Abstract
Background
Pharmacological treatment of atrial fibrillation (AF) in the setting of Brugada syndrome (BrS) is challenging. In addition, patients with BrS with an implantable cardioverter‐defibrillator (ICD) might experience inappropriate shocks for fast AF. Long‐term outcome of pulmonary vein isolation in BrS has not been well established yet, and it is still unclear whether pulmonary vein triggers are the only pathophysiological mechanism of AF in BrS. The aim of the study is to assess the long‐term outcomes in patients with BrS undergoing pulmonary vein isolation for paroxysmal AF compared with a matched cohort of patients without BrS.
Methods and Results
Sixty patients with BrS undergoing pulmonary vein isolation with cryoballoon catheter ablation for paroxysmal AF were matched with 60 patients without BrS, who underwent the same procedure. After a mean follow‐up of 58.2±31.7 months, freedom from atrial tachyarrhythmias was achieved in 61.7% in the BrS group and in 78.3% in the non‐BrS group (log‐rank P=0.047). In particular, freedom from AF was 76.7% in the first group and in 83.3% in the second (P=0.27), while freedom from atrial tachycardia/atrial flutter was 85% and 95% (P=0.057). In the BrS group, 29 patients (48.3%) had an ICD and 8 (27.6%) had a previous ICD‐inappropriate shock for fast AF. In the BrS cohort, ICD‐inappropriate interventions for AF were significantly reduced after ablation (3.4% versus 27.6%; P=0.01).
Conclusions
Pulmonary vein isolation in patients with BrS was associated with higher rate of arrhythmic recurrence. Despite this, catheter ablation significantly reduced inappropriate ICD interventions in BrS patients and can be considered a therapeutic strategy to prevent inappropriate device therapies.
Keywords: atrial fibrillation, Brugada syndrome, cryoballoon, inappropriate shock, pulmonary vein isolation
Subject Categories: Arrhythmias, Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator, Electrophysiology
Nonstandard Abbreviations and Acronyms
- ATa
atrial tachyarrhythmia
- BrS
Brugada syndrome
- CB‐A
cryoballoon catheter ablation
- PVI
pulmonary vein isolation
Clinical Perspective.
What Is New?
The rate of arrhythmic recurrences after pulmonary vein isolation (PVI) for paroxysmal atrial fibrillation in patients with Brugada syndrome (BrS) is significantly higher compared with a matched population without BrS undergoing the same procedure.
Implantable cardioverter‐defibrillator inappropriate interventions are significantly reduced after PVI in patients with BrS and paroxysmal atrial fibrillation.
What Are the Clinical Implications?
PVI for paroxysmal atrial fibrillation in patients with BrS is associated with a higher rate of arrhythmic recurrence compared with a matched population without BrS.
PVI significantly reduced the implantable cardioverter defibrillator inappropriate interventions in BrS and paroxysmal atrial fibrillation.
Despite the higher recurrence rate, PVI can be considered a therapeutic strategy to prevent inappropriate device implantable cardioverter defibrillator interventions in patients with BrS.
Brugada syndrome (BrS) is an inherited primary arrhythmia syndrome characterized by a peculiar ECG pattern and an increased risk of sudden cardiac death. 1 , 2 The most common supraventricular arrhythmia in patients with BrS is atrial fibrillation (AF), with a reported incidence between 9% and 53%. 3 , 4 , 5 Pharmacological treatment of AF in these patients is challenged by drug‐related risk of developing ventricular arrhythmias. 6 Pulmonary vein isolation (PVI) is the cornerstone of percutaneous transcatheter ablation for drug‐resistant paroxysmal AF. 7 , 8 However, it is still unclear whether pulmonary vein triggers are the underlying pathophysiological mechanism of AF in BrS, 3 , 9 , 10 , 11 , 12 and therefore, if PVI can be considered the ablation strategy target. Available data on PVI outcomes in this population are scarce. 13 , 14 , 15 Moreover, it has not been established yet if the long‐term efficacy of PVI is comparable to patients without BrS.
The aim of this study is to assess the very long‐term outcomes of PVI in a consecutive series of BrS patients with paroxysmal AF. Moreover, the ablation outcomes were compared with a propensity matched cohort of patients with paroxysmal AF undergoing PVI.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
All consecutive patients diagnosed with BrS are included in institutional registries and followed in a prospective fashion (UZ Brussel, Brussels: NCT05283759; Cardiocentro Ticino Institute, Lugano: CE TI 3476 Number 2019‐00754). All patients signed an informed consent that had been approved by each institutional review board. The study protocol was carried out in accordance with the ethical principles for medical research involving human subjects established by the Declaration of Helsinki and was approved by the local ethics committee. All patients with BrS were screened and included in the current study if they met the following inclusion criteria: (1) BrS diagnosed following current recommendations 16 ; (2) no evidence of structural heart disease; and (3) index PVI for symptomatic paroxysmal AF with cryoballoon catheter ablation (CB‐A). The study cohort consisted of 60 consecutive patients with BrS undergoing a PVI procedure for paroxysmal AF in the 2 centers between June 2012 and January 2019.
Paroxysmal AF was defined as AF that terminates spontaneously or with intervention within 7 days of onset. 17 Ablation outcomes in the study group were compared with those obtained in a retrospective control cohort of 654 patients with paroxysmal AF, who underwent PVI with CB‐A in the same period.
Patients were included in the propensity matching if they met the following criteria: (1) absence of spontaneous or ajmaline‐induced Brugada type 1 ECG or family history of BrS; (2) no evidence of structural heart disease; and (3) index PVI for symptomatic paroxysmal AF with CB‐A.
Propensity score matching was performed with a 1:1 ratio taking into account the following variables: (1) age; (2) sex; (3) left atrium size; and (4) presence of a cardiac implantable electronic device, defined as implantable cardioverter‐defibrillator (ICD) or insertable cardiac monitor.
Underlying structural cardiac abnormalities were excluded in all patients, in both groups, by means of echocardiography and heart computed tomography scan. Other noninvasive methods (such as stress test or nuclear magnetic resonance) or invasive methods (coronary angiography, left and right ventriculography) were used at the discretion of the treating physician.
Cryoballoon Ablation
The CB‐A procedure has been described in detail previously. 18 , 19 All procedures were performed under general anesthesia. Through a transseptal puncture, a 28‐mm cryoballoon (Arctic Front, Medtronic) was inserted over an inner‐lumen mapping catheter (Achieve, Medtronic) in the left atrium.
The cryoballoon was advanced, inflated, and positioned at each pulmonary vein ostium. Optimal vessel occlusion was defined by selective contrast injection. Once vessel occlusion was deemed satisfactory, delivery of cryoenergy to allow freezing was commenced. Standard cryothermal applications lasted 180 seconds. Our target temperature was −40 °C within the first 60 seconds. If the temperature did not attain this value, an extra freeze was delivered. To avoid phrenic nerve palsy, diaphragmatic stimulation was achieved by pacing the phrenic nerve during septal pulmonary vein ablation.
Postprocedural Management and Follow‐Up
After the procedure, a transthoracic echocardiography was performed before the discharge to exclude possible procedure‐related complications. Oral anticoagulation was restarted the same day of ablation and continued for at least 3 months. Patients were recommended to continue on the same antiarrhythmic drugs for at least 3 months after ablation followed by its discontinuation. After discharge, physical examination was scheduled with baseline ECG and 24‐hour Holter recordings at 3, 6, and 12 months. Patients with a cardiac implantable electronic device were followed up with home monitoring and with scheduled follow‐up of the device every 6 months. A blanking period of 90 days after ablation was taken into consideration. Any symptoms after ablation were deemed as deserving of additional Holter monitoring/device interrogation.
The primary end point of the study was defined as atrial tachyarrhythmia (ATa) occurrence. All ATa episodes >30 seconds, including recurrence of AF or occurrence of atrial tachycardia or atrial flutter were considered as arrhythmic recurrence. Follow‐up start was defined as the date of the PVI procedure for both groups. The secondary end point was defined as ICD‐inappropriate shocks in the BrS group. The date of ICD implantation and the date of the ablation procedure were considered as the date of follow‐up start before and after PVI, respectively.
Statistical Analysis
All variables were tested for normality with the Shapiro–Wilk test. Data are presented as mean±SD or as absolute numbers and percentages where appropriate. Comparison of continuous data between groups was performed with the Student t test for independent samples or with 1‐way ANOVA or with the Mann–Whitney test. The Pearson χ2 or the Fisher exact test were used to compare categorical variables. Propensity score matching was performed with a 1:1 ratio taking into account the following variables: (1) age, (2) sex, (3) left atrium size, and (4) presence of a cardiac implantable electronic device. For propensity score matching, the package MatchIt with the method nearest and a caliper width equal to 0.25 on R software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) was used. Recurrence‐free survival over time was calculated by the Kaplan–Meier method, log‐rank test, and Cox regression was used for comparison between groups. The proportional hazards assumption and influential observations for the Cox model were tested.
All tests were 2‐sided, and a P value of 0.05 was considered statistically significant. Analyses were performed with SPSS 23.0 statistical software (IBM Company, Chicago, IL) and with R software version 3.6.2 (R Foundation for Statistical Computing).
RESULTS
Baseline Characteristics
A total of 60 patients (32 men [53.3%]; mean age, 45.8±15.5 years) with BrS and paroxysmal AF were included in the study. Propensity score matching identified a subset of 60 patients with paroxysmal AF, without BrS. The 2 groups were well balanced, except for antiarrhythmic medications. Baseline patients characteristics are detailed in Table 1. All patients discontinued antiarrhythmic drugs. Seven patients (11.7%) presented with a spontaneous Brugada type 1 ECG, while in the remaining 53 patients (88.3%), BrS was diagnosed by ajmaline challenge. Nineteen patients (31.7%) had a family history of sudden cardiac death, 2 patients (3.3%) had a previous episode of aborted sudden cardiac arrest caused by ventricular fibrillation, and 11 patients (18.3%) reported a history of previous syncope. The genetic test was performed in 58 patients (96.7%) and SCN5A gene mutation was found in 9 of them (15%). Twenty‐nine patients (48.3%) were implanted with an ICD. Two out of 29 patients (6.9%) underwent ICD implantation in secondary prevention. Twenty‐four patients (82.8%) had a dual‐chamber defibrillator, and 5 patients (17.2%) had a single‐chamber defibrillator. Eight patients (27.6%) had at least 1 previous ICD‐inappropriate shock for AF with high‐rate ventricular response. Of these, 5 of 8 patients (62.5%) had a dual‐chamber defibrillator and 3 of 8 (37.6%) had a single‐chamber defibrillator (P=0.32). In 1 of 8 patients (3.4%), ventricular tachycardia zone detection with antitachycardia therapy (antitachycardia pacing followed by shock) was programmed; in 2 of 8 patients (25%), the ventricular fibrillation detection zone was >200 bpm, in 3 of 8 (37.5%) it was >214 bpm, and in 3 of 8 (37.5%) it was >222 bpm. After ablation, in the patient with the ventricular tachycardia detection zone activated, the device was reprogrammed by switching off the ventricular tachycardia zone; it was not able to avoid inappropriate ICD interventions because of AF episodes (ventricular rate in the ventricular fibrillation zone). In the other patients, there were no changes in programming the device after ablation.
Table 1.
Baseline Characteristics
| BrS group (60) | Non‐BrS group (60) | P value | |
|---|---|---|---|
| Age, y | 45.8±15.5 | 47.8±13.8 | 0.44 |
| Sex (male), n (%) | 32 (53.3) | 27 (45) | 0.36 |
| Race or ethnic group | |||
| White, n (%) | 56 (93.3) | 58 (96.6) | 0.68 |
| Black, n (%) | 1 (0.02) | 1 (0.02) | 1 |
| Asian, n (%) | 0 | 0 | NA |
| Other,* n (%) | 3 (0.05) | 1 (0.02) | 0.62 |
| BMI, kg/m2 | 24.96±4.5 | 26.14±5.4 | 0.26 |
| Hypertension, n (%) | 16 (26.7) | 14 (27.5) | 0.9 |
| Diabetes, n (%) | 2 (3.3) | 3 (5.9) | 0.52 |
| Dyslipidemia, n (%) | 4 (6.8) | 5 (9.8) | 0.56 |
| CHA2DS2VASc score | 0.92±0.91 | 1.02±0.84 | 0.54 |
| CIED, n (%)† | 29 (48.3) | 27 (45) | 0.71 |
| LVEF (%) | 59.61±3.9 | 60.65±4.3 | 0.17 |
| Left atrial diameter, mm | 38.3±6.9 | 40.2±6.2 | 0.11 |
| PR interval, ms | 169.9±32.6 | 160.5±21.5 | 0.097 |
| QRS interval, ms | 102.2±23.4 | 96.10±11.9 | 0.11 |
| Antiarrhythmic medications | |||
| Class IC, n (%) | 0 | 31 (57.4) | <0.001 |
| Class II, n (%) | 29 (49.2) | 25 (48.1) | 0.91 |
| Class III, n (%)‡ | 27 (45.8) | 4 (8.2) | <0.001 |
| Class IV, n (%) | 1 (1.7) | 0 | 0.36 |
BMI indicates body mass index; BrS, Brugada syndrome; CIED, cardiac implantable electronic device; and LVEF, left ventricular ejection fraction.
This category includes Middle East.
All devices were implantable cardioverter‐defibrillators in the BrS group and insertable cardiac monitors in the non‐BrS group.
Only sotalol was recorded.
Procedural Characteristics
Acute PVI was achieved in all veins using CB‐A, and no statistically significant differences in procedural outcomes were recorded between the 2 groups (Table 2). No major complications in the periprocedural period were reported—no deaths, cerebrovascular events, or groin vascular complications. Transient phrenic nerve palsy occurred in 2 patients, 1 for each group, with complete resolution before the end of the procedure.
Table 2.
Procedural Characteristics
| BrS group (60) | Non‐BrS group (60) | P value | |
|---|---|---|---|
| Cryoablation | |||
| Applications to achieve PVI | 4.87±0.98 | 4.5±0.92 | 0.09 |
| PVI, n (%) | 60 (100) | 60 (100) | |
| Duration single application, s | 190.7±12.9 | 188.2±18.3 | 0.4 |
| Time to PVI, s | 31.6±9.8 | 33.2±11.5 | 0.5 |
| Isolation temperature, °C | −28.03±6.7 | −30.8±7.4 | 0.08 |
| Minimum temperature, °C | −48.02±3.9 | −48.7±4.2 | 0.4 |
| Fluoroscopy time, min | 16.12±6.1 | 14.7±6.8 | 0.25 |
| Total procedure time, min | 62.16±13.5 | 59.2±14.6 | 0.25 |
BrS indicates Brugada syndrome; and PVI, pulmonary vein isolation.
Follow‐Up
After a mean follow‐up of 58.2±31.7 months (91% of patients are minimum 1 year follow‐up and 85% are minimum 2 years; 56.6±34.3 months in the BrS group and 59.9±29 in the non‐BrS group; P=0.58), freedom from ATas was achieved in 61.7% of patients in BrS group and in 78.3% in the non‐BrS group (log‐rank P=0.047).
In particular, freedom from AF was reached in 76.7% of patients in the first group and in 83.3% of patients in the second group (log‐rank P=0.27); freedom from atrial tachycardia/atrial flutter was 85% in the study group and 95% in the control group, respectively (log‐rank P=0.057). Kaplan–Meier survival curves showing each group's cumulative recurrence are depicted in Figures 1 and 2. At Cox regression analysis, BrS was a significant predictor of ATa occurrence (hazard ratio, 1.97 [95% CI, 1.01–3.9]; P=0.04). ICD‐inappropriate shocks for AF were recorded in 8 of 29 patients (27.6% at mean follow‐up of 32.8±21.5 months) before ablation versus 1 of 29 patients (3.4% at mean follow‐up of 58.5±24.9 months) after PVI (P=0.01; Figure 3); the former occurred 9 months after ablation in a patient who had experienced AF recurrence and inappropriate ICD interventions for AF before the PVI. The annual inappropriate shock rate was 5.67%/year before ablation versus 1.24%/year after ablation (P=0.03).
Figure 1. Kaplan–Meier curves of first atrial tachyarrhythmia recurrence after pulmonary vein isolation.
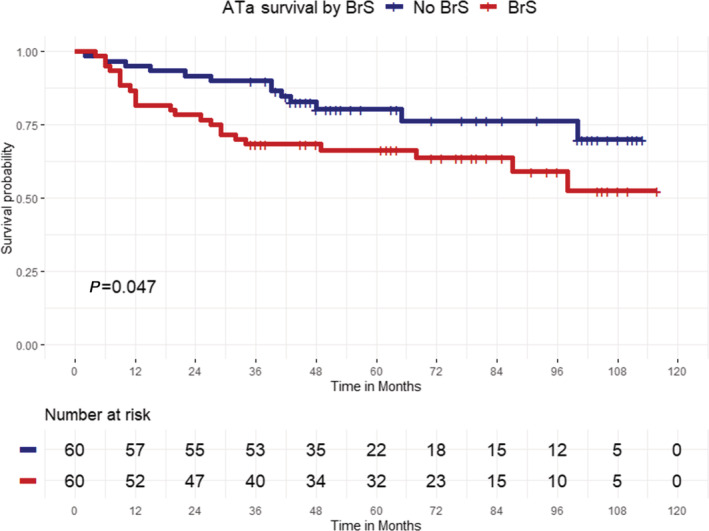
Atrial tachyarrhythmia (ATa)‐free survival was significantly higher in patients with Brugada syndrome (BrS, red curve) compared with a paroxysmal atrial fibrillation (AF) matched population (blue curve) without BrS (log‐rank P=0.047).
Figure 2. Kaplan–Meier curves of first atrial tachycardia/atrial flutter or atrial fibrillation recurrence.
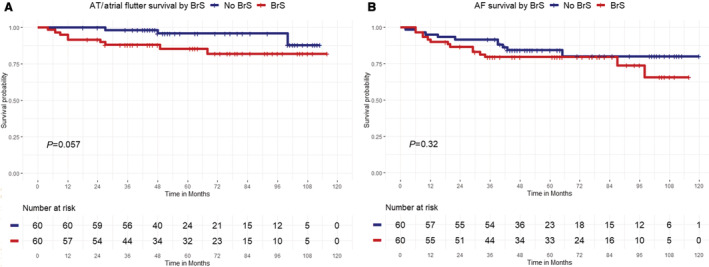
A, Atrial tachycardia (AT)/atrial flutter–free survival after pulmonary vein isolation (PVI) in Brugada syndrome (BrS) group (red curve) and non‐BrS group (blue curve) (log‐rank P=0.057). B, Atrial fibrillation (AF)–free survival after PVI in Brugada syndrome (BrS) group (red curve) and non‐BrS group (blue curve) (log‐rank P=0.27).
Figure 3. Bar plot showing implantable cardioverter defibrillator (ICD)‐inappropriate shocks before and after catheter ablation in the Brugada syndrome group.
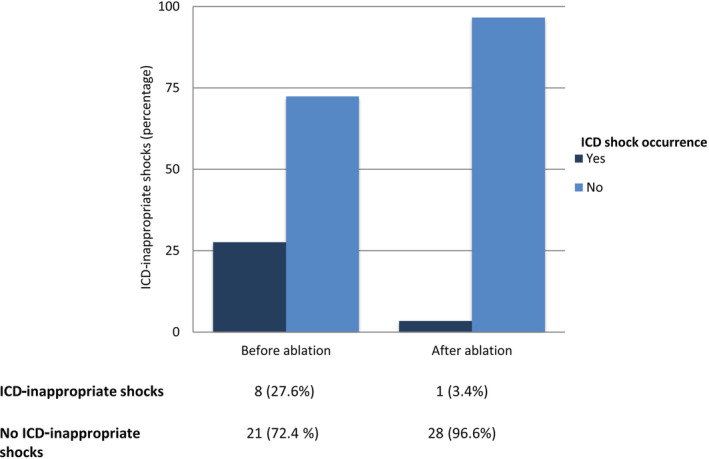
Pulmonary vein isolation resulted in a significant reduction of ICD–inappropriate interventions in patients with Brugada syndrome (P=0.01).
After a mean of 25.3±12.2 months, repeat ablation was performed in 24 (66.7%) of 36 patients who experienced recurrences: 14 of 23 patients (60.9%) in the BrS group and 10 of 13 patients (76.9%) in the control group (P=0.33). Of these, 9 of 14 patients (64.3%) in the study group and 7 of 10 patients (70%) in the second group exhibited pulmonary vein reconnection (P=0.77).
DISCUSSION
To the best of our knowledge, the current study reports on the largest population of patients with BrS and paroxysmal AF treated by catheter ablation with the longest follow‐up period (≈5 years). Furthermore, this is the first study reporting a long‐term comparison after PVI for paroxysmal AF between patients with BrS and patients without BrS.
The main findings of the study are (1) the rate of arrhythmic recurrences after PVI was significantly higher in patients with BrS compared with a paroxysmal AF matched population without BrS; (2) ICD‐inappropriate interventions were significantly reduced after PVI in patients with BrS and paroxysmal AF.
Role of AF Ablation in BrS
Pharmacological treatment for rhythm control of AF in the setting of BrS is challenging; indeed, class I antiarrhythmic drugs, with sodium channel–blocking properties, are not recommended in BrS because of a potential proarrhythmic effect. Moreover, AF in this population is often diagnosed at a young age, and other antiarrhythmic drugs like amiodarone are associated with well‐known side effects. 20 Furthermore, the use of beta‐blockers in BrS is debated. They have been reported to enhance transmural dispersion of repolarization, unmasking ST‐segment elevation 21 and to cause the initiation of ventricular fibrillation. 22 However, the long‐term intake of beta‐blockers was demonstrated to not aggravate the ECG parameters and clinical outcome in patients with BrS. 23 It may be explained by higher sympathetic tone and lower vagal tone at night in patients with symptomatic BrS. 24
Based on these premises, nonpharmacological treatment of AF should be considered an adequate therapeutic alternative for BrS patients. Available data on PVI outcomes in this population are scarce. In this study, only a considerably modest rate of patients with BrS (61.7%) were free of AF after an index PVI procedure by means of CB‐A technology during a 5‐year follow up and without antiarrhythmic drugs. Previous follow‐up analyses showed a freedom from AF after a single PVI in patients with BrS ranging from 67% to 92.9%. 13 , 14 , 15 , 25 Our group previously reported that in BrS, 7 of 9 patients (67%) were free from AF after a 2‐year follow‐up 14 ; conversely, in 2016, Kitamura et al 25 reported a higher freedom rate from AF (93%) after a mean follow‐up of 3.3 years in a population of 14 patients with BrS. These data can be explained by a shorter follow‐up and a smaller cohort compared with the current study.
Pathogenesis of AF in BrS
In the current study, although 61.7% of patients with BrS were free from ATa recurrence, this was significantly lower compared with a propensity score matched population. It could reflect the presence of other actors, besides pulmonary veins, in the genesis of AF in patients with BrS. Indeed, current understanding of the pathophysiology of AF in BrS includes an interplay of triggers, arrhythmogenic substrate, and modulating factors such as autonomic nervous system or inflammation. 12 A circadian pattern for AF has been described, where most episodes occur at night, suggesting a potential role for vagal tone in arrhythmogenesis 26 ; vagal stimulation might reduce atrial conduction velocity and shortens the effective refractory period, facilitating the induction of AF. Furthermore, atrial structural anomalies have been described. In their study on the relationship between AF and BrS, Kusano et al 26 elegantly concluded that the interatrial conduction delay was significantly increased in patients with a diagnosis of BrS and AF, indicating that global atrial myocardium conduction was impaired. Furthermore, an increase in atrial vulnerability has also been reported in BrS, 9 and a concealed abnormal atrial phenotype can even be detected by P‐wave analysis. 27 Therefore, abnormal atrial conduction could represent an electrophysiological basis for AF induction. Building on these premises, the arrhythmogenic substrate in BrS may not be restricted to the ventricular level, 28 and similar atrial changes could be the substrate for reentrant ATas. Reentrant arrhythmias need 2 fundamental conditions to occur: (1) an anatomical or a functional block and (2) an excitable gap throughout the circuit 29 ; atrial slow conduction might provide a milieu for reentrant ATas and may explain the higher occurrence of ATas observed in patients with BrS.
Inappropriate ICD Shocks
Inappropriate ICD interventions are a potential issue of ICD implantation in patients with BrS, affecting up to 19% of patients with an ICD. 30 , 31 In this setting, fast AF is the most common cause of inappropriate shocks, accounting for 45% of events, followed by lead fracture in 21%, T‐wave oversensing in 18%, and sinus tachycardia in 15% of cases. 31
Even if the outcome might be suboptimal if compared with patients without BrS, PVI is a possible approach to prevent inappropriate ICD therapy. Indeed, consistent with previous literature, 14 , 15 , 20 in our study a significant reduction in inappropriate device therapies for AF was recorded after ablation (from 27.6% to 3.4%; P=0.01).
Limitations
The study was a nonrandomized trial. In addition, although the use of a propensity score matching the potential for residual confounding factors or uncontrolled selection bias together with a possible temporal bias, cannot be excluded. The majority of patients with BrS included are White, and data cannot be generalized to other racial or ethnic groups. Moreover, non–pulmonary vein triggers were not routinely assessed. In the absence of long‐term ECG monitoring in all patients, the real arrhythmia recurrence rate may be underestimated. Finally, despite the fact that our data on ICD‐inappropriate shocks in BrS are consistent with previous literature, a regression to mean cannot be excluded.
CONCLUSIONS
Pulmonary vein isolation in patients with BrS was associated with a higher rate of long‐term arrhythmic recurrences if compared with a cohort without BrS. Catheter ablation significantly reduced inappropriate ICD interventions in patients with BrS; therefore, PVI could be considered a therapeutic strategy to prevent inappropriate device therapies in this category of patients. Further studies are warranted to establish the clinical role of PVI and pulmonary veins in the pathophysiology of AF in patients with BrS.
Sources of Funding
This research did not receive any specific funding.
Disclosures
Dr de Asmundis reports speaker fees for Medtronic, Biotronik, Biosense Webster, Abbott, and Boston Scientific; teaching honoraria from Medtronic, Biotronik, Abbott, and Boston Scientific; and proctoring honoraria from Medtronic, Abbott, and Biotronik. Dr Chierchia reports speaker fees for Medtronic, Biotronik, Biosense Webster, and Abbott; teaching honoraria from Medtronic and Biotronik; and proctoring honoraria from Medtronic. Dr Bisignani is consultant for Biotronik. Dr Conte has received a research grant (PZ00P3_180055) from the Swiss National Science Foundation. Dr Auricchio is a consultant with Boston Scientific, Backbeat, Biosense Webster, Cairdac, Corvia, Medtronic, Merit, Microport CRM, and Philips; participates in clinical trials sponsored by Boston Scientific, Medtronic, Microport CRM, and Philips; and has intellectual properties assigned to Boston Scientific, Biosense Webster, and Microport CRM. Dr Brugada reports consulting fees and speaker honoraria from Medtronic. The remaining authors have nothing to disclose.
For Sources of Funding and Disclosures, see page xxx.
Contributor Information
Antonio Bisignani, Email: abisignani@hotmail.it.
Carlo de Asmundis, Email: carlodeasmundis@me.com, Email: carlo.deasmundis@uzbrussel.be.
References
- 1. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-J [DOI] [PubMed] [Google Scholar]
- 2. Sieira J, Brugada P. The definition of the Brugada syndrome. Eur Heart J. 2017;38:3029–3034. doi: 10.1093/eurheartj/ehx490 [DOI] [PubMed] [Google Scholar]
- 3. Bordachar P, Reuter S, Garrigue S, Caï X, Hocini M, Jaïs P, Haïssaguerre M, Clementy J. Incidence, clinical implications and prognosis of atrial arrhythmias in Brugada syndrome. Eur Heart J. 2004;25:879–884. doi: 10.1016/j.ehj.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 4. Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, et al. Brugada syndrome; report of the second consensus conference; endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51 [DOI] [PubMed] [Google Scholar]
- 5. Letsas KP, Sideris A, Efremidis M, Pappas LK, Gavrielatos G, Filippatos GS, Kardaras F. Prevalence of paroxysmal atrial fibrillation in Brugada syndrome: a case series and a review of the literature. J Cardiovasc Med. 2007;8:803–806. doi: 10.2459/JCM.0b013e3280112b21 [DOI] [PubMed] [Google Scholar]
- 6. Giustetto C, Cerrato N, Gribaudo E, Scrocco C, Castagno D, Richiardi E, Giachino D, Bianchi F, Barbonaglia L, Ferraro A. Atrial fibrillation in a large population with Brugada electrocardiographic pattern: prevalence, management, and correlation with prognosis. Heart Rhythm. 2014;11:259–265. doi: 10.1016/j.hrthm.2013.10.043 [DOI] [PubMed] [Google Scholar]
- 7. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003 [DOI] [PubMed] [Google Scholar]
- 8. Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, et al. Circumferential RF ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619–2628. doi: 10.1161/01.cir.102.21.2619 [DOI] [PubMed] [Google Scholar]
- 9. Morita H, Kusano‐Fukushima K, Nagase S, Fujimoto Y, Hisamatsu K, Fujio H, Haraoka K, Kobayashi M, Morita ST, Nakamura K, et al. Atrial fibrillation and atrial vulnerability in patients with Brugada syndrome. J Am Coll Cardiol. 2002;40:1437–1444. doi: 10.1016/S0735-1097(02)02167-8 [DOI] [PubMed] [Google Scholar]
- 10. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST‐segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.CIR.100.15.1660 [DOI] [PubMed] [Google Scholar]
- 11. Wilde AA, Postema PG, Di Diego JM, Viskin S, Morita H, Fish JM, Antzelevitch C. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010;49:543–553. doi: 10.1016/j.yjmcc.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vlachos K, Mascia G, Martin CA, Bazoukis G, Frontera A, Cheniti G, Letsas KP, Efremidis M, Georgopoulos S, Gkalapis C, et al. Atrial fibrillation in Brugada syndrome: current perspectives. J Cardiovasc Electrophysiol. 2020;31:975–984. doi: 10.1111/jce.14361 [DOI] [PubMed] [Google Scholar]
- 13. Yamada T, Yoshida Y, Tsuboi N, Murakami Y, Okada T, McElderry HT, Yoshida N, Doppalapudi H, Epstein AE, Plumb VJ, et al. Efficacy of pulmonary vein isolation in paroxysmal atrial fibrillation patients with a Brugada electrogram. Circ J. 2008;72:281–286. doi: 10.1253/circj.72.281 [DOI] [PubMed] [Google Scholar]
- 14. Conte G, Chierchia GB, Wauters K, De Asmundis C, Sarkozy A, Levinstein M, Sieira J, Baltogiannis G, Di Giovanni G, Ciconte G, et al. Pulmonary vein isolation in patients with Brugada syndrome and atrial fibrillation: a 2‐year follow‐up. Europace. 2014;16:528–532. doi: 10.1093/europace/eut309 [DOI] [PubMed] [Google Scholar]
- 15. Mugnai G, Hünük B, Ströker E, Ruggiero D, Coutino‐Moreno HE, Takarada K, De Regibus V, Choudhury R, Abugattas de Torres JP, Moran D, et al. Long‐term outcome of pulmonary vein isolation in patients with paroxysmal atrial fibrillation and Brugada syndrome. Europace. 2018;20:548–554. doi: 10.1093/europace/euw428 [DOI] [PubMed] [Google Scholar]
- 16. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316 [DOI] [PubMed] [Google Scholar]
- 17. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 18. Bisignani A, Overeinder I, Kazawa S, Iacopino S, Cecchini F, Miraglia V, Osório TG, Boveda S, Bala G, Mugnai G, et al. Posterior box isolation as an adjunctive ablation strategy with the second‐generation cryoballoon for paroxysmal atrial fibrillation: a comparison with standard cryoballoon pulmonary vein isolation. J Interv Card Electrophysiol. 2021;61:313–319. doi: 10.1007/s10840-020-00812-z [DOI] [PubMed] [Google Scholar]
- 19. Bisignani A, Cecchini F, Mugnai G, Overeinder I, Sieira J, Osório TG, Miraglia V, Monaco C, Sofianos D, Boveda S, et al. Single procedural outcomes in the setting of percutaneous ablation for persistent atrial fibrillation: a propensity‐matched score comparison between different strategies. J Interv Card Electrophysiol. 2022;64:9–16. doi: 10.1007/s10840-021-00968-2 [DOI] [PubMed] [Google Scholar]
- 20. Wilson JS, Podrid PJ. Side effects from amiodarone. Am Heart J. 1991;121:158–171. doi: 10.1016/0002-8703(91)90969-O [DOI] [PubMed] [Google Scholar]
- 21. Yap YG, Behr ER, Camm AJ. Drug‐induced Brugada syndrome. Europace. 2009;11:989–994. doi: 10.1093/europace/eup114 [DOI] [PubMed] [Google Scholar]
- 22. Antzelevitch C, Yan GX, Ackerman MJ, Borggrefe M, Corrado D, Guo J, Gussak I, Hasdemir C, Horie M, Huikuri H, et al. J‐wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), the European Heart Rhythm Association (EHRA), the Heart Rhythm Society (HRS), and the Latin American Society of Cardiac Pacing and Electrophysiology (Sociedad Latinoamericana de Estimulacifin Cardíaca y Electrofisiología [SOLAECE]). Europace. 2017;19:665–694. doi: 10.1093/europace/euw235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamakura T, Wada M, Ishibashi K, Inoue YY, Miyamoto K, Okamura H, Nagase S, Noda T, Aiba T, Yasuda S, et al. Feasibility evaluation of long‐term use of beta‐blockers and calcium antagonists in patients with Brugada syndrome. Europace. 2018;20:f72–f76. doi: 10.1093/europace/eux198 [DOI] [PubMed] [Google Scholar]
- 24. Krittayaphong R, Veerakul G, Nademanee K, Kangkagate C. Heart rate variability in patients with Brugada syndrome in Thailand. Eur Heart J. 2003;24:1771–1778. doi: 10.1016/j.ehj.2003.06.005 [DOI] [PubMed] [Google Scholar]
- 25. Kitamura T, Fukamizu S, Kawamura I, Hojo R, Aoyama Y, Komiyama K, Nishizaki M, Hiraoka M, Sakurada H. Long‐term efficacy of catheter ablation for paroxysmal atrial fibrillation in patients with Brugada syndrome and an implantable cardioverter defibrillator to prevent inappropriate shock therapy. Heart Rhythm. 2016;13:1455–1459. doi: 10.1016/j.hrthm.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 26. Kusano KF, Taniyama M, Nakamura K, Miura D, Banba K, Nagase S, Morita H, Nishii N, Watanabe A, Tada T, et al. Atrial fibrillation in patients with Brugada syndrome relationships of gene mutation, electrophysiology, and clinical backgrounds. J Am Coll Cardiol. 2008;51:1169–1175. doi: 10.1016/j.jacc.2007.10.060 [DOI] [PubMed] [Google Scholar]
- 27. Conte G, Caputo ML, Volders PGA, Luca A, Mainardi L, Schotten U, Corino VDA, Regoli F, Zeemering S, Zink M, et al. Concealed abnormal atrial phenotype in patients with Brugada syndrome and no history of atrial fibrillation. Int J Cardiol. 2018;253:66–70. doi: 10.1016/j.ijcard.2017.09.214 [DOI] [PubMed] [Google Scholar]
- 28. Pannone L, Monaco C, Sorgente A, Vergara P, Calburean PA, Gauthey A, Bisignani A, Kazawa S, Strazdas A, Mojica J, et al. High‐density epicardial mapping in Brugada syndrome: depolarization and repolarization abnormalities. Heart Rhythm. 2022;19:397–404. doi: 10.1016/j.hrthm.2021.09.032 [DOI] [PubMed] [Google Scholar]
- 29. Antzelevitch C, Burashnikov A. Overview of basic mechanisms of cardiac arrhythmia. Card Electrophysiol Clin. 2011;3:23–45. doi: 10.1016/j.ccep.2010.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Auricchio A, Sterns LD, Schloss EJ, Gerritse B, Lexcen DR, Molan AM, Kurita T; PainFree SST Investigators . Performance evaluation of implantable cardioverter‐defibrillators with SmartShock technology in patients with inherited arrhythmogenic diseases. Int J Cardiol. 2022;350:36–40. doi: 10.1016/j.ijcard.2022.01.007 [DOI] [PubMed] [Google Scholar]
- 31. Conte G, Sieira J, Ciconte G, de Asmundis C, Chierchia GB, Baltogiannis G, Di Giovanni G, La Meir M, Wellens F, Czapla J, et al. Implantable cardioverter‐defibrillator therapy in Brugada syndrome: a 20‐year single‐center experience. J Am Coll Cardiol. 2015;65:879–888. doi: 10.1016/j.jacc.2014.12.031 [DOI] [PubMed] [Google Scholar]


