Dear Editor,
Monkeypox, a zoonotic virus from the poxviridae family and orthopoxvirus genus, has spread outside Africa since early May 2022 [1,2]. As it belongs to the same genus as smallpox, it has raised concerns worldwide. Virus endemicity is predominantly found in forest regions in western and central Africa [2]. The first case of human infection outside Africa was initially reported in the United States in 2003 when the individuals were exposed to infected prairie dogs [3]. As of July 8, 2022, the recent monkeypox outbreak has affected 7604 cases globally and predominantly in Europe [4].
The major animal reservoirs for the virus have yet to be identified. Nevertheless, it is believed that African squirrels and other rodents might be the primary reservoirs [2]. Human to animal disease transmission, a phenomenon referred to as reverse zoonosis, has been an issue during the SARS-CoV-2 and H1N1 pandemics [5]. The ongoing monkeypox epidemic comes with the caveat that reverse zoonosis may increase. Domestic animals living near their owners are more likely to become contaminated. Until now, dogs and ferrets appear to be invulnerable to the virus. Even though cats are susceptible to cowpox and zoonotic orthopoxvirus infection, monkeypox has not been reported in African cats.
Additionally, monkeypox might infect adult albino rabbits by natural means as well as neonates of rats and exotic pet mice in vitro. There is still controversy regarding the presence of non-domesticated animals prone to infection; however, rodents and squirrels are the most probable candidates [2]. Thus, scientists around the world must implement interdisciplinary strategies and investigate animal reservoirs of concerning zoonotic viruses such as monkeypox [6]. It is possible that asymptomatic individuals and children infected with monkeypox may pose a greater risk of transmitting the disease to domestic animals due to their increased engagement with them.
There is a possibility that wild animal populations vulnerable to reverse zoonoses may be reduced and even wiped out in the event of a reverse zoonosis. This might result in the area losing biological diversity and environmental equilibrium. Using conventional approaches, it may not be possible to recognize and eliminate reverse zoonoses successfully. This is due to the differences in risk factors and transmission mechanisms that depend on the animal type. Hence, reverse zoonoses must be controlled and minimized by utilizing modern techniques such as Artificial Intelligence. During the SARS-CoV-2 pandemic, it was suggested that contemporary technological advancements could be used to evaluate pet owners' movement patterns to determine their history of contact with the infection and inform them if they have been exposed to SARS-CoV-2 risk. Thus, they would be able to safeguard their pets from the possibility of contamination [7].
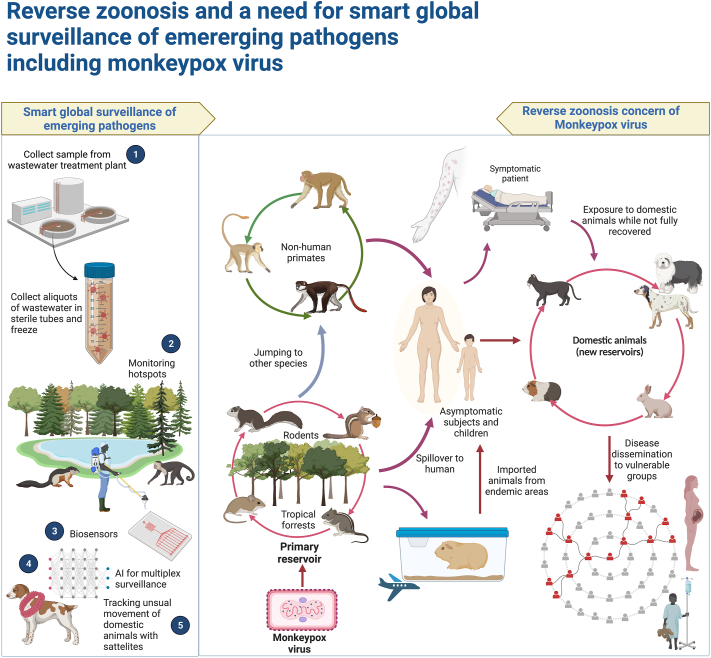
The 21st century has witnessed the emergence or reemergence of numerous pathogens that pose a significant threat to public health. SARS-CoV-2 pandemic revealed weaknesses of the current surveillance system in efficiently managing such outbreaks. In most cases, animals are the primary source of these outbreaks. Regular surveillance typically focuses on well-established illnesses. Nevertheless, the growing incidence of emerging outbreaks highlights the criticality of timely identifying pathogens before they reach humans. The most critical step to achieving these goals is establishing a system of surveillance that encompasses both animal and human populations along with optimizing current health care systems. A system of this type would utilize predetermined areas known as “hot spots” to identify and suppress any virus outbreak in human or animal populations before it has a chance to spread. This proactive approach would make it easier to anticipate future hazards and facilitate timely intervention. To address evolving challenges successfully, the establishment of a surveillance network that provides early notice on a global scale may be beneficial. This may require collaboration between international organizations, local and national partners, and funding organizations [8,9].
Another method of identifying pathogens quickly and effectively is to use biosensors. In addition to providing fast and affordable detection methods, biosensing technologies improve detection sensitivity. Biosensors that are precise, targeted, and transportable are required to combat the epidemics and pandemics of zoonotic pathogens [10]. Moreover, it may be possible to track the behavior and detect health problems of farm animals using GPS devices and sensors installed, for example, on their collars [7]. During the SARS-CoV-2 pandemic, RNA of the virus was detected in wastewater after the virus was shed in the feces of infected patients. This finding, coupled with the fact that the virus can survive in wastewater for up to a few days in an ideal environment, led to the suggestion of a new surveillance method. It has been proposed that it may be possible to prevent the spread of the disease by monitoring sewage flows and the viral RNA detection [11]. There is a possibility that these surveillance methods, which were introduced during the SARS-CoV-2 pandemic, may prove useful in the ongoing epidemic of monkeypox. There is, however, a need for further investigation.
In summary, reverse zoonosis, a concerning phenomenon that sparked worries during previous epidemics such as H1N1 and SARS-CoV-2, might become a threat as the monkeypox epidemic spreads. Therefore, we should be vigilant and take note of the failures of conventional surveillance systems. This will hopefully enable us to create a global surveillance system that adequately prepares us for future pandemics. Governments and international organizations must collaborate to develop such a system. It would probably be beneficial to evaluate some of the novel surveillance approaches developed during the SARS-CoV-2 pandemic in greater depth during the monkeypox epidemic to determine whether they will be effective. These proactive steps and the reverse zoonosis potential of the monkeypox virus are depicted in Fig. 1.
Fig. 1.
The prominence of monkeypox virus reverse zoonosis in domestic animal population and the necessity of a comprehensive global surveillance system for monitoring emerging pathogens through more advanced approaches.
Data availability statement
Not applicable
Funding statement
This work was not supported financially by any governmental or non-governmental organization.
Transparency declaration
The authors of this work declare no conflict of interests.
Ethics approval statement
Not applicable.
Patient consent statement
Not applicable.
Permission to reproduce material from other sources
Not applicable.
Clinical trial registration
Not applicable.
Authors’ contribution
AA framed the idea of the study and contributed to writing the first draft. AD helped with illustration, and with EA, searched the database, and finalized the manuscript content. All authors have read and confirmed the content of the manuscript for publication.
Contributor Information
Arya Afrooghe, Email: aryaafrooghe99@gmail.com.
Amirmasoud Rayati Damavandi, Email: amirmasoud.r@yahoo.com.
Elham Ahmadi, Email: elhaam.ahmadi98@gmail.com.
References
- 1.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 2.Haddad N. The presumed receptivity and susceptibility to monkeypox of European animal species. Infect Dis Now. 2022;52(5):294–298. doi: 10.1016/j.idnow.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds M.G., Yorita K.L., Kuehnert M.J., Davidson W.B., Huhn G.D., Holman R.C., et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194(6):773–780. doi: 10.1086/505880. [DOI] [PubMed] [Google Scholar]
- 4.https://map.monkeypox.global.health/country Available from:
- 5.Sooksawasdi Na Ayudhya S., Kuiken T. Reverse zoonosis of COVID-19: lessons from the 2009 influenza pandemic. Vet Pathol. 2021;58(2):234–242. doi: 10.1177/0300985820979843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty C., Bhattacharya M., Nandi S.S., Mohapatra R.K., Dhama K., Agoramoorthy G. Appearance and re-appearance of zoonotic disease during the pandemic period: long-term monitoring and analysis of zoonosis is crucial to confirm the animal origin of SARS-CoV-2 and monkeypox virus. Vet Q. 2022;42(1):119–124. doi: 10.1080/01652176.2022.2086718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia P., Dai S., Wu T., Yang S. New approaches to anticipate the risk of reverse zoonosis. Trends Ecol Evol. 2021;36(7):580–590. doi: 10.1016/j.tree.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aarestrup F.M., Bonten M., Koopmans M. Pandemics- one health preparedness for the next. Lancet Reg Health Eur. 2021;9:100210. doi: 10.1016/j.lanepe.2021.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll D., Morzaria S., Briand S., Johnson C.K., Morens D., Sumption K., et al. Preventing the next pandemic: the power of a global viral surveillance network. BMJ. 2021;372:n485. doi: 10.1136/bmj.n485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migueis S.D.C., Tavares A.P.M., Martins G.V., Frasco M.F., Sales M.G.F. Biosensors for European zoonotic agents: a current Portuguese perspective. Sensors (Basel) 2021;21(13) doi: 10.3390/s21134547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangkham S. A review on detection of SARS-CoV-2 RNA in wastewater in light of the current knowledge of treatment process for removal of viral fragments. J Environ Manage. 2021;299:113563. doi: 10.1016/j.jenvman.2021.113563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable