Abstract
Pigmentation abnormalities are reported in the spectrum of phenotypes associated with aging and in patients with mitochondrial DNA depletion syndrome (MDS). Yet, a relevant animal model that mimics these effects and would allow us to evaluate the detrimental aspects of mtDNA depletion on melanocyte function has not been described. Here, we characterize the pigmentary changes observed in the ears of a mtDNA-depleter mouse, which phenotypically includes accentuation of the periadnexal pseudonetwork, patchy hyper- and hypopigmentation, and reticular pigmentation. Histologically, these mice show increased epidermal pigmentation with patchy distribution, along with increased and highly dendritic melanocytes. These mtDNA-depleter mice mimic aspects of the cutaneous, pigmentary changes observed in humans with age-related senile lentigines as well as MDS. We suggest that this mouse model can serve as a novel resource for future interrogations of how mitochondrial dysfunction contributes to pigmentary skin disorders. The mtDNA-depleter mouse model also serves as a useful tool to identify novel agents capable of treating pigmentary changes associated with age-related mitochondrial dysfunction in humans.
Keywords: lentigines, melanocyte, mitochondria, mtDNA depletion, skin
1 |. INTRODUCTION
Studies have demonstrated a relationship between mitochondrial function and melanocyte physiology and pathology. Mitochondria physically tether to immature melanosomes providing a connection which promotes melanosome biogenesis (Daniele et al., 2014), and mitochondrial fusion enhances melanin synthesis (Kim et al., 2014; Snyder et al., 2005). The K+-dependent Na+/Ca2+ ion exchanger, SLC24A5, associates with the mitochondria in melanocytes where it regulates melanosomal Ca2+ homeostasis, and in humans, mutations in SLC24A5 are associated with both fair skin color and oculocutaneous albinism (Lamason et al., 2005; Stokowski et al., 2007; Wei et al., 2013; Zhang, Gong, Sviderskaya, Wei, & Li, 2019). The hypopigmentary, autoimmune disorder vitiligo is in part attributed to intrinsic melanocyte fragility that is associated with mitochondrial impairment and reduced ATP production (Dell’Anna et al., 2017). Changes in melanocyte function and skin and hair pigmentation have also been associated with alterations in mitochondrial DNA (mtDNA) content. A small, but notable, cohort of humans with mtDNA disorders exhibit poikiloderma, mottled or reticular pigmentation, hypo- or hyperpigmentation, or vitiligo (Bodemer et al., 1999; Flynn, Wee, & Lane, 1998). In healthy individuals experiencing gray hair, the mtDNA “common” deletion was detected at higher levels in actively graying hair follicles in comparison with those that are pigmented or unpigmented (Arck, 2006). However, despite a clear role for mitochondria in melanogenesis, and mitochondrial dysfunction in melanocyte pathologies of the skin and hair, few studies have directly addressed the consequence of mtDNA dysfunction on skin pigmentation in vivo. Previously, we developed an inducible, mtDNA-depleter mouse that expresses a dominant-negative mutation in the DNA polymerase γ gene, Polg (Singh, Schoeb, Bajpai, Slominski, & Singh, 2018). Using this approach, induction of mtDNA depletion in adult mice caused noticeable hyperpigmentation of the ears, and these mice provide an opportunity to characterize how mtDNA alterations affect non-follicular epidermal and dermal melanocytes.
The mtDNA depletion mouse model presented here expresses a tetracycline-inducible transgene containing a dominant-negative form of Polg (POLG1-DN), and a ubiquitously expressed, tetracycline- controlled transactivator (rtTA). Doxycycline administration results in depletion of mtDNA in various tissues including the skin (Singh et al., 2018). Animals start developing phenotypic changes similar to those experienced during aging including hair loss, hair graying, and skin wrinkles within two months of receiving doxycycline. For the following characterization, mice were divided into two groups: POLG1-DN+; rtTA- (Control, Con) and POLG1-DN+; rtTA+ (mtDNA-depleter, Dep). All mice received doxycycline in their diet, and we followed changes within the ears of these mice using photography and histological analysis (see Supplemental Methods).
MtDNA-depleter mice presented with a unique pattern of cutaneous pigmentary changes subsequent to induction with doxycycline (Figure 1a, Supplemental Figure S1). These changes ranged from hyperpigmentation of the ear and the dorsal surface of the feet (Figure 1a, i.-ii), punctate pigmentation on the volar aspect of the paws (Figure 1a, iii), pigmentation in a reticular pattern on the back of the mouse that was preceded by erythema and scaling (Figure 1a, iv.), and a similar reticular hyperpigmentation pattern on the perineal area of both males and females (Figure 1a, v; Supplemental Figure S1a). A time course of the ear revealed that mtDNA-depleter mice exhibit a noticeable change in pigmentation as early as two months on the doxycycline diet, and these changes continued to progress over time (Figure 1b). Two months after treatment, the ears of many of the mtDNA mice were darker than control animals and this phenotype transitioned to patchy hyper- and hypopigmentation after eight months of treatment in several animals (Figure 1b, Supplemental Figure S2). Pigmentary changes on the extremities were less obvious than those of the ear; however, similar observations were made. The dorsal aspect of the paws of mtDNA-depleter mice began to exhibit hair loss after two months of treatment, a phenotype reported previously (Singh et al., 2018), accompanied by increased cutaneous pigmentation that became reticular over time (Figure 1c; Supplemental Figure S1b). Pigmentary changes were also observed in the non-hairy volar aspect of the paw in one of the three male mtDNA mice in the form of punctate brown spots that darkened progressively (Figure 1d).
FIGURE 1.
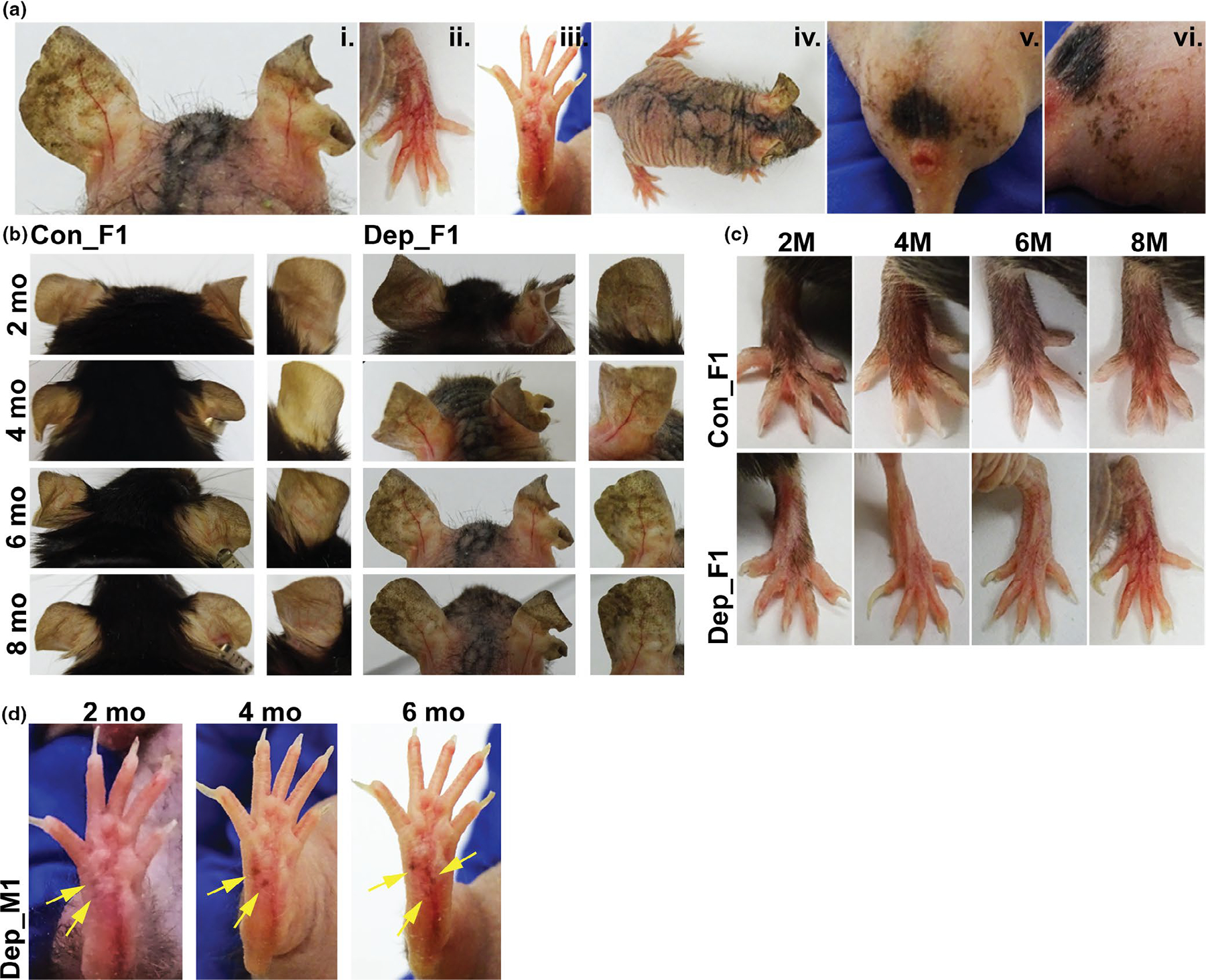
Cutaneous pigmentary changes observed in mtDNA-depleter mice. (a) The spectrum of pigmentary phenotypes observed in the mtDNA-depleter mouse model. Representative images taken of mtDNA-depleter mice after induction with doxycycline diet showing patchy hyperpigmentation on the ear at 8 months (i), reticular hyperpigmentation on the dorsal surface of the paws at 8 months (ii), punctate brown pigmentation on the volar aspect of footpads at 6 months (iii), reticular hyperpigmentation on the dorsal skin surface of a female mouse at 2.5 months after healing of erythematous scaly rash (iv), and reticular hyperpigmentation on the genital and scrotal area of a male mouse that started after 6 months of doxycycline induction (v,vi). (b-c) A time course of changes in ear (b and paw (c) pigmentation in control (Con) and mtDNA-depleter (Dep) female mice. Representative images taken of mice over 2, 4, 6 and 8 months showing no or minimal changes in the ear pigmentation pattern in the control mice while the ears in mtDNA-depleter mice start to show diffuse hyperpigmentation as early as 2 months of doxycycline induction that turns into darker, patchy pigmentation over time. Control mice exhibit no change on the dorsal surface of the paws where they retain normal body hair, whereas the mtDNA-depleter mice experience hair loss followed by increased patchy and reticular hyperpigmentation over time. (d) Images of a male mtDNA-depleter mouse with ectopic pigmentation (yellow arrows) that appears at 2 months of doxycycline induction on the volar aspect of the paw that continues to erupt and darken over time
Careful follow-up of the ear using whole-mount imaging revealed unique changes in the normal pigmentary pseudonetwork in both male and female mtDNA-depleter mice. The normal pigmentary pseudonetwork refers to diffuse skin pigmentation that is interrupted by non-pigmented adnexal openings, such as sebaceous glands and hair follicles, which is observed in control mice (Figure 2a). However, mtDNA-depleter mice revealed changes in this pseudonetwork pattern (Figure 2a–b). This included accentuation of the pseudonetwork pattern with enhanced perifollicular pigmentation (represented by Depleter_F3, F6), large areas of patchy pigmentation with aggregations of dark brown pigment (represented by Depleter_F1), and mottled areas of hyper- and hypopigmentation (represented by Depleter_F1, F3). Additionally, some mice exhibited broad areas where the normal pseudonetwork pattern of pigmentation is lost altogether (represented by Depleter_M2). Some mice also exhibited telangiectasia, a visible pattern of blood vessels that is considered a sign of extrinsic aging (Figure 2b, iii-iv). Interestingly, whole-mount images of tail skin show an overall decrease of pigment in mtDNA-depleter mice (Figure 2a). While tail skin, like the non-hairy skin of the ear and footpads, has a population of epidermal melanocytes (Glover et al., 2015), these results suggest that the ability of mtDNA depletion to produce epidermal melanocytosis may be influenced by anatomical location (e.g., different cell number or environment) or that the dosage of mtDNA depletion across skin tissues is varied.
FIGURE 2.
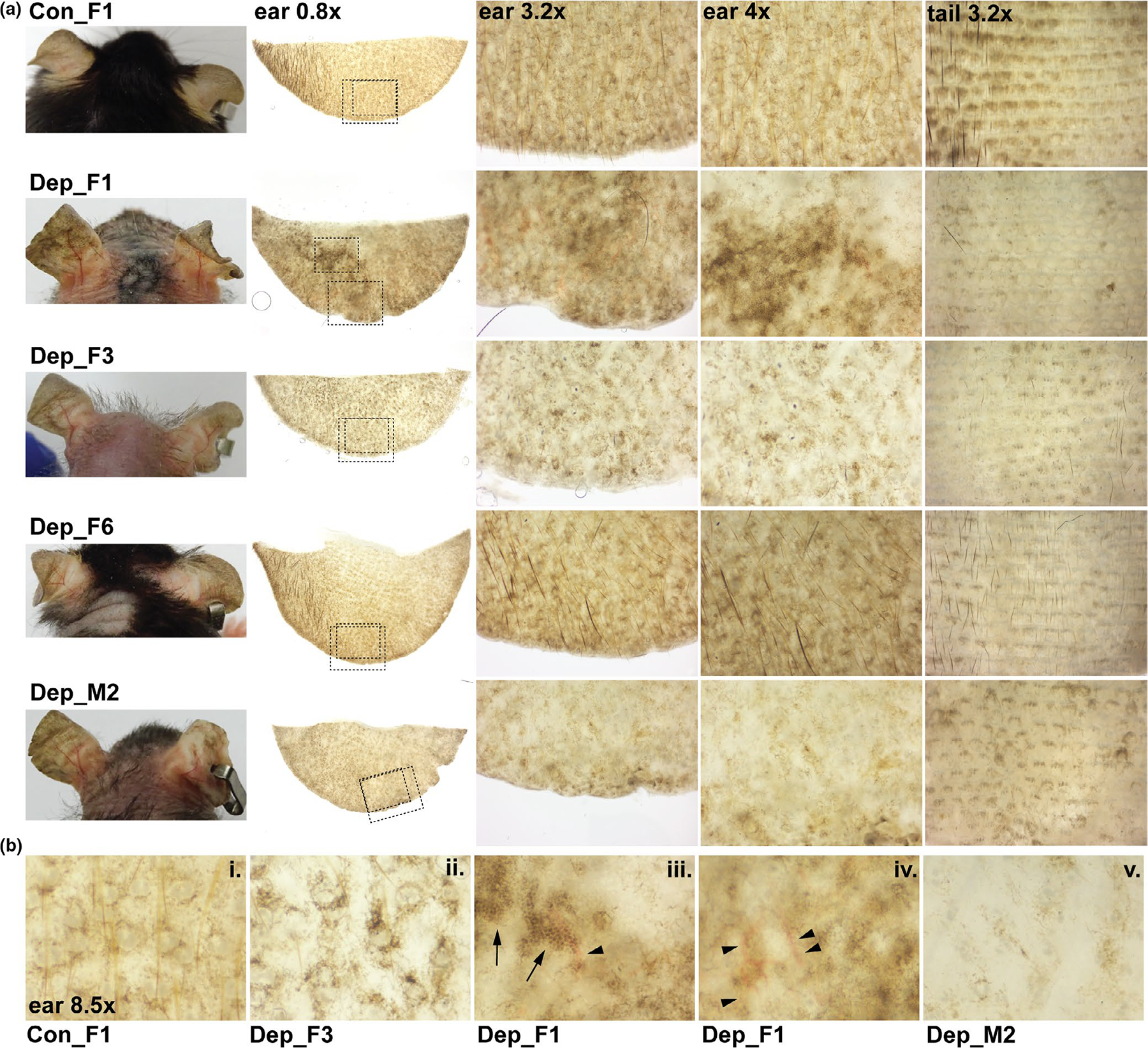
Changes in ear and tail pigmentation in mtDNA-depleter mice. (a) Representative images of mice and whole-mount images of ears and tails harvested from control (Con) and mtDNA-depleter female (F) and male (M) mice after 8 months on doxycycline diet. Ears were imaged on their posterior side. Tails were images with the epidermis facing upward. The dashed boxes in the whole-mount ear 0.8× images indicate the magnified regions shown in the ear 3.2× and ear 4x higher magnification images. (b) High magnification images (8.5×) highlighting the spectrum of pigmentary changes observed when comparing control (i) with mtDNA-depleter mice (ii-v). Control mice display the normal pseudonetwork pattern of pigmentation including symmetrical pigmentation around adnexal openings, such as sebaceous glands and hair follicles (i). MtDNA-depleter mice exhibit accentuation of perifollicular, interfollicular pigmentation (ii); coalesced hyperpigmented patches with mottled areas of hyper and hypo pigmentation and dark brown dots (arrows, iii); linear pattern of blood vessels (arrowheads, iii-iv); and some areas with significant loss of pigmentation (v)
Histological assessment of the ear, using transverse sections spanning the proximal base to the distal edge at the middle of the ear, revealed changes in both dermal and epidermal pigmentation (Figure 3). Within eosin-stained sections, control animals exhibited pigment that was loosely and evenly dispersed across the interfollicular space and intermittent cells containing pigment within the epidermis (Figure 3a, Con image). However, mtDNA-depleter mice exhibited a general redistribution of dermal pigment with areas of high and low pigmentation, more pronounced perifollicular pigmentation, and a general shift in dermal pigment toward the upper dermal layer (Figure 3a, Dep images). Epidermal pigment was observed in control animals, but generally restricted to what appeared to be rare, individual melanocytes (Figure 3a–b, Con images). MtDNA-depleter mice, on the other hand, presented with longer stretches of epidermal pigment with some areas exhibiting extensive basal and suprabasal pigmentation (Figure 3a–b, Dep images). These areas likely correspond to the patchy pigmentation pattern observed in the whole-mount ear images of some mtDNA-depleter animals (Figure 2a–b, Dep_F1). In comparison to control animals, high magnification images also reveal that mtDNA-depleter sections contain pigmented dermal cells that are generally darker, more granular, and spindle-shaped (Figure 3b, Dep_F1, F3), and pigmented epidermal cells that can be highly dendritic (Figure 3b, Dep_F3). Using digital analysis of unstained sections to quantify the percentage of pigmented area across each ear section, we discovered a notable decrease in the pigmented regions within the dermis of mtDNA-depleter mice (Figure 3c). However, unlike dermal pigment, epidermal pigment is increased strikingly in some mtDNA animals in comparison to control animals (Figure 3d).
FIGURE 3.
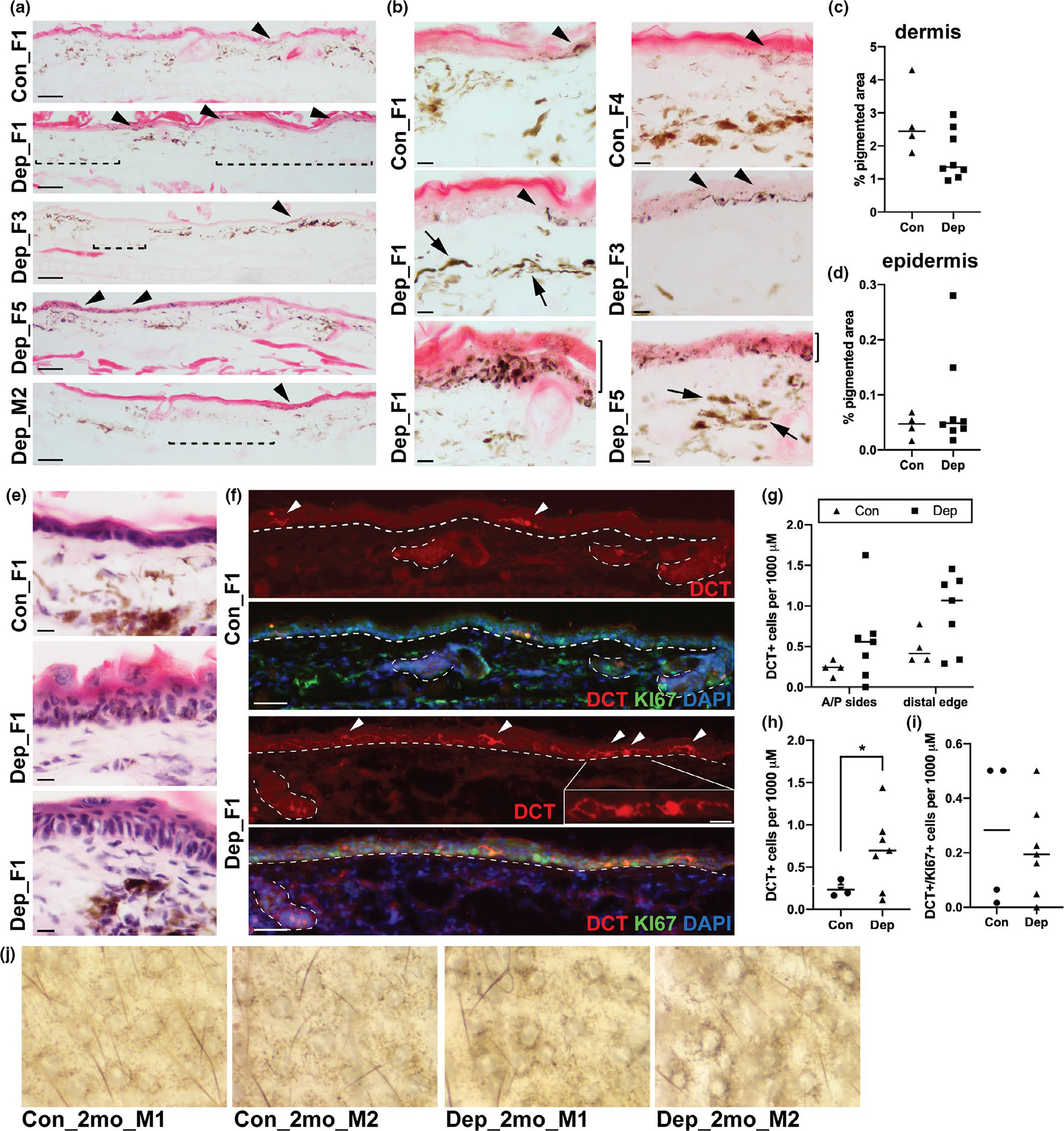
Differential pigment distribution, melanocyte morphology and color, and epidermal melanocyte number in mtDNA-depleter mouse model. (a) Representative images of eosin-stained skin sections taken from the ears of control (Con) and mtDNA-depleter (Dep) female (F) and male (M) mice after 6–8 months on doxycycline diet. Arrowheads indicate regions of epidermal pigment, and dashed brackets indicate regions of dermal hypomelanosis. Scale bar represents 100 mM. (b) Higher magnification images of similar eosin-stained sections as those shown in (a). Arrowheads indicate regions of epidermal pigment; note, highly dendritic cells indicated in Dep_F3. Arrows indicate darkly pigmented and spindle-shaped dermal cells. Solid brackets to the right of lowest panels (Dep_F1 and Dep_F3) indicate regions of basal and suprabasal epidermal pigment. Scale bar represents 10 mM. (c-d) Quantification of the area of pigmented dermis (c) and epidermis (d) observed in unstained skin sections taken from the ears of control and mtDNA-depleter mice described in (a). Each point represents one biological replicate, and the horizontal bar represents the mean of biological replicates for each condition. (e) Representative images of hematoxylin and eosin-stained skin sections taken from the ears of control and mtDNA-depleter mice demonstrating epidermal acanthosis, hyperkeratosis, and parakeratosis in Dep ears. Scale bar represents 10 mM. (f) Representative images of fluorescent immunolabeling of skin sections taken from the ears of control and mtDNA-depleter mice described in (a) and stained for the melanocyte marker DCT (red), proliferation marker KI67 (green), and DNA marker DAPI (blue). Arrowheads indicate epidermal melanocytes. Dashed lines outline the border between the epidermis and dermis and the hair follicles. Scale bar represents 100 mM. Boxed area in Dep_F1 is a higher magnification image of the region indicated, highlighting the dendricity of the epidermal melanocytes in the mtDNA-depleter mice. Scale bar in the high magnification image represents 25 mM. (g) Quantification of the DCT + positive cells observed in the sections described in (f), separated by region of the ear. Each point represents one biological replicate, and the horizontal bar represents the mean of biological replicates for each condition. (h-i) Quantification of the total DCT + cells (h) or DCT+/KI67 + cells (i) observed in the sections described in (f). Each point represents one biological replicate, and the horizontal bar represents the mean of biological replicates for each condition. *p-value < .05, Welch’s t test. Note, for the analyses presented in g-i, one mtDNA-depleter sample was omitted due to inadequate tissue quality for IHC. (j) Whole-mount images of ears of control (Con) and mtDNA-depleter male (M) mice after just 2 months on doxycycline diet
MtDNA-depleter mice are known to experience extensive epidermal hyperplasia across the dorsal skin of the body (Singh et al., 2018), and hematoxylin and eosin staining revealed similar, but less severe, acanthosis, hyperkeratosis and parakeratosis within the ear (Figure 3e). Based on this proliferative phenotype, we hypothesized that the obvious hyperpigmented epidermal plaques observed in some mtDNA animals may be the consequence of an increase in epidermal melanocytes. Using the melanocyte marker, dopachrome tautomerase (DCT), we found that only a small number of DCT + melanocytes are observed within the epidermis of control ears and these are dispersed infrequently along the anterior and posterior sides of the ear and slightly more concentrated at the edges of the ear (Figure 3f–G). Interestingly, DCT + cells can be seen staining melanocytes within the base of hair follicles, but does not mark the pigmented cells observed within the dermis. In mtDNA-depleter mice, however, the melanocyte population is elevated across both the anterior and posterior sides of the ear as well as at the edges of the ear (Figure 3f–G), and this leads to a statistically significant increase in the total pool of DCT + melanocytes within the epidermis of the entire ear section (Figure 3h). This suggests that the qualitative differences we observed in epidermal pigment in mtDNA-depleter mice (Figure 3a–b) can be attributed to an expanded epidermal melanocyte population and is a consequence of mtDNA depletion. However, despite numerous proliferative, KI67 + cells observed in keratinocytes of mtDNA-depleter mice, there was no change in the percentage of KI67 + epidermal melanocytes (DCT+/KI67+) when comparing control and mtDNA-depleter mice (Figure 3i). This suggests that epidermal ear melanocytes in mtDNA depleter mice may have been induced to proliferate at an earlier timepoint during treatment, or their etiology independent of hyperproliferation. In support of the former idea, that the ideal time to assess ear pigmentation in these mice is earlier, in ears of mice harvested at two months after initiating mtDNA depletion we observed significant changes in the pseudonetwork pattern in the mtDNA-depleter mice (n = 2, Figure 3j). We showed previously that two months of mtDNA depletion is sufficient to reduce mtDNA content by about 50% within whole skin and other body tissues (Singh et al., 2018). Thus this early phenotype supports a step-wise progression of pigmentary changes consequent to mtDNA depletion that initiates with accentuation of the pseudonetwork pattern that then leads to the patchy epidermal pigmentation and regional hypopigmentation observed after mtDNA-depleter mice are exposed to longer treatments with doxycycline.
Pigmentary changes have been observed in humans with MDS (Sreedhar, Aguilera-Aguirre, & Singh, 2020). Bodemer et al. (1999) reported clinical cases of mitochondrial dysfunction where reticulate hyperpigmentation in photoexposed areas was described in 3.6% of patients, which was often preceded by erythematous rashes on the hands and cheeks. A reticular pattern of pigmentation associated with scaly erythematous skin is also reported in Leigh syndrome, MELAS syndrome, and Pearson syndrome. Patches of hypomelanosis, reminiscent of the decreased regions of pigmentation we observed in some ears of our mtDNA-depleter mice, has also been reported in Kearns Sayre syndrome (Karpati, Carpenter, Larbrisseau, & Lafontaine, 1973; Simonsz, Bärlocher, & Rötig, 1992; Tulinius et al., 1995).
However, beyond mitochondrial diseases, there is also a strong link between mtDNA damage and photoaging. Previously it was proposed that mitochondrial genome alterations are associated with the extrinsic aging of the skin (Birch-machin, Tindall, Turner, Haldane, & Rees, 1998). The nuclear-encoded, mitochondrial DNA Polγ is the DNA replication and repair enzyme that maintains mtDNA levels and integrity which is critically essential for mitochondrial energy production (Bogenhagen, Pinz, & Perez-Jannotti, 2001). Polγ is highly susceptible to peroxynitrite attack and becomes nitrated and inactivated upon UV radiation in both human and murine keratinocytes (Bakthavatchalu et al., 2012; Dhar et al., 2018; Graziewicz, Day, & Copeland, 2002). The mutations in mtDNA upon UV exposure induce mitochondrial dysfunction that contributes to photoaging (Naidoo et al., 2018). Mutations in mtDNA are more frequent as mitochondria lack the nucleotide excision repair pathway that repairs the photoproducts resulting from UV radiation (Birch-Machin, Russell, & Latimer, 2013). Moreover, increased levels of large scale mtDNA deletions have been detected in photoaged human skin compared to sun-protected areas (Berneburg & Krutmann, 1998; Birch-machin et al., 1998; Yang, Lee, & Wei, 1995). These deletions may serve as an intracellular source for chronic oxidative stress that can lead to functional and structural alterations of the skin similar to those in photoaged human skin (Singh et al., 2018; Sreedhar et al., 2020).
The pigmentary changes observed in the ears of our mtDNA-depleter mice impressively resemble human senile lentigines (SL), a common pigmentation disorder that occurs in old age in both males and females commonly appearing at the fifth decade in life. SL progresses with aging, appears on the dorsa of hands, forearms, and face, and presents in the form of irregular patches that can coalesce into larger lesions and are frequently associated with focal hyperpigmentation and could be related to the level of sun exposure (Bastiaens, Hoefnagel, Westendorp, Vermeer, & Bouwes Bavinck, 2004; Haddad, Xu, & Medrano, 1998; Ortonne, 1990). It has also been shown that SL is associated with an increase in the number of melanocytes in lesional over perilesional epidermis, along with an increase in melanocyte cell size and elongation of their dendrites (Brenner & Hearing, 2008; Hodgson, 1963; Kadono, Manaka, Kawashima, Kobayashi, & Imokawa, 2001). Similarly, within mtDNA-depleter mice, we observe increased basal and suprabasal epidermal pigmentation which occurs in a patchy distribution that resembles the heterogeneity in skin pigmentation in SL, as well as increased epidermal melanocytes and melanocyte dendricity (Figure 3a–b). We also observe changes in the pigmented cells within the dermis of mtDNA-depleter mice, like increased granularity (Figure 3b), that resembles SL. Comparisons between SL and unaffected skin revealed the existence of dermal cells containing melanosomes and melanosome complexes, which are thought to be the result of melanin incontinence from the epidermis and phagocytosis by macrophages giving rise to melanophages (Braun-Falco & Schoefinius, 1971; Nagao, Ito, Suzuki, Satoh, & Iijima, 1986) More recently, melanin-containing cells in the dermis of SL skin were determined to be FXIIIa + dermal dendrocytes (Unver et al., 2006).
Along with cutaneous pigmentary changes, SL is associated with keratinocyte senescence and increased epidermal thickness (Shin, Park, Kim, & Kang, 2015). A contributing factor in SL development is thought to be the age-dependent decline in the activity of the nuclear DNA-encoded mitochondrial complex II, succinate dehydrogenase, predominantly in epidermal senescent cells (Bowman & Birch-Machin, 2016; Chang, 2016). Comparably, mtDNA-depleter mice also experience increased epidermal thickness, hyperplasia and hyperkeratosis with areas of para- and orthokeratosis (Singh et al., 2018), pointing to epidermal keratinocytes as potential contributors to mtDNA depletion-associated hyperpigmentation, again akin to that observed in SL. Deregulated secretion of growth factors like KGF, HGF, and SCF is involved in the onset and progression of ectopic pigmentation in SL, and SL is associated with intense upper dermal staining of these factors (Goorochurn et al., 2017; Kovacs et al., 2010). Other work on SL has highlighted EDN1 and SCF as key factors in the development of this disorder and indicates these growth factors as the molecular regulatory connection between melanocytes/keratinocytes and melanocytes/dermal fibroblasts (Hattori, Kawashima, Ichikawa, & Imokawa, 2004; Kadono et al., 2001). Interestingly, in mice, epidermal melanocytes and dermal melanocytes rely on different growth factors for their promotion and survival; epidermal melanocytosis can be induced with keratinocyte-driven expression of SCF but not HGF or EDNs (Aoki, Yamada, Hara, & Kunisada, 2009; Kunisada et al., 1998, 2000). This suggests that SCF may be a likely candidate for mediating pigmentary changes in response to keratinocyte or fibroblast-derived paracrine cues within the mtDNA-depleter mouse model. The dark-skinned mouse mutants Dsk2 and Dsk5, which exhibit pronounced melanocytosis in non-hairy epidermis, both exhibit hyperkeratosis prior to hyperpigmentation further supporting a connection between keratinocyte and melanocyte pathologies (Fitch, 2003). Future in vitro studies using keratinocytes isolated from mtDNA-depleter mice co-cultured with melanocytes will further address the relationship between these cell types after mtDNA depletion. This experimental approach would also be useful to investigate correlations between the dosage of mtDNA depletion and enhanced melanocyte proliferation or melanogenesis.
Mitochondrial dysfunction has been described as a “neglected component of skin disease” (Feichtinger, Sperl, Bauer, & Kofler, 2014), yet evidence for the role of the mitochondria in skin biology and pathology is growing (Sreedhar et al., 2020). Based on the characterization provided here, we propose that mtDNA-depleter mice can serve as useful model to further investigate the role of mitochondrial dysfunction particularly in age-related pigmentary disorders relevant to humans. In addition, previous work in this model demonstrated that repletion of mtDNA rescues much of the skin aging phenotypes observed in these mice (Singh et al., 2018). Although pigmentation was not assessed at that time, those results indicate that at least some of the detrimental effects of mtDNA depletion are reversible. Thus, mtDNA-depleter mice will permit screening for agents that enhance mitochondrial biogenesis and function in order to mitigate senile lentigines and improve skin aging in humans more broadly.
Supplementary Material
Significance.
The incidence of senile lentigines increases with age. Because mitochondrial dysfunction is a hallmark of aging, we examined the role of mitochondria in the development of lentigines using a novel mtDNA-depleter mouse model, which shows characteristics of aging. We demonstrate that mitochondrial dysfunction introduced by depletion of mtDNA induces pigmentation phenotypes reminiscent of human senile lentigines. We highlight the importance of mitochondria in the development of senile lentigines, the utility of the mtDNA-depleter mouse as a model to study mechanisms underlying pigmentary disorders, and the relevance of mitochondria as a target for therapeutics aimed at improving aging skin.
ACKNOWLEDGEMENTS
KKS is supported by NIH RO1 grant CA204430, and MLH by startup funding from the Department of Biology and College of Arts of Science at the University of Alabama, Birmingham.
Funding information
Department of Biology, University of Alabama, Birmingham; National Cancer Institute, Grant/Award Number: RO1 CA204430
Abbreviations:
- MDS
mitochondrial DNA depletion syndrome
- mtDNA
mitochondrial DNA
- DCT
dopachrome tautomerase
Footnotes
CONFLICT OF INTEREST
KKS is the scientific founder and Chief Scientific Advisor at Yuva Biosciences. KKS holds equity in Yuva Biosciences. The remaining authors claim no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- Aoki H, Yamada Y, Hara A, & Kunisada T (2009). Two distinct types of mouse melanocyte: Differential signaling requirement for the maintenance of non-cutaneous and dermal versus epidermal melanocytes. Development, 136, 2511–2521. 10.1242/dev.037168 [DOI] [PubMed] [Google Scholar]
- Arck PC, Overall R, Spatz K, Liezman C, Handjiski B, Klapp BF, … Peters EMJ (2006). Towards a “free radical theory of graying”: Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. The FASEB Journal, 20, 1567–1569. 10.1096/fj.05-4039fje [DOI] [PubMed] [Google Scholar]
- Bakthavatchalu V, Dey S, Xu Y, Noel T, Jungsuwadee P, Holley AK, … St Clair DK (2012). Manganese superoxide dismutase is a mitochondrial fidelity protein that protects Polγ against UV-induced inactivation. Oncogene, 31, 2129–2139. 10.1038/onc.2011.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaens M, Hoefnagel J, Westendorp R, Vermeer B-J, & Bouwes Bavinck JN (2004). Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Research, 17, 225–229. 10.1111/j.1600-0749.2004.00131.x [DOI] [PubMed] [Google Scholar]
- Berneburg M, & Krutmann J (1998). Mitochondrial DNA deletions in human skin reflect photo-rather than chronologic aging. The Journal of Investigative Dermatology, 111, 709–710. 10.1046/j.1523-1747.1998.00337.x [DOI] [PubMed] [Google Scholar]
- Birch-Machin MA, Russell EV, & Latimer JA (2013). Mitochondrial DNA damage as a biomarker for ultraviolet radiation exposure and oxidative stress. British Journal of Dermatology, 169(Suppl 2), 9–14. 10.1111/bjd.12207 [DOI] [PubMed] [Google Scholar]
- Birch-machin MA, Tindall M, Turner R, Haldane F, & Rees JL (1998). Mitochondrial DNA deletions in human skin reflect photo-rather than chronologic aging. Journal of Investigative Dermatology, 110, 149–152. 10.1046/j.1523-1747.1998.00099.x [DOI] [PubMed] [Google Scholar]
- Bodemer C, Rötig A, Rustin P, Cormier V, Niaudet P, Saudubray J-M, … de Prost Y (1999). Hair and skin disorders as signs of mitochondrial disease. Pediatrics, 103, 428–433. 10.1542/peds.103.2.428 [DOI] [PubMed] [Google Scholar]
- Bogenhagen DF, Pinz KG, & Perez-Jannotti RM (2001). Enzymology of mitochondrial base excision repair. Progress in Nucleic Acid Research and Molecular Biology, Base Excision Repair (pp. 257–271). Cambridge, MA: Academic Press. 10.1016/S0079-6603(01)68105-4 [DOI] [PubMed] [Google Scholar]
- Bowman A, & Birch-Machin MA (2016). Age-Dependent Decrease of Mitochondrial Complex II Activity in Human Skin Fibroblasts. The Journal of Investigative Dermatology, 136, 912–919. 10.1016/j.jid.2016.01.017 [DOI] [PubMed] [Google Scholar]
- Braun-Falco O, & Schoefinius HH (1971). Lentigo senilis. Review and studies. Hautarzt, 22, 277–283. [PubMed] [Google Scholar]
- Brenner M, & Hearing VJ (2008). Modifying skin pigmentation - approaches through intrinsic biochemistry and exogenous agents. Drug Discov Today Dis Mech, 5, e189–e199. 10.1016/j.ddmec.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ALS (2016). Expanding our understanding of human skin aging. The Journal of Investigative Dermatology, 136, 897–899. 10.1016/j.jid.2016.02.020 [DOI] [PubMed] [Google Scholar]
- Daniele T, Hurbain I, Vago R, Casari G, Raposo G, Tacchetti C, & Schiaffino MV (2014). Mitochondria and melanosomes establish physical contacts modulated by Mfn2 and involved in organelle biogenesis. Current Biology, 24, 393–403. 10.1016/j.cub.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Dell’Anna ML, Ottaviani M, Kovacs D, Mirabilii S, Brown DA, Cota C, … Picardo M (2017). Energetic mitochondrial failing in vitiligo and possible rescue by cardiolipin. Scientific Reports, 7, 13663. 10.1038/s41598-017-13961-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar SK, Bakthavatchalu V, Dhar B, Chen J, Tadahide I, Zhu H, … St Clair DK (2018). DNA polymerase gamma (Polγ) deficiency triggers a selective mTORC2 prosurvival autophagy response via mitochondria-mediated ROS signaling. Oncogene, 37, 6225–6242. 10.1038/s41388-018-0404-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feichtinger RG, Sperl W, Bauer JW, & Kofler B (2014). Mitochondrial dysfunction: A neglected component of skin diseases. Experimental Dermatology, 23, 607–614. 10.1111/exd.12484 [DOI] [PubMed] [Google Scholar]
- Fitch KR (2003). Genetics of dark skin in mice. Genes & Development, 17, 214–228. 10.1101/gad.1023703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn MK, Wee SA, & Lane AT (1998). Skin manifestations of mitochondrial DNA syndromes: Case report and review. Journal of the American Academy of Dermatology, 39, 819–823. 10.1016/s0190-9622(98)70356-1 [DOI] [PubMed] [Google Scholar]
- Glover JD, Knolle S, Wells KL, Liu D, Jackson IJ, Mort RL, & Headon DJ (2015). Maintenance of distinct melanocyte populations in the interfollicular epidermis. Pigment Cell & Melanoma Research, 28, 476–480. 10.1111/pcmr.12375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorochurn R, Viennet C, Tissot M, Locatelli F, Granger C, Varin-Blank N, … Roy CL (2017). Differential morphological and functional features of fibroblasts explanted from solar lentigo. British Journal of Dermatology, 177, e109–e111. 10.1111/bjd.15386 [DOI] [PubMed] [Google Scholar]
- Graziewicz MA, Day BJ, & Copeland WC (2002). The mitochondrial DNA polymerase as a target of oxidative damage. Nucleic Acids Research, 30, 2817–2824. 10.1093/nar/gkf392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad MM, Xu W, & Medrano EE (1998). Aging in epidermal melanocytes: Cell cycle genes and melanins. The Journal of Investigative Dermatology. Symposium Proceedings, 3, 36–40. 10.1038/jidsp.1998.9 [DOI] [PubMed] [Google Scholar]
- Hattori H, Kawashima M, Ichikawa Y, & Imokawa G (2004). The epidermal stem cell factor is over-expressed in lentigo senilis: Implication for the mechanism of hyperpigmentation. The Journal of Investigative Dermatology, 122, 1256–1265. 10.1111/j.0022-202X.2004.22503.x [DOI] [PubMed] [Google Scholar]
- Hodgson C (1963). Senile lentigo. Archives of Dermatology, 87, 197–207. 10.1001/archderm.1963.01590140059010 [DOI] [PubMed] [Google Scholar]
- Kadono S, Manaka I, Kawashima M, Kobayashi T, & Imokawa G (2001). The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. The Journal of Investigative Dermatology, 116, 571–577. 10.1046/j.1523-1747.2001.01296.x [DOI] [PubMed] [Google Scholar]
- Karpati G, Carpenter S, Larbrisseau A, & Lafontaine R (1973). The Kearns-Shy syndrome. A multisystem disease with mitochondrial abnormality demonstrated in skeletal muscle and skin. Journal of the Neurological Sciences, 19, 133–151. 10.1016/0022-510x(73)90158-5 [DOI] [PubMed] [Google Scholar]
- Kim ES, Park SJ, Goh M-J, Na Y-J, Jo DS, Jo YK, … Cho D-H (2014). Mitochondrial dynamics regulate melanogenesis through proteasomal degradation of MITF via ROS-ERK activation. Pigment Cell & Melanoma Research, 27, 1051–1062. 10.1111/pcmr.12298 [DOI] [PubMed] [Google Scholar]
- Kovacs D, Cardinali G, Aspite N, Cota C, Luzi F, Bellei B, … Picardo M (2010). Role of fibroblast-derived growth factors in regulating hyperpigmentation of solar lentigo. British Journal of Dermatology, 163, 1020–1027. 10.1111/j.1365-2133.2010.09946.x [DOI] [PubMed] [Google Scholar]
- Kunisada T, Lu S-Z, Yoshida H, Nishikawa S, Nishikawa S-I, Mizoguchi M, … Jack Longley B (1998). Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. The Journal of Experimental Medicine, 187, 1565–1573. 10.1084/jem.187.10.1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada T, Yamazaki H, Hirobe T, Kamei S, Omoteno M, Tagaya H, … Hayashi S-I (2000). Keratinocyte expression of transgenic hepatocyte growth factor affects melanocyte development, leading to dermal melanocytosis. Mechanisms of Development, 94, 67–78. 10.1016/S0925-4773(00)00308-7 [DOI] [PubMed] [Google Scholar]
- Lamason RL, Mohideen M-APK, Mest JR, Wong AC, Norton HL, Aros MC, …Cheng KC (2005). SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science, 310, 1782–1786. 10.1126/science.1116238 [DOI] [PubMed] [Google Scholar]
- Nagao S, Ito N, Suzuki M, Satoh N, & Iijima S (1986). Electron microscopic study of histological pigmentary incontinence–methods of melanosome translocation from the epidermis to the dermis. Fukushima Journal of Medical Science, 32, 141–153. [PubMed] [Google Scholar]
- Ortonne J-P (1990). Pigmentary changes of the ageing skin. British Journal of Dermatology, 122, 21–28. 10.1111/j.1365-2133.1990.tb16121.x [DOI] [PubMed] [Google Scholar]
- Shin J, Park J-Y, Kim SJ, & Kang HY (2015). Characteristics of keratinocytes in facial solar lentigo with flattened rete ridges: Comparison with melasma. Clinical and Experimental Dermatology, 40, 489–494. 10.1111/ced.12621 [DOI] [PubMed] [Google Scholar]
- Simonsz HJ, Bärlocher K, & Rötig A (1992). Kearns-Sayre’s syndrome developing in a boy who survived Pearson’s syndrome caused by mitochondrial DNA deletion. Documenta Ophthalmologica, 82, 73–79. 10.1007/BF00156996 [DOI] [PubMed] [Google Scholar]
- Singh B, Schoeb TR, Bajpai P, Slominski A, & Singh KK (2018). Reversing wrinkled skin and hair loss in mice by restoring mitochondrial function. Cell Death & Disease, 9, 735. 10.1038/s41419-018-0765-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JR, Hall A, Ni-Komatsu L, Khersonsky SM, Chang Y-T, & Orlow SJ (2005). Dissection of melanogenesis with small molecules identifies prohibitin as a regulator. Chemistry & Biology, 12, 477–484. 10.1016/j.chembiol.2005.02.014 [DOI] [PubMed] [Google Scholar]
- Sreedhar A, Aguilera-Aguirre L, & Singh KK (2020). Mitochondria in skin health, aging, and disease. Cell Death & Disease, 11, 10.1038/s41419-020-2649-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokowski RP, Pant PVK, Dadd T, Fereday A, Hinds DA, Jarman C, … Cox DR (2007). A genomewide association study of skin pigmentation in a South Asian population. American Journal of Human Genetics, 81, 1119–1132. 10.1086/522235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulinius MH, Oldfors A, Holme E, Larsson NG, Houshmand M, Fahleson P, … Kristiansson B (1995). Atypical presentation of multisystem disorders in two girls with mitochondrial DNA deletions. European Journal of Pediatrics, 154, 35–42. 10.1007/BF01972970 [DOI] [PubMed] [Google Scholar]
- Unver N, Freyschmidt-Paul P, Horster S, Wenck H, Stab F, Blatt T, & Elsasser H-P (2006). Alterations in the epidermal-dermal melanin axis and factor XIIIa melanophages in senile lentigo and ageing skin. British Journal of Dermatology, 155, 119–128. 10.1111/j.1365-2133.2006.07210.x [DOI] [PubMed] [Google Scholar]
- Wei A-H, Zang D-J, Zhang Z, Liu X-Z, He X, Yang L, … Li W (2013). Exome sequencing identifies SLC24A5 as a candidate gene for nonsyndromic oculocutaneous albinism. The Journal of Investigative Dermatology, 133, 1834–1840. 10.1038/jid.2013.49 [DOI] [PubMed] [Google Scholar]
- Yang JH, Lee HC, & Wei YH (1995). Photoageing-associated mitochondrial DNA length mutations in human skin. Archives of Dermatological Research, 287, 641–648. 10.1007/BF00371736 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Gong J, Sviderskaya EV, Wei A, & Li W (2019). Mitochondrial NCKX5 regulates melanosomal biogenesis and pigment production. Journal of Cell Science, 132, 10.1242/jcs.232009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


