Abstract
Congenital disorders of glycosylation (CDG) are a group of more than 160 inborn errors of metabolism affecting multiple pathways of protein and lipid glycosylation. Patients present with a wide range of symptoms, and therapies are only available for very few subtypes. Specific nutritional treatment options for certain CDG types include oral supplementation of monosaccharide sugars, manganese, uridine, or pyridoxine. Additional management includes specific diets (i. e. complex carbohydrate-, or ketogenic diet), iron supplementation and albumin infusions. We review the dietary management in CDG with a focus on two subgroups: N-linked glycosylation defects and GPI-anchor disorders.
Keywords: N-linked CDG, hypoglycemia, manganese, monosaccharide therapy, pyridoxin, GPI-anchor disorder
Congenital disorders of glycosylation
We expect an acceleration in the discovery of novel therapies in inborn errors of metabolism. One of the best examples is in Congenital disorders of glycosylation (CDG) (see Glossary), a rare disorder that affect the synthesis or processing of monosaccharide-chains, leading to decreased glycosylation and abnormal glycoforms attached to proteins and lipids [1,2,3,4]. Approximately 3–4% of the human genome participates in glycosylation pathways [5,6,7] resulting in close to 160 different types of CDG identified to date [8]. A commonly used biomarker for CDG is the glycosylated protein transferrin, which shows a distinct glycosylation profile in several, but far from all CDG (Box 1). The names of the individual CDG subtypes consist of the name of the causative gene, followed by “-CDG” (see Box 2). While many CDG are caused by enzyme deficiencies linked to the well conserved glycosylation machinery, for several CDG only the underlying gene defect is known, and we are only beginning to understand the molecular mechanisms linking the gene with the disease phenotype.
Box 1. Basic Biochemistry of Glycosylation:
Glycosylation is the synthesis of glycans, and their covalent enzymatic attachment to other molecules including proteins, lipids. From the two clinically most relevant pathways. N-linked glycosylation starts in the cytoplasm with monosaccharide activation, proceeding to oligosaccharide synthesis in the endoplasmatic reticulum (ER) and glycoprotein editing in the Golgi apparatus [3,30]. O-linked glycosylation occurs in the Golgi apparatus. Several CDG affect multiple pathways (combined protein glycosylation defects), and an emerging group involves lipid glycosylation and Glycosylphosphatidylinositol anchor synthesis [80]. The finding of hypoglycosylation and/or abnormal glycan structures is the hallmark of the disorders affecting N- and O-linked glycosylation [3]. Based on the localization of the defect within the cell, N-linked CDG are divided into type I and type II disorders: A type I CDG is caused by a defect of cytoplasmic activation, assembly, or transfer of the dolichol-linked glycan in the cytoplasm or the ER– resulting in a loss of entire glycan chains. It is characterized by an increase of di- and/or asialotransferrin (a transferrin molecule with one or no oligosaccharide chain attached to it, with only two or no distal sialic acid residues instead of four), which can be detected by a common screening method for CDG, the analysis of carbohydrate deficient transferrin (CDT) in the serum of the patients. A type II CDG indicates a glycan processing defect after the transfer of the glycan chain within the ER, or during the following remodeling process in the Golgi apparatus – resulting in truncated glycan chains and characterized by an increase of tri-, di-, mono- and/or asialo-transferrin in the CDT screening [3, 31]. The glycosylation process is linked to many essential pathways, including glycolysis, glycogenolysis, the Leloir pathway, nucleotide synthesis etc. The dietary concentration of monosaccharides regulates transporters which are used by the glycosylation machinery. The abundance of certain activated sugars (e.g., UDP sugars) are essential for normal glycosylation. The concentration of N-Acetylglucosamine (GlcNAc) reflects the active metabolism of carbohydrates, amino acids, and fats, indicating that nutrients are abundant [6,7].
Box 2. Genetic defects and corresponding enzymatic deficiencies in CDG amendable to dietary interventions.
MPI - mannose phosphate isomerase
Phosphomannose isomerase catalyzes the interconversion of fructose-6-phosphate and mannose-6-phosphate and plays a critical role in maintaining the supply of D-mannose derivatives, which are required for most glycosylation reactions.
ALG6 - alpha-1,3-glucosyltransferase
The encoded protein catalyzes the addition of the first glucose residue to the growing lipid-linked oligosaccharide precursor of N-linked glycosylation.
GFUS - GDP-L-fucose synthase
The encoded protein catalyzes the two-step epimerase and the reductase reactions in GDP-D-mannose metabolism, converting GDP-4-keto-6-D-deoxymannose to GDP-L-fucose. GDP-L-fucose is the substrate of several fucosyltransferases involved in the expression of many glycoconjugates.
PGM1- phosphoglucomutase 1
The encoded protein catalyzes the transfer of phosphate between the 1 and 6 positions of glucose.
PMM2 – phosphomannomutase 2
The encoded protein catalyzes the isomerization of mannose 6-phosphate to mannose 1-phosphate, which is a precursor to GDP-mannose necessary for the synthesis of dolichol-P-oligosaccharides.
MAN1B1 – mannosidase alpha class 1B member 1
The encoded protein converts Man9GlcNAc to Man8GlcNAc isomer B. It is required for N-glycan trimming to Man5–6GlcNAc2 in the endoplasmic-reticulum-associated degradation pathway.
SLC35C1 – solute carrier family 35 member C1
This gene encodes a GDP-fucose transporter that is found in the Golgi apparatus.
SLC35A2 – solute carrier family 35 member A2
The encoded protein transports UDP-galactose from the cytosol into Golgi vesicles, where it serves as a glycosyl donor for the generation of glycans.
SLC39A8 – solute carrier family 39 member 8
The encoded protein is glycosylated and found in the plasma membrane and mitochondria, and functions in the cellular import of zinc at the onset of inflammation.
TMEM165 – transmembrane protein 165
This gene encodes a predicted transmembrane protein with a perinuclear Golgi-like distribution in fibroblasts.
FUT8 – fucosyltransferase 8
The encoded protein catalyzes the transfer of fucose from GDP-fucose to N-linked type complex glycopeptides.
Patients diagnosed with CDG present with a multisystem phenotype affecting development, growth, liver function, endocrine regulation, and coagulation defects, etc [3,4,9].
At least five new N-linked CDG (disorder types which affect protein glycosylation), and seven GPI (glycosylphosphatidylinositol) anchor disorders (CDG types which affect lipid glycosylation), were identified in the last four years [8, 10, 11].
We expect an increase in the number of available treatments in CDG. While most CDG only have symptomatic and preventive treatment options, there are several specific nutritional therapies coming into clinical practice in CDG, and there are also several new therapeutic approaches on the horizon.
Nine N-linked glycosylation disorders, as well as some cases of GPI anchor disorders are potentially treatable. In this review we will discuss 1) common CDG symptoms that can be addressed by dietary therapies 2) nutrition management by diet in CDG, including complex carbohydrate diet or ketogenic diet and 3) disease specific supplementation of dietary sugars and other dietary measures (monosaccharides like D-galactose, mannose and L-fucose, nucleosides like uridine, trace elements including manganese and certain vitamins like pyridoxine).
Common CDG symptoms that can be addressed by dietary therapies
Multisystem manifestations in CDG may include the following (table 1):
Table 1:
Recommendations for dietary treatment of different CDG types and monitoring during therapy.
| CDG Type | CDG symptoms | Underlying mechanism | Dietary therapy | Safety labs during therapy, reported side effects | Refs |
|---|---|---|---|---|---|
| MPI-CDG (according to guidelines) | Multisystem phenotype: - Symptoms typically start in infancy - Hypoglycemia - Liver disease - Combined coagulopathy - Diarrhea, vomiting - Feeding difficulties - PLE - Failure to thrive |
Defect in mannose metabolism due to impaired function of the enzyme MPI |
Mannose therapy: - Bypasses the enzymatic defect - Improves glycosylation of functional proteins (coagulation, CDT) - Improves clinical symptoms: overall condition, normalization of hypoglycemia, vomiting and diarrhea, decreased risk of thrombosis and improved growth - First effects after a week of treatment Oral mannose: - Dose: 150–170 mg/kg/dose 4–5 x/d (max. 600–1200 mg/kg/d; max. 6 x/d) IV mannose: - Only in life-threatening conditions (when oral intake is not possible) - Max. dose: 1 g/kg/d (continuous infusion) - Combined with individualized IV glucose for prevention of hypoglycemia Other measures: - Alcohol abstinence - Parenteral nutrition in undernourished patients with chronic diarrhea or recurrent vomiting - Albumin infusions (20% solution) in patients with serum albumin <2 g/dL and edema - Hypoglycemia: IV glucose, glucagon, diazoxide, continuous/frequent feedings with complex carbohydrates - Complex carbohydrate diet for prevention of hypoglycemia |
Safety labs:
Oral mannose: Every 3 months: - Monitor for hemolysis (unconjugated bilirubin, whole blood count) - HbA1C - CDT IV mannose: - Monitor for hemolysis (unconjugated bilirubin, whole blood count) Side effects: Oral mannose: - Abdominal pain, diarrhea (consider dose adjustment) IV mannose: - Hemolysis - Neurological symptoms (seizures, stupor) |
[90] |
| PGM1-CDG (according to guidelines) | Multisystem phenotype: - Hypoglycemia (with possible seizures) - Cleft palate/bifid uvula (with possible feeding difficulties) - Elevated liver enzymes - Coagulopathy - Muscle weakness and exercise intolerance (possibly with rhabdomyolysis), motor delay - Cardiomyopathy - Symptoms typically start in infancy - |
Defect of the enzyme PGM1 (essential for glucose release from glycogen and glycosylation) with impaired interconversion of glucose-1-phosphate and glucose-6-phosphate (can be bypassed by supplementation of D-galactose) |
Oral D-galactose therapy: - Improves glycosylation and function of proteins (including CDT) - Improves clinical symptoms: liver function, coagulation, normalization of hypoglycemia, endocrinopathy, improves exercise intolerance and fatigue, reduced rhabdomyolysis - Dose: 1 g/kg/d (500–2500 mg/kg/d, max 50 g/d), divided into up to 6 doses/d Other measures: - Alcohol abstinence - Complex carbohydrate diet and frequent feedings for prevention of hypoglycemia - Uncooked cornstarch before bedtime (infants > 6 months) or modified cornstarch (children > 3 years) - Young infants: consider continuous tube feedings - Acute hypoglycemia: IV 10% dextrose bolus, followed by continuous glucose infusions (rate: 4–6 mg/kg/min for full term infants or 8–10 mg/kg/min for premature infants) - Consider: oral diazoxide in case of resistant hypoglycemia |
Safety labs:
Every 6 months: - Liver transaminases - ATIII - CK - CDT, N-glycans - Serum Gal-1-P, urine galactitol Side effects: - Increased excretion of galactitol in the urine |
[53] |
| SLC35C1-CDG/LADII | - Immunodeficiency, hyperleukocytosis - Developmental delay, intellectual disability - Short stature |
Impaired fucose transport into the Golgi apparatus due to defective GDP-fucose transporter, leading to impaired fucosylation of proteins and lipids |
Oral L-fucose:
- Improved fucosylation of glycosylated proteins - Normalization of neutrophil counts - Improved clinical symptoms: reduction of infection frequency and improvement of hyperleukocytosis - Effect on neurological issues and short stature still unclear - Dose: 400 mg/kg/d, divided into 23 doses (up to 1.2 g/kg/d in severe cases) |
Safety labs:
- Complete blood count, hemolysis parameters - H-antigen expression Side effects: - Bombay blood type: potential re-expression of H-antigen on red blood cells during fucose therapy - Monitor for autoimmune neutropenia and auto-hemolysis (if present, consider dose adjustment) |
, 57,63] |
| FUT8-CDG | - Developmental delay - muscular hypotonia - feeding problems - failure to thrive - respiratory issues |
Defective fucosylation of serum proteins |
Oral L-fucose:
- Clinical improvement - Increased fucosylation of glycosylated proteins - Dose: has been successfully done at a final dose of 825 mg/kg/d (divided into 5 doses) |
Side effects: - None noted |
[64] [65] [66] |
| GFUS-CDG | Neurological phenotype: - Developmental delay, intellectual disability - Microcephaly - Brain malformations - Feeding difficulties - Growth retardation - Coarse facial features |
Impaired de novo synthesis of GDP L fucose (needed for protein N- and Oglycosylation) |
Oral L-fucose:
Clinical improvement: - Improved neurological development - Mild effect on growth - Dose: Goal: 700 mg/kg/d, divided into 3 doses |
Side effects: - None noted (only one treated individual so far) Note: More research needed, no clinical studies have been done on L-fucose treatment of GFUS-CDG yet |
[10] |
| SLC35A2-CDG | Primarily neurological phenotype: - Infantile onset epilepsy - Developmental delay - Facial dysmorphism Also: - Liver abnormalities - Skeletal abnormalities - Failure to thrive |
Defective UDP-galactose transporter in the Golgi apparatus |
Oral galactose:
- Increasing the availability of the substrate for the defective transporter - improves growth, developmental progress and seizure control, as well as biochemical parameters and glycosylation - Dose: 1.5 g/kg/d (up to 3 g/kg/d), can be divided into up to 5 doses |
Safety labs:
- Monitor for galactosuria Side effects: - None reported Note: Improvement of glycosylation is not a reliable outcome parameter (due to existence of untreated patients with normal glycosylation and possibility of spontaneous improvement) |
[68] [67] |
| TMEM165-CDG | Severe multisystem phenotype: - Developmental delay - Skeletal dysplasia - Endocrine, hepatic, renal and cardiac symptoms |
Disrupted Golgi manganese homeostasis due to defective Golgi ion transporter, leading to secondary impairment of manganese-dependent galactosyltransferases in the Golgi with impaired galactosylation |
Oral D-galactose:
- Improves secondary glycosylation abnormalities - Biochemical improvement: Endocrine function (IGF1, IGFBP3), coagulation (APTT, ATIII), liver ALT - Improved N-glycosylation - Clinical improvement: overall wellbeing, increased activity - Dose: 1 g/kg/d |
Oral D-galactose: - Monitor for galactosuria Side effects: - None reported |
[73] |
| SLC39A8-CDG | Severe multisystem disorder with complex neurological phenotype: - Severe developmental delay - Severe seizures - Leigh-like MRI abnormalities - Hearing loss - Dwarfism |
Reduced manganese uptake into the cell due to defective membrane manganese transporter with secondary impairment of galactosyltransferases in the Golgi, resulting in impaired galactosylation and severely reduced manganese levels in blood and urine |
Combination of oral manganese-IIsulfate and D-galactose: - Leads to biochemical and clinical improvement: normalization of manganese levels, transferrin glycosylation, seizure cessation Oral manganese-II-sulfate: - Dose: 15–20 mg/kg body weight, divided into 5 doses Oral D-galactose: - Dose: 500–3750 mg/kg/d, divided into 5 doses Note: Additional uridine supplementation (dose: 150 mg/kg/d) may be beneficial to ensure sufficient supply of uridine for UDP-galactose synthesis. |
Safety labs:
- Monitor for galactosuria - Monitor blood Mn, Mg, Zn and iron levels Side effects: Manganese-II-sulfate: - potential risk of manganism D-Galactose: - None reported |
[70] [71] [72] |
| CAD deficiency | Severe neurometabolic disorder: - Early infantile epileptic encephalopathy - Global developmental delay - Dyserythropoietic anemia - Can be lethal during early childhood |
Defect in nucleotide sugar synthesis with impaired de novo pyrimidine production |
Oral uridine:
- Uses uridine salvage pathway to synthesize pyrimidines - Resolves anemia - Seizure cessation - Improved psychomotor development - Dose: 100 mg/kg/d (divided into 4 doses) |
Side effects: - None reported |
[74] [75] [76] [77] |
| GPI anchor disorders | Large group of disorders, often multisystem or primarily neurological phenotype: - Frequent: Hyperphosphatasia (sometimes hypophosphatasia) - Developmental delay - Seizures - Variable organ manifestations (dependent on type of disorder) |
Defective synthesis of GPI linkers, impaired binding of GPI linkers to proteins, or lack of processing of GPIlinked proteins in the Golgi (leading to reduced binding of proteins to the cell membrane with decreased activity/loss of function) |
Oral vitamin B6 (pyridoxine):
- Biochemical effect: increases availability of pyridoxal (activated form of pyridoxine), which can be transported across the blood-brain barrier - Clinical effect: decreased seizure activity in some patients - Dose: ~ 20 mg/kg Other measures: - Ketogenic diet: may improve seizures in some patients Sodium butyrate (PIGM-CDG): - Biochemical effect: inhibits histone deacetylation, increases PIGM transcription - Clinical effect: decreased seizure activity, improved development - Dose: 60–90 mg/kg/d (divided into 3 oral doses) |
Side effects: Oral vitamin B6: - None reported Sodium butyrate: - None reported |
[78] [79] [77,80] |
| PMM2-CDG | Multisystem (primarily neurological) phenotype: - Developmental delay, intellectual disability - Ataxia - Peripheral neuropathy - Brain malformations (cerebellar atrophy) - Abnormal facial features - Elevated liver transaminases - Coagulopathy - Feeding difficulties, failure to thrive - Endocrine abnormalities (hypoglycemia, growth retardation, impaired sexual development) - Retinitis pigmentosa |
Impaired function of PMM2 enzyme (resulting in decreased availability of Man-1-P for synthesis of GDPmannose), leading to impaired glycosylation in the ER | No approved specific therapy available yet. Therapies under development: - Acetazolamide: positive effect on speech and ataxia - Epalrestat: improves PMM2 enzyme activity - Hydrophobic Man-1-P (using liposomes as the delivery system) to increase intracellular availability of Man-1-P - Chaperone therapy Other measures: - Supplemental enteral feeds in undernourished patients - Parenteral nutrition (in undernourished patients with chronic diarrhea or recurrent vomiting) - Albumin infusions (20% solution) in patients with serum albumin <2 g/dL and edema - Constipation management |
[86] [91] |
Abbreviations: ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; ATIII, antithrombin III; CDG, congenital disorders of glycosylation; CK, creatine kinase; CDT, carbohydrate deficient transferrin; ER, endoplasmatic reticulum; Gal-1-P, galactose-1-phosphate; GDP, guanosine-diphosphate; GPI, glycosylphosphatidylinositol; HbA1C, hemoglobin A1C; IGF1, insulin-like growth factor 1; IGFBP3, insulin-like growth factor binding protein 3; IV, intravenous; Man-1-P, Mannose-1-phosphate; MRI, magnetic resonance imaging; PLE, protein-losing enteropathy; UDP, uridine-diphosphate
Malabsorption, diarrhea, and vomiting
Nutritional and growth assessment is the first step to design an individualized therapy. (Box 2). Malabsorption and diarrhea are a consequence of hypoglycosylation of the enterocytes, which can result in a reduced uptake of nutrients by the gut [12]. Mild or occasional diarrhea can be aggravated by a high osmolar diet, and in many cases lactose-containing foods, laxatives, as well as gastrointestinal procedures. These have the potential risk of dehydration, protein losing enteropathy (PLE), and mucosal damage. PLE is amenable by mannose supplementation in MPI-CDG (mannose-phosphate-isomerase deficiency)[13,14], but it can lead to life-threatening hypoalbuminemia in other CDG types (like ALG6-CDG, an N-glycosylation defect in the lipid linked oligosaccharide synthesis part of the pathway) without other treatments than supplementing albumin [15]. Vomiting and reflux are mostly significant problems in young patients, and often improve with age, but in severe cases may necessitate enteral or even parenteral feeding to be able to accomplish acceptable growth. Restricting gluten and/or lactose intake can significantly improve malabsorption [16]. Regular laboratory workup is recommended for iron studies, basic metabolic panel, liver function tests, and any suspected vitamin, mineral, and/or fatty acid deficiencies. Intravenous iron dextran might be necessary in severe cases of iron deficiency.
Constipation is also a common finding in patients with CDG, but could be a side effect of iron supplementation, insufficient hydration, or decreased mobility.
Liver involvement
Liver abnormalities (including transaminitis, cholestatic abnormalities and fatty liver disease) are common in CDG, especially because secretory glycosylation is performed in the liver. Elevated transaminases usually resolve within the first five years of life [17]. Liver dysfunction in a few cases might progress to fibrosis [18].
Albumin infusions are used in the case of severe hypalbuminemia with edema and/or ascites in the setting of critical liver disease. Chronic, long-term nutritional therapy is a daily restricted long chain fat intake (10–15% of total calories) with use of a formula high (>80%) in medium chain triglycerides.
Hypoglycemia
Most of the proteins regulating glucose homeo3stasis, and some of the insulin receptor proteins (for example, insulin receptor, IGF-1 receptor, IGF-2 and IGFBP-3) are glycosylated, and hypoglycemia and hyperinsulinism are common problems in CDG (including neonatal hypoglycemia). This is especially true for MPI-CDG; which almost always presents with hypoglycemia and hyperinsulinism [13, 14], and PGM1-CDG (phospho-gluco-mutase deficiency) [19], but also for PMM2-CDG (phospho-manno-mutase deficiency) [20]. ACTH (adrenocorticotropic hormone) related hypoglycemia in the context of adrenal insufficiency has been shown to occur in PMM2-CDG [21], however; hypoglycemia (glucose level <70mg/dl) may not always be a presenting sign. Consequently, morning levels of cortisol and ACTH should be checked for these patients at least annually since early detection and therapy can be lifesaving. To prevent hypoglycemic episodes, most patients need frequent, complex carbohydrate-containing meals, snacks, and/or enteral feeds.
Frequent infections and inflammation
Glycosylation is a highly energy consuming process. During the time of intercurrent infections glycosylation defects frequently become even more severe. As most of our immune cells’ surface (like our white blood cells) and our immunoglobulins are highly glycosylated, infections demand adequate nutrition and high caloric intake, potential G-tube feeding. Several CDG have immune deficiency, as a symptom [22]. SLC35C1-CDG (leukocyte adhesion deficiency type II, LAD II), for example, is a specific condition presenting with immunodeficiency that is frequently amendable by fucose therapy [23].
Bleeding predisposition and/or risk of thrombosis
Coagulation abnormalities can be present in many CDG types due to deficiency or decreased activity of coagulation factors [24,25], which can lead to acute vascular events and presents a major source of mortality. Besides preventive lifestyle measures and the potential use of the oral anticoagulant rivaroxaban, a factor Xa inhibitor [24,25], patients require adequate hydration at all times. This must be addressed in the dietary recommendations, especially if the patients are on acetazolamide treatment for their ataxia [91]. Coagulation parameters including Antithrombin-III (AT III) and Protein C levels (which have been associated with vascular complications) should be tested at least annually, and before surgeries to detect patients at risk [31].
Edema and pericardial effusion
Normal glycosylation of cell surface proteins is crucial for protein transport over membranes and fluid balance in different compartments of the body (including the pericardium and peritoneum) [26]. Enteral and parenteral nutrition, albumin infusions, and diuretics are often prescribed in a subgroup of patients who develop pericardial effusion [27, 28], or severe edema due to malabsorption, PLE, or hepatic disease (especially in PMM2-CDG) [29,30].
Patients in CDG show a relatively stable disease course, and third spacing generally gets better in older patients, especially in PMM2-CDG, but some clinical features might progress, including osteopenia, scoliosis, neuropathy, recurrent thrombotic events, mostly in in adult patients. There is a huge unmet need for novel therapies [32,33].
Nutrition Management by diet in CDG
Different diets can be useful in certain CDG patients, which are described in the following paragraphs. However, not all CDG patients respond well to these measures and an individualized approach is needed to find the right diet for each patient (Box 3).
Box 3. Nutrition assessment in CDG:
In this complex disorder we need a comprehensive approach for defining the nutrition state using the combination of medical, nutrition, and medication histories; physical examination; anthropometric measurements; and laboratory data. Assessing the patient for risk of malnutrition should be completed at each outpatient clinic visit. Careful measures and remeasures of suspected erroneous height measures will help prevent a false positive malnutrition flag. Alternative predictive equations, such as knee height, body segments, or ulna length, can be used as an estimation of height, in non-ambulatory patients[88].The nutrition focused physical exam focuses on changes to muscle, fat stores, fluid retention, and/or physical signs that can result from micronutrient efficiencies or excesses and provide guidelines for evaluating muscle and subcutaneous fat with this technique [89].
Many proteins that are involved in hormonal regulation are glycosylated, especially stimulating and releasing hormones, thyroid regulatory proteins, as well as hormonal growth factor binding proteins. Due to the impaired function of under-glycosylated hormonal substances in CDG, endocrine disturbance is very common and can lead to growth delay in many CDG patients. Importantly, when measuring growth parameters in CDG patients, growth delay should not always be interpreted as a result of a lack of nutrients unless there are other factors that point to malnutrition as the main reason for the growth delay. Frequently, CDG patients follow along their very own growth and weight curves, without the need for dietary adjustments.
Complex carbohydrate diet
To prevent hypoglycemic episodes, most patients need frequent, complex carbohydrate-containing meals, (bedtime) snacks, and/or enteral feeds. Patients over one year of age who cannot tolerate an overnight fast without hypoglycemia, especially when enteral feeds are not prescribed overnight, will benefit from uncooked cornstarch at 1.5–2.5 g UCCS/kg/dose, given at bedtime. Formulas containing maltodextrin and cornstarch as the carbohydrate sources are also beneficial [21,31,34].
Ketogenic diet
Patients who have intractable seizures often benefit from a ketogenic diet, which is characterized by a high fat to low carbohydrate ratio. The antiepileptic effects are assumed to be a result of an increased production of ketone bodies, which stimulates GABA (γ-amino butyric acid) production [35], as well as an increased intake of polyunsaturated fatty acids [36]. However, ketogenic diet may aggravate hypoglycemia in some patients [4], which means that close monitoring of patients started on ketogenic diet in an inpatient setting for a few days is recommended. Ketogenic diet is usually applied at a ratio of 4:1 or 3:1, administered as either foods only, formula only, or a combination of both. Ketogenic diet is most frequently used in GPI anchor defects [37], which often go along with seizures.
Notably, implementation of a (preventative) ketogenic diet in a patient who has previously been on an unrestricted diet, has no seizure activity, is mobile and without feeding problems can be challenging from a compliance perspective.
Caloric restriction by diet
Obesity is very rare in CDG, but has been described in MAN1B1-CDG, a multisystem disorder that is caused by defective mannosidase activity during the processing of glycoproteins. Daily caloric restriction may be needed in these patients due to an obsessive eating behavior with potential obesity [38]. The recommended diet should promote a very slow weight loss by decreasing total daily calories by about 50 kcal/day, while allowing the child to grow in length. Increasing physical activity will also help with normalizing the BMI.
Disease specific supplementation of dietary sugars and other dietary measures
CDG types that are known as being treatable by specific dietary therapies include PGM1-CDG, MPI-CDG, SLC35C1-CDG, SLC35A2-CDG (affecting the transport of the nucleotide sugar UDP-galactose into the Golgi compartment), TMEM165-CDG and SLC39A8-CDG (affecting the transport of manganese to the Golgi), FUT8-CDG and GFUS-CDG (disorders affecting the metabolism of the rare sugar; fucose), CAD deficiency and GPI anchor disorders, which will be further discussed in the upcoming sections.
Mannose in MPI-CDG
MPI-CDG is a CDG subtype with multisystem involvement that is due to a defect in mannose metabolism with symptoms typically starting in infancy. Most frequent symptoms include hypoglycemia, liver disease, combined coagulopathy, diarrhea, vomiting, feeding difficulties, and protein-losing enteropathy (PLE), often resulting in a failure to thrive. Oral mannose supplementation bypasses the enzymatic defect and was first tried in a patient in 1998 [39]. It is now an approved dietary supplement for the treatment of this disorder, both in the US and Europe [40] (see figure 1), and was found to improve biochemical factors (including improvement of the transferrin glycosylation profile) as well as clinical symptoms in MPI-CDG. The main clinical effects, which can already be observed after only a week of treatment, are the improvement of the patient’s overall condition, normalization of hypoglycemia, vomiting and diarrhea, decreased risk of thrombosis and improved growth [13] [40]. Possible side effects are abdominal pain and diarrhea, which can be addressed by a dose adjustment, or resolves spontaneously. The patient’s HbA1C level must be checked regularly to monitor for signs of a “mannose-diabetes”, which has been attributed to high mannose levels in humans [41], and lower the dose in case of elevation (> 5.6%). The recommended dose ranges between 150 and 170 mg/kg, given four to five times a day. Intravenous mannose is available as well and is dosed at 1 g/kg/d but should only be used in life-threatening emergencies and with extreme caution due to possible hematological (hemolysis) or neurological (seizures and stupor) side effects [42, 43]. Unfortunately, the hepatic manifestations of MPI-CDG are not always treatable by mannose therapy and some patients need to undergo liver transplantation in the case of severe liver fibrosis [18].
Fig 1: Therapy options for MPI-CDG, PGM1-CDG and PMM2-CDG.
In MPI-CDG, the impaired conversion of fructose-6-P to mannose-6-P due to the defect of the mannose-6-phosphate-isomerase can be addressed therapeutically by supplementation of mannose, which provides additional substrate after the biochemical block.
In PMM2-CDG, the conversion of mannose-6-phosphate to mannose-1-phosphate is impaired due to a defect of phosphomannomutase-2, resulting in decreased availability of GDP-mannose, which is used for glycosylation reactions in the endoplasmatic reticulum. Intravenous supplementation of lipophilic mannose-1-P, using liposomes as a carrier, could potentially bypass the metabolic block in PMM2-CDG and hereby, directly correct the glycosylation cascade in the cells. This approach is currently being investigated and might be a therapy option for PMM2-CDG in the future.
In PGM1-CDG, defective phosphoglucomutase-1 leads to impaired interconversion of glucose-1-phosphae and glucose-6-phosphate. Supplementation of oral D-galactose bypasses the metabolic block and hereby, directly increases the availability of substrate for glycosylation.
As a result of these therapeutical approaches, glycosylation of common proteins (for example, transferrin) is restored.
Severely affected infants may require parenteral nutrition due to malnourishment or recurrent vomiting and diarrhea. Intravenous albumin treatment may be necessary in the case of severe hypalbuminemia in the setting of PLE. Hypoglycemia often first has to be treated with intravenous glucose, glucagon and/or diazoxide, in addition to continuous or frequent feedings (and by adding complex carbohydrates to the diet), before the effect of mannose treatment is noticeable [44]. Importantly, certain situations need special attention: These include times of gastrointestinal tract infections, when the patient cannot guarantee adequate oral intake, or perioperative settings. Specific guidelines for the management and treatment of MPI-CDG are available [13].
D-galactose in PGM1-CDG
PGM1-CDG is based on a defect of the enzyme phosphoglucomutase-1 (PGM1), which is at the crossroads of the glycogen, glucose and galactose pathways and essential for adequate glucose release from glycogen and proper glycosylation. PGM1 is a critical enzyme for the interconversion of glucose-1-phosphate and glucose-6-phosphate, which is a step that can be bypassed by oral supplementation of D-galactose [34, 45]. Symptoms typically start in infancy and most frequently include hypoglycemia (possibly leading to seizures), cleft palate/bifid uvula (which can lead to feeding difficulties), elevated liver enzymes, coagulopathy, muscle weakness and exercise intolerance (often with rhabdomyolysis), and, in some cases, cardiomyopathy [45]. Oral D-galactose improves transferrin glycosylation and clinical symptoms, specifically liver function, coagulopathy, hypoglycemic episodes and endocrinopathy [46, 47] [48]. Oral D-galactose has a positive effect on exercise intolerance and fatigue and has been shown to reduce episodes of rhabdomyolysis by normalizing skeletal muscle substrate use from fat to carbohydrates during exercise [49]. Due to its multiple beneficial effects, oral supplementation of D-galactose is now a clinically used therapy for PGM1-CDG (see figure 1). However, it does not treat the cardiomyopathy observed in some patients [50, 51], which, in the worst case, may necessitate a heart transplant [52].
The recommended dose for oral D-galactose in PGM1-CDG is 1.0–1.5 g/kg/day (some of the younger patients might require up to 3.0 g/kg/day), in a single dose or divided in 4 doses per day [53]. The maximum safe dose that has been well tolerated without adverse effects is 50 g/day [47].
Additional to the specific therapy with D-galactose, oral administration of uncooked corn starch before bedtime (for infants over 6 months of age) or modified cornstarch (for children starting at 3 years of age) has been shown to help prevent morning hypoglycemia [53] [54], and frequent feedings, along with a complex carbohydrate diet are recommended in treating hyperinsulinemic hypoglycemia [55]. However, in infants presenting with acute hypoglycemia, a bolus of intravenous 10% dextrose, followed by continuous glucose infusions at a rate of 4 to 6 mg/kg/min (for full term infants) or 8 to 10 mg/kg/min (for premature infants) are recommended. Young infants may need continuous tube feedings to maintain normal blood glucose levels. If these measures are not sufficient, oral diazoxide should be considered. Patients with cleft palate should be followed for feeding difficulties and possible need for feeding assistance.
Some patients with PGM1-CDG develop an eating disorder due to the strict diet recommendations and risk for hypoglycemia for their young adulthood, which makes the management of the disease even more difficult. Specific guidelines for the management and treatment of PGM1-CDG are available [53].
Fucose in SLC35C1-CDG
SLC35C1-CDG, also known as Leukocyte adhesion deficiency type II (LADII), is due to a defective GDP-fucose transporter with impaired fucose transport into the Golgi apparatus. Fucose is a component of glycoproteins and glycolipids [56], and the disorder leads to impaired fucosylation of these glycoconjugates. Patients with SLC35C1-CDG have been described to respond to oral fucose supplementation with improved fucosylation of serum proteins and normalized neutrophil counts [57] (see figure 2). The main effects of the treatment seem to be a reduction of infection frequency and improvement of hyperleukocytosis, including re-expression of E- and P-selectin ligands. However, if fucose treatment has a significant effect on the neurological issues (i. e. psychomotor development) and the short stature of the patients is still unclear [57–60].
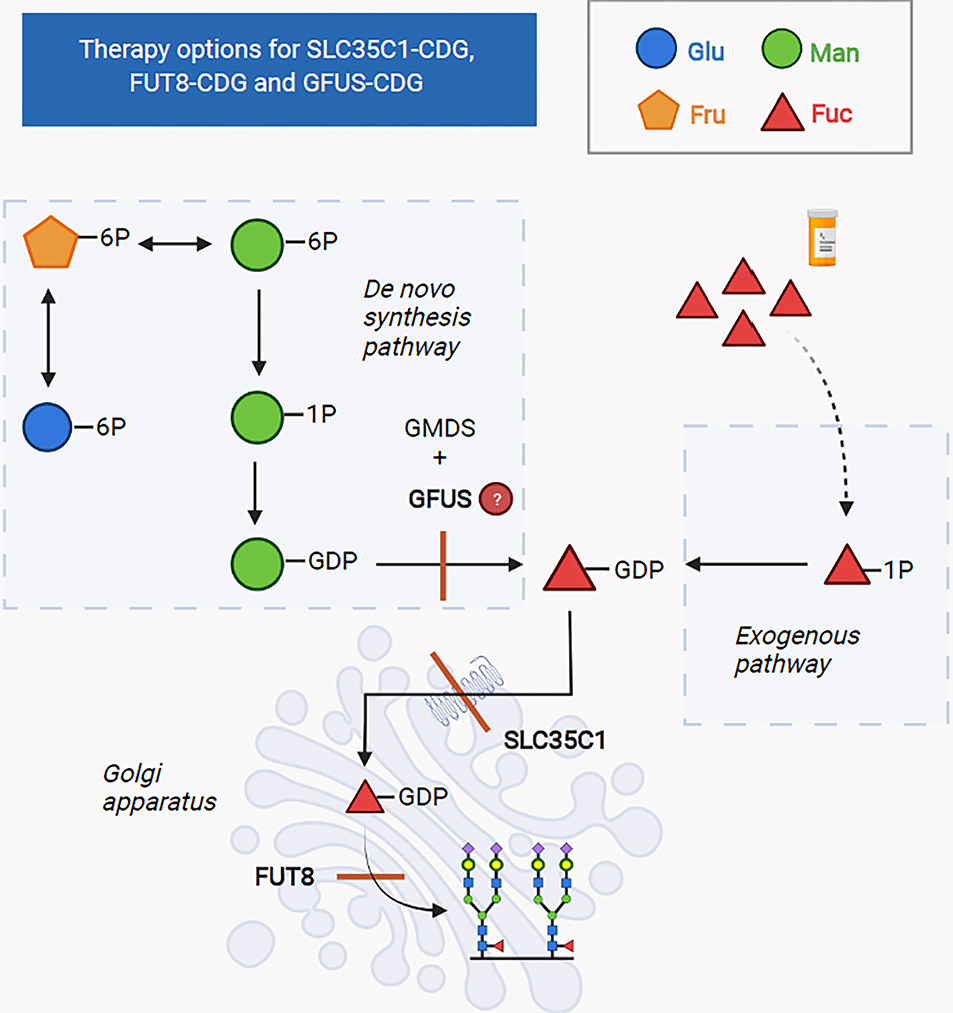
Fig 2: Therapy options for SLC35C1-CDG, FUT8-CDG and GFUS-CDG.
In SLC35C1-CDG, the defective transporter for GDP-fucose leads to a deficit of GDP-fucose in the Golgi apparatus and hereby, impairs fucosylation of proteins.
In FUT8-CDG the enzyme, α-1,6-fucosyltransferase, which adds fucose to glycans in the Golgi apparatus, is defective and due to this defect, fucosylation of serum proteins is impaired.
Both defects can be addressed therapeutically by oral supplementation of fucose, which increases the availability of substrate for the defective proteins through the exogenous pathway and hereby, restores fucosylation of glycosylated proteins.
GFUS-CDG is due to defective conversion of GDP-mannose to GDP-fucose and leads to impaired de novo synthesis of GDP-fucose. Like in SLC35C1-CDG and FUT8-CDG, increasing the availability of substrate for the defective enzyme by exogenous supplementation might also be a promising approach.
One more important aspect is the fact that the human ABO blood groups are based on protein glycosylation patterns [61] and that patients with SLC35C1-CDG lack the fucosylated H antigen (an oligosaccharide foundation for building the A and B antigens) on their red blood cells. This results in the so-called “Bombay blood type” (hh). Importantly, fucose therapy could, in theory, induce the re-expression of the H-antigen, which could potentially result in auto-hemolysis of red blood cells (in case anti-H-antigen antibodies are present in the blood of the patient) [57]. Consequently, after initiation of L-fucose therapy (see table 1) for SLC35C1-CDG, patients should receive regular laboratory workup to recognize early signs of an autoimmune reaction [62] [63].
L-Fucose supplementation in FUT8-CDG
This disorder, like SLC35C1-CDG, also results in defective fucosylation of serum proteins [64]. Main symptoms are developmental delay, muscular hypotonia, feeding problems, failure to thrive and respiratory issues [65]. Oral administration od L-fucose has reportedly led to clinical improvement in a pair of twins without noted adverse events [66], and fucosylation of glycosylated proteins was increased (see figure 2 and table 1).
Fucose in GFUS-CDG
GFUS-CDG is one of the most recently disorders described as treatable with fucose in one patient [10] [56]. The genetic defect responsible for the disease leads to impaired de novo synthesis of GDP-L-fucose, which is needed for both protein N- and O-glycosylation, and results in a disorder characterized by neurological problems including developmental delay, intellectual disability, microcephaly and brain malformations (Arnold Chiari malformation, abnormal corpus callosum), as well as feeding difficulties, growth retardation and coarse facial features. Based on the biochemical background and the observation that supplementation of the growth media with fucose improved protein fucosylation in the patient’s fibroblasts, supplementation of L-fucose was initiated in the patient (see figure 2). The treatment was well tolerated and had significant clinical effects on neurological development, as well as a mild effect on growth (see table 1). Since fucose therapy has only been studied in one individual patient with GFUS-CDG so far, more research is needed to confirm the described benefits.
D-galactose in SLC35A2-CDG
SLC35A2-CDG is a type of CDG that is inherited in an X-linked manner (however, most cases occur de novo) and results in a primarily neurological phenotype. The disorder is caused by a defective UDP-galactose transporter in the Golgi apparatus. Increasing the availability of the substrate by oral D-galactose supplementation at a dose of 1.5 g/kg/day (a dose up to 3 g/kg/day in younger patients have been tolerated without side effects) has been shown to improve growth, developmental progress, seizure control and biochemical parameters [67], as well as the transferrin glycosylation profile [68] (see figure 3 and table 1). However, improvement of glycosylation has not been proven to be a reliable outcome parameter, since there have also been untreated patients with normal glycosylation, as well as some patients that have shown spontaneous improvement of their glycosylation pattern even without treatment [67].
Fig 3: Therapy options for SLC39A8-CDG, SLC35A2-CDG and TMEM165-CDG.
SLC39A8-CDG is due to a defective manganese transporter in the cell membrane, which leads to reduced manganese uptake into the cell and impairment of manganese-dependent galactosyltransferases in the Golgi apparatus. Supplementation of manganese-II-sulfate increases the availability of substrate for the defective transporter, and additional supplementation of D-galactose ensures sufficient availability of galactose to ultimately restore galactosylation. TMEM165-CDG is due to a defective Golgi ion transporter, which leads to disrupted manganese homeostasis in the Golgi apparatus and ultimately, secondary impairment of manganese-dependent galactosyltransferases in the Golgi apparatus. The resulting impaired galactosylation can be addressed by oral supplementation of D-galactose.
SLC35A2-CDG is due to a defective UDP-galactose transporter in the Golgi apparatus. The resulting impairment in galactosylation can be improved by oral supplementation of D-galactose, which increases substrate availability for the defective transporter.
D-Galactose in TMEM165-CDG and SLC39A8-CDG, manganese in SLC39A8-CDG
Both SLC39A8-CDG and TMEM165-CDG are defects affecting manganese transport in the cell [69, 70, 71] [72, 73]. Since manganese is a crucial cofactor for glycosylation, a transport deficiency of this metal into the cell and into the Golgi apparatus will cause impaired function of galactosyltransferases and result in secondary glycosylation defects (specifically impaired galactosylation) with a type II CDG pattern.
SLC39A8-CDG is due to a defective manganese membrane transporter and leads to severely reduced manganese levels in blood (and urine), resulting in a secondary impairment of the function of manganese-dependent galactosyltransferases in the Golgi. Most patients have a severe multisystem disorder with severe developmental delay and epileptic encephalopathy. There are adult patients described as well, showing that survival to older age is possible. Treatment with manganese-sulfate at a dose of 15–20 mg/kg body weight [70] or a combination of manganese and galactose [71], with a galactose dose between 0.5 and 1 g/kg body weight per day, (doses up to 3.75g/kg/day have been also reported) reportedly produced both biochemical and clinical improvement including normalization of manganese levels, transferrin glycosylation and seizure cessation (see figure 3 and table 1). Additional uridine supplementation (a dose of 150 mg/kg bodyweight/d has been trialed) may be beneficial to ensure sufficient supply of uridine for UDP-galactose synthesis [72]. Importantly, monitoring of blood manganese levels has to be done regularly due to the potential danger of manganism.
Main symptoms of TMEM165-CDG are neurodevelopmental delay and skeletal dysplasia, along with endocrine, hepatic, renal and cardiac symptoms. Improving the availability of galactose by oral supplementation of D-galactose (1 g/kg body weight per day) has been done to address the secondary glycosylation abnormalities and has been shown to lead to biochemical improvement and improved N-glycosylation in two patients [73] (see table 1). Supplementation of manganese has been also shown to improve hypogalactosylation in vivo [73] in TMEM165-CDG.
Uridine in CAD deficiency
CAD deficiency is a defect in nucleotide sugar synthesis, which are important precursors for glycosylation [74]. Mutations in the CAD gene lead to impaired de novo pyrimidine production and result in a severe neurometabolic disorder with early infantile epileptic encephalopathy, global developmental delay and dyserythropoietic anemia [75]. When left untreated, this disorder can be lethal during early childhood. However, oral treatment with uridine using the uridine salvage pathway to synthesize pyrimidines has been reported as a safe therapeutic option for patients diagnosed with CAD deficiency, and resulted not only in resolved anemia but also significant clinical improvements including seizure cessation and improved psychomotor development [76] [77] (see table 1).
Vitamin B6 supplementation, butyrate therapy and ketogenic diet in GPI anchor disorders
One crucial and recently growing subgroup of CDG that has been shown to respond to vitamin supplementation are GPI anchor disorders [78, 79]. GPI is a glycophospholipid that is synthesized in the ER, attached to a protein, processed in the Golgi apparatus, and finally has the function to anchor different proteins to the cell membrane [79, 80, 88]. There are in total more than 150 human proteins linked to GPI, which all play crucial biological roles. Consequently, GPI plays an important role in embryogenesis, immune response, and neurogenesis [37, 87]. Importantly, GPI anchor disorders frequently present with hyperphosphatasia (or less frequently hypophosphatasia), as the enzyme alkaline phosphatase is normally anchored to the cell membrane by a GPI linker. When this linker is defective, binding of alkaline phosphatase is impaired, which can result in a loss of function [78].
Notably, it has recently been shown that in some cases, seizure activity in patients with GPI-anchor disorders can be positively influenced by therapy with vitamin B6 and its activated form, pyridoxal phosphate (PLP) [78] [79] (see table 1). This is thought to be due to the fact that the dephosphorylation of circulating pyridoxal 5-phosphate to pyridoxal, a vitamin B6 derivate that can cross the blood-brain barrier, is dependent on the enzymatic activity of functional alkaline phosphatase. Consequently, in the absence of functional alkaline phosphatase, this vitamin cannot fulfill its role within the brain, and seizures may respond to vitamin B6 administration. Oral vitamin B6 (pyridoxine) at a dose of 400 mg (20 mg/kg) has been tried for PIGO-CDG and has led to seizure cessation in this patient [79].
In PIGM-CDG, a disorder clinically characterized by intractable seizures, developmental delay, macrocephaly and infantile-onset cerebrovascular thrombotic events, sodium butyrate has been described to have a dramatic effect on seizures within only a few weeks, as well as developmental delay and general condition [80, 81], without notable side effects. This is thought to be due to the fact that sodium butyrate inhibits histone deacetylation, while histone hypoacetylation at the promoter of the PIGM gene has been shown to be the cause of the disease. Hence, sodium butyrate increases PIGM transcription and surface GPI expression. If it also has an effect on the risk of thrombosis in inherited GPI deficiency, it is not known. The currently recommended dose is 60–90 mg/kg/d, divided into 3 oral doses.
Another therapy that has been reported successful in GPI anchor disorders is the initiation of ketogenic diet [82], which has been discussed in more detail above.
Future strategies beyond dietary therapy
Management of CDG patients requires an approach which combines both nutritional and medical management, customized for the specific individual. However, not all CDG subtypes are amenable to dietary therapy.
Recently, several non-dietary therapeutic approaches are entering the phase of clinical trials, including a membrane permeable, hydrophobic Man-1-P using liposomal delivery system (intended to bypass the block in the biochemical pathway), as well as molecules activating phosphomannomutase (PMM) enzyme activity.
Supplementation with “activated” (phosphorylated) monosaccharide building blocks instead of free monosaccharides, when the biochemical step of adding or donating phosphorylated monosaccharides or sugar nucleotides is blocked, is a novel approach for therapy (see Clinician’s corner). However, this approach is challenging due to the aspects of compound stability, delivery to the right compartment(s), uptake into the cells, and the potential toxicity of phosphorylated compounds. Based on in vitro studies, hydrophobic mannose-1-phosphate derivates or liposomal delivery might prove to be safe and efficient in increasing glycosylation in PMM2-CDG [84] (see figure 1). The upcoming clinical trial with a liposomal form of mannose-1 phosphate in PMM2-CDG patient could be a proof-of-concept for this delivery method for other CDG as well, and turns the idea of supplementing monosaccharides by dietary measures into a non-dietary therapy, that delivers the activated monosaccharide directly into the bloodstream. Another potential way of improving glycosylation is the use of chaperone therapies, which improves the defective enzyme’s stability in in the whole body, and predicted to improve CNS function as well [85]. A novel avenue for CDG treatment is drug repurposing. For instance, epalrestat (an aldose reductase enzyme inhibitor) has been reported to improve PMM enzyme activity levels and shown to increase protein glycosylation by glycoproteomic studies [86]. It is also hypothesized to modify sugar fluxes and, by increasing glucose-1,6 biphosphate abundance, to stabilize the defective enzyme and increasing its activity [86].
Clinician’s Corner.
CDG is a group of more than 150 disorders with less than 100 cases reported per disease type and predicted incidence around 1:100 000
CDG affects the central nervous system, all organs and the endocrine and coagulation system, with mostly only supportive therapies
As most N-linked glycosylated proteins are produced by the liver, and any therapy which boosts substrate availability, and allows substrate activation (see phosphorylation and nucleotide sugars) could be successful in future CDG treatment
Providing glycans for the body in access activates internal salvage pathways and redirect sugar pools
Many glycosylation enzymes like PMM2 are regulated or stabilized by phosphorylated sugar pools, which could be used for therapy
Glycans are essential in other disease processes including CNS signaling, (auto)immunity and cancer, and CDG teaches us about novel treatment options in common disease (blocking glycosylation in cancer treatment, changing body pH by drugs to positively alter brain receptor function, GlcNAc supplement in autoimmune conditions etc)
Several clinical trials have been launched in CDG to trial different forms of monosaccharides, or repurposed drugs but gene therapy is only at the level of animal model trials
Concluding remarks
Patients with CDG require a multi-disciplinary team, including a dietician. Importantly, the management of CDG patients requires an approach which combines both nutritional and medical management, customized for the specific individual (e.g. individualized doses of monosaccharide therapy, ketogenic diet, sometimes in combination with other supplements, like pyridoxine, titrating the optimal manganese dose etc.). However, while these strategies are an important part of CDG patient treatment, not all subtypes are amenable to dietary therapy, and further research is needed to develop novel approaches to treat these disorders (see Outstanding Questions). In order to successfully achieve this, we need more data on the natural history of CDG [33, 83]. To get closer to this goal, a large collaborative effort including sites in the United States and Europe took an initiative in 2018 by launching natural history studies for clinical trial readiness (https://www.rarediseasesnetwork.org/fcdgc). The largest recruited patient group has the most common type of CDG, PMM2-CDG, which is one of the subtypes that still has no known FDA approved curative therapy. Past mannose supplementation trials did not show clear clinical benefits [83]. To assess improvement in glycosylation during dietary, as well as non-dietary measures, we need new, reliable biomarkers in many CDG. Using glycomics and glycoproteomics for monitoring the success of novel therapies have been shown to be essential in disorders of glycosylation. In the long run, our expanding knowledge on the effect of boosting secretory glycosylation (Box 4) will be especially helpful in the development of adenoassociated virus delivered, liver targeted gene therapies in metabolic disorders.
Outstanding questions.
How can we develop more efficient therapies, for the administration of active carbohydrate compounds to supply the specific missing building blocks of glycosylation?
How can new therapy reach the central nervous system as glycans do not cross the blood brain barrier?
Why are most animal (mice) models of CDG lethal, and how can we design viable models mirroring the true clinical phenotype?
Are early therapies able to reverse brain and peripheral nerve symptoms in CDG?
Would newborn screening improve the long-term outcome of glycosylation defects?
Is it safe and efficient to use nutritional intervention with high dose of monosaccharide intake during pregnancy in PMM2-CDG and PGM1-CDG?
Is the cardiomyopathy, which is therapy resistant in PGM1-CDG, related to the glycosylation defects or to a failure in energy metabolism?
Is the severe epilepsy in GPI anchor defects metabolic or developmental in origin?
Box 4. Is there cross-correction of glycosylation between different tissues?
We have growing experience with transplantation cases in CDG, especially liver transplantation cases in MPI-CDG, CCDC115-CDG, ATP6AP1 and ATP6AP2-CDG, restoring glycosylation for most of our glycosylated secretory proteins and significantly improving outcome beyond curing the liver disease aspect of the complex phenotype. But how is it possible that patients show improvement in not only their liver function, coagulation, and endocrine parameters? Several case reports suggest that intestinal absorption, alertness, muscle endurance and fatigue also improve after transplantation. Could this occur due to cross correction between nonhepatic and liver cells? As free glycans are not secreted, only more glycoproteins circulate in the whole body after liver transplantation. If cross correction does underlie the general improvement in these cases, this is most likely due to increased availability of monosaccharides and nucleotide sugars through the different internal salvage pathways, replenishing missing substrates for the glycosylation machinery. Would this mean that treatment with high glycan containing supplements, could be efficient in the treatment of different CDG? This possibility is supported by previous case reports like the positive effect of high milk intake in NANS-CDG, PGM1-CDG or using fractionated heparin in MPI-CDG.
Highlights.
CDG was discovered in 1979 and counts more than 150 disorders; until recent years, only MPI-CDG was treatable.
Altering sugar fluxes and activating internal monosaccharide salvage pathways have been recently shown efficacy in several other CDG affecting galactosylation and fucosylation.
There is still a huge unmet need to develop treatments for GPI anchor disorders, presenting with severe developmental delay and epilepsy.
New therapies, including chaperon and drug repurposing approaches, also affect glycosylation by altering sugar pools and stabilizing/activating defective enzymes like Epalrestat.
Unfortunately, gene therapy is still in early phases due to the lack of animal models in CDG.
Acknowledgements:
This work was funded by the grant titled Frontiers in Congenital Disorders of Glycosylation (1U54NS115198–01) from the National Institute of Neurological Diseases and Stroke (NINDS) and the National Center for Advancing Translational Sciences (NCATS), National Institute of Child Health and Human Development and the Rare Disorders Clinical Research Network (RDCRN), at the National Institute of Health.
Glossary
- Congenital disorders of glycosylation (CDG)
is a large group of rare, inherited disorders that affect the complex metabolic process called glycosylation
- Glycosylation
the process of creating, modifying and attaching sugar chains to proteins and lipids
- Glycoproteins
proteins with covalently attached sugar derivates, or sugar chains
- GPI anchor
a complex lipid attaching a sugar chain to the membrane anchoring a protein
- Liposomal delivery
biochemically manufactured molecules (activated monosaccharides) locked in a miniscule lipid vacuole preferentially administered in an infusion
- Monosaccharide
single-sugar building block used by metabolism
- MPI-CDG
a CDG type occurring due to pathogenic mutations in the gene MPI. CDG are named by the name of the affected gene followed by “-CDG.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sosicka P et al. (2021) Chemical Therapies for Congenital Disorders of Glycosylation. ACS Chem Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JH and Marquardt T (2021) Treatment Options in Congenital Disorders of Glycosylation. Frontiers in Genetics 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefeber DJ et al. (2011) How to find and diagnose a CDG due to defective N-glycosylation. J Inherit Metab Dis 34 (4), 849–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witters P et al. (2017) Nutritional Therapies in Congenital Disorders of Glycosylation (CDG). Nutrients 9 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schjoldager KT et al. (2020) Global view of human protein glycosylation pathways and functions. Nature Reviews Molecular Cell Biology 21 (12), 729–749 [DOI] [PubMed] [Google Scholar]
- 6.Reily C et al. (2019) Glycosylation in health and disease. Nat Rev Nephrol 15 (6), 346–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y et al. (2021) O-GlcNAcylation: the “stress and nutrition receptor” in cell stress response. Cell Stress Chaperones 26 (2), 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira CR et al. (2021) An international classification of inherited metabolic disorders (ICIMD). J Inherit Metab Dis 44 (1), 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verheijen J et al. (2020) Therapeutic approaches in Congenital Disorders of Glycosylation (CDG) involving N-linked glycosylation: an update. Genet Med 22 (2), 268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feichtinger RG et al. (2021) A spoonful of L-fucose-an efficient therapy for GFUS-CDG, a new glycosylation disorder. EMBO Mol Med 13 (9), e14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radenkovic S, et al. TRAPPC9-CDG: A novel congenital disorder of glycosylation with dysmorphic features and intellectual disability. Genet Med. 2022. Jan 15:S1098–3600(21)05471-X. [DOI] [PubMed] [Google Scholar]
- 12.Westphal V, Murch S, Kim S, et al. Reduced heparan sulphate accumulation in enterocytes contributes to protein-losing enteropathy in a congenital disorder of glycosylation. Am J Pathol 2000; 157:1917–25). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Čechová A et al. (2020) Consensus guideline for the diagnosis and management of mannose phosphate isomerase-congenital disorder of glycosylation. J Inherit Metab Dis 43 (4), 671–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lonlay P and Seta N (2009) The clinical spectrum of phosphomannose isomerase deficiency, with an evaluation of mannose treatment for CDG-Ib. Biochim Biophys Acta 1792 (9), 841–3 [DOI] [PubMed] [Google Scholar]
- 15.Morava E et al. (2016) ALG6-CDG: a recognizable phenotype with epilepsy, proximal muscle weakness, ataxia and behavioral and limb anomalies. J Inherit Metab Dis 39 (5), 713–723. [DOI] [PubMed] [Google Scholar]
- 16.Barone R et al. (2007) Borderline mental development in a congenital disorder of glycosylation (CDG) type Ia patient with multisystemic involvement (intermediate phenotype). J Inherit Metab Dis 30 (1), 107. [DOI] [PubMed] [Google Scholar]
- 17.Starosta RT, at al. (2021) Liver manifestations in a cohort of 39 patients with congenital disorders of glycosylation: pin-pointing the characteristics of liver injury and proposing recommendations for follow-up. Orphanet J Rare Dis.16(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen MCH et al. (2014) Successful Liver Transplantation and Long-Term Follow-up in a Patient With MPI-CDG. Pediatrics 134 (1), e279–e283 [DOI] [PubMed] [Google Scholar]
- 19.Zeevaert R et al. (2016) PGM1 deficiency diagnosed during an endocrine work-up of low IGF-1 mediated growth failure. Acta Clin Belg 71 (6), 435–437. [DOI] [PubMed] [Google Scholar]
- 20.Moravej H et al. (2020) Hypoglycemia in CDG patients due to PMM2 mutations: Follow up on hyperinsulinemic patients. JIMD Rep 51 (1), 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Čechová A, et al. (2021). Should patients with Phosphomannomutase 2-CDG (PMM2-CDG) be screened for adrenal insufficiency? Mol Genet Metab. 133(4):397–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascoal C et al. (2020) CDG and immune response: From bedside to bench and back. J Inherit Metab Dis 43 (1), 90–124. [DOI] [PubMed] [Google Scholar]
- 23.Hüllen A et al. (2021) Congenital disorders of glycosylation with defective fucosylation. J Inherit Metab Dis.44(6) 1441. [DOI] [PubMed] [Google Scholar]
- 24.Lefrère B et al. (2018) [Deep venous thrombosis treated by rivaroxaban in a young patient with type Ia carbohydrate-deficient glycoprotein (CDG) syndrome]. Ann Biol Clin (Paris) 76 (2), 217–223. [DOI] [PubMed] [Google Scholar]
- 25.Linssen M et al. (2013) Thrombotic complications in patients with PMM2-CDG. Mol Genet Metab 109 (1), 107–11.26 [DOI] [PubMed] [Google Scholar]
- 26.Brucker WJ. et al. An emerging role for endothelial barrier support therapy for congenital disorders of glycosylation. J Inherit Metab Dis. 2020. Jul;43(4):880–890. [DOI] [PubMed] [Google Scholar]
- 27.Marques-da-Silva D et al. (2017) Cardiac complications of congenital disorders of glycosylation (CDG): a systematic review of the literature. J Inherit Metab Dis 40 (5), 657–672. [DOI] [PubMed] [Google Scholar]
- 28.Bogdańska A et al. (2021) Clinical, biochemical and molecular phenotype of congenital disorders of glycosylation: long-term follow-up. Orphanet J Rare Dis 16 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noman K, et al. (2020). Clinical outcomes in an adult patient with mannose phosphate isomerase- congenital disorder of glycosylation who discontinued mannose therapy. Mol Genet Metab Rep.;25:100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam C, and Krasnewich DM. (2021) PMM2-CDG. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM, Amemiya A, editors. GeneReviews Seattle (WA): University of Washington, Seattle; 1993–2022. [Google Scholar]
- 31.Altassan R, et al. (2019) International clinical guidelines for the management of phosphomannomutase 2-congenital disorders of glycosylation: Diagnosis, treatment and follow up. J Inherit Metab Dis.42(1):5–28. [DOI] [PubMed] [Google Scholar]
- 32.Schiff M, et al. (2017) Clinical, laboratory and molecular findings and long-term follow-up data in 96 French patients with PMM2-CDG (phosphomannomutase 2-congenital disorder of glycosylation) and review of the literature. J Med Genet.54(12):843–851. [DOI] [PubMed] [Google Scholar]
- 33.Witters P et al. (2019) Long-term follow-up in PMM2-CDG: are we ready to start treatment trials? Genet Med 21 (5), 1181–1188 [DOI] [PubMed] [Google Scholar]
- 34.Conte F et al. (2020) Phosphoglucomutase-1 deficiency: Early presentation, metabolic management and detection in neonatal blood spots. Mol Genet Metab 131 (1–2), 135–146. [DOI] [PubMed] [Google Scholar]
- 35.Daci A et al. (2018) Individualizing Treatment Approaches for Epileptic Patients with Glucose Transporter Type1 (GLUT-1) Deficiency. Int J Mol Sci 19 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taha AY et al. (2010) Polyunsaturated fatty acids and epilepsy. Epilepsia 51 (8), 1348–58. [DOI] [PubMed] [Google Scholar]
- 37.Bayat A et al. (2021) Deep-Phenotyping the Less Severe Spectrum of PIGT Deficiency and Linking the Gene to Myoclonic Atonic Seizures. Front Genet 12, 663643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Scherpenzeel M et al. (2014) Diagnostic serum glycosylation profile in patients with intellectual disability as a result of MAN1B1 deficiency. Brain 137 (Pt 4), 1030–8. [DOI] [PubMed] [Google Scholar]
- 39.Niehues R et al. (1998) Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J Clin Invest 101 (7), 1414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brasil S et al. (2018) CDG Therapies: From Bench to Bedside. Int J Mol Sci 19 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma V et al. (2014) Mannose metabolism: more than meets the eye. Biochem Biophys Res Commun 453 (2), 220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroeder AS et al. (2010) Seizures and stupor during intravenous mannose therapy in a patient with CDG syndrome type 1b (MPI-CDG). J Inherit Metab Dis 33 Suppl 3, S497–502. [DOI] [PubMed] [Google Scholar]
- 43.Mention K et al. (2008) Development of liver disease despite mannose treatment in two patients with CDG-Ib. Mol Genet Metab 93 (1), 40–3. [DOI] [PubMed] [Google Scholar]
- 44.Babovic-Vuksanovic D et al. (1999) Severe hypoglycemia as a presenting symptom of carbohydrate-deficient glycoprotein syndrome. J Pediatr 135 (6), 775–81. [DOI] [PubMed] [Google Scholar]
- 45.Radenkovic S, et al. (2019). The Metabolic Map into the Pathomechanism and Treatment of PGM1-CDG. Am J Hum Genet. 2019 May 2;104(5):835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perales-Clemente E, et al. (2021). A new D-galactose treatment monitoring index for PGM1-CDG. J Inherit Metab Dis.44(5):1263–127147. [DOI] [PubMed] [Google Scholar]
- 47.Wong SY et al. (2017) Oral D-galactose supplementation in PGM1-CDG. Genet Med 19 (11), 1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrapers E et al. (2016) News on Clinical Details and Treatment in PGM1-CDG. JIMD Rep 26, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voermans NC et al. (2017) PGM1 deficiency: Substrate use during exercise and effect of treatment with galactose. Neuromuscul Disord 27 (4), 370–376. [DOI] [PubMed] [Google Scholar]
- 50.Nolting K et al. (2017) Limitations of galactose therapy in phosphoglucomutase 1 deficiency. Mol Genet Metab Rep 13, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donoghue SE et al. (2021) Galactose treatment of a PGM1 patient presenting with restrictive cardiomyopathy. JIMD Rep 57 (1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tegtmeyer LC et al. (2014) Multiple phenotypes in phosphoglucomutase 1 deficiency. N Engl J Med 370 (6), 533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altassan R et al. (2021) International consensus guidelines for phosphoglucomutase 1 deficiency (PGM1-CDG): Diagnosis, follow-up, and management. J Inherit Metab Dis 44 (1), 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokoi K et al. (2019) Disruption of the Responsible Gene in a Phosphoglucomutase 1 Deficiency Patient by Homozygous Chromosomal Inversion. JIMD Rep 43, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morava E (2014) Galactose supplementation in phosphoglucomutase-1 deficiency; review and outlook for a novel treatable CDG. Mol Genet Metab 112 (4), 275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becker DJ and Lowe JB (2003) Fucose: biosynthesis and biological function in mammals. Glycobiology 13 (7), 41R–53R. [DOI] [PubMed] [Google Scholar]
- 57.Marquardt T et al. (1999) Correction of leukocyte adhesion deficiency type II with oral fucose. Blood 94 (12), 3976–85. [PubMed] [Google Scholar]
- 58.Lühn K et al. (2001) The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet 28 (1), 69–72. [DOI] [PubMed] [Google Scholar]
- 59.Etzioni A et al. (2002) Leukocyte adhesion deficiency (LAD) type II/carbohydrate deficient glycoprotein (CDG) IIc founder effect and genotype/phenotype correlation. Am J Med Genet 110 (2), 131–5. [DOI] [PubMed] [Google Scholar]
- 60.Etzioni A and Tonetti M (2000) Fucose supplementation in leukocyte adhesion deficiency type II. Blood 95 (11), 3641–3. [PubMed] [Google Scholar]
- 61.Lee-Sundlov MM et al. (2020) Multifaceted role of glycosylation in transfusion medicine, platelets, and red blood cells. J Thromb Haemost 18 (7), 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hidalgo A et al. (2003) Insights into leukocyte adhesion deficiency type 2 from a novel mutation in the GDP-fucose transporter gene. Blood 101 (5), 1705–12. [DOI] [PubMed] [Google Scholar]
- 63.Shawn Tahata KR, Quage Marie, Boyer Suzanne, League Stacey, Kuman0vicks Attila, Abraham Roshini, Jacob Eapen, Menon Prem, Morava Eva (Forthcoming. 2021) Defining the mild variant of leukocyte adhesion deficiency type II (SLC35C1-congenital disorder of glycosylation) and response to L-fucose therapy: insights from two new families and review of the literature. American Journal of American Genetics Part A. doi: 10.1002/ajmg.a.62737 [DOI] [PubMed] [Google Scholar]
- 64.Ng BG et al. (2018) Biallelic Mutations in FUT8 Cause a Congenital Disorder of Glycosylation with Defective Fucosylation. Am J Hum Genet 102 (1), 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng BG et al. (2020) Expanding the molecular and clinical phenotypes of FUT8-CDG. J Inherit Metab Dis 43 (4), 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park JH et al. (2020) L-Fucose treatment of FUT8-CDG. Mol Genet Metab Rep 25, 100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Witters P et al. (2020) Clinical and biochemical improvement with galactose supplementation in SLC35A2-CDG. Genet Med 22 (6), 1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dörre K et al. (2015) A new case of UDP-galactose transporter deficiency (SLC35A2-CDG): molecular basis, clinical phenotype, and therapeutic approach. J Inherit Metab Dis 38 (5), 931–40. [DOI] [PubMed] [Google Scholar]
- 69.Foulquier F, et al. TMEM165 deficiency causes a congenital disorder of glycosylation. Am J Hum Genet. 2012. Jul 13;91(1):15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park JH et al. (2018) SLC39A8 deficiency: biochemical correction and major clinical improvement by manganese therapy. Genet Med 20 (2), 259–268. [DOI] [PubMed] [Google Scholar]
- 71.Riley LG et al. (2017) A SLC39A8 variant causes manganese deficiency, and glycosylation and mitochondrial disorders. J Inherit Metab Dis 40 (2), 261–269. [DOI] [PubMed] [Google Scholar]
- 72.Park JH et al. (2015) SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. Am J Hum Genet 97 (6), 894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morelle W et al. (2017) Galactose Supplementation in Patients With TMEM165-CDG Rescues the Glycosylation Defects. J Clin Endocrinol Metab 102 (4), 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ng BG et al. (2015) Biallelic mutations in CAD, impair de novo pyrimidine biosynthesis and decrease glycosylation precursors. Hum Mol Genet 24 (11), 3050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rymen D et al. (2020) Expanding the clinical and genetic spectrum of CAD deficiency: an epileptic encephalopathy treatable with uridine supplementation. Genet Med 22 (10), 1589–1597. [DOI] [PubMed] [Google Scholar]
- 76.Koch J et al. (2017) CAD mutations and uridine-responsive epileptic encephalopathy. Brain 140 (2), 279–286. [DOI] [PubMed] [Google Scholar]
- 77.Zhou L et al. (2020) A Patient With CAD Deficiency Responsive to Uridine and Literature Review. Front Neurol 11, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson MD et al. (2006) Hyperphosphatasia with neurologic deficit: a pyridoxine-responsive seizure disorder? Pediatr Neurol 34 (4), 303–7. [DOI] [PubMed] [Google Scholar]
- 79.Kuki I et al. (2013) Vitamin B6-responsive epilepsy due to inherited GPI deficiency. Neurology 81 (16), 1467–9. [DOI] [PubMed] [Google Scholar]
- 80.Almeida AM et al. (2007) Targeted therapy for inherited GPI deficiency. N Engl J Med 356 (16), 1641–7. [DOI] [PubMed] [Google Scholar]
- 81.Pode-Shakked B et al. (2019) Cerebral and portal vein thrombosis, macrocephaly and atypical absence seizures in Glycosylphosphatidyl inositol deficiency due to a PIGM promoter mutation. Mol Genet Metab 128 (1–2), 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joshi C et al. (2016) Ketogenic diet - A novel treatment for early epileptic encephalopathy due to PIGA deficiency. Brain Dev 38 (9), 848–51. [DOI] [PubMed] [Google Scholar]
- 83.Witters P, et al. (2021). Spontaneous improvement of carbohydrate-deficient transferrin in PMM2-CDG without mannose observed in CDG natural history study. Orphanet J Rare Dis. 16(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eklund EA et al. (2005) Hydrophobic Man-1-P derivatives correct abnormal glycosylation in Type I congenital disorder of glycosylation fibroblasts. Glycobiology 15 (11), 1084–93. [DOI] [PubMed] [Google Scholar]
- 85.Monticelli M et al. (2019) β-Glucose-1,6-Bisphosphate Stabilizes Pathological Phophomannomutase2 Mutants In Vitro and Represents a Lead Compound to Develop Pharmacological Chaperones for the Most Common Disorder of Glycosylation, PMM2-CDG. Int J Mol Sci 20 (17) :4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ligezka AN et al. (2021) Sorbitol Is a Severity Biomarker for PMM2-CDG with Therapeutic Implications. Ann Neurol 90 (6) 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nozaki M, et al. Developmental abnormalities of glycosylphosphatidylinositol-anchor-deficient embryos revealed by Cre/loxP system. Lab Invest. 1999. Mar;79(3):293–9. [PubMed] [Google Scholar]
- 88.Tarnowski MS et al. (2018) Height Prediction From Ulna Length of Critically Ill Patients. Nutr Clin Pract 33 (6), 887–892. [DOI] [PubMed] [Google Scholar]
- 89.Fischer M et al. (2015) Evaluation of muscle and fat loss as diagnostic criteria for malnutrition. Nutr Clin Pract 30 (2), 239–48. [DOI] [PubMed] [Google Scholar]
- 90.Čechová A, et al. (2020) Consensus guideline for the diagnosis and management of mannose phosphate isomerase-congenital disorder of glycosylation. J Inherit Metab Dis. 43(4):671–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martínez-Monseny AF et al. (2019) Acetazolamide safety and efficacy in cerebellar syndrome in PMM2 congenital disorder of glycosylation (PMM2-CDG). Ann Neurol. 85(5):740–751. [DOI] [PubMed] [Google Scholar]