Abstract
Objective:
Lavender oil is of a great economic importance. It has many biological and pharmacological activities. The present study aimed to identify the chemical constituents of the essential oil of Lavandula officinalis (LAEO) by using GC/MS analysis. Its genotoxicity, anti-genotoxicity and histopathological activities against the chemotherapeutic drug cyclophosphamide (CP) was investigated. The study also evaluated its anticancer activities against six human cancer cell lines: hepatocellular carcinoma (HepG2), Prostate (PC3), Lung carcinoma (A549), Skin cancer (A431), Colon cancer (HCT116) and Breast cancer (MCF7).
Methods:
The genotoxicity was determined using: micronucleus, chromosomal aberration, and comet assays. The histopathological study included liver. The examined groups were control negative, control plant, control positive (CP), and 3 combined groups received LAEO at different concentrations plus CP.
Results:
GC/MS analysis recorded 16 components. The principals were: linalool and linalyl acetate. The results indicated the safety of LAEO. It also attenuates genotoxicity and deleterious histopathological effects of CP in a dose-dependent manner. LAEO has a highly cytotoxic effect on HepG2 and A549 cell lines with 100% death at 100µg/ ml with IC50 67.8 and 12 µg/ ml, respectively. Its activity on other cell lines was weak.
Conclusion:
The essential oil of Lavandula officinalis has anticancer and anti-mutagenic effect.
Key Words: Lavandula officinalis, essential oil, cancer cell lines, cyclophosphamide, genotoxicity, histopathology
Introduction
The genus Lavandula, commonly known as lavender, is one of the important members of Lamiaceae (labiatae) family. Many species of this genus are highly aromatic due to the presence of essential oils (Lavender oil). Lavender oil especially is employed in many economic industries: perfumery, cosmetic, pharmaceutical and in the flavoring and fragrance industry (Prashar et al., 2004). It has a broad spectrum of biological and pharmacological activities thanks to its antioxidant, anti-inflammatory, antimicrobial, sedative and anxiolytic activities and know it is used to treat stress and depression (Kozics et al., 2017). Strong antioxidant properties were demonstrated by (Kozics et al., 2017) who reported that Lavender essential oil has a protective role against mutagen-induced DNA damage by increasing the levels of enzymatic and non-enzymatic antioxidants (GPx-glutathione peroxidase, SOD-superoxide dismutase, GSH-glutathione) in human hepatoma cell line HepG2 in vitro and rat hepatocytes in ex vivo. Lavender oil also has a strong free radical scavenging ability (Bouyahya et al., 2017). Anti-mutagenic properties were demonstrated by bacterial reverse mutation assay (Evandri et al., 2005). Cytotoxic properties of some lavender essential oils have been also demonstrated (Prashar et al., 2004; Woronuk et al., 2011). These activities have been attributed to the preeminence (superiority) of some monoterpenoids like linalool, linalyl acetate, eucalyptol and camphor in many lavender oils (Soulaimani et al., 2019).
Despite our rapidly growing knowledge about cancer, its treatment is still largely based on the use of chemotherapeutic drugs. In general chemo-therapy presents severe toxic side effects to all body organs and interacts with DNA. The poor selectivity of the antineoplastic drugs to malignant cells over normal cells is the main reason for their toxicity (Fahmy et al., 2020). Cyclophosphamide (endoxan, CP) is a chemotherapeutic drug used in the treatment of a wide range of cancers. It is also used as an immunosuppressive agent and in the organ transplantation (Fahmy et al., 2015). Many research studies showed that CP is a powerful mutagen and/or clastogen at all phylogenetic levels. It causes dose-related increase in genotoxic activities which described as cross links and strand breaks in DNA, gene mutations, chromosomal aberrations, sister chromatid exchanges in addition to increased DNA adduct formation (Melek et al., 2015; Fahmy et al., 2019). In the present study, CP is used as a positive genotoxic/mutagenic agent.
Pharmaceutical plants represent nowadays an important source of biologically active compounds that are used in many drugs (Diab et al., 2020).Till now; their effects on heritable material are unknown. So in the present study we decided to explore the chemical composition of Lavandula officinalis essential oil. Its mutagenic/anti-mutagenic, histopathological activity and its anticancer efficiency against some human cancer cell lines.
Materials and Methods
Chemicals
Cyclophosphamide (CP) was purchased from Sigma- Aldrich (St. Louis, MO, USA). All other chemicals used in extraction were purchased from ADWIC (Cairo, Egypt).
Plant material
Lavandula officinalis was collected from the Experimental Agricultural Station, Fac. Of Agriculture, Cairo University, Giza Governorate, during the season of 2019.
Chemical investigation of essential oils
Determination
The essential oils of Lavandula officinalis were obtained by the hydrodistillation method (Guenther, 1953).
Identification of the chemical composition of volatile oils Gas chromatography–mass spectrometry analysis (GC-MS)
The GC-MS system (Agilent Technologies) was equipped with gas chromatograph (7890B) and mass spectrometer detector (5977A) at Central Laboratories Network, National Research Centre, Cairo, Egypt. Samples were diluted with hexane (1:19, v/v). The GC wasequipped with HP-5MS column (30 m x 0.25 mm internal diameter and 0.25 μm film thickness). Analyses were carried out using helium as the carrier gas at a flow rate of 1.0 ml/min at a split ratio of 1:30, injection volume of 1 µl and the following temperature program: 40 °C for 1 min; rising at 4°C /min to 150°C and held for 6 min; rising at 4°C/min to 210°C and held for 1 min. The injector and detector were held at 280°C and 220°C, respectively. Mass spectra were obtained by electron ionization (EI) at 70 eV and using a spectral range of m/z 50-550. Identification of different constituents was determined by comparing the spectrum fragmentation pattern with those stored in Wiley and NIST Mass Spectral Library data.
Animals
Swiss male mice (Mus musculus) obtained from the animal house of the National Research Centre (Giza, Egypt) were used (9-12 weeks old). All suitable conditions were provided during the experiments, temperature (22±3oC), humidity (50±15%) and a light/dark photoperiod of 12 h. Food and water were provided ad libitum.
Experimental design
A total of 90 mice were divided randomly into six groups (15 animals/each). The treatment was carried out as follows:
Group 1: negative control (non- treated).
Group 2: received LAEO orally (0.8 mL /kg) for 5 successive days.
Group 3: A positive control group i.p treated with a single dose of CP (25 mg/kg).
Groups 4, 5 and 6: were orally administered LAEO at doses of 0.4, 0.6 and 0.8 mL/kg respectively for 5 successive days and i.p injected with CP at the last day of treatment. In all groups, samples were collected 24 h after the last treatment.
Cytogenetic analysis
Micronucleus Test
Micronuclei analysis from mice bone marrow was performed according the standard protocol of Schmid (1976) and OECD 474 guideline for testing chemicals (OECD, 2016). The slides were stained with May Grünwald-Giemsa protocol. Micronuclei were identified as dark blue stained structures in the cytoplasm of polychromatic erythrocytes (PE’s). The ratio of erythrocytes to nucleated cells was expressed as the percentage of PE’s/100 nucleated cells (PE᾽s + NE᾽s). Two hundred nucleated cells were counted/ each animal, five animals / treatment. Scoring was accomplished with light microscope under 1,000× magnification.
Chromosomal aberration analysis in mouse spleen cells
The protocol described by Amer et al., (1993) was followed. Colchicine (3 mg/kg) was i.p injected two hours before sacrificing. Spleen was removed and crushed in RPMI (1640) medium. Centrifugation was performed then the spleen cells were suspended in hypotonic solution (0.075 M) KCl and incubated for about 20 minutes at 37C. Cells were fixed twice in 3:1 methanol: glacial acetic acid. Slides were prepared and stained with 7% Giemsa in phosphate buffer pH 6.8. 100 well spread metaphases were scored/mice, 500/ each group, (2,000 ×magnification
Single-cell gel electrophoresis (Comet assay)
DNA damage were measured using alkaline comet assay described previously (Schlörmann and Glei, 2009). The slides were screened at 400× using fluorescent microscope (Zeiss, Germany) supplied with Axiocam 105 color digital camera, green excitation filter (510–560 nm) and barrier filter (590 nm). For counting the percentage of DNA damage, tail length (μm), and tail moment, 100 cells were analyzed each animal using Tritek comet score software (version 1.5., Sumerduck, VA22742).
Statistical analysis
The data obtained were statistically analyzed by one-way analysis of variance (ANOVA) test. Calculation of the inhibitory index was done according to Madrigal-Bujaidar et al., (1998).
Inhibitory index (II) = [1- (plant extract plus control positive – control negative) / (positive control – negative control)] ×100.
Cell viability
According to Shaker et al., (2015), cell viability was assessed by the mitochondrial dependent reduction of yellow MTT. This method was to determine the cytotoxic effect of extracts on HCT116 (colon cancer) , MCF-7 (breast cancer), A549 (lung cancer) and HepG2 (liver cancer) cell lines. A probit analysis was carried for IC50 and IC90 determination using SPSS 11 program.
Histopathological study
Pieces of the liver have fixed in 10% neutral buffered formalin for 24 hours. After dehydration, tissue samples have embedded in paraffin wax, sectioned at 5 μm and stained with hematoxylin and eosin.
Results
Chemical composition of volatile oils
Table 1 represents the chemical composition of Lavandula officinalis essential oil as indicated by GC/MS analysis. Sixty compounds constituting the essential oil have been identified. The major components were Linalool (38.59%), Linalyl acetate (37.04%), Triacetin (6.93%), D-Limonene (5.22 %), Eucalyptol (3.29%) and Camphor (2.91%). All other components represent in amount lower than 2.0 %.Monoterpene alcohols were the main class of compounds.
Table 1.
Chemical Composition of Lavandulaofficinalis Essential oil (LAEO) as Indicated by GC-MS Analysis
| No. | Compound | Rt | Concentration % |
|---|---|---|---|
| 1 | α-Pinene | 11.53 | 1.56 |
| 2 | β-Myrcene | 13.6 | 0.17 |
| 3 | Benzene, 1-methyl-3-(1-methylethyl) | 14.87 | 0.11 |
| 4 | D-Limonene | 15.05 | 5.22 |
| 5 | Eucalyptol | 15.11 | 3.29 |
| 6 | Cyclopentanol, 1,2-dimethyl-3-(1-methylethenyl)-, [1R-(1.alpha.,2.alpha.,3.beta | 17.41 | 0.16 |
| 7 | Linalool | 17.88 | 38.59 |
| 8 | Cyclopentanol, 1,2-dimethyl-3-(1-methylethenyl)-, [1R-(1.alpha.,2.beta.,3.alpha.)] | 18.63 | 0.34 |
| 9 | Camphor | 19.33 | 2.91 |
| 10 | α-Terpineol | 20.99 | 1.21 |
| 11 | Cyclohexanol, 1-methyl-4-(1-methylethylidene) | 21.21 | 0.15 |
| 12 | Isopulegol acetate | 22.38 | 0.22 |
| 13 | Linalyl acetate | 23.21 | 37.04 |
| 14 | 1,4-Hexadiene, 3-ethyl-4,5-dimethyl | 23.65 | 0.31 |
| 15 | Bornyl acetate | 24.23 | 1.81 |
| 16 | Triacetin | 26.32 | 6.93 |
Rt, retention time
Micronuclei in bone marrow cells
A detailed study concerning the effect of different groups on micro-nucleated polychromatic erythrocytes (MNPEs) was recorded in Table 2. The results demonstrated significant percentage of MNPEs after CP treatment that reached 8.96±0.63 vs 1.66±0.47 for control negative. Also the percentage of PEs/total counted cells was significantly increased after CP treatment indicating severe toxicity to bone marrow cells. Controversy, LAEO showed normal percentage of micronuclei and PEs. In addition, it attenuated the deleterious effects of CP.
Table 2.
Percentage of Polychromatic Erythrocytes (PEs) and PEs with Micronuclei Induced in Mouse Bone-Marrow Cells after Treatment with CP and LAEO
| Treatment and doses | No. and percentage of Pes | No. and percentage of MNPEs | Inhibitory index MNPEs (%) | ||
|---|---|---|---|---|---|
| No | Mean% ± S.E | No | Mean% ± S.E | ||
| I- Control (Non-treated) | 543 | 5.43±0.49 a | 9 | 1.66±0.47 a | __ |
| II.Control plant LAEO (0.8ml/kg) | 549 | 5.49±0.37 a | 8 | 1.46±0.51 a | __ |
| III. CP (25 mg/ kg) Positive control | 1639 | 16.39±0.52d | 147 | 8.96±0.63 d | __ |
| IV-VI. CP+ LAEO | |||||
| +0.4ml /kg | 1123 | 11.23±0.73 c | 74 | 6.58±0.53 c | 33 |
| +0.6ml /kg | 997 | 9.97±0.55 b | 56 | 5.61±0.75 b | 46 |
| +0.8ml /kg | 836 | 8.36± 0.51b | 45 | 5.38±0.39 b | 49 |
Such protective effect appeared in the combined groups (LAEO+CP) vs CP alone. Dose- related relationship was recorded.
Chromosomal aberration in mouse spleen cells
The results obtained in Table 3 showed that LAEO (0.8mL/Kg) had normal effect on chromosomes compared to the negative control. The percentage of abnormalities reached 37.20±1.02 after treatment with CP (highly significant) compared with 2.0±0.31 for control negative. The pre-administration of LAEO at the tested concentrations (0.4, 0.6 and 0.8 mL/kg) attenuated the mutagenic effect of CP in a dose-related manner. The inhibitory index reached 14, 42 and 66% respectively. The percentage of metaphases with multiple aberrations reduced in the groups treated with LAEO and CP (18.20, 11.20 and 8.40% respectively) in relation to CP alone (25.80%).
Table 3.
Frequency of Chromosomal Aberrations Induced in Mouse Spleen Cells after Treatment with CP and LAEO
| Treatment and doses | Total abnormal metaphases | No and (%) of metaphases with different types of chromosome aberrations |
Inhibitory index | ||||
|---|---|---|---|---|---|---|---|
| No | Mean% ± S.E | Gap | Fragment and/or Break and/or Break | Multiple aberrations | RT | ||
| I. Control (Non- treated) | 10 | 2.0±0.31a | 5 (1.0) | 5 (1.0) | - | - | - |
| II. Control plant LAEO (0.8 ml /kg) | 7 | 1.40±0.24a | 1 (0.20) | 6 (1.20) | - | - | - |
| III. CP (25mg/kg )Positive control | 186 | 37.20±1.02e | 3 (0.60) | 53 (10.60) | 129 (25.80) | 1 (0.20) | - |
| IV-VI . CP+LAEO | |||||||
| +0.4ml /kg | 161 | 32.20±0.8d | 9 (1.80) | 66 (13.20) | 91 (18.20) | 1 (0.20) | 14 |
| +0.6ml /kg | 112 | 22.40± 0.51c | 4 (0.80) | 50 (10.0) | 56 (11.20) | 2 (0.40) | 42 |
| +0.8 ml /kg | 70 | 14.0±0.71b | 5 (1.0) | 23 (4.60) | 42 (8.40) | - | 66 |
A total of 500 cells were analyzed (5 mice per group; 100 cells/mouse). RT, Robertsonian translocation; One way ANOVA–Tukey’s multiple comparisons test was used. The values having different superscript letters in each column are significantly different from one another.
DNA fragmentation by comet assay
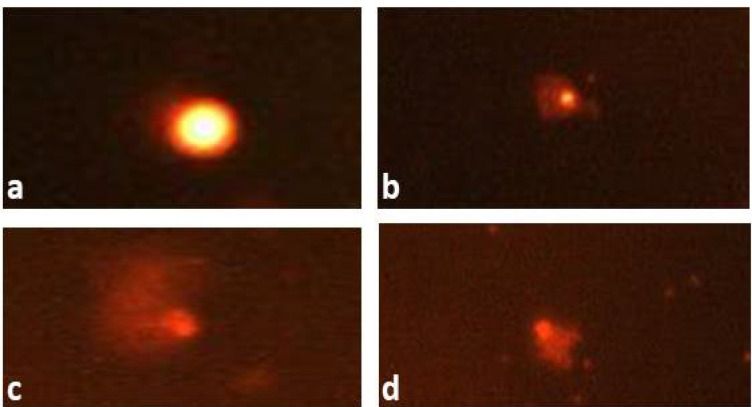
The results presented in Table 4 indicated that LAEO had a normal effect on the percentage of tail DNA (0.41±0.55) in comparison with the negative control that induced 0.48±0.61. A significant increase in tail DNA percentage 3.40±0.29 was detected after CP treatment (Figure 1). The percentage in DNA damage reached 12.58±0.62 with CP compared with 2.68±0.29 for the control. The pre-administration of LAEO at the tested concentrations for five days inhibited DNA damage and the tail DNA percentage induced by CP.
Table 4.
The Effect of CP and LAEO on the Level of DNA Fragmentation in Bone Marrow Cells Using Single Cell Gel Electrophoresis
| Treatment and dose | % of DNA damage | Tail length (µm) | Tail moment |
|---|---|---|---|
| I. Control (non-treated) | 2.68±0.29 a | 0.48± 0.61a | 0.06±0.34 a |
| II.Control plant LAEO (0.8ml/kg) | 2.59±0.25 a | 0.41±0.55 a | 0.09 ±0.37 a |
| III. CP (25mg/kg ) Positive control | 12.58±0.62 d | 3.40±0.29 d | 0.82±0.49 d |
| IV-VI . CP (25 mg/kg) + LAEO | |||
| +0.4ml / kg | 9.64±0.51 c | 2.98 ±0. 73 c | 0.45±0.79 c |
| +0.6ml / kg | 8.52±0. 60 c | 2.69 ±0.48 c | 0.22±0.61 b, c |
| +0.8ml / kg | 6.90 ± 0.49 b | 1.98±0.93 b | 0.17±0.48 b |
A total of 500 cells were analyzed (5 mice per group; 100 cells/mouse). One way ANOVA–Tukey’s multiple comparisons test was used. The values having different superscript letters in each column are significantly different from one another.
Figure 1.
Comet Pictures Representing (a) intact DNA, (b) moderately damaged DNA, and (c, d) extremely damaged DNA from mice bone-marrow treated with cyclophosphamide
Cell viability
LAEO was screened on 6 human cancer cell lines namely hepatocellular carcinoma (HepG2), Prostate (PC3), Lung carcinoma (A549), Skin cancer (A431), Colon cancer (HCT116) and Breast cancer (MCF7) to determent its anti-proliferative activity at 100µg/ ml and IC50. The results were displayed in Table 5 indicated that the LAEO has a highly cytotoxic effect on HepG2 and A549 cell lines with 100% death at 100µg/ ml with IC50 67.8 and 12 µg/ ml, respectively. While the activity on other cell lines was weak.
Table 5.
Percent Activity of LAEOat 100µg/ ml and Ic50 on Different Cell Lines
| HepG2 | PC3 | A549 | A431 | HCT116 | MCF7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 µg/ml |
IC50
µg/ ml |
100 µg/ml |
IC50
µg/ ml |
100 µg/ ml |
IC50
µg/ ml |
100 µg/ ml |
IC50
µg/ ml |
100 µg/ ml |
IC50
µg/ ml |
100 µg/ ml |
IC50
µg/ ml |
| 100 | 7.8 | 32.1 | ----- | 100 | 12 | 15.9 | ------ | 35.6 | ------ | 10.6 | ---- |
Histopathological results
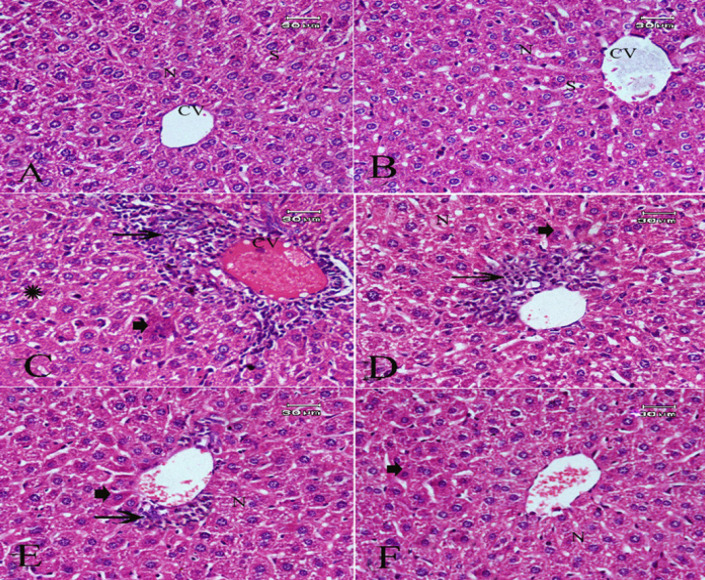
The liver sections of the control group or LAEO (0.8 mL/kg) showed normal histological architecture (Figures 2A and B).The results also revealed that CP at 25 mg/kg induced hepatocellular hydropic degeneration, necrosis, and mononuclear inflammatory leukocytes infiltrations with pyknotic nuclei (Figure 2C). LAEO ameliorated such alterations in a dose-dependent manner (Figs. 2D, E& F). The highest tested dose of LAEO greatly attenuated the histological lesions in almost liver cells with few necrotic cells and pyknotic nuclei (Figure 2F).
Figure 2.
Photomicrograph of H&E Staining Sections of Liver Showed: (A&B) Normal hepatic tissue architecture and cellular details of negative control group and LAEO treated group respectively. (C) Hepatocellular hydropic degeneration (thick arrow) , necrosis and mononuclear leukocytes infiltrations (thin arrow) with pyknotic nuclei of CP (positive control group). (D&E) Attenuated lesions in groups treated with cyclophosphamide (25mg/kg) and LAEO at the concentrations of 0.4 and 0.6 mg/kg respectively. (F) Greatly ameliorate effect in almost liver cells with few necrotic cells and pyknotic nuclei (thick arrow) in a group of animals treated with cyclophosphamide and LAEO at the highest concentration (0.8 mg/kg)
Discussion
Many researchers revealed an association between some serious diseases including cancer and the induction of mutation. The prevention of mutagen effects is of a great scientific interest to be studied.
Our results showed that LAEO had no effect on the induction of micronuleated polychromatic erythrocytes in mouse bone marrow or on chromosomal aberrations in mouse spleen cells after repeated treatment with the dose 0.8 mL/kg (highest tested dose). Also it had no damaging effect on bone-marrow DNA indicated by comet assay. Evandri et al., (2005) observed that lavender oil had no mutagenic activity using bacterial reverse mutation assay in Escherichia coli WP2 uvrA strain and Salmonella typhimurium TA98 and TA100 strains. Also the level of DNA strand breaks induced by lavender oil in HepG2 cells and primary rat hepatocytes as indicated by comet assay was normal compared with untreated control cells (Kozics et al., 2017).
Conversely, our results showed that oral pre-administration of LAEO at the tested concentrations (0.4, 0.6 and 0.8 ml/kg) for 5 days had the ability to reduce micronuclei, chromosomal aberration and DNA-damage induced by CP. The protective effect was dose-dependently. The same results observed by Evandri et al., (2005) who reported that lavender oil exerted strong anti-mutagenic activity. It was reduced the mutant colonies in the TA98 strain exposed to the direct mutagen 2-nitrofluorene in a concentration-dependent manner. Moreover, Kozics et al., (2017) observed that the essential oil extracted from Lavandula angustifolia had the ability to inhibit DNA damage induced by tert-butyl hydroperoxide (tBHP) and hydrogen peroxide (H2O2) as detected by comet assay in HepG2 cells and primary rat hepatocytes in vitro and in vivo. The authors mentioned that Lavender essential oil has strong antioxidant potential and free radical scavenging activity that plays a positive role in DNA repair system. The free radical scavenging activity of Lavender oil may be attributed to the polyphenolic compounds which have the ability to scavenge free radicals and enhance the DNA repair system or DNA synthesis (Kozics et al., 2017). The results indicated that the essential oil of Lavandula officinalis has a potent anticancer effect against HepG2 and A549 cell lines with 100% death at 100µg/ ml with IC50 67.8 and 12 µg/ ml, respectively. Other authors recorded a promising anticancer activities of lavender oils in different types of cancers (eg: Hodgkin’s lymphoma; human prostate cancer and A549, H1299, C6 cancer cells) through induction of apoptosis and necrosis (Dalilan et al., 2013. Zhao et al., 2017; Gezici 2018). Moreover, Gezici (2018) reported that lavender EOs could have possible usage for cancer treatment as an alternative anticancer agent.
Histopathological studies indicating that CP induced damage to liver. These results can be explained by the ability of CP to cause oxidative stress, increase the lipid peroxidation and decrease the anti-oxidant enzymes. The imbalance between antioxidant defense systems and the production of reactive oxygen species (ROS) could cause oxidative damage of biological cell macromolecules. The activation of antioxidant enzymes, such as GPX and SOD, could scavenge ROS and protect cells against oxidative stress (Xu et al., 2017). These can be explained the role of lavender oil in attenuating most of histopathological deleterious effects induced by CP.
GC-MS analysis of Lavandula officinalis essential oil showed that the major contents were: Linalool (38.9%), Linalyl acetate (37.04%), Triacetin (6.93%), D-Limonene (5.22 %), Eucalyptol (3.29%) and Camphor (2.91%). Linalool, a monoterpene alcohol, is a principle component of many aromatic plants. Linalool was reported to have many biological activities, including antioxidant, anti-inflammatory and analgesic effects. The anti-inflammatory effects of linalool in the cells have been associated with the modulation of pro-inflammatory cytokines and antioxidant enzymes. In particular, linalool reduced the levels of nuclear factor-erythroid 2, a regulator of antioxidant stress (Wu et al., 2014). Linalool was recorded to be effective as an antioxidant in guinea pig brains injected with H2O2, which is one of the major reagents used in antioxidant studies (Celik and Ozkaya, 2002). Also, in male Wister rats, linalool decreased the oxidative stress by increasing glutathione content and modulating malondialdehyde, a marker for lipid peroxidation (Mehri et al., 2015). Moreover, linalool had an analgesic effect in an animal model of acute pain induced by paclitaxel, a widely used chemotherapeutic agent (Katsuyama et al., 2012).
Linalyl acetate is also considered one of the principal components of many plant essential oils and was recorded to possess several biological and pharmacological activities. A number of linalool and linalyl acetate-producing species are used in traditional medicine systems to relieve symptoms and cure a variety of ailments (both acute and chronic). Moreover, Peana and Moretti (2002) revealed that linalool and linalyl acetate-producing species are potentially anti-inflammatory agents. Zhao et al., (2017) reported that Lavender angustifolia essential oil, and its major constituent’s linalool, and linalyl acetate showed strong anti-proliferative activity and apoptosis induction in human prostate cancer PC-3 cells (xenograft tumors). In addition, Elansary et al., (2018) suggested that oxygenated monoterpenes i.e. linalool and monoterpene hydrocarbons play an important role in the essential oil antioxidant, and anticancer activities.
Mitic´-Culafic et al., (2009) studied the protective effect of monoterpenes linalool, myrcene, and eucalyptol against the genotoxicity induced by t-butyl hydroperoxide (t-BOOH) using reverse mutation assay with Escherichia coli WP2 IC185 strain and its oxyR mutant IC202, and with comet assay in human hepatoma HepG2 and human B lymphoid NC-NC cells. The authors found that linalool and myrcene strongly suppressed t-BOOH induced mutagenesis. The author results also indicated that linalool, myrcene and eucalyptol have substantial protective effect against oxidative stress induced genotoxicity, which is predominately mediated by their radical scavenging activity.
D-limonene represents (5.22%) of the content of LAEO in the present study. D-limonene was previously demonstrated to have important immunomodulatory properties, including antitumor effects in addition to its potential antioxidant and anti inflammatory properties (Yu et al., 2017). Shah and Mehta (2018) evaluated the in vitro antioxidant effect of D-Limonene in comparison with Trolox using six different in vitro antioxidant assays: DPPH, FRAP, ABTS, hydroxyl radical scavenging assay, iron chelating and superoxide radical scavenging assay. The results indicated concentration-dependent reduction in free radical formation by D-Limonene in comparison with Trolox in all assays except iron chelating assay which suggests its promising role for cancer treatment.
Eucalyptol (1,8-cineole), a terpenoid oxide that showed antioxidant and anti-inflammatory effects in various diseases and might be a promising anticancer agent with anti-proliferative anti-metastatic activities (Rodenak-Kladniewa et al., 2020).
Our findings suggested that lavender oil has a strong anticancer activities which may be mediated through the induction of apoptosis. It also attenuated the genotoxic effect, DNA and histopathological damage of cyclophosphamide in mice by suppressing the oxidative stress pathway induced by CP.
In conclusion, we concluded that the antioxidant properties of the LAEO may account for its DNA-protective role and anticancer activities. LAEO is rich with many bioactive constituents and may serve as a promising candidate for future development.
Author Contribution Statement
Maha A. Fahmy; Designed and supervised the research, participate in practical work, wrote and reviewed the manuscript. Ayman A. Farghaly; Participate in research design, practical work, discussion of the manuscript, Entesar E. Hassan; Participate in research design, practical work, statistical evaluation, responsible for correspondence; Emad M. Hassan; Collection of plant material, extraction of plant under investigation, Participate in writing and review the paper. Zeinab M. Hassan; Participate in research design, participate in practical work; Khaled Mahmoud; participate in practical work, methodology. Enayat A. Omara; Participate in practical work and collection of samples. All authors reviewed and approved the final version of the manuscript.
Acknowledgments
This work is a part of the in house project of National Research Centre (NRC), Cairo, Egypt. NRC provided all necessary facilities to complete this work.
Funding statement
This work was supported by the Internal Project, National Research Centre (NRC), Dokki, Cairo, Egypt, under grant number: 12060167.
Ethical approval
This prospective study was reviewed and approved by the animal ethics committee of the National Research Centre, Cairo, Egypt and was carried out according to the National Institute of Health Guide (NIH) for the care and use of laboratory animal’s guidelines (approval number: 19 163)
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest
All the authors declare no conflict of interest.
References
- Amer SM, Ibrahim AAS, El-Sherbeny KM. Induction of chromosomal aberrations and sister chromatid exchange in vivo and in vitro by the insecticide cypermethrin. J Appl Toxicol. 1993;13:341–5. doi: 10.1002/jat.2550130508. [DOI] [PubMed] [Google Scholar]
- Bouyahya A, Et-Touysa A, Abrinib J, et al. Lavandulas toechas essential oil from Morocco as novel source of antileishmanial, antibacterial and antioxidant activities. Biocatal Agric Biotechnol. 2017;12:179–84. [Google Scholar]
- Celik S, Ozkaya A. Effects of intraperitoneally administered lipoic acid, vitamin E, and linalool on the level of total lipid and fatty acids in guinea pig brain with oxidative stress induced by H2O2. J Biochem Mol Biol. 2002;35:547–52. doi: 10.5483/bmbrep.2002.35.6.547. [DOI] [PubMed] [Google Scholar]
- Dalilan S, Rezaei-Tavirani M, Nabiuni M, et al. Aqueous Extract of Lavender Angustifolia Inhibits Lymphocytes Proliferation of Hodgkin’s Lymphoma Patients. Iran J Cancer Prev. 2013;6:201–8. [PMC free article] [PubMed] [Google Scholar]
- Diab KA, Fahmy MA, Zeinab M, et al. Inhibitory activity of black mulberry (Morusnigra) extract against testicular, liver, and kidney toxicity induced by paracetamol in mice. Mol Biol Rep. 2020;47:1733–49. doi: 10.1007/s11033-020-05265-1. [DOI] [PubMed] [Google Scholar]
- Elansary HO, Abdelgaleil SAM, Mahmoud EA, et al. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement Altern Med. 2018;18:214. doi: 10.1186/s12906-018-2262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evandri MG, Battinelli L, Daniele C, et al. The antimutagenic activity of Lavandula angustifolia (lavender) essential oil in the bacterial reverse mutation assay. Food Chem Toxicol. 2005;43:1381–7. doi: 10.1016/j.fct.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Fahmy MA, Abd-Alla HI, Hassan EE, et al. Genotoxicity and sperm defects induced by 5-FU in male mice and the possible protective role of Pentaslanceolata-iridoids. Mutat Res. 2020;2020:850– 503145. doi: 10.1016/j.mrgentox.2020.503145. [DOI] [PubMed] [Google Scholar]
- Fahmy MA, Farghaly AA, Hassan EE, et al. Fennel (Foeniculum vulgare) essential oil ameliorates DNA and histopathological damage induced by cyclophosphamide in mice. Bioscience Res. 2019;16:320–36. [Google Scholar]
- Fahmy MA, Hassan NHA, El-Fiky SA, et al. A mixture of honey bee products ameliorates the genotoxic side effects of cyclophosphamide. Asian Pac J Tropical Dis. 2015;5:638–44. [Google Scholar]
- Gezici S. Promising anticancer activity of lavender (Lavandula angustifolia Mill ) essential oil through induction of both apoptosis and necrosis. Ann Phytomedicine. 2018;7:38–45. [Google Scholar]
- Katsuyama S, Kuwahata H, Yagi T, et al. Intraplantar injection of linalool reduces paclitaxel-induced acute pain in mice. Biomed Res. 2012;33:175–81. doi: 10.2220/biomedres.33.175. [DOI] [PubMed] [Google Scholar]
- Kozics K, Srancikova A, Sedlackova E, et al. Antioxidant potential of essential oil from Lavandula angustifoliainin vitro and ex vivo cultured liver cells. Neoplasma. 2017;64:4. doi: 10.4149/neo_2017_401. [DOI] [PubMed] [Google Scholar]
- Madrigal-Bujaidar E, Diaz Barriga S, Cassani M, et al. In vivo and in vitro antigenotoxic effect of nordihydroguaiaretic acid against SCEs induced by methyl methanesulfonate. Mutat Res. 1998;419:163–8. doi: 10.1016/s1383-5718(98)00128-4. [DOI] [PubMed] [Google Scholar]
- Mehri S, Meshki MA, Hosseinzadeh H. Linalool as a neuroprotective agent against acrylamide-induced neurotoxicity in Wister rats. Drug Chem Toxicol. 2015;38:162–6. doi: 10.3109/01480545.2014.919585. [DOI] [PubMed] [Google Scholar]
- Melek FR, Aly FA, Kassem IAA, et al. Three further triterpenoid saponins from Gleditsia caspica fruits and protective effect of the total saponin fraction on cyclophosphamide-induced genotoxicity in mice. Z Naturforsch C. 2015;70:31–7. doi: 10.1515/znc-2014-4132. [DOI] [PubMed] [Google Scholar]
- Mitić-Culafić D, Zegura B, Nikolić BB, et al. Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem Toxicol. 2009;47:260–6. doi: 10.1016/j.fct.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Peana AT, Moretti MDL. Pharmacological activities and applications of Salvia sclarea and Salvia desoleana essential oils. Stud Nat Prod Chem. 2002;26:391–423. [Google Scholar]
- Prashar A, Locke IC, Evans CS. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Proliferation. 2004;37:221–9. doi: 10.1111/j.1365-2184.2004.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenak-Kladniewa B, Castroa MA, Crespoc R, et al. Anti-cancer mechanisms of linalool and 1, 8-cineole in non-small cell lung cancer A549 cells. Heliyon. 2020;6:e05639. doi: 10.1016/j.heliyon.2020.e05639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlörmann W, Glei M. Comet fluorescence in situ hybridization (Comet-FISH): Detection of DNA damage. Cold Spring Harbor Protocols. 2009;2009:1–6. doi: 10.1101/pdb.prot5220. [DOI] [PubMed] [Google Scholar]
- Shah BB, Mehta AA. In vitro evaluation of antioxidant activity of d–limonene. Asian J Pharm Pharmacol. 2018;4:883–7. [Google Scholar]
- Shaker YM, Omar MA, Mahmoud K, et al. Synthesis, in vitro and in vivo antitumor and antiviral activity of novel 1-substituted benzimidazole derivatives. J Enzyme Inhib Med Chem. 2015;30:826–45. doi: 10.3109/14756366.2014.979344. [DOI] [PubMed] [Google Scholar]
- Soulaimani B, Nafis A, Kasrati A, et al. Chemical composition, antimicrobial activity and synergistic potential of essential oil from endemic Lavandula maroccana (Mill ) South Afr J Botany. 2019;125:202–6. [Google Scholar]
- Woronuk G, Demissie Z, Rheault M, et al. Biosynthesis and therapeutic properties of Lavandula essential oil constituents. Planta Med. 2011;77:7–15. doi: 10.1055/s-0030-1250136. [DOI] [PubMed] [Google Scholar]
- Wu Q, Yu L, Qiu J, et al. Linalool attenuates lung inflammation induced by Pasteurell amultocidavia activating Nrf-2 signaling pathway. Int Immuno Pharm. 2014;21:456. doi: 10.1016/j.intimp.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Yu L, Yan J, Sun Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol Med Rep. 2017;15:2339–46. doi: 10.3892/mmr.2017.6241. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chen R, Wang Y, et al. In Vitro and In Vivo efficacy studies of Lavender angustifolia essential oil and its active constituents on the proliferation of human prostate cancer. Integr Cancer Ther. 2017;16:215–26. doi: 10.1177/1534735416645408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.