Abstract
The botanical product kratom produces opioid-like effects at high doses and is sometimes used for opioid replacement by individuals with opioid use disorder. Mitragynine, a major alkaloid contained in kratom leaves, has been shown to inhibit multiple cytochromes P450 (CYPs) in vitro, including CYP2D6 and CYP3A. As such, kratom may precipitate pharmacokinetic drug interactions when co-consumed with certain medications. We present a case of a patient taking 150 mg venlafaxine (CYP2D6/3A substrate), 300 mg quetiapine (CYP3A substrate), and a high amount of kratom (~90 g) daily. The patient presented to the emergency department with serotonin syndrome and corrected electrocardiogram abnormalities that may have been secondary to supratherapeutic exposure to venlafaxine and/or quetiapine. The patient’s symptoms resolved after discontinuation of venlafaxine and quetiapine. He was amenable to medication therapy for kratom discontinuation and successfully completed an at-home induction with buprenorphine/naloxone. This case report adds to the literature about potential pharmacokinetic kratom-drug interactions and suggests that buprenorphine/naloxone can facilitate recovery from kratom use disorder.
Keywords: buprenorphine, drug interaction, induction, kratom, naloxone, quetiapine, venlafaxine
The evergreen kratom tree (Mitragyna speciosa), which belongs to the coffee family, is native to Southeast Asia.1 Leaves of the kratom tree were traditionally chewed for their analgesic and stimulant effects. However, kratom use has increased internationally, leading to increased use as a recreational drug or for pain, opioid withdrawal, and opioid replacement. At low to moderate doses (1–5 g), kratom has stimulant-like effects, while at high doses (>8 g) has opioid-like effects.2 The mechanism of action of kratom is suspected to involve agonism of multiple opioid receptors, stimulation of adrenergic receptors, and somewhat undetermined actions on serotonin and dopamine receptors.1–3
Multiple phytochemicals within the kratom plant may alter the disposition of pharmaceutical medications in vivo. Two alkaloids present in the leaves – mitragynine, which is typically the most abundant, and 7-hydroxymitragynine – are perhaps the most extensively studied. The latter is also formed from mitragynine in vivo. These and other kratom alkaloids have been reported to inhibit the major drug metabolizing enzymes cytochrome P450 (CYP) 2D6 and CYP3A in vitro.4,5 These enzymes are responsible for metabolizing >50% of all drugs. Thus, kratom products may carry a risk of pharmacokinetic drug interactions when co-consumed with many medications.
Previously, a suspected pharmacokinetic interaction between kratom and the anti-psychotic quetiapine resulted in supratherapeutic systemic concentrations of quetiapine and death of the patient.6 Quetiapine undergoes extensive CYP3A-mediated first-pass metabolism. Thus, one potential mechanism is that one or more kratom constituents inhibited CYP3A, leading to toxic quetiapine concentrations.5 The following describes a case of potential kratom-drug interactions involving quetiapine, and the anti-depressant and dual CYP2D6/3A substrate venlafaxine.7,8 The presumed interactions produced serotonin syndrome and corrected QT (QTc) interval prolongation, both of which resolved after discontinuation of quetiapine and venlafaxine. Subsequently, the patient’s kratom use disorder was successfully treated with buprenorphine/naloxone using an at-home medication treatment protocol as described in Providers Clinical Support System guidelines.9
ETHICS APPROVAL
The patient provided written informed consent and HIPAA authorization to publish this case report. The Providence St. Joseph Health Human Research Protection Program determined that this case report did not meet the definition of research and did not require Institutional Review Board review as defined in the federal regulations.
CASE
A 36-year-old employed, Caucasian, single man presented to our ambulatory addictions clinic upon referral from his primary care provider. Two weeks prior, the patient had sought care at the emergency department due to “dizziness.” The only medications he reported at that time were venlafaxine 150 mg daily and quetiapine 300 mg nightly. Evaluation in the emergency department was notable for a high blood pressure (144/92 mm Hg), tachycardia (116 bpm), and elevated temperature (99.5 °F). Electrocardiogram showed sinus tachycardia (113 bpm) and a very prolonged QTc (563 msec). Urine toxicology, urinalysis, complete blood count with differential, comprehensive metabolic panel, acetaminophen level, salicylic acid level, and a computed tomography imaging study of the head without contrast were unremarkable. He left the emergency department against medical advice, but before leaving he was advised to discontinue venlafaxine and quetiapine due to concern for serotonin syndrome and prolonged QTc.
Ten days after this emergency department visit (Fig. 1A), the patient established care with his primary care provider and was no longer taking venlafaxine and quetiapine as recommended by the emergency department. Dizziness had resolved, vitals returned to normal, and repeat electrocardiogram demonstrated normal sinus rhythm (86 bpm) and QTc (408 msec). He was referred to our ambulatory addictions clinic for an initial evaluation of kratom use disorder 3 days later.
FIGURE 1.
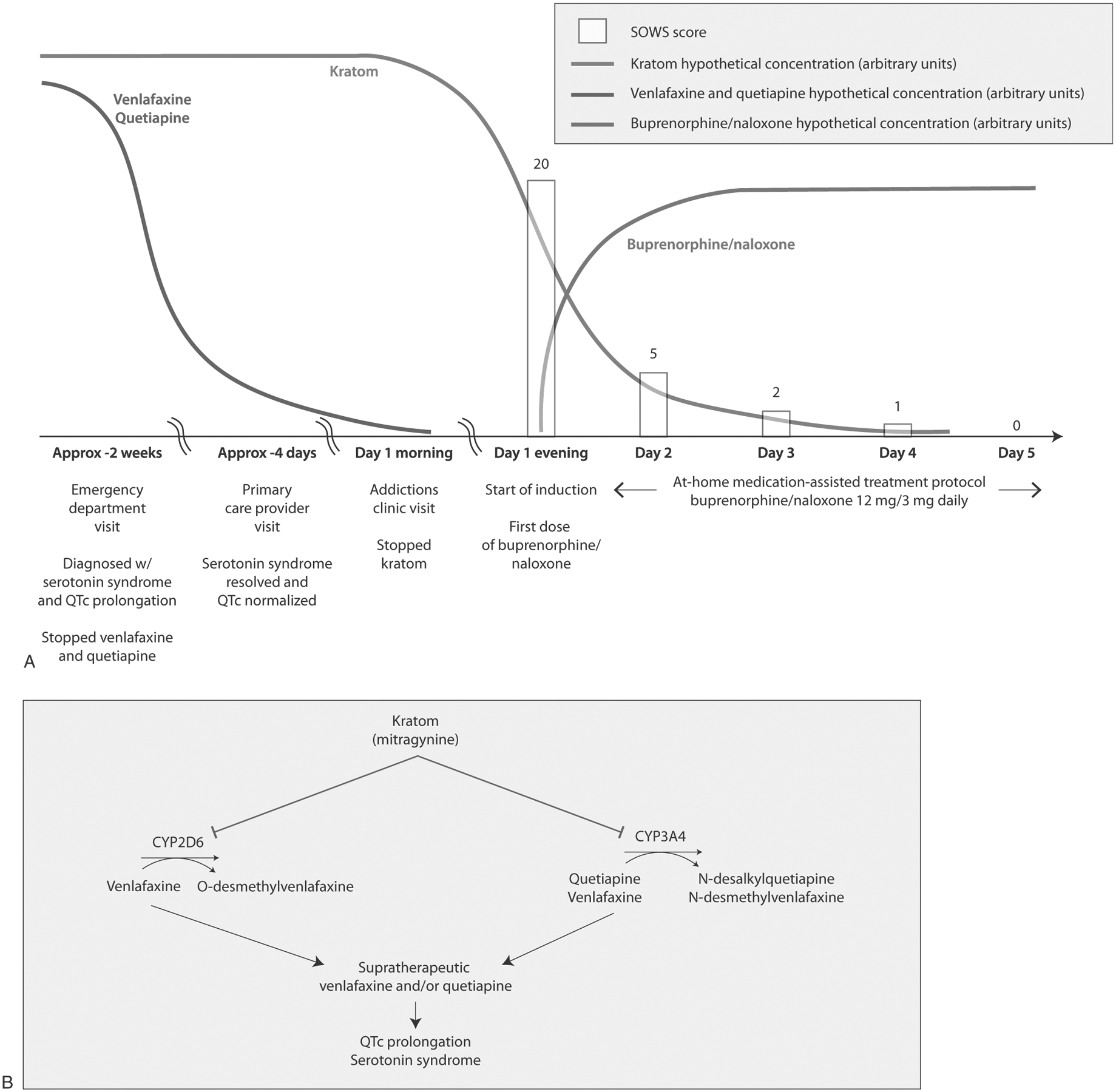
Buprenorphine/naloxone induction to support discontinuation of kratom and proposed mechanisms of pharmacokinetic kratom-drug interaction interactions. A, The timeline of this case and the buprenorphine/naloxone home induction is illustrated. A patient with kratom use disorder who consumed ~90 g kratom, 150 mg venlafaxine, and 300 mg quetiapine daily presented to the emergency department with serotonin syndrome and QTc interval prolongation. These symptoms resolved upon discontinuation of venlafaxine and quetiapine. The patient then visited a primary care provider, who referred him to an addictions clinic to assist with kratom cessation. The addictions clinic started him on a home induction with a titration to buprenorphine/naloxone 12 mg/3 mg total daily. SOWS assessments were conducted once daily, beginning with the first dose of buprenorphine/naloxone. The patient completed SOWS assessments through day 5 post home induction on buprenorphine/naloxone 12 mg/3 mg daily. B, Proposed mechanisms underlying potential interactions between the kratom alkaloid mitragynine, the CYP2D6/3A substrate venlafaxine, and the CYP3A4 substrate quetiapine. Mitragynine, typically the most abundant kratom alkaloid in kratom products, is a time-dependent inhibitor of CYP3A and a reversible inhibitor of CYP2D6.5 CYP2D6 is the major route of clearance for venlafaxine, whereas CYP3A is the major route of clearance for quetiapine and a minor route of clearance for venlafaxine.7,8,10 Quetiapine may have inhibited this minor route of venlafaxine clearance, although the resulting effect on venlafaxine concentrations is probably minor.16 Additionally, kratom alone can produce symptoms associated with serotonin syndrome and QTc interval prolongation.17
At the addictions clinic intake, he met criteria for additional substance use disorders, including alcohol (in early remission), opioids, and tobacco. His opioid use history consisted of misusing non-prescription opioids beginning at age 16 that progressed to intravenous heroin. He sought treatment and had success with buprenorphine/naloxone, resulting in 2 years of sobriety. Due to a change in his location, he had difficulty accessing buprenorphine/naloxone and learned online about kratom as a possible alternative. He began using kratom 11 years ago and increased his total daily kratom consumption to approximately 90 g (~0.75 g capsules × 120 capsules) taken in divided doses, costing him roughly $2250/month. The patient was amenable to an at-home induction of buprenorphine/naloxone to assist with kratom cessation. Approximately 12 hours after his last dose of kratom (15 capsules, ~11 g), he had a Subjective Opiate Withdrawal Scale (SOWS) of 20 and began the induction with oral buprenorphine/naloxone (8 mg/2 mg) (Fig. 1A). Two hours later, he started feeling restless and nauseated and took an additional 4 mg/1 mg as instructed. He continued taking buprenorphine/naloxone 12 mg/3 mg daily until his next appointment 1 week later. He reported cravings for kratom in the interim, so his buprenorphine/naloxone was increased to 16 mg/4 mg daily. He has been maintained at this dose and has abstained from kratom use for more than 10 months after his successful home induction with buprenorphine/naloxone.
DISCUSSION
This case illustrates the potential for kratom to precipitate pharmacokinetic interactions with drugs metabolized by CYP2D6 and CYP3A, plus adds to the list of reports in which a buprenorphine/naloxone protocol has been used successfully to treat kratom use disorder.9 Buprenorphine/naloxone is approved for the treatment of opioid use disorder. The idea of using similar regimens to wean patients from botanical opioid agonists is new,9 and our encouraging results support their effectiveness.
The patient’s initial presentation at the emergency department with both serotonin syndrome and QTc interval prolongation suggested that he was experiencing adverse events from venlafaxine and quetiapine, although direct effects of kratom cannot be ruled out. Infection, seizure, or acute ingestion of an illicit substance were within the differential; however, these events were less likely, as he was afebrile, complete blood count and urine toxicology were unremarkable, and no witnessed seizure or clear post-ictal state were described. Serotonin syndrome was suspected due to altered mental status, neuromuscular abnormalities, and autonomic hyperactivity, all of which resolved with cessation of quetiapine and venlafaxine.
Proposed mechanisms underlying the suspected kratom-drug interactions are provided (Fig. 1B). Briefly, by inhibiting CYP2D6 and CYP3A, kratom may have produced supratherapeutic concentrations of both drugs, resulting in serotonin syndrome and QTc interval prolongation. Venlafaxine is a CYP2D6 substrate and can produce serotonin toxicity when co-administered with other serotonergic medications and/or CYP2D6 inhibitors.7 CYP3A-mediated metabolism is a minor route of venlafaxine clearance but may be important in individuals with reduced function CYP2D6 genetic polymorphisms who rely on CYP3A for venlafaxine clearance.8 Quetiapine is a CYP3A substrate10 that can produce serotonin syndrome in combination with antidepressants,11,12 and although unlikely,13 may have contributed to the observed QTc interval prolongation. Likewise, while drug interactions with venlafaxine via CYP3A inhibition are unlikely,14,15 quetiapine may have inhibited this minor route of venlafaxine clearance as reported previously.16 Finally, kratom alone can produce both neurological and cardiovascular symptoms such as confusion, agitation, seizures, tachycardia, and conduction disturbances.17
The presumed CYP3A-mediated kratom-quetiapine interaction has been reported previously and was so severe that quetiapine reached fatally supratherapeutic concentrations (12 mg/L).6 CYP2D6-mediated kratom-drug interactions have been predicted but not observed clinically to date.5 However, the ultra-high total daily amount of kratom (~90 g) consumed by the current patient suggested that mitragynine and/or other kratom constituents achieved sufficient concentrations in the portal and/or systemic circulation to inhibit hepatic CYP2D6, and hepatic and/or intestinal CYP3A, further supporting that pharmacokinetic interactions occurred.
In addition to potential kratom-drug interactions, this report highlights the value of medication treatment for kratom use disorder. We elected to use a buprenorphine/naloxone regimen because this patient’s condition most closely resembled opioid use disorder. The principle underlying the treatment of kratom use disorder with buprenorphine/naloxone is the same as that for any other prescription or illicit opioid agonist. Buprenorphine is a potent, competitive, partial agonist of the μ opioid receptor.18 Thus, buprenorphine likely displaces the opioid agonists contained within the kratom plant while providing partial agonism, allowing the patient to cease using kratom. Typically, outpatient clinic appointments for buprenorphine/naloxone induction require hours of provider time and can be difficult to schedule, resulting in protracted wait times.19 The advantage of the protocol used in this case is that buprenorphine/naloxone induction can be completed at home. The at-home protocol was well tolerated by the patient, adding to the list of reports suggesting that providers should consider buprenorphine/naloxone protocols as treatment options for kratom use disorder.9
Although this case focuses on potential pharmacokinetic kratom-drug interactions involving venlafaxine and quetiapine, providers should be aware that kratom may interact with other CYP2D6 and CYP3A substrates, notably several opioids, anti-depressants, and other central nervous system-active drugs.2 Due to the increasing popularity of kratom, screening for kratom use when gathering medical histories is advised. Providers should probe the reason for use, dosage and frequency, discontinuation symptoms, and concomitant medications and/or supplements. We recommend asking patients if they are interested in discontinuing kratom, and if so, considering medication treatment with buprenorphine/naloxone. Further guidance for managing these patients is scarce, but at least 1 resource for providers is available from the Providers Clinical Support System.9
Acknowledgments
This work was supported in part by the National Institutes of Health National Center for Complementary and Integrative Health and Office of Dietary Supplements (U54 AT008909).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Eastlack SC, Cornett EM, Kaye AD. Kratom-pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9(1):55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792–799. [PubMed] [Google Scholar]
- 3.Vento AE, de Persis S, De Filippis S, et al. Case report: treatment of kratom use disorder with a classical tricyclic antidepressant. Front Psychiatry. 2021;12:640218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todd DA, Kellogg JJ, Wallace ED, et al. Chemical composition and biological effects of kratom (Mitragyna speciosa): in vitro studies with implications for efficacy and drug interactions. Sci Rep. 2020;10(1):19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanna RS, Tian DD, Cech NB, et al. Refined prediction of pharmacokinetic kratom-drug interactions: time-dependent inhibition considerations. J Pharmacol Exp Ther. 2021;376(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110–113. [DOI] [PubMed] [Google Scholar]
- 7.Fisher AA, Davis MW. Serotonin syndrome caused by selective serotonin reuptake-inhibitors-metoclopramide interaction. Ann Pharmacother. 2002;36(1):67–71. [DOI] [PubMed] [Google Scholar]
- 8.Lindh JD, Annas A, Meurling L, et al. Effect of ketoconazole on venlafaxine plasma concentrations in extensive and poor metabolisers of debrisoquine. Eur J Clin Pharmacol. 2003;59(5–6):401–406. [DOI] [PubMed] [Google Scholar]
- 9.Stanciu C, Penders T. Best practices in managing patients with kratom addiction. Published 2020. Available at: https://pcssnow.org/wp-content/uploads/2020/09/KRATOM-webinar-PCSS-2020-final-version-9.28.20-1.pdf. Accessed July 2, 2021.
- 10.DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40(7):509–522. [DOI] [PubMed] [Google Scholar]
- 11.Marlowe K, Schirgel D. Quetiapine and citalopram: aetiological significances in serotonin syndrome. N Z Med J. 2006;119(1237):U2058. [PubMed] [Google Scholar]
- 12.Kohen I, Gordon ML, Manu P. Serotonin syndrome in elderly patients treated for psychotic depression with atypical antipsychotics and anti-depressants: two case reports. CNS Spectr. 2007;12(8):596–598. [DOI] [PubMed] [Google Scholar]
- 13.Dube KM, DeGrado J, Hohlfelder B, et al. Evaluation of the effects of quetiapine on QTc prolongation in critically ill patients. J Pharm Pract. 2018;31(3):292–297. [DOI] [PubMed] [Google Scholar]
- 14.Levin GM, Nelson LA, DeVane CL, et al. A pharmacokinetic drug-drug interaction study of venlafaxine and indinavir. Psychopharmacol Bull. 2001;35(2):62–71. [PubMed] [Google Scholar]
- 15.Amchin J, Zarycranski W, Taylor KP, et al. Effect of venlafaxine on the pharmacokinetics of alprazolam. Psychopharmacol Bull. 1998;34(2):211–219. [PubMed] [Google Scholar]
- 16.Paulzen M, Schoretsanitis G, Hiemke C, et al. Reduced clearance of venlafaxine in a combined treatment with quetiapine. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:116–121. [DOI] [PubMed] [Google Scholar]
- 17.Post S, Spiller HA, Chounthirath T, et al. Kratom exposures reported to United States poison control centers: 2011–2017. Clin Toxicol (Phila). 2019;57(10):847–854. [DOI] [PubMed] [Google Scholar]
- 18.Shulman M, Wai JM, Nunes EV. Buprenorphine treatment for opioid use disorder: an overview. CNS Drugs. 2019;33(6):567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sevarino KA, Saxon AJ, Friedman M. Medically supervised opioid withdrawal during treatment for addiction. UpToDate. Published 2020. Available at: https://www.uptodate.com/contents/medically-supervised-opioid-withdrawal-during-treatment-for-addiction?search=kratom&source=search_result&selectedTitle=1~7&usage_type=default&display_rank=1#H292407430. Accessed June 15, 2021.


