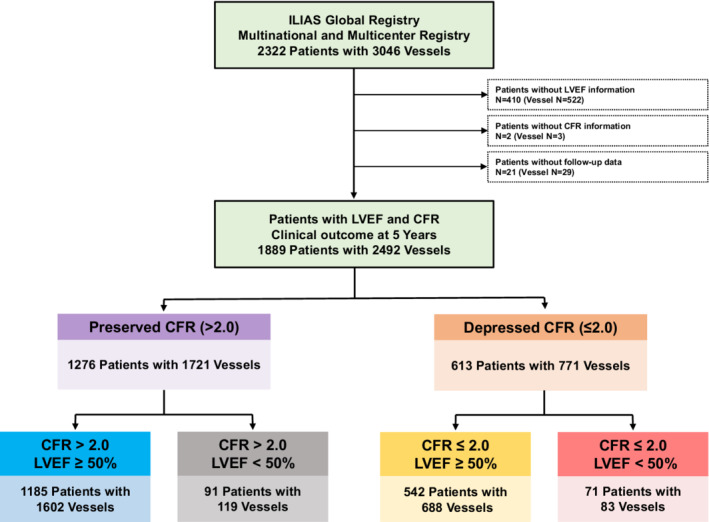
Study flow is presented. Among the total population of the ILIAS registry (2322 patients with 3046 vessels), patients with unavailable baseline LVEF or CFR data and without follow‐up data were excluded for the current study, leaving 1889 patients with 2492 vessels with clinical outcomes during 5 years of follow‐up. CFR indicates coronary flow reserve; ILIAS (Inclusive Invasive Physiological Assessment in Angina Syndromes) registry; LVEF, left ventricular ejection fraction; and TVF, target vessel failure.
Coronary Angiography and Intracoronary Physiologic Measurements
Coronary angiography and intracoronary physiologic assessment were performed using standard techniques. Angiographic views were obtained following the administration of intracoronary nitrates (100 or 200 μg). After diagnostic coronary angiography, an intracoronary physiological assessment was performed using a pressure–temperature sensor‐tipped guide wire (PressureWire; AbbottVascular, St. Paul, MN), a Doppler velocity‐equipped coronary guide wire (FloWire; Philips‐Volcano, San Diego, CA), or a dual pressure‐ and Doppler velocity‐equipped guide wire (ComboWire; Philips‐Volcano, San Diego, CA). Before physiologic assessment, intracoronary nitrates (100 or 200 μg) were administered, and the tip of the guide wire was positioned at the distal segment of the target vessel. Hyperemia was induced by an intravenous infusion of adenosine (140 μg/kg per min) or adenosine triphosphate (150 μg/kg per min) through a peripheral or central vein, an intracoronary bolus injection of adenosine (40–200 μg), or an intracoronary bolus injection of nicorandil (2 mg), according to local standards. 11 For the pressure and flow‐derived physiologic measurement, thermodilution or Doppler methods were used in 1569 vessels (63.0%) and 923 vessels (37.0%), respectively. FFR was calculated as the ratio between the mean proximal aortic (Pa) and mean distal coronary pressures (Pd) during maximal hyperemia. For the thermodilution method, resting and hyperemic thermodilution curves were obtained using 3 injections (4 mL each) of room‐temperature saline for derivation of resting and hyperemic mean transit times (Tmn). CFR was calculated as the ratio of hyperemic Tmn to resting Tmn. The index of microcirculatory resistance (IMR) was calculated as hyperemic Pd×hyperemic Tmn, and corrected using Yong's formula in case of FFR≤0.80 (corrected IMR=Pa×Tmn×([1.35×Pd/Pa]×0.32)). 12 For the Doppler method, basal and hyperemic average peak flow velocities (APV) were measured, and CFR was calculated as the ratio of hyperemic APV to resting APV. Basal microvascular resistance and hyperemic microvascular resistance were calculated as the ratio of resting Pd to resting APV and hyperemic Pd to hyperemic APV, respectively. 13 After all measurements were completed, the guide wire was pulled back to the guiding catheter. When the pressure drift was larger than >0.03 of an FFR unit, re‐equalization and repeated measurements were recommended.
Treatment, Patient Follow‐up, and Clinical Outcomes
Percutaneous coronary intervention (PCI) of the target vessel was recommended according to current guidelines at the time of procedure. However, the final decision was at the discretion of the operator. Optimal medical treatments with antiplatelet agents, statins, and antianginal medications were provided based on guidelines.
Follow‐up was performed by outpatient visits or telephone contact. The median follow‐up duration of the study population was 1140.0 days (interquartile range: 598.0–1826.0 days). The primary outcome was target‐vessel failure (TVF), which was defined as a composite of cardiac death, target vessel myocardial infarction (MI), and clinically driven target vessel revascularization. All clinical outcomes were defined according to the Academic Research Consortium report. 14 Periprocedural MI was not coded as a clinical event. All adverse clinical events were verified by assessing hospital records or contacting the primary cardiologist or general practitioner.
Statistical Analysis
Data were analyzed on a per‐patient basis for clinical characteristics and a per‐vessel basis for comparison of angiographic characteristics, physiologic indexes, and vessel‐specific clinical outcomes. For per‐vessel analyses, a generalized estimating equation was used to adjust intrasubject variability among vessels from the same patient. Correlation coefficients between LVEF and physiologic indexes were analyzed by Pearson or Spearman methods according to normality. The cumulative incidence of clinical events was presented as Kaplan–Meier estimate and compared using a log‐rank test. Multivariable marginal Cox proportional hazard regression was used to calculate adjusted hazard ratio (HR) and 95% CI to compare the risk of clinical events between groups. The restricted cubic spline model was fitted to assess for linearity between continuous variables and risk of clinical events. The assumption of proportionality was assessed by the Schoenfeld residuals and graphically by the log–log plot. The adjusted covariables were age, sex, diabetes, previous MI, clinical presentation, multivessel disease, target vessel intervention, pre‐PCI diameter stenosis, pre‐PCI FFR≤0.80, and increased microcirculatory resistance (IMR≥25 or hyperemic microvascular resistance≥2.5). The multivariable marginal Cox proportional hazard model was also used to identify independent predictors for TVF. Depressed CFR (≤2.0) and reduced LVEF (<50%) were added to previously listed covariables in this analysis. All probability values were 2‐sided, and P values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS 20.0 for Windows (SPSS‐PC, Chicago, IL) and R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline Characteristics of Study Population
Figure S1 shows the distribution of LVEF and physiologic indexes of the study population. Table 1 presents baseline patient and vessel characteristics according to LVEF. Among the 1889 patients with 2492 vessels, 162 patients (8.6%) with 202 vessels (8.1%) had LVEF <50% and 1727 patients (91.4%) with 2290 vessels (91.9%) had LVEF ≥50%. Mean LVEF value was 39.4±8.1 in the reduced LVEF group and 63.0±6.2 in the preserved LVEF group. Compared with the preserved LVEF group, the reduced LVEF group showed higher prevalence of men, diabetes, current smoking, previous MI, and previous PCI. There were no significant differences in angiographic lesion severity. In comparison of resting and hyperemic hemodynamics, patients with LV dysfunction showed significantly higher resting pressure‐rate‐product but similar hyperemic pressure‐rate‐product than those with preserved LV function (Table 1).
Table 1.
Comparison of Baseline Characteristics According to LVEF
| Total | LVEF ≥50% | LVEF <50% | P values | |
|---|---|---|---|---|
| Patient characteristics | 1889 | 1727 (91.4%) | 162 (8.6%) | |
| Demographics | ||||
| Age, y | 63.5±10.3 | 63.4±10.3 | 64.5±10.5 | 0.197 |
| Male | 1407 (74.6%) | 1274 (73.9%) | 133 (82.1%) | 0.028 |
| Body mass index, kg/m2 | 25.8±3.9 | 25.8±3.8 | 25.5±5.2 | 0.266 |
| Baseline LVEF (%) | 60.9±9.2 | 63.0±6.2 | 39.4±8.1 | |
| Cardiovascular risk factors | ||||
| Hypertension | 1132 (60.1%) | 1030 (59.8%) | 103 (63.0%) | 0.480 |
| Diabetes | 540 (28.6%) | 475 (27.6%) | 65 (40.1%) | 0.001 |
| Hyperlipidemia | 1194 (63.3%) | 1096 (63.5%) | 98 (60.5%) | 0.495 |
| Current smoker | 431 (23.1%) | 382 (22.4%) | 49 (30.6%) | 0.023 |
| Previous myocardial infarction | 355 (18.8%) | 283 (16.4%) | 72 (44.4%) | <0.001 |
| Previous percutaneous coronary intervention | 460 (27.2%) | 407 (26.1%) | 53 (39.6%) | 0.001 |
| Family history of coronary artery disease | 532 (30.2%) | 496 (30.8%) | 36 (23.8%) | 0.092 |
| Clinical presentation | 0.778 | |||
| Acute coronary syndrome | 227 (12.0%) | 206 (11.9%) | 21 (13.0%) | |
| Stable ischemic heart disease | 1658 (88.0%) | 1518 (88.1) | 140 (87.0%) | |
| Discharge medication | ||||
| Aspirin | 964 (72.7%) | 879 (71.9%) | 84 (83.2%) | 0.020 |
| P2Y12 inhibitor | 377 (47.5%) | 340 (46.2%) | 37 (63.8%) | 0.014 |
| β‐Blocker | 519 (39.2%) | 441 (36.1%) | 78 (77.2%) | <0.001 |
| RAAS blockade | 544 (41.1%) | 473 (38.7%) | 71 (70.3%) | <0.001 |
| Statin | 835 (63.1%) | 755 (61.7%) | 80 (79.2%) | 0.001 |
| Vessel characteristics | 2492 | 2290 (91.9%) | 202 (8.1%) | |
| Angiographic evaluation | ||||
| Multivessel disease | 895 (47.7%) | 825 (47.8%) | 70 (46.4%) | 0.804 |
| LM disease | 36 (1.7%) | 34 (1.7%) | 2 (1.1%) | 0.747 |
| Target vessel location | 0.349 | |||
| LAD | 1402 (56.8%) | 1295 (57.1%) | 107 (53.2%) | |
| LCX | 506 (20.5%) | 457 (20.2%) | 49 (24.4%) | |
| RCA | 560 (22.7%) | 515 (22.7%) | 45 (22.4%) | |
| Target vessel intervention | 675 (27.1%) | 606 (26.5%) | 69 (34.2%) | 0.023 |
| Quantitative coronary angiography | ||||
| Pre‐PCI reference vessel size, mm | 3.11±0.8 | 3.12±0.76 | 3.08±0.67 | 0.763 |
| Pre‐PCI diameter stenosis, % | 50.8±18.5 | 50.8±18.4 | 51.1±19.2 | 0.880 |
| Pre‐PCI lesion length, mm | 15.0±10.6 | 15.0±10.6 | 15.3±11.1 | 0.835 |
| Pre‐PCI physiologic indexes | ||||
| Measurement methods | 0.038 | |||
| Thermodilution method | 1569 (63.0%) | 1456 (63.6%) | 113 (55.9%) | |
| Doppler method | 923 (37.0%) | 834 (36.4%) | 89 (44.1%) | |
| Hemodynamics | ||||
| Resting heart rate, bpm | 68.5±11.7 | 68.2±11.6 | 73.5±13.1 | <0.001 |
| Hyperemic heart rate, bpm | 75.4±13.2 | 75.3±13.0 | 78.0±15.5 | 0.293 |
| Resting aortic pressure (Pa) | 97.2±15.1 | 97.4±15.1 | 94.6±15.3 | 0.017 |
| Resting distal coronary pressure (Pd) | 90.5±17.2 | 90.7±17.1 | 88.3±17.5 | 0.063 |
| Hyperemic aortic pressure (Pa) | 89.3±15.3 | 89.4±15.2 | 87.8±16.1 | 0.251 |
| Hyperemic distal coronary pressure (Pd) | 75.0±17.1 | 75.1±17.0 | 74.2±18.2 | 0.570 |
| Resting pressure‐rate product, mm Hg·bpm* | 6522.0 (5477.9–7722.8) | 6486.9 (5458.9–7722.0) | 7182.5 (5767.5–7992.0) | 0.044 |
| Hyperemic pressure‐rate product, mm Hg·bpm† | 6706.0 (5595.8–7728.0) | 6697.5 (5610.0–7728.0) | 6816.0 (5472.0–7980.0) | 0.688 |
| Indexes of epicardial coronary stenosis | ||||
| Resting Pd/Pa | 0.93±0.09 | 0.93±0.09 | 0.92±0.09 | 0.300 |
| FFR | 0.83±0.13 | 0.83±0.13 | 0.82±0.12 | 0.266 |
| Basal stenosis resistance | 0.26 (0.09–0.53) | 0.27 (0.09–0.54) | 0.22 (0.10–0.44) | 0.279 |
| Hyperemic stenosis resistance | 0.32 (0.15–0.63) | 0.32 (0.14–0.64) | 0.30 (0.18–0.55) | 0.739 |
| Flow‐derived indexes | ||||
| CFR | 2.7±1.2 | 2.7±1.2 | 2.4±1.2 | <0.001 |
| Thermodilution methods (N=1569) | ||||
| Resting Tmn | 0.68 (0.44–0.99) | 0.68 (0.45–1.00) | 0.52 (0.36–0.87) | 0.013 |
| Hyperemic Tmn | 0.24 (0.18–0.36) | 0.24 (0.18–0.36) | 0.22 (0.16–0.32) | 0.357 |
| IMR | 20.8±13.1 | 20.9±13.0 | 20.3±14.3 | 0.421 |
| Doppler methods (N=923) | ||||
| Resting APV | 17.1±7.3 | 16.9±7.1 | 18.9±8.9 | 0.045 |
| Hyperemic APV | 38.0±15.1 | 37.7±15.1 | 40.4±15.3 | 0.070 |
| BMR | 6.2±2.7 | 6.2±2.7 | 5.8±3.0 | 0.045 |
| HMR | 2.3±1.0 | 2.3±1.0 | 2.1±0.8 | 0.055 |
Data are expressed as number (%), mean±SD, or median (interquartile range). APV indicates averaged peak velocity; BMR, baseline microvascular resistance; CFR, coronary flow reserve; FFR, fractional flow reserve; HMR, hyperemic microvascular resistance; IMR, index of microcirculatory resistance; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main; LVEF, left ventricular ejection fraction; Pa, aortic pressure; PCI, percutaneous coronary intervention; Pd, distal coronary pressure; RCA, right coronary artery; RAAS, renin‐angiotensin‐aldosterone system; and Tmn, mean transit time.
Resting pressure‐rate product was calculated as resting heart rates (bpm) × resting aortic pressure (Pa, mm Hg).
Hyperemic pressure‐rate product was calculated as hyperemic heart rates (bpm) × hyperemic aortic pressure (Pa, mm Hg).
Table 2 shows baseline patient and vessel characteristics according to pre‐PCI CFR using a cut‐off value of 2.0. Among the total study population, 613 patients (32.5%) with 771 vessels (30.9%) had depressed CFR ≤2.0 and 1276 patients (67.5%) with 1721 vessels (69.1%) had preserved CFR >2.0. There was no significant difference in clinical characteristics between the 2 groups, except age. Regarding angiographic disease severity, patients with depressed CFR had higher proportions of multivessel disease, left main disease, and higher stenosis severity than those with preserved CFR. In addition, the depressed CFR group had lower pre‐PCI resting Pd/Pa and FFR values and a higher degree of microvascular resistance than the preserved CFR group.
Table 2.
Comparison of Baseline Characteristics According to CFR
| Total | CFR>2.0 | CFR ≤2.0 | P values | |
|---|---|---|---|---|
| Patient characteristics | 1889 | 1276 (67.5%) | 613 (32.5%) | |
| Demographics | ||||
| Age, y | 63.5±10.3 | 62.9±10.2 | 64.9±10.6 | <0.001 |
| Male | 1407 (74.6%) | 946 (74.3%) | 461 (75.2%) | 0.719 |
| Body mass index, kg/m2 | 25.8±3.9 | 25.8±4.0 | 25.9±3.8 | 0.348 |
| Baseline LVEF, % | 60.9±9.2 | 61.4±8.5 | 60.0±10.6 | 0.109 |
| Cardiovascular risk factors | ||||
| Hypertension | 1132 (60.1%) | 746 (58.6%) | 386 (63.1%) | 0.071 |
| Diabetes | 540 (28.6%) | 348 (27.4%) | 192 (31.3%) | 0.084 |
| Hyperlipidemia | 1194 (63.3%) | 802 (63.0%) | 392 (63.9%) | 0.712 |
| Current smoker | 431 (23.1%) | 296 (23.5%) | 135 (22.2%) | 0.557 |
| Previous myocardial infarction | 355 (18.8%) | 228 (17.9%) | 127 (20.7%) | 0.162 |
| Previous percutaneous coronary intervention | 460 (27.2%) | 332 (28.1%) | 128 (25.1%) | 0.235 |
| Family history of coronary artery disease | 532 (30.2%) | 357 (29.4%) | 175 (31.9%) | 0.327 |
| Clinical presentation | 0.051 | |||
| Acute coronary syndrome | 227 (12.0%) | 140 (11.0%) | 87 (14.2%) | |
| Stable ischemic heart disease | 1658 (88.0%) | 1134 (89.0%) | 524 (85.8%) | |
| Discharge medication | ||||
| Aspirin | 964 (72.7%) | 659 (70.6%) | 304 (77.9%) | 0.007 |
| P2Y12 inhibitor | 377 (47.5%) | 233 (44.2%) | 144 (53.9%) | 0.012 |
| β‐Blocker | 519 (39.2%) | 332 (35.5%) | 187 (47.9%) | <0.001 |
| RAAS blockade | 544 (41.1%) | 370 (39.6%) | 174 (44.6%) | 0.104 |
| Statin | 835 (63.1%) | 572 (61.2%) | 263 (67.4%) | 0.039 |
| Vessel characteristics | 2492 | 1721 (69.1%) | 771 (30.9%) | |
| Angiographic evaluation | ||||
| Multivessel disease | 895 (47.7%) | 579 (44.7%) | 316 (54.1%) | <0.001 |
| LM disease | 36 (1.7%) | 18 (1.2%) | 18 (2.7%) | 0.022 |
| Target vessel location | 0.450 | |||
| LAD | 1402 (56.8%) | 968 (56.7%) | 434 (57.0%) | |
| LCX | 506 (20.5%) | 341 (20.0%) | 165 (21.7%) | |
| RCA | 560 (22.7%) | 397 (23.3%) | 163 (21.4%) | |
| Target vessel intervention | 675 (27.1%) | 320 (18.6%) | 355 (46.0%) | <0.001 |
| Quantitative coronary angiography | ||||
| Pre‐PCI reference vessel size, mm | 3.11±0.8 | 3.15±0.73 | 3.03±0.78 | 0.006 |
| Pre‐PCI diameter stenosis, % | 50.8±18.5 | 48.5±17.9 | 55.9±18.8 | <0.001 |
| Pre‐PCI lesion length, mm | 15.0±10.6 | 14.8±10.3 | 16.0±11.1 | 0.156 |
| Pre‐PCI physiologic indexes | ||||
| Flow measurement | 0.153 | |||
| Thermodilution method | 1569 (63.0%) | 1100 (63.9%) | 469 (60.8%) | |
| Doppler method | 923 (37.0%) | 621 (36.1%) | 302 (39.2%) | |
| Hemodynamics | ||||
| Resting heart rate, bpm | 68.5±11.7 | 67.7±11.4 | 70.2±12.3 | <0.001 |
| Hyperemic heart rate, bpm | 75.4±13.2 | 74.8±12.8 | 76.9±13.8 | 0.028 |
| Resting aortic pressure (Pa) | 97.2±15.1 | 97.6±14.9 | 96.2±15.6 | 0.112 |
| Resting distal coronary pressure (Pd) | 90.5±17.2 | 92.9±15.6 | 85.2±19.2 | <0.001 |
| Hyperemic aortic pressure (Pa) | 89.3±15.3 | 89.8±15.0 | 88.2±15.8 | 0.018 |
| Hyperemic distal coronary pressure (Pd) | 75.0±17.1 | 77.7±15.5 | 68.9±18.9 | <0.001 |
| Resting pressure‐rate product, mm Hg·bpm* | 6522.0 (5477.9–7722.8) | 6455.9 (5460.5–7700.0) | 6714.0 (5570.2–7780.7) | 0.176 |
| Hyperemic pressure‐rate product, mm Hg·bpm† | 6706.0 (5595.8–7728.0) | 6714.0 (5570.2–7780.7) | 6694.0 (5621.8–7588.5) | 0.953 |
| Indexes of epicardial coronary stenosis | ||||
| Resting Pd/Pa | 0.93±0.09 | 0.95±0.05 | 0.88±0.13 | <0.001 |
| FFR | 0.83±0.13 | 0.86±0.10 | 0.77±0.16 | <0.001 |
| Basal stenotic resistance | 0.26 (0.09–0.53) | 0.22 (0.08–0.43) | 0.40 (0.18–0.93) | <0.001 |
| Hyperemic stenotic resistance | 0.32 (0.15–0.63) | 0.25 (0.11–0.43) | 0.58 (0.31–1.21) | <0.001 |
| Flow‐derived indexes | ||||
| CFR | 2.7±1.2 | 3.2±1.1 | 1.5±0.3 | |
| Thermodilution methods (N=1569) | ||||
| Resting Tmn | 0.68 (0.44–0.99) | 0.76 (0.54–1.05) | 0.42 (0.28–0.74) | <0.001 |
| Hyperemic Tmn | 0.24 (0.18–0.36) | 0.23 (0.17–0.31) | 0.31 (0.20–0.49) | <0.001 |
| IMR | 20.8±13.1 | 19.0±9.9 | 24.9±17.7 | <0.001 |
| Doppler methods (N=923) | ||||
| Basal APV | 17.1±7.3 | 15.7±6.0 | 20.0±8.8 | <0.001 |
| Hyperemic APV | 38.0±15.1 | 41.5±14.4 | 30.7±14.0 | <0.001 |
| BMR | 6.2±2.7 | 6.8±2.7 | 4.9±2.3 | <0.001 |
| HMR | 2.3±1.0 | 2.2±0.8 | 2.6±1.1 | <0.001 |
Data are expressed as number (%), mean±SD, or median (interquartile range). APV indicates averaged peak velocity; BMR, baseline microvascular resistance; bpm, beats per minute; CFR, coronary flow reserve; FFR, fractional flow reserve, HMR; hyperemic microvascular resistance; IMR, index of microcirculatory resistance; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main; LVEF, left ventricular ejection fraction; Pa, aortic pressure; PCI, percutaneous coronary intervention, Pd, distal coronary pressure; RAAS, renin‐angiotensin‐aldosterone system; RCA, right coronary artery; and Tmn, mean transit time.
Resting pressure‐rate product was calculated as resting heart rates (bpm) × resting aortic pressure (Pa, mm Hg).
Hyperemic pressure‐rate product was calculated as hyperemic heart rates (bpm) × hyperemic aortic pressure (Pa, mm Hg).
Association Between LVEF and Intracoronary Physiologic Indexes
Both CFR and resting Pd/Pa showed a weak correlation with LVEF (R=0.097, P<0.001 and R=0.050, P=0.029, respectively) Conversely, FFR showed no significant correlation with LVEF (R=0.038, P=0.095) (Figure S2). Compared with the preserved LVEF group, the reduced LVEF group had significantly lower CFR values (2.4±1.2 versus 2.7±1.2, P<0.001), which was originated from significantly higher resting coronary flow velocity, represented by lower resting Tmn and higher resting APV values (Table 1 and Figure 2). Conversely, pressure‐derived indexes for epicardial coronary stenosis, such as resting Pd/Pa, FFR, and basal and hyperemic stenosis resistance, were not significantly different between the 2 groups (Table 1 and Figure 3).
Figure 2. Comparison of flow‐derived physiologic indexes according to LVEF.
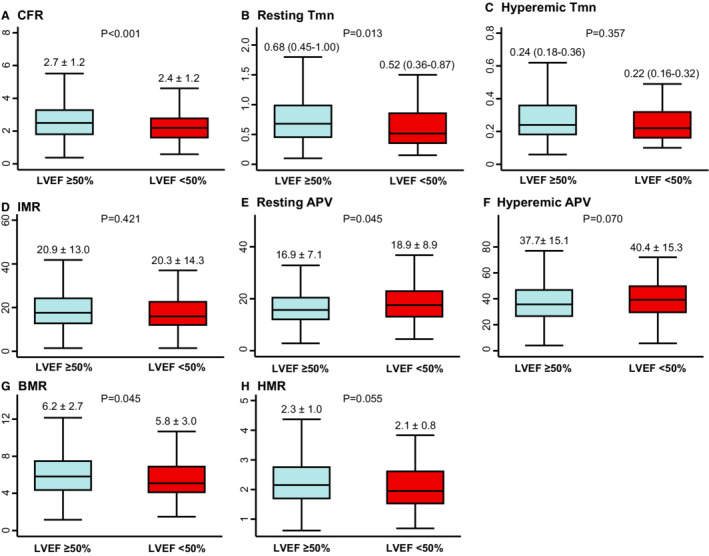
Comparison of A, CFR, B, Resting Tmn, C, Hyperemic Tmn, D, IMR, E, Resting APV, F, Hyperemic APV, G, BMR, H, HMR are shown according to LVEF. Data are expressed as mean±SD or median (interquartile range). APV indicates averaged peak velocity; BMR, basal microvascular resistance; CFR, coronary flow reserve; HMR, hyperemic microvascular resistance; IMR, index of microcirculatory resistance; LVEF, left ventricular ejection fraction; and Tmn, mean transit time.
Figure 3. Comparison of physiologic indexes of epicardial coronary stenosis according to LVEF.
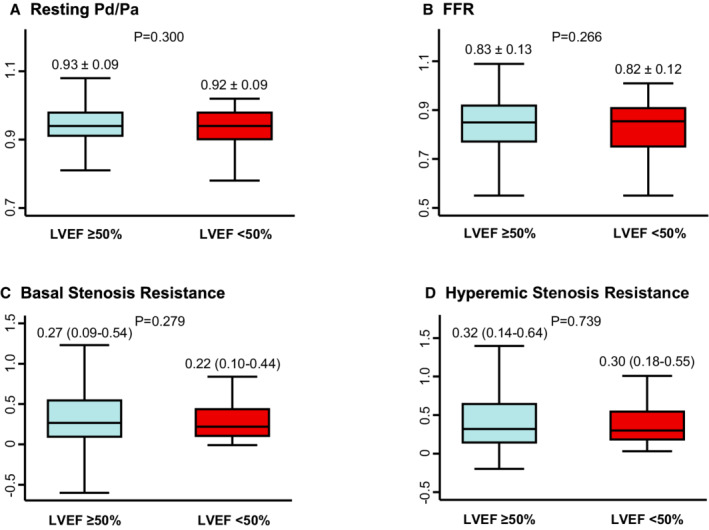
A, Resting Pd/Pa, B, FFR, C, Basal Stenosis Resistance, D, Hyperemic Stenosis Resistance are compared between patients with preserved and reduced LVEF. Data are expressed as mean±SD or median (interquartile range). FFR indicates fractional flow reserve; resting Pd/Pa, resting distal coronary pressure/aortic pressure; and LVEF, left ventricular ejection fraction.
Clinical Outcomes According to LVEF and CFR
Table 3 and Figure S3 present the cumulative incidence of TVF during 5 years of follow‐up. Both LVEF and CFR displayed linear inverse association with 5‐year risk of TVF as a continuous variable (Figure S4). Patients with reduced LVEF showed numerically higher risk of TVF than those with preserved LVEF (14.9% versus 9.2%, adjusted HR, 1.386 [95% CI, 0.677–2.840], P=0.372), mainly driven by significantly higher risk of target vessel MI (6.8% versus 1.6%, adjusted HR, 3.257 [95% CI, 1.044–10.167], P=0.042). Conversely, patients with depressed CFR had a significant higher risk of TVF than those with preserved CFR (14.6% versus 7.7%, adjusted HR, 2.080 [95% CI, 1.386–3.122], P<0.001). Patients with depressed CFR also showed a significantly higher risk of cardiac death, target vessel MI, and target vessel revascularization than those with preserved CFR (Table 3).
Table 3.
Clinical Outcomes Over 5 Years of Follow‐Up According to LVEF or CFR
| Clinical outcomes |
LVEF ≥50% (n=2290) |
LVEF <50% (n=202) |
Univariable HR (95% CI) | Multivariable HR* (95% CI)* | P value |
|---|---|---|---|---|---|
| Target vessel failure | 162 (9.2%) | 23 (14.9%) | 1.589 (0.961–2.628) | 1.386 (0.677–2.840) | 0.372 |
| All‐cause death | 80 (4.7%) | 14 (9.8%) | 1.960 (1.092–3.519) | 1.322 (0.550–3.178) | 0.533 |
| Cardiac death | 57 (3.4%) | 10 (6.9%) | 1.953 (0.965–3.952) | 1.265 (0.417–3.842) | 0.678 |
| Target vessel myocardial infarction | 28 (1.6%) | 10 (6.8%) | 4.037 (1.642–9.928) | 3.257 (1.044–10.167) | 0.042 |
| Target vessel revascularization | 116 (6.5%) | 9 (6.3%) | 0.875 (0.418–1.832) | 0.557 (0.159–1.957) | 0.362 |
| Clinical outcomes |
CFR >2.0 (n=1721) |
CFR ≤2.0 (n=771) |
Univariable HR (95% CI) | Multivariable HR* (95% CI)* | P value |
|---|---|---|---|---|---|
| Target vessel failure | 101 (7.7%) | 84 (14.6%) | 2.071 (1.528–2.806) | 2.080 (1.386–3.122) | <0.001 |
| All‐cause death | 45 (3.7%) | 49 (8.6%) | 2.738 (1.785–4.200) | 2.113 (1.215–3.677) | 0.008 |
| Cardiac death | 34 (2.7%) | 33 (6.3%) | 2.444 (1.472–4.057) | 1.906 (1.031–3.526) | 0.040 |
| Target vessel myocardial infarction | 20 (1.6%) | 18 (3.3%) | 2.237 (1.114–4.491) | 3.693 (1.348–10.116) | 0.011 |
| Target vessel revascularization | 66 (5.0%) | 59 (10.2%) | 2.213 (1.531–3.198) | 2.574 (1.557–4.255) | <0.001 |
CFR indicates coronary flow reserve; FFR, fractional flow reserve; HMR, hyperemic microvascular resistance; HR, hazard ratio; IMR, index of microcirculatory resistance; LVEF, left ventricular ejection fraction; and PCI, percutaneous coronary interventions.
Adjusted covariables were age, sex, diabetes, previous myocardial infarction, acute coronary syndrome, multivessel disease, target vessel intervention, pre‐PCI diameter stenosis, FFR ≤0.80, IMR ≥25, or HMR ≥2.5.
Prognostic Implications of LVEF and CFR
Table 4 and Figure 4 show clinical outcomes in the 4 groups stratified by CFR and LVEF. These 4 groups showed significantly different risk of TVF during 5 years of follow‐up (overall log‐rank P<0.001). The cumulative incidence of TVF was highest in the reduced LVEF and depressed CFR group (17.4%) and lowest in the preserved LVEF and CFR group (7.2%). The risk of TVF was similar between the reduced LVEF and preserved CFR group (13.1%) versus the preserved LVEF and depressed CFR group (14.2%). In the multivariable model, the independent predictors for TVF were depressed CFR, pre‐PCI FFR (≤0.80) and pre‐PCI diameter stenosis, but not LVEF (HR, 1.381 [95% CI, 0.681–2.800], P=0.371). Among them, depressed CFR was the most relevant independent predictor of TVF (HR, 2.081 [95% CI, 1.385–3.126], P<0.001) (Table 5).
Table 4.
Comparison of Clinical Outcomes Among 4 Groups Classified by LVEF and CFR
| Clinical outcomes | CFR>2.0 | CFR ≤2.0 | P value* | ||
|---|---|---|---|---|---|
|
LVEF ≥50% (n=1602) |
LVEF <50% (n=119) |
LVEF ≥50% (n=688) |
LVEF <50% (n=83) |
||
| Target vessel failure | 89 (7.2%) | 12 (13.1%) | 73 (14.2%) | 11 (17.4%) | <0.001 |
| All‐cause death | 38 (3.3%) | 7 (8.3%) | 42 (8.1%) | 7 (11.8%) | <0.001 |
| Cardiac death | 28 (2.4%) | 6 (6.8%) | 29 (6.2%) | 4 (7.1%) | <0.001 |
| Target vessel myocardial infarction | 17 (1.4%) | 3 (3.7%) | 11 (2.2%) | 7 (11.1%) | <0.001 |
| Target vessel revascularization | 60 (4.8%) | 6 (6.8%) | 56 (10.8%) | 3 (5.2%) | <0.001 |
CFR indicates coronary flow reserve; and LVEF, left ventricular ejection fraction.
Overall Log Rank P value.
Figure 4. Comparison of TVF According to LVEF and CFR.
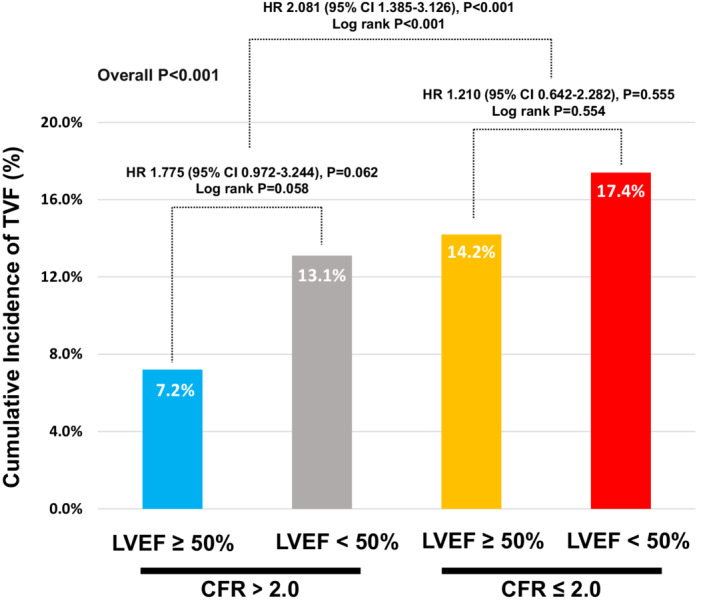
Cumulative incidence of TVF at 5 years are compared among 4 groups classified according to LVEF and CFR. Multivariable marginal Cox proportional hazard regression was used to calculate adjusted HR and 95% CI. The adjusted covariables were age, sex, diabetes, previous myocardial infarction, clinical presentation, multivessel disease, target vessel intervention, pre‐PCI diameter stenosis, pre‐PCI FFR≤0.80, and increased microcirculatory resistance (IMR≥25 or HMR≥2.5). CFR indicates coronary flow reserve; FFR, fractional flow reserve; HMR, hyperemic microvascular resistance; HR, hazard ratio; IMR, index of microvascular resistance; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; and TVF, target vessel failure.
Table 5.
Independent Predictors for Target Vessel Failure
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| CFR ≤2.0 | 2.070 (1.550–2.766) | <0.001 | 2.081 (1.385–3.126) | <0.001 |
| LVEF <50% | 1.589 (0.961–2.628) | 0.040 | 1.381 (0.681–2.800) | 0.371 |
| Age | 1.019 (1.001–1.037) | 0.035 | 1.022 (0.998–1.045) | 0.069 |
| Male | 1.368 (0.901–2.077) | 0.142 | 1.160 (0.681–1.977) | 0.586 |
| Diabetes | 1.739 (1.247–2.424) | 0.001 | 1.372 (0.871–2.160) | 0.172 |
| Previous myocardial infarction | 1.721 (1.194–2.481) | 0.004 | 1.446 (0.868–2.408) | 0.157 |
| Clinical presentation (ACS) | 1.190 (0.709–1.997) | 0.510 | 0.791 (0.399–1.568) | 0.502 |
| Multivessel disease | 2.049 (1.381–3.039) | <0.001 | 1.223 (0.741–2.017) | 0.431 |
| Target vessel intervention | 1.535 (1.126–2.092) | 0.007 | 0.646 (0.392–1.064) | 0.086 |
| Pre‐PCI diameter stenosis | 1.017 (1.008–1.025) | <0.001 | 1.016 (1.003–1.029) | 0.013 |
| Pre‐PCI FFR (≤0.80) | 2.721 (2.042–3.626) | <0.001 | 1.987 (1.273–3.101) | 0.003 |
| Microvascular dysfunction (IMR ≥25 or HMR ≥2.5) | 0.896 (0.631–1.273) | 0.541 | 0.860 (0.554–1.335) | 0.502 |
ACS indicates acute coronary syndrome; CFR, coronary flow reserve; FFR, fractional flow reserve; HMR, hyperemic microvascular resistance; HR, hazard ratio; IMR, index of microcirculatory resistance; LVEF, left ventricular ejection fraction; and PCI, percutaneous coronary intervention.
Subgroup Analysis According to Different Patterns of Resting and Hyperemic Coronary Flow Among Depressed CFR
Figure S5 presents the tertile distribution of resting or hyperemic surrogate marker of coronary flow (mean transit time, averaged peak velocity) among the depressed CFR cohort. To evaluate the prognostic impact of different patterns of resting and hyperemic flow among the depressed CFR cohort, we classified the depressed CFR cohort into 2 groups: (1) High resting flow and intermediate‐to‐high hyperemic flow; and (2) Low hyperemic flow and low‐to‐intermediate resting flow. Figure 5 shows the cumulative incidence of 5‐year TVF according to different patterns of depressed CFR. Regardless of different patterns in resting and hyperemic flow, the depressed CFR cohort showed significantly higher risk of TVF than the preserved CFR cohort.
Figure 5. Comparison of target vessel failure according to different patterns of depressed CFR.
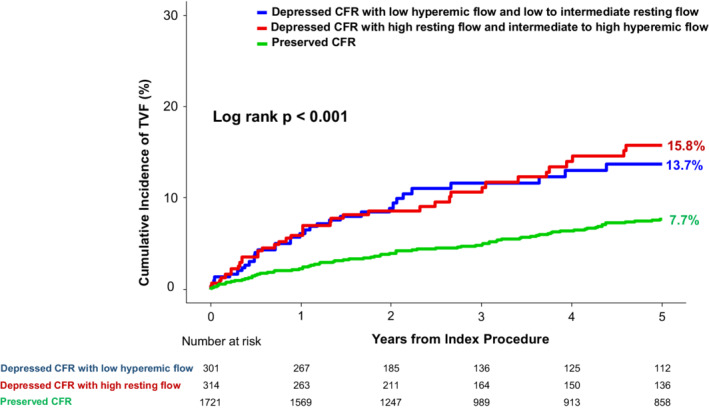
Cumulative incidence of TVF at 5 years is compared according to different patterns in resting and hyperemic coronary flow among depressed CFR cohort. In this analysis, 305 vessels with missing value in resting and hyperemic Tmn (19.4% of thermodilution technique) were excluded. Regardless of different patterns in resting and hyperemic flow, the depressed CFR cohort showed significantly higher risk of TVF than the preserved CFR cohort. CFR indicates coronary flow reserve; Tmn, mean transit time; and TVF, target vessel failure.
DISCUSSION
The current study evaluated the prognostic implications of coronary physiologic assessment in patients with reduced LVEF (<50%). Key findings are as follows. First, compared with patients with preserved LVEF, patients with reduced LVEF had increased resting coronary flow that led to lower CFR values, despite a similar degree of epicardial stenosis. Second, hyperemic physiologic indexes including FFR had no significant correlation with LVEF and were not significantly different between the 2 groups. Third, depressed CFR (≤2.0) was independently associated with an increased risk of TVF, regardless of LVEF. Fourth, patients with depressed CFR had a significantly higher risk of TVF than those with preserved CFR, regardless of different patterns of resting and hyperemic coronary flow.
Coronary Blood Flow and CFR in Reduced LVEF
Clinically, CFR represents the reserve of vasodilatory capacity in the reference vessel. 2 Besides significant epicardial coronary stenosis, CFR values can also be affected by either reduced hyperemic coronary flow because of microvascular dysfunction or increased resting coronary flow in the presence of disturbed coronary autoregulation, or both. 2 , 15 A few prior studies suggested that reduced hyperemic blood flow because of microvascular dysfunction could be a dominant cause of depressed CFR among patients with reduced LVEF. 16 , 17 In contrast, the results of the current study suggest that increased resting coronary flow and disturbed coronary autoregulation but not reduced hyperemic blood flow or microvascular dysfunction would be the predominant mechanism for the depressed CFR in patients with reduced LVEF. In the current study, patients with reduced LVEF showed significantly lower resting Tmn and higher resting APV values without difference in hyperemic Tmn or hyperemic APV values than those with preserved LVEF. Furthermore, there was no significant difference in IMR and hyperemic microvascular resistance values, suggesting a similar degree of microvascular dysfunction between the 2 groups. It would be important to notice that the extent, location, quantitative angiographic severity, and physiologic significance of epicardial CAD were similar between the 2 groups, excluding the possible contributions of the difference in epicardial CAD on these findings.
Disturbed coronary autoregulation is well described in patients with CAD. In normal coronary vasculature, coronary blood flow is maintained relatively constant by pressure‐flow autoregulation despite changes in coronary perfusion pressure, which is accomplished by adjustment of coronary microvascular resistance via various physiologic, metabolic, endothelial, and neuro‐hormonal mechanisms. 18 , 19 In the setting of decreased coronary perfusion pressure because of significant epicardial stenosis or elevated left ventricular end‐diastolic pressure, autoregulatory vasodilation of coronary arterioles occurs to maintain coronary blood flow and myocardial perfusion. 15 Although left ventricular end‐diastolic pressure or filling pressures were not available in the current registry, it could be hypothesized that the elevated left ventricular end‐diastolic pressure might have caused elevated resting flow and depressed CFR in patients with reduced LVEF. Furthermore, patients with reduced LVEF are likely to have an increased oxygen demand because of elevated resting heart rate and increased LV mass from the remodeling process, which could also lead to increase in the resting blood flow, and thus decrease in the CFR values. Significantly higher resting pressure‐rate‐product but similar hyperemic pressure‐rate‐product in the LV dysfunction group compared with the preserved LV function group further support this hypothesis.
Hyperemic Indexes in Reduced LVEF
As opposed to the resting flow indexes, the current study demonstrated that hyperemic indexes including FFR were not significantly different between patients with preserved and reduced LVEF. Although FFR has been used widely to determine the hemodynamic relevance of CAD in various clinical circumstances, some previous studies reported limited reliability of FFR measurement in patients with overt microvascular dysfunction. 2 , 20 , 21 This is because maximally achievable hyperemic flow decreases in the presence of overt microvascular dysfunction, which can lead to underestimation of FFR. 22 , 23 In the same context, accuracy of FFR measurements can be challenging in patients with reduced LVEF given the association between reduced LVEF and microvascular dysfunction. Furthermore, elevated venous pressures in patients with reduced LVEF can theoretically affect FFR measurement.
In the current study, FFR values did not correlate with LVEF and there was no significant difference in other hyperemic indexes such as hyperemic stenosis resistance, hyperemic Tmn, and hyperemic APV between patients with preserved and reduced LVEF. Furthermore, the reduced LVEF group did not show a significantly higher degree of microvascular dysfunction compared with the preserved LVEF group, manifested by similar IMR and hyperemic microvascular resistance values. These results suggest that maximally achievable hyperemic flow may not necessarily be affected by the presence of LV dysfunction, and that an FFR‐guided strategy would still be valuable in patients with reduced LVEF, consistent with prior studies showing clinical effectiveness of FFR‐guided decision making in this patient population. 9 , 24
Prognostic Implications of LVEF and CFR
Although both LVEF and CFR have been known to be independent predictors of poor outcomes, to the best of our knowledge, no published studies evaluated the combined prognostic impact of invasively measured CFR and LVEF. In the current study, patients with depressed CFR showed significantly higher cumulative incidence of TVF, its individual components, and all‐cause mortality than patients with preserved CFR during 5 years of follow‐up. Conversely, although patients with reduced LVEF showed numerically higher cumulative incidence of TVF and all‐cause mortality than patients with preserved LVEF, statistical significance disappeared after multivariable adjustment. In multivariable marginal Cox proportional hazard analysis, depressed CFR was the most powerful independent predictor of TVF followed by pre‐PCI FFR ≤0.80, but reduced LVEF was not. Although the current study included patients with midrange LVEF (40%–50%) in the reduced LVEF group, which might have mitigated the prognostic impact of LVEF, simply reduced LVEF without significant epicardial CAD or microvascular dysfunction would be less relevant to the risk of future adverse outcomes compared with CFR. Furthermore, it should be noted that patients with depressed CFR showed significantly higher risk of TVF than those with preserved CFR, irrespective of different patterns in resting and hyperemic coronary flow. These results support the prognostic relevance of depressed CFR despite heterogeneous underlying mechanisms such as disturbed autoregulatory processes in coronary circulation, 15 intraindividual variability in resting condition, 25 uncontrolled blood pressure or heart rate, 26 or coronary microcirculatory dysfunction. 5 Therefore, measurement of CFR in patients with reduced LVEF would provide a significant benefit in risk stratification and enable an individualized approach. Future studies are needed to find effective treatment strategies to improve the prognosis of these patients.
Study Limitations
Some limitations of the study should be acknowledged. First, measured CFR in our study may not represent true value of CFR, especially in patients with reduced LVEF, because of unidentified hemodynamic interference and the absence of repeat measurement of CFR. In previous studies, however, reproducibility of CFR measurement was excellent in cases without tachycardia, hypervolemia, and use of inotropic agent. 27 , 28 Second, the current registry did not collect LV end‐diastolic pressure, pulmonary capillary wedge pressure, or central venous pressure. This information represents the overall volume status of the patient, and an abnormal volume status might have affected the results of invasive physiologic assessment. Third, records of follow‐up optimal medical management were not available in our registry. Fourth, the exact cause of LV dysfunction was not revealed in our registry, which could affect the clinical outcomes. Finally, since this was a registry‐based observational study, blinding of neither the patient nor the physician to the results of physiologic evaluation was possible.
CONCLUSIONS
CFR was lower in patients with reduced LVEF because of increased resting coronary flow. Conversely, hyperemic coronary flow and FFR were not changed according to LVEF. Patients with depressed CFR showed significantly higher risk of TVF than did those with preserved CFR, regardless of LVEF. The current study supports the feasibility of the use of intracoronary physiologic indexes in patients with reduced LVEF.
Appendix
ILIAS Registry Investigators
Tim P. van de Hoef (Department of Cardiology, Amsterdam UMC – location AMC, Amsterdam; Department of Cardiology, Amsterdam UMC – location VUmc, Amsterdam; Department of Cardiology, NoordWest Ziekenhuisgroep, the Netherlands); Joo Myung Lee, Ki Hong Choi, David Hong (Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea); Seung Hun Lee (Division of Cardiology, Department of Internal Medicine, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea); Doosup Shin (Division of Cardiovascular Medicine, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa); Masahiro Hoshino, Tadashi Murai, Tsunekazu Kakuta (Tsuchiura Kyodo General Hospital, Department of Cardiology, Tsuchiura City, Japan); Bon Kwon Koo, Doyeon Hwang (Seoul National University Hospital, Department of Internal Medicine, Cardiovascular Center, Seoul, Korea); Coen K. M. Boerhout, Jan J. Piek (Department of Cardiology, Amsterdam UMC – location AMC, Amsterdam, the Netherlands); Guus A. de Waard, Steven A. J. Chamuleau, Koen Marques, Paul Knaapen (Department of Cardiology, Amsterdam UMC – location VUmc, Amsterdam, the Netherlands); Ji‐Hyun Jung (Sejong General Hospital, Sejong Heart Institute, Bucheon, Korea); Hernan Mejia‐Renteria, Javier Escaned (Hospital Clínico San Carlos, IDISSC, and Universidad Complutense de Madrid, Madrid, Spain); Mauro Echavarria‐Pinto (Hospital General ISSSTE Querétaro ‐ Facultad de Medicina, Universidad Autónoma de Querétaro, Querétaro, México); Martijn Meuwissen (Department of Cardiology, Amphia Hospital, Breda, the Netherlands); Hitoshi Matsuo (Gifu Heart Center, Department of Cardiovascular Medicine, Gifu, Japan); Maribel Madera‐Cambero (Tergooi Hospital, Department of Cardiology, Blaricum, the Netherlands); Ashkan Eftekhari, Evald H. Christiansen (Aarhus University Hospital, Department of Cardiology, Aarhus, Denmark); Mohamed AEffat (Division of Cardiovascular Health and Disease, University of Cincinnati, Cincinnati, Ohio); Joon‐Hyung Doh (Department of Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea); Rupak Banerjee (Department of Mechanical and Materials Engineering, University of Cincinnati, Veterans Affairs Medical Center, Cincinnati, Ohio); Hyun Kuk Kim (Department of Internal Medicine and Cardiovascular Center, Chosun University Hospital, University of Chosun College of Medicine, Gwangju, Korea); Chang‐Wook Nam (Department of Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea); Giampaolo Niccoli (University of Parma, Parma, Italy); Masafumi Nakayama (Toda Central General Hospital, Cardiovascular Center, Toda, Japan); Nobuhiro Tanaka (Tokyo Medical University Hachioji Medical Center, Department of Cardiology, Tokyo, Japan); Eun‐Seok Shin (Department of Cardiology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea); Niels Royen (Department of Cardiology, Radboud University Medical Center, Nijmegen, the Netherlands).
Sources of Funding
None.
Disclosures
JML received research grants from Abbott and Philips. TvdH has received speaker fees and institutional research grants from Abbott and Philips. MEP has received speaker fees from Abbott and Philips. NvR has received speaker fees and institutional research grants from Abbott and Philips. BKK has received institutional research grants from Abbott Vascular and Philips Volcano. JJP has received support as consultant for Philips/Volcano and has received institutional research grants from Philips. The remaining authors have no disclosures to report.
Supporting information
Figures S1‐S5
A complete list of the ILIAS Registry Investigators can be found in the Appendix at the end of the manuscript.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025841
For Sources of Funding and Disclosures, see page 14.
Contributor Information
Joo Myung Lee, Email: drone80@hanmail.net, Email: joomyung.lee@samsung.com.
The ILIAS Registry Investigators:
Tim P. van de Hoef, Joo Myung Lee, Ki Hong Choi, David Hong, Seung Hun Lee, Doosup Shin, Masahiro Hoshino, Tadashi Murai, Tsunekazu Kakuta, Bon Kwon Koo, Doyeon Hwang, Coen K. M. Boerhout, Jan J. Piek, Guus A. de Waard, Steven A. J. Chamuleau, Koen Marques, Paul Knaapen, Ji‐Hyun Jung, Hernan Mejia‐Renteria, Javier Escaned, Mauro Echavarria‐Pinto, Martijn Meuwissen, Hitoshi Matsuo, Maribel Madera‐Cambero, Ashkan Eftekhari, Evald H. Christiansen, Mohamed AEffat, Joon‐Hyung Doh, Rupak Banerjee, Hyun Kuk Kim, Chang‐Wook Nam, Giampaolo Niccoli, Masafumi Nakayama, Nobuhiro Tanaka, Eun‐Seok Shin, and Niels Royen
References
- 1. Gould KL, Kirkeeide RL, Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol. 1990;15:459–474. doi: 10.1016/s0735-1097(10)80078-6 [DOI] [PubMed] [Google Scholar]
- 2. van de Hoef TP, Siebes M, Spaan JA, Piek JJ. Fundamentals in clinical coronary physiology: why coronary flow is more important than coronary pressure. Eur Heart J. 2015;36:3312–3319a. doi: 10.1093/eurheartj/ehv235 [DOI] [PubMed] [Google Scholar]
- 3. van de Hoef TP, Bax M, Meuwissen M, Damman P, Delewi R, de Winter RJ, Koch KT, Schotborgh C, Henriques JP, Tijssen JG, et al. Impact of coronary microvascular function on long‐term cardiac mortality in patients with acute ST‐segment‐elevation myocardial infarction. Circ Cardiovasc Interv. 2013;6:207–215. doi: 10.1161/CIRCINTERVENTIONS.112.000168 [DOI] [PubMed] [Google Scholar]
- 4. van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, et al. Physiological basis and long‐term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–311. doi: 10.1161/CIRCINTERVENTIONS.113.001049 [DOI] [PubMed] [Google Scholar]
- 5. Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol. 2016;67:1158–1169. doi: 10.1016/j.jacc.2015.12.053 [DOI] [PubMed] [Google Scholar]
- 6. Lee JM, Choi KH, Hwang D, Park J, Jung JH, Kim HY, Jung HW, Cho YK, Yoon HJ, Song YB, et al. Prognostic implication of thermodilution coronary flow reserve in patients undergoing fractional flow reserve measurement. JACC Cardiovasc Interv. 2018;11:1423–1433. doi: 10.1016/j.jcin.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 7. Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, Osborne MT, Seidelmann SB, Vita T, Bibbo CF, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation. 2017;136:2325–2336. doi: 10.1161/CIRCULATIONAHA.117.029992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Triposkiadis F, Giamouzis G, Parissis J, Starling RC, Boudoulas H, Skoularigis J, Butler J, Filippatos G. Reframing the association and significance of co‐morbidities in heart failure. Eur J Heart Fail. 2016;18:744–758. doi: 10.1002/ejhf.600 [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi Y, Tonino PA, De Bruyne B, Yang HM, Lim HS, Pijls NH, Fearon WF, Investigators FS. The impact of left ventricular ejection fraction on fractional flow reserve: insights from the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) trial. Int J Cardiol. 2016;204:206–210. doi: 10.1016/j.ijcard.2015.11.169 [DOI] [PubMed] [Google Scholar]
- 10. Hoffman JI. Problems of coronary flow reserve. Ann Biomed Eng. 2000;28:884–896. doi: 10.1114/1.1308503 [DOI] [PubMed] [Google Scholar]
- 11. Toth GG, Johnson NP, Jeremias A, Pellicano M, Vranckx P, Fearon WF, Barbato E, Kern MJ, Pijls NH, De Bruyne B. Standardization of Fractional Flow Reserve Measurements. J Am Coll Cardiol. 2016;68:742–753. doi: 10.1016/j.jacc.2016.05.067 [DOI] [PubMed] [Google Scholar]
- 12. Yong AS, Layland J, Fearon WF, Ho M, Shah MG, Daniels D, Whitbourn R, Macisaac A, Kritharides L, Wilson A, et al. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv. 2013;6:53–58. doi: 10.1016/j.jcin.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 13. Sambuceti G, Marzilli M, Marraccini P, Schneider‐Eicke J, Gliozheni E, Parodi O, L'Abbate A. Coronary vasoconstriction during myocardial ischemia induced by rises in metabolic demand in patients with coronary artery disease. Circulation. 1997;95:2652–2659. doi: 10.1161/01.cir.95.12.2652 [DOI] [PubMed] [Google Scholar]
- 14. Garcia‐Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium‐2 consensus document. Circulation. 2018;137:2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289 [DOI] [PubMed] [Google Scholar]
- 15. van de Hoef TP, Bax M, Damman P, Delewi R, Hassell ME, Piek MA, Chamuleau SA, Voskuil M, van Eck‐Smit BL, Verberne HJ, et al. Impaired coronary autoregulation is associated with long‐term fatal events in patients with stable coronary artery disease. Circ Cardiovasc Interv. 2013;6:329–335. doi: 10.1161/CIRCINTERVENTIONS.113.000378 [DOI] [PubMed] [Google Scholar]
- 16. Skalidis EI, Parthenakis FI, Patrianakos AP, Hamilos MI, Vardas PE. Regional coronary flow and contractile reserve in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:2027–2032. doi: 10.1016/j.jacc.2004.08.051 [DOI] [PubMed] [Google Scholar]
- 17. Majmudar MD, Murthy VL, Shah RV, Kolli S, Mousavi N, Foster CR, Hainer J, Blankstein R, Dorbala S, Sitek A, et al. Quantification of coronary flow reserve in patients with ischaemic and non‐ischaemic cardiomyopathy and its association with clinical outcomes. Eur Heart J Cardiovasc Imaging. 2015;16:900–909. doi: 10.1093/ehjci/jev012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodwill AG, Dick GM, Kiel AM, Tune JD. Regulation of coronary blood flow. Compr Physiol. 2017;7:321–382. doi: 10.1002/cphy.c160016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mosher P, Ross J Jr, McFate PA, Shaw RF. Control of coronary blood flow by an autoregulatory mechanism. Circ Res. 1964;14:250–259. doi: 10.1161/01.res.14.3.250 [DOI] [PubMed] [Google Scholar]
- 20. van de Hoef TP, Nolte F, Echavarría‐Pinto M, van Lavieren MA, Damman P, Chamuleau SA, Voskuil M, Verberne HJ, Henriques JP, van Eck‐Smit BL, et al. Impact of hyperaemic microvascular resistance on fractional flow reserve measurements in patients with stable coronary artery disease: insights from combined stenosis and microvascular resistance assessment. Heart. 2014;100:951–959. doi: 10.1136/heartjnl-2013-305124 [DOI] [PubMed] [Google Scholar]
- 21. Murai T, Lee T, Yonetsu T, Isobe M, Kakuta T. Influence of microvascular resistance on fractional flow reserve after successful percutaneous coronary intervention. Catheter Cardiovasc Interv. 2015;85:585–592. doi: 10.1002/ccd.25499 [DOI] [PubMed] [Google Scholar]
- 22. McClish JC, Ragosta M, Powers ER, Barringhaus KG, Gimple LW, Fischer J, Garnett J, Siadaty M, Sarembock IJ, Samady H. Effect of acute myocardial infarction on the utility of fractional flow reserve for the physiologic assessment of the severity of coronary artery narrowing. Am J Cardiol. 2004;93:1102–1106. doi: 10.1016/j.amjcard.2004.01.035 [DOI] [PubMed] [Google Scholar]
- 23. Claeys MJ, Bosmans JM, Hendrix J, Vrints CJ. Reliability of fractional flow reserve measurements in patients with associated microvascular dysfunction: importance of flow on translesional pressure gradient. Catheter Cardiovasc Interv. 2001;54:427–434. doi: 10.1002/ccd.2005 [DOI] [PubMed] [Google Scholar]
- 24. Di Gioia G, De Bruyne B, Pellicano M, Bartunek J, Colaiori I, Fiordelisi A, Canciello G, Xaplanteris P, Fournier S, Katbeh A, et al. Fractional flow reserve in patients with reduced ejection fraction. Eur Heart J. 2020;41:1665–1672. doi: 10.1093/eurheartj/ehz571 [DOI] [PubMed] [Google Scholar]
- 25. Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–2061. doi: 10.1161/CIRCULATIONAHA.105.603522 [DOI] [PubMed] [Google Scholar]
- 26. Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012;5:430–440. doi: 10.1016/j.jcmg.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 27. McGinn AL, White CW, Wilson RF. Interstudy variability of coronary flow reserve. Influence of heart rate, arterial pressure, and ventricular preload. Circulation. 1990;81:1319–1330. doi: 10.1161/01.cir.81.4.1319 [DOI] [PubMed] [Google Scholar]
- 28. de Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94:1842–1849. doi: 10.1161/01.CIR.94.8.1842 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1‐S5


