Abstract
Mitochondrial dysfunction is one of the hallmarks of aging. Consistently mitochondrial DNA (mtDNA) copy number and function decline with age in various tissues. There is increasing evidence to support that mitochondrial dysfunction drives ovarian aging. A decreased mtDNA copy number is also reported during ovarian aging. However, the mitochondrial mechanisms contributing to ovarian aging and infertility are not fully understood. Additionally, investigations into mitochondrial therapies to rejuvenate oocyte quality, select viable embryos and improve mitochondrial function may help enhance fertility or extend reproductive longevity in the future. These therapies include the use of mitochondrial replacement techniques, quantification of mtDNA copy number, and various pharmacologic and lifestyle measures. This review aims to describe the key evidence and current knowledge of the role of mitochondria in ovarian aging and identify the emerging potential options for therapy to extend reproductive longevity and improve fertility.
Keywords: Mitochondrial dysfunction, Ovarian aging, Mitochondrial DNA, Reproductive longevity, Mitochondrial therapies, Infertility
1. . Introduction
The socioeconomic changes of the last century have significantly increased the participation of women in the work force, resulting in delayed childbearing worldwide. In the U.S., the mean age of first birth increased from 24.9 years in 2000 to 26.6 years in 2016. A major contributor to this shift is increasing birth rates among women in their 30’s and 40’s, according to Centers for Disease Control (CDC) data (Martin et al., 2018). A significant portion of these women experience infertility, at least in some part related to age.
Ovarian aging, whether obvious or occult, is often the only etiologic factor identified in women presenting with infertility. Women are born with a fixed number of oocytes that differentiate from premature ovum cells known as oogonia. Oogonia are produced in the female fetus by mitotic division of primordial germ cells, and peak at about 7 million by approximately 20 weeks of gestation. Oogonia then enter meiosis, giving rise to the primary oocytes, which undergo physiologic apoptosis and follicular atresia, resulting in only 1–2 million oocytes at birth and 400,000 by puberty (Vaskivuo et al., 2001). As women age, the ovaries undergo both quantitative and qualitative deterioration, and the former manifests as a diminished ovarian reserve (DOR) (Fig. 1). A low number of oocytes remaining in the ovary is generally indicated by an anti-Mullerian hormone (AMH) level of <1.1 ng/mL, follicle stimulating hormone (FSH) level of >10 IU/L, or an antral follicle count (AFC) of <5–7 total follicles. The qualitative aspect is reflected in oocyte quality or competence, which is defined by the oocyte’s ability to re-initiate the meiotic process and undergo fertilization and preimplantation (post-zygotic) development (Fig. 1).
Fig. 1.
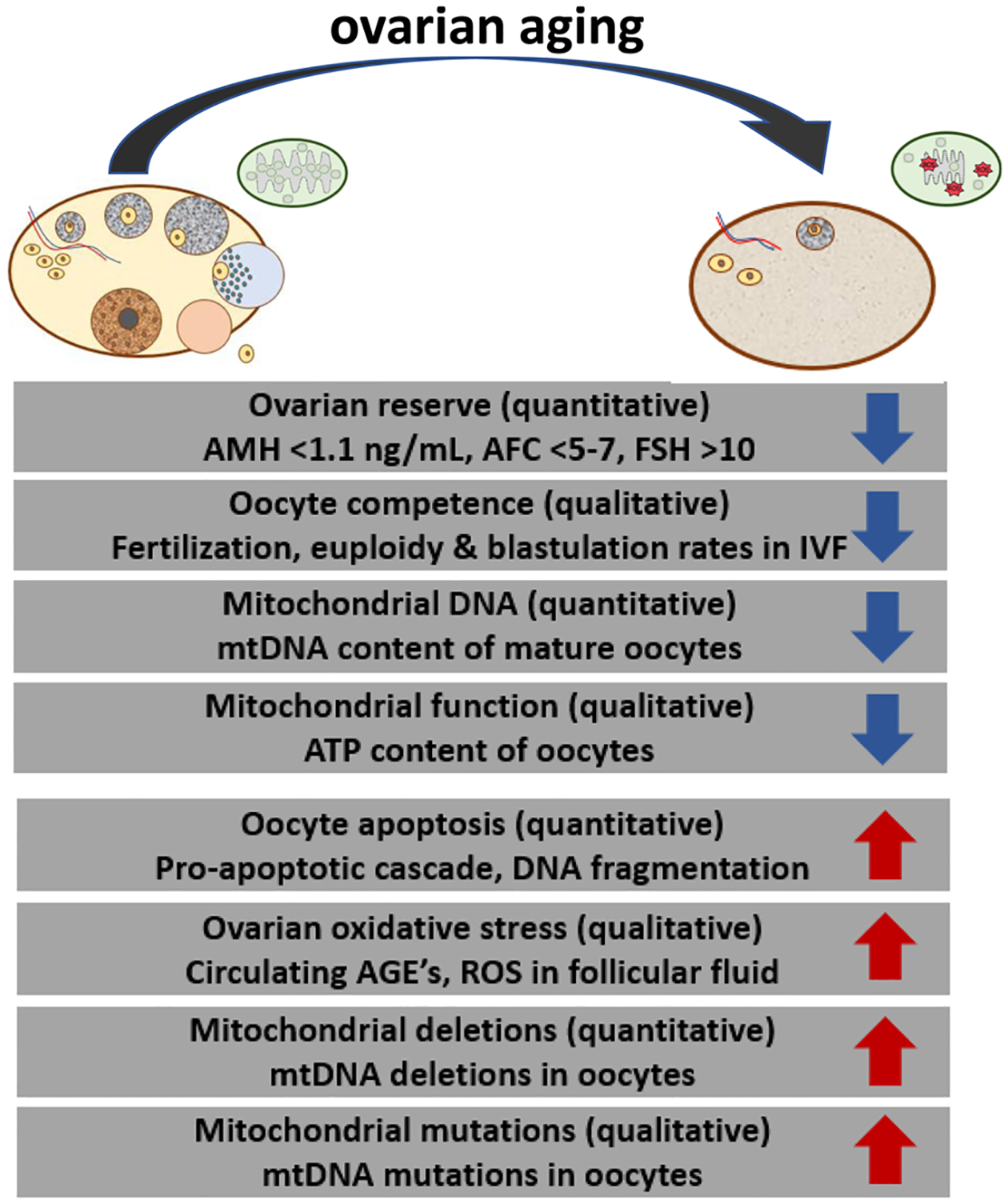
Representation of age-associated changes in the ovary and mitochondria. The aging process alters ovarian reserve (quantity) and oocyte competence (quality). Mitochondria demonstrate parallel changes, quantitatively and qualitatively. AMH, anti-Mullerian hormone; AFC, antral follicle count; FSH, follicle stimulating hormone; ROS, reactive oxygen species; IVF, in vitro fertilization; mtDNA, mitochondrial DNA; ATP, adenosine triphosphate; AGE, advanced glycation end-products.
The average age of menopause for a Caucasian woman in the U.S. is 51 years, although genetic and environmental factors result in significant variability (Broekmans et al., 2009), and in cases of primary ovarian insufficiency (POI), the onset of menopause is <40 years. Clinically, human ovarian aging often does not follow the same timeline as one’s biological age or the aging of other tissues. Nevertheless, ovarian aging beyond 40 years of age is considered a natural state of aging. Furthermore, many infertile women do not meet the diagnostic criteria for POI but do have evidence of early or occult ovarian aging based on laboratory criteria (May-Panloup et al., 2016).
Diminished ovarian reserve or oocyte quality may require in vitro fertilization (IVF). However, low quantity of oocytes, poor fertilization rates, or lack of embryo development often limit the efficacy of IVF and may therefore require the use of donor oocytes in some of these patients. Considering the desire of many women to pursue fertility at older ages, there is a need to reverse or attenuate human ovarian aging.
Although the potential mechanisms contributing to ovarian aging and infertility are not completely understood, there is increasing consensus implicating mitochondrial dysfunction, since oocytes generally have abundant mitochondria. Normally, mitochondria in the oocyte are generated during oogenesis, and then production ceases at the stage of the mature oocyte. The founding population of mitochondria in the human primordial germ cell is approximately 10–100, while the population in the mature oocyte is approximately several hundred thousand (St John, 2007). Post-fertilization, the number of mitochondria remains the same, as mitochondria are not replicated during the cleavage process. During cleavage, mitochondria are instead are distributed amongst the dividing cells, such that each blastomere receives a subset of the oocyte’s mitochondria (May-Panloup et al., 2005) (Fig. 2).
Fig. 2.

Schematic diagram of physiologic changes in mtDNA copy number from oogonia to blastocyst embryo. A minimum threshold for mtDNA copy number is necessary in the oocyte for fertilization (approximately 4000 copies) and for post-implantation development (approximately 45,000 copies). After fertilization, the mtDNA copy number within each cell decreases until it reaches the blastocyst stage. Around implantation, mitochondrial replication resumes in the blastomeres and total mitochondrial DNA copy number increases. GV, germinal vesicle; MI, meiosis I oocyte; MII meiosis II (mature) oocyte.
The proposed mechanisms through which mitochondria drive ovarian aging include accumulation of mtDNA mutations, mitochondrial dysfunction, impaired fusion and fission, altered membrane potential, altered metabolism, and defective electron transport chain (ETC) function (Wang et al., 2017), as well as quantitative issues such as decreased mtDNA content. The mechanism and degree of contribution of mitochondria to ovarian aging and reproductive longevity must be elucidated. In this review, we describe the role of mitochondria in ovarian aging, as well as its applications in reproductive longevity.
2. Mitochondria: basic biology and genetics
Mitochondria are the multifunctional powerhouse of cells, including human oocytes. Mitochondria generate ATP by oxidative phosphorylation (OXPHOS) through five complexes (Complex I-V) of the electron transport chain (St John, 2007; Singh and Costello, 2009). They harbor mitochondrial DNA (mtDNA) that contains 37 genes, of which 13 encode proteins involved in OXPHOS. The remaining proteins involved in OXPHOS are encoded by nuclear DNA (Fig. 3).
Fig. 3.

Mammalian mitochondrial electron transport chain subunit composition of oxidative phosphorylation (OXPHOS) complexes encoded by mtDNA and nuclear DNA. MtDNA, mitochondrial DNA; NAD, nicotinamide adenine dinucleotide; FAD, flavin adenine dinucleotide; CoQ10, coenzyme Q10; Cyt C, cytochrome C; ADP, adenosine diphosphate; ATP, adenosine triphosphate; NRF, nuclear respiratory factor; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1α; AMPK, adenosine monophosphate-activated protein kinase; SIRT3, sirtuin 3.
Because appropriate nuclear maturation in the oocyte, particularly maintenance of meiotic arrest prior to ovulation, is a cyclic adenosine monophosphate (cAMP)-dependent process (Sánchez and Smitz, 2012), the generation of sufficient adenosine triphosphate (ATP) by mitochondria is critical within and surrounding the oocyte during oogenesis. Another key function of mitochondria in the ovary is regulation of steroidogenesis. Transfer of cholesterol to the inner mitochondrial membrane by steroidogenic acute regulatory (StAR) protein is the rate-limiting step of ovarian steroidogenesis, which includes production of sex steroids. Estrogen produced by granulosa cells rises during folliculogenesis and is involved in triggering the luteinizing hormone surge prior to ovulation. Progesterone is involved in luteal support of the endometrium for pregnancy implantation (Speroff, 8th ed, 2011).
Multiple mechanisms of mitochondrial homeostasis appear to be impaired during ovarian aging. Mitochondria are major sites of reactive oxygen species (ROS) generation and are also susceptible to ROS-induced damage. Aging exacerbates ROS production and mtDNA damage (Wei and Lee, 2002). Chronic oxidative stress (OS) and exposure to ROS results in decreased ATP production, cell cycle arrest, and apoptosis. This process leads to dysfunctional metabolism and structural changes associated with ovarian aging in mice (Wilding et al., 2001; Ben-Meir et al., 2015). Mitochondrial swelling and vacuolization have been described and are associated with reduced ATP and mtDNA content in aged mouse and hamster oocytes (Müller-Höcker et al., 1996; Simsek-Duran et al., 2013) (Fig. 1).
Mitochondrial dynamics involve fission and fusion. Fission creates additional smaller mitochondria, which may be more capable of accelerating cell proliferation and generating ROS, while fusion results in enhanced communication with endoplasmic reticulum and dilutes accumulated mtDNA mutations and oxidized proteins (Archer, 2013). Fission and fusion defects have significant implications with regard to ovarian aging. Oocyte-specific deletion of the mitochondrial fusion protein Mitofusin (Mfn1) in C57BL/6 mice leads to accelerated depletion of ovarian follicular reserve. The assessment of ovarian reserve in mice was based on a reduced number of ovarian follicles by 6 months of age and reduced AMH by 2 months of age compared to wild-type mice. RNA sequencing analysis identified increases in pro-apoptotic gene expression of caspase 6 (CASP6) and cytochrome c (CYCS), whose mRNA levels were confirmed greatly increased in Mfn1−/− mouse oocytes. RNA sequencing also revealed increased ceramide biosynthesis enzymes in mice with the Mfn1 deletion. Ceramide, a membrane sphingolipid involved in apoptosis and cell cycle arrest, was increased in Mfn1−/− mice oocytes based on immunofluorescence, identifying it as a likely contributor to the mechanism of diminished ovarian reserve in this mouse model. Treatment of mice with ceramide synthesis inhibitor (myriocin) daily for 21 consecutive days improved follicular growth and allowed development of antral follicles, partially rescuing the reproductive phenotype (Zhang et al., 2019).
Deletion of oocyte-specific mitochondrial fission protein (Dynamin-related protein 1) Drp1 led to decreased oocyte quality due to maturation defects. In Drp1 knockout mice, fewer oocytes completed germinal vesicle breakdown, which occurs at resumption of meiosis I and is a marker of nuclear maturation (Udagawa et al., 2014). Fission is a component of the process of mitochondrial biogenesis, which occurs during oocyte maturation. The ovulated oocyte derives the cellular energy for fertilization and embryogenesis from mitochondria (Chiaratti et al., 2018). Thus it is likely that Drp1, or any defect impairing mitochondrial biogenesis during oocyte maturation, represents a mechanism affecting oocyte competence. (Fig. 4).
Fig. 4.
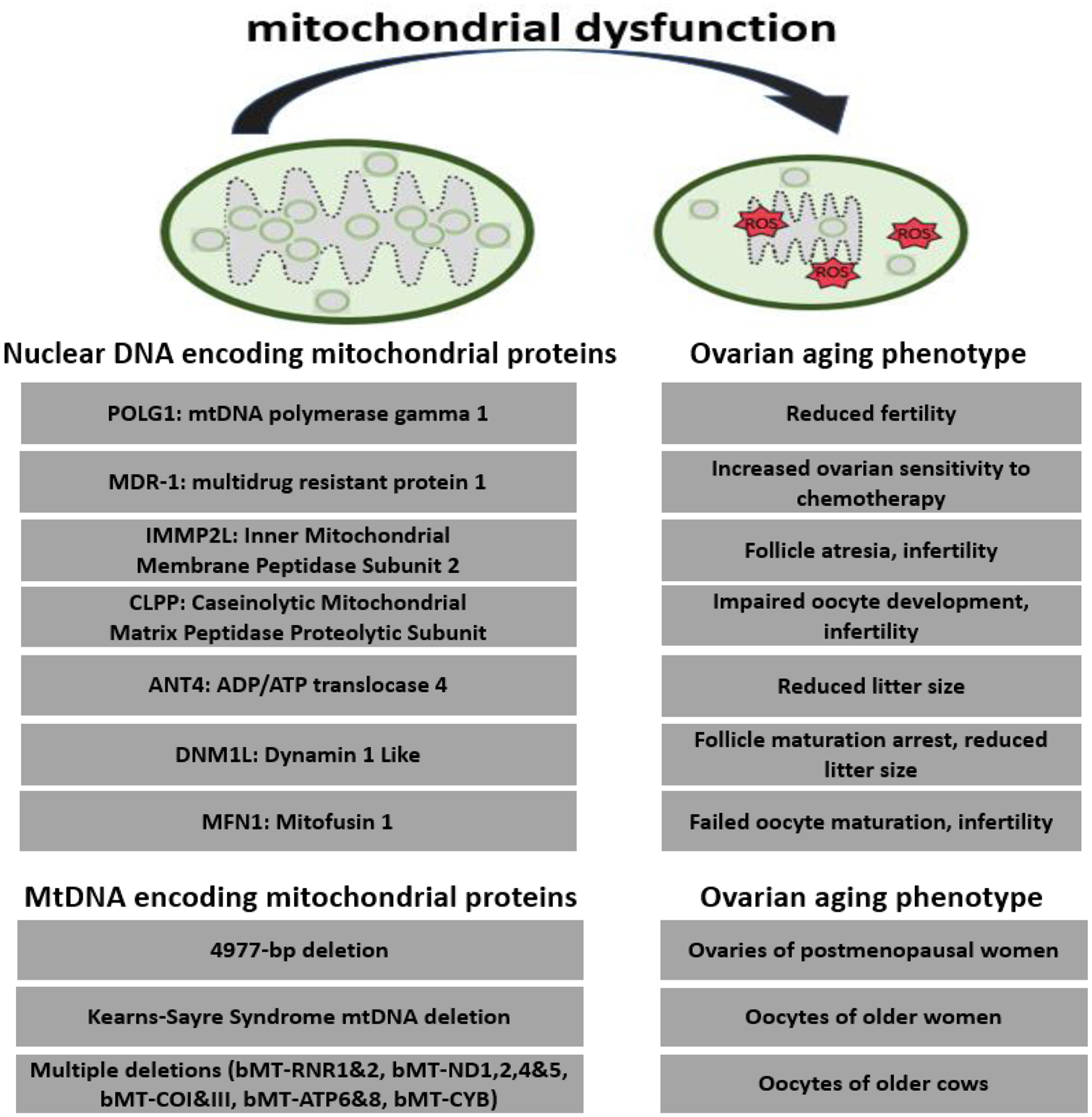
Mutation in these nuclear genes are associated with mitochondrial dysfunction and accelerated ovarian aging (mouse model). Mutations in these mitochondrial genes are associated with ovarian aging (multiple species). There is mitochondrial-to-nuclear crosstalk, which induces phenotypic effects on ovarian aging. ROS, Reactive Oxygen Species. bMT, bovine mitochondria; RNR, ribonucleotide reductase; ND1, NADH-ubiquinone oxidoreductase chain 1; cytochrome oxidase subunit 1; ATP, adenosine triphosphate; CYB, Cytochrome B.
The capacity for mitochondrial biogenesis is a crucial and complex homeostatic mechanism. It requires coordination of both mitochondrial and nuclear genomes. It is dependent on nuclear genes that encode peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1α (PGC-1α) and PGC-1β, estrogen-related receptor (ERR), and nuclear respiratory factor-1 and −2 (NRF-1, 2). It also involves genes in the sirtuin (SIRT) family, SIRT3, SIRT4, and SIRT5, which localize to mitochondria. Of the sirtuins, SIRT3 is the most well characterized, and has been found to interact with PGC-1α, a key regulator of mitochondrial biogenesis, suppress intracellular ROS, and possibly regulate lifespan and aging phenotypes (Lombard et al., 2011). Additionally, AMP-activated protein kinase (AMPK) promotes mitochondrial biogenesis and is a central regulator in mitochondrial homeostasis. Particularly, AMPK communicates with PGC-1α through multiple mechanisms (Herzig and Shaw, 2018). Mitochondrial biogenesis is regulated by mitophagy, the selective autophagic elimination of damaged mitochondria. Mitophagy is mediated by AMPK (Herzig and Shaw, 2018), as well as the PTEN-induced kinase 1 (PINK1)–Parkin pathway (Wang et al., 2017).
Mitochondrial biogenesis is critically important to ovarian function. There is a tremendous surge in mitochondrial biogenesis during oocyte development from the immature germinal vesicle stage to the mature oocyte. At the time of fertilization, a single egg contains hundreds of thousands of mitochondria that provide sufficient ATP to allow fertilization and sustain embryogenesis until implantation when mitochondrial replication resumes in the blastocyst (Fig. 2). Decreased mitochondrial biogenesis, as indicated by lower mtDNA content, is routinely observed during POI, DOR, and physiological ovarian aging. The lowest levels of mtDNA are found in POI patients, followed by the poor responders to IVF, and the normal responders to IVF (Bonomi et al., 2012). In humans with reduced ovarian reserve undergoing in vitro fertilization (IVF), Sirtuin 3 (SIRT3) active protein co-localized to mitochondria in follicular granulosa and cumulus cells, and SIRT3 mRNA levels were decreased in DOR compared to control women (Pacella-Ince et al., 2014). PGC-1α expression was lower in cumulus cells and accompanied by decreased mtDNA content in cumulus and oocyte cells in women with DOR compared to those with normal ovarian reserve (NOR) (Boucret et al., 2015). Such associations add support to the theory that insufficient mitochondrial biogenesis during oocyte maturation may contribute to poor oocyte competence in IVF (May-Panloup et al., 2005).
Another homeostatic function of mitochondria is removal of misfolded and aggregated protein by mitochondrial proteases, such as Caseinolytic Peptidase P (Clpp). This “proteostasis” is essential to maintain protein homeostasis and mitochondrial quality. The absence of Clpp in mice resulted in prematurely decreased ovarian follicles and AMH at 6 months of age, which was associated with increased mammalian target of rapamycin (mTOR) pathway activation based on gene sequencing and confirmed with western analysis of mTOR proteins. Increased ROS and decreased expression of mitochondrial fusion genes in Clpp−/− mouse oocytes suggests that abnormal protein homeostasis affects other mitochondrial functions as well (Wang et al., 2018).
Genetic variations in mtDNA affect ovarian aging. MtDNA is polymorphic and various haplogroups are associated with either protection or increased risk of disease. For example, the JT haplogroup is associated with longevity and decreased risk of premature DOR by 66% compared to other haplogroups (May-Panloup et al., 2014). Women carrying the JT haplogroup show significantly higher mean antral follicle count (AFC), and significantly lower mean follicle stimulating hormone (FSH) and estradiol levels compared to those with other haplogroups (May-Panloup et al., 2014). MtDNA inheritance is also important, as it is normally maternally inherited (Rojansky et al., 2016). Paternal mtDNA inheritance has been reported in some cases, and interestingly presence of paternal mtDNA is linked to poor oocyte quality (St John et al., 2000).
3. Mitochondria in ovarian aging
Mitochondrial dysfunction induces ovarian aging. The mtDNA dysfunction may be either quantitative, such as mtDNA copy number and mtDNA deletions, or qualitative, such as strand breaks, point mutations and oxidative damage. However, the relationship is complex, and the mechanisms are not completely understood. In some cases, mitochondrial damage is likely collateral, occurring as a result of a primary disease or inflammatory process that has overwhelmed mitochondrial capacity to dispose of oxidative by-products. This is likely the case, for example, in aging, obesity, diabetes, and other states of chronic inflammation. In other cases, such as congenital mutations affecting mitochondrial or nuclear DNA, mitochondrial dysfunction is the primary catalyst for inflammation and premature aging (Park et al., 2004). In either case, there is a need to define the mechanisms by which mitochondrial dysfunction and depletion occur because correction of these deficits is likely to attenuate the end-outcome of premature aging. To date, we do not have a strong evidence-based pharmacologic method to effectively rejuvenate mitochondria in patients with premature ovarian aging.
3.1. Qualitative mitochondrial DNA dysfunction in ovarian aging
Qualitative mtDNA dysfunction includes mutations, oxidative bases, and strand breaks and is relevant to ovarian aging. Somatic mtDNA mutations accumulate with time in cells, eroding cellular function (Trifunovic et al., 2004; Kujoth et al., 2005; Schriner et al., 2005) and leading to apoptosis. Therefore, the accumulation of mtDNA mutations can be regarded as a “biological clock” (Wallace, 2005). Mitochondrial DNA may be more susceptible to mutational burden due to its proximity to the ETC and ROS generated thereof, as it has been observed to have a mutation rate 25 times higher than nuclear DNA (Lynch, 2006). In the ovary, the oocyte pool is constituted during embryonic development. The primary oocytes are arrested at prophase I in the primordial follicle during fetal life. The mid-cycle luteinizing hormone (LH) surge, which first occurs after puberty, triggers meiotic progression to the mature Metaphase II (MII) oocyte (Edwards et al., 1996). During this long quiescent period of meiotic arrest, mtDNA mutations associated with aging may arise (May-Panloup et al., 2016).
Experimental introduction of mtDNA mutations into animal models results in premature aging, particularly ovarian aging. The mtDNA mutator mouse model, which harbors a mutated mtDNA polymerase gamma (POLG), which is deficient in proofreading capabilities (Trifunovic et al., 2004), demonstrates premature aging and profound reduction in ovarian reserve and fertility associated with the mtDNA mutational load. In this mouse model, mutations in maternally inherited mtDNA are associated with reduced fecundity based on litter sizes, but this low fecundity was reversed with re-introduction of wild type maternal mtDNA through breeding experiments (Ross et al., 2013). These animal models suggest that a combination of acquired and inherited mtDNA mutations contribute to ovarian aging and infertility in mice.
In humans, the role of mtDNA mutations in ovarian aging is less extensively studied, but likely contributory. For carriers of maternally inherited mtDNA mutations, ovarian reserve may be diminished. Women undergoing IVF who are carriers of pathogenic mtDNA mutations, such as those mutations involved in Leigh Syndrome and mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS), demonstrate decreased ovarian reserve based on lower AMH, lower AFC, and lower number of oocytes retrieved, when compared to healthy volunteer oocyte donors (Kang et al., 2016). It has been proposed that follicular atresia serves to eliminate primary oocytes with a significant mutational load and may explain some cases of DOR or primary ovarian insufficiency (POI) (May-Panloup et al., 2016). Other evidence suggests the degree of variation in the mitochondrial genomes existing in the human oocyte may increase with advancing maternal age (Steuerwald et al., 2000).
There is also an association with ovarian aging with acquired (somatic) mtDNA mutations in humans. The mechanism of such acquired mtDNA mutations has not been definitively proven, but may result either from oxidative damage, or from the inherent error rate of mtDNA polymerase gamma (POLG) (Larsson, 2010). Human oocytes harbor mitochondrial point mutations (Brenner, 1998), and these may preferentially accumulate in aged oocytes. In an analysis of human oocytes for the T414 G transversion mutation in mitochondria, only 4.4% of oocyte from younger patients exhibited this mutation, as compared to 39.5% of oocytes from older patients (Barritt et al., 2000).
3.2. Quantitative mitochondrial DNA dysfunction in ovarian aging
Quantitative mitochondrial dysfunction includes mtDNA copy number and mtDNA deletions. MtDNA is coated by the mitochondrial transcriptional factor A (TFAM), a histone-like protein that preserves mtDNA homeostasis by regulating transcription initiation and mtDNA copy number, thus conferring stability and protection to the mitochondrial genome (Campbell et al., 2012). Differential binding of TFAM to mtDNA regions prone to oxidation may occur and may be involved in regulation of quantitative mtDNA dysfunction (Chimienti et al., 2019). Studies support the role of decreased mtDNA copy number in reproductive aging.
In animal studies, lower mtDNA copy number is associated with aging. Old mice with age-related decreased fecundity had significantly less mtDNA in their oocytes compared to younger mice (Kushnir et al., 2012). Similarly, oocytes from older cows have lower mtDNA content than oocytes from younger cows (Iwata et al., 2011). In mice with a single copy TFAM gene deletion, the decline in mtDNA copy number in the oocytes showed that a critical threshold of mtDNA copies is necessary for post-implantation development (Chiaratti and Meirelles, 2010) (Fig. 2).
In humans, the mtDNA content of oocytes is a determining factor for oocyte fertilization and embryonic development (May-Panloup et al., 2005). It is significantly lower in oocytes of older women or those with DOR compared to that in younger women or those with normal ovarian reserve (NOR) (Konstantinidis et al., 2014). The mtDNA copy number is also lower in the unfertilized oocytes from women with infertility problems (Santos et al., 2006). Due to the positive correlation between oocyte mtDNA copy number and developmental competence, increasing the oocyte mitochondrial mass through pharmacologic means may have a positive impact on fertility treatment.
Mitochondrial DNA deletions are another example of a quantitative dysfunction associated with ovarian aging. In humans, increased frequency of a 4977 base-pair deletion in post-menopausal women indicates accumulation of this deletion around the post-menopausal period (Kitagawa et al., 1993) (Fig. 4). Furthermore, unfertilized MII oocytes retrieved from IVF patients showed that the mean age of patients harboring mtDNA deletions was greater (38 years old) than that of patients without mtDNA deletions (31 years old) (Keefe et al., 1995), suggesting accumulation of mtDNA deletions with advancing maternal age.
3.3. Mitochondrial oxidative stress in ovarian aging
Any disruption in the homeostatic functions of mitochondria, such as ROS neutralization and disposal of dysfunctional mitochondria (mitophagy), can cause aging and infertility. ROS are the by-products of mitochondrial OXPHOS and include superoxide, hydroxyl, and hydrogen peroxide. In addition, reactive nitrogen species, such as peroxynitrite and nitric oxide, are also known to cause oxidative damage (Galli et al., 2005). ROS react with the surrounding proteins, lipids, and DNA, leading to mutations and macromolecular damage. In response to such OS, the expression levels of antioxidant enzymes and other substances (e.g., intracellular proteins and certain vitamins) are rapidly increased (Rani et al., 2016; Birben et al., 2012). Under physiological conditions, the production and neutralization of ROS are balanced. However, excessive ROS generation may overwhelm the cellular antioxidant defenses, resulting in oxidative stress, damage, and premature aging of ovaries (Fig. 1).
The ovary and oviduct utilize ROS-scavenging enzymes, such as Peroxiredoxin 3 (Prdx3) and Thioredoxin 2 (Txn2), which are specifically localized within mitochondria (Pedrajas et al., 2000). In a study of physiologic aging in C57BL/6 wild-type mice, ovarian Prdx3 and Txn2 mRNA expression decreased significantly with age. This was associated with significant age-related changes in oxidatively damaged granulosa and theca cells within ovarian follicles based on immunostaining for oxidative damage markers (Lim and Luderer, 2011).
In humans, high levels of oxidative stress within the ovary, follicular fluid, granulosa, and cumulus cells correlate with follicular atresia and poor oocyte quality, as well as decreased oocyte fertilization, embryo development and fertility (Ávila et al., 2016) (Fig. 1). In a study on granulosa cells (GC) from IVF patients, the gene expression levels of the antioxidant enzyme superoxide dismutase (SOD) was found to be lower in the cells of older versus younger women, indicating reduced defense against ROS with ovarian aging (Tatone et al., 2006). Furthermore, the expression levels of the H2O2 neutralizing antioxidant enzyme Prdx4 is lower in the ovarian tissues of peri-menopausal women than in young women, underscoring the age-dependent decrease in ROS-neutralizing capacity in the ovary (Qian et al., 2016). Aldh3A2, an enzyme that detoxifies lipid peroxidation products, increases in response to oxidative stress (Demozay et al., 2004). Aldh3A2 levels in the granulosa lutein (GL) cells of IVF patients increases with age (González-Fernández et al., 2016), and is negatively correlated to FSH-receptor expression, as well as the number of total and mature oocytes obtained during ovarian stimulation (Palumbo et al., 2014). Down-regulation of FSH-receptor expression during oxidative stress is therefore a potential mechanism of ovarian aging.
Lifestyle and several environmental factors such as cigarette smoke, alcohol, exposure to chemicals, and radiation have been linked oxidative stress with ovarian aging (Paszkowski, 2002; De Ziegler et al., 2013; Schrieks et al., 2013; Martini et al., 2016). In addition, bisphenol A, present in some plastic containers and canned food linings, is known to increase oxidative stress in rodent germ cells (Wu et al., 2013) and is associated with lower implantation rates in humans (Ehrlich et al., 2012). Furthermore, advanced glycation end-products (AGEs) that accumulate in the bloodstream with age also promote OS (Fig. 1). AGEs are further increased by high blood sugar as seen in diabetes and ingestion of charred foods (grilling, barbeque), and are associated with reduced follicular response and conception (Jinno et al., 2011). Obesity is increasingly being considered as a state of high oxidative stress (Matsuda and Shimomura, 2013), and is known to reduce the frequency of pregnancy and live birth (Luke et al., 2011). Regular exercise can reduce excess weight and OS and is therefore recommended to all patients seeking fertility treatment (Meldrum et al., 2016). Physical activity before IVF has been shown to improve the IVF clinical pregnancy rate among obese patients (Palomba et al., 2014). A diet low in saturated fat and high in fruits and vegetables may have antioxidant effects, and correlates negatively with ROS levels in follicular fluid and positively with clinical pregnancy rate in IVF (Bedaiwy et al., 2012). The evidence to date, although limited, supports that lifestyle modification to minimize oxidative stress preconception is associated with improved pregnancy outcomes.
3.4. Other factors involved in premature ovarian aging
Premature ovarian aging is a complex process, and many other factors contribute either separately or in conjunction with mitochondrial dysfunction. These mechanisms include systemic issues, such as genetic variants or mutations, X-chromosomal syndromes, environmental and lifestyle conditions such as smoking, autoimmune diseases, infectious causes, drug toxicity, and hormone receptor variants or mutations (Broekmans et al., 2009). It has been estimated that “menopausal age” heritability ranges from 30 to 85% when looking at associations between sister pairs (Wicks et al., 2004). While some gene mutations have been identified as direct causes of POI such as GDF9 mutations (Skillern and Rajkovic, 2008), the genetics of menopausal age are complex, and the downstream mechanisms not well understood. Other mechanisms include factors within the ovary that disable metabolism of ROS, enhance oocyte apoptosis pathways, disrupt ovarian steroidogenesis and thus follicle maturation, or alter expression of genes involved in follicle development. For example, in Turner’s Syndrome, follicle depletion leading to POI occurs by accelerated apoptosis of primordial follicles with 50–70% of human fetal oocytes staining positive for terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL), versus only 3–7% of control oocytes (Modi et al., 2003). In galactosemia, a disease typically caused by a deficiency in the enzyme galactose-1-phosphate uridylyl transferase (GALT), women develop POI in over 80% of cases. In this disorder, it is proposed that metabolites, such as methylglyoxal and galactitol accumulate, while glutathione in the redox cycle is exhausted, leading to cellular stress and apoptosis of ovarian cells (Fridovich-Keil et al., 2011). Although some gene mutations, such as POLG, clearly implicate mitochondrial dysfunction as the etiology for POI, it is unclear to what degree mitochondrial dysfunction contributes to POI in general. Some studies have not shown any correlation between patient age and number of mtDNA deletions or rearrangements in the oocytes (Brenner, 1998; Barritt, 1999), and therefore other factors in premature ovarian aging must be accounted for in the evaluation of POI or DOR.
4. Mitochondrial dysfunction perturbs oocyte-cumulus crosstalk in the ovary
The primary follicle consists of a primary oocyte surrounded by somatic granulosa cells (GCs). During follicular and oocyte development, the GCs proliferate and differentiate into the two cell types, cumulus granulosa cells (CGCs) and mural GCs (Fig. 5). The CGCs are in close proximity to the oocyte and are involved in the oocyte’s growth and maturation, while the mural granulosa cells (MGCs) regulate steroidogenic activity and apoptosis (Wang et al., 2013). Within the ovarian follicle, there is bi-directional communication between the oocyte and the CGCs, which is essential for oocyte competence, proper embryo-genesis, and the transportation of glucose, lipid, proteins, and metabolites through gap junctions. Since oocytes cannot optimally utilize glucose (Biggers et al., 1967; Sutton-McDowall et al., 2010), CGCs metabolize the bulk of glucose consumed by the cumulus oocyte complex and supply metabolic intermediates like pyruvate, mainly via glycolysis, to the oocytes (Sutton-McDowall et al., 2010). The CGCs also transport amino acids and nucleotides to the growing oocyte for the synthesis of proteins and ribosomal and messenger RNAs (May-Panloup et al., 2016). Mitochondrial dysfunction may perturb metabolite trafficking leading to poor oocyte quality, ovarian aging, and infertility (Wang et al., 2009).
Fig. 5.
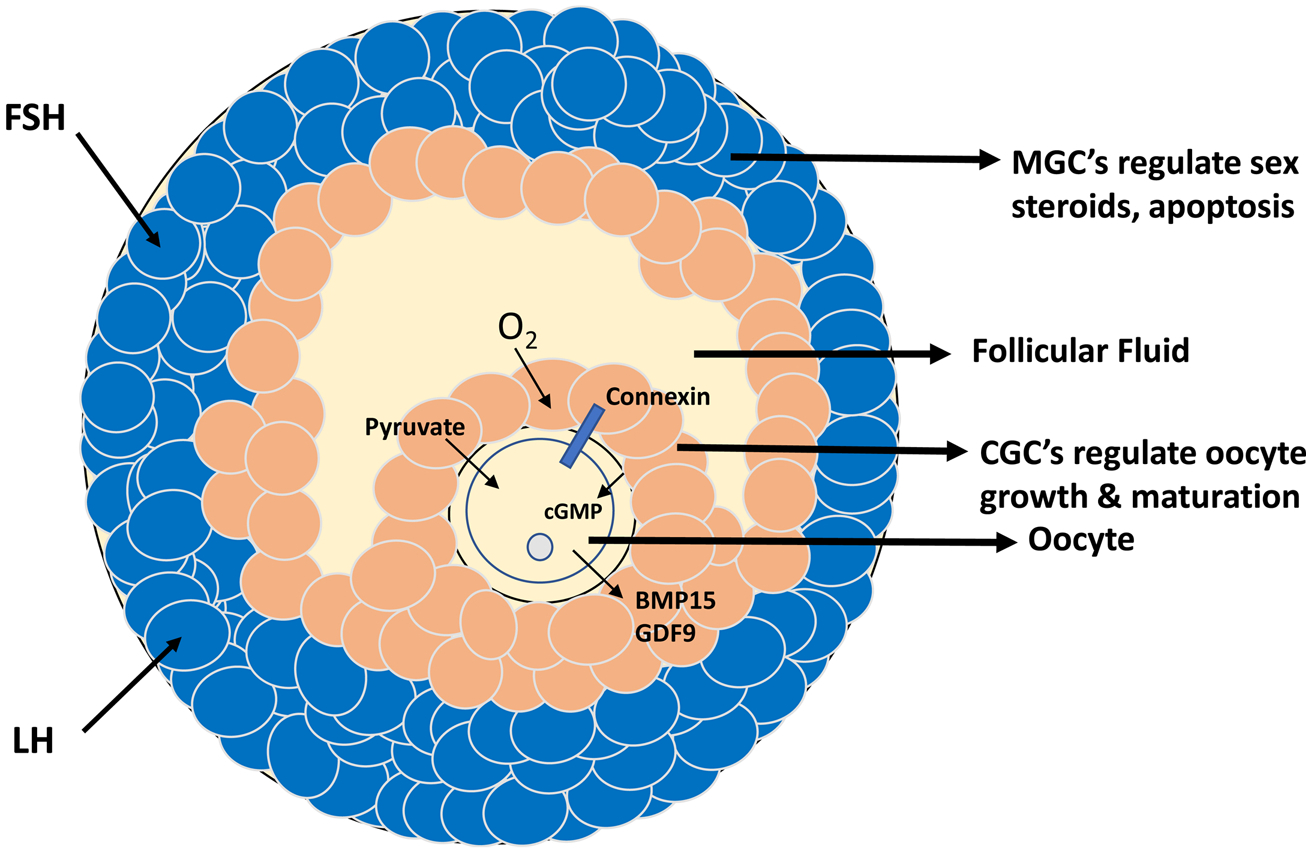
Diagram of an ovarian preovulatory follicle depicting the cumulus oocyte complex and bidirectional crosstalk between cumulus granulosa cells and the oocyte, perturbation of which contributes to ovarian aging and infertility. FSH, follicle stimulating hormone; LH, luteinizing hormone; cGMP, cyclic guanosine monophosphate; BMP15, bone morphogenetic protein 15; GDF9, growth differentiation factor 9; MGC, mural granulosa cell; CGC, cumulus granulosa cell.
5. Mitochondrial dysfunction in disorders linked to ovarian aging
Genetic disorders associated with primary ovarian insufficiency (POI) are linked to mitochondrial dysfunction (Tiosano et al., 2019), but few studies have focused on the mitochondria of the defective human oocytes or ovarian tissue. Turner’s Syndrome (TS) is a genetic cause of POI due to monosomy of the X chromosome. Recent studies suggest that changes in DNA methylation in TS patients may influence mitochondrial biogenesis (Álvarez-Nava and Lanes, 2018). Honda et al. (2005) analyzed the mitochondrial genome of a TS patient and detected 4 syndrome-specific mutations, indicating a possible interaction between sex chromosome aberrations and mtDNA (Honda et al., 2015).
Fragile X Syndrome is another potential genetic cause of POI due to triplet repeat mutations in the FMR1 gene (Man et al., 2017). Shen et al. showed that deficiency of the FMR protein in neurons results in altered expression of mitochondrial genes, fragmented mitochondria, impaired mitochondrial function and increased oxidative stress (Shen et al., 2019). Several studies have linked Fragile X Syndrome and Premutation to mitochondrial dysfunction (Ross-Inta et al., 2010). Interestingly, mice with fragile X premutation (FXPM) show reduced mtDNA content, and display decreased corpora lutea, increased atresia of large antral follicles, and reduced fertility (Conca Dioguardi et al., 2016). Thus, it is likely that the FMR1 induction of mitochondrial dysfunction contributes to ovarian aging. It would be interesting to investigate the effect of Fragile X premutation on the mitochondrial content of oocytes in women undergoing IVF.
6. Ovarian dysfunction in disorders linked to mitochondrial diseases
POLG encodes the mtDNA polymerase, and mutations in this gene have been linked to mitochondrial dysfunction (Stumpf et al., 2013). The phenotypic spectrum of POLG mutations is broad, including progressive external ophthalmoplegia (POE), spinocerebellar ataxia with epilepsy (SCAE), and MELAS. In genome-wide association studies in humans, POLG mutations were linked to POI in isolation and associated with other neurologic conditions (Day et al., 2015) (Fig. 4). However, the effect of these mutations on ovarian mitochondrial dysfunction needs to be further elucidated. Furthermore, the mice containing mutations in the mtDNA polymerase gamma (PolgA) show ovarian failure and reduced fertility (Ross et al., 2013).
Mutations in the IMP gene encoding mitochondrial inner membrane peptidase cause abnormal processing of mitochondrial membrane proteins leading to female infertility (Fig. 4). Mitochondrial dysfunction in these mice was associated with defective follicle maturation, degeneration of follicles, poor oocyte yield after superovulation (Lu et al., 2008). Additionally, the MDR-1 gene encoding mitochondrial ABC transporter has been associated with increased susceptibility to cyclophosphamide-induced ovarian dysfunction and infertility. (Brayboy et al., 2013, Clark et al., 2019) (Fig. 4).
7. Approaches to mitigate ovarian aging and extend reproductive longevity
There are limited treatment modalities at present for improving ovarian reserve and oocyte quality in women. Hence, further research is needed to devise novel therapeutic strategies that will translate into improved reproductive outcomes in patients.
7.1. Mitochondrial nutrient therapy
Available treatments for DOR in infertility patients include the administration of Coenzyme Q10 (CoQ10), Dehydroepiandrosterone (DHEA), vitamins, such as vitamin C and D, and dietary or supplemental isoflavones. Although these bioactive factors may have some therapeutic effect on DOR, no study has unequivocally supported their routine use. CoQ10 is a component of the mitochondrial ETC that has been shown to mitigate mitochondrial dysfunction in infertile mice (Ben-Meir et al., 2015). A 2014 study in humans compared the post-meiotic aneuploidy rates in embryos of IVF patients treated with 600 mg per day CoQ10 or placebo and reported 46.5% and 62.8% aneuploidy rates in CoQ10 and control groups, respectively. However, the study was stopped due to concerns about polar body biopsy on embryos and thus did not achieve statistical significance (Bentov et al., 2014). Some clinicians nevertheless recommend CoQ10 for female infertility patients with DOR. DHEA is a weak androgen produced in the adrenal glands and ovaries. Although initially thought to alleviate DOR by improving the ovarian hormonal milieu, a recent study indicates that DHEA also protects mitochondria and reduces apoptosis in human GCs (Tsui et al., 2017). A randomized prospective control trial was performed in 2012 on poor responders that were given three doses of 25 mg DHEA or placebo daily for 12 weeks prior to IVF. The required doses of recombinant FSH were lower, peak estradiol levels higher, and clinical pregnancy rate significantly higher in the DHEA-treated group (20.9% vs 15.2%, p = 0.048) (Moawad and Shaeer, 2012). However, due to lack of adequately powered randomized controlled trials and side effects, such as hirsutism and acne, DHEA is still not routinely recommended (Fouany and Sharara, 2013). Phytoestrogens are less commonly used to mitigate DOR in fertility clinics. Vanegas et al. reported a positive correlation between self-reported dietary soy isoflavone intake and IVF live birth rates (Vanegas et al., 2015), but a prospective study found no correlation between urinary isoflavone levels and fecundity of couples trying to conceive (Mumford et al., 2014). Therefore, phytoestrogens are also not routinely recommended in clinical practice.
Other pharmacologic agents are still investigational. Resveratrol is a natural polyphenol synthesized by plants and found in certain foods (grapes, nuts, berries) and red wine. It has been associated with upregulation of sirtuin (SIRT) 1 expression in the ovaries and protection from oxidative stress. Resveratrol prolonged ovarian lifespan in rats (Chen et al., 2010). However, in humans, it was potentially detrimental and not recommended for routine clinical practice, as women undergoing IVF cycles taking 200 mg per day of oral resveratrol were retrospectively found to have decreased clinical pregnancy rates (Ochiai and Kuroda, 2019). Instead, resveratrol has been added to culture media for in vitro maturation of germinal vesicle stage (immature) human oocytes with some success. Resveratrol-exposed oocytes were more likely to reach maturity, and of those that did reach maturity, immunofluorescence confirmed higher immunoreactivity of mitochondria (Liu et al., 2018). Thus, resveratrol may hold some promise in improving mitochondrial function in human oocytes, but its exact therapeutic use and safety remains to be determined.
C-phyocyanin (PC) is another investigational agent, which has not previously been studied in humans. Mice studies on the reproductive performance of galactose-induced aged mice after superovulation found that PC prevented oocyte fragmentation and aneuploidy by maintaining cytoskeletal integrity. PC also upregulated the antioxidant genes, increased SOD activity, decreased malondialdehyde (MDA) content, and normalized mitochondrial distribution (Li et al., 2016).
7.2. Mitochondria for embryo selection
Embryos on day 5 to 6 of development are characterized by an inner cell mass, trophoblast, and blastocoel, and are referred to as blastocysts. Most IVF clinics prefer to transfer blastocyst embryos, rather than cleavage-stage embryos (day 2 to 3 of development). During an IVF cycle, multiple blastocysts may be produced, but typically the number recommended for transfer is between 1 (in the youngest patients with the best quality blastocysts) and 3 (in the oldest patients with the worst quality blastocysts). This introduces the issue of selecting the optimum quality blastocyst(s). Embryos are conventionally graded based on morphology (expansion, inner cell mass, trophectoderm quality) and ploidy. Preimplantation genetic testing for aneuploidy (PGT-A) is routinely used in clinics to select healthy embryos. However, normal morphology and euploidy do not always ensure pregnancy. Therefore, the mitochondrial content of embryos can be an additional tool for selecting an optimal embryo.
It is worth noting that the selection tools used in IVF tend to be useful in patients with a good prognosis, i.e. those who generate the most embryos and are least likely to need additional interventions (Orvieto and Gleicher, 2016). Although mitochondria are emerging as a potential embryo selection tool in IVF, their efficacy and utility remain to be determined. As previously discussed in this review, ovarian aging affects oocyte mitochondria, and the lower mtDNA content in aged oocytes may correspond to decreased fertilizability and capacity for early development (May-Panloup et al., 2005). MtDNA replication does not occur in the embryo until the blastocyst stage. The amount of mtDNA derived from the mature oocyte is divided at each cell division, such that the mtDNA content per cell decreases until the blastocyst stage (Pikó and Taylor, 1987). Therefore, as opposed to oocytes, healthier blastomeres (cells from blastocysts) tend to have a lower mtDNA content, which is considered favorable in terms of predicting blastocyst viability (Fragouli et al., 2015). Euploid blastocysts that implanted after transfer had significantly lower mtDNA content than non-implanted ones. Furthermore, mtDNA copy number was significantly higher in embryos derived from older women and in aneuploid embryos. The authors introduced a concept of “threshold of non-viability,” or the relative mtDNA quantity above which none of the embryos implanted (Fragouli et al., 2015).
Although it seems counterintuitive that optimal embryos generate lower mtDNA, Fragouli et al. have proposed that a stressed embryo may induce mitochondrial biogenesis earlier during its development. Thus, early mitochondrial activation may be a compensatory mechanism in response to suboptimal environmental or genetic conditions (Fragouli et al., 2015). Consistent with these findings, Ravichandran et al. confirmed the association between low mtDNA content of blastocysts and improved implantation rates in a large blinded retrospective study. However, when the relative mtDNA “threshold of non-viability” was assessed, only a small percentage (9.2%) of blastocysts had mtDNA content above that threshold. In addition, certain clinics had a higher percentage of embryos meeting the non-viability threshold than others (Ravichandran et al., 2017). Cecchino and Garcia-Velasco (2019) discussed that mtDNA non-viability threshold is a potential embryology lab quality indicator, and fertility clinics generally should not produce many embryos with mtDNA levels at the highest end of the spectrum (Cecchino and Garcia-Velasco, 2019). A blinded prospective non-selection study by Fragouli et al. in 2017 assessed 199 euploid blastocysts to evaluate the clinical impact of mtDNA as a selection tool. They found that all implanted embryos had normal to low mtDNA content, but 16% of the non-implanted embryos had elevated mtDNA content (Fragouli et al., 2017). Since 84% of the non-implanting embryos had normal mtDNA content, we can surmise that while high mtDNA content may serve as a marker of non-viability, normal mtDNA content is not a useful predictor of viability. Further studies are needed to better elucidate the role of using mitochondrial “cut-offs” as an embryo selection tool in human IVF.
7.3. Mitochondrial replacement therapy
Mitochondria have gained considerable attention in IVF procedures as a tool for improving oocyte competence. Mitochondrial replacement therapy (MRT) using donor ooplasm, which has been used to treat inherited mitochondrial disease, is now an investigational technique to treat poor egg quality in IVF. This approach is termed “oocyte rejuvenation” (Labarta et al., 2019a). In the future, it may be considered an option for IVF patients to rejuvenate their own oocytes rather than, or prior to, using donor oocytes.
Patients with signs of ovarian aging tend to have lower mtDNA copy number in their oocytes (May-Panloup et al., 2005; Duran et al., 2011). In addition, oocytes with fertilization issues (not explained by a severe male factor) also tend to have lower mtDNA content (Reynier et al., 2001; Santos et al., 2006). This correlation between low mtDNA content and poor oocyte quality prompted an investigation into MRT for oocyte rejuvenation. In 1998, Cohen et al. reported the first case of a human pregnancy and live birth following MRT through the transfer of donor ooplasm into the oocytes of a patient with repeated embryo implantation failure (Cohen et al., 1997). Although this approach subsequently resulted in 25 live births, prospective follow-up studies showed concern for mtDNA heteroplasmy in the offspring and the potential risk of mitochondrial disease (Brenner et al., 2000). In 2001, the FDA suspended donor ooplasm transfer in clinics until these safety concerns are addressed, a policy that remains in place today.
Partial ooplasm transfer has been performed either by electrofusion or direct ooplasmic injection, with the latter being the preferred technique due to higher normal fertilization rates (Cohen, 1998). Direct injection is performed via a modified intracytoplasmic sperm injection (ICSI) procedure, wherein both the sperm and 5 %–10 % of the donor ooplasm are simultaneously injected into the recipient oocyte. The children born using this technique are currently between 13–18 years of age. A follow-up study on 17 children was conducted in 2016 by Chen et al., and revealed no health issues (Chen et al., 2016). However, the effects of partial cytoplasm transfer have not been fully assessed since alternative MRTs are now being used, namely those that replace the entire ooplasm rather than only a small portion (nuclear transfer), and autonomous germline mitochondrial energy transfer (AUGMENT).
AUGMENT involves obtaining the ovarian cortex via laparoscopy, isolating the mitochondria from oogonial stem cells (OSC’s), and transferring them into the recipient oocyte during ICSI (Cecchino et al., 2018). The first birth from AUGMENT technology occurred in 2015 in Canada (Labarta et al., 2019b). Since then, multiple centers across different countries have used this technique. In 2018, a well-designed triple-blind randomized control trial was performed by IVI-RMA Valencia and showed that AUGMENT did not improve pregnancy rates or clinical outcomes (Labarta et al., 2018). Therefore, it is unlikely to be continued in clinical practice and is not considered standard of care.
Other MRT approaches are being explored at the early experimental stages. The CRISPR/Cas9, gene editing technology, can potentially repair mutant mitochondria in women with ovarian aging but has ethical concerns related to gene manipulation. The use of stem cells for the generation of young mitochondria has been theorized, but no studies have been conducted so far. The addition of antioxidants to the culture media of developing embryos in mice is currently being explored (Fragouli et al., 2019). While the theoretical basis for MRT in improving oocyte quality is solid, more clinical data is needed to validate its safety and efficacy.
7.4. Nuclear transfer therapy
Following concerns of mtDNA heteroplasmy raised by the FDA in 2001, nuclear genome transfer techniques and autonomous germline mitochondrial energy transfer (AUGMENT) were developed (Woods and Tilly, 2015). Nuclear genome transfer techniques include oocyte spindle transfer, germinal vesicle (GV) transfer, and pronuclear transfer (PNT). For oocyte spindle transfer, the maternal nucleus (spindle) is removed from the oocyte and transferred into the enucleated donor oocyte. Although this is considered by many as third party reproduction (as with any use of donor gametes), experts do not consider the child to ethically have three parents (de Zeigler et al., 2019). Zhang et al. reported the first live birth using this technique in 2016. The embryo transfer and live birth took place in an affiliated clinic in Mexico. The indication for this approach was to prevent transmission of the rare mitochondrial disease Leigh’s Syndrome, which results in severe neurological disorder, psychomotor regression, and death by age 2–3 years of age (Zhang et al., 2017). The FDA has still not approved MRT in the US (Singh and Naviaux, 2001). However, since 2015 the UK has allowed spindle transfer and PNT solely for treating mitochondrial disease (Alikani et al., 2017). A recent pilot trial at the Institute of Life in Athens, Greece, used spindle transfer specifically for the treatment of DOR and infertility (rather than mitochondrial disease), and this resulted in a live birth in April of 2019 (Kostaras et al., 2019).
GV transfer in immature oocytes has been proposed to improve meiotic resumption and allow oocyte maturation and is a viable option for women with DOR who do not produce mature oocytes or quality embryos for IVF (Zhang, 2015). It is technically less invasive since the GV can be easily observed under a microscope and is still protected by the nuclear membrane. It is, however, limited by the subsequent need for in vitro maturation (IVM) and likely high mtDNA carryover from the original cytoplasm due to proximity of mitochondria to the GV (Sathananthan, 1997). Nevertheless, live births have been reported using this technique (Nagy et al., 1996).
PNT involves the transfer of pronuclei from one zygote to another. This is achieved by the simultaneous fertilization of the donor egg (from the mitochondrial donor) with the intended male partner’s sperm, and that of the maternal oocytes with the corresponding sperm. Both sets of zygotes are allowed to develop to the pronuclei (2 P N) phase, following which the pronuclei are removed from the zygotes derived from donor eggs and replaced with that of zygotes derived from maternal eggs. This method generated a triplet pregnancy in China in 2003, which underwent fetal reduction, but the two remaining fetuses died of respiratory distress and cord prolapse (Ishii and Hibino, 2018). Since this technique involves the destruction of one embryo set (the donor eggs fertilized with the intended male partner sperm), it has some ethical concerns and therefore has not been pursued extensively.
Polar body nuclear transfer (PBNT) is another strategy for MRT or enhancing oocyte competence in infertile patients. It involves the transfer of the polar body from MII oocytes into enucleated donor cytoplasm. Although blastocysts have been generated using this technique, the PBNT oocytes developed into blastocysts less frequently than controls (42% vs. 75%) (Ma et al., 2017). No live births from this technique have yet been reported.
8. Conclusion
Mitochondria play a key role in ovarian aging and reproductive longevity. However, the mechanisms and degree to which mitochondria contribute to ovarian function are not fully understood. Environmental factors, lifestyle, and genetic susceptibility all contribute to ovarian aging. As women age, the ability of ovarian cells to neutralize oxidative stress diminishes, resulting in a steady decline in oocyte quality. Although lifestyle and pharmacological interventions may be helpful, additional potent therapeutic approaches are needed to enhance the clinical benefits. Mitochondrial replacement therapy (MRT) via ooplasm injection and nuclear transfer techniques in IVF continues to be explored as a method to obtain quality embryos and avoid transmission of mitochondrial disease, but further studies are needed prior to its incorporation into routine clinical practice for treatment of DOR or age-related infertility. The use of mtDNA content as a selection tool for embryo viability in IVF may be valuable in certain clinical scenarios in the future. Additional research is needed to better understand the mechanisms of mitochondrial dysfunction in ovarian aging and the consequences thereof, such as diminished ovarian reserve and embryonic aneuploidy based on meiotic errors. Future research should focus on the development of mitochondria as targets to prevent, slow or reverse the process of ovarian aging, and thereby increase reproductive longevity.
Acknowledgements
We thank the members of Singh laboratory for their help with the writing of this manuscript.
Funding
KKS and JLC acknowledge the grant support from the Department of Obstetrics and Gynecology, University of Alabama at Birmingham. KKS is also supported by an NIH grant R01CA204430.
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Alikani M, Fauser BCJ, García-Valesco JA, Simpson JL, Johnson MH, 2017. First birth following spindle transfer for mitochondrial replacement therapy: hope and trepidation. Reprod. Biomed. Online 34, 333–336. 10.1016/j.rbmo.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Álvarez-Nava F, Lanes R, 2018. Epigenetics in Turner syndrome. Clin. Epigenetics 10, 45. 10.1186/s13148-018-0477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, 2013. Mitochondrial Dynamics — Mitochondrial Fission and Fusion in Human Diseases. N. Engl. J. Med 369, 2236–2251. 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- Ávila J, González-Fernández R, Rotoli D, Hernández J, Palumbo A, 2016. Oxidative Stress in Granulosa-Lutein Cells From In Vitro Fertilization Patients. Reprod. Sci 23, 1656–1661. 10.1177/1933719116674077. [DOI] [PubMed] [Google Scholar]
- Barritt JA, 1999. Mitochondrial DNA rearrangements in human oocytes and embryos. Mol. Hum. Reprod 5, 927–933. 10.1093/molehr/5.10.927. [DOI] [PubMed] [Google Scholar]
- Barritt JA, Cohen J, Brenner CA, 2000. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reprod. Biomed. Online 1, 96–100. 10.1016/S1472-6483(10)61946-3. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Elnashar SA, Goldberg JM, Sharma R, Mascha EJ, Arrigain S, Agarwal A, Falcone T, 2012. Effect of follicular fluid oxidative stress parameters on intracytoplasmic sperm injection outcome. Gynecol. Endocrinol 28, 51–55. 10.3109/09513590.2011.579652. [DOI] [PubMed] [Google Scholar]
- Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Naranian T, Chi M, Wang Y, Bentov Y, Alexis J, Meriano J, Sung H-K, Gasser DL, Moley KH, Hekimi S, Casper RF, Jurisicova A, 2015. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 14, 887–895. 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentov Y, Hannam T, Jurisicova A, Esfandiari N, Casper RF, 2014. Coenzyme Q10 Supplementation and Oocyte Aneuploidy in Women Undergoing IVF-ICSI Treatment. Clin. Med. Insights Reprod. Heal 8 10.4137/CMRH.S14681. CMRH. S14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O, 2012. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J 5, 9–19. 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomi M, Somigliana E, Cacciatore C, Busnelli M, Rossetti R, Bonetti S, Paffoni A, Mari D, Ragni G, Persani L, 2012. Blood Cell Mitochondrial DNA Content and Premature Ovarian Aging. PLoS One 7, e42423. 10.1371/journal.pone.0042423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucret L, Chao de la Barca JM, Moriniere C, Desquiret V, Ferre-L’Hotellier V, Descamps P, Marcaillou C, Reynier P, Procaccio V, May-Panloup P, 2015. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum. Reprod 30, 1653–1664. 10.1093/humrep/dev114. [DOI] [PubMed] [Google Scholar]
- Brayboy LM, Oulhen N, Witmyer J, Robins J, Carson S, Wessel GM, 2013. Multidrug-resistant transport activity protects oocytes from chemotherapeutic agents and changes during oocyte maturation. Fertil. Steril 100, 1428–1435. 10.1016/j.fertnstert.2013.07.002 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C, 1998. Mitochondrial DNA deletion in human oocytes and embryos. Mol. Hum. Reprod 4, 887–892. 10.1093/molehr/4.9.887. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Barritt JA, Willadsen S, Cohen J, 2000. Mitochondrial DNA heteroplasmy after human ooplasmic transplantation. Fertil. Steril 74, 573–578. 10.1016/S0015-0282(00)00681-6. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC, 2009. Ovarian Aging: Mechanisms and Clinical Consequences. Endocr. Rev 30, 465–493. 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- Campbell CT, Kolesar JE, Kaufman BA, 2012. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta - Gene Regul. Mech 1819, 921–929. 10.1016/j.bbagrm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Cecchino GN, Garcia-Velasco JA, 2019. Mitochondrial DNA copy number as a predictor of embryo viability. Fertil. Steril 111, 205–211. 10.1016/j.fertnstert.2018.11.021. [DOI] [PubMed] [Google Scholar]
- Cecchino GN, Seli E, Alves da Motta EL, García-Velasco JA, 2018. The role of mitochondrial activity in female fertility and assisted reproductive technologies: overview and current insights. Reprod. Biomed. Online 36, 686–697. 10.1016/j.rbmo.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Chen SH, Pascale C, Jackson M, Szvetecz MA, Cohen J, 2016. A limited survey-based uncontrolled follow-up study of children born after ooplasmic transplantation in a single centre. Reprod. Biomed. Online 33, 737–744. 10.1016/j.rbmo.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Chen Z-G, Luo L-L, Xu J-J, Zhuang X-L, Kong X-X, Fu Y-C, 2010. Effects of plant polyphenols on ovarian follicular reserve in aging rats. This paper is one of a selection of papers published in this special issue entitled “Second International Symposium on Recent Advances in Basic, Clinical, and Social Medicine” and has. Biochem. Cell Biol 88, 737–745. 10.1139/O10-012. [DOI] [PubMed] [Google Scholar]
- Chiaratti MR, Garcia BM, Carvalho KF, Macabelli CH, Ribeiro F.K. da S., Zangirolamo AF, Sarapião FD, Seneda MM, Meirelles FV, Guimarães FEG, Machado TS, 2018. Oocyte mitochondria: role on fertility and disease transmission. Anim. Reprod 15, 231–238. 10.21451/1984-3143-AR2018-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaratti MR, Meirelles FV, 2010. Mitochondrial DNA Copy Number, a Marker of Viability for Oocytes1. Biol. Reprod 83, 1–2. 10.1095/biolreprod.110.084269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimienti G, Picca A, Fracasso F, Marzetti E, Calvani R, Leeuwenburgh C, Russo F, Lezza AMS, Pesce V, 2019. Differences in Liver TFAM Binding to mtDNA and mtDNA Damage between Aged and Extremely Aged Rats. Int. J. Mol. Sci 20, 2601. 10.3390/ijms20102601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H, Knapik LO, Zhang Z, Wu X, Naik MT, Oulhen N, Wessel GM, Brayboy LM, 2019. Dysfunctional MDR-1 disrupts mitochondrial homeostasis in the oocyte and ovary. Sci. Rep 9, 9616. 10.1038/s41598-019-46025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1998. Ooplasmic transfer in mature human oocytes. Mol. Hum. Reprod 4, 269–280. 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- Cohen J, Scott R, Schimmel T, Levron J, Willadsen S, 1997. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet 350, 186–187. 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- Conca Dioguardi C, Uslu B, Haynes M, Kurus M, Gul M, Miao D-Q, De Santis L, Ferrari M, Bellone S, Santin A, Giulivi C, Hoffman G, Usdin K, Johnson J, 2016. Granulosa cell and oocyte mitochondrial abnormalities in a mouse model of fragile X primary ovarian insufficiency. Mol. Hum. Reprod 22, 384–396. 10.1093/molehr/gaw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, Stolk L, Finucane HK, Sulem P, Bulik-Sullivan B, Esko T, Johnson AD, Elks CE, Franceschini N, He C, Altmaier E, Brody JA, Franke LL, Huffman JE, Keller MF, McArdle PF, Nutile T, Porcu E, Robino A, Rose LM, Schick UM, Smith JA, Teumer A, Traglia M, Vuckovic D, Yao J, Zhao W, Albrecht E, Amin N, Corre T, Hottenga J-J, Mangino M, Smith AV, Tanaka T, Abecasis GR, Andrulis IL, Anton-Culver H, Antoniou AC, Arndt V, Arnold AM, Barbieri C, Beckmann MW, Beeghly-Fadiel A, Benitez J, Bernstein L, Bielinski SJ, Blomqvist C, Boerwinkle E, Bogdanova NV, Bojesen SE, Bolla MK, Borresen-Dale A-L, Boutin TS, Brauch H, Brenner H, Brüning T, Burwinkel B, Campbell A, Campbell H, Chanock SJ, Chapman JR, Chen Y-DI, Chenevix-Trench G, Couch FJ, Coviello AD, Cox A, Czene K, Darabi H, De Vivo I, Demerath EW, Dennis J, Devilee P, Dörk T, Dos-Santos-Silva I, Dunning AM, Eicher JD, Fasching PA, Faul JD, Figueroa J, Flesch-Janys D, Gandin I, Garcia ME, García-Closas M, Giles GG, Girotto GG, Goldberg MS, González-Neira A, Goodarzi MO, Grove ML, Gudbjartsson DF, Guénel P, Guo X, Haiman CA, Hall P, Hamann U, Henderson BE, Hocking LJ, Hofman A, Homuth G, Hooning MJ, Hopper JL, Hu FB, Huang J, Humphreys K, Hunter DJ, Jakubowska A, Jones SE, Kabisch M, Karasik D, Knight JA, Kolcic I, Kooperberg C, Kosma V-M, Kriebel J, Kristensen V, Lambrechts D, Langenberg C, Li J, Li X, Lindström S, Liu Y, Luan J, Lubinski J, Mägi R, Mannermaa A, Manz J, Margolin S, Marten J, Martin NG, Masciullo C, Meindl A, Michailidou K, Mihailov E, Milani L, Milne RL, Müller-Nurasyid M, Nalls M, Neale BM, Nevanlinna H, Neven P, Newman AB, Nordestgaard BG, Olson JE, Padmanabhan S, Peterlongo P, Peters U, Petersmann A, Peto J, Pharoah PDP, Pirastu NN, Pirie A, Pistis G, Polasek O, Porteous D, Psaty BM, Pylkäs K, Radice P, Raffel LJ, Rivadeneira F, Rudan I, Rudolph A, Ruggiero D, Sala CF, Sanna S, Sawyer EJ, Schlessinger D, Schmidt MK, Schmidt F, Schmutzler RK, Schoemaker MJ, Scott RA, Seynaeve CM, Simard J, Sorice R, Southey MC, Stöckl D, Strauch K, Swerdlow A, Taylor KD, Thorsteinsdottir U, Toland AE, Tomlinson I, Truong T, Tryggvadottir L, Turner ST, Vozzi D, Wang Q, Wellons M, Willemsen G, Wilson JF, Winqvist R, Wolffenbuttel BBHR, Wright AF, Yannoukakos D, Zemunik T, Zheng W, Zygmunt M, Bergmann S, Boomsma DI, Buring JE, Ferrucci L, Montgomery GW, Gudnason V, Spector TD, van Duijn CM, Alizadeh BZ, Ciullo M, Crisponi L, Easton DF, Gasparini PP, Gieger C, Harris TB, Hayward C, Kardia SLR, Kraft P, McKnight B, Metspalu A, Morrison AC, Reiner AP, Ridker PM, Rotter JI, Toniolo D, Uitterlinden AG, Ulivi S, Völzke H, Wareham NJ, Weir DR, Yerges-Armstrong LM, Price AL, Stefansson K, Visser JA, Ong KK, Chang-Claude J, Murabito JM, Perry JRB, Murray A, 2015. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat. Genet 47, 1294–1303. 10.1038/ng.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeigler D, Fragouli E, Costa-Borges N, Mitalipov S, Scott R, Barnhardt K, 2019. Journal club global live on the role of mitochondria in reproduction. 2nd Scientific Symposium on Assisted Reproduction. [Google Scholar]
- de Ziegler D, Santulli P, Seroka A, Decanter C, Meldrum DR, Chapron C, 2013. In women, the reproductive harm of toxins such as tobacco smoke is reversible in 6 months: basis for the “olive tree” hypothesis. Fertil. Steril 100, 927–928. 10.1016/j.fertnstert.2013.05.043. [DOI] [PubMed] [Google Scholar]
- Demozay D, Rocchi S, Mas J-C, Grillo S, Pirola L, Chavey C, Van Obberghen E, 2004. Fatty Aldehyde Dehydrogenase. J. Biol. Chem 279, 6261–6270. 10.1074/jbc.M312062200. [DOI] [PubMed] [Google Scholar]
- Duran HE, Simsek-Duran F, Oehninger SC, Jones HW, Castora FJ, 2011. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil. Steril 96, 384–388. 10.1016/j.fertnstert.2011.05.056. [DOI] [PubMed] [Google Scholar]
- Edwards RG, Lobo R, Bouchard P, 1996. Time to revolutionize ovarian stimulation. Hum. Reprod 11, 917–919. 10.1093/oxfordjournals.humrep.a019317. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, Ye X, Petrozza JC, Wright D, Hauser R, 2012. Urinary Bisphenol A Concentrations and Implantation Failure among Women Undergoing in Vitro Fertilization. Environ. Health Perspect 120, 978–983. 10.1289/ehp.1104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouany MR, Sharara FI, 2013. Is there a role for DHEA supplementation in women with diminished ovarian reserve? J. Assist. Reprod. Genet 30, 1239–1244. 10.1007/s10815-013-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragouli E, McCaffrey C, Ravichandran K, Spath K, Grifo JA, Munné S, Wells D, 2017. Clinical implications of mitochondrial DNA quantification on pregnancy outcomes: a blinded prospective non-selection study. Hum. Reprod 32, 2340–2347. 10.1093/humrep/dex292. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel C-E, Kokocinski F, Cohen J, Munne S, Wells D, 2015. Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential. PLOS Genet 11, e1005241 10.1371/journal.pgen.1005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli F, Piroddi M, Annetti C, Aisa C, Floridi E, Floridi A, 2005. Oxidative Stress and Reactive Oxygen Species, in: Cardiovascular Disorders in Hemodialysis KARGER, Basel: 240–260. 10.1159/000085686. [DOI] [PubMed] [Google Scholar]
- González-Fernández R, Hernández J, Martín-Vasallo P, Puopolo M, Palumbo A, Ávila J, 2016. Expression Levels of the Oxidative Stress Response Gene ALDH3A2 in Granulosa-Lutein Cells Are Related to Female Age and Infertility Diagnosis. Reprod. Sci 23, 604–609. 10.1177/1933719115607996. [DOI] [PubMed] [Google Scholar]
- Herzig S, Shaw RJ, 2018. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol 19, 121–135. 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Hashimoto R, Muramatsu H, Iwabuchi Y, Tatsuzawa C, Yano S, Sugano Y, 2015. The mitochondrial DNA polymorphisms in chromosomal aberration detected by next generation sequence. Forensic Sci. Int. Genet. Suppl. Ser 5, e375–e377. 10.1016/j.fsigss.2015.09.149. [DOI] [Google Scholar]
- Ishii T, Hibino Y, 2018. Mitochondrial manipulation in fertility clinics: Regulation and responsibility. Reprod. Biomed. Soc. Online 5, 93–109. 10.1016/j.rbms.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, Monji Y, 2011. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod. Fertil. Dev 23, 424. 10.1071/RD10133. [DOI] [PubMed] [Google Scholar]
- Jinno M, Takeuchi M, Watanabe A, Teruya K, Hirohama J, Eguchi N, Miyazaki A, 2011. Advanced glycation end-products accumulation compromises embryonic development and achievement of pregnancy by assisted reproductive technology. Hum. Reprod 26, 604–610. 10.1093/humrep/deq388. [DOI] [PubMed] [Google Scholar]
- Kang E, Wu J, Gutierrez NM, Koski A, Tippner-Hedges R, Agaronyan K, Platero-Luengo A, Martinez-Redondo P, Ma H, Lee Y, Hayama T, Van Dyken C, Wang X, Luo S, Ahmed R, Li Y, Ji D, Kayali R, Cinnioglu C, Olson S, Jensen J, Battaglia D, Lee D, Wu D, Huang T, Wolf DP, Temiakov D, Belmonte JCI, Amato P, Mitalipov S, 2016. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature 540, 270–275. 10.1038/nature20592. [DOI] [PubMed] [Google Scholar]
- Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S, 1995. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil. Steril 64, 577–583. [PubMed] [Google Scholar]
- Kitagawa T, Suganuma N, Nawa A, Kikkawa F, Tanaka M, Ozawa T, Tomoda Y, 1993. Rapid Accumulation of Deleted Mitochondrial Deoxyribonucleic Acid in Postmenopausal Ovaries. Biol. Reprod 49, 730–736. 10.1095/biolreprod49.4.730. [DOI] [PubMed] [Google Scholar]
- Konstantinidis M, Alfarawati S, Hurd D, Paolucci M, Shovelton J, Fragouli E, Wells D, 2014. Simultaneous assessment of aneuploidy, polymorphisms, and mitochondrial DNA content in human polar bodies and embryos with the use of a novel microarray platform. Fertil. Steril 102, 1385–1392. 10.1016/j.fertnstert.2014.07.1233. [DOI] [PubMed] [Google Scholar]
- Kostaras K, Costa-Borges N, Psathas P, Calderon G, Nikitos E, 2019. ISRCTN Registry, Spindle transfer for the treatment of infertility problems associated to poor egg quality: a pilot trial 10.1186/ISRCTN11455145. [DOI] [Google Scholar]
- Kushnir VA, Ludaway T, Russ RB, Fields EJ, Koczor C, Lewis W, 2012. Reproductive aging is associated with decreased mitochondrial abundance and altered structure in murine oocytes. J. Assist. Reprod. Genet 29, 637–642. 10.1007/s10815-012-9771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarta E, de los Santos MJ, Escribá MJ, Pellicer A, Herraiz S, 2019a. Mitochondria as a tool for oocyte rejuvenation. Fertil. Steril 111, 219–226. 10.1016/j.fertnstert.2018.10.036. [DOI] [PubMed] [Google Scholar]
- Labarta E, de los Santos MJ, Herraiz S, Escribá MJ, Marzal A, Buigues A, Pellicer A, 2019b. Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization—a randomized pilot study. Fertil. Steril 111, 86–96. 10.1016/j.fertnstert.2018.09.023. [DOI] [PubMed] [Google Scholar]
- Larsson N-G, 2010. Somatic Mitochondrial DNA Mutations in Mammalian Aging. Annu. Rev. Biochem 79, 683–706. 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- Li Y-J, Han Z, Ge L, Zhou C-J, Zhao Y-F, Wang D-H, Ren J, Niu X-X, Liang C-G, 2016. C-phycocyanin protects against low fertility by inhibiting reactive oxygen species in aging mice. Oncotarget 7. 10.18632/oncotarget.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Luderer U, 2011. Oxidative Damage Increases and Antioxidant Gene Expression Decreases with Aging in the Mouse Ovary. Biol. Reprod 84, 775–782. 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M-J, Sun A-G, Zhao S-G, Liu H, Ma S-Y, Li M, Huai Y-X, Zhao H, Liu HB, 2018. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil. Steril 109, 900–907. 10.1016/j.fertnstert.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Tishkoff DX, Bao J, 2011. Mitochondrial Sirtuins in the Regulation of Mitochondrial Activity and Metabolic Adaptation, pp. 163–188. 10.1007/978-3-642-21631-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Poirier C, Gaspar T, Gratzke C, Harrison W, Busija D, Matzuk MM, Andersson K-E, Overbeek PA, Bishop CE, 2008. A Mutation in the Inner Mitochondrial Membrane Peptidase 2-Like Gene (Immp2l) Affects Mitochondrial Function and Impairs Fertility in Mice1. Biol. Reprod 78, 601–610. 10.1095/biolreprod.107.065987. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Missmer SA, Bukulmez O, Leach R, Stern JE, 2011. The effect of increasing obesity on the response to and outcome of assisted reproductive technology: a national study. Fertil. Steril 96, 820–825. 10.1016/j.fertnstert.2011.07.1100. [DOI] [PubMed] [Google Scholar]
- Lynch M, 2006. Mutation Pressure and the Evolution of Organelle Genomic Architecture. Science (80-.) 311, 1727–1730. 10.1126/science.1118884. [DOI] [PubMed] [Google Scholar]
- Ma H, O’Neil RC, Marti Gutierrez N, Hariharan M, Zhang ZZ, He Y, Cinnioglu C, Kayali R, Kang E, Lee Y, Hayama T, Koski A, Nery J, Castanon R, Tippner-Hedges R, Ahmed R, Van Dyken C, Li Y, Olson S, Battaglia D, Lee DM, Wu DH, Amato P, Wolf DP, Ecker JR, Mitalipov S, 2017. Functional Human Oocytes Generated by Transfer of Polar Body Genomes. Cell Stem Cell 20, 112–119. 10.1016/j.stem.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man L, Lekovich J, Rosenwaks Z, Gerhardt J, 2017. Fragile X-Associated Diminished Ovarian Reserve and Primary Ovarian Insufficiency from Molecular Mechanisms to Clinical Manifestations. Front. Mol. Neurosci 10 10.3389/fnmol.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Hamilton B, Osterman M, Driscoll A, Drake P, 2018. CDC National Vital Statistics Report [PubMed]
- Martini D, Del Bo’ C, Tassotti M, Riso P, Del Rio D, Brighenti F, Porrini M, 2016. Coffee Consumption and Oxidative Stress: A Review of Human Intervention Studies. Molecules 21, 979. 10.3390/molecules21080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I, 2013. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract 7, e330–e341. 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- May-Panloup P, Chrétien MF, Jacques C, Vasseur C, Malthiéry Y, Reynier P, 2005. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum. Reprod 20, 593–597. 10.1093/humrep/deh667. [DOI] [PubMed] [Google Scholar]
- May-Panloup P, Desquiret V, Moriniére C, Ferré-L’Hôtellier V, Lemerle S, Boucret L, Lehais S, Chao de la Barca JM, Descamps P, Procaccio V, Reynier P, 2014. Mitochondrial macro-haplogroup JT may play a protective role in ovarian ageing. Mitochondrion 18, 1–6. 10.1016/j.mito.2014.08.002. [DOI] [PubMed] [Google Scholar]
- May-Panloup P, Boucret L, Chao de la Barca J-M, Desquiret-Dumas V, Ferré-L’Hotellier V, Morinière C, Descamps P, Procaccio V, Reynier P, 2016. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum. Reprod. Update 22, 725–743. 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- Meldrum DR, Casper RF, Diez-Juan A, Simon C, Domar AD, Frydman R, 2016. Aging and the environment affect gamete and embryo potential: can we intervene? Fertil. Steril 105, 548–559. 10.1016/j.fertnstert.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Moawad A, Shaeer M, 2012. Long-term androgen priming by use of dehydroepiandrosterone (DHEA) improves IVF outcome in poor-responder patients. A randomized controlled study. Middle East Fertil. Soc. J 17, 268–274. 10.1016/j.mefs.2012.11.002. [DOI] [Google Scholar]
- Müller-Höcker J, Schäfer S, Weis S, Münscher C, Strowitzki T, 1996. Morphological-cytochemical and molecular genetic analyses of mitochondria in isolated human oocytes in the reproductive age. Mol. Hum. Reprod 2, 951–958. 10.1093/molehr/2.12.951. [DOI] [PubMed] [Google Scholar]
- Mumford SL, Sundaram R, Schisterman EF, Sweeney AM, Barr DB, Rybak ME, Maisog JM, Parker DL, Pfeiffer CM, Louis GMB, 2014. Higher Urinary Lignan Concentrations in Women but Not Men Are Positively Associated with Shorter Time to Pregnancy. J. Nutr 144, 352–358. 10.3945/jn.113.184820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy ZP, Cecile J, Liu J, Loccufier A, Devroey P, Van Steirteghem A, 1996. Pregnancy and birth after intracytoplasmic sperm injection of in vitro matured germinal-vesicle stage oocytes: case report**Supported by grants by the Belgian Fund for Medical Research, Brussels, Belgium. Fertil. Steril 65, 1047–1050. 10.1016/S0015-0282(16)58285-5. [DOI] [PubMed] [Google Scholar]
- Ochiai A, Kuroda K, 2019. Preconception resveratrol intake against infertility: Friend or foe? Reprod. Med. Biol 10.1002/rmb2.12303rmb2.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvieto R, Gleicher N, 2016. Should preimplantation genetic screening (PGS) be implemented to routine IVF practice? J. Assist. Reprod. Genet 33, 1445–1448. 10.1007/s10815-016-0801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacella-Ince L, Zander-Fox D, Lane M, 2014. Mitochondrial SIRT3 and its target glutamate dehydrogenase are altered in follicular cells of women with reduced ovarian reserve or advanced maternal age. Hum. Reprod 29, 1490–1499. 10.1093/humrep/deu071. [DOI] [PubMed] [Google Scholar]
- Palomba S, Falbo A, Valli B, Morini D, Villani MT, Nicoli A, La Sala GB, 2014. Physical activity before IVF and ICSI cycles in infertile obese women: an observational cohort study. Reprod. Biomed. Online 29, 72–79. 10.1016/j.rbmo.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Rotoli D, Gonzalez-Fernandez R, Hernandez J, Avila J, 2014. Oxidative stress affects FSH response in human granulosa-lutein cells. Hum. Reprod 29, i308. [Google Scholar]
- Park SY, Choi B, Cheon H, Pak YK, Kulawiec M, Singh KK, Lee M-S, 2004. Cellular aging of mitochondrial DNA-depleted cells. Biochem. Biophys. Res. Commun 325, 1399–1405. 10.1016/j.bbrc.2004.10.182. [DOI] [PubMed] [Google Scholar]
- Paszkowski T, 2002. Smoking induces oxidative stress inside the Graafian follicle. Hum. Reprod 17, 921–925. 10.1093/humrep/17.4.921. [DOI] [PubMed] [Google Scholar]
- Pedrajas JR, Miranda-Vizuete A, Javanmardy N, Gustafsson J-Å, Spyrou G, 2000. Mitochondria of Saccharomyces cerevisiae Contain One-conserved Cysteine Type Peroxiredoxin with Thioredoxin Peroxidase Activity. J. Biol. Chem 275, 16296–16301. 10.1074/jbc.275.21.16296. [DOI] [PubMed] [Google Scholar]
- Pikó L, Taylor KD, 1987. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev. Biol 123, 364–374. 10.1016/0012-1606(87)90395-2. [DOI] [PubMed] [Google Scholar]
- Qian Y, Shao L, Yuan C, Jiang C-Y, Liu J, Gao C, Gao L, Cui Y-G, Jiang S-W, Liu J-Y, Meng Y, 2016. Implication of Differential Peroxiredoxin 4 Expression with Age in Ovaries of Mouse and Human for Ovarian Aging. Curr. Mol. Med 16, 243–251. 10.2174/1566524016666160225151647. [DOI] [PubMed] [Google Scholar]
- Rani V, Deep G, Singh RK, Palle K, Yadav UCS, 2016. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci 148, 183–193. 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Ravichandran K, McCaffrey C, Grifo J, Morales A, Perloe M, Munne S, Wells D, Fragouli E, 2017. Mitochondrial DNA quantification as a tool for embryo viability assessment: retrospective analysis of data from single euploid blastocyst transfers. Hum. Reprod 32, 1282–1292. 10.1093/humrep/dex070. [DOI] [PubMed] [Google Scholar]
- Reynier P, May-Panloup P, Chretien M-F, Morgan CJ, Jean M, Savagner F, Barriere P, Malthiery Y, 2001. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol. Hum. Reprod 7, 425–429. 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- Rojansky R, Cha M-Y, Chan DC, 2016. Elimination of paternal mitochondria in mouse embryos occurs through autophagic degradation dependent on PARKIN and MUL1. Elife 5. 10.7554/eLife.17896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JM, Stewart JB, Hagström E, Brené S, Mourier A, Coppotelli G, Freyer C, Lagouge M, Hoffer BJ, Olson L, Larsson N-G, 2013. Germline mitochondrial DNA mutations aggravate ageing and can impair brain development. Nature 501, 412–415. 10.1038/nature12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Inta C, Omanska-Klusek A, Wong S, Barrow C, Garcia-Arocena D, Iwahashi C, Berry-Kravis E, Hagerman RJ, Hagerman PJ, Giulivi C, 2010. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem. J 429, 545–552. 10.1042/BJ20091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez F, Smitz J, 2012. Molecular control of oogenesis. Biochim. Biophys. Acta - Mol. Basis Dis 1822, 1896–1912. 10.1016/j.bbadis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Santos TA, El Shourbagy S, St. John JC, 2006. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil. Steril 85, 584–591. 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Sathananthan AH, 1997. Ultrastructure of the human egg. Hum. Cell 10, 21–38. [PubMed] [Google Scholar]
- Schrieks IC, van den Berg R, Sierksma A, Beulens JWJ, Vaes WHJ, Hendriks HFJ, 2013. Effect of Red Wine Consumption on Biomarkers of Oxidative Stress. Alcohol 48, 153–159. 10.1093/alcalc/ags086. [DOI] [PubMed] [Google Scholar]
- Shen M, Wang F, Li M, Sah N, Stockton ME, Tidei JJ, Gao Y, Korabelnikov T, Kannan S, Vevea JD, Chapman ER, Bhattacharyya A, van Praag H, Zhao X, 2019. Reduced mitochondrial fusion and Huntingtin levels contribute to impaired dendritic maturation and behavioral deficits in Fmr1-mutant mice. Nat. Neurosci 22, 386–400. 10.1038/s41593-019-0338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek-Duran F, Li F, Ford W, Swanson RJ, Jones HW, Castora FJ, 2013. Age-Associated Metabolic and Morphologic Changes in Mitochondria of Individual Mouse and Hamster Oocytes. PLoS One 8, e64955. 10.1371/journal.pone.0064955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Naviaux RK, 2001. Need for public debate about fertility treatments: manipulations of the mitochondrial germline must be openly debated and followed up. Nature 413, 347. [DOI] [PubMed] [Google Scholar]
- Singh Keshav K., Costello L, 2009. Mitochondria and Cancer Springer, New York, New York, NY. 10.1007/978-0-387-84835-8. [DOI] [Google Scholar]
- Skillern A, Rajkovic A, 2008. Recent Developments in Identifying Genetic Determinants of Premature Ovarian Failure. Sex. Dev 2, 228–243. 10.1159/000152039. [DOI] [PubMed] [Google Scholar]
- St John J, 2007. The Mitochondrion in the Germline and Early Development
- St John J, Sakkas D, Dimitriadi K, Barnes A, Maclin V, Ramey J, Barratt C, Jonge C De, 2000. Failure of elimination of paternal mitochondrial DNA in abnormal embryos. Lancet 355, 200. 10.1016/S0140-6736(99)03842-8. [DOI] [PubMed] [Google Scholar]
- Steuerwald N, Barritt JA, Adler R, Malter H, Schimmel T, Cohen J, Brenner CA, 2000. Quantification of mtDNA in single oocytes, polar bodies and subcellular components by real-time rapid cycle fluorescence monitored PCR. Zygote 8, 209–215. 10.1017/S0967199400001003. [DOI] [PubMed] [Google Scholar]
- Stumpf JD, Saneto RP, Copeland WC, 2013. Clinical and Molecular Features of POLG-Related Mitochondrial Disease. Cold Spring Harb. Perspect. Biol 5, a011395. 10.1101/cshperspect.a011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F, 2006. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. MHR Basic Sci. Reprod. Med 12, 655–660. 10.1093/molehr/gal080. [DOI] [PubMed] [Google Scholar]
- Tiosano D, Mears JA, Buchner DA, 2019. Mitochondrial Dysfunction in Primary Ovarian Insufficiency. Endocrinology 160, 2353–2366. 10.1210/en.2019-00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson N-G, 2004. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423. 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Tsui K-H, Wang P-H, Lin L-T, Li C-J, 2017. DHEA protects mitochondria against dual modes of apoptosis and necroptosis in human granulosa HO23 cells. Reproduction 154, 101–110. 10.1530/REP-17-0016. [DOI] [PubMed] [Google Scholar]
- Udagawa O, Ishihara T, Maeda M, Matsunaga Y, Tsukamoto S, Kawano N, Miyado K, Shitara H, Yokota S, Nomura M, Mihara K, Mizushima N, Ishihara N, 2014. Mitochondrial Fission Factor Drp1 Maintains Oocyte Quality via Dynamic Rearrangement of Multiple Organelles. Curr. Biol 24, 2451–2458. 10.1016/j.cub.2014.08.060. [DOI] [PubMed] [Google Scholar]
- Vanegas JC, Afeiche MC, Gaskins AJ, Mínguez-Alarcón L, Williams PL, Wright DL, Toth TL, Hauser R, Chavarro JE, 2015. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil. Steril 103, 749–755. 10.1016/j.fertnstert.2014.12.104 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaskivuo TE, Anttonen M, Herva R, Billig H, Dorland M, te Velde ER, Stenbäck F, Heikinheimo M, Tapanainen JS, 2001. Survival of Human Ovarian Follicles from Fetal to Adult Life: Apoptosis, Apoptosis-Related Proteins, and Transcription Factor GATA-4 1. J. Clin. Endocrinol. Metab 86, 3421–3429. 10.1210/jcem.86.7.7679. [DOI] [PubMed] [Google Scholar]
- Wallace DC, 2005. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annual Review of Genetics 39, 359–407. 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang D, Zou X, Xu C, 2009. Mitochondrial functions on oocytes and preimplantation embryos. J. Zhejiang Univ. Sci. B 10, 483–492. 10.1631/jzus.B0820379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Leader A, Tsang BK, 2013. Follicular stage-dependent regulation of apoptosis and steroidogenesis by prohibitin in rat granulosa cells. J. Ovarian Res 6, 23. 10.1186/1757-2215-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Babayev E, Jiang Z, Li G, Zhang M, Esencan E, Horvath T, Seli E, 2018. Mitochondrial unfolded protein response gene Clpp is required to maintain ovarian follicular reserve during aging, for oocyte competence, and development of pre-implantation embryos. Aging Cell 17, e12784. 10.1111/acel.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zhang M, Jiang Z, Seli E, 2017. Mitochondrial dysfunction and ovarian aging. Am. J. Reprod. Immunol 77, e12651 10.1111/aji.12651. [DOI] [PubMed] [Google Scholar]
- Wei Y-H, Lee H-C, 2002. Oxidative Stress, Mitochondrial DNA Mutation, and Impairment of Antioxidant Enzymes in Aging. Exp. Biol. Med 227, 671–682. 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- Wicks J, Treloar SA, Martin NG, 2004. Using Identity-by-descent Information in Affected Sib Pairs to Increase the Efficiency of Genetic Association Studies. Twin Res 7, 211–216. 10.1375/136905204323016195. [DOI] [PubMed] [Google Scholar]
- Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G, 2001. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod 16, 909–917. 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- Woods D, Tilly J, 2015. Autologous Germline Mitochondrial Energy Transfer (AUGMENT) in Human Assisted Reproduction. Semin. Reprod. Med 33, 410–421. 10.1055/s-0035-1567826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-J, Liu C, Duan W-X, Xu S-C, He M-D, Chen C-H, Wang Y, Zhou Z, Yu Z-P, Zhang L, Chen Y, 2013. Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat. Res. Toxicol. Environ. Mutagen 752, 57–67. 10.1016/j.mrgentox.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Zhang J, 2015. Revisiting Germinal Vesicle Transfer as a Treatment for Aneuploidy in Infertile Women with Diminished Ovarian Reserve. J. Assist. Reprod. Genet 32, 313–317. 10.1007/s10815-014-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu H, Luo S, Lu Z, Chávez-Badiola A, Liu Z, Yang M, Merhi Z, Silber SJ, Munné S, Konstantinidis M, Wells D, Tang JJ, Huang T, 2017. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod. Biomed. Online 34, 361–368. 10.1016/j.rbmo.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott R, Horvath T, Seli E, 2019. Mitofusin 2 plays a role in oocyte and follicle development and is required to maintain ovarian follicular reserve during reproductive aging. Aging (Albany. NY) 11, 3919–3938. 10.18632/aging.102024. [DOI] [PMC free article] [PubMed] [Google Scholar]


