Abstract
Objective:
Angiogenesis is new blood vessels formations that are necessary for certain physiological and pathologic conditions. Almond oil represents adjuvant therapies for numerous health benefits; It has ability for prevent the formation angiogenesis in tumour, due to its high concentration of unsaturated fatty acids, polyphenols, flavonoids and other ingredients content. Aspirin is non-steroidal ant-inflammatory drug; significantly reduce the angiogenesis of cancer. The aim of the study is to investigate the role of Prunus dulcis oil alone and in combination with aspirin, as an anti-angiogenic.
Method:
A sequential concentrations ex-vivo rat aorta ring assay, investigate the anti-angiogenic activity of Prunus dulcisoil in vivo. After three days of incubation, small holes were created on the fine pinpoint. Acute toxicity study was evaluated after administration of Prunus dulcis oil via intraperitoneal route.
Results:
the obtained results displayed that the serial concentration dose-response has a significant inhibition effect of blood vessels growth when compared to the negative control (DMSO 1%), the dose depended percentage of inhibition, sweet almond oil in combination with aspirin synergism effect to inhibition growth blood vessels as anti-angiogenic activity. The zone of inhibition was been measured as the mean of inhibition area of blood vessel on eggs in millimetre (mm) ± standard deviation. Conclusion: Almond oil dose has inhibition effect on cancer and can be used as (anti-angiogenesis), the activity of almond oil with Aspirin synergism can significantly reduce blood vessel’s growth in rate aorta ring and CAM assay.
Key Words: Antiangiogenesis, CAM, rat aorta rings, sweet almond oil, aspirin
Introduction
Angiogenesis is the formation of new blood vessels to meet the needs of the cells. Endothelial cells can play a significant role in either normal or pathological conditions by being stimulated to multiply ((Jiang et al., 2020). This process is in a state of balance between activation and inhibition, but during diseases, such as tumour growth, development, and metastasis, an imbalance occurs. When angiogenesis is stimulated, the balance between inducers and inhibitors of angiogenic factors is disrupted, resulting in angiogenesis disorder. (Carmeliet, 2005)Teleanu et al., 2020). Many studies have discovered that compounds in herbal derivatives play a major role in traditional medicine for several ailments and that suppressing angiogenesis may be a cancer treatment.
Almond (Prunus dulcis Miller, D. A. Webb), also known as sweet almond is a Rosaceae family member, which has long been recognized as a source of essential nutrients and as healthy food (Çelik et al., 2019). Aspirin belongs to a class of non-steroidal anti-inflammatory drugs (NSAID; Desborough and Keeling, 2017). It inhibits prostaglandin synthesis by non-selective inhibition of cyclooxygenase enzymes (COX1 and COX2). Also, the drug has anti-cancer activity through inhibition of COX2 (Zumwalt et al., 2017), and has pharmacological activities such as anti-metastasis and antiangiogenesis in both in vitro and in vivo assays (El-Khashab, 2021). The objective of this study was to determine the antiangiogenic activity of sweet almond (Prunus dulcis) oil alone and in combination with aspirin using in vitro and in vivo assays.
Materials and Methods
Source of experimental animals and ethical approval
The animals used in this study were obtained from the Animal House Facility, College of Pharmacy, University of Baghdad, Baghdad, Iraq. The animals were male Sprague Dawley rats aged 12 to 14 weeks. While the temperature was kept between 28 and 30 oC, all of the animals were allowed full access to food and tap water. Ethical approval was obtained from the Animal Ethical Committee at the College of Medicine, Al-Nahrain University, Baghdad, Iraq.
Source of sweet almonds
The sweet almonds used in this study were obtained from the
Extraction of sweet almond oil
Oil was extracted from the sweet almonds using regular method of oil extraction
Analysis of the antiangiogenic effect of sweet almond oil on rat aorta rings
Ex vivo antiangiogenic activity of sweet almond oil alone and in combination with aspirin was tested on rat aortic rings using the protocol described by Brown et al. (1996).With a salt solution containing 2.5 g/ml amphotericin B, the cleaned thoracic aorta of the rat was cross-sectioned into thin rings of aorta about 1 mm in thickness (Sigma, St. Louis, MO). To avoid fibrinolysis of vascular fragments, one ring was placed in the center of each well of a 48-well plate containing 500 µl of medium M199, a basal medium containing fibrinogen, L-glutamine, and aprotinin at 3 mg/mL, 1 % Wt/V, and 5 g/mL, respectively.Following a 90-minute incubation at 37 °C, an additional 500 mL of M199 medium containing 20% v/v fetal bovine serum (FBS), L-glutamine at 2 mM (1%), aminocaproic acid at 1 mg/mL (1%), amphotericin B at 2.5 g/mL, and gentamicin at 60 g/mL was poured on top of the solidified bottom layer. To make different concentrations, the sweet almond oil was dissolved in 10 mg/1ml dimethyl sulfoxide (DMSO) as a stock, and then doubly diluted with anM199 growth medium. Sweet almond oil alone (6.25-200 µg/mL range), and oil in combination with aspirin (200+50, 100+50, 50+50, respectively) were each tested three times with each concentration of oil alone, with six replicates in the oil combination with aspirin.After 4 days of incubation at 37 °C in a 5% CO2 incubator, the top layer of the medium was replaced with a new one containing only the oil, followed by the addition of aspirin. On day four of the experiment, a new fresh medium was applied to the negative control cultures, which was a fresh medium without sweet almond oil. The degree of sprouting micro-vessel growth was measured on day 5 according to Nicosia et al.(1997). Using a Leica inverted phase-contrast microscope (Leica, Germany) with a piece of fast imaging equipment to capture photos and calculatethem using the software programs.At least 30 points of the distance between each ring were measured.The results of inhibition by 50% (IC50 [n = ]) were provided. Suramin was used as a positive control.The fraction of blood vessels that were inhibited was calculated using the formula:
| (1) |
where Ao=distance of blood vessels growth as µm and A=distance of blood vessels growth in the control (µm) (Brown et al., 1993).
Chorioallantoic membrane (CAM) assay on sweet almond oil
Fertilized chicken eggs were purchased from the Baghdad Research Centre. The eggs were incubated at 37 °C with 60% relative humidity. The antiangiogenic effect of sweet almond oil was investigated in vivo using the chorioallantoic membrane (CAM) assay (West & Burbridge, 2009).Five-day-old fertilized eggs were bought from the local hatchery. On the third day of incubation, 5 mL of albumin was aspirated, and the eggs were then incubated horizontally to allow the CAM to detach from the eggshell. Sweet almond oil was prepared in 1.2% agarose discs at a concentration of 100µg/ disc in the egg. Discs containing the vehicle (DMSO) only were used as a negative control. A small hole was made in the shell, and the discs were then placed straight into the CAM eggs. The embryos were incubated at 37°C for 48 hours after the little opening was sealed with sterilized surgical tape. Under a dissecting microscope, the CAM assay was photographed, and the blood arteries of each CAM were determined. The results are presented as a mean percentage of inhibition of the control ± SD, (n = 4)(Lokman et al., 2012).
Lethal dose 50 test on experimental animals treated with sweet almond oil
The acute toxicity was determined following the intraperitoneal administration of sweet almond oil to 35 male Swiss albino mice with weights ranging from 20 to 25 g each. Comparably and randomly, the animals were assigned to one control group and six treatment groups.Before the experiment, all animals were given water and food, and they were given seven days to acclimatize to the laboratory environment. After depriving the animals of food but allowing them to drink water for an overnight period, the control group was given a vehicle (normal saline) 3ml/kg IP, whereas the treatment groups were given IP sweet almond oil, which was prepared by dissolving it in the doses as follows: 10, 5, 2.5, 1.25, 0.625 g/kg, to determine any death or changes in general behaviour and further physiological activities.After administering the sweet almond oil, the animals were observed constantly for the first 4 hours and then every 24 hours for the next 48 hours until the 14th day (Alsina-Sanchis et al., 2021).
Statistical analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS; version 21). Analysis of Variance (ANOVA) was used to compare the level of significance between the means.
Results
Antiangiogenic activity of sweet almond oil on rat aorta rings
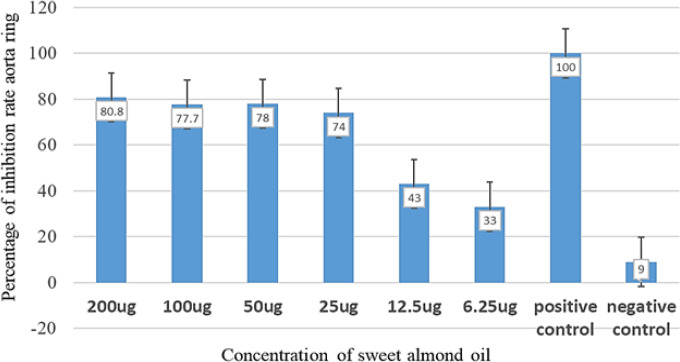
When the antiangiogenic activity of different concentrations of sweet almond oil was tested against the blood vessel growth of rat aorta, the data were presented as a mean percentage change in blood vessels growing on the rat aorta ± standard deviation. In comparison to other concentrations, the 200 g/ml concentration significantly inhibited blood vessel growth on the 5th day (potent antiangiogenic activity) as illustrated in Figure 1. Blood vessel growth was decreased by the 200 g/ml of sweet almond oil by 80.8±0.646, whereas blood vessel growth was inhibited by 77.7±1, and 78±1.087, respectively, at the two remaining concentrations of 100 and 50 g/ml. There was a significant difference (P<0.05) in blood vessel inhibitions of sweet almond oil among 200, 100, and 50 g/ml, as well as the negative control. Similarly, there was a significant difference (P<0.05) in the blood vessel inhibition period among 200, 100, and 50 g/ml, as well as the negative control. Suramin, the positive control, had a higher percentage of inhibition of vessel formation than sweet almond oil at 200 g/ml. Suramin at 100 g/ml, used as a positive control, completely prevented blood vessel growth. However, there was no significant (P<0.05) difference between the 100 and 50 µg/ml. The percentage of inhibition was dose-dependent. After 5 days, different concentrations of almond oil were measured in comparison to the negative control (DMSO) and positive control (suramin) that prevented blood vessel growth.
Figure 1.
The Antiangiogenic Activity of Sweet Almond Oil on Blood Vessel Growth of Rat Aorta
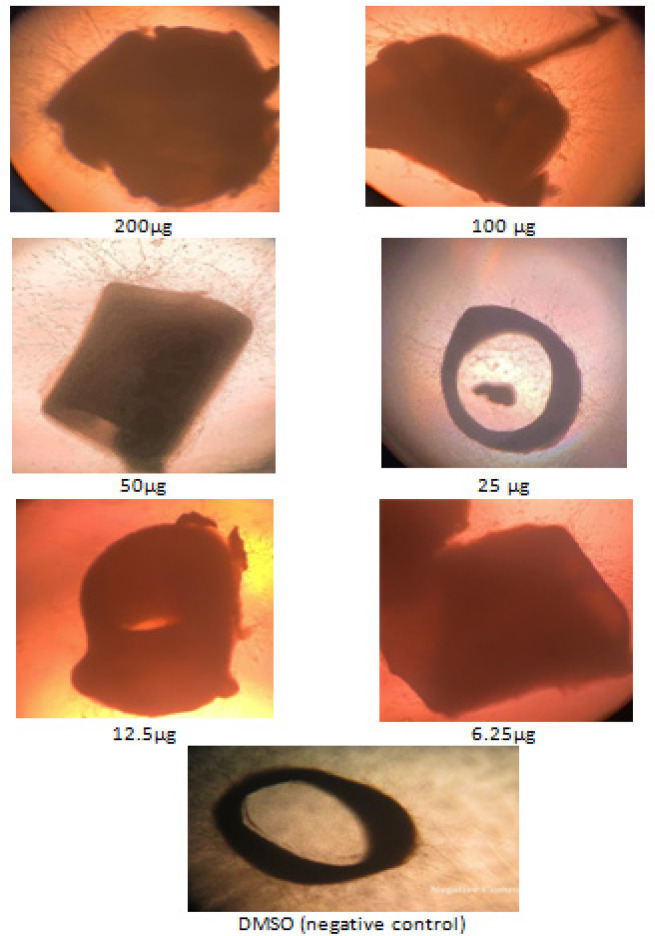
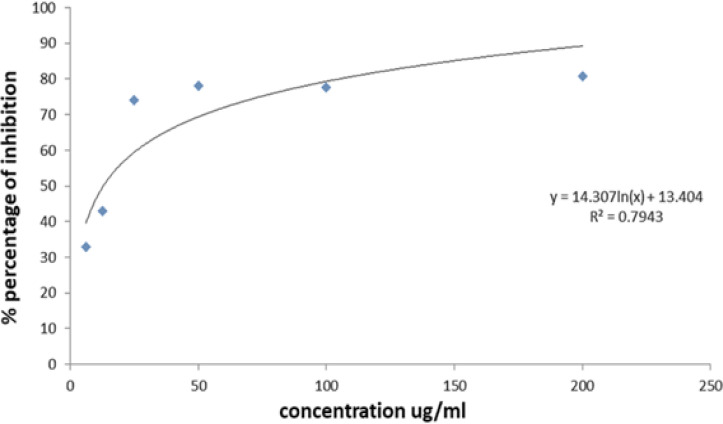
The concentrations utilized in this experiment were 200, 100, 50, 25, 12.5, and 6.25 g/ml. In comparison to the other oils, 200 g/ml had the highest antiangiogenic action till day five of the experiment (Figure 2). However, when compared to the negative control, 100 and 50 g/ml resulted insignificant (P<0.05) inhibition of blood vessels, although at a lower level than the positive control.Figure 3 depicts the dose-response curve of sweet almond oil applied to the rat aorta at various concentrations. Six different concentrations were used, which ranged from 200, 100, 50, 25, 12.5 to 6.25 µg /ml.The concentration that inhibited 50% of blood vessels(IC50 value) was determined by the logarithmic regression equation as: Y= 29.327ln(x) – 50.695 equating to =12.90µg/ml; where Y= the percentageof inhibition %; X=concentration of sweet almond oil.The IC50 was determined using a linear regression equation because the results indicated significant dose-dependent inhibitions with the sweet almond oil in combination with aspirin.
Figure 2.
Aorta Rings Treated with Different Concentrations of Sweet Almond Oil and the Negative Control
Figure 3.
Dose-response Curve of Sweet Almond Oil on Rat Aorta Rrings
Effects of sweet almond oil alone, and in combination with aspirin
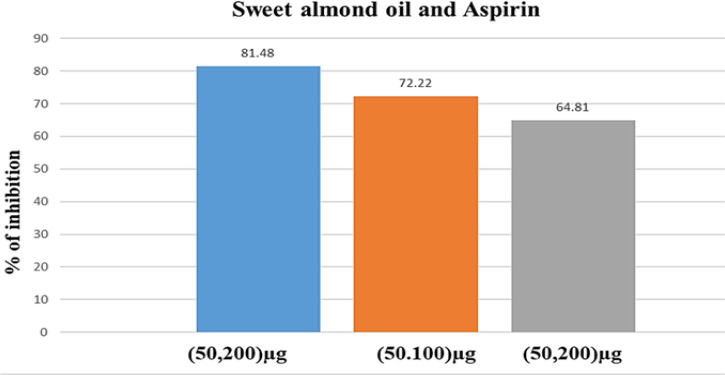
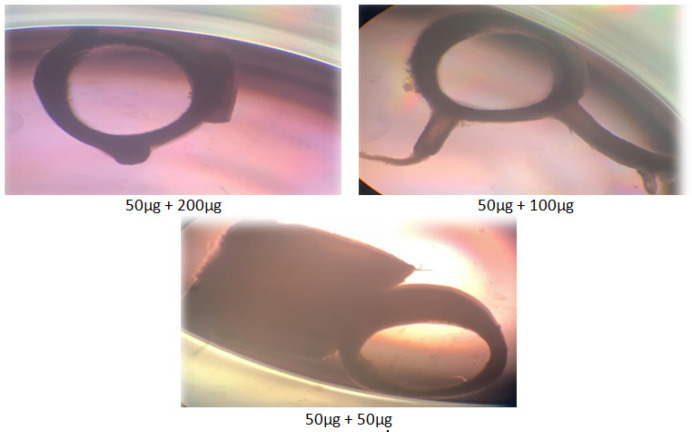
The effect of sweet almond oil (200, 100, and 50 g/ml) in combination with 50 g/ml aspirin on rat aorta blood vessel growth revealed a synergistic effect as shown in Figure 4a. The combination case significantly inhibited blood vessel growth at day five when compared to sweet almond oil alone, with percentage inhibition values of 81.48±0.82, 72.22±1.05, 64.81±0.75 for the 200, 100, and 50 g/ml, respectively. Figure 4b shows the effects of various combinations of oil and aspirin on the rat aorta rings. Similarly, Table 1 summarizes the inhibitory effects of the various concentrations of oil in combination with aspirin on the aorta rings of rats, showing an antiangiogenic activity.
Figure 4A.
The Effect of Sweet Almond Oil (200,100, and 50 µg/ml) in Combination with Aspirin (50 µg/ml) on Blood Vessel Growth
Figure 4B.
Images of the Aorta Rings Treated with Sweet Almond Oil and Aspirin
Table 1.
Inhibition Percentages of Sweet Almond Oil in Combination with Aspirin on the Aorta Rings of Rats
| Concentration of Aspirin (µg/ml) |
Concentration sweet almond oil (µg/ml) |
% inhibition + SD |
|---|---|---|
| 50 | 200 | 81.48 +0.82 |
| 100 | 72.22 +1.05 | |
| 50 | 64.81+0.75 | |
| IC50= 8.348 µg/ml | ||
The outcome of the chicken embryo chorioallantoic membrane assay
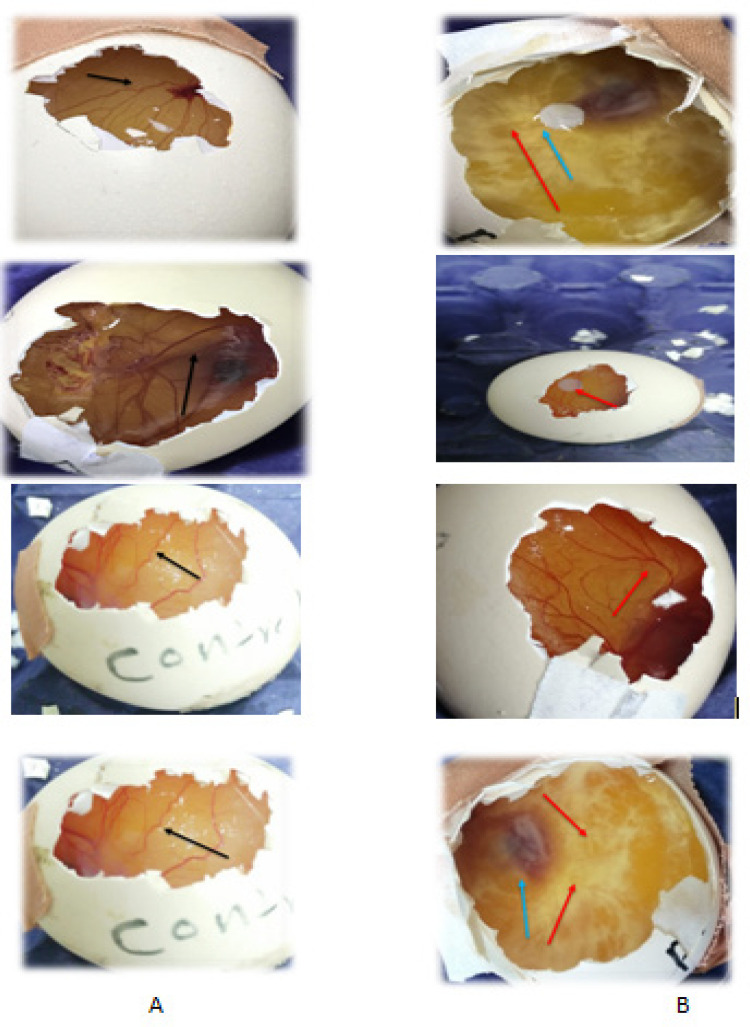
In an in vivo rat aorta antiangiogenesis study, 200 and 100 g/ml sweet almond oil demonstrated a strong antiangiogenic effect (Sahib et al., 2009). Therefore, the CAM essay has become one of the numerous classical in vivo model assays for analyzing angiogenesis (Ribatti, 2010).Because the inhibitory zones are greater than 10 mm in diameter, the score is three plus. This effect is presented in Table 2. In the chicken embryo chorioallantoic membrane assay (Figure 5), suppression of blood vessel growth is divided into two categories: (A) a negative control CAM assay, and (B) sweet almond oil treatment groups in the CAM assay. Table 2 and Figure 5 reveal that the CAM treated with sweet almond oil had a significant(P<0.05) antiangiogenic effect. From beneath the disc, a large number of vessels were populated, resulting in 10 mg of delectable almond oil. The vessels were very sparse and difficult to recognize, despite their light yellow appearance.
Table 2.
Zone of Blood Vessel Inhibition in an in vivo Chick Chorioallantoic Membrane Assay at day 7
| Egg sample | Zone area inhibition (mm) | Score |
|---|---|---|
| 1 | 16 | +++ |
| 2 | 14 | +++ |
| 3 | 8 | + |
| 4 | 9 | ++ |
| Mean ±SD | 11.75± 3.344 | ++ + |
Figure 5.
Chick Chorioallantoic Membrane (CAM) Assay. A, Control egg; B, Treated eggs with sweet almond oil; Black marker, Growth of blood vessels; Blue marker, Sweet almond oil; Red marker, Zone of inhibition
Analysis of the lethal dose 50 test on animals treated with sweet almond oil
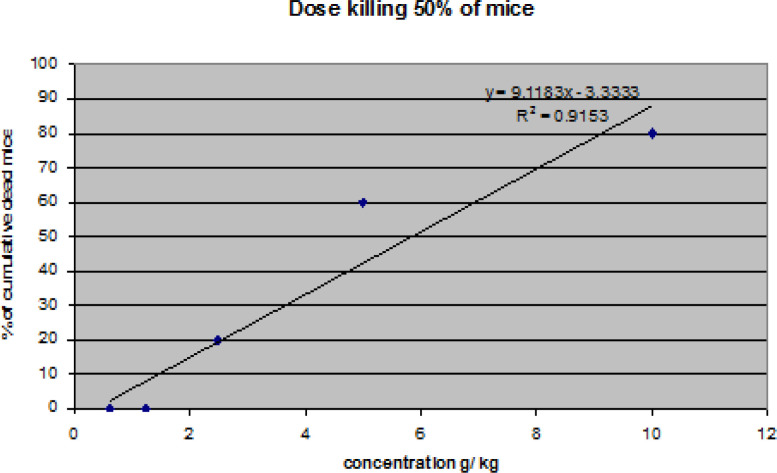
When each group of mice was given (IP) a different concentration (10, 5, 2.5, 1.25, 0.625, or 0.312 g/kg) of sweet almond oil, the weights of the groups of animals before and after treatment are presented in Table 3. Male mice were given a lethal dose of sweet almond oil that killed 50% of them (LD50). The LD50 was 5.849g/kg, which was calculated using the equation Y= 9.1183 x -3.3333. The dose-response curve is shown in Figure 6.The numbers of dead mice and those who survived until the 14th day of the experiment for male albino mice are reported in Table 4. Each group of mice was given different concentrations of sweet almond oil. The higher mortality rate in mice was 10 at a dose of 5 g/kg, indicating that this dose is more toxic than the other doses examined.
Table 3.
The Weight of Experimental Animals before and after Administration with Sweet Almond Oil
| Group | Weight of mice before treatment with sweet almond oil (g) | Weight of mice after treatment with sweet almond oil (g) |
|---|---|---|
| 1 | 25 | 30 |
| 2 | 28 | 29 |
| 3 | 26 | 28 |
| 4 | 25 | 25.5 |
| 5 | 25 | 25 |
| 6 | 27 | 27.5 |
| Control | 25 | 25 |
| P value> 0.05 non-significant change in weight | ||
Figure 6.
Dose Response Curve of Sweet Almond Oil on Treated Male Mice Group
Table 4.
Percentage of Death of Male Mice Administered with Various Doses of Sweet Almond Oil
| Group | No. of mice | Congentration of sweet almond oil (g/kg) | No. of dead mice | % of cumulative dead mice |
|---|---|---|---|---|
| 1 | 5 | 10 | 4 | 80 |
| 2 | 5 | 5 | 3 | 60 |
| 3 | 5 | 2.5 | 1 | 20 |
| 4 | 5 | 1.25 | 0 | 0 |
| 5 | 5 | 0.625 | 0 | 0 |
| 6 | 5 | 0.312 | 0 | 0 |
| 7 | 5 | Control | 0 | 0 |
Discussion
Cancer is a common and difficult disease to treat, with an increase in annual deaths, necessitating the search for a natural alternative therapy that acts as an antiangiogenic agent. Angiogenesis is required for cancer growth, and the use of new strategies based on angiogenesis inhibition by phytochemical extracts from plants such as sweet almond oil is gaining research interest. This study confirmed previous studies of the role of sweet almond oil in reducing cancer risk (Monagas et al., 2007).In recent years, many researchers have used the rat aortic ring as an ex vivo assay to investigate the antiangiogenic activities in full or partial organ culture. To establish the activity of the antiangiogenesis assay and understand the mechanism, it was required to analyze the sequential concentration of sweet almond oil against rat aorta in an ex vivo experiment. The aim of the present study was to investigate if the quantities of sweet almond oil have an antiangiogenic effect alone or in combination with aspirin.
In this study, sweet almond oil alone at a concentration of 200µg/ml was observed to have higher antiangiogenic activity on rat aorta rings compared to the other concentrations. Meanwhile, a combination of sweet almondoilwith aspirin resulted in a higher IC50value of 8.348µg/ml than the sweet almondoil alone, which had an IC50value of 12.90 µg/ml.Sweet almond oil has been reported to be rich in tocopherols and vitamin E. Tocopherols are powerful antioxidants that may reduce oxidative stress-induced DNA damage (Jiang, 2014). Also, they are known for their anti-inflammatory, anti-cancer properties due to inhibition of cyclo-oxygenases (COX-1 and -2), and 5-lipoxygenase (5-LOX).
Apoptosis, anti-proliferation, and anti-angiogenesis are all enhanced by various forms of vitamin E and metabolites. COX-2 overexpression has been linked to cancer and has been shown to reduce apoptosis in colon cancer cells(Sun et al., 2002). COX-1 also promotes angiogenesis and tumorigenesis.The present study aimed to determine the mechanism of action in which herbs containing vitamin E in combination with NSAIDs have synergistic and protective effects against cancer development, angiogenesis, and inflammation. Therefore, sweet almond oil, which is high in vitamin E and tocopherol, was used (Çelik et al., 2019; Karimi et al., 2021) in combination with aspirin to determine synergistic activity.
The properties of sweet almond oil could also be due to the presence of various phytochemicals, such as polyphenols of phenolic acids (caffeic acid, ferulic acid, P-coumaric acid, and vanillic acid), flavonoids (anthocyanidins, isoflavones, flavonols, and flavanones), and phytosterol (Woyengo et al., 2009). Additionally, stilbenes, vitamin E, and omega-9 have protective effects against cancer (Graf et al., 2005), which have a synergistic effect with aspirin. Therefore, almond oil may have a protective effect against the proliferation of tumors (Filiz Mericli et al., 2017).All the concentrations of sweet almond oil alone have dose-dependentantiangiogenic activity at a concentration of 200ug/ml,which resulted in more potent antiangiogenic activity. However, sweet almond possesses antiangiogenic activity when used alone or in combination with aspirin (synergism effect). The dose-response curve of sweet almond oil alone and in combination with aspirin was tested against the rat aorta antiangiogenic assay to get a sense of the oil’s safety (selective toxicity). When the IC50 values decreases, the safety of the test compound decreases and vice versa. The IC50for blood vessel outgrowth was within acceptable limits (Fang et al., 2012).
The advantages of the CAM assay are that it is a sensitive, practical, easy, and inexpensive in vivo assay for determining the antiangiogenic potential of each drug and herbal product. The assay not only provides information on a compound’s activity but also indicates toxicity effects in vivo(Chopra et al., 1986). The current research used the CAM assay, which is one of many standardsin vivo methods for studying angiogenesis production (Ribatti, 2010). As shown in Table 2, the zones of inhibition area are greater than 10 mm (score three-plus), whereas the score one-plus was less effective, depending on the condition (Lokman et al., 2012). This observation is confirmed by the results obtained in Figure 5, which showed the blood vessel growth inhibition in the control and the treated blood vessels of CAM. The CAM inhibition of blood vessels was treated with a disc containing 10 mg of sweet almond oil that was applied to the area with numerous blood vessels, resulting in strong antiangiogenic action, a reduction in the number of blood vessels, and a pale yellow look of the vessels.In acute toxicity experiments, sweet almond oil is well tolerated up to 5 g/kg, and in sub-acute toxicity studies, administration of the oil over a longer period did not appear to have any negative effects on behavior. The acute toxicity investigation of sweet almond oil revealed that mortality was recorded in the treatment groups of albino rats at 14 days lC50of 5.849g/kg from the linear equation at a dose of 10 5g/kg.Some signs of toxicity like dizziness and inactivity were noticed initially, and weight loss was also observed. This could suggest the deleterious effect of sweet almond oil is hepatotoxic.
In conclusion, According to the findings of this study, sweet almond oil, alone or in combination with aspirin, has a promising effect against tumor cells as a target for angiogenesis-related or chemotherapy-related disorders. Sweet almond oil, which contains flavonoids and polyphenols, significantly inhibited the formation of blood vessels in CAM. Furthermore, the chemical groups investigated were found to inhibit angiogenesis, or the growth of tumor cells. Finally, sweet almonds are edible but toxic at doses of 5 and 10 g/kg.
Author Contribution Statement
Zainab K. Ali (ZA): Conceptualization, Methodology, Data curation, Formal analysis, Writing - original draft. Hayder B Sahib (HS): Supervision, Validation, Writing – review & editing.
Acknowledgements
The authors would like to acknowledge Professor Dr. Ahmed R. Abu Raghif, head of the Department of Pharmacology, College of Medicine, Al-Nahrain University, for offering the logic support to finish this work.
Approval
This paper is part of the dissertation of the corresponding author at the College of Medicine at Al-Nahrain University in Partial Fulfilment of the Requirements for the Degree of PhD in Science in Pharmacology.
Ethic statement
Handling of the animals and the experimental design were carried out based on the guidelines reported in “Guide for the care and use of laboratory animal” and according to the protocol approved by the Al-Nahrain University/college of medicine, Baghdad, Iraq with the ethical clearance number 08/mmm126/03/2022.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Study Registration
There is no registration number or support for this project.
Conflict of interest
The authors declare that they have no competing interests.
References
- Alsina-Sanchis E, Mülfarth R, Moll I, et al. Intraperitoneal oil application causes local inflammation with depletion of resident peritoneal macrophages. Mol Cancer Res. 2021;19:288–300. doi: 10.1158/1541-7786.MCR-20-0650. [DOI] [PubMed] [Google Scholar]
- Brown KJ, Maynes SF, Bezos A, et al. A novel in vitro assay for human angiogenesis. Lab Invest. 1996;75:539–55. [PubMed] [Google Scholar]
- Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–35. [PubMed] [Google Scholar]
- Çelik F, Balta MF, Erci\csli S, et al. Tocopherol contents of almond genetic resources from Eastern and Western Turkey. Erwerbs-Obstbau. 2019;61:257–62. [Google Scholar]
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants (including the supplement), Council Sci. New Delhi, India: Ind. Res; 1986. [Google Scholar]
- Desborough MJR, Keeling DM. The aspirin story--from willow to wonder drug. Br J Haematol. 2017;177:674–83. doi: 10.1111/bjh.14520. [DOI] [PubMed] [Google Scholar]
- El-Khashab IH. Antiangiogenic and Proapoptotic Activities of Atorvastatin and Ganoderma lucidum in Tumor Mouse Model via VEGF and Caspase-3 Pathways. Asian Pac J Cancer Prev. 2021;22:1095–1104. doi: 10.31557/APJCP.2021.22.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Peng F, Yi T, et al. Biological activity and safety of Tripterygium extract prepared by sodium carbonate extraction. Molecules. 2012;17:11113–23. doi: 10.3390/molecules170911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food. 2005;8:281–90. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang J, Deng X, et al. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39:1–19. doi: 10.1186/s13046-020-01709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi Z, Firouzi M, Dadmehr M, et al. Almond as a nutraceutical and therapeutic agent in Persian medicine and modern phytotherapy: A narrative review. Phyther Res. 2021;35:2997–3012. doi: 10.1002/ptr.7006. [DOI] [PubMed] [Google Scholar]
- Lokman NA, Elder ASF, Ricciardelli C, Oehler MK. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int J Mol Sci. 2012;13:9959–70. doi: 10.3390/ijms13089959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mericli F, Becer E, Kabaday\i H, et al. Fatty acid composition and anticancer activity in colon carcinoma cell lines of Prunus dulcis seed oil. Pharm Biol. 2017;55:1239–48. doi: 10.1080/13880209.2017.1296003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monagas M, Garrido I, Lebrón-Aguilar R, et al. Almond (Prunus dulcis (Mill ) DA Webb) skins as a potential source of bioactive polyphenols. J Agric Food Chem. 2007;55:8498–8507. doi: 10.1021/jf071780z. [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Lin YJ, Hazelton D, Qian X. Endogenous regulation of angiogenesis in the rat aorta model Role of vascular endothelial growth factor. Am J Pathol. 1997;151:1379. [PMC free article] [PubMed] [Google Scholar]
- Ribatti D. The chick embryo chorioallantoic membrane as an in vivo assay to study antiangiogenesis. Pharmaceuticals. 2010;3:482–513. doi: 10.3390/ph3030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Tang XM, Half E, Kuo MT, Sinicrope FA. Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res. 2002;62:6323–8. [PubMed] [Google Scholar]
- Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med. 2020;9:84. doi: 10.3390/jcm9010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyengo TA, Ramprasath VR, Jones PJH. Anticancer effects of phytosterols. Eur J Clin Nutr. 2009;63:813–20. doi: 10.1038/ejcn.2009.29. [DOI] [PubMed] [Google Scholar]
- Zumwalt TJ, Wodarz D, Komarova NL, et al. Aspirin-induced chemoprevention and response kinetics are enhanced by PIK3CA mutations in colorectal cancer cells. Cancer Prev Res. 2017;10:208–18. doi: 10.1158/1940-6207.CAPR-16-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.