Abstract
Anti-interleukin (IL)-1 agents have been developed for the treatment of autoinflammatory and rheumatic conditions, where overproduction of IL-1 is an important pathophysiologic process. IL-1α and IL-1β are the most studied members of the IL-1 family of cytokines and have the strongest proinflammatory effects. A naturally occurring antagonist (IL-1Ra) mitigates their proinflammatory effects. Overproduction of both IL-1α (released by inflamed/damaged pericardial cells) and IL-1β (released by inflammatory cells) is now a well-recognized therapeutic target in patients with recurrent idiopathic pericarditis. Currently, there are three available anti-IL-1 agents: anakinra (recombinant human IL-1Ra), rilonacept (a soluble decoy receptor ‘trap’, binding both IL-1α and IL-1β), and canakinumab (human monoclonal anti-IL-1β antibody). For patients with corticosteroid-dependent and colchicine-resistant recurrent pericarditis with evidence of systemic inflammation, as evidenced by elevated C-reactive protein, the efficacy and safety of anakinra (2 mg/kg/day up to 100 mg/day subcutaneously usually for at least 6 months, then tapered) and rilonacept (320 mg subcutaneously for the first day followed by 160 mg subcutaneously weekly) have been clearly demonstrated in observational studies and randomized controlled clinical trials. Severe side effects are rare and discontinuation rates are very low (<4%). The most common reported side effect is injection site reactions (>50% of patients). In this article, we describe the historical and pathophysiological background and provide a comprehensive review of these agents, which appear to be the most significant advance in medical therapy of recurrent pericarditis in the last 5 years.
Keywords: Pericarditis, Anakinra, Rilonacept, Interleukin-1
Graphical Abstract
Introduction
Recurrent pericarditis is one of the most common and troublesome complications of pericarditis, affecting 15–30% of patients after an initial episode, and >40% after a first recurrence.1,2 Moreover, 5–10% of patients with recurrent pericarditis develop multiple recurrences that are particularly difficult to treat and often refractory to conventional anti-inflammatory drugs.
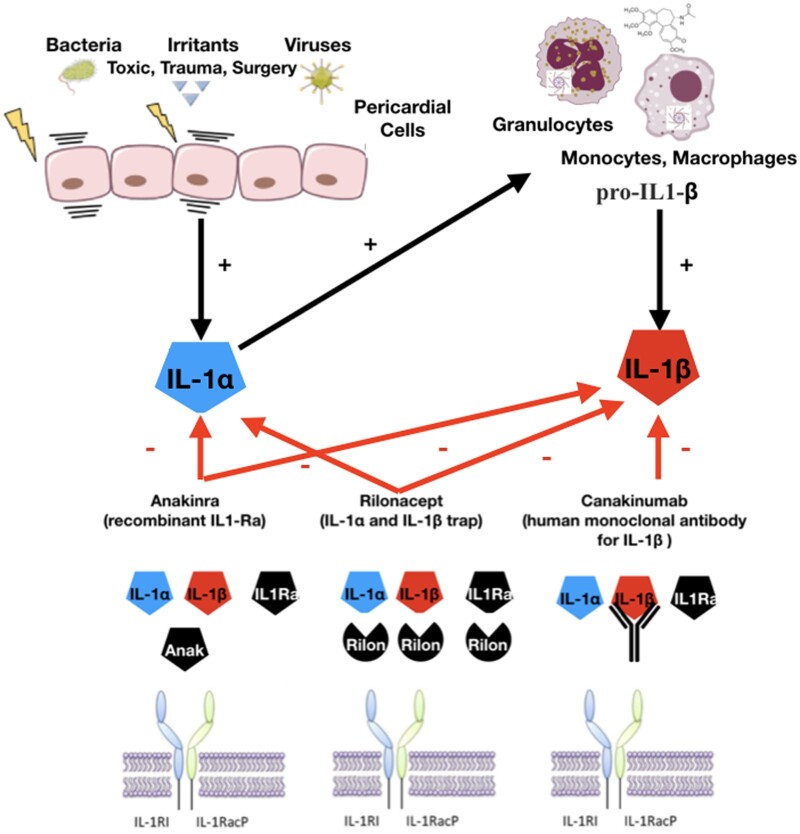
Based on current evidence, pericarditis should be considered an inflammatory reaction to various stimuli, including chemical/physical and infectious, with viral infection being a common aetiology. Interaction of pathogens or irritants with toll-like receptors leads to an increased transcription of proinflammatory genes, including those needed for assembly of the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing 3 (NLRP3) inflammasome, which leads to activation of interleukin-1 (IL-1; autoinflammatory pathway).3 This pathogenetic scheme has been confirmed indirectly by the beneficial effect of both colchicine (an indirect inhibitor of the NLRP3 inflammasome)1,2 and specific IL-1 blockers in patients with recurrent pericarditis (Graphical Abstract).4,5
Graphical Abstract.
Mechanism of action of available IL-1 agents for pericarditis: (1) anakinra (Anak) is a recombinant IL-1 receptor antagonist blocking either IL-1α or IL-1β; (2) rilonacept is a fusion protein forming a trap that binds IL-1α or IL-1β and IL1Ra; (3) canakinumab is an IgG1 human monoclonal antibody targeted at IL-1β.
The aim of this review is to provide a focused primer for clinicians on the use of anti-IL-1 agents for pericarditis, including their historical background, how they were developed, their mechanism of action, dosing, side effects, and practical tips for clinical use.
Historical background
The first report of the use of anti-IL-1 agents in pericarditis was by paediatricians from the Gaslini Institute (Genova, Italy) in 2009. They described the first cases of children with recurrent pericarditis who were successfully treated with the recombinant IL‐1 receptor antagonist anakinra (Kineret ®, Swedish Orphan Biovitrum, Stockholm, Sweden). These patients were all corticosteroid dependent and had fever and evidence of systemic inflammation based on elevated C-reactive protein (CRP).6 Anakinra administered subcutaneously was associated with very rapid disappearance of symptoms and normalization of CRP and other acute‐phase reactants. Continuous treatment with anakinra allowed rapid tapering and then discontinuation of corticosteroid treatment. At a mean follow-up of 6 months from the initial administration of anakinra, none of the patients had experienced a new disease relapse.
Most patients had idiopathic recurrent pericarditis, which has many features in common with autoinflammatory diseases (periodic fever, elevation of markers of inflammation, and serositis with pericarditis). Recurrent episodes of apparently unprovoked inflammation are the most characteristic feature of autoinflammatory diseases, and familial Mediterranean fever is a well‐known cause of recurrent pericarditis.7 Moreover, familial cases of recurrent idiopathic pericarditis have been described, with 6% reported to carry a mutation in the TNFRSF1A gene responsible for tumour necrosis factor receptor-associated periodic syndrome.8
Based on these favourable experiences in the paediatric setting, anakinra was then used in adults. One of the first descriptions of the use of anakinra in adult patients was published in 2012 by Vassilopoulos et al.9 They reported successful use in cases of drug-resistant and corticosteroid-dependent recurrent pericarditis with elevation of inflammatory markers. The medication was well tolerated in all but one patient, who exhibited a reversible elevation of transaminases. This is in agreement with the literature in rheumatoid arthritis patients, where anakinra has demonstrated an excellent safety profile, even after prolonged administration.9
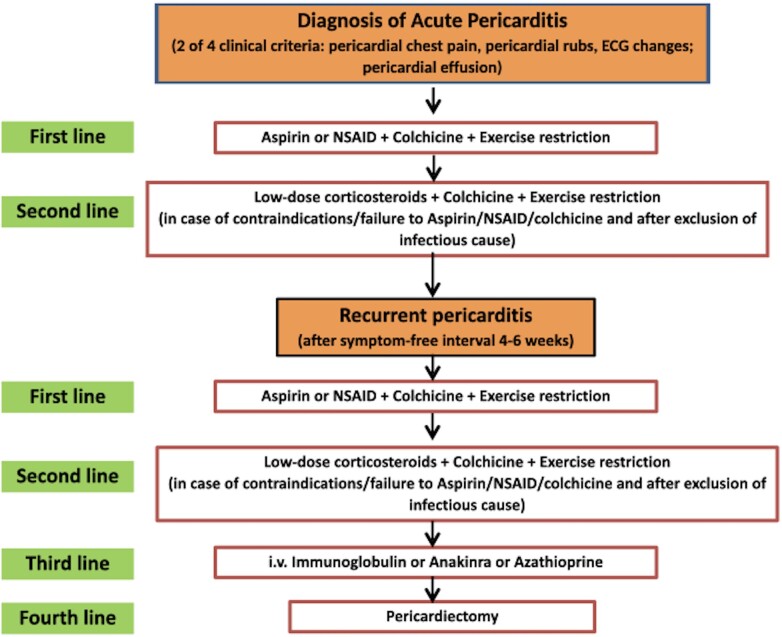
Based on this preliminary experience with anakinra, the 2015 European Society of Cardiology (ESC) guidelines on the management of pericardial diseases gave a class IIb recommendation (level of evidence C) for the use of anakinra in patients with recurrent pericarditis and corticosteroid dependence not responsive to colchicine, as a third-line treatment after non-steroidal anti-inflammatory drug and corticosteroid failure.10 The same level of recommendation was provided for other alternative third-line options for recurrent pericarditis,10 specifically, azathioprine,11 and intravenous human immunoglobulins,12 although based on weaker evidence from case reports and expert opinion without randomized trials. In some institutions (especially in the USA), pericardiectomy may be an earlier preferred option instead of third-line options, mainly after many failed attempts to discontinue steroid therapy. Additional clinical trials are warranted to better address this issue and other evidence-based treatments (Figure 1).
Figure 1.
Management algorithm for the treatment of recurrent pericarditis with first-line agents (non-steroidal anti-inflammatory drugs and colchicine), second-line drugs (low-dose corticosteroids and colchicine), third-line options (anakinra, azathioprine, and intravenous human immunoglobulins), and fourth-line treatment (pericardiectomy) according to the 2015 ESC guidelines.10 In some institutions (especially in the US), pericardiectomy may be an earlier preferred option instead of taking injection of anti-IL-1 agents, after many failed attempts to be off steroid therapy. Additional clinical trials are warranted to better address this issue.
A subsequent systematic review of cases with refractory recurrent idiopathic pericarditis reported clinical experience with anakinra in 34 patients (20 men, mean age 26.8 years).13 The mean disease duration was 31 months and the number of recurrences was 8.2. Anakinra was generally administered as a daily subcutaneous injection of 100 mg daily with a mean full-dose duration of 9.2 months. CRP normalized within 7.1 days, and corticosteroids were withdrawn within 62 days. During an average 28.3-month follow-up, 8 (23.5%) out of 34 patients were disease free off treatment, after having received anakinra for an average of 10.4 months.13
This initial evidence provided the rationale to conduct randomized trials,14–16 and establish an international registry.17
Mechanism of action and anti-IL-1 agents
Mechanism of action
IL-1 is a master cytokine which is released after the activation of the NLRP3 inflammasome. The IL-1 family is a group of 11 cytokines that plays a central role in the regulation of the inflammatory response to infections or sterile insults. The discovery of these cytokines began with studies on the pathogenesis of fever in experimental animal models. IL-1 was the name given to the products derived from macrophages. In 1985, two distinct proteins sharing human IL-1 activity were identified: IL-1α and IL-1β. These remain the most studied members of the family and possess the strongest proinflammatory effects.18,19
IL-1 is a pivotal cytokine that induces a complex network of other proinflammatory cytokines and regulates and initiates inflammatory responses by expression of integrins on leukocytes and endothelial cells. An important effect of IL-1 is the induction of fever in the thermoregulatory centre in the hypothalamus, as well as induction of hyperalgesia (increased pain sensitivity), vasodilatation, and hypotension.18–20 A naturally occurring IL-1α and IL-1β receptor antagonist (IL-1Ra) mitigates their inflammatory effects by competing for the same receptor. Both IL-1α and IL-1β bind to the same IL-1 receptor (IL-1R).18–20
IL-1α is produced as a precursor protein and is constitutively stored in the cytoplasm of cells of mesenchymal origin and in epithelial cells. Pericardial cells produce and store this cytokine. Pericardial injury by either infectious (e.g. bacteria, viruses) or non-infectious agents (e.g. irritants, toxic agents, trauma) is responsible for the release of IL-1α and damage-associated molecular pattern (DAMP) and pathogen-associated molecular patterns (PAMPs), which activate inflammatory cells (Figure 2).19–21 IL-1α also stimulates the transcription of IL-1β from monocytes and acts as an amplifier of inflammation by recruiting additional inflammatory cells. In contrast, IL-1β is synthetized as a precursor form only after stimulation. Its expression is induced by transcription factor NF-kB after exposure to DAMPs and alarmins. The active form of IL-1β is generated in monocytes after the activation of the inflammasome, a cytosolic macromolecular structure composed of an adaptor protein, procaspase 1, and a sensor molecule. The sensor molecule contains a nucleotide-binding oligomerization domain-like receptor (Nod-like receptor, NLR). When this sensor molecule is activated by DAMPs or PAMPs, the precursor pro-IL-1β is cleaved to active IL-1β. Colchicine blocks tubulin polymerization affecting microtubule function that is necessary for the assembly of the inflammasome and acts as a non-specific inhibitor of the inflammasome.22
Figure 2.
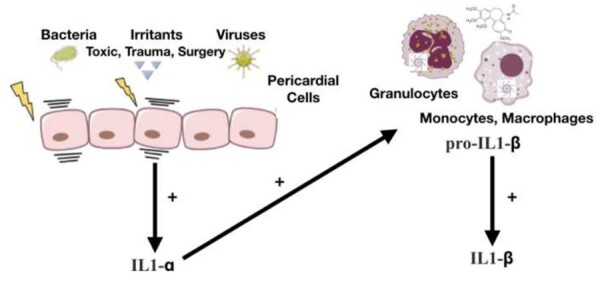
Pericardial injury by either infectious (e.g. bacteria, viruses) or non-infectious agents (e.g. irritants, toxic agents, trauma) is responsible for the release of IL-1α by pericardial cells and damage-associated molecular pattern and pathogen-associated molecular pattern release. Damage-associated molecular patterns and pathogen-associated molecular patterns trigger the activation of nuclear factor kB that leads to gene transcription of pro-inflammatory mediators and the activation of the inflammasome leading to the generation of activated pro-inflammatory cytokines such as IL-1 (target of anti-IL-1 agents). Colchicine is able to concentrate in white blood cells and interferes with microtubule function. Another anti-inflammatory mechanism is related to the non-specific inhibition of the inflammasome (the assembly of the inflammasome is allowed by microtubules).
Anti-IL-1 agents
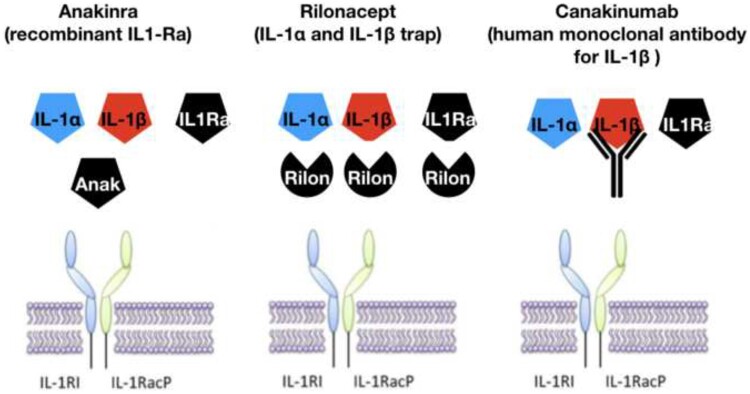
Several different anti-IL-1 agents have been developed (Table 1 and Figure 3). Anakinra (Kineret ®, Swedish Orphan Biovitrum) is a recombinant human IL-1Ra and is currently registered for the treatment of rheumatoid arthritis and cryopyrin-associated periodic syndromes, which are characterized by overproduction of IL-1, and, as discussed, has more recently been used for corticosteroid-dependent and colchicine-resistant recurrent pericarditis.13,14,17,23 Anakinra interferes with both IL-1α and IL-1β. Rilonacept, originally developed by Regeneron Pharmaceuticals, Inc, is a dimeric fusion protein consisting of the ligand-binding domains of the extracellular portions of the human IL-1 receptor component (IL-1RI) and IL-1 receptor accessory protein (IL-1RAcP) linked in-line to the Fc portion of human immunoglobulin G1.21 Rilonacept operates as a soluble decoy receptor or ‘trap’, which binds IL-1α or IL-1β and prevents engagement with the cell-surface receptor for IL-1, thus inhibiting IL-1 activity.21
Table 1.
Anti-IL-1 agents in clinical use
| Anakinra | Rilonacept | Canakinumab | |
|---|---|---|---|
| Form | Recombinant human IL-1Ra | IL-1α and IL-1β trap | Human monoclonal antibody anti-IL-1β |
| IL-1 target | IL-1α and IL-1β | IL-1α and IL-1β | IL-1β |
| Half-life | 4–6 h | 7 days | 26 days |
| Administration | SC or IV | SC | SC |
| Dosing | Every day | Weekly | Every 1–2 months |
| Dose adjustment for renal failure | Yes | No | No |
| Anti-inflammatory potency | +++ | ++ | +++ |
| FDA-approved indications | CAPS, RA | CAPS | CAPS, TRAPS, FMF, HIDS/MVK, systemic juvenile IA, adult-onset Still disease |
| EMA-approved indications | CAPS, systemic juvenile IA, adult-onset Still disease, RA | None | CAPS, TRAPS, FMF, HIDS/MVK, systemic juvenile IA, adult-onset Still disease, gout flare |
| Adverse Events | Injection site reactions, hepatitis, infections | Injection site reactions, infections, neutropenia, hyperlipidemia | Injection site reactions, infections, neutropenia |
CAPS, cryopyrin-associated periodic syndromes; FMF, familial Mediterranean fever; HIDS, hyperimmunoglobulin D syndrome; IA, idiopathic arthritis; IV, intravenous; MVK, mevalonate kinase deficiency; RA, rheumatoid arthritis; SC, subcutaneous; TRAPS, tumor necrosis factor receptor-associated periodic syndrome.
Figure 3.
Mechanism of action of available IL-1 agents in current clinical practice: (i) anakinra (Anak) is a recombinant IL-1 receptor antagonist blocking either IL-1α or IL-1β; (ii) rilonacept is a fusion protein consisting of extracellular domains of IL-1 receptor 1 (IL-1R1) and IL-1 receptor accessory protein (IL-1RacP; both IL-1R components) attached to each arm of an IgG Fc portion forming a trap that binds IL-1α or IL-1β and IL-1Ra; and (iii) canakinumab is an IgG1 human monoclonal antibody targeted at IL-1β. All anti-IL-1 agents interfere with the binding of IL-1 with its membrane receptor.
Canakinumab, a monoclonal antibody that selectively blocks IL-1β, is a third anti-IL-1 agent. Its use has been only anecdotally reported to treat pericarditis.24–26 It is best known in cardiovascular medicine because of the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS),27 a randomized, double-blind trial, involving 10 061 patients with previous myocardial infarction and a high-sensitivity CRP level >2 mg/L. CANTOS is an important proof of concept trial, which showed that an anti-IL-1 agent reduces cardiovascular risk solely because of its anti-inflammatory effect, with no effects on lipids.27
In 2018, a first, very small series of patients with recurrent pericarditis treated with canakinumab was published. Results were mixed, with responses in 2 out of 3 cases and inability to achieve prolonged control of the disease in one case.24 Subsequent case reports also provided mixed results.25,26 Overall, canakinumab failed to achieve stable control of pericarditis in most case reports of recurrent pericarditis.24–26 This may reflect selective blocking of IL-1β.
Evidence from clinical trials and registries: safety of anti-IL-1 agents
Clinical trials and registries
The first and thus far only investigator-initiated randomized, placebo-controlled trial of anakinra for corticosteroid-dependent and colchicine-resistant recurrent pericarditis was the Anakinra-Treatment of Recurrent Idiopathic Pericarditis (AIRTRIP) trial.14
The AIRTRIP trial was a double-blind, placebo-controlled, randomized withdrawal trial including an open-label treatment with anakinra followed by a double-blind withdrawal phase with anakinra or placebo until recurrent pericarditis occurred. The study hypothesis was based on the striking efficacy reported in children and adults, requiring only 21 patients to be randomized for adequate power. To be enrolled in this study, an increased level of CRP was mandatory. Anakinra was administered at 2 mg/kg per day, up to 100 mg, for 2 months. All patients responded with the resolution of pericarditis and were randomized to continue anakinra (n = 11) or switch to placebo (n = 10) for 6 months or until a pericarditis recurrence. After a median follow-up of 14 months, recurrent pericarditis occurred in 9 of 10 patients (90%; incidence rate, 2.06% of patients per year) assigned to placebo and 2 of 11 patients (18.2%; incidence rate, 0.11% of patients per year) assigned to anakinra, for an incidence rate difference of −1.95% (95% CI, −3.3% to −0.6%). During anakinra treatment, 20 of 21 patients (94.8%) experienced transient local skin reactions, 1 (4.8%) herpes zoster, 3 (14.3%) transaminase elevation, and 1 (4.8%) ischaemic optic neuropathy. No patient permanently discontinued the active drug.
The efficacy and safety of anakinra for corticosteroid-dependent and colchicine-resistant pericarditis was also confirmed in a real-world international registry, the International Registry of Anakinra for Pericarditis (IRAP).17 In the IRAP registry, investigators enrolled consecutive patients with recurrent pericarditis who were corticosteroid-dependent and colchicine-resistant for treatment with anakinra. The primary outcome was pericarditis recurrence rate after treatment. Secondary outcomes included emergency department visits, hospitalzations, corticosteroid use, and adverse events. Among 224 patients (46 ± 14 years old, 63% women, 75% idiopathic aetiology, 13% post-cardiac injury syndrome), the median duration of disease was 17 months (interquartile range 9-33). Most patients had elevated CRP (91%) and pericardial effusion (88%). After a median treatment of 6 months, pericarditis recurrences were reduced six-fold (from 2.33 to 0.39 per patient per year), emergency department admissions were reduced 11-fold (from 1.08 to 0.10 per patient per year), and hospitalizations were reduced seven-fold (from 0.99 to 0.13 per patient per year). Corticosteroid use was decreased by anakinra (from 80% to 27% of patients; P < 0.001). No serious adverse events were recorded; adverse events consisted mostly of transient skin reactions (38%) at the injection site. Adverse events led to discontinuation in 3% of cases. A full-dose treatment duration of over 3 months followed by a tapering period of over 3 months were the therapeutic schemes associated with a lower risk of recurrence.17
A new anti-IL-1 agent, rilonacept (Arcalyst ®, Regeneron/Kiniksa, USA) was more recently tested in patients with recurrent pericarditis. Rilonacept is a soluble chimeric fusion protein that functions as a decoy receptor, inhibiting the function of both IL-1α and IL-1β. In a sponsored open-label phase II study,15 25 adult patients with idiopathic or postpericardiotomy recurrent pericarditis were enrolled. They were either symptomatic (≥2 pericarditis recurrences) or corticosteroid-dependent (≥2 prior recurrences). Patients received rilonacept 320 mg Sc load and then 160 mg SC weekly during a 6-week base treatment period followed by an optional 18-week on-treatment extension period. Rilonacept led to rapid and sustained improvement in pain, inflammation (CRP and pericarditis manifestations), and health-related quality of life.15
These positive findings provided the rationale for a sponsored phase 3 trial, the RHAPSODY trial.16 RHAPSODY was a multicentre, double-blind, event-driven, randomized-withdrawal trial of rilonacept in patients with acute symptoms of recurrent pericarditis (as assessed on a patient-reported scale) and systemic inflammation (as shown by elevated CRP levels). Patients presenting with a pericarditis recurrence while receiving standard therapy were enrolled in a 12-week run-in period, during which rilonacept (320 mg SC for the first day followed by 160 mg SC weekly) was initiated and background medications were discontinued. All patients had a clinical response (i.e. met prespecified response criteria) and were then randomly assigned in a 1:1 ratio to receive continued rilonacept monotherapy (n = 30) or placebo (n = 31), administered subcutaneously once weekly. The primary efficacy endpoint was the time to the first pericarditis recurrence. The median time to the first adjudicated recurrence in the placebo group was 8.6 weeks [95% confidence interval (CI), 4.0–11.7; hazard ratio in a Cox proportional-hazards model, 0.04; 95% CI, 0.01–0.18; P < 0.001 by the log-rank test]. During this period, 2 of 30 patients (7%) in the rilonacept group had a pericarditis recurrence, as compared with 23 of 31 patients (74%) in the placebo group. In the run-in period, 4 (13%) patients had adverse events leading to discontinuation of rilonacept therapy. The most common adverse events with rilonacept were injection site reactions and upper respiratory tract infections.16
Safety of anti-IL-1 agents
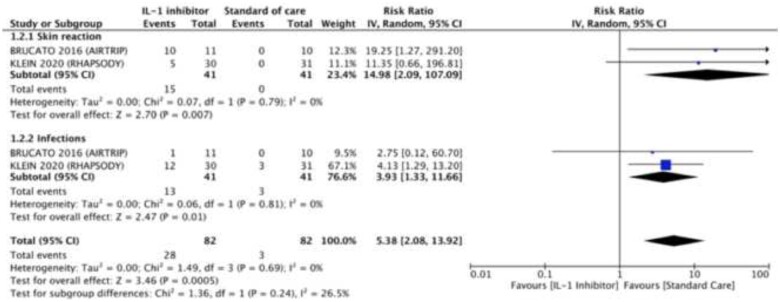
Anti-IL-1 agents are in general safe drugs, which have now been prescribed for years. If we consider only randomized controlled clinical trials, anti-IL-1 agents have been associated with an increased risk of any adverse events compared to placebo [risk ratio (RR) 5.38, 95% CI 2.08–13.92, I2 = 0%], including injection site reactions (RR 14.98, 95% CI 2.09–107.09, I2 = 0%) and infections (RR 3.93, 95% CI 1.33–11.66; Figure 4).14,16,28
Figure 4.
Drug-related adverse events in patients treated with or without anti-IL-1 agents.
The most common reported side effect is injection site reactions, which occur in the majority of patients treated with anakinra (>50%),14,17 a significant proportion of those treated with rilonacept (up to 60%),15,16 and approximately 10% of those treated with canakinumab.24–27 Patients receiving anakinra usually report stinging during injection, but injection site reactions usually occur after 1–2 weeks of therapy.14,17,23 Multiple prior injection sites may simultaneously become red and painful (Figure 5). These adverse events usually resolve within 1–2 months. They are typically transient and do not evolve. In order to minimize and resolve this side effect, it is recommended to allow the syringe to come to room temperature before injection (the drug is stored in a refrigerator at 2–8°C). Oral antihistamines and topical corticosteroids can be used for treatment. It is important to inform the patients of this common adverse event and reassure them that the reaction is transient and passes without sequelae in the large majority of patients.14,17,23
Figure 5.
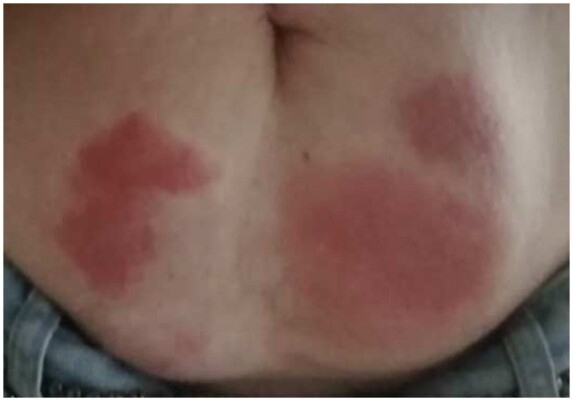
A representative case of intense injection site reaction after 1 week of therapy with anakinra (see text for additional explanations).
The second most common side effect is infections (especially upper respiratory or skin infections). Based on all of its indications, anakinra is associated with a lower risk of infections (3–5%)14,17,23 compared with rilonacept15,16 and canakinumab.24–27,29 Infections reported with anakinra or rilonacept in patients with pericarditis have, for the most part, been mild respiratory or skin infections and not due to opportunistic pathogens.14–17,23Arthralgias and myalgias have been reported in 6–12% of patients.15,17,23Elevation of transaminases is reported in at least 4% of cases and rarely associated with hepatitis,14–17,24 while additional laboratory findings include neutropenia and leukopenia (1–3%),14,17,23,24 and elevation of non-fasting blood lipids (up to 8% in patients treated with rilonacept).15,16 These laboratory abnormalities are generally mild and not clinically significant. No significant differences in the overall incidence of malignancies in patients exposed or unexposed to anti-tumour necrosis factor α or anakinra treatment were found. The same applied to the risk of recurrent malignancies.30
On this basis, measurement of blood cell count and transaminases is recommended at least at baseline before starting these drugs, and at 1, 3, and 6 months, and then according to the duration of therapy and possible clinical events. A permanent discontinuation of the drug because of adverse events is uncommon and reported in up to 3% of patients treated with anakinra and rilonacept.16,17,23 The main reported side effects are summarized in Table 2.
Table 2.
Reported adverse events of anti-intereukin-1 agents in clinical use for pericarditis
| Anakinra14,17,23 | Rilonacept15,16 | Canakinumab24–27,29 | |
|---|---|---|---|
| Severe side effectsa | <1–4% | Up to 6% | Up to 12% |
| Injection site reactions | 38–95% | 34–60% | <10% |
| Infections | 3–5% | Variable* | 3–7% |
| Elevation of transaminases | 3–14% | Up to 4% | 2–4% |
| Arthralgias, myalgias | 6–8% | Up to 12% | 2% |
| Elevation of blood lipids | Possibleb | Up to 8% | NR |
| Neutropenia or leukopenia | 1–3% | Possiblea | <1% |
| Permanent discontinuation due to adverse events | 3% | 3% | NR |
NR, not reported.
Depending on treatment times up to 40% (RHAPSODY trial) and usually mild to moderate.
Reported severe side effects included an ischaemic optic neuropathy in the AIRTRIP trial14 that was judged to be probably unrelated to treatment; skin reactions and intolerable arthralgias and myalgias leading to drug discontinuation were reported in the IRAP registry17; squamous-cell carcinoma, alopecia, extrinsic allergic alveolitis, erythema, and hypersensitivity reactions were reported in the RHAPSODY trial.16 Fatal infections and sepsis were the main adverse events reported in the CANTOS trial.27
Adverse events reported without a precise quantification.
Current indications, therapeutic scheme, and monitoring
Current indications
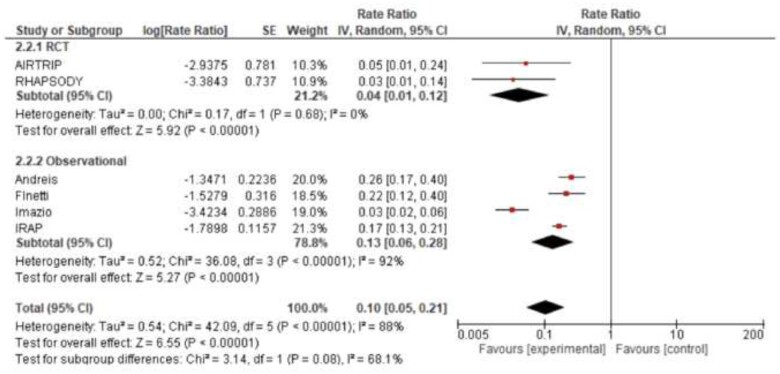
Current 2015 ESC guidelines give a class IIb recommendation (level of evidence C) for the use of anakinra in patients with recurrent pericarditis and corticosteroid dependence, not responsive to colchicine, based on limited case reports available at that time.10 However, subsequent research has expanded to larger observational studies and the first randomized controlled clinical trial. The strongest evidence to support the use of anti-IL-1 agents in pericarditis is for cases with recurrent pericarditis with corticosteroid dependence (unable to taper or withdraw corticosteroids) and colchicine resistance (recurrences despite use of colchicine). Including the larger observational studies,15,17,23,31 and randomized controlled clinical trials,14,16 after a median follow-up of 14 months (interquartile range 12–39), patients receiving anti-IL-1 agents (anakinra or rilonacept) had a significantly lower incidence rate ratio (IRR) for recurrent pericarditis (IRR 0.06, 95% CI 0.03–0.14, I2 = 95%) compared to patients receiving placebo and/or standard medical therapy (Figure 6). Anti-IL-1 agents reduced the risk of recurrent pericarditis both in the subgroup of randomized controlled clinical trials (IRR 0.04, 95% CI 0.01–0.12, I2 = 0%) and observational studies (IRR 0.07, 95% CI 0.03–0.17, I2 = 95%). On this basis, current evidence supports upgrade of current recommendations for the use of anti-IL-1 agents (anakinra or rilonacept) in patients with recurrent pericarditis, not responsive to colchicine and other conventional anti-inflammatory therapies (including non-steroidal anti-inflammatory drugs and colchicine), and with elevation of CRP. These agents should be recommended as a first-line treatment option for these patients, especially those with >1 recurrence.
Figure 6.
Recurrent pericarditis assessed as incidence rate ratio in patients treated with or without anti-IL-1 agents.
Therapeutic scheme and monitoring
Currently, two anti-IL-1 agents (anakinra and rilonacept) have been proven to be efficacious and safe in the setting of recurrent pericarditis with corticosteroid dependence and colchicine resistance. Both anakinra and rilonacept provide combined IL-1α and IL-1β antagonism, which seems important to achieve optimal control of the inflammatory mechanisms in pericarditis, as compared with selective IL-1β antagonism (canakinumab).
Anti-IL-1 treatments should be commenced in a hospital or clinic environment with a first dose provided under medical supervision and then continued on an outpatient basis with scheduled follow-up blood tests and visits. Before initiating treatment, it is recommended to measure a complete blood count, renal function, and transaminases. Females of childbearing potential should be screened for pregnancy and counselled appropriately with respect to issues related to pregnancy and lactation (see below). Screening for latent tuberculosis, hepatitis B and C and human immunodeficiency virus (HIV) should be performed. The most important issue is latent tuberculosis, and appropriate discussion with an infectious disease expert should be done in individual cases to assess the use of prophylactic antituberculous therapy for those with a positive TB Quantiferon test. IL-1 inhibitors generally do not appear to complicate these conditions and, when treatment is urgently indicated, it may be reasonable to initiate therapy while waiting screening results.20
Anakinra
Anakinra is administered SC without a loading dose (usual dose for adults 1–2 up to 100 mg/kg/day), and it is rapidly efficacious with pain relief within hours in patients with pericarditis.14,17 No major safety issues were reported in cases in which anakinra was used during pregnancy in women with autoinflammatory diseases; however, due to the limited total number of exposed pregnancies, the use of anti-IL-1 agents should be considered in pregnancy when the risk–benefit assessment of maintaining disease control justifies the potential risk to the foetus.
Anakinra is a large molecule, and it is unlikely that significant amounts of this medication will be transferred to breast milk, but notably IL-1Ra is a normal component of human milk where it may play a role as an anti-inflammatory agent.32,33
The minimal duration of therapy should be 3 months according to the IRAP registry, where an increased rate of relapses was documented for therapies lasting for <3 months.17 The usual duration of therapies is 6–12 months but longer durations may be required to ensure prolonged remissions. The drug can also be given IV if necessary. It has a high rate of injection site reactions of mild to moderate intensity in >50–60% of cases. These usually can be managed without treatment discontinuation with systemic antihistamines and topical corticosteroids. The shorter half-life (compared with rilonacept) provides the advantage of a faster reversibility of the effect (especially useful for acutely ill patients), but has the disadvantages of a daily administration and need for tapering. Abrupt discontinuation of anakinra is followed by recurrences in the short term in 50–70% of cases.17,34 Thus, tapering should be strongly recommended in patients treated with anakinra. The best tapering scheme is unknown. It has been proposed to start by reducing the weekly dosing schedule by one administration every week. As an alternative, the drug can be provided every other day at full dose at the beginning (usually for 3 months) then with half doses at the end of tapering (additional 1–3 months).17
Rilonacept
Rilonacept is administered SC with a loading dose of 320 mg on the first day (or 4.4 mg/kg if <18 years of age) followed by 160 mg weekly (or 2.2 mg/kg if <18 years of age). The relief of pain is seen within a few days.15,16 The minimal duration of therapy should be 6–8 months and varied according to the clinical response. Due to weekly administration, skin reactions are less common. At present, relevant data for rilonacept are not available in regard to the need for tapering. Due to the longer half-life, it is plausible that tapering may be less relevant in patients treated with this drug. The suggested therapeutic schemes are summarized in Table 3. No information is available concerning rilonacept in pregnancy and breast-feeding.
Table 3.
Therapeutic schemes and monitoring of anti-IL-1 agents in pericarditis
| Anakinra | Rilonacept | Canakinumab | |
|---|---|---|---|
| Loading dose | Not recommended | 320 mg SC on first day (or 4.4 mg/kg if <18 years of age) | Not recommended |
| Maintenance dose | 1–2 mg/kg/day up to 100 mg/day SC (usual dose for adults) | 160 mg weekly SC (or 2.2 mg/kg if <18 years of age) | 4–5 mg/kg SC usually every 4 weeks; for patients >40 kg usually 150 mg every 4 weeks; if inadequate response after 7 days may repeat dose and increase to 300 mg every 4 weeks |
| Duration | 3–6 months (usual), longer times in specific cases | At least 6–8 months | At least for 6–12 months |
| Tapering | Suggested for at least 3–6 monthsa | Probably not necessary | Probably not necessary |
| Monitoringb | Blood cell count, transaminases, renal function, blood lipids | Blood cell count, transaminases, renal function, blood lipids | Blood cell count, transaminases, renal function, blood lipids |
| Pregnancy | It can be used through conception | No data | Limited data; alternative drug to be preferred |
| Lactation | Compatible | No data | Limited data |
Different possible tapering schemes: reducing the dose of a single daily dose every week, as alternative with full dose every other day for 1–3 months and then half dose every other day for 1–3 months.
Be aware of possible increased risk of infections (especially respiratory and skin infections).
Use of imaging to guide therapy and monitoring
Cardiac magnetic resonance (CMR) allows objective identification of pericardial inflammation by means of evidence of pericardial thickening, oedema, and late gadolinium enhancement, since the inflamed pericardium is thickened and neovascularized.35,36 On this basis, CMR guided therapy may be a useful option, especially in cases where clinical evaluation and blood testing is insufficient to clearly determine the remission state (e.g. symptomatic patients with non-elevated markers of inflammation).35,36 Additional studies are ongoing and needed to better clarify the role of CMR in these settings.
Monitoring is required with blood cell count, transaminases, renal function, and blood lipids at baseline, and then during follow-up at least after 1 month, and then every 3 months. A careful follow-up is needed also to address the possible increased risk of infections (especially respiratory and skin infections).
Information for patients
Patients with complicated and/or refractory recurrent pericarditis should be ideally referred to cardiologists with special expertise in the management of pericarditis and the use of anti-IL-1 agents.
Prescription and dosing
The use of anti-IL-1 agents is currently off label for the treatment of pericarditis and informed consent is requested in these patients (in Italy anakinra can be prescribed for patients with corticosteroid-dependent and colchicine-resistant recurrent pericarditis within the National Healthcare System). Rilonacept has been currently approved by the FDA in the USA, but still under evaluation in Europe. Appropriate information should be provided on indications, possible side effects, and expected outcomes. Moreover, specific information should be provided on reproductive issues, pregnancy, lactation, and vaccination. An additional issue is related to the compatibility of these therapies with SARS-CoV-2 infection and vaccination.
Side effects
Although generally well tolerated, possible side effects should be anticipated, especially injection site skin reactions. Advice should be given on their usual timing after 1–2 weeks of therapy, and how to cope with these side effects. Patients should also be advised about the need for monitoring of blood tests during therapy after 1 month and then at least every 3 months.
Pregnancy and lactation
These are specific issues to be discussed with women of childbearing potential. The largest experience of use in the setting of pregnancy and lactation is with anakinra. Based on limited information, anakinra may be continued through conception in women with rheumatic and musculoskeletal diseases who are planning a pregnancy and are not able to use alternative therapies, although it is suggested to plan during a period of quiescent/low disease activity.32,33,37
Based on limited information, use of anakinra may be continued in males with rheumatic and musculoskeletal diseases who are planning to father a child.33
For women, the drug can be used through conception and it is also safe during lactation. However, there are no data or very limited data with other anti-IL-1 agents during pregnancy and lactation.
Immunizations and COVID-19
Patients should be brought up to date with all immunizations before initiating therapy with anti-IL-1 or other immunomodulatory agents; live vaccines should not be given concurrently. There are no data available concerning the effects of therapy on vaccination or secondary transmission of live vaccines in patients receiving therapy. Another issue is related specifically to COVID-19 vaccines. The vaccine stimulates production of antibodies that protect patients from COVID-19. It is not possible to become infected with COVID-19 from receiving the vaccine, and it is safe to receive it regardless of other immunosuppressive medications. Patients with pericarditis on immunomodulatory medications were not included in the COVID-19 vaccine clinical trials, which are similar to trials for other vaccines. However, we strongly recommend the COVID-19 vaccine, and this issue should be discussed further with patients and referral physicians. There is a possibility that the effect of the vaccine may be partially blunted by the use of these drugs, but there are no available data at present.
The last important consideration is that during the COVID-19 pandemic anti-IL-1 agents as well as colchicine are safe in patients with pericarditis and are probably also useful for COVID-19 management in patients who display pericardial involvement.38
Conclusions and future perspectives
Anti-IL-1 agents appear to be a major advance in medical therapy of recurrent pericarditis. These drugs represent a paradigm shift in the treatment of recurrent pericarditis, allowing more targeted and personalized therapy for patients showing evidence of systemic inflammation (e.g. fever and/or CRP elevation).39 Selection of patients remains very important; not all subjects with pericardial diseases are good candidates for anti-IL-1 agents, especially if evidence of systemic inflammation is absent. At present, although data are limited, there is evidence of efficacy of these agents in patients with other aetiologies besides idiopathic recurrent pericarditis (e.g. postpericardiotomy syndrome and systemic autoimmune and autoinflammatory diseases). The AIRTRIP14 and RHAPSODY16 trials enrolled patients with idiopathic recurrent pericarditis or postpericardiotomy syndromes, while 11% of patients in the IRAP registry15 had systemic autoimmune or autoinflammatory diseases.
Current pooled data suggest that anti-IL-1 agents should be a first option for corticosteroid-dependent and colchicine-resistant recurrent pericarditis with the evidence of systemic inflammation by means of elevated CRP. This will very likely translate into an upgraded recommendation for these agents in future guidelines.
What are the remaining questions to be addressed? First, the best duration of therapy and the need for tapering remain to be investigated: 6–8 months appear to be an optimal duration for a first therapeutic cycle, but some patients may require longer treatments to prevent additional recurrences. The second question is whether tapering is indicated and what is the best scheme to follow. Tapering is generally recommended with these agents, especially with anakinra. However, the best tapering regimen should be addressed in additional clinical studies. The third question is whether these drugs can be used before corticosteroids in order to avoid their side effects, and whether they are efficacious as monotherapy without colchicine. The use of anti-IL-1 agents as alternative drugs should be addressed in randomized trials, since these agents may offer the opportunity to replace corticosteroids, if efficacy and safety are confirmed. Lastly, regarding the possibility of replacing colchicine with anti-IL-1 agents, this is certainly an option. However, these agents may be synergistic in interfering with the inflammasome, i.e. non-specific inhibition by colchicine and specific inhibition by anti-IL-1 agents, when combination therapy is needed to control the disease.
Contributors
All authors contributed to the planning, conduct, reporting and drafting of the work.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Massimo Imazio, Head of Cardiology, Cardiothoracic Department, University Hospital “Santa Maria della Misericordia”, ASUFC, Piazzale Santa Maria della Misericordia 15, Udine 33100, Italy.
George Lazaros, 1st Cardiology Clinic, National and Kapodistrian University of Athens, School of Medicine, Hippokration General Hospital, Athens, Greece.
Marco Gattorno, Center for Autoinflammatory Diseases and Immunodeficiencies, IRCCS G. Gaslini, Genova, Italy.
Martin LeWinter, Cardiology Unit, University of Vermont Medical Center, Burlington, VT, USA.
Antonio Abbate, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, USA.
Antonio Brucato, Department of Biomedical and Clinical Sciences “Sacco”, Fatebenefratelli Hospital, Università di Milano, Milan, Italy.
Allan Klein, Center for the Diagnosis and Treatment of Pericardial Diseases, Section of Cardiovascular Imaging, Department of Cardiovascular Medicine, Heart, Vascular, and Thoracic Institute, Cleveland Clinic, USA.
References
- 1. Imazio M, Brucato A, Cemin R, Ferrua S, Maggiolini S, Beqaraj F, Demarie D, Forno D, Ferro S, Maestroni S, Belli R, Trinchero R, Spodick DH, Adler Y; ICAP Investigators . A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013;369:1522–1528. [DOI] [PubMed] [Google Scholar]
- 2. Imazio M, Belli R, Brucato A, Cemin R, Ferrua S, Beqaraj F, Demarie D, Ferro S, Forno D, Maestroni S, Cumetti D, Varbella F, Trinchero R, Spodick DH, Adler Y. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. Lancet 2014;383:2232–2237. [DOI] [PubMed] [Google Scholar]
- 3. Bonaventura A, Vecchié A, Mauro AG, Brucato AL, Imazio M, Abbate A. An update on the pathophysiology of acute and recurrent pericarditis. Panminerva Med 2020. doi:10.23736/S0031-0808.20.04205-6. [DOI] [PubMed] [Google Scholar]
- 4. Lopalco G, Rigante D, Cantarini L, Imazio M, Lopalco A, Emmi G, Venerito V, Fornaro M, Frediani B, Nivuori M, Brucato A, Iannone F. The autoinflammatory side of recurrent pericarditis: enlightening the pathogenesis for a more rational treatment. Trends Cardiovasc Med 2020. doi:10.1016/j.tcm.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 5. Khayata M, Shah NP, Verma BR, Giugni AS, Alkharabsheh S, Asher CR, Imazio M, Klein AL. Usefulness of Interleukin-1 Receptor Antagonists in Patients With Recurrent Pericarditis. Am J Cardiol 2020;127:184–190. [DOI] [PubMed] [Google Scholar]
- 6. Picco P, Brisca G, Traverso F, Loy A, Gattorno M, Martini A. Successful treatment of idiopathic recurrent pericarditis in children with interleukin-1beta receptor antagonist (anakinra): an unrecognized autoinflammatory disease? Arthritis Rheum 2009;60:264–268. [DOI] [PubMed] [Google Scholar]
- 7. Tutar HE, Imamoglu A, Kendirli T, Akar E, Atalay S, Akar N. Isolated recurrent pericarditis in a patient with familial Mediterranean fever. Eur J Pediatr 2001;160:264–265. [DOI] [PubMed] [Google Scholar]
- 8. Cantarini L, Lucherini OM, Brucato A, Barone L, Cumetti D, Iacoponi F, Rigante D, Brambilla G, Penco S, Brizi MG, Patrosso MC, Valesini G, Frediani B, Galeazzi M, Cimaz R, Paolazzi G, Vitale A, Imazio M. Clues to detect tumor necrosis factor receptor-associated periodic syndrome (TRAPS) among patients with idiopathic recurrent acute pericarditis: results of a multicentre study. Clin Res Cardiol 2012;101:525–531. [DOI] [PubMed] [Google Scholar]
- 9. Vassilopoulos D, Lazaros G, Tsioufis C, Vasileiou P, Stefanadis C, Pectasides D. Successful treatment of adult patients with idiopathic recurrent pericarditis with an interleukin-1 receptor antagonist (anakinra). Int J Cardiol 2012;160:66–68. [DOI] [PubMed] [Google Scholar]
- 10. Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W; ESD Group . 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC). Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vianello F, Cinetto F, Cavraro M, Battisti A, Castelli M, Imbergamo S, Marcolongo R. Azathioprine in isolated recurrent pericarditis: a single centre experience. Int J Cardiol 2011;147:477–478. [DOI] [PubMed] [Google Scholar]
- 12. Imazio M, Lazaros G, Picardi E, Vasileiou P, Carraro M, Tousoulis D, Belli R, Gaita F. Intravenous human immunoglobulins for refractory recurrent pericarditis: a systematic review of all published cases. J Cardiovasc Med (Hagerstown) 2016;17:263–269. [DOI] [PubMed] [Google Scholar]
- 13. Lazaros G, Imazio M, Brucato A, Vassilopoulos D, Vasileiou P, Gattorno M, Tousoulis D, Martini A. Anakinra: an emerging option for refractory idiopathic recurrent pericarditis: a systematic review of published evidence. J Cardiovasc Med (Hagerstown) 2016;17:256–262. [DOI] [PubMed] [Google Scholar]
- 14. Brucato A, Imazio M, Gattorno M, Lazaros G, Maestroni S, Carraro M, Finetti M, Cumetti D, Carobbio A, Ruperto N, Marcolongo R, Lorini M, Rimini A, Valenti A, Erre GL, Sormani MP, Belli R, Gaita F, Martini A. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA 2016;316:1906–1912. [DOI] [PubMed] [Google Scholar]
- 15. Klein AL, Lin D, Cremer PC, Nasir S, Luis SA, Abbate A, Ertel A, LeWinter M, Beutler A, Fang F, Paolini JF. Efficacy and safety of rilonacept for recurrent pericarditis: results from a phase II clinical trial. Heart 2021;107:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klein AL, Imazio M, Cremer P, Brucato A, Abbate A, Fang F, Insalaco A, LeWinter M, Lewis BS, Lin D, Luis SA, Nicholls SJ, Pano A, Wheeler A, Paolini JF; RHAPSODY Investigators . Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med 2021;384:31–41. [DOI] [PubMed] [Google Scholar]
- 17. Imazio M, Andreis A, De Ferrari GM, Cremer PC, Mardigyan V, Maestroni S, Luis SA, Lopalco G, Emmi G, Lotan D, Marcolongo R, Lazaros G, De Biasio M, Cantarini L, Dagna L, Cercek AC, Pivetta E, Varma B, Berkson L, Tombetti E, Iannone F, Prisco D, Caforio ALP, Vassilopoulos D, Tousoulis D, De Luca G, Giustetto C, Rinaldi M, Oh JK, Klein AL, Brucato A, Adler Y. Anakinra for corticosteroid-dependent and colchicine-resistant pericarditis: the IRAP (International Registry of Anakinra for Pericarditis) study. Eur J Prev Cardiol 2020;27:956–964. [DOI] [PubMed] [Google Scholar]
- 18. Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity 2019;50:778–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buckley LF, Viscusi MM, Van Tassell BW, Abbate A. Interleukin-1 blockade for the treatment of pericarditis. Eur Heart J Cardiovasc Pharmacother 2018;4:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nigrovic PA. Interleukin 1 inhibitors: Biology, principles of use, and adverse events. www.uptodate.com (6 January 2020).
- 21. Klein AL, Imazio M, Brucato A, Cremer P, LeWinter M, Abbate A, Lin D, Martini A, Beutler A, Chang S, Fang F, Gervais A, Perrin R, Paolini JF. RHAPSODY: rationale for and design of a pivotal Phase 3 trial to assess efficacy and safety of rilonacept, an interleukin-1α and interleukin-1β trap, in patients with recurrent pericarditis. Am Heart J 2020;228:81–90. [DOI] [PubMed] [Google Scholar]
- 22. Andreis A, Imazio M, De Ferrari GM. Colchicine for the treatment of cardiovascular diseases: old drug, new targets. J Cardiovasc Med (Hagerstown) 2021;22:1–8. [DOI] [PubMed] [Google Scholar]
- 23. Andreis A, Imazio M, Giustetto C, Brucato A, Adler Y, De Ferrari GM. Anakinra for constrictive pericarditis associated with incessant or recurrent pericarditis. Heart 2020;106:1561–1565. [DOI] [PubMed] [Google Scholar]
- 24. Kougkas N, Fanouriakis A, Papalopoulos I, Bertsias G, Avgoustidis N, Repa A, Sidiropoulos P. Canakinumab for recurrent rheumatic disease associated-pericarditis: a case series with long-term follow-up. Rheumatology (Oxford) 2018;57:1494–1495. [DOI] [PubMed] [Google Scholar]
- 25. Epçaçan S, Sahin S, Kasapcopur O. Anaphylactic reaction to anakinra in a child with steroid-dependent idiopathic recurrent pericarditis and successful management with canakinumab. Cardiol Young 2019;29:549–551. [DOI] [PubMed] [Google Scholar]
- 26. Signa S, D'Alessandro M, Consolini R, Miniaci A, Bustaffa M, Longo C, Tosca MA, Bizzi M, Caorsi R, Mendonça LO, Pession A, Ravelli A, Gattorno M. Failure of anti interleukin-1 β monoclonal antibody in the treatment of recurrent pericarditis in two children. Pediatr Rheumatol Online J 2020;18:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 28. Imazio M, Andreis A, Piroli F, Lazaros G, Gattorno M, Lewinter M, Klein AL, Brucato A. Anti-interleukin 1 agents for the treatment of recurrent pericarditis: a systematic review and meta-analysis. Heart 2021. doi:10.1136/heartjnl-2020-318869. [DOI] [PubMed] [Google Scholar]
- 29. De Benedetti F, Gattorno M, Anton J, Ben-Chetrit E, Frenkel J, Hoffman HM, Koné-Paut I, Lachmann HJ, Ozen S, Simon A, Zeft A, Calvo Penades I, Moutschen M, Quartier P, Kasapcopur O, Shcherbina A, Hofer M, Hashkes PJ, Van der Hilst J, Hara R, Bujan-Rivas S, Constantin T, Gul A, Livneh A, Brogan P, Cattalini M, Obici L, Lheritier K, Speziale A, Junge G. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med 2018;378:1908–1919. [DOI] [PubMed] [Google Scholar]
- 30. Strangfeld A, Hierse F, Rau R, Burmester GR, Krummel-Lorenz B, Demary W, Listing J, Zink A. Risk of incident or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in the German biologics register RABBIT. Arthritis Res Ther 2010;12:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Finetti M, Insalaco A, Cantarini L, Meini A, Breda L, Alessio M, D'Alessandro M, Picco P, Martini A, Gattorno M. Long-term efficacy of interleukin-1 receptor antagonist (anakinra) in corticosteroid-dependent and colchicine-resistant recurrent pericarditis. J Pediatr 2014;164:1425–31.e1. [DOI] [PubMed] [Google Scholar]
- 32. Bermas BL. Safety of rheumatic disease medication use during pregnancy and lactation. www.uptodate.com (28 March 2021).
- 33. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, Marder W, Guyatt G, Branch DW, Buyon J, Christopher-Stine L, Crow-Hercher R, Cush J, Druzin M, Kavanaugh A, Laskin CA, Plante L, Salmon J, Simard J, Somers EC, Steen V, Tedeschi SK, Vinet E, White CW, Yazdany J, Barbhaiya M, Bettendorf B, Eudy A, Jayatilleke A, Shah AA, Sullivan N, Tarter LL, Birru Talabi M, Turgunbaev M, Turner A, D'Anci KE. 2020 American College of Rheumatology Guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2020;72:529–556. [DOI] [PubMed] [Google Scholar]
- 34. Lazaros G, Vasileiou P, Koutsianas C, Antonatou K, Stefanadis C, Pectasides D, Vassilopoulos D. Anakinra for the management of resistant idiopathic recurrent pericarditis. Initial experience in 10 adult cases. Ann Rheum Dis 2014;73:2215–2217. [DOI] [PubMed] [Google Scholar]
- 35. Imazio M, Pivetta E, Palacio Restrepo S, Sormani P, Pedrotti P, Quarta G, Brucato A, Bubbico E, Dal Corso M, Milazzo A, Quattrocchi G, Andriani M, Lobetti Bodoni L, Davini O, Sironi S, Giannattasio C, Giustetto C, Bogaert J, Adler Y, Bucciarelli Ducci C, De Ferrari GM. Usefulness of cardiac magnetic resonance for recurrent pericarditis. Am J Cardiol 2020;125:146–151. [DOI] [PubMed] [Google Scholar]
- 36. Chetrit M, Xu B, Kwon DH, Ramchand J, Rodriguez RE, Tan CD, Jellis CL, Johnston DR, Renapurkar RD, Cremer PC, Klein AL. Imaging-guided therapies for pericardial diseases. JACC Cardiovasc Imaging 2020;13:1422–1437. [DOI] [PubMed] [Google Scholar]
- 37. Brucato A, Pluymaekers N, Tombetti E, Rampello S, Maestroni S, Lucianetti M, Valenti A, Adler Y, Imazio M. Management of idiopathic recurrent pericarditis during pregnancy. Int J Cardiol 2019;282:60–65. [DOI] [PubMed] [Google Scholar]
- 38. Imazio M, Brucato A, Lazaros G, Andreis A, Scarsi M, Klein A, De Ferrari GM, Adler Y. Anti-inflammatory therapies for pericardial diseases in the COVID-19 pandemic: safety and potentiality. J Cardiovasc Med (Hagerstown) 2020;21:625–629. [DOI] [PubMed] [Google Scholar]
- 39. Imazio M. Anti-IL-1 agents: a paradigm shift in medical therapy for recurrent pericarditis? Heart 2020;107:438–440. [DOI] [PubMed] [Google Scholar]