Abstract
Introduction
Immune checkpoint pathway markers induce immune tolerance to non-small cell lung cancer (NSCLC). Therapeutic antibodies targeting the PD-1/PD-L1 pathway have demonstrated efficacy in tumors expressing relatively high PD-L1. Minimally invasive endobronchial ultrasound guided fine needle aspiration (EBUS-FNA) allows for patients with inoperable tumors or comorbidities to attain a confirmatory diagnosis. The aims of this study include determining if PD-L1 testing is equivalent on cytology and biopsy/resection specimens at different tumor proportion score (TPS) cutoffs and for different NSCLC subtypes.
Materials and Methods
Data was retrospectively collected on patients with paired NSCLC cytology and surgical resection specimens between 5/4/2007-5/4/2017. The FDA-approved Dako PD-L1 immunohistochemistry (IHC) 22C3 pharmDx™ Kit was performed on paired cytology cell block and biopsy/resection specimens and PD-L1 tumor proportion scores (TPS) were recorded. Statistical analysis of categorical and continuous variables was performed using SAS 9.4.
Results
53 paired cytology/resection samples (27 adenocarcinomas, 25 squamous cell carcinomas (SqCC), and 1 unclassified) were analyzed. Supposing the resection reflects true PD-L1 expression, the cytology method showed 73.3% sensitivity, 65.2% specificity, 73.3% positive predictive value (PPV), 65.2% negative predictive value (NPV), and overall agreement of 69.5%. For PD-L1 high expression (≥ 50%), the cytology method demonstrated overall agreement of 79.2%. Overall agreement between methods was 81.5% and 76% in cases of adenocarcinoma and squamous cell carcinoma, respectively.
Discussion/Conclusions
NSCLC cytology samples from EBUS-FNA are suitable for PD-L1 testing, especially using a high PD-L1 expression cutoff (≥ 50%) and in adenocarcinomas.
Keywords: PD-L1, checkpoint inhibitor, non-small cell lung cancer, FNA, EBUS-FNA
Introduction
Lung cancer is the most common fatal cancer worldwide with approximately 80% of lung cancer patients presenting with locally advanced or metastatic disease.1 The molecular characterization of non-small cell lung cancers (NSCLC) has enabled the identification of oncologic subsets associated with immune checkpoint pathway markers exhibiting host immune tolerance and carrying implications for therapeutic treatments and prognosis.2 An immune checkpoint protein, programmed death ligand-1 (PD-L1) abrogates the immune system’s T-cell response through interaction with PD-1. PD-L1 is expressed in 24-60% of NSCLC, with expression enabling tumor cells to evade the host immune system.3 Tumor cell PD-L1 assessment via immunohistochemistry (PD-L1 IHC) on surgical pathology (biopsy/resection) specimens is the standard for companion diagnostic testing and assists in determining which tumors may respond to therapeutic antibodies targeting the PD-1/PD-L1 pathway (checkpoint blockade inhibitors).4
Therapeutic antibodies targeting the PD-1/PD-L1 pathway have demonstrated therapeutic efficacy in tumors with relatively high PD-L1 expression.1,4-8 Interestingly, despite absent or low PD-L1 IHC, some patients respond to anti-PD-1/PD-L1 therapies.1,9 Proposed contributing factors to this phenomenon include: 1) the inherent biological characteristics of PD-L1 and 2) the technicalities of the PD-L1 IHC stains. Biologically, PD-L1 expression may be altered by dynamic induction of its expression, intra- and inter-tumoral expression heterogeneity,10 and modulated expression levels in response to concomitant therapies or the tumor microenvironment1,11,12. Technical factors affecting PD-L1 IHC staining include tumor fixation time, inter-clone affinity for the epitope(s), and varied signal amplification methods.1,11 Because of these factors, the Food and Drug Administration (FDA)-approved companion diagnostic IHC tests are tightly regulated. Despite the aforementioned nuances with IHC staining, advantages include low cost, widespread availability, rapid turnaround time, and direct signal visualization.1 In recognition of both the utility and limitations of PD-L1 immunohistochemistry, the FDA and other organizations have devoted effort to compare the performance and staining patterns of different PD-L1 antibody clones and those offered by different vendors.13,14 The goal is to strengthen the comparability, reproducibility, and predictive capacity of PD-L1 testing across different treatment centers.
Given the advanced stage of the majority of patients with NSCLC at presentation, minimally invasive diagnostic techniques, such as endobronchial ultrasound guided fine needle aspiration (EBUS-FNA), allow for patients with inoperable tumors or complicating comorbidities to attain a confirmatory diagnosis without the need for surgical mediastinoscopy or thoracotomy. Similarly, liquid-based cytology specimens collected through less-invasive means (e.g. thoracentesis in patients with malignant pleural effusions) have proven useful for both diagnostic and molecular testing purposes.15 Molecular analysis of EBUS-FNA-derived cytology specimens has been shown to detect EGFR, KRAS, and ALK mutations, despite the relatively low tumor burden in this sample type.11 However, NSCLC cytology specimens are not currently FDA approved for PD-L1 IHC testing. Validation of PD-L1 IHC staining on formalin fixed, paraffin embedded, cellblock material from EBUS-FNA procedures would enable prognostic and theragnostic evaluation from a single, less invasive procedure.
The availability of PD-L1 IHC testing on samples acquired through minimally invasive methods is desired as it expands the patient population that could potentially benefit from this personalized therapy.16 The primary aim of this study is to determine PD-L1 tumor proportion score (TPS) concordance between cytology and surgical pathology specimens. Our secondary aims include the determination of the TPS agreement between cytology and surgical specimens stratified by PD-L1 expression systems (two-tier and three-tier) and NSCLC subtypes, specifically adenocarcinoma and squamous cell carcinoma (SqCC).
Materials and Methods
Following Institutional Review Board (IRB) approval, a retrospective search of our institution’s laboratory information system (LIS) from 5/4/2007-5/4/2017 was undertaken to identify patients with paired, treatment naïve NSCLC cytology and surgical pathology (biopsy/resection) specimens. Patients with unpaired specimens, diagnoses other than NSCLC, inadequate material for a diagnosis, and inadequate material for IHC stains were excluded from the study. Data collected on each participant included lung cancer histologic type and any existing PD-L1 IHC results.
Once the cohort was formed, two pathologists reviewed the lung cancer surgical resection and the cytology cell block specimens for 1) confirmation of diagnosis/classification and 2) to determine if sufficient material (100+ tumor cells) existed for PD-L 1 IHC. Cell block preparation over the course of the study period followed the cytology laboratory’s standard operating procedure which varies by specimen quantity. Needle rinses and dedicated passes from EBUS-FNA procedures are collected in 10% neutral buffered formalin. Specimens with >50 mL of fluid were processed using the thrombin sediment cell block method, and specimens with <50 mL of fluid were processed using thrombin to create a sediment cell block and PreservCyt to create an additional ThinPrep preparation. All cytology materials used for this study were ultimately formalin fixed, paraffin embedded. PD-L1 IHC was performed using the FDA-approved Dako PD-L1 IHC 22C3 pharmDx™ Kit for surgical specimens which lacked prior routine PD-L1 IHC and for all paired cytology specimens. The same two pathologist reviewed PD-L1 IHC in conjunction with a hematoxylin and eosin (H&E) stained slide to determine the level of PD-L1 expression, expressed as the tumor proportion score (TPS).
For statistical analysis, categorical variables were summarized with frequency counts and percentages. Continuous variables were summarized with mean/median/standard deviation/IQR/minimum and maximum. Significance was assessed at alpha = 0.05. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC). Based on the TPS, specimens were classified into two categories (positive and negative) or three categories (negative, <1% low positive, 1%-49% and high positive, 50-100%). PD-L1 expression on cytology and surgical resection specimens was cross-tabulated to calculate sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Agreement of TPS between cytology specimens and surgical specimens was examined using simple Kappa statistics (two-category expression) or weighted Kappa statistics (three-category expression) based on a commonly cited scale.17 Bland-Altman plots were used to assess the magnitude of disagreement, including error and bias of the two methods on measuring TPS score. Intraclass correlation coefficients (ICC), a measure of reliability of the two measurements, with the respective 95% confidence interval, were calculated.
Results
A search of the LIS for patients with lung cancer identified 517 patients. After exclusion criteria was addressed, 53 patients with corresponding surgical excision and EBUS-FNA cytology cases were included in the study. Of the 53 patients, 27 (50.9%) had adenocarcinoma, 25 (47.2%) had SqCC, and 1 (1.9%) had an unclassified tumor. The surgical specimens were composed of 36 biopsies (68%) and 17 resections (32%) and study specimen sources included 49 lung, 2 lymph node, and 2 pleura sites. The results that follow suppose that the surgical biopsy/resection specimen reflects true PD-L1 expression when comparing EBUS-FNA cytology.
Table 1 summarizes the results of PD-L1 expression on paired cytology and surgical specimens using a two-tiered system (<1% TPS, negative and ≥1% TPS, positive). The Kappa coefficient is 0.386 (95% CI [0.135, 0.636]), indicating there is fair agreement in measuring TPS between EBUS-FNA cytology and surgical resection specimens. Table 2 summarizes the results of PD-L1 expression on paired cytology and surgical specimens using a three-tiered system (<1% TPS, negative; 1-49%, low expression positive; 50-100%, high expression positive. The Kappa coefficient is 0.452 (95% CI [0.245, 0.658])), indicating there is moderate agreement between the EBUS-FNA cytology and surgical specimens. Table 3 focuses on PD-L1 high expression positive (≥ 50%) on paired cytology and surgical specimens. The Kappa coefficient is 0.526 (95% CI [0.291, 0.761]), indicating that there is moderate agreement of high expression positive between surgical specimens and EBUS-FNA cytology.
Table 1:
PD-L1 expression using the cytological method stratified by the surgical method with tumor proportion score (percentage of PD-L1 expressed by tumor cells) expression using the two-category system where <1% is graded as PD-L1 negative and >/=1% is graded as PD-L1 positive.
| Cytology | Surgery-Positive | Surgery-Negative | Total |
|---|---|---|---|
| Overall NSCLC | n=30 | n=23 | n=53 |
| Positive | 22 (73.3%) | 8 (34.8%) | 30 (56.6%) |
| Negative | 8 (26.7%) | 15 (65.2%) | 23 (43.4%) |
| Adenocarcinoma | n=16 | n=11 | n=27 |
| Positive | 12 (75%) | 2 (18.2%) | 14 (51.9%) |
| Negative | 4 (25%) | 9 (81.8%) | 13 (48.1%) |
| SqCC | n=14 | n=11 | n=25 |
| Positive | 10 (71.4%) | 6 (54.5%) | 16 (64%) |
| Negative | 4 (28.6%) | 5 (45.5%) | 9 (36%) |
Abbreviations: NSCLC-non-small cell lung cancer; SqCC-squamous cell carcinoma
Table 2:
PD-L1 expression using the cytological method stratified by the surgical method with tumor proportion score (percentage of PD-L1 expressed by tumor cells) expression using the three-category system where <1% is graded as PD-L1 negative, 1-49% as low PD-L1 positive, and >/=50% is graded as high PD-L1 positive.
| Cytology | Surgery- High positive |
Surgery- Low positive |
Surgery-Negative | Total |
|---|---|---|---|---|
| Overall NSCLC | n=20 | n=10 | n=23 | n=53 |
| High positive | 11 (55%) | 0 (0%) | 2 (8.7%) | 12 (24.5%) |
| Low Positive | 4 (20%) | 7 (70%) | 6 (26.1%) | 17 (32.1%) |
| Negative | 5 (25%) | 3 (30%) | 15 (65.2%) | 23 (43.4%) |
| Adenocarcinoma | n=12 | n=4 | n=11 | n=27 |
| High positive | 7 (58.3%) | 0 (0%) | 0 (0%) | 7 (25.9%) |
| Low Positive | 2 (16.7%) | 3 (75%) | 2 (18.2%) | 7 (25.9%) |
| Negative | 3 (25%) | 1 (25%) | 9 (81.8%) | 13 (48.1%) |
| SqCC | n=8 | n=6 | n=11 | n=25 |
| High positive | 4 (50%) | 0 (0%) | 2 (18.2%) | 6 (24%) |
| Low Positive | 2 (25%0 | 4 (66.7%) | 4 (36.4%) | 10 (40%) |
| Negative | 2 (25%) | 2 (33.3%) | 5 (45.5%) | 9 (36%) |
Abbreviations: NSCLC-non-small cell lung cancer; SqCC-squamous cell carcinoma
Table 3:
PD-L1 expression using the cytological method stratified by the surgical method with tumor proportion score (percentage of PD-L1 expressed by tumor cells) expression using the high expression (>/= 50% cutoff) as positive.
| Cytology | Surgery- Positive |
Surgery- Negative |
Total |
|---|---|---|---|
| Overall NSCLC | n=20 | n=33 | n=53 |
| Positive | 11 (55%) | 2 (6.1%) | 13 (24.5%) |
| Negative | 9 (45%) | 31 (93.9%) | 40 (75.5%) |
| Adenocarcinoma | n=12 | n=15 | n=27 |
| Positive | 7 (58.3%) | 0 (0%) | 7 (25.9%) |
| Negative | 5 (41.7%) | 15 (100%) | 20 (74.1%) |
| SqCC | n=8 | n=17 | n=25 |
| Positive | 4 (50%) | 2 (11.8%) | 6 (24%) |
| Negative | 4 (50%) | 15 (88.2%) | 19 (76%) |
Abbreviations: NSCLC-non-small cell lung cancer; SqCC-squamous cell carcinoma
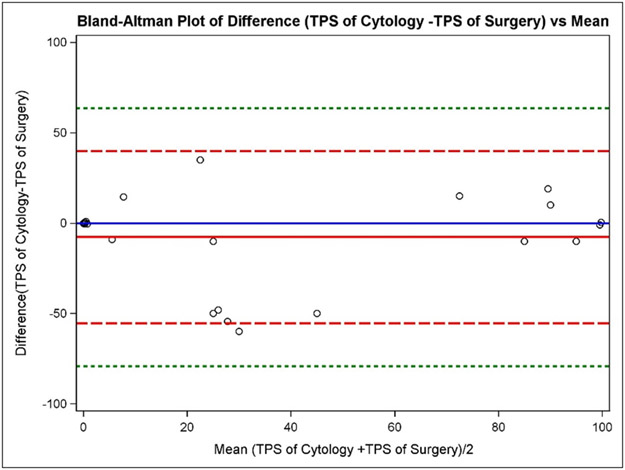
A Bland-Altman plot was used to determine the direction of bias between surgical resection and EBUS-FNA cytology (Figure 1). The plot illustrates that the mean of the difference (Cytology TPS – Surgical TPS) is very close to the (Cytology TPS – Surgical TPS) =0 line, indicating that Cytology TPS measures are, on average, similar to Surgical TPS specimen measures. Moreover, when the PD-L1 TPS is >60% or <10%, the surgical resections and EBUS-FNAs are more likely to agree with each other. The intraclass correlation coefficient (ICC) of TPS by the two sample types is 0.669 (95% CI [0.488, 0.794]).
Figure 1: Bland-Altman Plot of difference in tumor proportion score (TPS) of cytological versus surgical method compared to the mean.
Bland-Altman plot used to show the difference between the methods (Cytologic TPS – Surgical TPS) against the average (Cytologic TPS – Surgical TPS)/2 with the addition of a horizontal line at (Cytologic TPS – Surgical TPS) =0 (solid blue line), the mean of the difference (Cytologic TPS – Surgical TPS) (solid red line), dotted lines at ± 2SD (dashed red lines), and dotted lines at ± 3SD (dashed green lines). The 2 standard deviation limits (dashed red lines) provide an estimate of where 95% of the differences should lie if the differences are normally distributed. Plotting the horizontal line at (Cytologic TPS – Surgical TPS) =0 and comparing the location of the horizontal line at the mean of the difference (Cytologic TPS – Surgical TPS) aids in determining bias in one direction or another. In this case, the mean of the difference (Cytologic TPS – Surgical TPS) is very close to the (Cytologic TPS – Surgical TPS) =0 line, indicating that TPS of Cytology measures are, on average, similar to TPS of Surgery. When TPS is greater than 60% or less than 10%, the two methods are more likely to agree with each other.
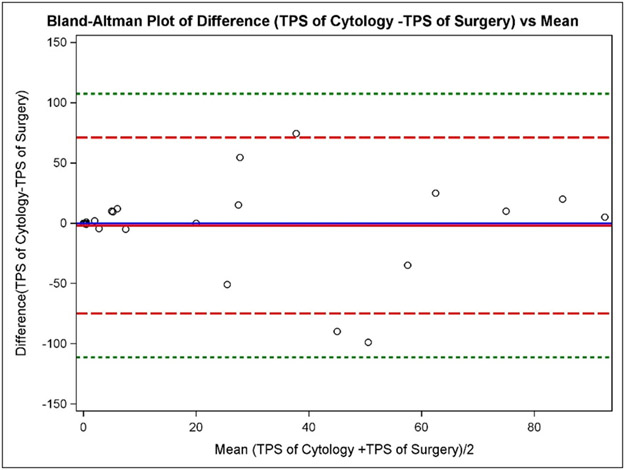
Patients were further divided into two groups based on the NSCLC tumor type (adenocarcinoma and SqCC) and results of PD-L1 expression graded by the two-tiered system, three-tiered system, and using high expression positive (>/=50%) cutoff; these are summarized in Tables 1-3, respectively. For lung adenocarcinomas, two-tiered PD-L1 expression on EBUS-FNA cytology and surgical resection is summarized in Table 1. The sensitivity of the cytology method is 75%, the specificity is 81.1%, the PPV is 85.7%, the NPV is 69.2%, and the overall agreement is 77.8%. The Kappa coefficient is 0.553 (95% CI [0.241, 0.865]), indicating that there is moderate agreement between the surgical resection and EBUS-FNA cytology. Three-tiered PD-L1 expression of adenocarcinomas on EBUS-FNA cytology and surgical resection is summarized in Table 2. The weighted Kappa coefficient is 0.580 (95% CI [0.325, 0.835]), again indicating that there is moderate agreement. Focusing on PD-L1 high expression positive (>/= 50%) in lung adenocarcinomas, EBUS-FNA and surgical resection data is summarized in Table 3. The Kappa coefficient is 0.609 (95% CI [0.324, 0.894]), indicating that there is substantial agreement. A Bland-Altman plot (Figure 2) shows the mean of the difference (Cytology TPS–Surgical TPS) is very close to the (Cytology TPS – Surgical TPS) =0 line, indicating that Cytology TPS measures are, on average, similar to Surgical TPS of PD-L1 expression in adenocarcinoma. The ICC of TPS by the two methods is 0.819 (95% CI [0.641, 0.913]) in cases of adenocarcinoma.
Figure 2: Bland-Altman plot of the difference in the tumor proportion score (TPS) of the cytological versus surgical methods compared to the mean in adenocarcinoma specimens.
Bland-Altman plot used to show the difference between the methods (Cytologic TPS – Surgical TPS) against the average (Cytologic TPS – Surgical TPS)/2 with the addition of a horizontal line at (Cytologic TPS – Surgical TPS) =0 (solid blue line), the mean of the difference (Cytologic TPS – Surgical TPS) (solid red line), dotted lines at ± 2SD (dashed red lines), and dotted lines at ± 3SD (dashed green lines). The mean of the difference (Cytologic TPS – Surgical TPS) is very close to the (Cytologic TPS – Surgical TPS) =0 line, indicating that TPS of cytology measures are, on average, similar to TPS of surgery.
For SqCC specimens, PD-L1 expression using the cytological method stratified by expression using the surgical method is summarized in Table 1. The sensitivity of the cytology method is 71.4%, the specificity is 45.5%, the PPV is 62.5%, the NPV is 55.6%, and the overall agreement is 60.0%. The Kappa coefficient is 0.172 (95% CI [−0.212, 0.557]) and since the lower limit of the 95% confidence interval of the Kappa coefficient goes below 0, it indicates that there is poor agreement between the two methods when evaluating TPS in SqCC specimens. Using the three-category PD-L1 expression system, the cytological method stratified by expression using the surgical method is summarized in Table 2 for SqCC cases. The weighted Kappa is 0.281 (95% CI [−0.045, 0.606]) and since the lower limit of the 95% confidence interval of the Kappa coefficient goes below 0, again, it indicates that there is poor agreement between the two methods when evaluating TPS in SqCC specimen. Focusing on PD-L1 high expression (>/= 50%), expression using the cytological method stratified by expression using the surgical method is summarized in Table 3. The sensitivity of the cytology method is 50.0%, the specificity is 88.2%, the PPV is 66.7%, the NPV is 79.0%, and the overall agreement is 76.0%. The Kappa coefficient is 0.409 (95% CI [0.022, 0.797]), indicating that there is fair agreement between the two methods. A Bland-Altman plot (Figure 3) shows the mean of the difference (Cytology TPS–Surgical TPS) is very close to the (Cytology TPS – Surgical TPS) =0 line, indicating that TPS of cytology measures are, on average, similar to TPS of surgical measures of PD-L1 expression in SqCC. The ICC of TPS by the two methods is 0.452 (95% CI [0.077, 0.715]) in cases of SqCC.
Figure 3: Bland-Altman plot of the difference in the tumor proportion score (TPS) of the cytological versus surgical methods compared to the mean in squamous cell carcinoma specimens.
Bland-Altman plot used to show the difference between the methods (Cytologic TPS – Surgical TPS) against the average (Cytologic TPS – Surgical TPS)/2 with the addition of a horizontal line at (Cytologic TPS – Surgical TPS) =0 (solid blue line), the mean of the difference (Cytologic TPS – Surgical TPS) (solid red line), dotted lines at ± 2SD (dashed red lines), and dotted lines at ± 3SD (dashed green lines). The mean of the difference (Cytologic TPS – Surgical TPS) is very close to the (Cytologic TPS – Surgical TPS) =0 line, indicating that TPS of cytology measures are, on average, similar to TPS of surgery.
Of the 14 discordant cases, 5 were adenocarcinoma, and 9 were SqCC. The tissue types of the surgical pathology samples with discordant cytology cases included 7 resection specimens and 7 biopsy specimens. All of the surgical samples in the discordant case cohort were from lung tissue. The 6 false negative surgical samples (PD-L1 negative surgical specimens with corresponding PD-L1 positive biopsy specimens) were composed of 3 biopsy and 3 resection specimens. The TPS of these specimens on cytology was 14.8% on average (range 1-55) (Table 4).
Table 4:
Discordant cytology and surgical pathology specimens by PD-L1 expression result, sample source, and surgical specimen type.
| Tumor Subtype (SqCC = squamous cell carcinoma) |
PD-L1 expression: Cytology Result (P=positive; N=negative) |
Cytology TPS |
PD-L1 expression: Surgical Pathology Result (P=positive; N=negative) |
Surgical Pathology TPS |
Surgical Pathology Specimen Source |
Surgical Pathology Specimen Type |
|---|---|---|---|---|---|---|
| Adenocarcinoma | N | <1 | P | 1 | Lung | Resection |
| Adenocarcinoma | N | 0 | P | 50 | Lung | Biopsy |
| Adenocarcinoma | N | 0 | P | 60 | Lung | Biopsy |
| SqCC | P | 10 | N | 0 | Lung | Biopsy |
| SqCC | N | <1 | P | 5 | Lung | Biopsy |
| Adenocarcinoma | N | <1 | P | 55 | Lung | Resection |
| Adenocarcinoma | P | 1 | N | 0 | Lung | Biopsy |
| SqCC | N | 0 | P | 1 | Lung | Resection |
| SqCC | N | 0 | P | 90 | Lung | Resection |
| SqCC | N | 0 | P | 51 | Lung | Biopsy |
| SqCC | P | 12 | N | 0 | Lung | Biopsy |
| SqCC | P | 1 | N | 0 | Lung | Resection |
| SqCC | P | 55 | N | <1 | Lung | Resection |
| SqCC | P | 10 | N | <1 | Lung | Resection |
Discussion
Currently, core biopsies and surgical resection specimens are FDA approved for testing using the Dako PD-L1 IHC 22C3 pharmDx™ Kit. Similarly, other PD-L1 companion diagnostic IHC tests were also approved on this specimen type, and cytology specimens fall outside of that approval. PD-L1 checkpoint inhibitors are indicated for first and second-line therapy in patients with advanced stage NSCLC or chemotherapy resistant/refractory patients, respectively.18 Patients with treatment refractory or advanced stage NSCLC are medically vulnerable, often with numerous comorbidities limiting both their ability to tolerate and the clinical appropriateness of surgical resections. As such, minimally invasive tissue sampling methods, including EBUS-FNA, are greatly preferable to surgery when collecting samples for ancillary studies to predict response to immunotherapy.
Several studies have demonstrated varying degrees of correlation between PD-L1 expression in paired surgical and cytology samples, ranging from 0.63 to 0.78 concordance/agreement. However, these included a mixture of EBUS-FNA and exfoliative fluid cytology specimens.19 EBUS-FNA sampling targets a small portion of tumor cells and this may compromise PD-L1 expression assessment compared to surgical resection or even pleural fluid specimens in tumors with more robust expression heterogeneity.20
Data from the current study indicates that tumor heterogeneity may be greater in lung SqCC versus lung adenocarcinoma as concordance between SqCC surgical pathology and EBUS-FNA cytology specimens was poor (kappa coefficient 0.172) versus moderate in adenocarcinoma (kappa coefficient 0.553). Tumors with <10% or >60% TPS may reflect less tumor heterogeneity based on the higher agreement between surgical and cytology specimens with these TPS levels. Furthermore, there may be less intra- and inter-observer variability in TPS assessment at the far ends of the PD-L1 expression spectrum. These findings suggest there may be PD-L1 expression ranges in which cytologic evaluation provides a more accurate assessment of overall PD-L1 expression and is in line with that which would be seen in a surgical resection specimen of the same tumor. Whereas we saw an increase in concordance rates of PD-L1 expression from 69.8% for >/=1% to 79.2% for >/=50%, Sakata et al reported a decrease in concordance using a >50% cutoff (82%) compared to a >/=1% cutoff (87%).21 These conflicting findings highlight the need for further studies to establish clinically and biologically relevant TPS cutoff values for PD-L1 IHC on EBUS-FNA samples. Apart from tumor subtype possibly contributing to disagreement between cytology and surgical specimens, tumor grade may have played a role as the discrepant cases were predominantly moderately-poorly differentiated. Cell block tumor cellularity and architecture did not seem to contribute to discrepant PD-L1 staining between cytology and surgical specimens.
The difference in TPS agreement between surgical and cytology samples by NSCLC subtype was not as marked in a study by Ilie et al.1 Using the ASL48 PD-L1 laboratory derived test on biopsy and cytology samples, they showed intraclass correlation coefficients of 0.864 for adenocarcinoma (n=48) and 0.962 for SqCC (n=22).22 This may be due to their series’ surgical specimens being limited to biopsies as opposed to larger resection specimens, as well as the few number of patients with SqCC included in this and our study. The hypothesis that tumor heterogeneity is a potential contributing factor to discordant staining would suggest larger surgical samples would aid in the detection of PD-L1 positive tumor cells that were not detected on cytology samples. However, in our series, only 8/14 discordant cases showed positive PD-L1 staining in the surgical specimen compared to negative staining in the cytology specimen. Surprisingly, 6/14 surgical specimens were PD-L1 negative when the cytology specimens were PD-L1 positive, despite their larger size with more potential tumor cells to stain. Another factor that may have contributed to disagreement between cytology and surgical specimens was time between procurement of each of the specimens since longer intervals may have allowed for rapidly dividing tumor cells to mutate and alter their PD-L1 expression pattern. Only 4/14 discordant cases were taken more than 1 year apart. Furthermore, various chemotherapeutic treatments taken by patients between sampling events may have also altered PD-L1 expression patterns between specimens. Specific treatment data was unavailable to analyze in this cohort. Based on the findings in our study, however, the leading objective factor that contributed to disagreement between the cytology and surgical specimens included tumor subtype with SqCC showing greater disagreement. Interestingly, all discordant cases were samples of the lung tumors directly, compared to lymph node or pleural metastases. Therefore, our data does not support the notion that metastases show greater variation in PD-L1 expression than primary tumor samples.
Potential limitations of the current study include the limited number of patients with paired, PD-L1 targeted inhibitor treatment naïve surgical and cytology specimens available with sufficient material (minimum of 100 cells) for PD-L1 IHC testing. Further, the inclusion of both core biopsies and resection specimens in our surgical pathology cohort may have exaggerated the discordance between surgical and cytology specimens.
The results of the current study demonstrate there is fair agreement in measuring PD-L1 expression between surgical pathology and cytology specimens using a two-tiered expression system (negative or positive) and moderate agreement using a three-tiered expression system (negative, low positive, or high positive). The agreement between the two specimen types is much better when TPS is >/=50%. When results are stratified by NSCLC subtype, the agreement between cytology and surgical specimens is moderate for adenocarcinoma compared to poor agreement in SqCC. The significance of the higher concordance between cytology and surgical pathology specimens with a TPS >/=50% and with adenocarcinomas may warrant caution when evaluating PD-L1 expression on SqCC cytology specimens and in cases with a TPS <50% as these may not be reflective of PD-L1 expression in the tumor as a whole. In such scenarios, it may be advisable to repeat PD-L1 testing on surgical pathology specimens if patients proceed to resection for a more accurate TPS to better guide anti-PD-1 immunotherapy. For TPS >/=50%, it is important to note that EBUS-FNA specimens were rarely false positive compared to surgical specimens (Table 3). However, about 45% EBUS-FNA specimens were false negative compared to surgical specimens. Hence, in the clinical practice, negative EBUS-FNA specimens may need to be investigated by surgical biopsies whenever feasible. In addition, our study highlights the clinically important revelation that cytology specimens obtained from a minimally invasive procedure such as EBUS-FNA, provide a reasonable first approach for determining both oncologic subtype diagnosis and PD-L1 expression, particularly when in patients whose specimens are found to be consistent with a TPS>50% and/or adenocarcinoma subtype, and can eliminate the need for obtaining more invasive surgical resection specimens in this vulnerable patient population. Overall, the fair agreement in PD-L1 expression between surgical and EBUS-FNA cytology specimens will enable the use of cytology specimens to evaluate patients for personalized treatment options with PD-L1 targeted therapy.
Funding:
The authors express gratitude to the Lung Cancer Initiative of North Carolina for their generous funding of this project. The funds they provided were used to cover the costs of PDL1 immunohistochemical stains conducted on concurrent specimens which had not been stained in the routine clinical work-up of the subjects outside the study. The Lung Cancer Initiative of North Carolina did not partake in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Declarations of interest: none
References
- 1.Ilie M, Hofman V, Dietel M, Soria JC, Hofman P. Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. 2016;468(5):511–525. [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Zhang Y, Parra E, et al. Multiplatform-based Molecular Subtypes of Non-Small Cell Lung Cancer. Oncogene. 2017;36(10):1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 Expression in Lung Cancer. J Thorac Oncol. 2016;11(7):964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwok G, Yau TCC, Chiu JW, Tse E, Kwong Y-L. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016;12(11):2777–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 6.Jr PNA, Mello RAD, Hall P, Tadokoro H, Lopes GdL. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9(6):499–506. [DOI] [PubMed] [Google Scholar]
- 7.de Mello RA, Veloso AF, Esrom Catarina P, Nadine S, Antoniou G. Potential role of immunotherapy in advanced non-small-cell lung cancer. Onco Targets Ther. 2017;10:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilleminault L, Carmier D, Heuze-Vourc'h N, Diot P, Pichon E. [Immunotherapy in non-small cell lung cancer: inhibition of PD1/PDL1 pathway]. Rev Pneumol Clin. 2015;71(1):44–56. [DOI] [PubMed] [Google Scholar]
- 9.PNA Jr, Santoro IL, Tadokoro H, et al. The role of PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: a network meta-analysis. Immunotherapy. 2016;8(4):479–488. [DOI] [PubMed] [Google Scholar]
- 10.Casadevall D, Clavé S, Taus Á, et al. Heterogeneity of Tumor and Immune Cell PD-L1 Expression and Lymphocyte Counts in Surgical NSCLC Samples. Clin Lung Cancer. 2017;18(6):682–691.e685. [DOI] [PubMed] [Google Scholar]
- 11.Hiley CT, Le Quesne J, Santis G, et al. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. The Lancet. 2016;388(10048):1002–1011. [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature reviews Cancer. 2016;16(5):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12(2):208–222. [DOI] [PubMed] [Google Scholar]
- 14.Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol. 2018;13(9):1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martini M, Capodimonti S, Cenci T, et al. To Obtain More With Less: Cytologic Samples With Ancillary Molecular Techniques-The Useful Role of Liquid-Based Cytology. Arch Pathol Lab Med. 2018;142(3):299–307. [DOI] [PubMed] [Google Scholar]
- 16.Lehman JM, Gwin ME, Massion PP. Immunotherapy and Targeted Therapy for Small Cell Lung Cancer: There Is Hope. Curr Oncol Rep. 2017;19(7):49. [DOI] [PubMed] [Google Scholar]
- 17.Landis JR KG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 18.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. The Lancet Oncology. 2016;17(11):1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833 [DOI] [PubMed] [Google Scholar]
- 21.Grosu HB, Arriola A, Stewart J, et al. PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology. 2019;24(12):1198–1203. [DOI] [PubMed] [Google Scholar]
- 22.Russell-Goldman E, Kravets S, Dahlberg SE, Sholl LM, Vivero M. Cytologic-histologic correlation of programmed death-ligand 1 immunohistochemistry in lung carcinomas. Cancer Cytopathol. 2018;126(4):253–263. [DOI] [PubMed] [Google Scholar]