Abstract
Background:
Tumor-infiltrating lymphocytes (TILs) are assessed by the ratio of the area of lymphocytes infiltrating the stroma. TILs are important in breast cancer and malignant melanoma and are being established as a marker of prognosis and sensitivity to chemotherapy. This has resulted in various therapies being developed in fields such as breast cancer. However, the evaluation of TILs in head and neck squamous cell carcinoma (HNSCC) is not progressing, and the prognosis is still poor. Thus, investigating whether or not the evaluation of TILs is also effective in HNSCC and prognoses can be predicted with just biopsy samples alone is required.
Methods:
This study included 153 patients who were diagnosed with HNSCC between January 2010 and December 2019, underwent treatment, and could be followed up thereafter at our institution.
Results:
TILs, overall survival (OS), and progression-free survival (PFS) were evaluated in all patients, the chemoradiotherapy arm, and the surgery arm. The cut-off value for TILs was 50%. In all patients, OS was 69.8% and 40.2% (P = 0.01) and PFS was 58.4% and 31.6% (P = 0.003) in the high and low TIL groups, respectively. Multivariate analyses revealed that TILs independently predicted prognosis. In the chemoradiotherapy arm, OS was 70.8% and 31.6% (P = 0.012) and PFS was 63.4% and 20.3% (P = 0.001) in the high and low TIL groups, respectively. No significant differences were noted in the surgery arm.
Conclusions:
In HNSCC, TILs can be used as a prognosis predictor and chemoradiotherapy biomarker. Assessments can be performed just with hematoxylin–eosin staining and is very simple. This will greatly contribute to report personalized therapy progress. Further evaluations and, thus, prospective clinical multicenter trials are needed to use TILs in clinical practice for HNSCC.
Key Words: Tumor, infiltrating lymphocytes, head and neck squamous cell carcinoma, histopathology, prognostic
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy affecting approximately 600,000 new patients worldwide (Ferlay et al., 2015; Torre et al., 2015). Surgical resection, radiation therapy, and chemotherapy have been used, but survival has not substantially improved over several decades (Wiegand et al., 2015). Immunotherapy has received huge attention with the advent of immune checkpoint inhibitors. Tumor microenvironmental factors are critical to the success of the combinations of immunotherapy, chemotherapy, and radiation therapy, and tumor-infiltrating lymphocytes (TILs) are attracting increasing attention. Immune cells existing among TILs were recently recognized to coexist with and not attack cancer cells, and antibody therapies that inhibit programmed cell death protein-1 (PD-1) and programmed cell death-ligand 1 (PD-L1), which are involved in this state of coexistence, have been developed.
In 1992, Aaltomaa et al., (1992) reported that TILs are prognostic factors of invasive breast cancer and are immune responses to tumor growth. Yamaguchi et al., (2012) have reported that complete response rates were high in patients with high TILs in the arm receiving neoadjuvant chemotherapy. Moreover, Denkert et al., (2010) have reported that TILs were prognostic predictors in a large-scale clinical trial. Ono et al., (2012) have reported that TILs were also correlated with therapeutic response in triple-negative breast cancer. Studies on TILs in breast cancer are much advanced as described above, and it is becoming clear that TILs are important factors for predicting prognosis and showing treatment efficacy. TILs include CD8+ T, CD4+ T, and B cells. Furthermore, regulatory T cells and molecules such as cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and PD-1 are expressed around tumors, making the situation extremely complicated. TILs are also being evaluated in several solid tumors, including melanoma (Clark et al., 1989) and non-small cell lung cancer (Remark et al., 2015). Additionally, evaluations are being performed in oropharyngeal and laryngeal cancers in HNSCC (Uppaluri et al., 2008; Vassilakopoulon et al., 2016).
However, according to Mariusz et al., majority of the studies on HNSCC focused on TIL immunohistology, whereas a few studies focused on the hematoxylin–eosin (HE) staining of TILs (Książek et al., 2019). Recently, Nghia et al. focused on TIL subtypes in HNSCC (Nguyen et al., 2016).
Many retrospective studies in breast cancer have shown that TIL evaluation via HE staining is useful for predicting prognosis and drug therapy response. However, the issue is how to evaluate TILs. Because the assessment methods and cut-off values varied among reports, in 2014, an International Working Group developed guidelines regarding them (Salgado et al., 2015). The standardization of the TIL evaluation methods has just started, and further development and modifications are expected in the future.
Studies on TILs in HNSCC are currently lagging behind those in breast cancer and melanoma. TILs evaluation by HE staining in HNSCC is not yet clear and has not reached the evaluation level similar to that in breast cancer. Even in breast cancer, the American Society of Oncology guidelines state that TILs represent a promising biomarker for predicting prognosis and therapeutic response, but should not yet be used in adjuvant treatment for early breast cancer (Hendry et al., 2017). Therefore, clinical data still need to be accrued, and the International Working Group is in the process of standardizing TIL evaluations. In this study, we evaluated the 5-year overall survival (OS) and progression-free survival (PFS) of 153 patients with HNSCC who were diagnosed and treated at our institution based on the proportion of mononuclear cells in the stroma using only HE-stained sections of biopsy samples and not by immunohistological evaluations of TILs.
Discussion
Patients
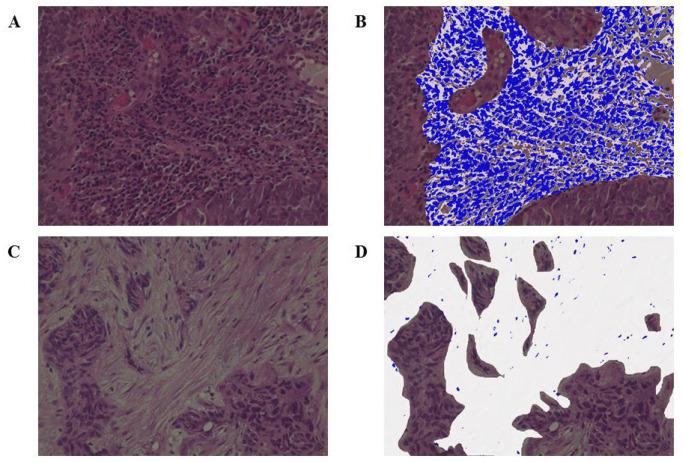
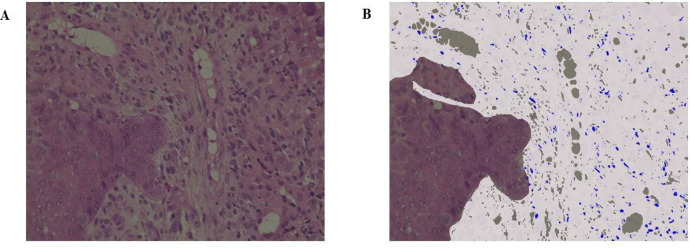
This study included 153 patients diagnosed with HNSCC at our institution over a 10-year period from January 2010 to December 2019 (Table 1). Patients who were diagnosed, treated, and followed up at our institution were included. The median follow-up period was 34 months (range: 2–60 months). With respect to treatment methods, chemoradiotherapy (such as combination of cisplatin, docetaxel, and cetuximab; no drugs as per the patient’s performance status; etc.) was administered to 72 patients and surgical treatment was performed for 81 patients. The radiation dose was determined by the radiologist and ranged between 60 and 70 Gy. Patients who received any neoadjuvant chemotherapy and those with multiple cancers were excluded from surgery. Medical records and pathology specimens were retrospectively reviewed. The endpoints of this study were 5-year OS and 5-year PFS, and the TIL value, which is calculated by the ratio of area occupied by the mononuclear cells in the stroma, was evaluated by age, gender, T classification, N classification, and HE staining. The TNM classification was based on image evaluations before treatment and followed the General Rules for Clinical Studies on Head and Neck Cancer, 6th edition (Japan Society for Head and Neck Cancer. General rules for clinical studies on head and neck cancer. Kanahara Publishing Group. Tokyo, Japan, 2018). The Japan Society for Head and Neck Cancer follows the UICC TNM Classification. The International Immuno-Oncology Biomarker Working Group defines a TIL value of ≥50% for lymphocyte predominant breast cancer (LPBC) that is often accompanied by lymphocyte infiltration and has reported this group as having a good prognosis (Denkert et al., 2010; Salgado et al., 2015). A cut-off value of 50% was set based on the LPBC definition because thresholds related to prognosis and clinical response have not yet been set, and groups were divided into high TIL (Figure 1a) and low TIL (Figure 1b) groups. Among the subjects, 47 and 106 patients with high and low TILs, respectively, were noted. Percentages were determined after discussions between two expert pathologists who were blinded to clinical information. The values of TILs were measured using a fluorescence microscope, BZ-X800 (Keyence, Osaka, Japan), and high (Figure 1c) and low (Figure 1d) TIL classification of patients was confirmed. Those that exceeded and did not exceed 50% in both 200× and 400× magnifications were classified under the high and low TIL groups, respectively. The measurement sites and methods for TILs were performed following the recommendations of the International Immuno-Oncology Biomarker Working Group.
Table 1.
Patient Characteristics
| Characteristic | Values | |
|---|---|---|
| Age (year) | 65.7 ± 11.0 | |
| Gender (male/female) | 124/29 | |
| T stage | T1 | 14 (9%) |
| T2 | 60 (39%) | |
| T3 | 33 (22%) | |
| T4 | 46 (30%) | |
| N stage | N0 | 44 (29%) |
| N1 | 15 (10%) | |
| N2 | 91 (59%) | |
| N3 | 3 (2%) | |
| M stage | M0 | 153 (100%) |
| Stage | I | 11 (7%) |
| II | 22 (14%) | |
| III | 17 (11%) | |
| IV | 103 (67%) | |
| Tumor sites | Tongue | 32 (21%) |
| Nasopharynx | 9 (6%) | |
| Oropharynx | 55 (36%) | |
| Hypopharynx | 43 (28%) | |
| Gingiva | 14 (9%) |
The majority of the patients (78%) had stage 3 and 4 advanced cancers.
Figure 1.
(a), The stromal infiltration of numerous lymphocytes could be confirmed; (b), Evaluation by fluorescence microscopy. The white portion is the stroma and the blue portion represents lymphocytes. Those with >50% TIL were in the high TIL group; (c), Interstitial lymphocytic infiltration was uncommon; (d), Evaluation by fluorescence microscopy. Those with <10% TIL were in the low TIL group (all were observed under 400× magnification). TIL, tumor-infiltrating lymphocyte
Medical records were recommended and consent was obtained from patients before using pretreatment clinical samples. Furthermore, this study adhered to the principles of the Declaration of Helsinki.
Histopathological diagnosis
All samples were fixed in formalin and embedded in paraffin, and pathological diagnoses were made using HE samples.
Statistical analysis
Survival was estimated using the Kaplan–Meier method, and significance was tested using log-rank tests. P < 0.05 was considered to show a significant difference. In addition, a multivariate analysis was performed using a Cox proportional hazard regression analysis if a significant difference was observed. IBM SPSS, ver. 21 was used for statistical processing (IBM Inc., Armonk, NY, USA). Values with P < 0.05 were defined as significantly significant.
Results
This study included 153 patients with an average age of 65.7 ± 11 years (124 males and 29 females). Tumor sites included the tongue (32 patients, 21%), nasopharynx (9 patients, 6%), oropharynx (55 patients, 36%), hypopharynx (43 patients, 28%), and gingiva (14 patients, 9%). Of the patients, 33 (21%) and 120 (78%) had stage 1–2 and 3–4 cancer, showing that the majority of patients had advanced cancer (Table 1).
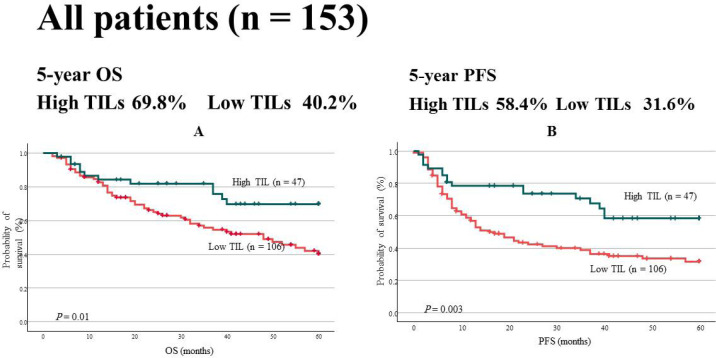
The 5-year OS and PFS rates for all HNSCC patients were significantly higher in the high TIL group. OS was 69.8% and 40.2% in the high and low TIL groups (P = 0.01), respectively, and the PFS was 58.4% and 31.6% in the high and low TIL groups (P = 0.003; Figure 2), respectively. Multivariate analysis of OS demonstrated that TILs were a strong independent prognostic factor (P = 0.033; hazard ratio [HR], 0.495; 95% confidence interval [CI], 0.259–0.944). Similarly, TILs were identified as an independent prognostic factor in the multivariate analysis of PFS (P = 0.005; HR, 0.458; 95% CI, 0.267–0.793; Table 2).
Figure 2.
(a), Five-year OS. The probability of survival in the high TIL group was significantly higher than that in the low TIL group (69.8% vs. 40.2%, P = 0.01); (b), Five-year PFS. Similarly, the probability of survival was significantly high in the high TIL group (58.4% vs. 31.6%, P = 0.003)
Table 2.
Univariate and Multivariate Analyses for Overall Survival (A) and Progression-Free Survival (B) (Cox Proportional Hazard Model)
| A | Univariate analysis | Multivariate analysis | P value | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | |||
| Age | <65 vs. ≥65 | 1.592 | 0.961–2.638 | 0.071 | 1.669 | 0.992–2.808 | 0.054 |
| Gender | Female vs. male | 1.302 | 0.664–2.554 | 0.443 | 1.377 | 0.699–2.713 | 0.355 |
| T stage | T1–2 vs. T3–4 | 2.065 | 1.235–3.455 | 0.006** | 1.563 | 0.897–2.722 | 0.115 |
| N stage | N0 vs. N1–3 | 1.75 | 0.968–3.165 | 0.064 | 1.795 | 0.947–3.402 | 0.073 |
| Stage | I–II vs. III–IV | 1.792 | 0.913–3.518 | 0.09 | |||
| TIL | Low vs. high | 0.452 | 0.241–0.846 | 0.013* | 0.495 | 0.259–0.944 | 0.033* |
| B | Univariate analysis | Multivariate analysis | P value | ||||
| HR | 95% CI | P value | HR | 95% CI | |||
| Age | <65 vs. ≥65 | 1.125 | 0.730–1.734 | 0.593 | 1.163 | 0.741–1.826 | 0.51 |
| Gender | Female vs. male | 1.358 | 0.737–2.503 | 0.327 | 1.44 | 0.778–2.666 | 0.245 |
| T stage | T1–2 vs. T3–4 | 1.524 | 0.983–2.362 | 0.06 | 1.207 | 0.752–1.936 | 0.437 |
| N stage | N0 vs. N1–3 | 1.464 | 0.885–2.420 | 0.137 | 1.523 | 0.883–2.627 | 0.13 |
| Stage | I–II vs. III–IV | 1.369 | 0.783-2.393 | 0.271 | |||
| TIL | Low vs. high | 0.456 | 0.267–0.778 | 0.004** | 0.458 | 0.264–0.793 | 0.005** |
According to multivariate analyses, TILs are independent prognostic factors for both OS (P = 0.033; HR, 0.495, 95% CI, 0.259–0.944) and PFS (P = 0.005; HR, 0.458; 95% CI, 0.264–0.793); CI, confidence interval; HR, hazard ratio; TIL, tumor-infiltrating lymphocyte
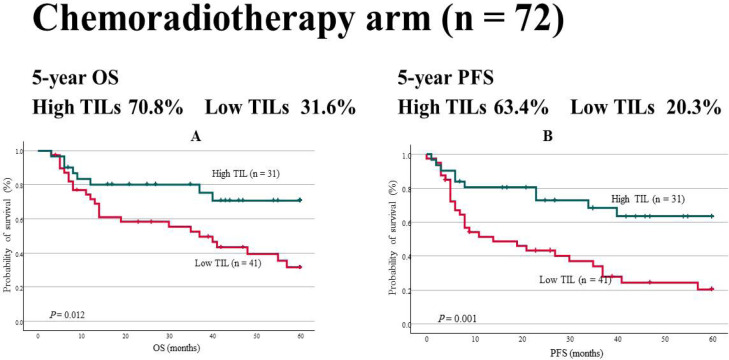
In addition, the chemoradiotherapy and surgery arms were evaluated. Of the 72 patients treated by chemoradiotherapy, 31 and 41 were in the high and low TIL groups, respectively. The age was 66.0 ± 9.1 years, and 63 and 9 subjects were males and females, respectively. The tumor site was the tongue (2 patients, 3%), nasopharynx (9 patients, 13%), oropharynx (40 patients, 56%), hypopharynx (19 patients, 19%), and gingiva (2 patients, 3%). Moreover, 9% and 91% of the patients had stage 1-2 and 3-4 cancer, respectively. OS was 70.8% and 31.6% in the high and low TIL groups, respectively (P = 0.012). PFS was 63.4% and 20.3% in the high and low TIL groups, respectively (P = 0.001; Figure 3). TILs were an independent factor for PFS in the multivariate analyses (P = 0.019; HR, 0.386; 95% CI, 0.174–0.856; Table 3).
Figure 3.
OS in the Chemoradiotherapy Arm. For both (a) OS and (b) PFS, prognosis was good in the high TIL group: OS (70.8% vs. 31.6%, P = 0.012), PFS (63.4% vs. 20.3%, P = 0.001)
Table 3.
Univariate and Multivariate Analyses for Over Survival (A) and Progression-Free Survival (B) (Cox Proportional Hazard Model)
| A | Univariate analysis | Multivariate analysis | P value | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | |||
| Age | <65 vs. ≥65 | 2.606 | 1.124–6.043 | 0.026* | 1.75 | 0.704–4.351 | 0.229 |
| Gender | Female vs. male | 1.498 | 0.456–4.920 | 0.505 | 1.399 | 0.421–4.653 | 0.584 |
| T stage | T1–2 vs. T3–4 | 3.269 | 1.464–7.303 | 0.004** | 2.314 | 0.953–5.620 | 0.064 |
| N stage | N0 vs. N1–3 | 1.393 | 0.488–3.980 | 0.536 | 1.291 | 0.433–3.852 | 0.647 |
| Stage | I–II vs. III–IV | 4.232 | 0.576–31.072 | 0.156 | |||
| TIL | Low vs. high | 0.378 | 0.170–0.842 | 0.017* | 0.583 | 0.244–1.392 | 0.224 |
| B | Univariate analysis | Multivariate analysis | P value | ||||
| HR | 95% CI | P value | HR | 95% CI | |||
| Age | <65 vs. ≥65 | 1.745 | 0.868–3.510 | 0.118 | 1.193 | 0.550–2.590 | 0.655 |
| Gender | Female vs. male | 2.04 | 0.627–6.636 | 0.236 | 1.922 | 0.589–6.265 | 0.279 |
| T stage | T1–2 vs. T3–4 | 1.994 | 1.021–3.893 | 0.043* | 1.272 | 0.584–2.774 | 0.545 |
| N stage | N0 vs. N1–3 | 1.27 | 0.496–3.250 | 0.618 | 1.297 | 0.487–3.452 | 0.603 |
| Stage | I–II vs. III–IV | 2.321 | 0.559–9.640 | 0.247 | |||
| TIL | Low vs. high | 0.332 | 0.161–0.683 | 0.003** | 0.386 | 0.174–0.856 | 0.019* |
According to multivariate analyses, TILs are independent prognostic factors for PFS (P = 0.019; HR, 0.386; 95% CI, 0.174–0.856); CI, confidence interval; HR, hazard ratio; TIL, tumor-infiltrating lymphocyte
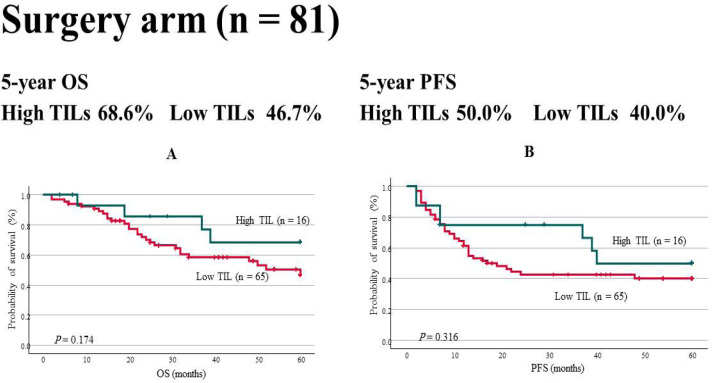
Furthermore, OS and PFS were also evaluated in the 81 patients treated by surgery. The tumor sites were the tongue (30 patients, 37%), oropharynx (15 patients, 19%), hypopharynx (24 patients, 30%), and gingiva (12 patients, 15%). Those with stage 1–2 and 3–4 accounted for 33% and 67%, respectively, showing the presence of more number of patients with advanced cancer. In the surgery arm, OS (68.6% vs. 46.7%, P = 0.174) and PFS (50.0% vs. 40.0%, P = 0.316) were not found to be significantly different (Figure 4).
Figure 4.
OS in the Surgery Arm. Neither (a) OS (68.6% vs. 46.7%, P = 0.174) nor (b) PFS (50.0% vs. 40.0%, P = 0.316) showed significant differences
Eight patients had distant metastases at the time of diagnosis; they were not included in the present statistics. All these patients were treated with cisplatin or cetuximab combined with radiotherapy, but the prognosis was poor. TILs were low in the primary lesions of all patients with an average of 8.1% (Figure 5).
Figure 5.
TILs in Patients with Distant Metastases. The mean value of TILs was 8.1% with (a) minimal interstitial lymphocyte infiltration. (b) The TIL value was only 2% in one patient (400× magnification). TIL, tumor-infiltrating lymphocyte
Discussion
The current retrospective study demonstrated that higher TILs were associated with a better HNSCC prognosis. Multivariate analyses also revealed that TILs were strong independent prognostic factors. In particular, patients with high TILs treated with chemoradiotherapy had a very good survival probability. Moreover, no significant differences were noted in survival probability between the high and low TIL groups in the surgery arm. Therefore, TILs were found to be useful as prognostic biomarkers of chemoradiotherapy and significantly contribute to improving prognoses and individualizing treatment. In addition, being able to predict prognosis using only biopsy samples will have a great impact on future treatment.
High TIL expressions are a favorable prognostic factor in various solid tumors, including ovarian cancer (Zhang et al., 2003), hepatocellular carcinoma (Wada et al., 1998), colorectal cancer (Teng et al., 2015), and urothelial cancer (Horn et al., 2016). The results of the current study also suggest that high TIL expression is a favorable prognostic factor in HNSCC. In recent years, immune checkpoint inhibitors (e.g., nivolumab and pembrolizumab) have been approved for the treatment of various cancers, and research in the field of immunity is progressing at a very fast pace. TILs include CD8+ T cells (killer T cells, cytotoxic T cells), CD4+ T cells (helper T cells), and B cells. Regulatory T cells are cells that express CTLA-4, PD-1, etc. and control immune responses. They express Foxp3, and although a consensus has not been achieved, some studies have shown that the CD8+/Foxp3+ ratio is a predictor of prognosis (Miyashita et al., 2014). Ngiha et al. performed a detailed investigation of these TIL subtypes in HNSCC (Nguyen et al., 2016). Those that are CD4-, CD8-, and FoxP3-positive have shown good survival probabilities with chemoradiotherapy. This is consistent with the finding that chemoradiotherapy is highly effective in patients with high TIL expressions, although subtypes were not investigated in the current study. In addition, these authors evaluated patients who underwent surgery. The only group where significant differences were observed was the CD8-positive group, and no significant differences were noted for those who were positive for other markers such as CD4, Foxp3, and CD68. This is also consistent with the findings of the current study that the TIL expression was not associated with differences in surgical treatment. In addition, human papillomavirus-positive tumors were also explained as having more lymphocyte infiltration, which contributed to higher chemoradiotherapy sensitivity. In HNSCC, many studies focus on subtypes rather than TILs (Spector et al., 2019). The immune system complexity may complicate TIL assessment. Moreover, one issue with evaluating TILs is that the assessment depends on which tumor part is observed and how these are evaluated. This study was conducted following the guidelines provided by the International Working Group and International Immuno-Oncology Biomarker Working Group, but the cut-off value for TILs has not been determined yet. Mariusz et al. set the cut-off at 30% in their investigation of oral squamous cell cancer (Książek et al., 2019). Breast cancer patients with TILs of ≥50% have been defined as having LPBC, and these patients are considered to have a good prognosis (Morita et al., 2016). Thus, the cut-off value for this study was set as 50%. It is expected that that the evaluation method will be standardized in the process of accruing more patients in the future.
The present study revealed that high TIL expression is a good prognostic factor and that the chemoradiotherapy effect can be highly expected in this group. Although no significant differences were seen in the surgery arm, additional treatments (e.g., chemoradiotherapy) after surgery in patients with high TIL expression are expected to improve HNSCC prognosis, which is usually poor otherwise. Moreover, TILs in patients with distant metastases were very low. The number of patients included in the study was less; therefore, more patients must be included in further investigations. Although a need to standardize treatment exists, individualized treatments are also needed. TILs can be evaluated at any institution by a pathologist using HE staining without the need for special techniques (e.g., immunostaining), thereby saving costs and time. Moreover, predicting prognosis with just biopsy samples is also considered an important aspect. In HNSCC, the evaluation of TILs is expected to lead to prognoses prediction and treatment individualization and thereby improve prognoses. This was a single-center, retrospective, nonrandomized study, and a multicenter, prospective study should be conducted to demonstrate the usefulness of TILs in HNSCC.
The current study showed that investigating TILs in HNSCC is also highly beneficial. TILs can be used as a chemoradiotherapy sensitivity marker. No significant differences based on surgical treatment were noted, but some type of adjuvant treatment in the high TIL group after surgery may contribute to improved survival. Moreover, the evaluation of TILs was found to be feasible with just HE staining, and it is essential to standardize and establishing a simple evaluation method. In the future, multicenter, prospective clinical trials will be necessary to clarify the true utility of TIL.
Author Contribution Statement
YK and YO designed the outline of the study. HS, YK, MM, HH, KS, SS, MS, and AI conducted the experiments and data analysis. HS, YK, and YO were involved in the preparation of manuscript. HS and YK wrote the manuscript. KS, SS, TY, and YO supervised the study and contributed to data interpretation and manuscript revision. YK and YO confirm the authenticity of all raw data. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors are grateful to Mr. Yusuke Ono and Ms. Reiko Ito for their technical assistance and Ms. Eriko Kumagai for her secretarial work.
Funding Statement
This work was supported in part by JSPS KAKENHI (grant numbers: 18K09311 to YK and 19K07497 to YO).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Informed consent was obtained from all patients. The possibility that the specimen may be used for clinical research is clearly stated and documented in the medical record. All procedures used in this research were approved by the Ethical Committee of Akita University Hospital.
Patient consent for publication
Informed consent has been obtained from all patients.
Statement of conflict of interest
The authors declare that they have no conflicts of interest.
References
- Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28:859–64. doi: 10.1016/0959-8049(92)90134-n. [DOI] [PubMed] [Google Scholar]
- Clark Jr WH, Elder DE, Guerry IV D, et al. Model predicting survival in stage 1 melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- Denkert C, Loibl S, Noske A, et al. Tumor associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- Ferlay , J , Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for standardized method from the international immunooncology biomarkers working group: part 1: assessing the host immune response, TILs in invasive in breast carcinoma and ductal carcinoma insitu, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24:235–51. doi: 10.1097/PAP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn T, Laus J, Seitz AK, et al. The prognostic effect of tumor infiltrating lymphocytic subpopulations in bladder cancer. World J Urol. 2016;34:181–7. doi: 10.1007/s00345-015-1615-3. [DOI] [PubMed] [Google Scholar]
- Japan Society for Head and Neck Cancer. General rules for clinical studies on head and neck cancer. Kanahara Publishing Group; [Google Scholar]
- Książek M, Lewandowski B, Brodowski R, et al. The prognostic significance of tumor infiltrating lymphocytes in oral squamous cell carcinoma. Pol J Pathol. 2019;70:277–85. doi: 10.5114/pjp.2019.93130. [DOI] [PubMed] [Google Scholar]
- Miyashita M, Sasano H, Tamaki K, et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple- negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2014;148:525–34. doi: 10.1007/s10549-014-3197-y. [DOI] [PubMed] [Google Scholar]
- Morita M, Yamaguchi R, Tanaka M, et al. Two progressive pathways of microinvasive carcinoma: low-grade luminal pathway and high-grade HER2 pathway based on high tumor-infiltrating lymphocytes. J Clin Pathol. 2016;69:890–8. doi: 10.1136/jclinpath-2015-203506. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Bellile E, Thomas D, et al. Tumor infiltrating lymphocytes and survival patients with head and neck squamous cell carcinoma. Head Neck. 2016;38:1074–84. doi: 10.1002/hed.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Tsuda H, Shimizu C, et al. Tumor infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple negative breast cancer. Breast Cancer Res Treat. 2012;132:793–805. doi: 10.1007/s10549-011-1554-7. [DOI] [PubMed] [Google Scholar]
- Remark R, Becker C, Gomez JE, et al. The non-small cell lung cancer immune contexture: A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med. 2015;26:377–90. doi: 10.1164/rccm.201409-1671PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado R, Denkert C, Demaria S, et al. International TIL Working Group 2014 The evaluation of tumor infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector ME, Bellile E, Amlani L, et al. Prognotic value of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2019;145:1012–9. doi: 10.1001/jamaoto.2019.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F, Mu D, Meng X, et al. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemotherapy and its clinical utility for rectal cancer. Am J Cancer Res. 2015;5:2064–74. [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Uppaluri R, Dunn GP, Lewis JS. Focus on TILs: Prognostic significant of tumor infiltrating lymphocytes in head and neck cancer. Cancer Immun. 2008;8:16. [PMC free article] [PubMed] [Google Scholar]
- Vassilakopoulon M, Avgeris M, Velcheti V, et al. Evaluation of PD-L1 expression and associated tumor infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22:704–13. doi: 10.1158/1078-0432.CCR-15-1543. [DOI] [PubMed] [Google Scholar]
- Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–14. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- Wiegand S, Zimmermann A, Whilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499–502. [PubMed] [Google Scholar]
- Yamaguchi R, Tanaka M, Yano A, et al. Tumor-infiltlating lymphocytes are important pathologic predictots for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol. 2012;43:1688–94. doi: 10.1016/j.humpath.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovalian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.