Graphical abstract
Global health and human well-being are continuously challenged austerely by the ongoing coronavirus disease 2019 (COVID-19) pandemic. Considering the high number of patients who recovered from COVID-19 worldwide, the health status and the sequelae after recovery, not only in the acute phase of infection, deserve comprehensive attention. Evidence that patients with COVID-19 still have long-term symptoms 1–6 months after hospital discharge indicates that COVID-19 infection can affect multiple organ systems, including the heart, liver, kidneys, muscle, digestive, and nervous functions, not just the respiratory system [1], [2], [3]. Furthermore, potential risk factors associated with long-term outcomes for convalescents have also been identified, such as sex, age, body mass index, and disease severity [4], [5]. However, only a few studies have focused on the self-body perception of convalescents and assessed physical function indicators during a longer recovery period.
Here, a follow-up cohort of the first COVID-19 survivors during the pandemic was designed and the prognosis and recovery were assessed in a group of patients with COVID-19 semiannually from 6 months to 2 years post-discharge. During the last three follow-up visits, epidemiological questionnaires were used to investigate the subjective feelings of convalescents, whereas blood samples were collected for clinical laboratory assessments, including 55 indicators of hemocyte subgroups and multiple organ-related biochemistry. This study potentially provides a more comprehensive understanding of the COVID-19 prognosis (see Supplementary materials online for detailed methods).
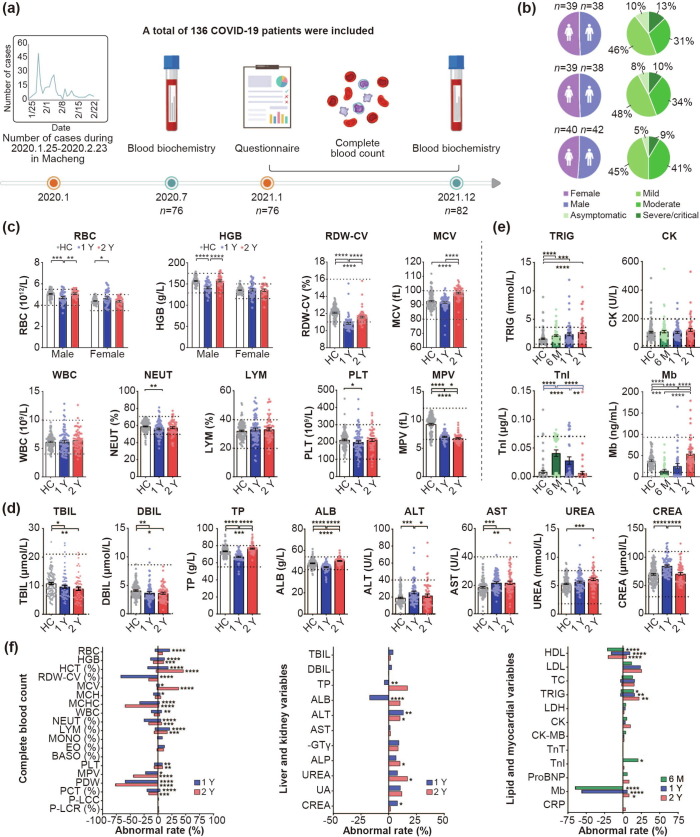
Participants included in this study were patients with COVID-19 (n = 136) infected at the early stages of the pandemic in early 2020 in Macheng, Hubei, China, and a sample of controls without COVID-19 (n = 125). Of the healthy controls, 35 were from the same residential community where the patients reside, and the other 90 were recruited as clear controls in Beijing at the end of 2020, where no cases were reported. Participants in the three follow-ups included asymptomatic, mild, moderate, severe, and critical cases diagnosed during their acute phase (Table S1 online and Fig. 1 ). The study was approved by the Ethics Committee of National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention (No. IVDC2020-021). Written informed consent was obtained from all participants (or legal guardians of minors).
Fig. 1.
Flow diagram and blood test results of the COVID-19 convalescents during 6 months, 1 year, and 2 years follow-ups. (a) Study flow diagram. From January 25 to February 23, 2020, 253 patients with COVID-19 were confirmed in Macheng, Hubei Province, China, of whom 136 were included in the study. During the first follow-up (July 23–28, 2020), 77 participants were involved. Blood biochemical examinations about lipid and myocardial function (n = 76) were performed and some of these indicators were tested among parts of the convalescents (n = 34) because of insufficient blood samples. Until the second follow-up (January 15–16, 2021), 23 and 23 participants were lost to follow-up and were newly included (n = 77), respectively. Questionnaire and complete blood count (n = 76), and blood biochemical examinations about lipid and myocardial function (n = 63) were performed. During the third follow-up (December 21–24, 2021), 46 and 36 participants were successfully followed up and newly enrolled, respectively (n = 82). Questionnaire (n = 80) and all blood examinations (n = 59) were performed. (b) Sex composition and clinical types of participants. (c–d) Comparison of blood cell counts (c) and liver- and kidney-related variables (d) between 1- and 2-year COVID-19 convalescents and healthy controls. HC: healthy control (white, n = 125). 1 Y: About 1 year post disease onset (blue, n = 76); 2 Y: About 2 years post disease onset (red, n = 59). (e) Lipid and myocardial-related variables of 6-month, 1- and 2-year COVID-19 convalescents were compared with healthy controls. 6 M: 6-month (green, n = 76 for TRIG and CK, n = 34 for TnI and Mb due to insufficient blood sample), 1-year (blue, n = 63 for TRIG and CK, n = 34 for TnI and Mb), 2-year COVID-19 convalescents (red, n = 59) and healthy controls (white, n = 125 for TRIG and CK, n = 75 for TnI and Mb). Each point corresponds to an individual, and bar values represent the mean and standard error of the mean. The dashed lines indicate the normal reference range issued by the testing institution. Approximately >25% (28.8%, 23.2%) of healthy controls corresponding to the ALB and TRIG indicators did not meet the reference range; thus, the normal range of these indicators was set as the 5%–95% percentile range of the healthy controls. (f) The normal rate of each indicator of the convalescents. The right side of the line at 0 is the proportion higher than the reference normal values issued by the testing institution, whereas the left side means lower. The asterisk indicates a significant abnormal rate compared with the corresponding healthy controls. Mann–Whitney U test was used for (c–e) and Chi-square or Fisher’s exact test was used for (f). Two-tailed P-values were calculated. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. RBC, red blood cell; HGB, hemoglobin; RDW-CV, red-blood-cell distribution width-coefficient of variation; MCV, mean corpuscular volume; WBC, white blood cell; NEUT, neutrophil; LYM, lymphocyte; PLT, platelet; MPV, mean platelet volume; TBIL, total bilirubin; DBIL, direct bilirubin; TP, total protein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CREA, creatinine; TRIG, triglyceride; CK, creatine kinase; TnI, troponin I; Mb, myoglobin; HCT, hematocrit; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MONO, monocyte; EO, eosinophil; BASO, basophils; IBIL, indirect bilirubin; GLO, globulin; A/G, albumin/globulin; AST/ALT, aspartate aminotransferase/alanine aminotransferase ratio; γ-GT, gamma-glutamyl transferase; ALP, alkaline phosphatase; UA, uric acid; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TC, total cholesterol; LDH, lactate dehydrogenase; CK-MB, creatine kinase isoenzyme; TnT, troponin T; ProBNP, pro-brain natriuretic peptide; CRP, C-reactive protein.
The questionnaire includes 30 indicators focused on COVID-19 convalescent symptoms 1 year and 2 years post-discharge. About 60.5% (46/76, 95% confidence interval [CI]: 49.5%–71.5%) of COVID-19 convalescents still reported at least one symptom 1 year post-recovery, and 47.5% (38/80, 95% CI: 36.6%–58.4%) reported symptoms at 2 years. At 1 year, the discomforts mainly include fatigue (25/76, 33.9%), myasthenia (19/76, 25.0%), hair loss (17/76, 22.4%), and sleep difficulties (14/76, 18.4%), which are similar to data from previous COVID-19 1-year long-term follow-up [6]. A longitudinal cohort study of COVID-19 survivors showed that 68% of patients had at least one sequelae symptom at 6 months, which decreased to 49% at 12 months post-COVID-19 infection [7]. Simultaneously, inpatient medical records of participants were collected in the acute phase, and results showed that the proportion of patients with underlying diseases was much lower than those with discomfort during the follow-up (Table S2 online). Furthermore, we found that 22 (28.9%) 1-year convalescents presented circulatory symptoms (chill, sweat, palpitations, etc.), 19 (25.0%) physical pain (joint pain, myalgia, chest pain, or headache), and 16 (21.1%) respiratory system-related symptoms (chest distress, shortness of breath, etc.). At 2 years, fatigue (15/80, 18.8%), muscle weakness (7/80, 8.8%), and hair loss (7/80, 8.8%) ratios were significantly lower (P = 0.0431, 0.0065, and 0.0185, respectively). Most 2-year symptoms decreased compared with 1 year (Table 1 ). In general, alopecia can be attributed to systemic diseases, febrile disease, drugs, stressful events, and nutritional deficiencies, among others [8]. This form of chronic alopecia might be caused by multiple factors, including physical infection, psychosocial factors, and even drug therapy [9]. A systematic review of psychiatric effects of the COVID-19 pandemic showed a series of manifestations of mental symptoms about the disease complications, such as stigma, forgetfulness, or traumatic memories of severe illness among people infected with COVID-19 [10]. Especially, the so-called “stress-sensitive” scalp conditions can be affected by increased psychosocial pressures.
Table 1.
Symptoms of the COVID-19 convalescents during 1 year and 2 years follow-ups.
| Item | 1 year | 2 years | P-valuea |
|---|---|---|---|
| n = 76 | n = 80 | ||
| Post-discharge discomfort,bn (%) | 46 (60.5) | 38 (47.5) | 0.103 |
| Fatigue | 25 (33.9) | 15 (18.8) | 0.043c |
| Muscle weakness | 19 (25.0) | 7 (8.8) | 0.007c |
| Hair loss | 17 (22.4) | 7 (8.8) | 0.019c |
| Sleep difficulties | 14 (18.4) | 17 (21.3) | 0.658 |
| Hypertension | 12 (15.8) | 10 (12.5) | 0.555 |
| Decreased appetite | 3 (3.9) | 4 (5.0) | 1.000 |
| Taste disorder | 1 (1.3) | 3 (3.8) | 0.621 |
| Smell disorder | 1 (1.3) | 6 (7.5) | 0.117 |
| mMRC score,dn (%) | |||
| 0 | 45 (59.2) | 60 (75.0) | 0.036c |
| 1 | 27 (35.5) | 15 (18.8) | 0.018c |
| 2 | 3 (3.9) | 5 (6.3) | 0.720 |
| 3 | 1 (1.3) | 0 | 0.487 |
| 4 | 0 | 0 | Not applicable |
| Pain, n (%) | 19 (25.0) | 10 (12.5) | 0.045c |
| Joint pain | 7 (9.2) | 2 (2.5) | 0.092 |
| Myalgia | 6 (7.9) | 2 (2.5) | 0.159 |
| Chest pain | 5 (6.6) | 1 (1.3) | 0.110 |
| Headache | 2 (2.6) | 6 (7.5) | 0.278 |
| Circulatory symptoms, n (%) | 22 (28.9) | 11 (13.8) | 0.020c |
| Feeling cold | 17 (22.4) | 3 (3.8) | 0.001c |
| Excessive sweating when mild exercise/rest/sleep | 11 (14.5) | 7 (8.8) | 0.263 |
| Palpitations | 6 (7.9) | 0 | 0.012c |
| Arrhythmia | 2 (2.6) | 3 (3.8) | 1.000 |
| Respiratory symptoms, n (%) | 16 (21.1) | 24 (30.0) | 0.201 |
| Chest distress | 10 (13.2) | 6 (7.5) | 0.244 |
| Shortness of breath | 6 (7.9) | 7 (8.8) | 0.847 |
| Cough | 4 (5.3) | 8 (10.0) | 0.267 |
| Sore throat or foreign body sensation | 4 (5.3) | 2 (2.5) | 0.434 |
| Sensitive to dust | 0 | 1 (1.3) | 1.000 |
| Gastrointestinal symptoms, n (%) | 9 (11.8) | 2 (2.5) | 0.023c |
| Hematochezia | 3 (3.9) | 0 | 0.113 |
| Constipation | 3 (3.9) | 1 (1.3) | 0.358 |
| Nausea | 2 (2.6) | 1 (1.3) | 0.613 |
| Hemolymphatic symptoms, n (%) | 7 (9.2) | 0 | 0.006c |
| Gum bleeding | 6 (7.9) | 0 | 0.012c |
| Jaundice | 2 (2.6) | 0 | 0.236 |
| Urinary symptoms, n (%) | 6 (7.9) | 3 (3.8) | 0.319 |
| Copious urine | 3 (4.0) | 1 (1.3) | 0.358 |
| Dysuria | 1 (1.3) | 1 (1.3) | 1.000 |
| Urinary calculi | 1 (1.3) | 0 | 0.487 |
| Limb edema | 1 (1.3) | 0 | 0.487 |
| Proteinuria | 0 | 1(1.3) | 1.000 |
| Comorbidities, n (%) | 23 (30.3) | 16 (20.0) | 0.139 |
| Hypertension | 10 (13.2) | 8 (10.0) | 0.537 |
| Diabetes | 5 (6.6) | 4 (5.0) | 0.741 |
| Chronic gastric disease | 3 (3.9) | 2 (2.5) | 0.676 |
| Chronic pharyngitis | 3 (3.9) | 1 (1.3) | 0.358 |
| Chronic liver disease | 2 (2.6) | 2 (2.5) | 1.000 |
| Cardia-cerebrovascular diseases | 2 (2.6) | 2 (2.5) | 1.000 |
| Lung cancer | 2 (2.6) | 2 (2.5) | 1.000 |
P value of Chi-square/Fisher's exact test between 1 year and 2 years.
Overall proportion of convalescents who reported at least one discomfort after discharge.
There are significant differences between the two time points (α = 0.05).
mMRC score: modified British Medical Research Council score. Percentages may not add up to exactly 100% due to rounding. The higher the score (range 0–4), the more severe the dyspnea.
In our study, most hematologic indicators related to blood cell counts of COVID-19 convalescents are in a relatively normal state, such as most of the white blood cell and platelet counts. Numerically, the red blood cell and most related indicators (hemoglobin, red blood cell distribution width-coefficient of variation, mean corpuscular hemoglobin) showed statistical differences between the convalescents and healthy controls, and the 2-year indicators were closer to the healthy controls than 1 year. The overall abnormal rates of red blood cell counts were 26.4% (20/76) and 8.5% (5/59) in 1- and 2-year convalescents, with a statistical difference (P = 0.0149). However, compared with 1 year, the 2-year proportion of mean corpuscular volume above the normal range significantly increased from 2.6% (2/76) to 37.3% (22/59) (P < 0.0001). As for the white blood cells, the total count in convalescents was not different from that of healthy controls and was basically within the normal range. The abnormal white blood cell count rate decreased from 18.4% (14/76) to 10.2% (6/59). Most platelet values were within the normal range and showed no difference between 1 year and 2 years. Regarding the abnormal rates, 1- and 2-year platelet abnormalities were similar, i.e., 14.5% (11/76) and 15.3% (9/59), respectively. The percentage of mean platelet volume below the normal range at 2 years (22.4%,17/76) was higher than in 1 year (44.1%, 26/59) (P = 0.0092) (Fig. 1c,f and Table S3 online). Several reports indicated that white blood cells, lymphocytes, neutrophils, red blood cells, hemoglobin, and platelets of patients initially decrease in the acute phase of COVID-19 infection, followed by increased leukocytes and neutrophils [11]. At 6 months post-discharge, Huang et al. [12] found that peripheral blood lymphocyte counts in the recovered patients were generally >0.8 × 109/L. In our patients with 1- and 2-year convalescents, although neutrophils were still lower than the normal level in some patients, they were fully recovered at 2 years compared with that at 1 year, and the lymphocyte count was also within the normal range (0.8 × 109/L–4 × 109/L). For reference, our previous study has shown that both severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2)-specific CD4+ and CD8+ T lymphocytes of convalescents lasted at least 1 year [13]. Indicators related to red blood cells also showed recovery. Most abnormal parameters in the acute stage became normal (white blood cell, lymphocyte, and platelet) (Fig. S1a online). In this sense, most patients’ prognoses are assumed to be relatively good as the “cytokine storm” caused by viral infection may be subsiding or has subsided.
As for liver- and kidney function-related laboratory factors, the total and direct bilirubin, blood uric acid, aspartate aminotransferase, and gamma-glutamyl transferase were basically within the normal values in the COVID-19 convalescents for both 1 and 2 years. The total protein and albumin levels were lower at 1 year (P < 0.0001), but were closer to the level of healthy controls at 2 years. Alanine aminotransferase and creatinine levels were also significantly recovered from 1 to 2 years, and the abnormal rate (all higher) decreased from 13.2% (10/76) to 10.2% (6/59), and from 7.9% (6/76) to 1.7% (1/59), respectively. Although the rate of urea abnormality at 2 years was higher than that in healthy controls (P = 0.0257), no difference was observed from that at 1-year recovery phase (Fig. 1d, f and Table S3 online). Qing et al. [14] found that 12%–25% of the surveyed patients with COVID-19 had elevated markers during hospitalization. We found that some cases of liver and kidney injury post-COVID-19 infection may have lasted for a year or two, and fortunately are recovering (Fig.S1b online). It is hypothesized that direct viral injury or drug-induced injury might lead to organ dysfunction in hospitalized patients.
In blood lipid and myocardial indicators, triglyceride values at 6 months, 1 year, and 2 years gradually increased and were significantly higher in the patient group than that in healthy controls (P < 0.05), with abnormal rates of 17.1% (13/76), 19.1% (12/63), and 22.0% (13/59), respectively. The average troponin I level shows a downward trend from 6 months to 2 years, with abnormal (higher) rates of 20.6% (7/76) at 6 months, 0 at 1 year, and 1.7% (1/59) at 2 years. The overall myoglobin trend gradually increases over time with the abnormal lower rates of 64.7% (22/34) at 6 months, 55.9% (19/34) and higher 5.9% (2/63) at 1 year, and 8.5% (5/59) at 2 years (Fig. 1e, f and Table S4 online). Myocardial injury and inflammatory response-related indicators, such as creatine kinase, lactate dehydrogenase, troponin I, pro-brain natriuretic peptide, and C-reactive protein, are significantly higher in patients with COVID-19 at an acute stage. In our study, continuous follow-up data showed that the majority of 1- and 2-year COVID-19 convalescents had normalized these indicators, particularly troponin I. Some participants with critical troponin I levels in 6 months gradually confluent with healthy levels at 1 and 2 years. During the dynamic tracking of some biochemical markers at 6 months to 2 years post-COVID-19, the conclusion of abnormal lipid metabolism and myocardial function impairment was confirmed during the recovery period (Fig. S1c online), which verified the conclusion of a proteomic analysis by Chen et al. [15]. In particular, triglyceride levels in convalescents tended to deviate more from normal over time.
To investigate the potential impact of the disease severity of the patients during the acute phase on the duration and health status of after recovery, we conducted stratified analysis and the results showed that few significant differences were found among the patients with different severity (Fig. S2 online).
Since the COVID-19 outbreak, the clinical characterization of COVID-19 convalescents showed a certain level of sequelae persistence [2]. We gathered characteristics and laboratory test results from the first group of patients with COVID-19 during the pandemic in their convalescent stage from 6 months to 2 years post-recovery. Overall, the convalescents had well physical recovery from their illness, and most of convalescent indicators were within the normal range, with some markers observed moving closer to healthy levels over time from 1 to 2 years post-recovery. However, some convalescents still had physical symptoms and showed incomplete recovery of some blood parameters (e.g., triglyceride, myoglobin, alkaline phosphatase).
Our study has several limitations. First, although over 50% of the patients in Macheng have been recruited within our convalescent cohort in the study, the sample size is still small, especially considering the ones who fully participated in all three consecutive follow-up visits. However, when participants at all three consecutive follow-up visits were extracted for additional analysis, findings were consistent with that of the existing results (Table S5, Fig. S3 online). Second, the healthy controls in this study did not match well with the COVID-19 convalescents, especially the concomitant diseases. Nevertheless, this study mainly used the before-after self-comparison based on the longitudinal results involving 6 months to 24 months. Furthermore, we used both data from the healthy populations from the local community of Macheng and Beijing where no epidemic was reported in early 2020. Third, the presence or absence of symptoms was investigated solely based on the questionnaire.
This study involved a cohort of COVID-19 convalescents who recovered from the first wave of the pandemic and examined both symptoms and accessible laboratory tests. We found that during the period from 6 months to 2 year-recovery, most of the health indicators recovered, but some did not fully return to normal. Through the long-term follow-up, this study provides new insights into the prognosis of COVID-19 patients. Broader follow-up populations of COVID-19 and long-term longitudinal studies are needed to further address the continuing effects of SARS-COV-2 in convalescents.
Author contributions
Guizhen Wu, William J. Liu, and George F. Gao designed and supervised the study. Hao Lin, Xueyuan Liu, Jie Zhang, and Yaxin Guo designed the questionnaire. Jie Zhang, Xueyuan Liu, Maoshun Liu, Lei Li, Jinmin Tian, and Yaxin Guo collected the samples and conducted field investigations. Heqiang Sun, Jie Zhang, Xueyuan Liu, Hao Lin, and Maoshun Liu conducted the sample processing and testing. Hao Lin, Xueyuan Liu, Lei Li, Maoshun Liu, Jinmin Tian, Jinxian Gan, Zhangfu Chen, Xin Wang, Ying Lin, and Danni Zhang conducted data entry. Shaobo Dong, Yaning Liu, Xiaoshan Zhang, Peipei Liu, Ke Xu, Xiangtian Zhou, and Hao Liang provided technical support and investigation assistance. Hao Lin, William J. Liu, and Xueyuan Liu analyzed and interpreted data. Hao Lin, William J. Liu, and Heqiang Sun wrote the initial draft of the manuscript. All authors contributed intellectually and approved the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82161148008 and 81971501), the National Key Research and Development Program of China (2021YFC2301400, 2020YFA0708103, and 2021YFC0863300), and the Excellent Young Scientist Program of the National Natural Science Foundation of China (81822040). We thank Prof. Cuiling Xu for data interpretation assistance.
Biographies

Hao Lin obtained her MPH degree in 2022 at National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention. She graduated from Tongji Medical College, Huazhong University of Science and Technology in 2019, majoring in Preventive Medicine. She received her law degree from Wuhan University in 2019. Her research interest is public health and health statistics.

Xueyuan Liu is a Ph.D. candidate at the School of Public Health, Cheeloo College of Medicine, Shandong University. In 2018, she graduated from the School of Public Health, Qingdao University, with a B.S. degree in Preventive Medicine. Her current interest is development of vaccines against emerging infectious diseases.

Heqiang Sun is an associate chief physician at PLA General Hospital. He accepted the research training at Clinical Laboratory Medicine Department, the Third Military Medical University and Immunology Department, Duke University, and obtained his Ph.D. degree in 2018. His research interest includes infection and immunity, especially H. pylori-associated CD4+ T-cell response.

Jie Zhang obtained her M.S. degree in 2017 at Chinese Academy of Agricultural Sciences, and received her M.D. degree in 2021 from National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention. Her research interest is T-cell immunity in viral diseases.

George F. Gao obtained his DPhil degree from Oxford University, UK, and did his postdoc work at both Oxford University and Harvard University. His research interest includes pathogenic microorganism transmission across hosts, infection mechanisms, and host cellular immunity, as well as public health policy and global health strategy.

William J. Liu graduated from the School of Public Health, Peking University Health Science Center in 2005, majoring in Preventive Medicine. He received his Ph.D. degree from the Institute of Microbiology, Chinese Academy of Sciences (CAS) in 2010. Later on, he underwent postdoctoral training at the Institute of Biophysics, CAS and the Yale School of Medicine. His research interest includes the prevention and control of emerging and re-emerging viral diseases including influenza and COVID-19, as well as T-cell immunity to viruses.

Guizhen Wu is the chief expert on biosafety at Chinese Center for Disease Control and Prevention. She received her B.S. degree in Medicine from Peking University and her M.S. degree from China University of Political Science and Law. Her research interest includes the prevention and control of infectious diseases and the management of laboratory biosafety.
Footnotes
Supplementary materials to this short communication can be found online at https://doi.org/10.1016/j.scib.2022.06.025.
Appendix A. Supplementary materials
The following are the Supplementary data to this article:
References
- 1.Taquet M., Dercon Q., Luciano S., et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773. doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5):e2111417. doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynberg E., van Willigen H.D.G., Dijkstra M., et al. Evolution of COVID-19 symptoms during the first 12 months after illness onset. Clin Infect Dis. 2021:ciab759. doi: 10.1093/cid/ciab759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izcovich A., Ragusa M.A., Tortosa F., et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15(11):e0241955. doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klok F.A., Boon G.J.A.M., Barco S., et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56(1):2001494. doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L., Yao Q., Gu X., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S.W., Juhasz M., Mobasher P., et al. A systematic review of topical finasteride in the treatment of androgenetic alopecia in men and women. J Drugs Dermatol. 2018;17(4):457–463. [PMC free article] [PubMed] [Google Scholar]
- 9.Trüeb R.M., Dutra Rezende H., Gavazzoni Dias M.F.R. What can the hair tell us about COVID-19? Exp Dermatol. 2021;30(2):288–290. doi: 10.1111/exd.14259. [DOI] [PubMed] [Google Scholar]
- 10.Rogers J.P., Chesney E., Oliver D., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu G., Wang J. Dynamic changes in routine blood parameters of a severe COVID-19 case. Clin Chim Acta. 2020;508:98–102. doi: 10.1016/j.cca.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J., Lin H., Ye B., et al. One-year sustained cellular and humoral immunities of COVID-19 convalescents. Clin Infect Dis. 2021:ciab884. doi: 10.1093/cid/ciab884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Q., Huang D., Yu H., et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y., Zhang N., Zhang J., et al. Immune response pattern across the asymptomatic, symptomatic and convalescent periods of COVID-19. Biochim Biophys Acta Proteins Proteom. 2022;1870(2):140736. doi: 10.1016/j.bbapap.2021.140736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.