Abstract
The dependency on non-renewable fossil fuels as an energy source has drastically increased global temperatures. Their continuous use poses a great threat to the existing energy reserves. Therefore, the energy sector has taken a turn toward developing eco-friendly, sustainable energy generation by using sustainable lignocellulosic wastes, such as rice straw (RS). For lignocellulosic waste to be utilized as an efficient energy source, it needs to be broken down into less complex forms by pretreatment processes, such as alkaline pretreatment using NaOH. Varied NaOH concentrations (0.5%,1.0%,1.5%,2%) for alkaline pretreatment of RS were used for the holocellulose generation. Amongst the four NaOH concentrations tested, RS-1.5% exhibited higher holocellulose generation of 80.1%, whereas 0.5%, 1 5 and 2% pointed 71.9%, 73.8%, and 78.5% holocellulose generation, respectively. Further, microbial fuel cells (MFCs) were tested for voltage generation by utilizing holocellulose generated from untreated (RS-0%) and mildly alkaline pretreated RS (RS-1.5%) as a feedstock. The MFC voltage and maximum power generation using RS-0% were 194 mV and 167 mW/m2, respectively. With RS-1.5%, the voltage and maximum power generation were 556 mV and 583 mW/m2, respectively. The power density of RS-1.5% was three-fold higher than that of RS-0%. The increase in MFC power generation suggests that alkaline pretreatment plays a crucial role in enhancing the overall performance.
Keywords: Alkaline pretreatment, Bioenergy, Lignocellulose waste, Microbial fuel cells, Rice straw
Introduction
The increasing population growth, rise in global temperatures due to greenhouse gases, changes in lifestyle and industrial innovations have significantly increased energy utilization. These energy demands were forecasted to increase by 56% by 2040 [1, 2]. Traditional techniques for generating energy from fossil fuels have negative implications for global warming. Furthermore, the exhaustion of fossil fuel supply can result in economic and political turmoil. Therefore, researchers are searching for alternative sustainable renewable energy sources to combat the predicted impacts. Owing to their abundance in nature, lignocellulosic or agricultural wastes can be used for sustainable and renewable energy generation [3]. Rice straw (RS) is one of the most abundantly available wastes used for bioenergy production. In 2017, the annual global estimate of RS as waste was 730 metric tons. However, the energy potential of RS has not been aggressively explored [4]. It is often burned in open farmlands to minimize labor costs and thus raises enormous environmental (e.g., increase in GHGs) and human risk factors [5]. Therefore, utilizing RS for a bioenergy production process that does not require post-product treatments, such as electricity generation from microbial fuel cells (MFCs), can be beneficial to circumvent the energy crisis [6].
Moreover, previous studies on MFCs have pointed out the eco-friendly approaches and economically feasible bioelectricity-generating techniques over other conventional processes [7–9] using RS. However, one of the major hurdles in using RS as feedstock in biological processes is its composition. RS’s lignin, cellulose, and hemicellulose content were approximately 12%, 38%, and 25%, respectively [10]. Among these components, lignin is rigid and is composed of phenolics, which bind to cellulose and hemicellulose and resemble a three-dimensional (3D) structure. Furthermore, lignin also assists in protecting hemicellulose and cellulose from harsh conditions, providing support for plants and resistance to microbial enzymes/attacks. Hemicellulose and cellulose are both known as holocellulose. As a feedstock for biological processes, lignocellulosic waste, such as RS, is mainly dependent on the holocellulose percentage, as these are degradable sugars utilized for energy generation [11] in biological processes.
To expose the hemicellulose and cellulose of RS as feedstock for microbes, the breakdown of lignin is required. Therefore, several studies have attempted delignification by following physical, chemical, and biological pretreatment processes or by combining these processes. To use RS as a feedstock in any biological process (e.g., MFCs or anaerobic digestion (AD)), selective pretreatment should minimize the loss of hemicellulose and cellulose while simultaneously avoiding size reduction and generating fewer inhibitors during the breakdown of lignin. This is also beneficial if the selected pretreatment process is economical, energy-efficient, easy to operate, and has low downstream operational costs. Among the stated pretreatment processes, chemical pretreatment using alkaline systems (e.g., NaOH) has proven effective for delignification [12]. NaOH pretreatment also concurrently assisted in solubilizing hemicellulose and generating reducing sugars and other carbohydrates. Moreover, mild alkaline pretreatment increased the surface area by swelling, thereby assisting biodegradation. It is also noted that the generation of inhibitory compounds such as phenols and furfurals is low in alkaline pretreatment processes [13].
MFCs are bioelectrochemical systems in which electrical energy is generated through the oxidation of organics using self-sustainable regenerative biocatalysts at the anode. The electrons generated at the anode of the MFC by the breakdown of organics are passed to the cathode via an external circuit. The difference between the anode and cathode potentials is noted as the voltage. Recently, MFC application has made tremendous improvements in treating organics from waste and lignocellulosic wastes with concurrent electricity generation [14, 15]. Moreover, most studies employing RS as feedstock are pursued in integration with one or more energy platforms like biohydrogen, biomethane, and bioethanol generation. However, these products require a downstream process to improve their product quality. Whereas the bioelectricity generated from MFCs employing the RS as a substrate does not require any downstream process, which minimizes the overall cost of the operation. The alkaline pretreatment using NaOH is one of the most effective chemical pretreatment processes to break the lignin in RS. A 6% NaOH concentration has led to a 716% increase in biogas generation than control [16]. However, to the best of our knowledge, no previous studies have employed alkaline pretreatment of RS in connection with MFCs for power generation. Few researchers have used untreated RS as a feedstock in MFCs [17] with lower power generations. Therefore, this study tested the influence of NaOH alkaline pretreatment on RS to incorporate as a feedstock in MFC. Prior to submission of RS to MFC, various NaOH (0.5%, 1%, 1.5%, and 2% w/v) concentrations were tested for optimal conditions to generate holocellulose. The optimized NaOH pretreated RS (RS-1.5%) with higher holocellulose was subjected to single-chamber air cathode MFCs for voltage generation, and untreated RS (RS-0%) was used as a control.
Materials and Methods
Pretreatment of Rice Straw
Oryza sativa (RS) was obtained from Phygen Co., Ltd. (Korea). The collected RS was dried for 24 h at 60 °C and milled using a laboratory hammer mill. The milled RS was passed through a sieve to separate the particles and collect them within a range of 2–6 mm. Milled RS was pretreated with various concentrations of NaOH (0.5%, 1%, 1.5%, and 2% w/v) in a ratio of 1:20. The mixture was incubated at 37 °C and filtered after 3 h. The filtered residue was neutralized using multiple washes with distilled water. The residue was then dried at 60 °C for 24 h. In this stduy, the mildly alkaline pretreated RSs with varied concentrations of NaOH were labeled as RS-0.5, RS-1.0%, RS-1.5%, and RS-2.0%. The untreated RS was labeled as RS-0%. The pretreated RS was stored in an airtight container for further use in MFC and analysis.
Anodic Bioelectrogenic Mixed Consortium
In this study, the AD effluent was used as a seed culture to enrich the anaerobic electrogenic consortia on the anode of the MFC for power generation. Food waste leachate acted as the feedstock for the AD system. The AD effluent was centrifuged at 5000 rpm at 30 °C, and the pellet was cleaned three times with 50 mM phosphate buffer. After washing, the pellet collected from the AD effluent was supplemented with synthetic wastewater (SW) prepared as described in previous MFC studies [6].
MFC Construction and Operation
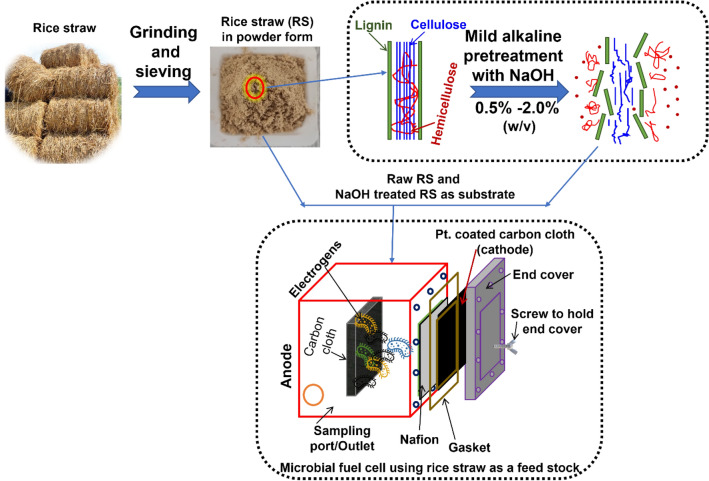
Two single chamber MFCs were selected to test the voltage generation using untreated (RS-0%) and mildly alkaline pretreated RS (RS-1.5%) as feedstock for the present study (Fig. 1). The anodic chamber was designed to have working volumes of 300 mL and 200 mL, respectively. Furthermore, 1 g/L of RS-0% and RS-1.5% were used as a substrates in MFC. Carbon cloth acted as an anode, whereas Pt.-coated carbon cloth (10%, wet proofed) was utilized as the cathode (10 cm2). The electrodes were separated using a pretreated proton exchange membrane (PEM, Nafion117, Fuel cell store, USA) [18]. The MFC was equipped with an external resistor of 1000Ω. To maintain anaerobic conditions, the MFC was degassed with nitrogen gas before the operation and sealed with silica gel to minimize oxygen diffusion.
Fig. 1.
Schematic representation of MFC operation using raw rice straw (RS) and mild alkaline pretreated RS as a substrate for power generation
Before MFC operation, the microflora in the pellet collected from the AD and suspended in SW was introduced into the anodic chamber with glucose as the substrate. After stable voltage generation was recorded using SW in repetitive cycles, RS-0% and RS-1.5% were introduced to the MFC as a substrate. The influent pH of the anolyte was changed to neutral (7.0); all analyses were performed in repetitive cycles, and the average values were recorded. Volatile fatty acids (VFAs), chemical oxygen demand (COD), and pH were studied for the MFC influent and effluent liquid fractions using standard APHA methods [19].
Analysis and Calculations
The voltage generation in MFC using the RS-0% and RS-1.5% was noted using a digital multimeter against 1000Ω resistor. Polarization studies were performed by varying the external resistance from 5000 to 5Ω. The voltage was computed as power and current (I) using Ohms’ law (Current = Voltage/Resistor size) [20]. In our previous studies, the total internal resistance of the MFC was estimated from the I-V plot of polarization plot. Columbic efficiency was measured based on the COD removal, as reported in the literature [21, 22].
Results and Discussion
Impact of Pretreatment on RS Composition
Holocellulose serves as a substrate for the growth of electrogenic microbes. In this study, holocellulose constituted 68.8% (hemicellulose, 28.6%; cellulose, 40.2%) of the untreated dry RS (RS-0%). Subsequently, the lignin content was 20.6%. The composition of lignocellulosic content in RS varies depending on nutrient availability, soil type, and crop harvest season. Changes in the lignocellulosic composition of RS after pretreatment are reported in Table 1, along with the raw RS content. NaOH pretreatment of RS resulted in decreased lignin and increased cellulose contents. Among the four NaOH pretreatments, maximum cellulose content was recorded for RS-2.0% (48.3%); whereas the cellulose contents of RS-1.5%, RS-1.0%, and RS-0.5% were 45.3%, 42.6%, and 41.8%, respectively. In contrast, maximum lignin solubilization leading to high holocellulose content of 80.1% was observed with RS-1.5%; whereas at similar conditions, the lignin content was 6.3%. Compared with RS-0%, pretreated RS-1.5% exhibited an 11.3% increase in holocellulose, with a decrease of 14.3% in the lignin component. These differences in composition could have been due to lignin degradation with alkaline pretreatment. Previous studies have shown that NaOH involvement leads to breaking glycosidic linkages and ester bonds in lignocellulose present in the cell wall matrix [2]. This cleavage of glycosidic linkages leads to modification of the lignin structure and breakdown of the lignin–hemicellulose complex, which further leads to saponification, causing swelling and decrystallization of cellulose.
Table 1.
Changes in the composition of RS by employing different alkaline pretreatment
| NaOH Pretreatment (% w/v) | Lignin (%) | Hemicellulose (%) | Cellulose (%) | Holocellulose (%) |
|---|---|---|---|---|
| RS-0% (Raw RS) | 20.6 ± 0.98 | 28.6 ± 1.23 | 40.2 ± 1.75 | 68.8 ± 2.2 |
| RS-0.5% | 15.2 ± 0.58 | 30.2 ± 1.42 | 41.6 ± 1.89 | 71.9 ± 2.6 |
| RS-1.0% | 14.3 ± 0.66 | 31.2 ± 1.34 | 42.6 ± 2.32 | 73.8 ± 2.5 |
| RS-1.5% | 6.31 ± 0.31 | 34.8 ± 1.55 | 45.3 ± 0.52 | 80.1 ± 1.8 |
| RS-2.0% | 7.32 ± 0.35 | 30.2 ± 1.12 | 48.3 ± 1.74 | 78.5 ± 2.1 |
Bioelectricity Generation in MFC Using RS as a Feedstock
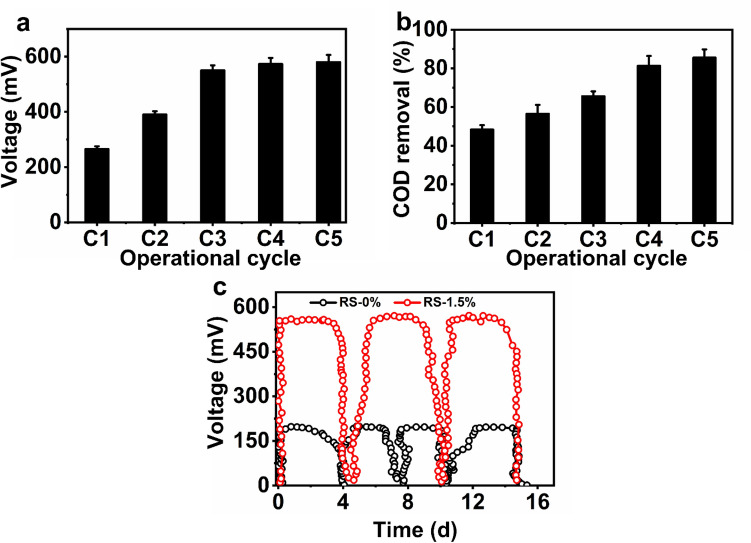
Initially, both single-chamber MFCs were managed in fed-batch mode with glucose (2 g/L) as a feedstock and SW as an anolyte for attaining stable power generation at neutral pH. Previous studies have established that complex substrates such as glucose can enrich bioelectrogens and utilize complex substrates [14]. During stabilization, an enhancement in MFC voltage generation with each operative cycle was noted. This indicated the enrichment of bioelectrogens on the anode electrode (Fig. 2). Simultaneously, the increase in the COD removal percentage with each operational cycle corroborates the bioelectrogenic development of microbes at the MFC anode. The maximum voltage generation noted with glucose as a substrate was approximately 580 mV, with 1000Ω of external resistance. The voltage generation increased from 266 to 580 mV with an increase in the operational cycle (Fig. 2a). Similarly, the COD removal percentage also varied from 48.3 to 85.5% (Fig. 2b). After noting a similar stable voltage generation, glucose was replaced with 1 g/L of RS-0% and mildly alkaline treated RS-1.5% as a substrate (Fig. 2c). In this study, RS-1.5% was chosen based on high holocellulose generation observed after treatment with NaOH. The maximum voltages generated using RS-0% and RS-1.5% were 194 mV and 556 mV, respectively. The high voltage generated with pretreated RS could have been due to more holocellulose content, which is considered a readily available sugar for bioelectrogens and other microbes, due to increased metabolic activity.
Fig. 2.
Bioelectrochemical profile of single chamber air cathode MFC.Voltage generation a and COD removal b were noted in MFC during the initial acclimatization period to grow bioelectrogenic microbes on the anode. Voltage generation b in MFC by utilizing the raw RS (RS-0%) and mild alkaline pretreated RS (RS-1.5%) as a substrate
Polarization Curves of MFC-Using RS as a Feedstock
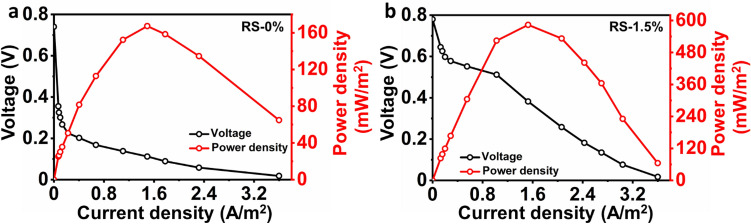
After noting repeatable, stable voltage generation, both MFCs using RS-0% and RS-1.5% were subjected to polarization analysis with varying external resistances. With the decrease in external resistance, a pronounced voltage decrease and an increase in current were noted. In addition, higher power densities were noted, with MFC employing RS-1.5% as a substrate over RS-0%, owing to the employment of pretreatment, resulting in higher holocellulose generation (Fig. 3). The MFCs using RS-0% and RS-1.5% exhibited comparable open circuit voltage (OCVs) of approximately 740 and 760 mV, respectively. The use of mildly alkaline pretreated RS-1.5% as a feedstock in MFC displayed a power density of 583 mW/m2 at a current density of 1.528 A/m2. MFC with RS-0% has pointed to a power density of only 167 mW/m2 at a current density of 1.49 A/m2. The maximum power densities with RS-1.5% were 3.48-fold higher than RS-0%. This increase can be attributed to the readily available sugars by employing pretreatment. Similarly, a 1.34 fold increase in maximum voltage generation is noted in MFC operating with rice bran and employing pretreatment using hydrodynamic cavitation [23]. Correspondingly, the operation of AD with alkaline pretreated RS has exhibited a 225.6% increase in methane yield due to the accelerated pre-hydrolysis [24]. Overall, pretreatment of lignocellulosic and agricultural wastes is essential in attaining higher production yields. The internal resistance (Rin) of the MFCs was determined from the I-V plot of polarization plot. MFC with RS-1.5% exhibited an internal Rin of 85Ω, whereas the control MFC, i.e., RS-0%, pointed to a Rin of 146Ω. The Rin values were similar to the external resistance at which the highest power generation was observed. The disparity in the maximum power generation and Rin in both MFCs can be attributed to variations in the bioelectrogenic environment on the anode.
Fig. 3.
Polarization curves of single chamber air cathodes MFCs using 1 g/L of raw RS (RS-0%) a and mild alkaline pretreated RS (RS-1.5%) b as a substrate
The maximum power generation noted with RS-0% in this study was similar to that reported by previous studies using a double chamber MFC. In their studies, the power densities with RS-0% were 119 mW/m2 [25] and 190 mW/m2 [26]. In other studies on air cathode MFCs, corn stover and corn stover remaining solids after pretreatment displayed power densities of 331 mW/m2 and 406 mW/m2, respectively [27]. A Membrane-less MFC operation using the lignocellulose wastes like a banana peel and corn bran has exhibited a maximum power generation of only 23.75 and 22.03 mW/m2, respectively [28]. Also use of cassava peel extracts has exhibited a maximum power generation of only 155 mW/m3 [29]. The use of other lignocellulosic hydrolysates from agricultural waste like cashew apple juice and barley straw noted a maximum power generation of only 31.5 and 52.8 mW/m2, respectively [30, 31]. Taken together, these studies suggest that pretreatment of lignocellulosic feedstocks is essential to enhance the overall performance of MFCs. Compared to other studies, the power generation differences in this study are due to variations in operational conditions like employment of pretreatment type and microbial communities in the anode and reactor configurations (ex: single chamber air cathode MFC vs. double chamber MFC.
Organics Reduction and VFAs Variation in MFC-Using RS as a Feedstock
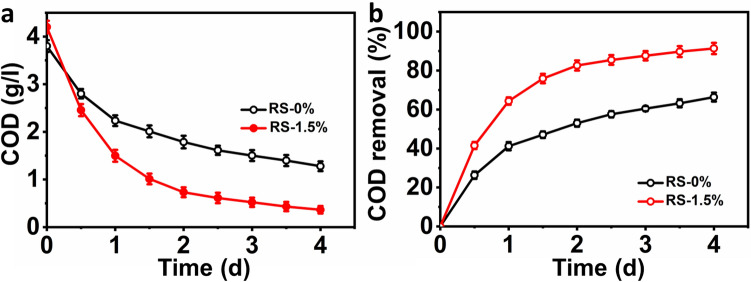
The oxidation of organics for the metabolic activity of bioelectrogenic microbes during MFC operation was noted as the COD removal. Both MFCs using RS-0% and RS-1.5% demonstrated a decrease in COD with increased operational time (Fig. 4). After four days of operation, the maximum reduction in COD was noted in the MFC treated with pretreated RS. MFC using the RS-0% had a COD concentration of 3.8 g/L. By the end of the operation, this was decreased to 1.27 g/L with a COD removal percentage of 66.3%. At the same time, MFC using RS-1.5% pointed to a COD removal percentage of 91.4%, with initial and final COD concentrations of 4.21 and 0.36 g/L, respectively. In this study, the variation in initial COD concentration might be due to the disparity in the sugars generated by the pretreatment. The COD removal noted with MFC using RS-1.5% was similar to that reported by other studies using SW and acetate as substrates [32, 33]. The COD removal noted using RS-0% was comparable to that reported by earlier studies of MFCs employing raw RS as a feedstock. In their studies, the maximum COD percentage was 72% after 15 days of operation using raw RS as a substrate [22].
Fig. 4.
Effluent COD concentrations a and removal percentages b were noted in MFC by varying the type of rice straw used as a substrate
Furthermore, the columbic efficiency (CE) of the MFC was assessed based on the COD removal, as stated in the literature [25]. The CEs of MFCs using RS-0% and RS-1.5% were 22.6% and 78.6%, respectively. In addition, the variation in VFAs was measured for the MFC employing RS-0% and RS-1.5% as substrates. VFA generation was found to be higher with the pretreatment process. MFC using RS-1.5% exhibited a VFA concentration of 14.8 mg/L. Using raw RS, the VFA was 9.8 mg/L. This difference may be due to the generation of reducing sugars after pretreatment and utilization by bioelectrogens. Although the initial pH was adjusted to 7.1, owing to the increase in VFA concentration, the effluent pH of the MFC also varied. The effluent pH of MFC using RS-0% and RS-1.5% were 7.3 and 7.6, respectively. pH is one of the crucial parameters that can alter an MFC’s overall performance. Therefore, further studies are required to control the pH in MFCs for field application and to simultaneously enhance RS degradation.
Generation of Reducing Sugars During the use of RS in MFC
During MFC operation, lignocellulosic compounds in RS can be hydrolyzed to simple sugars for bioelectrogens. The degradation of RS can be quantified in terms of reducing sugar removal. Figure 5 shows the variation in reducing sugar profile noted in MFC using RS-0% and RS-1.5%. Initially, the concentration of reducing sugars was low. At 2.5 days of MFC operation, the reducing concentration was higher, and a continuing decline was observed by the end of the operation. The operation of MFC using RS-0% exhibited a maximum reducing sugar concentration of 0.16 mg/L at 2.5 days of operation. The reducing sugar concentration decreased to 0.064 mg/. Similarly, MFCs using RS-1.5% as a substrate exhibited a maximum reducing sugar concentration of 0.201 mg/L, which further decreased to 0.025 mg/L. A similar profile in reducing sugar generation has been noted in other studies on double-chamber MFC-usiung raw RS [25].
Fig. 5.
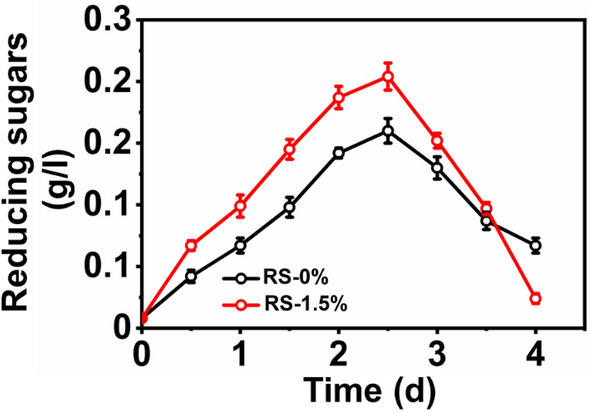
Variation in the concentrations of reducing sugars noted during the operation of MFC-treated RS
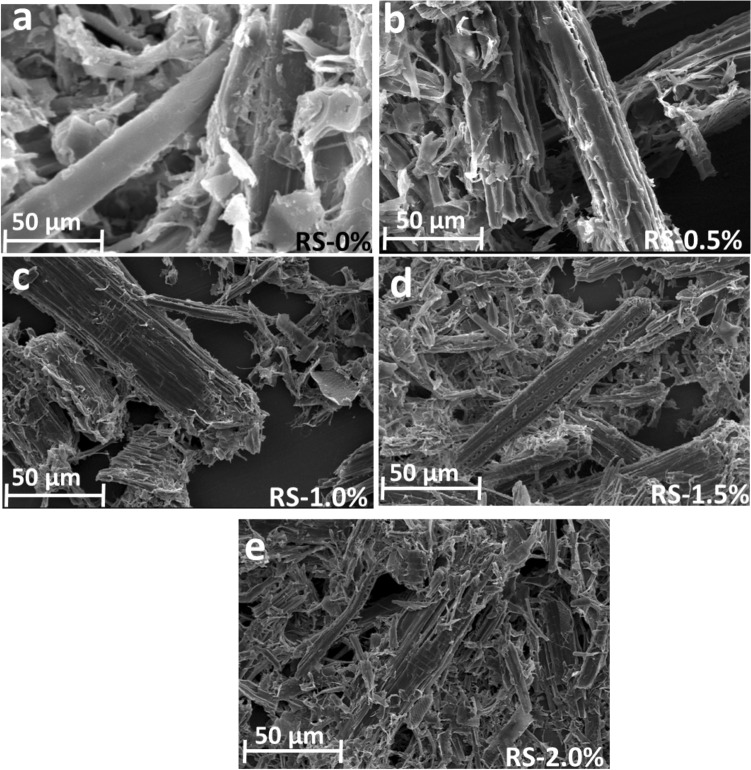
Surface Characterization of Raw and Pretreated RS Usingscanning Electro Microscopy ( SEM)
To understand the surface characterization of RS after pretreatment, SEM analysis was performed (Fig. 6). Raw RS exhibited a dense structure as they had intact plant cell wall components, such as the epidermis, vascular bundles, and parenchyma, adhering to the bundle surface. With NaOH pretreatment, deformed cell structures were observed along with the cellulose fibers. It was also visible that the epidermis and vascular bundles of RS were significantly altered. It is known that NaOH pretreatment of RS can induce the breakdown of lignin with exposure to the distinctive components of silica, breakdown of the intramolecular H-bonding RS structure, and release of pectin and hemicellulose. Moreover, the shape of RS also changed by swelling, with more exposure to cellulose fibers on the surface, which could have been due to the breaking of RS lignin and liposoluble components. Overall, the morphological analysis of RS through SEM revealed that NaOH pretreatment resulted in the avalability of more cellulose than raw RS. Microbes can use the availability of cellulose on the surface of RS as a substrate to meet their metabolic needs.
Fig. 6.
Scanning electron microscopy (SEM) images of raw RS a and mild alkaline pretreated RS b-e with NaOH
In general, MFCs are the potential low energy consuming, higher efficient, and cost-effective alternative to AD [34–36]. However, the product revenue for bioelectricity generation is questionable in MFC, considering the more significant capital costs and comparative edge in the race with AD. Also, significant environmental issues in terms of GHGs emissions have been raised during the processing (ex: burnout) of RS. As an alternative eco-friendly to society and economical way out, the biovolarization of RS as feedstock for electricity generation has risen due to the inessential downstream processing. Using RS as feedstock in other biological processes (ex: biohydrogen, biomethane, bioethanol) requires a downstream process. However, the problems associated with collecting and pre-processing (ex: pretreatment using NaOH) of RS for industrial applications should be considered. Therefore the low cost of RS (10 $/ton) and NaOH (325 $/ton) for pretreatment can increase the ecological gains and cover the capital costs related to MFCs operation. From 1 g of RS with a 1.5% NaOH pretreatment, 0.583 mW of electricity is generated. By scaling up MFC, this power generation can be increased; using 1 ton of RS, 583 W of bioelectricity can be harvested using MFC with a possibility of concurrent wastewater treatment.
Conclusion
This study demonstrates the efficient use of RS as a substrate in MFCs for electricity generation. The difference in holocellulose percentage was noted with variations in the mild alkaline treatment, and a higher amount of holocellulose was noted in RS using 1.5% NaOH. Better performance in MFC was noted with pretreated RS-1.5% over raw RS-0%. The maximum power generation using RS-1.5% (583 mW/m2) was three-fold higher than thst using RS-0% (167 mW/m2). Further studies are required to understand the microbial community variation with changes in RS type and NaOH recovery to minimize the environmental impact.
Acknowledgements
The Basic Science Research Program supported this research through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (NRF-2021R1I1A1A01060963 and 2021H1D3A2A01099705). This paper was supported by Konkuk University Researcher Fund in 2020.
Author Contributions
SK: conceptualization, data curation, writing and reviewing original draft, AB: validation, RP: validation, CL: validation, RL: validation, AK: validation, DD: validation, VCK: validation, monitoring and funding acquisition, JKL: validation, monitoring and funding acquisition.
Declarations
Conflict of interests
The authors affirm that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vipin C. Kalia, Email: vckaliaku@gmail.com
Jung-Kul Lee, Email: jkrhee@konkuk.ac.kr.
References
- 1.U. EIA (2013) EIA projects world energy consumption will increase 56% by 2040. https://www.eia.gov/todayinenergy/detail.php?id=12251. Accessed 05 Feb 2022
- 2.Sabeeh M, Zeshan LR, Maryam A. Effect of alkaline and alkaline-photocatalytic pretreatment on characteristics and biogas production of rice straw. Bioresour Technol. 2020;309:123449. doi: 10.1016/j.biortech.2020.123449. [DOI] [PubMed] [Google Scholar]
- 3.Darmawan A, Fitrianto A, Aziz M, Tokimatsu K. Integrated system of rice production and electricity generation. Appl Energy. 2018;220:672–680. doi: 10.1016/j.apenergy.2018.03.098. [DOI] [Google Scholar]
- 4.Swain M, Singh A, Sharma A, Tuli D. Bioethanol production from rice-and wheat straw: an overview. Bioeth Prod Food Crops. 2019 doi: 10.1016/B978-0-12-813766-6.00011-4. [DOI] [Google Scholar]
- 5.Fang Z, Deng W, Zhang Y, Ding X, Tang M, Liu T, Hu Q, Zhu M, Wang Z, Yang W. Open burning of rice, corn and wheat straws: primary emissions, photochemical aging, and secondary organic aerosol formation. Atmos Chem and Phy. 2017;17:14821–14839. doi: 10.5194/acp-17-14821-2017. [DOI] [Google Scholar]
- 6.Kondaveeti S, Mohanakrishna G, Pagolu R, Kim I, Kalia V, Lee J. Bioelectrogenesis from raw algal biomass through microbial fuel cells: effect of acetate as co-substrate. Indian J Microbiol. 2019;59:22–26. doi: 10.1007/s12088-018-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondaveeti S, Mohanakrishna G, Kumar A, Lai C, Lee J, Kalia V. Exploitation of citrus peel extract as a feedstock for power generation in microbial fuel cell (MFC) Indian J Microbiol. 2019;59:476–481. doi: 10.1007/s12088-019-00829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondaveeti S, Mohanakrishna G, Lee J, Kalia V. Methane as a substrate for energy generation using microbial fuel cells. Indian J Microbiol. 2019;59:121–124. doi: 10.1007/s12088-018-0765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondaveeti S, Patel S, Woo J, Wee J, Kim S, Al-Raoush R, Kim I, Kalia V, Lee J. Characterization of cellobiohydrolases from schizophyllum commune KMJ820. Indian J Microbiol. 2020;60:160–166. doi: 10.1007/s12088-019-00843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama S, Matsumura Y (2008) The Asian biomass handbook: a guide for biomass production and utilization. The Japan Institute of Energy 1(ed) pp 61–62
- 11.Saritha M, AroraLata A. Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J Microbiol. 2012;52:122–130. doi: 10.1007/s12088-011-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mankar A, Pandey A, Modak A, Pant K. Pretreatment of lignocellulosic biomass: a review on recent advances. Bioresour Technol. 2021;334:125235. doi: 10.1016/j.biortech.2021.125235. [DOI] [PubMed] [Google Scholar]
- 13.Moradi F, Amiri H, Soleimanian-Zad S, Ehsani M, Karimi K. Improvement of acetone, butanol and ethanol production from rice straw by acid and alkaline pretreatments. Fuel. 2013;112:8–13. doi: 10.1016/j.fuel.2013.05.011. [DOI] [Google Scholar]
- 14.Rabaey K, Lissens G, Siciliano S, Verstraete W. A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechnol Lett. 2003;25:1531–1535. doi: 10.1023/A:1025484009367. [DOI] [PubMed] [Google Scholar]
- 15.Peera S, Maiyalagan T, Liu C, Ashmath S, Lee T, Jiang Z, Mao S. A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells. Int J Hydrog Energy. 2021;46:3056–3089. doi: 10.1016/j.ijhydene.2020.07.252. [DOI] [Google Scholar]
- 16.Yu Q, Liu R, Li K, Ma R. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew Sust Energ Rev. 2019;107:51–58. doi: 10.1016/j.rser.2019.02.020. [DOI] [Google Scholar]
- 17.Savla N, Pandit S, Mathuriya A, Gupta P, Khanna N, Babu KS. Recent advances in bioelectricity generation through the simultaneous valorization of lignocellulosic biomass and wastewater treatment in microbial fuel cell. Sustain Energy Technol Assess. 2021;48:101572. doi: 10.1016/j.seta.2021.101572. [DOI] [Google Scholar]
- 18.Kondaveeti S, Park G, Shanmugam R, Pagolu R, Patel S, Bisht A, Kim D, Kang Y, Lee J-K. Investigating the role of metals loaded on nitrogen-doped carbon-nanotube electrodes in electroenzymatic alcohol dehydrogenation. Appl Catal B. 2022;307:121195. doi: 10.1016/j.apcatb.2022.121195. [DOI] [Google Scholar]
- 19.APHA . Standard methods for the examination of water and wastewater. Washington DC: American Public Health Association; 2005. [Google Scholar]
- 20.Kondaveeti S, Choi K, Kakarla R, Min B. Microalgae Scenedesmus obliquus as renewable biomass feedstock for electricity generation in microbial fuel cells (MFCs) Front Environ Sci Eng. 2014;8:784–791. doi: 10.1007/s11783-013-0590-4. [DOI] [Google Scholar]
- 21.Kondaveeti S, Seelam J, Mohanakrishna G. Anodic electron transfer mechanism in bioelectrochemical systems. In: Das D, editor. Microbial Fuel Cell: A bioelectrochemical system that converts waste to watts. Cham: Springer International Publishing; 2018. pp. 87–100. [Google Scholar]
- 22.Kondaveeti S, Lee S, Park H, Min B. Specific enrichment of different Geobacter sp. in anode biofilm by varying interspatial distance of electrodes in air-cathode microbial fuel cell (MFC) Electrochim Acta. 2020;331:135388. doi: 10.1016/j.electacta.2019.135388. [DOI] [Google Scholar]
- 23.Yoshimura Y, Nakashima K, Kato M, Inoue K, Okazaki F, Soyama H, Kawasaki S. Electricity generation from rice bran by a microbial fuel cell and the influence of hydrodynamic cavitation pretreatment. ACS Omega. 2018;3(11):15267–15271. doi: 10.1021/acsomega.8b02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mothe S, Polisetty R. Review on anaerobic digestion of rice straw for biogas production. Environ Sci Pollut Res. 2021;28:24455–24469. doi: 10.1007/s11356-020-08762-9. [DOI] [PubMed] [Google Scholar]
- 25.Flimban S, Hassan S, Rahman M, Oh S. The effect of Nafion membrane fouling on the power generation of a microbial fuel cell. Int J Hydrog Energy. 2020;45:13643–13651. doi: 10.1016/j.ijhydene.2018.02.097. [DOI] [Google Scholar]
- 26.Gurung A, Oh S. Rice straw as a potential biomass for generation of bioelectrical energy using microbial fuel cells (MFCs) Energ Source-Part A. 2015;37:2625–2631. doi: 10.1080/15567036.2012.728678. [DOI] [Google Scholar]
- 27.Wang X, Feng Y, Wang H, Qu Y, Yu Y, Ren N, Li N, Wang E, Lee H, Logan B. Bioaugmentation for electricity generation from corn stover biomass using microbial fuel cells. Environ Sci Technol. 2009;43:6088–6093. doi: 10.1021/es900391b. [DOI] [PubMed] [Google Scholar]
- 28.Makhtar M, Tajarudin A. Electricity generation using membrane-less microbial fuel cell powered by sludge supplemented with lignocellulosic waste. Int J Energ Res. 2020;44(4):3260–3265. doi: 10.1002/er.5151. [DOI] [Google Scholar]
- 29.Adekunle A, Raghavan V. Evaluation of the suitability and performance of cassava waste (peel) extracts in a microbial fuel cell for supplementary and sustainable energy production. Waste Manage Res. 2017;35:47–55. doi: 10.1177/0734242X16670487. [DOI] [PubMed] [Google Scholar]
- 30.Priya D, Setty P. Cashew apple juice as substrate for microbial fuel cell. Fuel. 2019;246:75–78. doi: 10.1016/j.fuel.2019.02.100. [DOI] [Google Scholar]
- 31.Gurav R, Bhatia K, Choi T, Kim H, Song H, Park S, Lee S, Lee H, Kim S, Yoon J, Yang Y. Utilization of different lignocellulosic hydrolysates as carbon source for electricity generation using novel Shewanella marisflavi BBL25. J Cleaner Prod. 2020;277:124084. doi: 10.1016/j.jclepro.2020.124084. [DOI] [Google Scholar]
- 32.Kondaveeti S, Moon J, Min B. Optimum spacing between electrodes in an air-cathode single chamber microbial fuel cell with a low-cost polypropylene separator. Bioprocess Biosyst Eng. 2017;40:1851–1858. doi: 10.1007/s00449-017-1838-3. [DOI] [PubMed] [Google Scholar]
- 33.Khater D, El-Khatib K, Hassan H. Microbial diversity structure in acetate single chamber microbial fuel cell for electricity generation. J Genet Eng Biotechnol. 2017;15:127–137. doi: 10.1016/j.jgeb.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel S, Gupta R, Kondaveeti S, Otari S, Kumar A, Kalia VC, Lee J-K. Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour Technol. 2020;315:123791. doi: 10.1016/j.biortech.2020.123791. [DOI] [PubMed] [Google Scholar]
- 35.Patel S, Gupta R, Kalia VC, Lee J-K. Integrating anaerobic digestion of potato peels to methanol production by methanotrophs immobilized on banana leaves. Bioresour Technol. 2021;323:124550. doi: 10.1016/j.biortech.2020.124550. [DOI] [PubMed] [Google Scholar]
- 36.Patel SKS, Das D, Kim C, Cho K, Kalia VC, Lee J-K. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew Sust Energ Rev. 2021;150:111491. doi: 10.1016/j.rser.2021.111491. [DOI] [Google Scholar]