Abstract
Background
Oral anticoagulation (OAC) therapy prevents morbidity and mortality in nonvalvular atrial fibrillation; whether location of diagnosis influences OAC uptake or adherence is unknown.
Methods and Results
Retrospective cohort study (2008–2019), identifying adults with incident nonvalvular atrial fibrillation across health care settings (emergency department, hospital, outpatient) at high risk of stroke. OAC uptake and adherence via proportion of days covered for direct OACs and time in therapeutic range for warfarin were measured. Proportion of days covered was categorized as low (0–39%), intermediate (40–79%), and high (80–100%). Warfarin control was defined as time in therapeutic range ≥65%. All‐cause mortality was examined at a 3‐year landmark. Among 75 389 patients with nonvalvular atrial fibrillation (47.0% women, mean 77.4 years), 19.7% were diagnosed in the emergency department, 59.1% in the hospital, and 21.2% in the outpatient setting. Ninety‐day OAC uptake was 51.6% in the emergency department, 50.9% in the hospital, and 67.9% in the outpatient setting (P<0.0001). High direct OAC adherence increased from 64.9% to 80.3% in the emergency department, 64.3% to 81.7% in the hospital, and 70.9% to 88.6% in the outpatient setting over time (P values for trend <0.0001). Warfarin control was 40.3% overall and remained unchanged. In multivariable analysis, outpatient diagnosis compared with the hospital was associated with greater OAC uptake (odds ratio [OR], 1.79; [95% CI, 1.72–1.87]) and direct OAC (OR, 1.42; [95% CI, 1.27–1.59]) and warfarin (OR, 1.49; [95% CI, 1.36–1.63]) adherence. Varying or persistently low adherence was associated with a poor prognosis, especially for warfarin.
Conclusions
Locale of nonvalvular atrial fibrillation diagnosis is associated with varying OAC uptake and adherence. Interventions specific to health care settings are needed to improve stroke prevention.
Keywords: adherence, anticoagulation, atrial fibrillation, health care settings, uptake
Subject Categories: Atrial Fibrillation, Primary Prevention, Anticoagulants, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- DOAC
direct oral anticoagulant
- NVAF
nonvalvular atrial fibrillation
- OAC
oral anticoagulation
- TTR
time in therapeutic range
Clinical Perspective.
What Is New?
Driven by greater direct oral anticoagulant use, oral anticoagulant (OAC) uptake and adherence has increased over time but remains poor overall, with a third of eligible patients with nonvalvular atrial fibrillation not being prescribed OAC therapy, primarily in the emergency department and the hospital.
Patients with OAC adherence that varies or is persistently low have increased mortality, especially with warfarin.
What Are the Clinical Implications?
Several risk factors, including location of diagnosis, may identify patients at high risk for OAC nonadherence who may have improved mortality with prescription of direct OAC instead of warfarin.
Atrial fibrillation (AF), the most common cardiac arrhythmia, 1 is a leading cause of stroke in older populations. 2 , 3 , 4 , 5 Stroke caused by AF causes longer‐term morbidity, greater health care resource use compared with stroke unrelated to AF, and higher mortality. 6 , 7 , 8 , 9 , 10 , 11 Oral anticoagulant (OAC) therapy is highly effective for stroke prevention in AF with a relative risk reduction of ≈60% with warfarin compared with placebo and 20% with direct oral anticoagulants (DOACs) compared with warfarin. 12 , 13 However, treatment adherence is critical to reducing the associated risk of stroke. 14 Because of convenience in administration together with comparable or greater effectiveness and safety, DOACs have emerged as a preferred choice to warfarin for stroke prevention. 15 , 16 , 17 Notwithstanding these advantages, warfarin represents anywhere from a quarter to a third of all OAC prescriptions. 18 , 19
Many studies have assessed whether the advantages of DOACs over warfarin for stroke prevention have translated into increased uptake in clinical practice and found DOAC uptake increased over time. 18 , 19 , 20 , 21 , 22 , 23 , 24 However, uptake trends vary significantly between studies, which may be attributable to differences in study periods and locations of diagnoses. 18 , 19 , 20 , 21 , 22 , 23 , 24 Although adherence to DOACs has been shown to be higher than warfarin, studies were limited by warfarin prescription use rather than the more accurate measure of time in therapeutic range (TTR). 25 , 26 , 27 , 28 Importantly, there is a paucity of data characterizing patterns in uptake and adherence over time, especially after widespread availability of DOACs. In addition, these studies were limited by lack of stratification by location of diagnosis. There are data to suggest that AF epidemiology is changing, with fewer patients with incident AF being managed in the hospital 29 , 30 , 31 ; therefore, evaluating uptake and adherence patterns across health care settings may help to identify care gaps and opportunities to address them.
Accordingly, we evaluated uptake and adherence patterns to DOACs and warfarin (including laboratory data) for patients with incident nonvalvular AF (NVAF) at high risk for stroke across various health care settings (hospital, emergency department [ED], and outpatient setting) over a 10‐year period. We also examined predictors of uptake and adherence and the effect of adherence patterns on mortality.
METHODS
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Alberta Health Services at sporab@ualberta.ca.
Study Population
We identified all adult patients discharged from the ED or hospital or who had at least 2 outpatient visits at least 30 days apart within a year 32 with a new primary or secondary diagnosis of NVAF (International Classification of Diseases, Tenth Revision [ICD‐10] code I48) and at high risk of stroke (CHA2DS2‐VASc >2 in men and >3 in women) in Alberta, Canada, between April 1, 2008, and March 31, 2019. We used a washout period of 5 years to identify incident AF. 32 Patients with a prior AF diagnosis in any diagnostic position were excluded. Patients with <5 years of lead data were not excluded but represent only 4.7% of the cohort. The cohort consisted of 75 389 patients with NVAF (Figure S1). The uptake analysis examined 64 373 patients with at least a 90‐day follow‐up to ascertain OAC uptake. The adherence analysis examined the 24 876 patients with OAC uptake within 90 days and diagnosis after April 1, 2012, to have international normalized ratio laboratory data available. Mortality analysis was performed at a 3‐year landmark in the 6482 patients who had at least 3 years’ completed follow‐up to establish a pattern of adherence, and then a time‐to‐event mortality analysis was performed with additional subsequent follow‐up of up to 3 years. The median follow‐up time was 1.5 years in the time‐to‐event analysis.
Data Sources
We conducted a retrospective population‐based study linking the following administrative databases: (1) the Discharge Abstract Database, which records the most responsible and up to 24 other diagnoses for all acute‐care hospitalizations; (2) the National Ambulatory Care Reporting System, which captures all visits to any ED and hospital‐based specialist outpatient visits and includes up to 10 diagnosis fields per visit; (3) the Practitioner Claims Database, which captures non–hospital‐based physician office visits; (4) the Population Registry (demographic and geographic information); (5) the Pharmaceutical Information Network (PIN) (drug codes in Table S2) from outpatient pharmacies for patients of all ages (DOACs were introduced to the provincial formulary in 2012); and (6) the Alberta Vital Statistics Database, which records all deaths in the province.
Data Elements and Variables
We identified comorbidities (heart failure, hypertension, diabetes, stroke/transient ischemic attack, peripheral artery disease, coronary artery disease, major bleeding, anemia, thrombocytopenia, excess alcohol, prior falls, and chronic kidney disease) as present using validated ICD codes, if they were documented in any of the aforementioned databases during the 5 years before incident AF diagnosis (definition in Data S1 and codes in Table S1). 33 The urban/rural location of patient residence was established using the Alberta Health Postal Code Translation File and was linked to postal codes of residence from Statistics Canada dissemination areas. Socioeconomic effects were estimated using the Pampalon Material Deprivation Index based on census dissemination areas. 34 The Pampalon Material Deprivation Index considers education, employment, solitary living, marital separation, and single‐parent family rates, in addition to average income, to assign quintiles of material deprivation.
Uptake and Adherence
Uptake of warfarin and DOACs was identified as the first dispensation within 90 days after discharge. Adherence was measured as the proportion of days covered for DOACs. Warfarin control was based on TTR. 35 Proportion of days covered was calculated for DOACs adherence starting at the first dispensation within 90 days of discharge and followed for 365 days unless censored by death, switching OACs, out‐migration, or the study end date. Proportion of days covered for DOAC was categorized as low (0–39%), intermediate (40–79%), and high (80–100%). 36 An international normalized ratio between 2.0 and 3.0 was used to define the therapeutic range for TTR. TTR was calculated for warfarin control starting at the first warfarin dispensation within 90 days of discharge and followed for 365 days unless censored by death, switching OACs, out‐migration, or the study end date. International normalized ratio values between lab tests, up to 56 days apart, were linearly interpolated using Rosendaal’s method. 35 Warfarin control was defined as TTR of ≥65% in those days with a measured or interpolated international normalized ratio value. 37 , 38
Outcomes
The primary outcomes were temporal trends in OAC uptake and adherence for incident NVAF patients at high risk of stroke across health care settings. The secondary outcomes were predictors of uptake and adherence and 3‐year all‐cause mortality.
Statistical Analysis
Descriptive statistics are presented as means and SD for continuous variables and count with percentages for categorical variables, as appropriate, unless otherwise specified. Chi‐square tests were used to compare between locations. Tests for trends used the Cochran‐Armitage or Jonckheere‐Terpstra test, as appropriate. All‐cause mortality after a 3‐year landmark was described using Kaplan‐Meier curves, and comparisons used the log‐rank statistic. Logistic regression models for OAC uptake and high adherence were adjusted for locale of diagnosis, age category (18–64, 65–74, and ≥75 years), sex, urban/rural location, material deprivation, history of major bleeding, anemia, thrombocytopenia, history of falls, and chronic kidney disease. Cox proportional hazards models for all‐cause mortality after the 3‐year landmark adjusted for the same covariates above and the previous 3‐year adherence category censoring occurred at the earliest of the end of follow‐up or out‐migration. For the 3‐year landmark mortality model, the 3 annual adherence categories were aggregated to always high, always low, and varies. Statistical analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, NC).
Ethics
This study was approved by the University of Alberta Health Research Ethics Board (Pro00083729), including waiving the need for individual patient informed consent.
RESULTS
Baseline Characteristics
Of the total 75 389 adults with incident NVAF at high risk of stroke, 14 816 (19.7%) were first diagnosed in the ED, 44 564 (59.1%) in the hospital, and 16 009 (21.2%) in the outpatient setting (Table). Overall, the mean age was 77.4 (SD, 10.7) years old and 47.0% were women. Patients diagnosed in the hospital were older and had higher comorbidity burden; patients diagnosed in the ED were more likely to be women who live in a rural setting; and patients diagnosed in the outpatient setting were less likely to be materially deprived. The risk factors contributing most often to the CHA2DS2‐VASc score were hypertension and coronary artery disease in the hospital, while in the ED and outpatient setting, it was hypertension and diabetes.
Table Table. .
Baseline Demographics, Stratified by Setting Where NVAF Was First Diagnosed
| Total, n (%) | Setting | P value | |||
|---|---|---|---|---|---|
| ED, n (%) | Hospital, n (%) | Outpatient, n (%) | |||
| Sample | 75 389 (100.0) | 14 816 (19.7) | 44 564 (59.1) | 16 009 (21.2) | |
| Female | 35 457 (47.0) | 7371 (49.8) | 20 957 (47.0) | 7129 (44.5) | <0.0001 |
| Age, y | |||||
| Mean±SD | 77.4 (10.7) | 76.4 (10.7) | 78.0 (11.0) | 76.3 (9.9) | <0.0001 |
| 18–64 | 8357 (11.1) | 1727 (11.7) | 5021 (11.3) | 1609 (10.1) | <0.0001 |
| 65–74 | 18 673 (24.8) | 4095 (27.6) | 10 093 (22.6) | 4485 (28.0) | |
| ≥75 | 48 359 (64.1) | 8994 (60.7) | 29 450 (66.1) | 9915 (61.9) | |
| Rural | 20 651 (27.4) | 4471 (30.2) | 12 283 (27.6) | 3897 (24.3) | <0.0001 |
| Material deprivation quintiles | |||||
| 1—least deprived | 11 335 (15.0) | 2238 (15.1) | 6356 (14.3) | 2741 (17.1) | <0.0001 |
| 2 | 11 081 (14.7) | 2090 (14.1) | 6368 (14.3) | 2623 (16.4) | |
| 3 | 12 779 (17.0) | 2556 (17.3) | 7414 (16.6) | 2809 (17.5) | |
| 4 | 15 806 (21.0) | 3159 (21.3) | 9395 (21.1) | 3252 (20.3) | |
| 5—most deprived* | 15 959 (21.2) | 3266 (22.0) | 9654 (21.7) | 3039 (19.0) | |
| Heart failure | 20 926 (27.8) | 2559 (17.3) | 16 131 (36.2) | 2236 (14.0) | <0.0001 |
| Hypertension | 61 912 (82.1) | 11 916 (80.4) | 37 521 (84.2) | 12 475 (77.9) | <0.0001 |
| Diabetes | 24 969 (33.1) | 4589 (31.0) | 15 706 (35.2) | 4674 (29.2) | <0.0001 |
| Stroke/transient ischemic attack | 11 668 (15.5) | 1768 (11.9) | 8183 (18.4) | 1717 (10.7) | <0.0001 |
| Peripheral artery disease | 7505 (10.0) | 1128 (7.6) | 5315 (11.9) | 1062 (6.6) | <0.0001 |
| Coronary artery disease | 26 280 (34.9) | 4130 (27.9) | 17 847 (40.0) | 4303 (26.9) | <0.0001 |
| Major bleeding | 2858 (3.8) | 432 (2.9) | 2015 (4.5) | 411 (2.6) | <0.0001 |
| Anemia | 16 584 (22.0) | 2185 (14.7) | 12 366 (27.7) | 2033 (12.7) | <0.0001 |
| Thrombocytopenia | 1720 (2.3) | 186 (1.3) | 1365 (3.1) | 169 (1.1) | <0.0001 |
| Excess alcohol | 3070 (4.1) | 421 (2.8) | 2342 (5.3) | 307 (1.9) | <0.0001 |
| Falls | 19 291 (25.6) | 3296 (22.2) | 13 216 (29.7) | 2779 (17.4) | <0.0001 |
| Chronic kidney disease | 7924 (10.5) | 1032 (7.0) | 5962 (13.4) | 930 (5.8) | <0.0001 |
| Liver disease | 2189 (2.9) | 307 (2.1) | 1613 (3.6) | 269 (1.7) | <0.0001 |
| Cancer | 13 232 (17.6) | 2094 (14.1) | 9172 (20.6) | 1966 (12.3) | <0.0001 |
ED indicates emergency department; and NVAF, nonvalvular atrial fibrillation.
Deprivation could not be determined in 8429 (11.2%) patients.
Uptake of OAC
The overall 90‐day OAC uptake was 55.2% and varied significantly by locations with 51.7% in the ED, 50.9% in the hospital, and 67.9% in the outpatient setting (P<0.0001; Figure 1). Over the 10‐year study period, OAC uptake increased from 44.3% to 67.0% overall, 39.3% to 67.8% in the ED, 39.6% to 64.5% in the hospital, and 60.0% to 71.1% in the outpatient setting (Figure 1; P value for trend <0.0001, respectively). During this time, DOAC uptake increased from 0% to 51.6%, and warfarin decreased from 44.3% to 15.4%. Overall, 17.7% of patients initially prescribed warfarin switched over to a DOAC within 1 year, while 5.6% of patients prescribed a DOAC changed to warfarin (Figure S2).
Figure 1. Uptake of OAC at 90 days over study period in patients at high stroke risk stratified by locale.
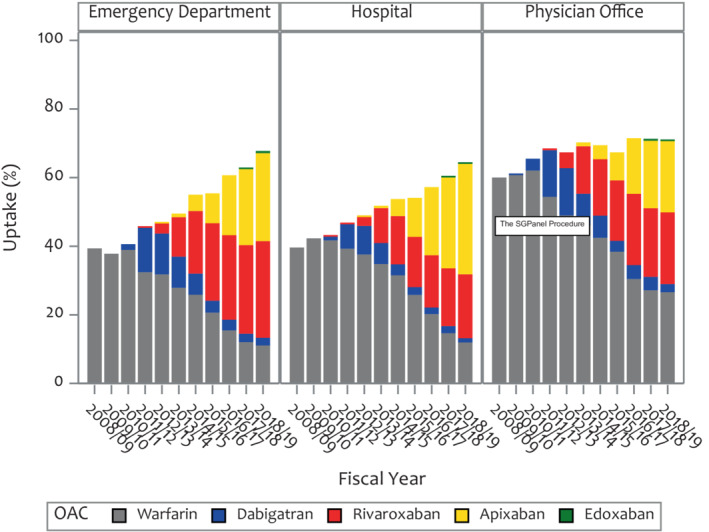
OAC indicates oral anticoagulant.
Predictors of OAC uptake are shown in Figure S3. In multivariable analysis, the strongest predictor of OAC uptake was diagnosis in the outpatient setting (odds ratio [OR], 1.79, [95% CI, 1.72–1.87]) (P<0.0001). Age categories 65 to 74 (OR, 1.20; [95% CI, 1.14–1.27]) and ≥75 (OR, 1.21; [95% CI, 1.15–1.28]), and quintiles of material deprivation were independent predictors of OAC uptake. Female sex and comorbidities including prior major bleed, history of thrombocytopenia, cancer, liver disease, excess alcohol, prior falls, and chronic kidney disease were associated with a lower odds of OAC uptake.
Adherence Patterns to DOAC Across Health Care Settings
Overall, high adherence to DOACs occurred in 76.5% of patients, intermediate in 14.0%, and low in 9.5%. Between the 2012 and 2018 fiscal years, high adherence increased from 66.6% to 82.8%, and low DOAC adherence decreased from 15.2% to 5.7% (P value for trend <0.0001).
Trends in adherence to DOAC therapy across health care settings are shown in Figure 2. Overall, high adherence to DOACs occurred in 74.0% of patients diagnosed in the ED, 75.0% in the hospital, and 81.6% in the outpatient setting (P<0.0001). Over the study period, there was a significant increase in high adherence to DOAC therapy across all health care settings. High adherence to DOACs increased from 64.9% to 80.3% in the ED, 64.3% to 81.7% in the hospital, and 70.9% to 88.6% in the outpatient setting (P<0.001, respectively).
Figure 2. Oral anticoagulation adherence stratified by locale.
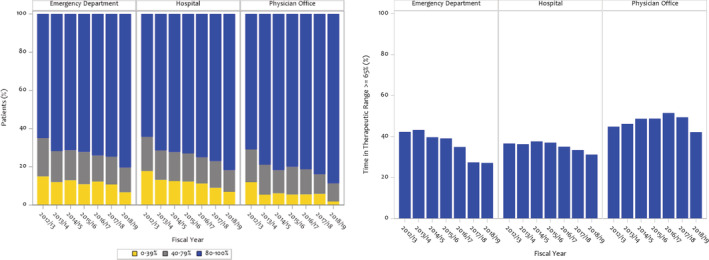
DOAC adherence by proportion of days covered in patients at high stroke risk stratified by locale (left). Warfarin control by time‐in‐therapeutic range ≥65% in patients at high stroke risk stratified by locale (right). DOAC indicates direct oral anticoagulant.
Changes in adherence categories over a 3‐year period following the first DOAC prescription are shown in Figure S4 and Table S3. Between consecutive years, for patients with NVAF who had high or low adherence after the first year of DOAC initiation, the vast majority (87–92%) remained in the same adherence category in the next year.
Predictors of DOAC adherence are shown in Figure 3. After adjusting for confounders, there was a 42% higher odds of high DOAC adherence when NVAF was diagnosed in the outpatient setting (95% CI, 1.27–1.58) compared with the hospital setting and 16% higher odds for female sex (95% CI, 1.06–1.27). There was a 10% decreased odds of DOAC adherence if diagnosed in the ED (95% CI, 0.81–0.99) and a 41% and 38% increased odds for those aged 65 to 74 and ≥75, respectively, compared with age 18 to 64.
Figure 3. Predictors of OAC adherence.
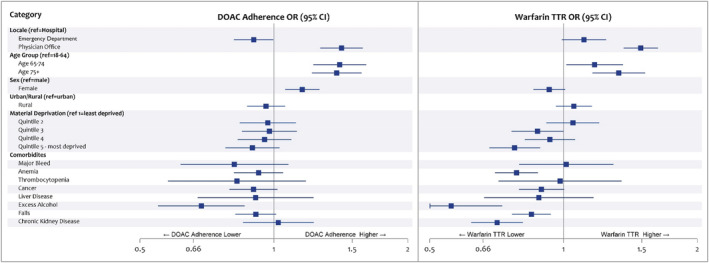
Odds ratios (ORs) and 95% CIs for factors affecting high DOAC adherence and high warfarin adherence. ORs and 95% CIs for factors affecting high DOAC adherence and high warfarin adherence. Predictors of DOAC and warfarin adherence with references are given on the left and right, respectively. DOAC adherence was assessed with proportion of days covered, while warfarin adherence was assessed with TTR. DOAC indicates direct oral anticoagulant; ED, emergency department; OAC, oral anticoagulant; and TTR, time in therapeutic range.
Warfarin Control Patterns Across Health Care Settings
Warfarin control was ≥65% in 40.3% of patients taking warfarin. Trends in warfarin control across health care settings are shown in Figure 2. When NVAF was diagnosed in the ED, warfarin control was 38.2%, 36.1% in the hospital, and 47.5% in the outpatient setting. Over time, warfarin control decreased in ED‐diagnosed patients, while it remained unchanged in the hospital and outpatient setting (P value for trends <0.0003, 0.12, and 0.24, respectively).
Changes in control categories over a 3‐year period following first warfarin prescription are shown in Figure S5 and Table S3. For patients with TTR ≥65% in the first year, ≈25% had suboptimal warfarin control in the subsequent year. While those who were suboptimal in the first year, 47.8% had TTR ≥65% in the second year and 41.1% in the third year. Trends were similar across different locations of diagnosis.
Predictors of warfarin control are shown in Figure 3. Diagnosis in the outpatient setting was associated with a 49% greater odds of warfarin control (95% CI, 1.37–1.63) compared with the hospital setting and a 22% lower odds for the most materially deprived patients (95% CI, 0.68–0.89) compared with the least.
Mortality
Overall, 935 (14.4%) patients with OAC update died in the 3 years after the landmark period. Kaplan‐Meier curves for 3‐year mortality according to OAC adherence/control categories (always high, always low, varies) are shown in Figure 4. For DOACs, patients with NVAF with both variable and low adherence had a worse prognosis. For warfarin, patients with variable control had fewer deaths than those with persistently low TTR. Predictors associated with all‐cause mortality are shown in Figure S6.
Figure 4. All‐cause mortality by 3‐year adherence category.
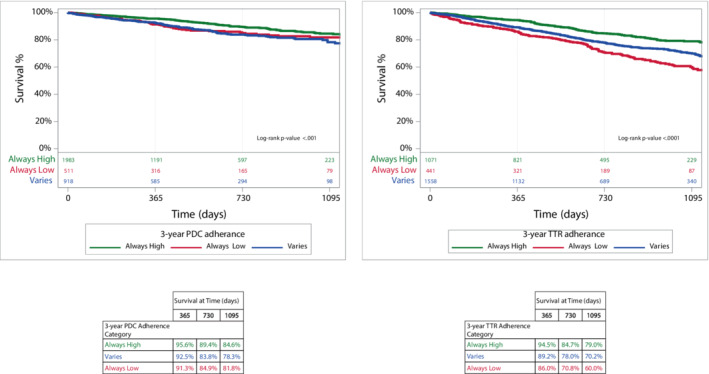
Kaplan–Meier curves of all‐cause mortality by adherence category is shown for DOACs and warfarin on the left and right, respectively. DOAC indicates direct oral anticoagulants; PDC, proportion of days covered; and TTR, time in therapeutic range.
DISCUSSION
In this population‐based study of patients with incident NVAF at increased risk of stroke and recommended to be anticoagulated in current guidelines, we found OAC uptake has increased over time across all health care settings and is driven by higher DOAC use. Three‐quarters of patients were found to have a high adherence to DOAC therapy, and this increased over time and across health care settings. Patients first diagnosed in the outpatient setting were more likely to have OAC uptake and to have high adherence to DOACs compared with the hospital setting after adjusting for confounders. In patients managed with warfarin, control was suboptimal and declined over time in the ED setting but was unchanged in other settings. Variable or persistently low adherence had a poor prognosis, particularly for warfarin.
Observational studies comparing OAC uptake in the pre‐ and post‐DOAC eras using administrative data have shown increased uptake (by ≈50%) in both incident and prevalent NVAF across a variety of health care settings. 18 , 20 , 21 We observed a similar increase in OAC uptake from 44.3% in 2008 to 67.0% in 2019, corresponding to a relative increase of 51%. Our study builds on this work by demonstrating that OAC uptake increased over time across all locales of diagnosis with a 51% relative increase overall; 72% in the ED, 63% in the hospital, and 19% in the outpatient setting. This finding is in the context of a narrowing difference in uptake between ED and hospital diagnoses compared with patients diagnosed in outpatient clinics over time. 39 Patients in the hospital were more likely to have coronary artery disease and may have been prescribed antiplatelet agents, which may cause providers to be less likely to prescribe OAC therapy because of greater bleeding risk. These differences by location of diagnosis are becoming increasingly important as the epidemiology of NVAF changes over time and patients with newly diagnosed AF are seen in health care settings other than the hospital. 29 This first point of contact provides an important opportunity to evaluate eligibility for stroke prevention therapy. Regardless of location, one‐third of patients were not started on OACs, even in 2018, the final year of the study. Efforts targeted to further understand this care gap are needed.
Comparative studies of incident and prevalent AF across all locations of diagnosis found an ≈20% relative greater adherence to DOACs than warfarin. 25 , 26 , 27 , 28 Although these previous studies did not report OAC adherence by location and did not use TTR for warfarin, 25 , 26 , 27 , 28 , 40 , 41 our data demonstrate OAC adherence, both for DOACs and warfarin, was overall highest in patients diagnosed in the outpatient setting. Importantly, warfarin control was poor regardless of setting. One possible explanation for the difference with respect to location may be limited follow‐up for patients diagnosed in the ED or the hospital. Prior work has demonstrated that patients who are diagnosed with AF in the ED are more likely to receive follow‐up if an established family physician is identified. 42 A major factor in adherence is physician communication and patient understanding of OACs. 43 More research is needed to better understand how both may serve as potential barriers to adherence and to help develop mitigating strategies. 42 Patient health literacy, which is associated with medication adherence, disease status, and outcomes, 44 , 45 should be assessed in each patient. 46 Further studies, including randomized controlled trials, are needed to determine whether provision of educational material on the importance of OAC adherence tailored to patient‐specific health literacy can improve adherence. In addition, recommending and tracking OAC adherence discussions as part of AF quality measures may further reduce this care gap.
There are limited real‐world, population‐level data on warfarin control using TTR for incident NVAF. 47 We found that the proportion of patients who exhibited adequate warfarin control (TTR ≥65%) in our study was low, at 40%. Similarly, a previous study in the same geographic location as our study reported only 41% of patients exhibited adequate control with warfarin, 48 while another study in Sweden found a 57% control rate (TTR ≥70%). The Swedish study did not restrict its patients to incident NVAF and included only patients >65 years old, which may explain the higher adherence in their cohort. Indeed, younger patients are more likely to have poor adherence to warfarin. 49 The increased adherence observed in the Swedish study may also be attributable to improved continuity of primary care. 48 Nevertheless, we found that low warfarin control persisted over time and across location of diagnosis. Although definitions of adherence to warfarin and DOAC differed, the most adherent warfarin group had higher all‐cause mortality compared with the most adherent DOAC group. Further research is needed to better understand this finding.
Although patients diagnosed in the outpatient setting had greater rates of warfarin control, the overall level was still quite low, suggesting that there may be factors unrelated to physician follow‐up. The reasons for poor warfarin control (ie, lack of defined follow‐up, labile dietary, interacting medications, alternating dosing regimens, transportation barriers, and unstable living conditions) have been previously described. 49 , 50 , 51 Identification of modifiable factors that portend poor warfarin control is important to recognize patients who may not be optimal candidates for warfarin therapy. In those that are prescribed warfarin, integrated AF care teams and quality improvement programs can improve OAC use. 52 , 53 These findings further reinforce guideline recommendations for first‐line DOACs over warfarin. 15 , 16 , 17
Previous studies of real‐world OAC adherence and outcomes found that low adherence was associated with higher rates of all‐cause mortality. 25 , 54 , 55 , 56 We found similar results in our cohort, with an increase in mortality in patients with low adherence. Our study also presents novel findings with respect to how adherence patterns change over time. We demonstrate the importance of having high adherence whether it is DOACs or warfarin during the first year after NVAF diagnosis and its relationship to mortality.
Our study has some limitations that warrant discussion. Diagnoses were based on administrative data, and misclassification or underdiagnosis may exist. However, the ICD codes used to identify AF have been previously validated with reasonable accuracy. 57 Other patient factors, for example, cognitive issues, lack of social supports, and transportation difficulties, may have influenced uptake and adherence but were not available in administrative databases. We assumed a TTR of ≥65% as controlled on the basis of prior clinical thresholds, 36 , 37 but higher benchmarks may be warranted. Adherence to DOAC therapy in our study was not directly assessed but was inferred from patients’ filled prescriptions via PIN profile. We used proportion of days covered as a measure of DOAC adherence, as this has been previously published when using administrative claims data. 36 Our data did not include prescription of antiplatelet agents; more research is needed to determine whether the use of antiplatelet agents may affect OAC prescription across all health care settings. Our data did not separate general practitioner from specialist outpatient visits. Further work is needed to better understand practice patterns for OAC uptake and adherence according to urban/rural location and practitioner type. Our study only included patients in the province of Alberta and may not be generalizable to other health care systems.
In this population‐based study, we found OAC uptake and adherence increased over time, while warfarin control remained suboptimal across all health care settings. AF diagnosis in outpatient settings is associated with higher OAC uptake and adherence. Poor OAC adherence is associated with a higher mortality, especially for warfarin. These data support guideline recommendations for first‐line DOAC therapy. Strategies aimed at improving OAC uptake and adherence across all health care settings where AF diagnosis occurs are needed.
Sources of Funding
Drs Sandhu and Kaul are supported by a Servier Alberta Innovation Health Fund grant for this work. The funder did not have access to data, and was not involved in the analysis, interpretation, or writing of the manuscript.
Disclosures
Dr McAlister is supported by the AHS Chair in Cardiovascular Outcomes Research. Dr Goodman reports research grant support (eg, steering committee or data and safety monitoring committee) or speaker/consulting honoraria (eg, advisory boards) from Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi‐Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, HLS Therapeutics, JAMP Pharma, Merck, Novartis, Novo Nordisk A/C, Pendopharm/Pharmascience, Pfizer, Regeneron, Sanofi, Servier, and Valeo Pharma; and salary support/honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Centre for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Centre, PERFUSE Research Institute, and the TIMI Study Group (Brigham Health). Dr Sandhu reports research grant support from Pfizer and Servier. The other authors have no relevant financial interests to declare.
Supporting information
Data S1
Tables S1–S3
Figures S1–S6
Acknowledgments
Data were extracted from the Alberta Health Services Enterprise Data Warehouse with support provided by AbSPORU Data and Research Services platform, which is funded by Canadian Institutes of Health Research, Alberta Innovates, University Hospital Foundation, University of Alberta, University of Calgary, and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of Alberta Health Services or any of the funders.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024868
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y‐H, McAnulty JH Jr, Zheng Z‐J, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.STR.22.8.983 [DOI] [PubMed] [Google Scholar]
- 3. Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I–III clinical trials. Stroke. 1999;30:1223–1229. doi: 10.1161/01.STR.30.6.1223 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . The top 10 causes of death. 2018.
- 5. Wolf PA, Dawber TR, Thomas HE, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham Study. Neurology. 1978;28:973–977. doi: 10.1212/WNL.28.10.973 [DOI] [PubMed] [Google Scholar]
- 6. Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, Carolei A. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population‐based study. Stroke. 2005;36:1115–1119. doi: 10.1161/01.STR.0000166053.83476.4a [DOI] [PubMed] [Google Scholar]
- 7. Wolf PA, Mitchell JB, Baker CS, Kannel WB, D'Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. doi: 10.1001/archinte.158.3.229 [DOI] [PubMed] [Google Scholar]
- 8. Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, Singer DE. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. doi: 10.1056/NEJMoa022913 [DOI] [PubMed] [Google Scholar]
- 9. Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population‐based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.STR.31.5.1062 [DOI] [PubMed] [Google Scholar]
- 10. Penado S, Cano M, Acha O, Hernández JL, Riancho JA. Atrial fibrillation as a risk factor for stroke recurrence. Am J Med. 2003;114:206–210. doi: 10.1016/S0002-9343(02)01479-1 [DOI] [PubMed] [Google Scholar]
- 11. Li X, Tse VC, Au‐Doung LW, Wong IC, Chan EW. The impact of ischaemic stroke on atrial fibrillation‐related healthcare cost: a systematic review. Europace. 2017;19:937–947. doi: 10.1093/europace/euw093 [DOI] [PubMed] [Google Scholar]
- 12. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003 [DOI] [PubMed] [Google Scholar]
- 13. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 14. Hernandez I, He M, Chen N, Brooks MM, Saba S, Gellad WF. Trajectories of oral anticoagulation adherence among Medicare beneficiaries newly diagnosed with atrial fibrillation. J Am Heart Assoc. 2019;8:e011427. doi: 10.1161/JAHA.118.011427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 16. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS) the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 17. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–1948. doi: 10.1016/j.cjca.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 18. Camm AJ, Accetta G, Ambrosio G, Atar D, Bassand J‐P, Berge E, Cools F, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. 2017;103:307–314. doi: 10.1136/heartjnl-2016-309832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marzec LN, Wang J, Shah ND, Chan PS, Ting HH, Gosch KL, Hsu JC, Maddox TM. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69:2475–2484. doi: 10.1016/j.jacc.2017.03.540 [DOI] [PubMed] [Google Scholar]
- 20. Gadsbøll K, Staerk L, Fosbøl EL, Sindet‐Pedersen C, Gundlund A, Lip GY, Gislason GH, Olesen JB. Increased use of oral anticoagulants in patients with atrial fibrillation: temporal trends from 2005 to 2015 in Denmark. Eur Heart J. 2017;38:899–906. doi: 10.1093/eurheartj/ehw658 [DOI] [PubMed] [Google Scholar]
- 21. Kezerle L, Tsadok MA, Berliner Senderey A, Hoshen M, Leventer‐Roberts M, Reges O, Leibowitz M, Haim M. Use of oral anticoagulation therapy in the first 3 months after the diagnosis of atrial fibrillation in Israel: a population‐based study. J Cardiovasc Electrophysiol. 2020;31:1356–1363. doi: 10.1111/jce.14452 [DOI] [PubMed] [Google Scholar]
- 22. Admassie E, Chalmers L, Bereznicki LR. Changes in oral anticoagulant prescribing for stroke prevention in patients with atrial fibrillation. Am J Cardiol. 2017;120:1133–1138. doi: 10.1016/j.amjcard.2017.06.055 [DOI] [PubMed] [Google Scholar]
- 23. Narita N, Okumura K, Kinjo T, Mikami J, Tsushima K, Takahashi R, Noro M, Hashimoto A, Sasaki T, Takaki M, et al. Trends in prevalence of non‐valvular atrial fibrillation and anticoagulation therapy in a Japanese region—analysis using the National Health Insurance Database—. Circulation. 2020;84:706–713. doi: 10.1253/circj.CJ-18-0989 [DOI] [PubMed] [Google Scholar]
- 24. Brown JD, Shewale AR, Dherange P, Talbert JC. A comparison of oral anticoagulant use for atrial fibrillation in the pre‐and post‐DOAC eras. Drugs Aging. 2016;33:427–436. doi: 10.1007/s40266-016-0369-y [DOI] [PubMed] [Google Scholar]
- 25. Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, Gersh BJ, Shah ND, Noseworthy PA. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5:e003074. doi: 10.1161/JAHA.115.003074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McHorney CA, Ashton V, Laliberté F, Germain G, Wynant W, Crivera C, Schein JR, Lefebvre P, Peterson ED. Adherence to rivaroxaban compared with other oral anticoagulant agents among patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2017;23:980–988. doi: 10.18553/jmcp.2017.23.9.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sørensen R, Jamie Nielsen B, Langtved Pallisgaard J, Ji‐Young Lee C, Torp‐Pedersen C. Adherence with oral anticoagulation in non‐valvular atrial fibrillation: a comparison of vitamin K antagonists and non‐vitamin K antagonists. Eur Heart J Cardiovasc Pharmacother. 2017;3:151–156. doi: 10.1093/ehjcvp/pvw048 [DOI] [PubMed] [Google Scholar]
- 28. Banerjee A, Benedetto V, Gichuru P, Burnell J, Antoniou S, Schilling RJ, Strain WD, Ryan R, Watkins C, Marshall T, et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: a population‐based study. Heart. 2020;106:119–126. doi: 10.1136/heartjnl-2019-315307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. The epidemiology of atrial fibrillation in adults depends on locale of diagnosis. Am Heart J. 2011;161:986–992. doi: 10.1016/j.ahj.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 30. Atzema CL, Austin PC, Miller E, Chong AS, Yun L, Dorian P. A population‐based description of atrial fibrillation in the emergency department, 2002 to 2010. Ann Emerg Med. 2013;62:570–577. doi: 10.1016/j.annemergmed.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 31. Rozen G, Hosseini SM, Kaadan MI, Biton Y, Heist EK, Vangel M, Mansour MC, Ruskin JN. Emergency department visits for atrial fibrillation in the United States: trends in admission rates and economic burden from 2007 to 2014. J Am Heart Assoc. 2018;7:e009024. doi: 10.1161/JAHA.118.009024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hawkins NM, Daniele PR, Humphries KH, Ezekowitz JA, McAlister FA, Sandhu RK, Kaul P. Empirical insights when defining the population burden of atrial fibrillation and oral anticoagulation utilization using administrative data. Can J Cardiol. 2019;35:1412–1415. doi: 10.1016/j.cjca.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 33. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 34. Pampalon R, Hamel D, Gamache P, Raymond G. A deprivation index for health planning in Canada. Chronic Dis Can. 2009;29:178–191. doi: 10.24095/hpcdp.29.4.05 [DOI] [PubMed] [Google Scholar]
- 35. Rosendaal F, Cannegieter S, Van der Meer F, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. J Thromb Haemost. 1993;70:236–239. doi: 10.1055/s-0038-1651587 [DOI] [PubMed] [Google Scholar]
- 36. Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut‐point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25:2303–2310. doi: 10.1185/03007990903126833 [DOI] [PubMed] [Google Scholar]
- 37. Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Healey JS, Yusuf S. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–2037. doi: 10.1161/CIRCULATIONAHA.107.750000 [DOI] [PubMed] [Google Scholar]
- 38. Senoo K, Lip GY. Female sex, time in therapeutic range, and clinical outcomes in atrial fibrillation patients taking warfarin. Stroke. 2016;47:1665–1668. doi: 10.1161/STROKEAHA.116.013173 [DOI] [PubMed] [Google Scholar]
- 39. Kea B, Waites BT, Lin A, Raitt M, Vinson DR, Ari N, Welle L, Sill A, Button D, Sun BC. Practice gap in atrial fibrillation oral anticoagulation prescribing at emergency department home discharge. West J Emerg Med. 2020;21:924. doi: 10.5811/westjem.2020.3.45135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Forslund T, Wettermark B, Hjemdahl P. Comparison of treatment persistence with different oral anticoagulants in patients with atrial fibrillation. Eur J Clin Pharmacol. 2016;72:329–338. doi: 10.1007/s00228-015-1983-z [DOI] [PubMed] [Google Scholar]
- 41. Beyer‐Westendorf J, Ehlken B, Evers T. Real‐world persistence and adherence to oral anticoagulation for stroke risk reduction in patients with atrial fibrillation. Europace. 2016;18:1150–1157. doi: 10.1093/europace/euv421 [DOI] [PubMed] [Google Scholar]
- 42. Atzema CL, Yu B, Ivers N, Rochon P, Lee DS, Schull MJ, Austin PC. Incident atrial fibrillation in the emergency department in Ontario: a population‐based retrospective cohort study of follow‐up care. CMAJ Open. 2015;3:E182–E191. doi: 10.9778/cmajo.20140099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mazor KM, Baril J, Dugan E, Spencer F, Burgwinkle P, Gurwitz JH. Patient education about anticoagulant medication: is narrative evidence or statistical evidence more effective? Patient Educ Couns. 2007;69:145–157. doi: 10.1016/j.pec.2007.08.010 [DOI] [PubMed] [Google Scholar]
- 44. Peterson PN, Shetterly SM, Clarke CL, Bekelman DB, Chan PS, Allen LA, Matlock DD, Magid DJ, Masoudi FA. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305:1695–1701. doi: 10.1001/jama.2011.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu J‐R, Holmes GM, DeWalt DA, Macabasco‐O'Connell A, Bibbins‐Domingo K, Ruo B, Baker DW, Schillinger D, Weinberger M, Broucksou KA, et al. Low literacy is associated with increased risk of hospitalization and death among individuals with heart failure. J Gen Intern Med. 2013;28:1174–1180. doi: 10.1007/s11606-013-2394-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aliot E, Breithardt G, Brugada J, Camm J, Lip GY, Vardas PE, Wagner M; AWareness AF, Group RE . An international survey of physician and patient understanding, perception, and attitudes to atrial fibrillation and its contribution to cardiovascular disease morbidity and mortality. Europace. 2010;12:626–633. doi: 10.1093/europace/euq109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Björck F, Renlund H, Lip GY, Wester P, Svensson PJ, Själander A. Outcomes in a warfarin‐treated population with atrial fibrillation. JAMA Cardiol. 2016;1:172–180. doi: 10.1001/jamacardio.2016.0199 [DOI] [PubMed] [Google Scholar]
- 48. McAlister FA, Wiebe N, Hemmelgarn BR. Time in therapeutic range and stability over time for warfarin users in clinical practice: a retrospective cohort study using linked routinely collected health data in Alberta, Canada. BMJ Open. 2018;8:e016980. doi: 10.1136/bmjopen-2017-016980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe‐TT2R2 score. Chest. 2013;144:1555–1563. doi: 10.1378/chest.13-0054 [DOI] [PubMed] [Google Scholar]
- 50. Orensky IA, Holdford DA. Predictors of noncompliance with warfarin therapy in an outpatient anticoagulation clinic. Pharmacotherapy. 2005;25:1801–1808. doi: 10.1592/phco.2005.25.12.1801 [DOI] [PubMed] [Google Scholar]
- 51. Witt DM, Nieuwlaat R, Clark NP, Ansell J, Holbrook A, Skov J, Shehab N, Mock J, Myers T, Dentali F, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2:3257–3291. doi: 10.1182/bloodadvances.2018024893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vinereanu D, Lopes RD, Bahit MC, Xavier D, Jiang J, Al‐Khalidi HR, He W, Xian Y, Ciobanu AO, Kamath DY, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT‐AF): an international, cluster‐randomised trial. Lancet. 2017;390:1737–1746. doi: 10.1016/S0140-6736(17)32165-7 [DOI] [PubMed] [Google Scholar]
- 53. Piccini JP, Allred J, Bunch TJ, Deering TF, Di Biase L, Hussein AA, Lewis WR, Mittal S, Natale A, Osorio J, et al. Rationale, considerations, and goals for atrial fibrillation centers of excellence: a Heart Rhythm Society perspective. Heart Rhythm. 2020;17:1804–1832. doi: 10.1016/j.hrthm.2020.04.033 [DOI] [PubMed] [Google Scholar]
- 54. Gabet A, Chatignoux E, Grave C, Vallée A, Tuppin P, Béjot Y, Olié V. Stroke incidence and death in atrial fibrillation patients newly treated with direct oral anticoagulants. Clin Epidemiol. 2021;13:131–140. doi: 10.2147/CLEP.S290707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Borne RT, O'Donnell C, Turakhia MP, Varosy PD, Jackevicius CA, Marzec LN, Masoudi FA, Hess PL, Maddox TM, Ho PM. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the Veterans Health Administration. BMC Cardiovasc Disord. 2017;17:1–7. doi: 10.1186/s12872-017-0671-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Krittayaphong R, Winijkul A, Kunjara‐Na‐Ayudhya R, Apiyasawat S, Siriwattana K, Kanjanarutjawiwat W, Dutsadeevettakul S, Lip GY; COOL‐AF Investigators . Adherence to anticoagulant guideline for atrial fibrillation improves outcomes in Asian population: the COOL‐AF Registry. Stroke. 2020;51:1772–1780. doi: 10.1161/STROKEAHA.120.029295 [DOI] [PubMed] [Google Scholar]
- 57. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21:141–147. doi: 10.1002/pds.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figures S1–S6


