Abstract
Background
A single dose of small interfering RNA (siRNA) targeting liver angiotensinogen eliminates hepatic angiotensinogen and lowers blood pressure. Angiotensinogen elimination raises concerns for clinical application because an angiotensin rise is needed to maintain perfusion pressure during hypovolemia. Here, we investigated whether conventional vasopressors can raise arterial pressure after angiotensinogen depletion.
Methods and Results
Spontaneously hypertensive rats on a low‐salt diet were treated with siRNA (10 mg/kg fortnightly) for 4 weeks, supplemented during the final 2 weeks with fludrocortisone (6 mg/kg per day), the α‐adrenergic agonist midodrine (4 mg/kg per day), or a high‐salt diet (all groups n=6–7). Pressor responsiveness to angiotensin II and norepinephrine was assessed before and after siRNA administration. Blood pressure was measured via radiotelemetry. Depletion of liver angiotensinogen by siRNA lowered plasma angiotensinogen concentrations by 99.2±0.1% and mean arterial pressure by 19 mm Hg. siRNA‐mediated blood pressure lowering was rapidly reversed by intravenous angiotensin II or norepinephrine, or gradually reversed by fludrocortisone or high salt intake. Midodrine had no effect. Unexpectedly, fludrocortisone partially restored plasma angiotensinogen concentrations in siRNA‐treated rats, and nearly abolished plasma renin concentrations. To investigate whether this angiotensinogen originated from nonhepatic sources, fludrocortisone was administered to mice lacking hepatic angiotensinogen. Fludrocortisone did not increase angiotensinogen in these mice, implying that the rise in angiotensinogen in the siRNA‐treated rats must have depended on the liver, most likely reflecting diminished cleavage by renin.
Conclusions
Intact pressor responsiveness to conventional vasopressors provides pharmacological means to regulate the blood pressure–lowering effect of angiotensinogen siRNA and may support future therapeutic implementation of siRNA.
Keywords: adipose tissue, glucocorticoid receptor, renin, α‐adrenergic receptor
Subject Categories: Hypertension, ACE/Angiotension Receptors/Renin Angiotensin System, Basic Science Research, Mechanisms
Nonstandard Abbreviations and Acronyms
- eWAT
epididymal white adipose tissue
- RAS
renin‐angiotensin system
- SHR
spontaneously hypertensive rat
Clinical Perspective.
What Is New?
Intravenous angiotensin II or norepinephrine can acutely reverse low blood pressure in spontaneously hypertensive rats treated with small interfering RNA (siRNA) targeting liver angiotensinogen, whereas fludrocortisone treatment or high salt intake gradually increased blood pressure.
Although fludrocortisone partially restored the suppressed angiotensinogen concentrations in siRNA‐treated rats, this was not attributable to diminished effectivity of the siRNA or angiotensinogen release from extrahepatic sites, but rather the consequence of the fact that fludrocortisone abolished renin.
What Are the Clinical Implications?
Intact pressor responsiveness to conventional vasopressors may support therapeutic implementation of siRNA by providing pharmacological means to regulate the blood pressure–lowering effect of angiotensinogen siRNA.
Treatment of hypertension effectively prevents death and disability but remains challenging in many patients. 1 , 2 Blood pressure control may be improved by treating patients with small interfering RNA (siRNA) targeted to liver angiotensinogen. In a preclinical, proof‐of‐principle study, the antihypertensive effect of angiotensinogen siRNA was similar to that of conventional angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers. 3 Potential benefits of siRNA over currently available antihypertensive drugs include better treatment adherence because of the low frequency at which siRNA needs to be dosed, and because storage of siRNA in hepatic endosomes ensures prolonged target depletion that lasts for weeks to months after a single subcutaneous injection. 4 Resistance to renin‐angiotensin system (RAS)‐escape phenomena may represent an additional benefit; reconstitution of angiotensin II appears finite during siRNA treatment, because siRNA leaves little angiotensinogen, and the remainder is depleted because of accelerated consumption by the compensatory rise in renin that inevitably follows blood pressure lowering. 3 However, angiotensinogen depletion may also hinder broad therapeutic implementation of angiotensinogen siRNA, because acute RAS activation may be needed to maintain adequate arterial pressure and tissue perfusion during shock and other conditions that cause hypotension. 5 Therefore, we investigated whether conventional vasopressors can reverse siRNA‐mediated blood pressure lowering and raise arterial pressure when angiotensinogen is depleted. The blood pressure‐lowering effect of angiotensinogen siRNA was maximized in spontaneously hypertensive rats by limiting dietary sodium intake, an effect observed previously for other RAS blockers. 6 Full reversal of siRNA‐mediated blood pressure lowering was rapidly achieved with intravenous norepinephrine or angiotensin II, or gradually achieved by increasing dietary sodium intake or fludrocortisone. These findings may support therapeutic implementation of siRNA by providing pharmacological means to control the antihypertensive effect of angiotensinogen siRNA. Finally, because fludrocortisone upregulated angiotensinogen, we additionally investigated the origin of this angiotensinogen, making use of mice with genetic ablation of hepatic angiotensinogen.
METHODS
The authors declare that all supporting data are available within the article and its supplemental files.
Rat Studies
Male, 11‐week‐old, spontaneously hypertensive rats (SHRs; Janvier Labs, Le Genest‐Saint‐Isle, France) were studied at the Erasmus MC (Rotterdam, the Netherlands) under the regulation of the Animal Care Committee (protocol 16‐511‐02). Rats were housed individually and maintained on a 12‐hour light–dark cycle with access to standard rat laboratory diet and tap water ad libitum. Radiotelemetry transmitters (HD‐S10; Data Sciences International, St. Paul, MN) were implanted to continuously measure blood pressure, heart rate, and activity. 7 Buprenorphine (0.5 mg/kg SC) was given 1 hour before and 6 hours after surgery for perioperative analgesia, followed by twice‐daily doses up until 2 days after surgery. After a 2‐week recovery period and 3 subsequent days of baseline telemetry recordings (at 10‐minute intervals), we replaced standard rat laboratory diet (0.4% NaCl) by a low‐salt diet (<0.03% NaCl; Ssniff, Soest, Germany). Dietary sodium intake was restricted to potentiate the antihypertensive effect of angiotensinogen siRNA. Angiotensinogen siRNA (10 mg/kg SC injection; Alnylam Pharmaceuticals, Cambridge, MA) was given on day 7 and 21. From day 21 onward, rats were randomized for the final 2 weeks to fludrocortisone (n=7; 6 mg/kg per day; Sigma‐Aldrich, Zwijndrecht, the Netherlands) 8 or midodrine (n=6; 4 mg/kg per day; Cayman Chemicals, Ann Arbor, MI) 9 , 10 treatment, or the low‐salt diet was replaced by a high‐salt diet (n=6; 4% NaCl; Ssniff, Soest, Germany). Fludrocortisone and midodrine were dissolved in a mixture of polyethylene glycol 400 (85%) and dimethyl sulfoxide (15%; both from Sigma‐Aldrich) and administered subcutaneously via an osmotic minipump (2ML2; Alzet, Cupertino, CA). To exclude the possibility that the effect of midodrine was impaired by subcutaneous delivery, the administration interval of these rats was extended by 4 days in which rats received 8 mg/kg per day dissolved in the drinking water.
Just before both doses of siRNA (day 7 and 21), we measured the vasoconstrictive response (radiotelemetry at 10‐second intervals) to intravenous bolus injections of ascending doses of angiotensin II (n=11; 0.05, 0.5, and 5 μg/kg; Sigma‐Aldrich) and norepinephrine (n=8; 0.1, 1.0, and 10 μg/kg; Sigma‐Aldrich) given at 10‐minute intervals in rats anesthetized by inhalation of isoflurane. Baseline arterial pressure was established 30 seconds before each bolus.
During the study, blood samples were collected from the lateral tail vein (on day 5 and 19) and from the portal vein at termination. Blood was supplemented with EDTA (final concentration 1.8 mg/mL) and centrifuged at 3000g to obtain plasma. At termination, the livers, kidneys, and adipose tissue (epididymal, inguinal, and brown) were excised and snap‐frozen in liquid nitrogen for gene expression and protein analysis. Livers and kidneys from male, 16‐week‐old SHRs (n=3, Janvier Labs; protocol number 16‐511‐01) were used for comparison. 3 Adipose tissues from only 2 out of 6 midodrine‐treated rats were available for downstream experiments because of a technical failure.
Oligonucleotide Synthesis
The angiotensinogen siRNA consisted of a chemically modified antisense strand with sequence UUGAUUUUUGCCCAGGAUAGCUC, hybridized with a chemically modified sense strand of sequence GCUAUCCUGGGCAAAAAUCAA. Oligonucleotides were synthesized as described previously. 3 To ensure selective and efficient delivery to hepatocytes, a triantennary N‐acetylgalactosamine, a high‐affinity ligand for the hepatocyte‐specific asialoglycoprotein receptor, was attached to the 3′ end of the sense strand. 11
Mouse Studies
Male, 7‐ to 10‐week‐old angiotensinogen floxed (termed Agt f/f)×albumin‐Cre−/− (hepAGT+/+) and Agt f/f×albumin‐Cre+/− (hepAGT−/−) mice developed in an low‐density lipoprotein receptor −/− background 12 (weight 18–25 g) were studied at the University of Kentucky under the regulation of the Institutional Animal Care and Use Committee (protocol number 2018–2968). Genotypes were determined before weaning by Cre polymerase chain reaction and validated after termination by genetic services of Transnetyx blindly. Mice were group‐housed and maintained on a 14:10‐hour light–dark cycle with access to standard laboratory diet and drinking water from a reverse osmosis system (pH 6.0–6.2) ad libitum. Mice were treated for 4 weeks with fludrocortisone (12 mg/kg per day, dissolved in a mixture of polyethylene glycol‐400 (85%) and DMSO (15%), all from MilliporeSigma) or vehicle (solvent only) administered subcutaneously via an osmotic minipump (model 2004; Durect). Minipumps were implanted on the right flank of each mouse, incisions were closed with surgical staples, and a topical anesthetic cream (LMX4; Ferndale Laboratories, Ferndale, MI) was applied immediately after surgery and 3 to 4 hours later to relieve pain. Systolic blood pressure was measured in conscious mice using a noninvasive tail‐cuff system (BP‐2000 blood pressure analysis system; Visitech Systems, Apex, NC) following our standard protocol. 13 After acclimatization on day 7, 9, and 11 after pump implantation, blood pressure was measured for 5 consecutive days (days 11–15 and days 20–24). The mean from the final 3 days of measurements was used for statistical analysis. Before termination, mice were kept in metabolic cages (Techniplast, West Chester, PA) from 6 pm until 8 am (14 hours) to collect urine. Blood samples were collected at termination from the right cardiac ventricle, supplemented with EDTA, and centrifuged at 3000g to obtain plasma. Because fludrocortisone in rats induces the biggest rise in angiotensinogen in epididymal adipose tissue, we limited our tissue analysis in mice to liver and epididymal adipose tissue, which were excised and snap‐frozen in liquid nitrogen for gene expression and protein analysis.
Biochemical Measurements
In both rat and mouse plasma, intact angiotensinogen concentrations were measured by enzyme‐kinetic assay as the maximum quantity of angiotensin I generated during incubation, at pH 7.4 and 37 °C, with rat kidney renin in the presence of a mixture of angiotensin‐converting enzyme, angiotensinase, and serine protease inhibitors. 14 , 15 The lower limit of detection of this assay was 0.2 nmol/L. Active plasma renin concentrations in rat plasma were measured by enzyme‐kinetic assay, by quantifying angiotensin I generation in the presence of excess sheep angiotensinogen (lower limit of detection 0.17 ng angiotensin I/mL per hour). 14 In the cases that measurements were at or below the lower limit of detection, this limit was applied to allow for statistical analysis.
Quantitative Polymerase Chain Reaction
Total RNA was isolated from snap‐frozen livers, kidneys, epididymal adipose tissue, inguinal adipose tissue, and brown adipose tissue from rats, and liver and epididymal adipose tissue from mice using TRI Reagent (Sigma‐Aldrich) and reverse transcribed into cDNA using the QuantiTect Reverse Transcription Kit (Qiagen, Venlo, the Netherlands). cDNA was amplified in triplicate in 40 cycles (denaturation at 95 °C for 3 minutes, thermal cycling at 95 °C for 3 seconds, annealing/extension at 60 °C for 20 seconds) followed by a melt curve with a CFX384 (Bio‐Rad, Veenendaal, the Netherlands) using Kapa SYBR Fast (Kapa Biosystems). The ΔΔCt method was used for relative quantification of Agt mRNA expression levels, using the geometric mean of β2‐microglobulin (B2M) and β‐actin (ActB) for normalization. The intron‐spanning oligonucleotide primers were designed with National Center for Biotechnology Information Primer‐Basic Local Alignment Search Tool. Primer sequences: Agt, CCAGCACGACTTCCTGACTT (forward), GCAGGTTGTAGGATCCCCGA (reverse); B2M, ATGGCTCGCTCGGTGACCG (forward), TGGGGAGTTTTCTGAATGGCAAGCA (reverse); Actb, GGGAAATCGTGCGTGACATT (forward), GCGGCAGTGGCCATCTC (reverse).
Western Blotting
The specificity of an angiotensinogen antibody (Immuno‐Biological Laboratories, Fujioka, Japan) has been validated previously, by demonstrating a band at ≈53 kDa in tissues obtained from wild‐type but not in tissues from hepAGT−/− mice. 16 Snap‐frozen livers, kidneys, epididymal adipose tissue, inguinal adipose tissue, and brown adipose tissue of rats as well as livers and epididymal adipose tissue from mice were homogenized on ice in a buffer containing 0.3 mol/L sucrose, 50 mmol/L Tris–HCl pH 7.5, 1 mmol/L EDTA, 1 mmol/L EGTA, 50 mmol/L sodium fluoride, 1 mmol/L DTT, and 1 mmol/L PMSF supplemented with protease inhibitors. Subsequently, 20 to 50 μg of protein (DC protein assay kit; Bio‐Rad) was separated by electrophoresis on a Criterion TGX precast protein gel (Bio‐Rad) and transferred to a membrane using the Trans‐Blot Turbo Transfer System (Bio‐Rad). Membranes were blocked with 5% bovine serum albumin in Tris‐buffered saline containing 0.1% Tween‐20, followed by incubation overnight at 4 °C with a primary antibody directed against angiotensinogen (1:100; Immuno‐Biological Laboratories). After washing, blots were incubated with an anti‐rabbit horseradish peroxidase‐conjugated secondary antibody (1:3000; Bio‐Rad). Signals were detected by chemiluminescence (Clarity Western ECL substrate; Bio‐Rad) and quantified using ImageQuant LAS 4000 (GE Healthcare, Diegem, Belgium). Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH; 1:5000; GeneTex, Irvine, CA) was used for normalization of protein levels.
Statistical Analysis
Data are represented as mean±SEM. All analyses were performed using Prism version 8.0 (GraphPad Software, La Jolla, CA). Data obtained at a single point in time were analyzed with a 1‐way ANOVA, after log‐transformation for plasma angiotensinogen and active plasma renin concentration to obtain equal variances. A repeated‐measures ANOVA was used for data obtained at multiple points in time. Post hoc correction according to Dunnett or Bonferroni was performed in case of multiple comparisons. Univariate linear associations were assessed by calculation of Pearson coefficient of correlation. Area under the curve was defined as positive deviation from baseline arterial pressure established before each intravenous vasopressor dose and analyzed by a paired t test. Two‐tailed P<0.05 was considered statistically significant.
RESULTS
Angiotensinogen siRNA‐Mediated Blood Pressure Lowering Was Rapidly Reversed by Intravenous Angiotensin II or Norepinephrine, and Gradually Restored by Fludrocortisone or High Dietary Sodium Intake
Baseline mean arterial pressure (MAP; 136±3 mm Hg) was lowered to 130±2 mm Hg by reducing dietary sodium intake (P<0.0001 versus baseline), and to 117±2 mm Hg by addition of angiotensinogen siRNA (P<0.0001 versus low salt; Figure 1A; systolic and diastolic blood pressures are provided in Figure S1A and S1B). Blood pressure lowering was accompanied by a compensatory increase in heart rate (Figure 1B). siRNA‐mediated blood pressure lowering could be reversed rapidly using intravenous vasopressors or gradually using subcutaneous or oral vasopressor‐sparing strategies. In response to intravenous bolus injections of ascending doses of angiotensin II, MAP increased by, respectively, 11±3, 44±5, and 73±4 mm Hg before siRNA (Figure 1C). During siRNA treatment, responses were increased to 25±4 (P<0.05), 52±6 (not significant), and 85±5 (P<0.05) mm Hg. The α‐adrenergic agonist norepinephrine caused a vasoconstrictive response that was similar before and during siRNA administration, although during siRNA, MAP did not fully return to baseline after the highest dose of norepinephrine (P<0.05; Figure 1D). Both angiotensin II and norepinephrine caused transient increases in heart rate, and these responses were not altered by siRNA (Figure S2). Increasing dietary sodium intake increased MAP by 8±0.4 mm Hg on the first day of administration (P<0.0001) and fully restored MAP on the fourth day (Figure 1A). In contrast, the rise in MAP induced by subcutaneous administration of the corticosteroid fludrocortisone became significant only on the fifth day of treatment (P<0.05), and MAP was fully restored on the seventh day (Figure 1A). Restoration of MAP by fludrocortisone or by increasing dietary sodium intake decreased heart rate during the final 3 days of measurements to an average of 36±8 and 28±7 bpm below baseline level (both P<0.05; Figure 1B). Surprisingly, the α‐adrenergic agonist midodrine could neither increase blood pressure when administered subcutaneously nor when a double dose was administered orally (Figure 1A). None of the treatments affected locomotor activity (Figure S1). Only fludrocortisone impaired food consumption and physiological weight gain; rats administered fludrocortisone lost 26±5% of their body weight over a 2‐week treatment period (Table). Throughout the study period, there were no indications of renal failure, because plasma creatinine and urea levels remained low (Table).
Figure 1. Pressor responsiveness of small interfering RNA (siRNA)‐administered rats.
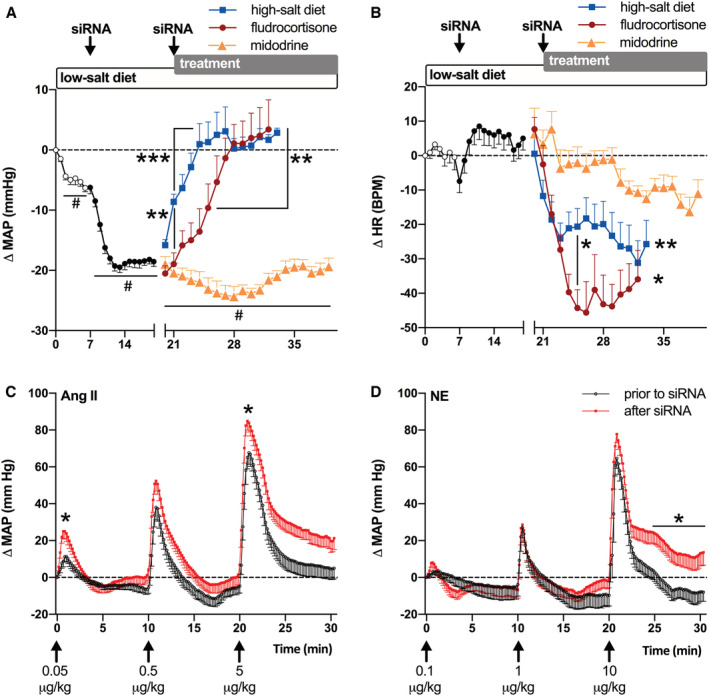
Change from baseline (A) mean arterial pressure (Δ MAP) and (B) heart rate (Δ HR) in spontaneously hypertensive rats on a low‐salt diet, treated with siRNA on day 7 and 21. From day 21, siRNA was supplemented with fludrocortisone or midodrine, or the low‐salt diet was replaced with a high‐salt diet (n=6–7 per group). Midodrine administration was extended by 4 days, in which a double oral dose was given. Data (mean±SEM) were analyzed using a 2‐way ANOVA and post hoc Dunnet (# P≤0.0001 vs baseline (days −2 to 0); *P≤0.05, **P≤0.01, ***P≤0.001 vs siRNA (days 18–20) or otherwise indicated in the graph. C and D, Pressor responses to IV bolus injections of angiotensin (Ang) II (n=11) or norepinephrine (NE; n=8) before siRNA (day 7) or after siRNA (day 21). Data are represented as mean±SEM. Area under the curve (defined as positive deviation from baseline arterial pressure established before each vasopressor dose) was analyzed using a paired Student t test (*P≤0.05 vs before siRNA). BPM indicates beats per minute.
Table.
Development of Body Weight and Renal Function Parameters
| 0 wk | 1 wk | 3 wk | 5 wk | |||
|---|---|---|---|---|---|---|
| Fludrocortisone | High‐salt diet | Midodrine | ||||
| N | 19 | 19 | 19 | 7 | 6 | 6 |
| Body weight, g | 325±13 | 347±22* | 368±20* | 269±44† | 413±15‡ | 379±12† |
| ∆ Treatment, g | … | … | … | −26±12% | +7±3% | +6±1% |
| Creatinine, mg/dL | … | 0.22±0.03 | 0.29±0.07 | 0.20±0.11 | 0.31±0.05 | 0.36±0.04 |
| Urea, mg/dL | … | 42±7 | 46±5 | 53±10 | 38±4 | 52±5 |
Spontaneously hypertensive rats on a low‐salt diet were dosed with small interfering RNA after 1 week and again after 3 weeks. From week 3 onward, treatment was supplemented with fludrocortisone or midodrine, or the low‐salt diet was replaced with a high‐salt diet (n=6–7 per group). Data represented as mean±SD were analyzed using a repeated measures ANOVA and post hoc Bonferroni.
P≤0.0001 vs 0 weeks.
P≤0.01.
P≤0.001 vs 3 weeks.
Fludrocortisone Partially Restored the Concentrations of Plasma Angiotensinogen
Angiotensinogen siRNA decreased the plasma angiotensinogen concentrations by 99.2±0.1%, to 5±1 nmol/L (Figure 2A). Angiotensinogen remained low during midodrine administration (8±1 nmol/L) and high dietary sodium intake (14±3 nmol/L), but increased during fludrocortisone administration to 122±26 nmol/L (ie, 20±5% of pre‐siRNA levels; P<0.0001 versus siRNA alone). Active plasma renin concentration increased 5‐fold in response to siRNA‐mediated blood pressure lowering (P<0.0001), and kept on rising during midodrine administration (P<0.01 versus siRNA alone; Figure 2B). Active plasma renin concentration decreased when blood pressure was restored by fludrocortisone (P<0.01) or by increasing dietary sodium intake (P<0.05).
Figure 2. Plasma AGT (angiotensinogen) and renin concentrations.
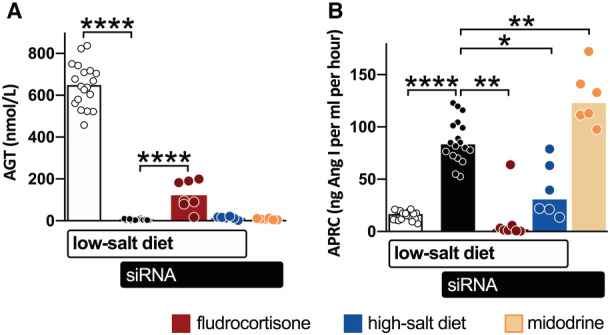
Plasma levels of AGT (A) and active plasma renin concentrations (APRC) (B) of spontaneously hypertensive rats on a low‐salt diet (open bars), treated with small interfering RNA (siRNA) (black bars), supplemented with fludrocortisone (red bars) or midodrine (yellow bars), or the low‐salt diet was replaced with a high‐salt diet (blue bars; n=6–7 per group) during the final 2 weeks. Data, represented as mean±SEM, were analyzed after log transformation using repeated measures ANOVA and post hoc Bonferroni (*P≤0.05, **P≤0.01, ****P≤0.0001).
Fludrocortisone Increased Angiotensinogen in Adipose Tissue
The rise in plasma angiotensinogen concentrations induced by fludrocortisone during liver‐targeted angiotensinogen siRNA treatment raised the possibility that it originated from nonhepatic sources. If drugs can stimulate release of angiotensinogen from nonhepatic sources, this might interfere with the antihypertensive efficacy of liver‐targeted siRNA. To this end, we quantified angiotensinogen in liver and tissues in which Agt is additionally transcribed (ie, the kidney and adipose tissue). 17 , 18 In SHRs treated with siRNA plus high salt or midodrine, liver Agt mRNA abundance was 0.6±0.2% and 0.7±0.1% of the abundance of control SHRs (Figure 3A). After fludrocortisone, this percentage doubled to 1.5±0.2% (P<0.05 versus both). Hepatic angiotensinogen protein concentrations displayed a similar trend, although the rise after fludrocortisone was not significant (Figure 3B). We have reported previously that renal Agt mRNA abundance in SHRs is ≈1 to 2 orders of magnitude lower than hepatic Agt mRNA abundance, and is not affected by liver‐targeted angiotensinogen siRNA. 3 Here, renal Agt mRNA abundance in all 3 groups was again >10‐fold below that in the liver of control SHRs (with no difference between any of the groups), whereas renal angiotensinogen protein was virtually undetectable in all groups. Remarkably, fludrocortisone increased Agt mRNA abundance in epididymal white adipose tissue (eWAT), inguinal white adipose tissue, and brown adipose tissue (P<0.05 versus high salt or midodrine). As a consequence, Agt mRNA abundance in these tissues resembled that in the liver of control SHRs (Figure 3A). Likewise, angiotensinogen protein abundance in eWAT, inguinal white adipose tissue, and brown adipose tissue was higher in fludrocortisone‐treated rats than in high salt‐ or midodrine‐administered rats (P<0.05 for all; Figure 3C). Only angiotensinogen in inguinal white adipose tissue correlated with plasma angiotensinogen (r=0.87; P=0.01; data not shown). In summary, fludrocortisone upregulated angiotensinogen in 3 different types of adipose tissue, raising the possibility that the rise in plasma angiotensinogen concentrations in fludrocortisone‐administered rats originated from these sites.
Figure 3. AGT (angiotensinogen) mRNA and protein abundance.
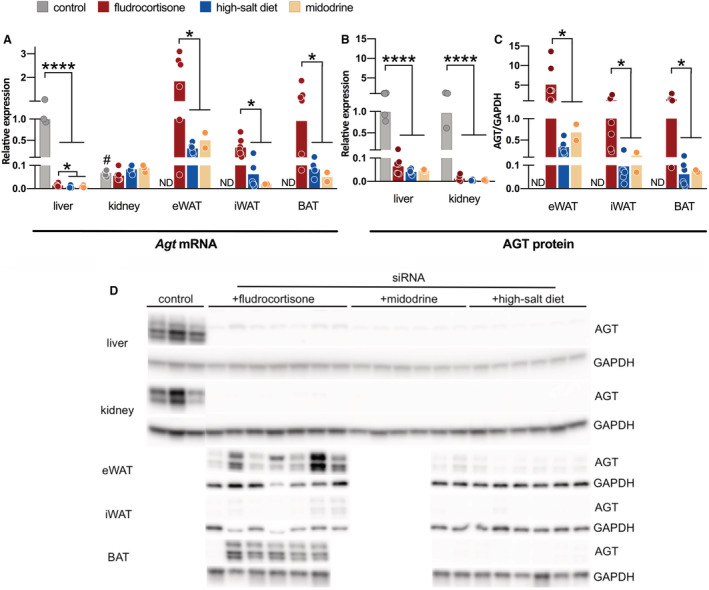
A, Agt (angiotensinogen)mRNA abundance (mean±SEM) in selected tissues from spontaneously hypertensive rats (SHRs) administered with fludrocortisone (n=7), high‐salt diet (n=6), or midodrine (n=2) are all represented relative to Agt mRNA abundance in livers of untreated SHRs (control, n=3). B, AGT (angiotensinogen) protein abundance (mean±SEM) in the livers and kidneys are represented relative to AGT abundance in the livers or kidneys of untreated SHRs. To also compare AGT protein abundance in adipose tissues we show in (C) the ratio of AGT protein to GAPDH protein. D, Immunoblots. Data were analyzed using a Student t test (# P≤0.0001 vs SHR liver) or using a 1‐way ANOVA and post hoc Dunnett (*P≤0.05, ****P≤0.0001). BAT indicates brown adipose tissue; eWAT, epididymal white adipose tissue; iWAT, inguinal white adipose tissue; and ND, not determined.
Fludrocortisone Did Not Increase Plasma Angiotensinogen Concentrations in Mice With Hepatocyte‐Specific Deficiency of Angiotensinogen
To further investigate whether fludrocortisone induced angiotensinogen release from nonhepatic sources, we evaluated its effect in mice deficient for hepatocyte angiotensinogen and their floxed littermate controls that did not express Cre (n=7–14 per group). Genetic ablation of hepatocyte angiotensinogen depleted angiotensinogen protein concomitantly from the liver, plasma, and eWAT (P<0.0001 for all; Figure 4A through 4C). Fludrocortisone increased blood pressure similarly in mice deficient for liver angiotensinogen and their controls (P<0.01 for both; Figure 4D). However, both plasma and hepatic angiotensinogen decreased during fludrocortisone administration in controls, whereas no change occurred in hepatocyte angiotensinogen‐deficient mice (Figure 4A and 4B). Fludrocortisone increased eWAT Agt mRNA abundance in control mice by 4‐fold (P=0.07) and in mice with hepatocyte angiotensinogen deficiency by 3‐fold (P<0.05; Figure S3), and this resulted in parallel rises in eWAT angiotensinogen protein (by 2‐fold, P=0.12 and 6‐fold, P<0.01; Figure 4C). Angiotensinogen protein in eWAT did not correlate with plasma angiotensinogen concentrations (data not shown). As in rats, fludrocortisone impaired physiological weight gain; body weight in control mice and hepatocyte angiotensinogen‐deficient mice increased during the 4‐week study period by 16±3% and 12±1%, respectively (P<0.01), whereas in the fludrocortisone‐administered mice, body weight remained unaltered (changes of 1±3% and 1±2%, respectively, P=not significant). Fludrocortisone similarly increased urine production (from 0.9±0.1 and 1.8±0.4 mL per 14 hours in control mice and hepatocyte angiotensinogen deficient mice to 3.5±0.9 and 3.5±0.7 mL per 14 hours, respectively; P<0.01 for both) and drinking behavior (data not shown) in both groups.
Figure 4. Effect of fludrocortisone in mice deficient for hepatic AGT (angiotensinogen).
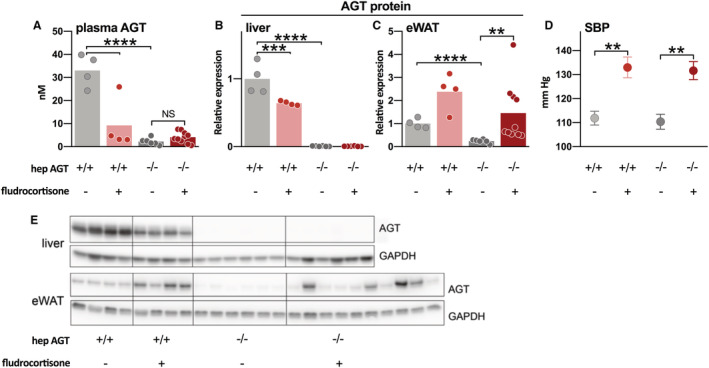
A, AGT protein abundance in the liver, (B) plasma AGT concentrations, (C) AGT protein abundance in epididymal white adipose tissue (eWAT), (D) systolic blood pressure (SBP), and (E) representative immunoblots of mice deficient for liver angiotensinogen (hepAGT−/−) and floxed mice not expressing Cre (hepAGT+/+) that were administered with either vehicle or fludrocortisone as indicated. Data (mean±SEM of 7–14 per group) were analyzed using a 1‐way ANOVA and post hoc Bonferroni (**P≤0.01, ***P≤0.001, ****P≤0.0001). NS indicates not significant.
DISCUSSION
This study revealed that the angiotensinogen siRNA‐mediated drop in blood pressure can be counteracted acutely by infusing the vasopressors norepinephrine or angiotensin II, and chronically by vasopressor‐sparing strategies such as high salt and fludrocortisone, but not midodrine. This is important information, because angiotensinogen depletion may not only improve blood pressure control by preventing RAS reactivation, but it may also prevent the rapid rise in RAS activity that is required to maintain perfusion pressure during hypovolemia. Thus, knowledge should be available on what to do in case of severe blood pressure lowering under conditions of angiotensinogen depletion. Although norepinephrine currently is the first‐line vasopressor for the treatment of shock, 19 recent studies also support a role for angiotensin II. 20 Whereas it was expected that the pressor responses to norepinephrine were not affected by siRNA, the increased reactivity of siRNA‐treated rats to angiotensin II observed in this study is not surprising, given that a reduction in angiotensin II is likely to increase the sensitivity of angiotensin II type 1 receptors to angiotensin II. Surprisingly, high doses of subcutaneous or oral midodrine did not increase blood pressure. We have no explanation as to why midodrine administration failed; lower doses than those applied here have been shown to massively increase blood pressure in rats of the same genetic background, 9 and norepinephrine, which also raises blood pressure by stimulating α‐adrenoceptors, did increase blood pressure. Rats treated with fludrocortisone lost a significant amount of weight, consistent with previous studies showing that higher doses of fludrocortisone induce anorexia in rats, 21 , 22 a phenomenon that does not occur in humans. 23
Consistent with observations in hepatocyte‐specific Agt knockout mice, 17 , 24 hepatocyte‐specific deficiency of Agt gene expression by siRNA almost eliminated plasma angiotensinogen concentrations. Because of its liver specificity, the angiotensinogen siRNA did not affect Agt gene expression at extrahepatic sites, including the kidney, inguinal white adipose tissue, eWAT, brown adipose tissue, and the brain. 16 However, at all of these sites, despite local mRNA abundance, angiotensinogen protein turned out to be largely, if not completely, liver derived, and thus local angiotensin generation disappeared after siRNA administration. 16 The current study confirmed this view, at least when siRNA administration was combined with either high salt intake or midodrine. However, unexpectedly, plasma angiotensinogen concentrations were partially restored when siRNA‐treated rats were administered fludrocortisone. It is unlikely that this rise was caused by a decline in the efficacy of siRNA over time, because angiotensinogen concentrations remained low during midodrine administration and high sodium intake. Remarkably, fludrocortisone increased Agt mRNA abundance in all tissue except the kidney, and the highest rise was observed in eWAT. These mRNA increases were accompanied by parallel rises in angiotensinogen protein in these tissues. These findings raise 2 questions: (1) Does the rise in plasma angiotensinogen concentrations underlie the rise in blood pressure during fludrocortisone administration? (2) Do the elevated angiotensinogen concentrations in plasma originate from extrahepatic sources?
To address these questions, we made use of mice in which hepatic angiotensinogen was genetically ablated and their littermate controls. As in rats exposed to liver‐specific angiotensinogen siRNA, 16 the eWAT angiotensinogen protein content in hepatocyte angiotensinogen‐deficient mice was greatly reduced. This confirms that also in mice, eWAT angiotensinogen is predominantly derived from the liver, at least under normal circumstances. 25 Fludrocortisone increased blood pressure similarly in hepatocyte angiotensinogen‐deficient and control mice, but was unable to upregulate plasma angiotensinogen and even reduced plasma angiotensinogen concentrations in control mice, in spite of upregulated Agt mRNA and angiotensinogen protein abundance in eWAT in both strains. In hepatocyte angiotensinogen‐deficient mice, the increase in eWAT after fludrocortisone was of similar magnitude as that in the siRNA‐treated rats. Fludrocortisone also reduced body weight to the same degree in mice as in rats. Taken together, the effects of fludrocortisone in mice on blood pressure, body weight, and eWAT angiotensinogen fully resemble those in rats, except for the rise in plasma angiotensinogen that occurred in rats but not in mice. From this we conclude (1) that the effects of fludrocortisone on blood pressure occur independently of plasma angiotensinogen, and (2) that the rise in plasma angiotensinogen observed in rats after this drug is not adipose tissue‐derived.
The mechanism by which fludrocortisone raises blood pressure, if not involving angiotensinogen upregulation, most likely relates to its capacity to stimulate mineralocorticoid and glucocorticoid receptors. 26 In rats, glucocorticoid effects predominate, 21 whereas in humans 27 and mice, 28 fludrocortisone mainly stimulates the mineralocorticoid receptor. The increased drinking behavior in mice agrees with this concept. Because glucocorticoids increase the transcription rate of Agt in the liver 29 and adipose tissue 30 (but not the kidney 31 ), the upregulation of angiotensinogen could simply be the consequence of glucocorticoid receptor stimulation, without necessarily contributing to its hypertensive effect. Both in humans and rats, glucocorticoid excess associates with increased plasma angiotensinogen concentrations. 32 , 33 Simultaneously, in control mice, fludrocortisone decreased hepatic angiotensinogen production. To what degree this is the consequence of simultaneous mineralocorticoid receptor stimulation remains to be determined.
This leaves the question why fludrocortisone upregulated plasma angiotensinogen concentrations in siRNA‐administered rats. Its lack of effect on the plasma angiotensinogen concentration in mice deficient for liver angiotensinogen despite the upregulation of eWAT angiotensinogen synthesis strongly argues against release from extrahepatic (adipose tissue) sites, and suggests a hepatic source. However, Agt mRNA abundance increased only marginally (although significantly) in the liver. A further possibility is that the massive reduction in renin after fludrocortisone (potentially related to its mineralocorticoid receptor‐stimulatory effect) allowed a significant reduction in angiotensinogen metabolism. Such renin suppression after fludrocortisone has been described before in both rats 34 and humans. 35 Particularly at low angiotensinogen levels (when angiotensinogen is no longer in the Michaelis‐Menten constant range), rises in renin are capable of decreasing angiotensinogen even further, and the opposite may occur when renin disappears, particularly if hepatic angiotensinogen synthesis modestly increases.
PERSPECTIVE
In conclusion, deletion of liver angiotensinogen by a single dose of siRNA virtually abolishes plasma angiotensinogen and lowers blood pressure for weeks to months in preclinical models. We showed that conventional vasopressor and vasopressor‐sparing strategies can reverse siRNA‐mediated blood pressure lowering, although plasma angiotensinogen concentrations are almost deleted. A rise in plasma angiotensinogen concentrations may occur during fludrocortisone administration, but this does not drive the mechanism by which fludrocortisone raises blood pressure. That pressor responsiveness remained intact during siRNA administration provides a pharmacological escape to raise arterial pressure and maintain perfusion pressure during shock or other conditions that induce hypotension. Obviously, additional studies should now evaluate this approach during hypovolemia induced by hemorrhage or sepsis. These findings in preclinical models may support the therapeutic implementation of angiotensinogen siRNA in the future.
Sources of Funding
This work was partially supported by Alnylam Pharmaceuticals. Dr Mirabito Colafella was supported by a National Health and Medical Research Council of Australia CJ Martin Fellowship (number 1112125). Dr Ren was supported by a National Natural Science Foundation of China (grant number 81900668).
Disclosures
Drs Nioi and Foster are employees of Alnylam Pharmaceuticals. Dr Danser received a grant from Alnylam Pharmaceuticals, which has partially supported this work. The other authors report no conflicts.
Supporting information
Figures S1–S3
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 2. Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, et al. Success and predictors of blood pressure control in diverse north american settings: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x [DOI] [PubMed] [Google Scholar]
- 3. Uijl E, Mirabito Colafella KM, Sun Y, Ren L, van Veghel R, Garrelds IM, de Vries R, Poglitsch M, Zlatev I, Kim JB, et al. Strong and sustained antihypertensive effect of small interfering RNA targeting liver angiotensinogen. Hypertension. 2019;73:1249–1257. doi: 10.1161/HYPERTENSIONAHA.119.12703 [DOI] [PubMed] [Google Scholar]
- 4. Brown CR, Gupta S, Qin J, Racie T, He G, Lentini S, Malone R, Yu M, Matsuda S, Shulga‐Morskaya S, et al. Investigating the pharmacodynamic durability of GalNAc‐siRNA conjugates. Nucleic Acids Res. 2020;48:11827–11844. doi: 10.1093/nar/gkaa670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beaty O III, Sloop CH, Schmid HE Jr, Buckalew VM Jr. Renin response and angiotensinogen control during graded hemorrhage and shock in the dog. Am J Physiol. 1976;231:1300–1307. doi: 10.1152/ajplegacy.1976.231.4.1300 [DOI] [PubMed] [Google Scholar]
- 6. MacGregor GA, Markandu ND, Singer DR, Cappuccio FP, Shore AC, Sagnella GA. Moderate sodium restriction with angiotensin converting enzyme inhibitor in essential hypertension: a double blind study. Br Med J (Clin Res Ed). 1987;294:531–534. doi: 10.1136/bmj.294.6571.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Esch JH, Moltzer E, van Veghel R, Garrelds IM, Leijten F, Bouhuizen AM, Danser AH. Beneficial cardiac effects of the renin inhibitor aliskiren in spontaneously hypertensive rats. J Hypertens. 2010;28:2145–2155. doi: 10.1097/HJH.0b013e32833d01ae [DOI] [PubMed] [Google Scholar]
- 8. Kammerl MC, Richthammer W, Kurtz A, Kramer BK. Angiotensin II feedback is a regulator of renocortical renin, COX‐2, and nNOS expression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1613–R1617. doi: 10.1152/ajpregu.00464.2001 [DOI] [PubMed] [Google Scholar]
- 9. Dabasaki T, Shimojo M, Ishikawa H, Uemura A. Anti‐hypotensive effects of m6434, an orally active alpha 1‐adrenoceptor agonist, in rats. Jpn J Pharmacol. 1992;59:145–150. doi: 10.1254/jjp.59.145 [DOI] [PubMed] [Google Scholar]
- 10. Sansoe G, Aragno M, Mastrocola R, Parola M. Dose‐dependency of clonidine's effects in ascitic cirrhotic rats: comparison with alpha1‐adrenergic agonist midodrine. Liver Int. 2016;36:205–211. doi: 10.1111/liv.12905 [DOI] [PubMed] [Google Scholar]
- 11. Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kel'in AV, Milstein S, et al. Multivalent N‐acetylgalactosamine‐conjugated siRNA localizes in hepatocytes and elicits robust RNAi‐mediated gene silencing. J Am Chem Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a [DOI] [PubMed] [Google Scholar]
- 12. Lu H, Wu C, Howatt DA, Balakrishnan A, Moorleghen JJ, Chen X, Zhao M, Graham MJ, Mullick AE, Crooke RM, et al. Angiotensinogen exerts effects independent of angiotensin II. Arterioscler Thromb Vasc Biol. 2016;36:256–265. doi: 10.1161/ATVBAHA.115.306740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daugherty A, Rateri D, Hong L, Balakrishnan A. Measuring blood pressure in mice using volume pressure recording, a tail‐cuff method. J Vis Exp. 2009;27:1291. doi: 10.3791/1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Lannoy LM, Danser AH, van Kats JP, Schoemaker RG, Saxena PR, Schalekamp MA. Renin‐angiotensin system components in the interstitial fluid of the isolated perfused rat heart. Local production of angiotensin I. Hypertension. 1997;29:1240–1251. doi: 10.1161/01.HYP.29.6.1240 [DOI] [PubMed] [Google Scholar]
- 15. van den Heuvel M, Batenburg WW, Jainandunsing S, Garrelds IM, van Gool JM, Feelders RA, van den Meiracker AH, Danser AH. Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin‐angiotensin‐aldosterone system activity and the efficacy of renin‐angiotensin‐aldosterone system blockade in the kidney. J Hypertens. 2011;29:2147–2155. doi: 10.1097/HJH.0b013e32834bbcbf [DOI] [PubMed] [Google Scholar]
- 16. Uijl E, Ren L, Mirabito Colafella KM, van Veghel R, Garrelds IM, Domenig O, Poglitsch M, Zlatev I, Kim JB, Huang S, et al. No evidence for brain renin‐angiotensin system activation during DOCA‐salt hypertension. Clin Sci (Lond). 2021;135:259–274. doi: 10.1042/CS20201239 [DOI] [PubMed] [Google Scholar]
- 17. Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koizumi M, Niimura F, Fukagawa M, Matsusaka T. Adipocytes do not significantly contribute to plasma angiotensinogen. J Renin Angiotensin Aldosterone Syst. 2016;17:1470320316672348. doi: 10.1177/1470320316672348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118 [DOI] [PubMed] [Google Scholar]
- 20. Papazisi O, Palmen M, Danser AHJ. The use of angiotensin II for the treatment of post‐cardiopulmonary bypass vasoplegia. Cardiovasc Drugs Ther. 2020. Online ahead of print. doi: 10.1007/s10557-020-07098-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knowlton AI, Loeb EN, Stoerk HC. Effect of synthetic analogues of hydrocortisone on the blood pressure of adrenalectomized rats on sodium restriction. Endocrinology. 1957;60:768–777. doi: 10.1210/endo-60-6-768 [DOI] [PubMed] [Google Scholar]
- 22. Simpson CW, Dicara LV, Wolf G. Glucocorticoid anorexia in rats. Pharmacol Biochem Behav. 1974;2:19–25. doi: 10.1016/0091-3057(74)90130-0 [DOI] [PubMed] [Google Scholar]
- 23. Distler A, Philipp T, Lüth B, Wucherer G. Studies on the mechanism of mineralocorticoid‐induced blood pressure increase in man. Clin Sci (Lond). 1979;57(suppl 5):303s–305s. doi: 10.1042/cs057303s [DOI] [PubMed] [Google Scholar]
- 24. Wu C, Xu Y, Lu H, Howatt DA, Balakrishnan A, Moorleghen JJ, Vander Kooi CW, Cassis LA, Wang JA, Daugherty A. Cys18‐Cys137 disulfide bond in mouse angiotensinogen does not affect AngII‐dependent functions in vivo. Hypertension. 2015;65:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cruz‐López EO, Uijl E, Danser AHJ. Perivascular adipose tissue in vascular function: does locally synthesized angiotensinogen play a role? J Cardiovasc Pharmacol. 2021;78:S53–S62. doi: 10.1097/FJC.0000000000001027 [DOI] [PubMed] [Google Scholar]
- 26. Whitworth JA, Butkus A, Coghlan JP, Denton DA, Mills EH, Spence CD, Scoggins BA. 9‐alpha‐fluorocortisol‐induced hypertension: a review. J Hypertens. 1986;4:133–139. doi: 10.1097/00004872-198604000-00002 [DOI] [PubMed] [Google Scholar]
- 27. Chobanian AV, Burrows BA, Hollander W. Body fluid and electrolyte composition in arterial hypertension. II. Studies in mineralocorticoid hypertension. J Clin Invest. 1961;40:416–422. doi: 10.1172/JCI104269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nizar JM, Shepard BD, Vo VT, Bhalla V. Renal tubule insulin receptor modestly promotes elevated blood pressure and markedly stimulates glucose reabsorption. JCI Insight. 2018;3:e95107. doi: 10.1172/jci.insight.95107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klett C, Ganten D, Hellmann W, Kaling M, Ryffel GU, Weimar‐Ehl T, Hackenthal E. Regulation of hepatic angiotensinogen synthesis and secretion by steroid hormones. Endocrinology. 1992;130:3660–3668. doi: 10.1210/endo.130.6.1597163 [DOI] [PubMed] [Google Scholar]
- 30. Aubert J, Darimont C, Safonova I, Ailhaud G, Negrel R. Regulation by glucocorticoids of angiotensinogen gene expression and secretion in adipose cells. Biochem J. 1997;328(Pt 2):701–706. doi: 10.1042/bj3280701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalinyak JE, Perlman AJ. Tissue‐specific regulation of angiotensinogen mrna accumulation by dexamethasone. J Biol Chem. 1987;262:460–464. doi: 10.1016/S0021-9258(19)75949-3 [DOI] [PubMed] [Google Scholar]
- 32. Krakoff LR. Measurement of plasma renin substrate by radioimmunoassay of angiotensin. I. Concentration in syndromes associated with steroid excess. J Clin Endocrinol Metab. 1973;37:110–117. doi: 10.1210/jcem-37-1-110 [DOI] [PubMed] [Google Scholar]
- 33. van der Pas R, van Esch JH, de Bruin C, Danser AH, Pereira AM, Zelissen PM, Netea‐Maier R, Sprij‐Mooij DM, van den Berg‐Garrelds IM, van Schaik RH, et al. Cushing's disease and hypertension: in vivo and in vitro study of the role of the renin‐angiotensin‐aldosterone system and effects of medical therapy. Eur J Endocrinol. 2014;170:181–191. doi: 10.1530/EJE-13-0477 [DOI] [PubMed] [Google Scholar]
- 34. Hepp R, Garbade K, Oster P, Gross F. Arterial hypertension induced by 9 alpha‐fluorocortisol in rats. Acta Endocrinol. 1974;75:539–549. doi: 10.1530/acta.0.0750539 [DOI] [PubMed] [Google Scholar]
- 35. Whitworth JA, Saines D, Thatcher R. Differential blood pressure and metabolic effects of 9 alpha‐fluorocortisol in man. Clin Exp Pharmacol Physiol. 1983;10:351–354. doi: 10.1111/j.1440-1681.1983.tb00211.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S3


