Abstract
Background
Fetal echocardiography has been the mainstay of fetal arrhythmia diagnosis; however, fetal magnetocardiography (fMCG) has recently become clinically available. We sought to determine to what extent fMCG contributed to the precision and accuracy of fetal arrhythmia diagnosis and risk assessment, and in turn, how this altered pregnancy management.
Methods and Results
We reviewed fMCG tracings and medical records of 215 pregnancies referred to the Biomagnetism Laboratory, UW‐Madison, over the last 10 years, because of fetal arrhythmia or risk of arrhythmia. We compared referral diagnosis and treatment with fMCG diagnosis using a rating scale and restricted our review to the 144 subjects from the tachycardia, bradycardia/AV block, and familial long QT syndrome categories. Additional fMCG findings beyond those of the referring echocardiogram, or an alternative diagnosis were seen in 117/144 (81%), and 81 (56%) were critical changes. Eight (5.5%) had resolution of arrhythmia before fMCG. At least moderate changes in management were seen in 109/144 (76%) fetuses, of which 35/144 (24%) were major. The most diverse fMCG presentation was long QT syndrome, present in all 3 referral categories. Four of 5 stillbirths were seen with long QT syndrome. Nine fetuses showed torsades de pointes ventricular tachycardia, of which only 2 were recognized before fMCG.
Conclusions
FMCG has a significant impact on prenatal diagnosis and management of arrhythmias or familial arrhythmia risk, which cannot be fully met by existing technology. The combination of fMCG and fetal echocardiography in fetal care centers will be needed in the future to optimize care.
Keywords: electrocardiography, fetal arrhythmia, fetal echocardiography, fetus, long QT syndrome, magnetocardiography
Subject Categories: Arrhythmias
Nonstandard Abbreviations and Acronyms
- AVB
atrioventricular block
- BAB
blocked atrial bigeminy
- fMCG
fetal magnetocardiography
- LQTS
long QT syndrome
- PVCs
premature ventricular contractions
- SSA/Ro
Sjogren's antibody A
- SVT
supraventricular tachycardia
- TdP
torsades de pointes ventricular tachycardia
- TWA
T wave alternans
Clinical Perspective.
What Is New?
Fetal magnetocardiography (fMCG) is an advanced technology similar to postnatal electrocardiography or Holter monitoring.
In 144 high‐risk pregnant subjects with or at risk for fetal arrhythmias related to tachycardia, atrioventricular block, and long QT syndrome, fMCG supplemented and/or changed the clinical diagnosis in over half of fetuses studied by (1) dispelling ambiguity of echocardiographic rhythm diagnosis, and/or (2) uncovering new unsuspected rhythm or conduction abnormalities.
What Are the Clinical Implications?
fMCG findings led to major clinical fetal–maternal management changes in one quarter of patients, often resulting in a safer maternal care plan.
Seven of 9 cases of torsades de pointes were not detected by echocardiography before fMCG.
Correct fetal rhythm diagnosis provided by a combination of fMCG and fetal echocardiography, in the future, may improve maternal–fetal outcomes.
Fetal magnetocardiography (fMCG) is a promising technology that safely and noninvasively records the natural electromagnetic signal of the fetal heart. 1 , 2 , 3 New optically pumped magnetometer sensors, and person‐sized magnetic shields have lowered technology cost and will likely soon facilitate broader adoption. 4 , 5 Like postnatal ECG, fMCG allows precise assessment of cardiac time intervals (RR, P, PR, QRS, QT, U, and QTc), signal characteristics, and diverse rhythm patterns. The Scientific Statement of the American Heart Association supports the use of fMCG to assess cardiac conduction and rhythm in fetuses with known or suspected conduction system abnormalities, a Category 2a recommendation (ie, benefits>>risks) and stated that no other technique is as direct or precise. 1 Publications over the past decade have shown the efficacy of fMCG for diagnosis and for predicting prognosis of serious fetal arrhythmias, 3 , 6 , 7 , 8 , 9 , 10 , 11 , 12 yet to date, no study has systematically compared fMCG and referral diagnosis, as defined by fetal echocardiography or referral ultrasound.
fMCG has several other characteristics that allow it to better define fetal rhythm disorders. Unlike echocardiography that is stored as short clips, fMCG allows Holter‐like continuous beat‐to‐beat recordings over time. It also defines both depolarization and repolarization. The aim of this study was to compare referral diagnosis with the diagnosis subsequently made by fMCG to evaluate the additional findings as well as drastic changes in diagnosis, and further, to assess the impact of fMCG on risk stratification and on clinical management, since precise analysis of rhythm disorders and diagnosis‐driven treatments might lead to better clinical management and outcomes.
Methods
Transparency and Openness
Because of the sensitive nature of the data collected for this study, involving a designated vulnerable fetal population, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols and within similar areas of research, may be sent to Ronald T Wakai, PhD, Director Biomagnetism Laboratory, University of Wisconsin Department of Medical Physics, Wisconsin Institute of Medical Research, Room L1 1005, 1111 Highland Ave, Madison, WI 53705–2275.
Patients
This was a single‐center retrospective study of women who participated in 2 National Institutes of Health–funded studies and who were at least 18 years of age and 16 or more weeks pregnant. Pregnant subjects were referred to the University of Wisconsin Biomagnetism laboratory over the past 10 years, either because of a fetal arrhythmia or because the pregnancy was at risk of a fetal arrhythmia because of either maternal anti‐Ro/SSA (Sjogren's antibody A positive) antibodies or a heritable arrhythmia syndrome. Both singleton and twin pregnancies were included. Informed consent for inclusion into the study was obtained from each participant. The time from referral to evaluation for those with active arrhythmias was nearly always under 1 week, and often within 24 hours.
Subjects with minor arrhythmias (premature atrial contractions, SSA antibody without block) and predominantly sinus rhythm were not included. Fetuses thought to have transient decelerations were also not included. Twenty‐four fetuses with congenital heart disease will be considered elsewhere, because of their unique findings in conduction and repolarization.
The 3 largest groups are reported here. These subjects were categorized based on their referral diagnosis or family history. The groups were the following:
-
1.
fetal tachycardia (n=31)
-
2.
sustained bradycardia, (n=74), subdivided into
sinus bradycardia (n=34).
nonisoimmune congenital atrioventricular block (AVB) (n=15).
SSA/Ro‐associated AVB (n=25), and
-
3.
familial LQTS (long QT syndrome) (n=39).
Subjects were assigned to only 1 category for statistical assessment. Although referral diagnoses were not assigned by us, we believe most centers used the standard American College of Obstetricians and Gynecologists definitions of bradycardia (<110/min) and tachycardia (>180/min), with the exception of certain slow tachycardias, which may have been defined based on ventriculoatrial or atrioventricular interval. Many centers used gestation‐based fetal heart rates (HRs) to define those fetuses under the third or over the 95th percentile for gestation.
fMCG Measurements
fMCG was acquired by using a superconducting quantum interference device biomagnetometer (Tristan Technologies, San Diego, CA), a US Food and Drug Administration 510K approved technology. Superconducting quantum interference devices are passive detectors that do not emit energy or magnetic fields. Each of 7 vector sensors allowed assessment of the X, Y, and Z magnetic field components (21 total channels). The studies were performed in a magnetically shielded room that attenuates magnetic interference from external sources. The fetal magnetocardiographic tracings were recorded using a previously published standardized protocol. 3 Of the 18 subjects with maternal LQTS, 9 women had implantable cardioverter‐defibrillators and adequate diagnostic fMCG tracings were obtained in all. Limited 2D/Doppler echocardiogram for assessment of VV and mechanical PR intervals were obtained in the laboratory to validate the rhythm. In cases where the findings were normal by both fMCG and in‐laboratory echocardiogram, it was assumed the arrhythmia had spontaneously resolved, and they were not considered to have a change in diagnosis (because it was not related to fMCG findings). Medical records were requested and reviewed, including in most cases neonatal records. The study was approved under the UW‐Madison Institutional Review Board and carried out under 2 US Food and Drug Administration Investigational Device Exemption studies. Twenty‐seven subjects in the familial and 11 subjects with de novo LQTS have been previously reported, but not related to their change in diagnosis or management. 9 , 13
Data Collection and Analysis
Signal processing was required to separate the fMCG from the maternal MCG and other interferences. 14 , 15 , 16 A minimum of 4 ten‐minute runs were recorded in each subject. The data were used to generate a subject report, consisting of fetal actocardiograms (fetal activity and HR tracings), signal‐averaged waveforms, 5‐second rhythm strips, and extended (Holter‐like) rhythm tracings. Waveform intervals in normal fetuses, recently published, provided the basis for determination of abnormal prolongation or shortening. 16
Categories in Diagnosis and Management
Change in Diagnosis
Algorithms were developed by our group and approved by the US Food and Drug Administration for use in our 2 investigational device exemption studies (Figure 1, Table 1). A “Critical” change in diagnosis was identified if fMCG found a different or an additional unsuspected clinically significant diagnosis that was not recognized at the time of referral, or if a more precise assessment led to identification of risk factors such as prolonged QTc, Wolff‐Parkinson‐White pattern, or bundle branch block. Only 1 designation of critical change per subject was counted even if >1 was present.
Figure 1. Patients and categories.
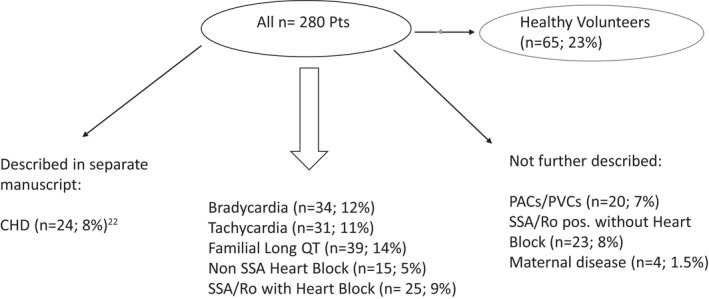
CHD indicates congenital heart disease; PACs/PVCs, premature atrial contractions/premature ventricular contractions; Pts, patients; and SSA/Ro pos, Sjogren's antibody A positive.
Table 1.
Categories and Degrees in the Change in Management
| Clinical information | Moderate change | Major change |
|---|---|---|
| Prenatal surveillance | Moderate change in level of surveillance | Major increase or decrease in surveillance because of a new (or dispelled) life‐threatening arrhythmia |
| Medications (either added, altered, withdrawn, or avoided) | Low‐risk and short‐term medications added, altered, or withdrawn | Higher‐risk or longer‐term medications added, altered, or withdrawn |
| Delivery planning | New team in delivery plans | New high‐acuity fetal and neonatal team and delivery site; major transitional care needed, potentially including in the delivery room, need for urgent hospitalization or delivery, need for major fetal or neonatal intervention (intramuscular digoxin,etc) |
| Prognosis of disease | Moderate increase or decrease, arrhythmia has the potential to cause severe morbidity if new recommendations are not observed | Unsuspected risk of fetal death identified or risk dispelled |
| Level of follow‐up and neonatal care | Moderate increase or decrease in care (potential NICU care, genetic testing, noninvasive EP testing) | Protracted NICU care, postnatal electrophysiologic testing, pacemaker, open heart surgery, postmortem autopsy, or other |
EP indicates electrophysiology; and NICU, neonatal intensive care unit.
No Changes or Negative Studies
For those referred for family history of risk of inherited arrhythmia, we elected to assume the null hypothesis, that the fetus was not affected, even though many have a 50:50 risk of the disease. Therefore, if the fMCG was normal in a fetus at risk for LQTS but without symptoms before referral, that would not represent a change in diagnosis. Conversely, a positive fMCG finding of QTc>500 ms was a critical change in diagnosis (new diagnosis) given the life‐long implications of a positive diagnosis.
A “negative” study might also be because of spontaneous regression or treatment that took place before study of the subject. In these cases, a change in diagnosis was not assigned; however, changes in management (such as discontinuation of dexamethasone after a normal study) were assigned. Down‐ranking severity (for example, from 2° AVB to blocked atrial bigeminy) in some cases had a major benefit to the patient: less need for visits to, or delivery at, a tertiary center, which often provided psychological relief to the subject.
Change in Management
To qualify as a change in management (shown in the text in italics for clarity), the recommendations must have directly followed from the diagnostic findings by fMCG that showed either a more or a less serious condition than suspected at referral. The degree of change was categorized as “moderate” or “major” (Table 1). To qualify as a major change, the change in management must have significantly impacted the dose or choice of medications, the monitoring, or the prognosis. These were sometimes complete reversals of the treatment regimen initiated before referral, because of a complete change in diagnosis. Examples of changes in diagnosis and management can be found in Data S1.
Statistical Analysis
Standard descriptive statistics were used for baseline subject characteristics. Because the patient groups were so different from each other, they were individually described to highlight their unique features. Bradycardia was grouped as 1 category. Only 1 primary diagnostic category was assigned for each subject, and this was based on the primary referral diagnosis; however, some subjects had multiple diagnoses.
Results
Baseline Characteristics
The baseline characteristics of the 215 original patients are shown in Table 2. None of the 3 subgroups reported here had a significant difference in maternal age, or race/ethnicity from the main group.
Table 2.
Baseline Characteristics of the Mother
| Mother | No. | Mean time (±SD) | % |
|---|---|---|---|
| Maternal age (y) | 215* | 28 ± 5.3 y | |
| Gestational age (wks) | 215 | 29 ± 4.6 wks | |
| White race | 164 | 76 | |
| African American | 17 | 7 | |
| Hispanic (white or black race) | 20 | 9 | |
| Asian | 13 | 6 | |
| More than 1 race | 1 | 0.4 | |
| Antiarrhythmic therapy | 48 | 22 | |
| Steroid therapy | 34 | 15 |
Excludes healthy volunteers.
Overview of the Changes in Diagnosis and Management
Of the 144 subjects in these 3 categories, 81 had critical changes in diagnosis.
An overview of all changes in diagnosis, and the subsequent changes in management in the 3 major categories are shown in Table 3. This included silent conduction abnormalities (bundle branch block, QTc prolongation), which in general could not have been diagnosed by echocardiography.
Table 3.
Changes in Diagnosis and Management
| Fetal tachycardia | Fetal sinus bradycardia | Nonisoimmune AVB | SSA/Ro pos with AVB | Familial LQTS | |
|---|---|---|---|---|---|
| All pts., No. | 31 | 34 | 15 | 25 | 39 |
| Changes in diagnosis | |||||
| Spontaneous resolution, No. of pts. (%) | 2 (6) | 4 (11) | 2 (13) | … | … |
|
Any change in diagnosis, No. of pts.(%) |
25 (80) | 29 (85) | 13 (86) | 25 (100) | 25 (64) |
|
Critical change in diagnosis, No. of pts.(%) |
11 (35) | 23 (67) | 13 (86) | 9 (36) | 25 (64) |
| Changes in management | |||||
| Moderate change in management, No. of pts. (%) | 13 (41) | 17 (50) | 3 (20) | 19 (76) | 22 (39) |
| Major change in management, No. of pts.(%) | 10 (32) | 8 (23) | 9 (60) | 4 (16) | 3 (7) |
AVB indicates atrioventricular block; LQTS long QT syndrome; Pts., patients; and SSA/Ro, Sjogren's antibody A positive.
Results of Changes in Diagnosis and Management According to the Referral Diagnosis
Fetal Tachycardia
Thirty‐one fetuses were referred for fetal tachycardia. Of those, 3 were specifically referred for supraventricular tachycardia (SVT), 5 for atrial flutter, 2 for ventricular tachycardia, and the others were unspecified 1:1 SVT or sinus tachycardia. Thirteen (38%) were taking antiarrhythmic medications at the time of the fMCG.
In 25 cases (87%), fMCG showed additional findings. Twenty‐three (67%) had a critical change in diagnosis. The most important changes included persistent (n=8) and intermittent (n=1) atrial ectopic tachycardia, atrioventricular re‐entry tachycardia (n=1), sinus bradycardia (n=1), 1°AVB (n=1), multiple mechanism tachycardia (n=6), QTc prolongation in 2, junctional ectopic tachycardia (n=1), and torsades de pointes ventricular tachycardia (TdP) (n=2).
A moderate or major change in management resulted from the findings in 23 (74%) patients. Moderate changes included adjustment of drug dosages up or down (n=14) and much closer surveillance (n=3). Major changes in 10 (37%) included recommending introduction of new antiarrhythmic therapy (n=8) with sotalol 3, digoxin/sotalol 1, magnesium/propranolol 2, amiodarone 1, and digoxin 1 (although this last patient opted for no medication).
Fetal Bradycardia
Sinus Bradycardia
Thirty‐four cases were referred for unspecified fetal bradycardia. Four showed a spontaneous return of sinus rhythm.
In 29 (85%) a change in diagnosis was found.
Concerning the change in diagnosis, complex atrial rhythms were found in 5 fetuses, blocked atrial bigeminy (BAB) in another 5, and intermittent AVB in 1. In 9 fetuses, QTc prolongation, related to previously undiagnosed LQTS, was found; torsades was found in 2, both with QRS and T wave alternans (TWA) as well as late premature ventricular contractions (PVCs). Fetal demise occurred in these 2 fetuses (de novo LQTS); all others had sinus bradycardia with additional findings such as short PR interval (ectopic atrial rhythm).
Twenty‐five of 34 (73%) also had a change in management, with moderate changes in 17 subjects (50%) and major in 8 (23%) subjects. These changes included closer surveillance and change in prognosis. Concerning the major changes (23%), 3 fetuses were recommended to wean or stop short‐course dexamethasone therapy after an alternate diagnosis (BAB instead of 2° AVB) was found and before SSA results, and 2 fetuses had severe LQTS with torsades and were started on transplacental therapy with magnesium and β‐blockers.
Nonisoimmune AVB
Fifteen fetuses were referred for what was thought to be SSA negative AVB (14 for 2° AVB, 1 for 3° AVB). Two resolved and were probably either transient AVB or BAB.
Thirteen of 15 fetuses had a change in diagnosis as fMCG showed more precision.
Changes of diagnosis included PVCs (n=1), severe QTc prolongation with TdP (n=2), blocked atrial quadrigeminy and trigeminy in 1 fetus each, and finally BAB with ectopy (n=3). (Figures 2A through 2D, 3A through 3D, 4A and 4B). Twelve fetuses had a change in management of which 9 were major, including closer surveillance and LQTS protocol. In 6 fetuses transplacental management was added (magnesium 3, high‐dose propranolol 2, mexiletine 2, lidocaine 1), 3 had withdrawal of short‐course dexamethasone (pending SSA results). Two fetuses died in utero despite treatment. Both had 2°AVB because of de novo LQTS.
Figure 2. Aortic Doppler in a 26‐week gestational age fetus during TdP ventricular tachycardia.
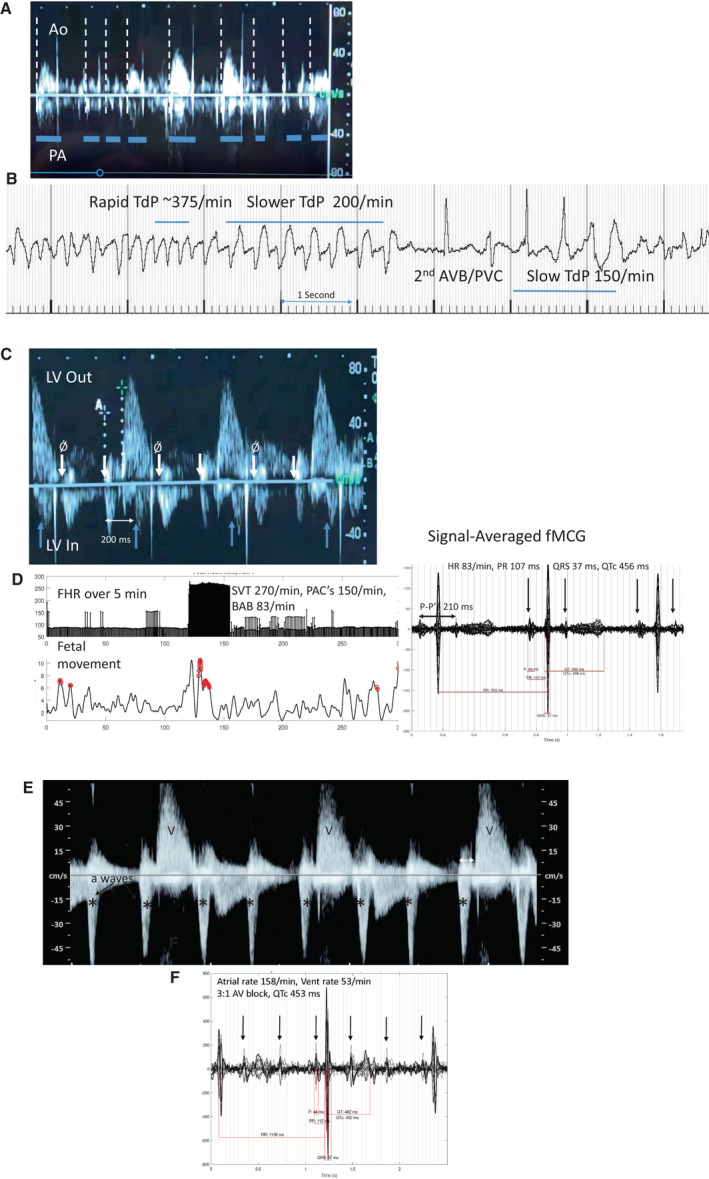
A, Doppler flow velocity onsets for pulmonary artery (downward) and aorta (upward) are not the same, suggesting bundle branch block. Hatched lines mark each systolic onset. Multiple valve clicks can be seen. Duration of systole (horizontal lines) and velocity of aortic output are also variable and attenuated in TdP, useful echo/Doppler features during evaluation, along with ventricular dysfunction. The rate of TdP is often underestimated by Doppler because of the severe and variable diminution of systolic output. B, Fetal magnetocardiography from this fetus. TdP was present 90% of the time during the recording session, alternating with 2°AVB, but because it was “normal‐rate” TdP between 150 and 200 bpm, it was not recognized as TdP by echocardiography/Doppler, and the higher rate was thought to be sinus with ectopy. C, Blocked atrial bigeminy (BAB); Doppler tracings in a 29 6/7‐week‐gestation fetus referred for suspected 2°AVB. The subject had been started on dexamethasone, but SSA antibodies were later noted to be normal. The mPR was 125 ms. Pseudo “a” waves (Ǿ arrow) were nearly equally spaced with “a” waves, suggesting 2°AV block. The real blocked ectopic velocities were discordant, because of their re‐entrant nature. During the time frame of the aortic flow velocity, but in a downward direction is the atrial “a” velocity corresponding to the p prime on fMCG (upright arrows mark onsets). These do not conduct to the ventricle, resulting in BAB with a rate of 83/min. a‐a' is ≈200 ms, closely correlating to the p–p' of 210 ms by fMCG. D, HR trend graph, actogram, and (right) 20‐s signal‐averaged tracing during BAB. Not detected by fetal echocardiography was the brief SVT episode at 270 beats/min. Stopping dexamethasone, and managing the SVT were major changes in management. E, Doppler tracing from referring hospital on a 22 3/7‐week‐gestation fetus thought to have third‐degree AV block. F, fMCG signal‐averaged tracing demonstrates a stable PR interval consistent with 3:1, 2°AVB. The subject was SSA negative. The cause of the AV block is unclear, because the QTc was normal, and postnatal genetic testing for LQTS showed no pathologic variant.*denotea atrial "A" waves. Ao indicates aortic outflow; AV, atrioventricular; AVB, atrioventricular block; FHR, fetal heart rate; fMCG, fetal magnetocardiography; LQTS, long QT syndrome; LV Out, left ventricular outflow; LV In, left ventricular inflow; mPR, mechanical PR interval; PA, pulmonary artery outflow; PACs, premature atrial contractions; PVC, premature ventricular contraction; SSA, Sjogren's antibody A; SVT, supraventricular tachycardia; TdP, torsades de pointes; and V, ventricular "V" waves by Doppler.
Figure 3. 3.5‐s signal averaged tracing from the 26‐week fetus in Figure 2.
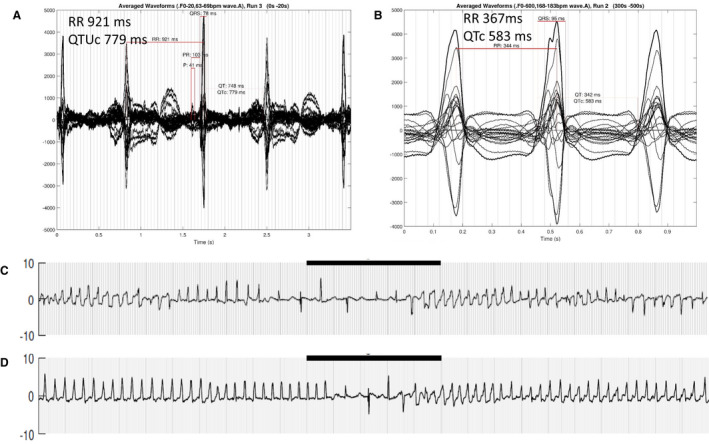
A, functional 2:1 AV block and marked QTU prolongation. B, 1‐s signal averaged tracing during monomorphic VT showing a very stable pattern of VT, unlike that seen typically with long QT syndrome. This fetus had both LQT1 and LQT2. The fetus required β‐blocker therapy pre‐ and postnatally, and a pacemaker and left stellate ganglionectomy after delivery. VT indicates ventricular tachycardia. C, Same fetus with periods of typical polymorphic torsades morphology. Black bar = 5 s. D, Same fetus, same run, showing the monomorphic‐appearing VT. Neither form of VT was recognized before fMCG, largely because of the relatively slow rate (163 beats/min), which was thought to be sinus rhythm. Both forms of VT responded in utero to maternal high‐dose propranolol therapy at 320 mg/d. AV indicates atrioventricular; LQT ‐ Long QT; TWA, T wave alternans; and VT, ventricular tachycardia.
Figure 4. Blocked Atrial Bigeminy (BAB) and 2:1 AV Block by fMCG.
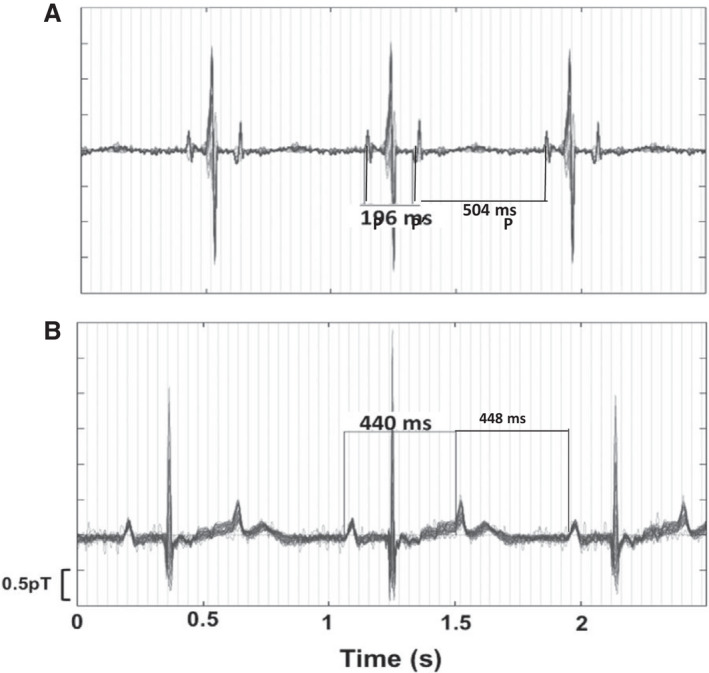
A, Typical blocked atrial bigeminy with P–P′ 196 ms with morphologically different P and P′. In this setting of very short P–P′, the functional antegrade AV block results in stable ventricular rates of ≈75–90/min. AV indicates atrioventricular; and fMCG, fetal magnetocardiography. B, In true second‐degree AV block, the P waves are usually morphologically similar, and there is often slight ventriculophasic sinus arrhythmia, where the P–P′ with the QRS between is slightly shorter than the P′‐P.
SSA Ro Pregnancies With Heart Block
Forty‐eight fetuses were referred because of SSA Ro isoimmunization; 22 (45%) of these were referred with known or suspected AVB. Three additional fetuses were found to have block at the time of fMCG.
The changes in diagnosis included a worse degree of AVB (n=3), PVCs (n=7), additional escape rhythms (n=2), junctional ectopic tachycardia or VT (n=4), and severe QTc prolongation (n=3).
One fetus with third‐degree AVB with a ventricular rate of 80 beats/min and brief ventricular tachycardia had fetal demise despite treatment.
Twenty‐three fetuses had a change in management. Four of 23 (17%) were major. Four had a change in medication, and 19 needed much closer surveillance or a change in delivery site and acuity. Examples of these changes included anticipation of need for delivery room care or early pacemaker implantation, addition of terbutaline, and treatment of QTc prolongation. Repeat fMCG procedures were common in this group.
Familial LQTS
Thirty‐nine patients were referred with familial LQTS in a first‐degree relative to the fetus (mother, father, sibling). QTc>500 ms was found in 25 (64%) fetuses. In 24 of 39 (62%) LQTS was confirmed after birth by positive genetic testing, with 1 (2.5%) false positive.
Familial Long QT–Positive Fetuses
Of the 24 (62%) patients with LQTS genetically confirmed postnatally, 9 had fetal QTc prolongation only, 10 had additional fetal sinus bradycardia, 1 had bradycardia and 2°AVB, 1 had a 2°AVB only during decelerations, and finally 3 fetuses (12.5%) had TdP. None of the babies died pre‐ or postnatally, consistent with the better prognosis for those with familial LQTS, compared with de novo LQTS. 9 Major changes in management were found in 3 (drug therapy, change in surveillance, and delivery planning). TdP was treated in these 3 pregnancies with magnesium, transplacental ß‐blocker, and subsequently mexiletine. A proarrhythmic effect after mexiletine was seen in 1 KCNH2 pregnancy (more frequent fetal TdP and QTc lengthening in both mother and fetus), which would not have been detected with fetal echocardiography alone.
The remaining 21 pregnancies with fetal LQTS had a moderate change in management and were recommended to follow the LQTS protocol. 3 When the fetal QTc was normal in the other 14 of 39, postnatal ECG was concordant in all; however, despite normal MCG and normal neonatal ECG, 1 infant was genetically positive for the familial LQTS1 (false negative).
Summary and Importance of the Most Critical Findings
Critical Change in Diagnosis
In 81 of 144 (56%), a critical change in diagnosis by fMCG was found (Table 3).
Thus, a critical change in diagnosis was mainly found in the category of nonisoimmune AVB, followed by familial LQTS and fetal sinus bradycardia (Table 3). Nine fetuses (from all 3 major groups) showed TdP, of which only 2 were recognized before fMCG. Four of 5 stillbirths (and 1 neonatal death) were seen with de novo LQTS (the fifth had SSA‐associated AVB). Of the LQTS fetuses that died in utero, only 1 was receiving appropriate antiarrhythmic treatment before referral, and all received transplacental treatment for TdP following fMCG (magnesium and propranolol 4, addition of lidocaine 2/mexiletine 1). Two had concomitant atrial flutter, and all stillborn fetuses had hydrops. In addition to TdP and atrial flutter, other LQTS‐related arrhythmias were also underdetected at referral, including 3:1 second‐degree AVB, late PVCs, and both electrical and mechanical TWA. One familial LQT2 subject experienced a pro‐arrhythmic response to combination therapy after addition of mexiletine, with QTc lengthening in both mother and fetus, and more frequent fetal TdP. The treatment regimen was then altered from nadolol/mexiletine to long‐acting propranolol. The infant was liveborn and is currently 18 months old.
In addition to familial LQTS, QTc prolongation (>500 ms) was seen in 11/34 (32%) with fetal sinus bradycardia, 2/31 (6%) with fetal tachycardia, 3/15 (20%) with nonisoimmune AVB, and 3/25 (12%) with SSA Ro positive AVB.
Other changes in diagnosis besides those in the LQTS group were also critical. We found BAB instead of 2°AVB in 5, of which 3 had been started on dexamethasone. It was subsequently discontinued based on the fMCG diagnosis. Fetal HR alone was not enough to exclude tachyarrhythmias since some fetuses had rates well below 200 bpm. Junctional ectopic tachycardia with hydrops presented at a rate of 168 bpm, and TdP mimicked sinus tachycardia or sinus rhythm with ectopy during transitions from 2°AVB. This was a common reason for its underdetection since clinicians can assume that a rate increase correlates with a lesser degree of AVB, and not initiation of TdP. At these times, by Doppler, the aortic valve outflow did not consistently produce a systolic flow velocity during rapid irregular TdP, and this led to the appearance of a slower rate (Figure 2A and 2B).
Major Changes in Management
Major changes in management occurred in all 3 groups. These have been described within each section above and were most common with tachycardia (n=10, 37%), sinus bradycardia (n=8, 25%), nonisoimmune heart block (n=9, 60%), SSA Ro heart block (n=4, 20%). and familial LQTS (n=3, 7%).
Discussion
Key Findings Related to the Referral Diagnosis and Management
fMCG demonstrated an important change in diagnosis in 81 of 144 subjects (56%) in the 3 groups, leading to a major change in management in 35, or 24% overall. The most notable changes were of BAB mistaken for 2°AVB and failure to diagnose life‐threatening TdP because of the inability to recognize LQTS‐associated rhythms (Figure 2A and 2B, 2E and 2F, 3A through 3D), especially in cases of de novo LQTS, where family history cannot be used to narrow the differential diagnosis. This was largely because of the very diverse presentation of LQTS as described in the next paragraph. The highest degree of critical change was found in the nonisoimmune heart block category. Common reasons for critical changes in diagnosis were that the fMCG detected a new clinically significant rhythm or conduction finding. In many cases, the intrinsic limitations in echocardiography for detecting bundle branch block or QTc prolongation were exemplified in this study, demonstrating the need to implement both echocardiography and fMCG diagnostic modalities in fetal care centers.
Long QT Syndrome
fMCG has unrivaled capability for evaluation of fetal LQTS. As recently reported by our group and others, the presentation is quite different from that seen in older patients, and is more severe, especially for de novo LQTS, 9 , 17 , 18 which often presented with unexplained hydrops and atypical tachy‐brady arrhythmias that were often attributed to more common fetal arrhythmias. Such complex presentations included combinations of sinus bradycardia, 2:1 and 3:1 AVB, late PVCs, QRS‐ and TWA, TdP, slow VT (120–160 bpm), 9 and atrial flutter, thus presenting a confusing picture to the fetal echocardiographer. We have also recently reported mechanical alternans by echocardiography in association with TWA. Mechanical alternans, late PVCs, and 2:1 and 3:1 AVB should be detectable echocardiographically. TdP is often missed by echocardiogram, as was seen in 7 of 9 cases. It can present with heart rates similar to that of sinus rhythm (120–160 beats/min) or may have very brief salvos that elude echocardiogram/Doppler detection. LQTS can also present with severe functional AVB. In a recent study, Strand and colleagues described 3:1 2° AVB with ventricular rates in the 50s, which was thought to be 3° AVB. 9 Careful initial echocardiographic examination, to locate repetitive stable PR interval patterns, is needed to identify 3:1 atrioventricular conduction (Figure 2). Nonisoimmune 2:1 or 3:1 AVB warrants fMCG evaluation because TdP was seen in 3 of 14 (21%) fetuses with 2°AVB in this study and 4/7 (57%) in the study of Strand et al. 9 In this current series, 2 subjects from the tachycardia group, 2 from the bradycardia group, 2 from the nonisoimmune AVB group, and another 3 from the familial LQTS group had fetal TdP.
Treatment of TdP may need to be tailored to the specific type of LQTS, if it is known, as well as to whether the mother is currently taking antiarrhythmic medications herself. It is possible to effectively treat both de novo LQTS as well as familial LQTS with TdP through magnesium repletion and by administering transplacental antiarrhythmic therapy for TdP (magnesium, propranolol, lidocaine, and mexiletine), and this may be indicated even when the fetus is near term gestation. 7 TdP has been shown to be associated with QTc>620 ms, PVCs, and QRS‐ or TWA. 9 Thus far no familial cases of fetal LQTS with TdP have died with treatment, but mortality for the denovo group remains almost 45%.
Fetal Bradycardia Groups
Gestation‐based normative fetal heart rate measures should become standard practice at each obstetrical visit. As noted by Cuneo et al, 13 11 of 13 LQTS cases would have been missed if gestation‐based normative fetal HR values had not been used. Sinus bradycardia was seen in 14 of 18 LQT1 cases <36 weeks gestational age, as well as those with double mutations, but was inconsistently seen in LQT2 and LQT3. 19 Familial sinus bradycardia syndromes, such as HCN4, can present with fetal bradycardia, and QTc can be prolonged in these cases as well. 8 fMCG sometimes critically reversed the presumed diagnosis of 2° AVB, when blocked atrial bigeminy or poly‐geminy was identified. In fetuses with complete AVB, fMCG was useful for detection of nonreactive fetal HR tracings, a wide‐QRS escape rhythm, QTc prolongation, and PVCs or junctional ectopic tachycardia/VT. These findings are important because they may impact decision‐making for pacemaker implantation.
Fetal Tachycardia
Tachycardias that are slower than 200 beats/min were more likely to be referred for fMCG, because of less perceived need for immediate treatment; thus atrial ectopic tachycardia was overrepresented. 20 It was often difficult by echocardiography/Doppler to differentiate sinus tachycardia from atrial ectopic tachycardia. Rapid re‐entrant tachycardias are more recognizable echocardiographically. Tachyarrhythmias may coexist, making the diagnosis even more difficult, such as SVT and atrial flutter, 20 or TdP and atrial flutter. Likewise, they can be paroxysmal and brief like junctional ectopic tachycardia, and some TdP, requiring continuous recording to detect. fMCG demonstrated drug effect on fetal cardiac time intervals during transplacental therapy. This allowed modification of dosing and abandonment of toxic, ineffective, or proarrhythmic therapies.
Non‐LQTS–Related QTc Prolongation
In this series, QTc prolongation (QTc>500 ms) not only accompanied familial and de novo LQTS but was also seen in other conditions. QTc prolongation may accompany myocarditis, maternal nutritional deficiency states, or maternal use of QT‐prolonging medications (www.qtdrugs.org). Since many of these risks are modifiable, knowing the QTc in association with an arrhythmia is advantageous, though in the case of sinus bradycardia, or fetuses with prominent U waves producing QTUc prolongation, it can be a source of overdiagnosis of LQTS. We recently reported our method for distinguishing benign U waves from pathologic U waves. 21
Limitations
It is likely that pregnancies with more severe arrhythmias were referred for fMCG. The perceived clinical need for referral may also have been higher in certain categories (familial LQTS). Fetal echocardiography and fMCG are complementary and synergistic techniques, each having strengths and limitations in the diagnosis of fetal arrhythmias.
Conclusions
fMCG has a significant impact on prenatal diagnosis and management of arrhythmias or familial arrhythmia risk, which cannot be fully met by existing technology. The combination of fMCG and fetal echocardiography in fetal care centers will be needed in the future to optimize care. The advances in low‐cost optically pumped magnetometers and breakthroughs in shielding technologies will soon make high‐quality fMCG available. In the interim, most minor arrhythmias, such as transient ectopic beats, easily managed SVT, or isoimmune AVB do not need to be referred to an fMCG center; however, certain groups such as those with a family history of LQTS, unexplained nonimmune hydrops fetalis, a prior history of multiple stillbirths, complex tachy‐brady arrhythmias, acute‐onset AVB with junctional ectopic tachycardia or VT, and SVT where there is poor response to antiarrhythmic treatment may benefit, and these patients may wish to participate in one of the existing clinical studies (Clinical Trials.Gov NCT #3047161 or NCT #03775954).
Sources of Funding
NIH RO1HL063174 (Wakai), and NIH RO1HL143485 (Strasburger), and grants from the Dr Scholl Foundation, Chicago, IL (Strasburger).
Disclosures
Wacker‐Gussmann A: None; Strasburger, JF: Grants (listed above). Dr Strasburger is currently a member of the writing committee for the 2022 Heart Rhythm Society Consensus Statement on Arrhythmia Management in Pregnancy; Wakai, RT: Grants (listed above).
Supporting information
Data S1
Acknowledgments
The authors wish to thank William T. Lutter, PhD, Gretchen K. Eckstein, RN, BSN, UW Clinical Trials, Sarah Bitant, PhD, Suhong Yu, PhD, and Tan Phan, MS for their considerable contribution. We also wish to thank the Pediatric Cardiologists, and Maternal‐Fetal‐Medicine specialists from across the United States for supporting this work through their referrals. The first two authors shared equally in the research and writing of this manuscript.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025224
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Donofrio MT, Moon‐Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, Huhta JC, Jonas RA, Krishnan A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d [DOI] [PubMed] [Google Scholar]
- 2. Strasburger JF, Wakai RT. Fetal cardiac arrhythmia detection and in utero therapy. Nat Rev Cardiol. 2010;7:277–290. doi: 10.1038/nrcardio.2010.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wacker‐Gussmann A, Strasburger JF, Cuneo BF, Wakai RT. Diagnosis and treatment of fetal arrhythmia. Am J Perinatol. 2014;31:617–628. doi: 10.1055/s-0034-1372430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strand S, Lutter W, Strasburger JF, Shah V, Baffa O, Wakai RT. Low‐cost fetal magnetocardiography: a comparison of superconducting quantum interference device and optically pumped magnetometers. J Am Heart Assoc. 2019;8:e013436. doi: 10.1161/JAHA.119.013436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sulai IA, DeLand ZJ, Bulatowicz MD, Wahl CP, Wakai RT, Walker TG. Characterizing atomic magnetic gradiometers for fetal magnetocardiography. Rev Sci Instrum. 2019;90:085003. doi: 10.1063/1.5091007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters C, Wacker‐Gussmann A, Strasburger JF, Cuneo BF, Gotteiner NL, Gulecyuz M, Wakai RT. Electrophysiologic features of fetal ventricular aneurysms and diverticula. Prenat Diagn. 2015;35:129–136. doi: 10.1002/pd.4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wacker‐Gussmann A, Wakai RT, Strasburger JF. Importance of fetal arrhythmias to the neonatologist and pediatrician. Neoreviews. 2016;17:e568–e578. doi: 10.1542/neo.17-10-e568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wacker‐Gussmann A, Oberhoffer‐Fritz R, Westphal DS, Hessling G, Wakai RT, Strasburger JF. The missense variant p.(Gly482Arg) in HCN4 is responsible for fetal tachy‐bradycardia syndrome. Heart Rhythm Case Rep. 2020;6:352–356. doi: 10.1016/j.hrcr.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strand S, Strasburger JF, Cuneo BF, Wakai RT. Complex and novel arrhythmias precede stillbirth in fetuses with De novo long QT syndrome. Circ Arrhythm Electrophysiol. 2020;13:e008082. doi: 10.1161/CIRCEP.119.008082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao H, Cuneo BF, Strasburger JF, Huhta JC, Gotteiner NL, Wakai RT. Electrophysiological characteristics of fetal atrioventricular block. J Am Coll Cardiol. 2008;51:77–84. doi: 10.1016/j.jacc.2007.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wacker‐Gussmann A, Strasburger JF, Cuneo BF, Wiggins DL, Gotteiner NL, Wakai RT. Fetal arrhythmias associated with cardiac rhabdomyomas. Heart Rhythm. 2014;11:677–683. doi: 10.1016/j.hrthm.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiggins DL, Strasburger JF, Gotteiner NL, Cuneo B, Wakai RT. Magnetophysiologic and echocardiographic comparison of blocked atrial bigeminy and 2:1 atrioventricular block in the fetus. Heart Rhythm. 2013;10:1192–1198. doi: 10.1016/j.hrthm.2013.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuneo BF, Strasburger JF, Yu S, Horigome H, Hosono T, Kandori A, Wakai RT. In utero diagnosis of long QT syndrome by magnetocardiography. Circulation. 2013;128:2183–2191. doi: 10.1161/CIRCULATIONAHA.113.004840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen M, Van Veen BD, Wakai RT. Linear minimum mean‐square error filtering for evoked responses: application to fetal MEG. IEEE Trans Biomed Eng. 2006;53:959–963. doi: 10.1109/TBME.2006.872822 [DOI] [PubMed] [Google Scholar]
- 15. Leuthold A, Wakai RT, Martin CB. Noninvasive in utero assessment of PR and QRS intervals from the fetal magnetocardiogram. Early Hum Dev. 1999;54:235–243. doi: 10.1016/S0378-3782(98)00100-5 [DOI] [PubMed] [Google Scholar]
- 16. Strand SA, Strasburger JF, Wakai RT. Fetal magnetocardiogram waveform characteristics. Physiol Meas. 2019;40:035002. doi: 10.1088/1361-6579/ab0a2c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moore JP, Gallotti RG, Shannon KM, Bos JM, Sadeghi E, Strasburger JF, Wakai RT, Horigome H, Clur SA, Hill AC, et al. Genotype predicts outcomes in fetuses and neonates with severe congenital long QT syndrome. JACC Clin Electrophysiol. 2020;6:1561–1570. doi: 10.1016/j.jacep.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crotti L, Tester DJ, White WM, Bartos DC, Insolia R, Besana A, Kunic JD, Will ML, Velasco EJ, Bair JJ, et al. Long QT syndrome‐associated mutations in intrauterine fetal death. JAMA. 2013;309:1473–1482. doi: 10.1001/jama.2013.3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cuneo BF, Kaizer AM, Clur SA, Swan H, Herberg U, Winbo A, Rydberg A, Haugaa K, Etheridge S, Ackerman MJ, et al. Mothers with long QT syndrome are at increased risk for fetal death: findings from a multicenter international study. Am J Obstet Gynecol. 2020;222:263.e1–263.e11. doi: 10.1016/j.ajog.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 20. Wacker‐Gussmann A, Strasburger JF, Srinivasan S, Cuneo BF, Lutter W, Wakai RT. Fetal atrial flutter: electrophysiology and associations with rhythms involving an accessory pathway. J Am Heart Assoc. 2016;5:e003673. doi: 10.1161/JAHA.116.003673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strand S, Strasburger JF, Lutter WJ, Wakai RT. Repolarization predictors of fetal long QT syndrome. Heart Rhythm O2. 2020;1:200–205. doi: 10.1016/j.hroo.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wacker‐Gussmann A, Strasburger JF, Wakai RT. Fetal magnetocardiography alters diagnosis and management in fetal congenital heart disease and cardiomyopathy. J Am Coll Cardiol EP. 2022. doi: 10.1016/j.jacep.2022.04.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


