Abstract
Objective:
The co-infection of HCV/CMV may accelerate the progression of liver diseases and worsen responsiveness to IFN treatment. The Direct-acting antiviral agents (DAAs), currently approved therapy for HCV, may cause a transient change in immune status, favoring the reactivation of other viruses. The current study aims to evaluate the impact of DAAs treatment on the reactivation of latent CMV in HCV patients.
Methods:
The serological IgG, IgM Abs against CMV were detected by ELISA on192 HCV patients. The seronegative CMV IgM patients received (sofosbuvir/daclatasvir) regimen, then the CMV reactivation was examined by measuring the CMV IgM by ELISA and CMV DNA by real-time PCR.
Results:
The serological data revealed that all patients were positive for CMV IgG (100%) while (64%) patients were positive for CMV IgM. The seronegative CMV IgM (36%) received the DAAs protocol. The sustained virological response was monitored by measuring the HCV RNA viremia in the patient sera. The serological data revealed that 28.6% of patients had a reactivation of CMV, while 18.5% of patients had detectable CMV DNA viremia. Moreover, there was a significant improvement in liver function as well as a decrease in FIB-4 and APRI scores at EOT. SVR was reached 97.4% among the total studied patients (N= 192).
Conclusion:
CMV co-infection has no impact on the response rate to DAAs. However, the CMV reactivation might have occurred after the complete eradication of HCV by DAAs.
Key Words: Herpes virus, reactivation, direct-acting antiviral, Hepatitis C virus
Introduction
The liver is the main target organ for the hepatitis C virus HCV. The majority of people infected with HCV do not clear the infection on their own, leading to a stage of chronic infection. Chronic infection with Hepatitis C is a major public health problem that affecting 1% of the world’s population and causing long-term liver inflammation, which leads to advanced liver diseases such as liver fibrosis, cirrhosis, and HCC (Cooke et al., 2019). HCV infects approximately 71 million people worldwide, but only 20–30% of those infected develop liver cirrhosis, and only 1–4% of cirrhotic patients develop HCC each year (Gower et al., 2014). Egypt has a high incidence of chronic HCV infection, with an estimated 15% of the population infected, with HCV genotype 4 (Guerra et al., 2012; Shiha et al., 2020). Lately, the standard of care (SOC) for HCV infected patients was a combination of pegylated interferon plus ribavirin, given for 24- to 48-weeks based on HCV genotype (Alexopoulou and Karayiannis, 2015). However, the response rate of the SOC is limited based on the ethnicity and HCV genotyping. The current standard therapy for hepatitis C is quite effective in patients with HCV genotype 2 or 3 infection, resulting in a sustained virologic response in approximately 80%-90% of treated patients. However, about 50 percent of treated HCV infected patients with genotypes 1 or 4 can achieve SVR (El-Awady et al., 2003; Aman et al., 2012). Then, subsequent progresses in the HCV treatment field have been occurred and leading to the discovery of the DAAs. The DAAs therapy showed multiple advantages
over (SOC) such as low duration of therapy to 12-24 weeks, reduced side effects and enhanced outcomes, with a high rate of sustained virological response (85-95%) than SOC treatment (Breban et al., 2014). Despite their potency, a recent study found that DAAs treatment could reactivate Hepatitis B virus (HBV) infection in HBV/HCV co-infected patients (Fabbri et al., 2017). Perello et al also found that herpes virus reactivation occurs in DAA-treated patients (Perello et al., 2016). Marocco et al also documented herpetic reactivations in four HIV-HCV co-infected patients within three to eight weeks of initiating DAA medication (Marocco et al., 2016). Immune changes produced by HCV eradication have been linked to down-regulation of interferon (IFN)-stimulated genes, which can lead to the reactivation of other viruses like herpes virus. CMV is one of the most omnipresent herpes viruses, with an incidence of up to 60-80% in humans. The virus produces a life-long latent infection upon primary infection. During the suppression of the immune system induced by drugs, autoimmune diseases, and /or bacterial infection ,viral reactivation may be occurred (Mohamed et al., 2017). CMV latency and reactivation appear to be a complicated process that lacks a full understanding of the pathways that are triggered by clinical variables (Grinde, 2013). CMV / HCV co-infection among Egyptian patients was reported to be around 68 % higher than other populations (Tabll et al., 2011). It was reported that the co-infection of CMV /HCV decreased the response rate to interferon therapy (Bader el-Din et al., 2011) and to block many inflammatory cytokines in HCV patients (El-Meguid et al., 2020). In some patients, treatment with a DAA may cause a sudden change in immune status, favoring the reactivation of Herpes Virus (HV). However, the exact mechanisms of HV reactivation in patients treated with DAAs during the early stages of HCV clearance are unknown (Ghweil and Helal, 2019). Patients treated with DAA may encounter unexpected events, necessitating additional research to approve or rebut causality and identify actual incidences; a broader scope and dedicated designs are needed to assess unexpected risks of direct-acting antiviral treatment. In the current study we aim to evaluate the impact of DAAs treatment on CMV reactivation in chronic HCV patients at the end of treatment, as well as the treatment response towards DAAs in HCV/CMV co-infection.
Materials and Methods
Patient cohort
The prospective study was conducted at General Fayoum hospital on one hundred and ninety two Egyptian patients with CHC treated with DAAs between July 2018 and October 2019. All eligible patients were enrolled in this study based on the inclusion criteria: age ≥ 18 years, HCV RNA positivity, only treatment-naïve patients. Patients with hepatitis B virus (HBV) co-infection, decompensated liver cirrhosis, inadequately controlled diabetes mellitus (HbA1c >9%), hepatocellular carcinoma, or extra-hepatic malignancy were excluded from the study.
All the recruited patients have seropositive for anti-HCV IgG antibodies and serum HCV, The clinical examination, and routine laboratory workup at baseline, at end of treatment (EOT) (W12) and 12 weeks after EOT (W24) have been performed for all patients. According to the approved treatment recommendations, all patients were treated daily with Sofosbuvir (400 mg) and Daclatasvir (60 mg) regimens for 12 weeks.
Ethics
The study was conducted in accordance with the Helsinki Declaration and approved by the Faculty of Medicine’s Department of Ethical Committee, Fayoum University (#IRB –D-153). Before starting treatment and collecting blood samples, signed written informed consent was taken from all patients.
Assessment of scores liver fibrosis
The degree of fibrosis in all patients was assessed by FIB-4 and APRI scores at the beginning of treatment with DAAs, at the EOT and 3 months after EOT.
FIB4 score
Sterling’s formula was used to calculate the FIB4 score: Age (years) × AST (IU/l) / PLT count (×109/l) × ALT (IU/l)) (Martínez et al., 2011). For advanced fibrosis, the FIB4 cut-off of more than 3.25 had a positive predictive value (PPV) of 65% and the cut-off of less than 1.45 had a negative predictive value (NPV) of 90% for advanced fibrosis and cirrhosis. Three stages of the FIB-4 index were classified as follows: No significant fibrosis (FIB-4 <1.45), Mild to significant fibrosis (FIB-4 from 1.45 to 3.25) and advanced fibrosis (FIB-4 ≥ 3.25) (Sterling et al., 2006).
Aspartate aminotransferase-to-platelet ratio index (APRI)
Wai’s formula was used to calculate the APRI score: (AST/upper limit of normal) / PLT count (expressed as PLTs ×109/l) ×100 (Wai et al., 2003). A cut-off of APRI greater than 1.0 predicts cirrhosis, whereas a cut-off greater than 0.7 predicts hepatic fibrosis.
Detection of HCV RNA by real time PCR
Extraction of HCV-RNA from sera samples was assessed using QIAamp Viral RNA kit (Qiagen; Cat. No. 52904) according to standard manufacturer’s instructions, then the detection of HCV confirmed by one-step, real time RT-PCR, by using Artus HCV QS RGQ Kit (Qiagen) following the manufacturer’s protocols. The thermal profile of amplification is: initial incubation for 30 min at 51°C, a second step of 10 min at 95°C, followed by 50 cycles of 30 s at 95°C and 1 min at 60°C, followed by 40 cycles at 95°C for 15 s, 60°C for 1 min and 72°C for 30 s. Fluorescence signal detection was performed at annealing/extension step of each cycle Amplification will perform using Rotor Gene real-time PCR (Qiagen, Santa Clarita, CA).
Follow up
Clinical follow up was implemented at Tropical Medicine department, Fayoum University and Fayoum specialized center for viral hepatitis treatment. All patients were followed up during treatment, at the EOT and 12 weeks after end of treatment.
The CMV IgM Ab concentration was assessed to evaluate the reactivation of CMV after DAAs treatment. Furthermore, for patients with positive CMV IgM, the CMV DNA was evaluated by quantitative PCR amplification.
Cytomegalovirus IgG/IgM antibodies detection by ELISA
Sera samples were tested with Commercial ELISA IMMUNOLAB Cytomegaly IgG/IgM kits (Gmbh, Kassel, Germany) for CMV-specific IgG and IgM Abs, in accordance with the procedure described by the manufacturer. In brief, the diluted sera and calibrators are pipetted into the wells of the microtiter plate coated with a monoclonal antibody specific for the tested proteins (IgG and IgM). Plates were incubated for 1 hour, rinsed with diluted washing buffer, and then anti-human-IgG peroxidase or anti-human-IgM peroxidase conjugate is added and incubated for 30 minutes. Plates were then washed and treated with substrate (TMB) for 20 minutes, causing a blue dye to form in the wells. After that, the stop solution is applied, and the color is changed from blue to yellow and absorbance was measured at 450 nm using an ELx800 Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VE, USA).
Sera protein concentration of (IgG and IgM) was calculated by comparing absorbance of the sample to the standard curve. The concentration of the IgG or IgM abs is directly proportional to the intensity of the color.
Amplification of CMV DNA by Real Time PCR
Whole blood was withdrawn from all subjects and genomic DNA was extracted using genomic DNA Extraction kit (QIAGEN, Germantown, MD, USA) as recommended by the manufacturer. Detection of CMV viral load was carried out using the Artus CMV PCR assay according to the manufacturer’s instructions (QIAGEN). The following conditions were used for the amplification reactions: initial incubation for 10 min at 95°C, followed by 45 cycles of 95°C for 15 sec and 55°C for 1 min. The real-time Rotor Gene PCR was used for amplification (Qiagen, Santa Clarita, CA). Samples were reported as positive samples with detectable viruses quantified at >150 copies/ml.
Statistical analysis
The collected data was organized, tabulated and statistically analyzed using SPSS software statistical computer package version 22 (SPSS Inc, USA). For quantitative data, the mean, standard deviation (SD) and range were calculated. Independent t-test was used to test the differences between any two groups of patients. Repeated measures ANOVA was used in comparing between the readings of study variables at different times. For qualitative data the number and percent distribution was calculated, chi-squared test was used as test of significance. Significance was adopted at P ≤ 0.05.
Results
Patient Characteristics
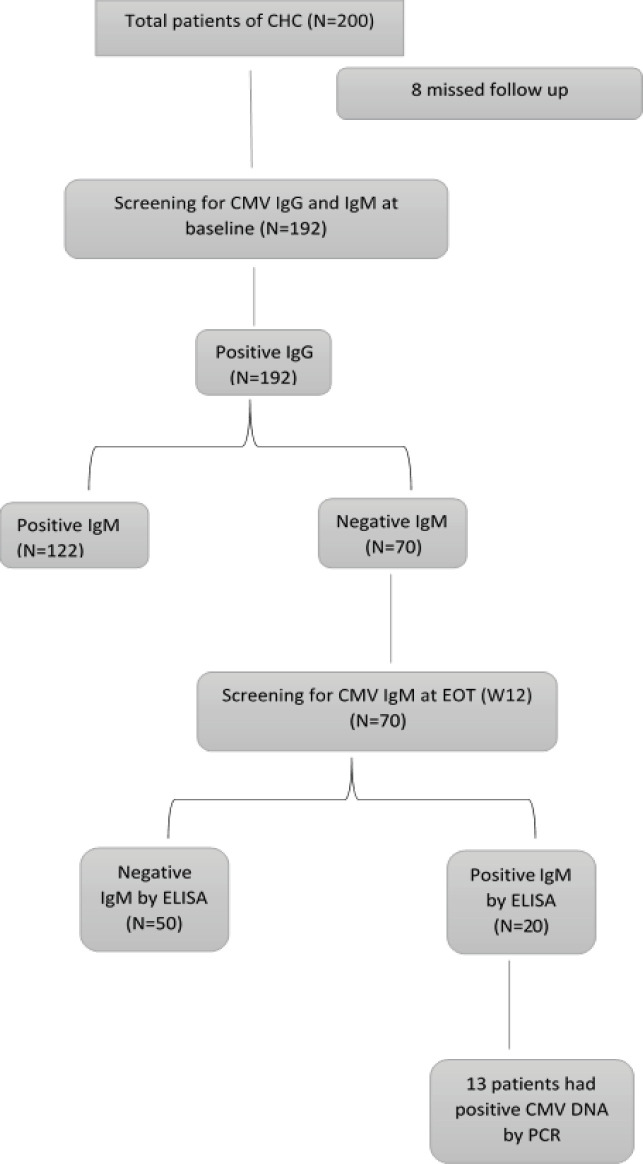
Initially, 200 patients with CHC will be enrolled in this study, eight patients were excluded from the analysis, and 192 patients were screened for CMV IgG, IgM Abs before the DAAs treatment. All patients were positive for CMV IgG (100%) while 122 patients were positive for CMV IgM (64%), and 70 patients had negative CMV IgM (36%) as illustrated in Figure 1. Then the study was conducted on the seventy patients with CHC who had past exposure to CMV infection at baseline (W0), and did not show recent infection. The treatment regimen was (SOF-DCV) for three months. Patients were followed up at EOT (W12) and retested for CMV IgM to evaluate the CMV reactivation. Reactivation of CMV was confirmed by CMV DNA PCR amplification.
Figure 1.
Flow Chart of Patients Included in the Study
Clinical characteristic of enrolled patients
The laboratory investigations of all CHC patients (at baseline (W0), at end of treatment (EOT) (W12), and after 12 weeks of EOT (W24)) were outlined in Table 1. The data revealed that there was statistically significant increase in PLT count and albumin level after treatment (W12 and 24) relative to baseline. Moreover, significant decrease in liver enzymes, Bilirubin, INR, HbA1C was observed after the treatment (W12 and 24) when compared to baseline. We have noticed a remarkable improvement in the fibrosis grades through the FIB4 and APRI scores after the treatment (W12 and 24) compared to baseline.
Table 1.
Comparison of Clinical Investigations before and after Treatment (WO, W12 &W24) among All Studied Patients (N=192)
| Clinical parameters | Baseline (W0) Mean ±SD |
Week 12 Mean ±SD |
Mean Change (%) (baseline vs. 12) P-value |
Week 24 Mean ±SD |
Mean change (%) (12 vs. 24) P-value |
Mean change (%) (baseline vs. 24) P-value |
|---|---|---|---|---|---|---|
| HGB ͣ (g/dl) | 12.85±1.63 | 12.64±1.51 | 1.6 | 12.98±1.58 | 2.7 | 1 |
| <0.0001 | <0.0001 | 0.005 | ||||
| WBCs ͣ (×103/mm3) | 6.04 ±1.69 | 6.03±1.65 | -0.2 | 6.05±1.56 | 0.3 | 0.2 |
| 0.996 | 0.892 | 0.99 | ||||
| PLT ͣ (×103/mm3) | 199.39 ±66.33 | 210.87±69.89 | 5.8 | 213.63±69.46 | 1.3 | 7.1 |
| <0.0001 | 0.174 | <0.0001 | ||||
| AST ͣ (U/L) | 57.93 ±36.29 | 31.77±12.5 | -45.2 | 27.87±10.36 | -12.3 | -51.9 |
| <0.0001 | <0.0001 | <0.0001 | ||||
| ALT ͣ (U/L) | 56.76± 43.87 | 29.61±14.2 | -47.8 | 27.17±17.68 | -8.2 | -52.1 |
| <0.0001 | 0.178 | <0.0001 | ||||
| Bilirubin (mg/dl) | 0.87 ±0.45 | 0.7±0.39 | -19.5 | 0.66±0.37 | -5.7 | -24.1 |
| <0.0001 | 0.002 | <0.0001 | ||||
| Albumin ͣ (g/dl) | 4.01 ±0.43 | 4.1±0.44 | 2.2 | 4.14± 0.42 | 1 | 3.2 |
| 0.001 | 0.001 | <0.0001 | ||||
| INR ͣ | 1.07 ±0.11 | 1.05±0.09 | -1.9 | 1.04 ±0.08 | -1 | -2.8 |
| 0.007 | 0.01 | <0.0001 | ||||
| HbA1C ͣ | 7.55± 0.87 | 7.14±0.86 | -5.4 | 7 ±0.82 | -2 | -7.3 |
| <0.0001 | <0.0001 | <0.0001 | ||||
| FIB4 ͣ | 2.35 ±2.12 | 1.59±1.19 | 32.3 | 1.42 ±1.12 | -10.7 | -39.6 |
| <0.0001 | <0.0001 | <0.0001 | ||||
| APRI ͣ | 0.7 ±0.63 | 0.35±0.24 | -50 | 0.3 ±0.22 | -14.3 | -57.1 |
| <0.0001 | <0.0001 | <0.0001 |
Data are expressed in Mean (±SD). HGB, hemoglobin; WBC, white blood cell count; PLT, platelet; ALT, alanine transaminase;, AST, aspartate transaminase; INR, international normalized ratio; Bold values are significant (Significant if P value <0.05and highly significant if the P value <0.001).
Comparison between patients with reactivated CMV and latent CMV at end of treatment (W12)
Comparison between patients with reactivated CMV and latent CMV at EOT regarding baseline socio-demographic characteristics, laboratory investigations are shown in Table 2. Age, BMI (Body mass index), WBCs (white blood cells counts) albumin, Bilirubin as well as AFP (Alfa fetoprotein) did not show any significant difference between Reactivated CMV patients and latent CMV patients. Reactivated CMV patients showed significantly elevated levels in platelets count compared to latent CMV patients. Also, Reactivated CMV patients showed significantly lower of hemoglobin level and liver enzymes (ALT& AST). Moreover, (65%) of CMV Reactivated patients were females.
Table 2.
Characteristics in Patients with Reactivated or Latent CMV
| Clinical parameters | Latent CMV (N=50) | Reactivated CMV (N=20) | P-value |
|---|---|---|---|
| Mean ±SD | Mean ±SD | ||
| Age ͣ (years) | 47.46± 14.9 | 44.9±13.84 | 0.51 |
| BMI ͣ | 26.61± 4.97 | 27.36±6.14 | 0.598 |
| Sex (male/ female) | 34/16 | 13-Jul | 0.016 |
| HB ͣ (g/dl) | 13.3 ±1.88 | 12.44±1.24 | 0.031 |
| WBCs ͣ (×103/mm3) | 5.81 ±1.73 | 6.06±2.04 | 0.596 |
| PLT ͣ (×103/mm3) | 186.98 ±69.12 | 220.6±56.14 | 0.04 |
| ALT ͣ (ULN:50 U/L) | 65.27 ±42.79 | 44.48±22.84 | 0.011 |
| AST ͣ (ULN:50 U/L) | 66.91± 42.5 | 43.88±16.17 | 0.002 |
| Bilirubin ͣ (mg/dl) | 0.87 ±0.52 | 0.86±0.57 | 0.945 |
| Albumin ͣ (g/dl) | 4.04± 0.43 | 4.06±0.34 | 0.837 |
| AFP a (ng/dl) | 7.05 ±13.72 | 7.6± 10.03 | 0.871 |
| HCV PCR at (W0) (IU) | 964653.9± 1696192 | 1151189±1270159 | 0.659 |
| CMV DNA at (W12) (copies/ml) | ˂150 | N=7 N=13 ˂150 3228.85±2289.44 |
0.067 |
| FIB4 ͣ | 2.75± 2.68 | 1.42±0.59 | 0.002 |
| APRI ͣ | 0.86± 0.7 | 0.41±0.18 | <0.0001 |
ͣ Data are given in mean (M) and standard of deviation (SD); BMI, body mass index; HB, Hemoglobin; WBC, White Blood Cells; PLT, Platelet; ALT, Alanine Transaminase; AST, Aspartate Transaminase; AFP, Alfa fetoprotein; Bold values are significant (Significant if P value <0.05 and highly significant if the P value <0.001).
In addition, HCV RNA levels prior to treatment were high in both reactivated CMV and latent patients. CMV DNA viremia was higher in reactivated CMV patients compared to latent patients at EOT (W12). Patients with reactivated CMV had remarkable lower FIB-4 and APRI scores (1.42 &0.41 respectively) compared to latent CMV patients (2.75 and 0.86 respectively) as shown in table (2).
Impact of CMV infection on response to DAAs treatment
The efficiency of DAAs regimen in treating CHC infection was evaluated at the end of the12th week after the completion of therapy. The rate of SVR among total studied patients was 97.4%.
The efficiency of DAAs was evaluated based on the CMV reactivation before and after treatment (W0 and W12). Patients with active CMV at the beginning of treatment (W0) have shown that the SVR was 98.4% while it was 95.7% in patients with latent CMV. Furthermore, Patients with active CMV at the EOT (W12) have shown that the SVR was 100% while it was 94% in patients with latent CMV as shown in table 3. The present data revealed that the presence of active CMV infection either before or after treatment had no effect on the response to DAAs treatment.
Table 3.
SVR and Relapse Rates in the Studied Patients
| Variable | (N) of patients studied | SVR | |
|---|---|---|---|
| N | % | ||
| Total patients studied | 192 | 187 | 97.40% |
| Patients with positive CMV IgM at W0 | 122 | 120 | 98.40% |
| Patients with negative CMV IgM at W0 | 70 | 67 | 95.70% |
| Reactivated CMV at EOT (W12) | 20 | 20 | 100% |
| latent CMV at EOT (W12) | 50 | 47 | 94% |
Impact of DAAs on CMV reactivation
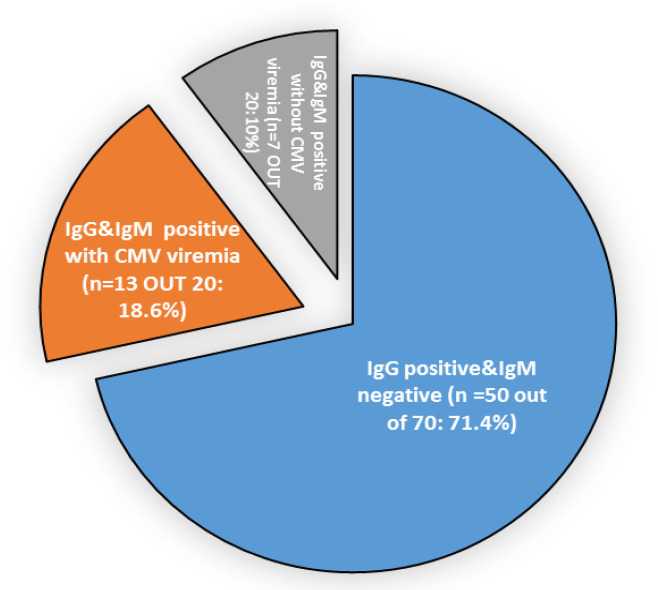
The current study focused on the latent CMV patients to evaluate the role of DAAs to activate the CMV through the immune system dysregulation. At the EOT, the latent CMV patients were reevaluated for the appearance of CMV-IgM. We found that CMV was reactivated in twenty patients (20/70: 28.6%), and these data were confirmed by real time PCR only 13 patients had detectable CMV viremia by Real time PCR (13/20) as illustrated in Figure 2.
Figure 2.
The Pie Chart Represents the IgG and IgM Levels in Latent CMV Patients at EOT (N=70). The circulated IgG and IgM Abs were detected in HCV patients with latent CMV (n=50), reactivated CMV infection (n=20) by ELISA. Active CMV replication was confirmed by real-time PCR in 13 patients
These findings reflect that the CMV Reactivation might be occurred after complete eradication of HCV by DAAs.
Discussion
Direct-Acting Antivirals (DAAs) have been used efficiently to treat chronic hepatitis C virus infections (Asselah et al., 2018). Even though DAAs are effective, they have many adverse outcomes that can be serious and life-threatening (Nappi et al., 2017). According to previous studies, reactivation of the herpes virus might be occurred after DAA therapy in HCV patients (Perello et al., 2016; El Kassas et al., 2017; Ghweil and Helal, 2019). As the Herpes virus and CMV are considered as long life infection, the latent infection of CMV is characterized by the virus inability to replicate and produce a new virion. However, the virus can be reactivated due to several stimulus as well as immunosuppression status. Several studies reported a link between CMV and cancer patients, as well as a role of CMV on the development of breast cancer (Sepahvand et al., 2019; Nakhaie et al., 2021). Further studies are needed to understand the exact mechanisms involved in the reactivation and/or latency of HV involved pathways to differentiate between the reactivation and latency of HV.
To our knowledge, there had been no previous published reports evaluated the occurrence of CMV reactivation in CHC patients previously treated with DAAs.
Therefore, the main objective of the current study is to determine the impact of DAAs on the reactivation of CMV in CHC infected patients and the effect of CMV co-infection on the response to DAAs treatment. All the recruited patients are CMV-IgG seropositive. While the CMV-IgM was detected in approximately 63.5% of patients (N=122) indicating the CMV recent infection. The current study focused on the patients who had negative CMV-IgM indicating the latent infection (N=70, 36.5%). The obtained serological results were consistent with Mohamed et al who indicated the prevalence of HCMV Abs (IgG and IgM) in CHC patients (91.7% and 28.3% respectively) (Mohamed et al., 2017).
In the present study, CMV reactivation after the DAAs treatment has been observed in 20 cases of chronic HCV (CMV IgM positive). The serological data have been confirmed by the real time PCR to evaluate the CMV replication and showed that only 13 patients had detectable CMV viremia. This finding has been observed by several studies and reported reactivation of herpes virus after DAAs treatment (Perello et al., 2016; El Kassas et al., 2017; Ghweil and Helal, 2019).
Besides, no risk factors for CMV reactivation had been detected in the recruited cases such as post-transplantation states, psychological/physical stress, and concurrent infections or known immunocompromised status.
The possible mechanisms of CMV reactivation might include: Firstly, the suppressive influence of HCV on CMV, which is abolished following HCV clearance by DAAs, or a potential direct effect of DAAs therapy in generating a pathological inflammatory response in the setting of immune system restoration and hence CMV reactivation and this copes with Hengst et al who have indicated that chronic HCV infection disrupts the milieu of soluble inflammatory mediators even after viral clearance (Hengst et al., 2016). Therefore, HCV cure did not lead to full immunological restitution (Hengst et al., 2016). Secondly, the Down-regulation of IFN-stimulated genes, which were key elements of antiviral defense, occurs after HCV clearance (Meissner et al., 2014). Thirdly, the presence of the IRIS (immune reconstitution inflammatory response) is a pathologic inflammatory response to earlier latent herpes virus in the context of enhanced immune function following DAA treatment (Tsang and Samaranayake, 2010). Changes in the immune system occurring after starting DAAs could play a role in reactivating CMV. However, the precise mechanisms involved in CMV reactivation are not clear in the early stages of HCV clearance in patients treated with DAAs. We deduce that there may be an increased incidence of CMV reactivation among patients on IFN-free regimens. Therefore, in order to ensure early and prompt management of such cases, a high index of clinical suspicion may be required, particularly in immunosuppressed patients.
Moreover, we revealed that the existence of active CMV infection prior to treatment did not affect response to DAAs therapy (IFN –Free regimen). The relationship between the treatment response and the preexistence of CMV was not well established. Patients with active CMV had an SVR of 98.4% at the beginning of treatment, while those with latent CMV had an SVR of 95.7%. However, several studies used IFN based regimen showed a strong correlation between the CMV and treatment response. Mohamed et al., (2017) stated that HCMV is one of the independent aspects that may significantly influence response of CHC patients to combined therapy with PEG-IFN and RBV. Bader El-Din et al reported that positive CMV patients treated with PEG-IFN therapy had a 20.87 times higher probability of non-response than negative CMV patients (Bader el-Din et al., 2011). This disparity is attributed to the difference in the regimen used for treatment.
Many evidences approved the improvement of liver function parameters as well as liver fibrosis after DAAs treatment. In the current study, at the EOT a significant amelioration of liver enzymes, albumin level, PLT count and coagulation profile have been remarked. Additionally, substantial decline in fibrosis level (FIB4 and APRI), were observed. This finding is consistent with the data observed by Mehta et al., (2018), Elsharkawy et al., (2017) and Giannini et al., (2019) who found that the DAAs treatment had a remarkable regression of liver fibrosis and improvement of liver biochemical profile. Regarding the response rate to DAAs, we have found that the SVR has been achieved in 187 (97.4%) patients in agreement with Omar et al who found that SVR rate was 95% in 18,378 HCV Egyptian patients(Omar et al., 2018).
Finally we conclude that the CMV reactivation occurred in 28.6% of patients at the end of DAAs treatment. As a result, we can deduce that IFN-free regimens are linked to an increased risk of CMV reactivation, but more evidence is needed to confirm this finding. Moreover, the CMV co-infection had no effect on the DAAs treatment outcome. Our patient cohort had an SVR rate of 97.4 %, and their clinical profiles had been improved at the end of treatment. Further investigations are required to understand the clinical and immunological consequences of HCV removal throughout medication with DAAs. Furthermore, the impact of interferon-free regimen on viral latency or reactivation should be investigated to manage the treatment strategy in HCV/CMV co-infected patients. The main limitation of the study is the small sample size; and the diagnosis of the latent CMV infection before treatment relied only on the serological test.
Abbreviations
AFP, Alfa fetoprotein; ALT, Alanine amino transferase; APRI, AST to Platelet Ratio Index; AST, Aspartate Transaminase; CHC, chronic hepatitis C; CMV, Cytomegalovirus; DAAs, Direct-acting antiviral agents; DCV, daclatasvir; EOT, end of treatment; FIB, Fibrosis-4 ; HBV, Hepatitis B Virus; HCC, Hepatocellular Carcinoma; HCV, Hepatitis C virus; HIV, human immunodeficiency virus ; HV, herpes virus; IFN, interferon; PCR, polymerase chain reaction; PEG, Pegylated; PLT, Platelet; RBV, ribavirin; SOC, standard of care; SOF, sofosbuvir; SVR, Sustained Virologic Response.
Author Contribution Statement
Reham DAWOOD: Project administration, Data curation, Resources, Funding acquisition, Investigation, Methodology, Validation, and Writing-review & editing. Ahmed Gomaa: Investigation, Validation and Supervision. Mai Abd El Meguid: Data curation, Methodology, and Validation, Resources, Writing- original draft, and Writing- review & editing. Essam Hassan: Resources, Software and Investigation. Ghada Salum: Data curation, Methodology, Investigation, Visualization. Hany Fares: Formal analysis and Software. Mostafa El Awady: Investigation, Validation and supervision. Eman Fares: Clinical examination and patient recruitment, Writing – original draft. Gamal Esmat: Conceptualization, Investigation, Validation and Supervision.
Acknowledgments
We would like to thank Reham DAWOOD for her financial support of the current research through the STDF- Egypt / Science and Technology Development Fund (Grant No. 15002).
Funding statement
The funding source (i.e. STDF) was only responsible for the financial aspects of the project and has no functional role in aspects like (study design; in the collection, analysis and interpretation of data; in the writing of the report; and making decision to submit the article for publication).
Ethical approval
This research was approved by the Faculty of Medicine’s Department, Fayoum University (#IRB –D-153). All patients signed informed consent before any study related procedures.
Availability of the data
All the data used to support the current study are included within the article.
Conflict of interest
No competing financial interests exist.
References
- Alexopoulou A, Karayiannis P. Interferon-based combination treatment for chronic hepatitis C in the era of direct acting antivirals. Ann Gastroenterol. 2015;28:55–65. [PMC free article] [PubMed] [Google Scholar]
- Aman W, Mousa S, Shiha G, et al. Current status and future directions in the management of chronic hepatitis C. Virol J. 2012;9:57. doi: 10.1186/1743-422X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselah T, Reesink H, Gerstoft J, et al. Efficacy of elbasvir and grazoprevir in participants with hepatitis C virus genotype 4 infection: A pooled analysis. Liver Int. 2018;38:1583–91. doi: 10.1111/liv.13727. [DOI] [PubMed] [Google Scholar]
- Bader el-Din NG, Abd el-Meguid M, Tabll AA, et al. Human cytomegalovirus infection inhibits response of chronic hepatitis-C-virus-infected patients to interferon-based therapy. J Gastroenterol Hepatol. 2011;26:55–62. doi: 10.1111/j.1440-1746.2010.06319.x. [DOI] [PubMed] [Google Scholar]
- Breban R, Arafa N, Leroy S, et al. Effect of preventive and curative interventions on hepatitis C virus transmission in Egypt (ANRS 1211): a modelling study. Lancet Glob Health. 2014;2:541–9. doi: 10.1016/S2214-109X(14)70188-3. [DOI] [PubMed] [Google Scholar]
- Cooke GS, Andrieux-Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4:135–84. doi: 10.1016/S2468-1253(18)30270-X. [DOI] [PubMed] [Google Scholar]
- El-Awady MK, Abdel Rahman MM, Ismail SM, et al. Prediction of relapse after interferon therapy in hepatitis C virus-infected patients by the use of triple assay. J Gastroenterol Hepatol. 2003;18:68–73. doi: 10.1046/j.1440-1746.2003.02919.x. [DOI] [PubMed] [Google Scholar]
- El-Meguid MA, Dawood RM, Ibrahim MK, et al. Reactivation of human cytomegalovirus inhibits expression of liver fibrosis related cytokines in patients chronically infected with hepatitis C virus genotype 4a. Microb Pathog. 2020;2020:104596. doi: 10.1016/j.micpath.2020.104596. [DOI] [PubMed] [Google Scholar]
- El Kassas M, Wifi MN, Mahdy R, et al. Herpes Zoster reactivation in patients with chronic hepatitis C under treatment with directly acting antiviral agents: A case series. Arab J Gastroenterol. 2017;18:39–41. doi: 10.1016/j.ajg.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Elsharkawy A, Alem SA, Fouad R, et al. Changes in liver stiffness measurements and fibrosis scores following sofosbuvir based treatment regimens without interferon. J Gastroenterol Hepatol. 2017;32:1624–30. doi: 10.1111/jgh.13758. [DOI] [PubMed] [Google Scholar]
- Fabbri G, Mastrorosa I, Vergori A, et al. Reactivation of occult HBV infection in an HIV/HCV Co-infected patient successfully treated with sofosbuvir/ledipasvir: a case report and review of the literature. BMC Infect Dis. 2017;17:182. doi: 10.1186/s12879-017-2287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghweil AA, Helal MM. Reactivation of herpesvirus in patients with hepatitis C treated with direct-acting antiviral agents. Infect Drug Resist. 2019;12:759–62. doi: 10.2147/IDR.S184598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini EG, Crespi M, Demarzo M, et al. Improvement in hepatitis C virus patients with advanced, compensated liver disease after sustained virological response to direct acting antivirals. Eur J Clin Invest. 2019;49:e13056. doi: 10.1111/eci.13056. [DOI] [PubMed] [Google Scholar]
- Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Grinde B. Herpesviruses: latency and reactivation - viral strategies and host response. J Oral Microbiol. 2013:5. doi: 10.3402/jom.v5i0.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra J, Garenne M, Mohamed MK, et al. HCV burden of infection in Egypt: results from a nationwide survey. J Viral Hepat. 2012;19:560–7. doi: 10.1111/j.1365-2893.2011.01576.x. [DOI] [PubMed] [Google Scholar]
- Hengst J, Falk CS, Schlaphoff V, et al. Direct-acting antiviral-induced hepatitis C virus clearance does not completely restore the altered cytokine and chemokine milieu in patients with chronic Hepatitis C. J Infect Dis. 2016;214:1965–74. doi: 10.1093/infdis/jiw457. [DOI] [PubMed] [Google Scholar]
- Marocco R, Lichtner M, Tieghi T, et al. Herpes virus reactivation after initiation of interferon-free antiviral agents in HIV-HCV-coinfected subjects: a new immune restoration disease? Antivir Ther. 2016;21:739–42. doi: 10.3851/IMP3072. [DOI] [PubMed] [Google Scholar]
- Martínez SM, Crespo G, Navasa M, et al. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325–35. doi: 10.1002/hep.24013. [DOI] [PubMed] [Google Scholar]
- Mehta R, Kabrawala M, Nandwani S, et al. Safety and efficacy of sofosbuvir and daclatasvir for hepatitis C virus infection in patients with β-thalassemia major. J Clin Exp Hepatol. 2018;8:3–6. doi: 10.1016/j.jceh.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner EG, Wu D, Osinusi A, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest. 2014;124:3352–63. doi: 10.1172/JCI75938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed H, El-Amin MM, Abdeen H, et al. Cytomegalovirus infection among patients with chronic hepatitis C virus and its relation to hepatitis C virus load. Afro-Egypt J Infect Endemic Dis. 2017;7:102–8. [Google Scholar]
- Nakhaie M, Charostad J, Azaran A, et al. Molecular and serological prevalence of HCMV in Iranian patients with breast cancer. Asian Pac J Cancer Prev. 2021;22:2011–6. doi: 10.31557/APJCP.2021.22.7.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi A, Perrella A, Bellopede P, et al. Safety of new DAAs for chronic HCV infection in a real life experience: role of a surveillance network based on clinician and hospital pharmacist. Infect Agents Cancer. 2017;12:12. doi: 10.1186/s13027-017-0119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar H, El Akel W, Elbaz T, et al. Generic daclatasvir plus sofosbuvir, with or without ribavirin, in treatment of chronic hepatitis C: real-world results from 18 378 patients in Egypt. Aliment Pharmacol Ther. 2018;47:421–31. doi: 10.1111/apt.14428. [DOI] [PubMed] [Google Scholar]
- Perello MC, Fernandez-Carrillo C, Londono MC, et al. Reactivation of herpesvirus in patients with hepatitis C treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol. 2016;14:1662–6 e1. doi: 10.1016/j.cgh.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Sepahvand P, Makvandi M, Samarbafzadeh A, et al. Human cytomegalovirus DNA among women with breast cancer. Asian Pac J Cancer Prev. 2019;20:2275–9. doi: 10.31557/APJCP.2019.20.8.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiha G, Soliman R, Mikhail NNH, et al. An educate, test and treat model towards elimination of hepatitis C infection in Egypt: Feasibility and effectiveness in 73 villages. J Hepatol. 2020;72:658–69. doi: 10.1016/j.jhep.2019.11.004. [DOI] [PubMed] [Google Scholar]
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- Tabll A, Shoman S, Ghanem H, et al. Assessment of human cytomegalovirus co-infection in Egyptian chronic HCV patients. Virol J. 2011;8:343. doi: 10.1186/1743-422X-8-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CS, Samaranayake LP. Immune reconstitution inflammatory syndrome after highly active antiretroviral therapy: a review. Oral Dis. 2010;16:248–56. doi: 10.1111/j.1601-0825.2009.01628.x. [DOI] [PubMed] [Google Scholar]
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]