Abstract
The nephrotic syndrome consists of heavy proteinuria with hypoalbuminemia. These are the clinical manifestations of several rare kidney disease. Although the population incidence is low (an estimated incidence of three cases per 100 000 patient-years), nephrotic syndrome has been associated with a range of complications including cardiovascular and thromboembolic events, acute kidney injury or systemic infections. These complications are generated by a combination of increased protein urinary losses and greater liver protein synthesis. The current paper aims to present pathophysiological mechanisms and current therapeutic recommendations for hyperlipidemia, acute kidney injury and other complications associated with nephrotic syndrome.
Keywords:nephrotic syndrome, hyperlipidemia, statins, AKI, infection risk, drug pharmacokinetic.
INTRODUCTION
Nephrotic syndrome (NS) is defined by heavy proteinuria (more than 3.5 g per day in adults or 40 mg/m2 per hour in children), due to severe alteration in the glomerular filtration barrier of various causes and hypoalbuminemia (<3.5 g/dL). Both criteria are mandatory for diagnosis (1). Edema, hyperlipidemia, etc are commonly associated with NS but are not essential to its definition. They can be seen as consequences of heavy proteinuria and are part of the clinical presentation of many, but not all, patients with NS – for this reason, they are not regarded as part of the current definition, and therefore, like other authors (2), we preferred to use the term "complications" instead of "consequences".
Edema is the most frequent complication; hyperlipidemia depends on hepatic protein synthesis in response to hypoproteinemia and the hypercoagulable state is maintained by a combination of imbalance between antithrombo- tic-prothrombotic mechanisms, abnormal platelet activation and defective fibrinolysis. Acute kidney injury (AKI) could complicate the course of NS, especially in older male patients, while bacterial infections are more often seen in the pediatric population. Drugs used for pathogenic or symptomatic treatment may also induce or exacerbate various complications, which are beyond the scope of the present review.
Acute kidney injury
Acute kidney injury has been mainly observed in adult patients with idiopathic NS, with an incidence varying between 24-47% (3-5). Risk factors for AKI included age over 60 years, male patients, hypertension, severe nephrotic edema, i.e., heavy proteinuria (more than 10 g/day), low serum albumin (1-2 g/dL) and massive edema (1, 3, 6). Large series of histopathological studies from 1993 to 2017 reported minimal change disease (MCD) to be the main substrate of NS in patients with AKI, mainly in elderly ones (2). The average time between the onset of NS and AKI is four weeks, but sometimes it can occur simultaneously with a very rapid remission (4). In our experience, AKI was diagnosed at presentation in a large proportion of nephrotic patients (42%) (3).
The pathogenesis of AKI in NS remains uncertain. Given the different clinical presentation, various mechanisms can be identified. Hypovolemia seams to play a predominant role, especially in “underfill” patients, who describe symptoms like nausea, vomiting, diarrhea, tachycardia, low blood pressure. Diuretics may worsen this picture, but blood volume restauration may correct the imbalance. Sometimes, especially in the elderly, preexisting arteriolar lesions promote worsening of the initial lesions, making a complete recovery of renal function unlikely.
Another postulated mechanism is the impaired filtration pressure due to tubular obstruction by protein casts in case of massive proteinuria. This mechanism appears to be associated with sudden onset (or relapses) of NS. The histological evidence of numerous large protein casts in dilated cortical tubules containing varying proportions of albumin and globulin, but no Tamm- Horsfall protein supports this mechanism (7, 8). Also, interstitial edema associated with severe NS ("nephrosarca") can induce tubular collapse. Recovery of renal function was reported after diuretic therapy.
Ischemic tubular damage in adult onset MCD, with severe NS and expanded extracellular fluid ("overfill patients"), can be caused by elevated endothelin 1 (ET-1). Abnormal regulation of inflammatory cytokines involved in MCD pathogenesis has been shown to induce local ET-1 expression. Enhanced ET-1 expression following transient ischemia induced by diuretic therapy could also produce glomerular and mesangial cell contraction, reducing the glomerular filtration rate (9).
NS. Acute kidney injury can be precipitated by either drug-induced glomerular hemodynamic alteration (ACEI/ARB, calcineurin inhibitors, nonsteroidal anti-inflammatory drugs), interstitial nephritis (NSAID, proton pump inhibitors, diuretics, antibiotics), or contrast-induced nephropathy (especially iodinated high-osmolality contrast media).
Other uncommon causes include renal vein thrombosis (more frequent in membranous nephropathy) or another superimposed lesion (ANCA vasculitis or anti-glomerular basement membrane nephritis associated with membranous nephropathy, or lupus nephritis).
Most patients recover to normal renal function; the mean time to recovery of renal function increases with AKI severity: 20 days for patients with AKI risk (AKI R) versus 30 days for patients with AKI failure (AKI F) RIFLE classes. Also, the recovery of complete renal function in three months was 92% for AKI R versus 65% for AKI F classes (1). However, AKI does not seem to influence either the kidney or patients' outcomes (3).
Hyperlipidemia
Nephrotic syndrome is associated with a significant alteration in lipid and lipoprotein metabolism. The underlying mechanism is complex and seems to be related to hypoalbuminemia rather than proteinuria. There are marked elevations in serum LDL cholesterol, VLDL, IDL and lipoprotein (a) [Lp (a)], accompanied by a low or normal HDL level, alteration in the composition of different lipoproteins such as cholesterol to triglycerides, free cholesterol to cholesterol ester or phospholipid to protein ratios. VLDL and chylomicron clearance is impaired. All these result in an accumulation of atherogenic particles, potentially increasing the cardiovascular (CV) risk and kidney disease progression.
Pathogenesis of hyperlipidemia in the nephrotic syndrome
The NS hypercholesterolemia is explained by the increased biosynthesis and decreased catabolism of cholesterol. These two processes are controlled by 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) – the rate-limiting enzyme in cholesterol synthesis – and cholesterol-7 alpha-hydroxylase – the rate-limiting enzyme of cholesterol catabolism. Both are regulated by the intracellular free cholesterol concentration, which in NS does not increase despite severe hypercholesterolemia. This discrepancy may be partly explained by the impaired hepatic cholesterol uptake due to acquired LDL receptor (LDLr) deficiency, but also by up-regulation of hepatic acyl-coenzyme A (CoA):cholesterol acyltransferase (ACAT) (which catalyzes cholesterol esterification) activity.
The overexpression of hepatic ACAT is primarily due to proteinuria; it plays a special role in both packaging cholesterol in apoB-100 and release of VLDL by hepatocytes. Also, by increasing cholesterol esterification, ACAT allows its accumulation in macrophages and vascular tissue, hence foam cell generation and atherosclerosis.
The acquired LDLr deficit is due to a posttranslational and posttranscriptional mechanism. The LDL receptor has two important posttranslational regulators: proprotein convertase subtilisin kexin type 9 (PCSK9) and inducible degrader of the LDLr (IDOL). The first one, PCSK9, a serine protease produced by the hepatocyte, intestine and kidney, is upregulated in NS primarily in relation to proteinuria; in addition, PCSK9 interferes with the action of CD36 (receptor class B type 3 – SR-B3), a multifunctional receptor for cholesterol influx and efflux, expressed by several cells (macrophage, cardiomyocyte, adipocyte) (10), which intervenes in foam cell formation and atherosclerosis, but also contributes to lipid utilization by muscle, energy storage in adipocytes by binding and promoting cellular uptake of long-chain fatty acids and triglycerides. Accordingly, CD36 degradation by PCSK9 contributes to hypertriglyceridemia, elevated serum fatty acids and reduced adipose tissue mass in nephrotic patients (11, 12).
High lipoprotein (a) [Lp(a)] level in NS is reported because increased liver synthesis is a risk factor for atherosclerosis and TEs in the general population. Lp(a) is formed from an LDL particle to which apoA is covalently bound. ApoA gene has a high polymorphism, explaining the high (more than 1 000 times) interindividual variations in Lp(a) levels. This is important because routine methods used to measure LDL do not distinguish between LDL- and Lp(a)-derived cholesterol and statins decrease only LDL cholesterol. Thus, high Lp(a) levels could be a cause of statin resistance of hypercholesterolemia in NS.
HDL plays the leading role in reverse cholesterol transport from extrahepatic tissues to the liver, thus providing cardiovascular protection, complemented by anti-inflammatory, antioxidant and anti-thrombotic activities. In NS, HDL is normal or slightly reduced, but the HDL:total cholesterol ratio is low as compared to healthy individuals. Also, the maturation of cholesterol ester-poor HDL particle to cholesterol ester-rich HDL is altered. These disturbances are caused by the NS-associated alteration of various proteins involved in HDL metabolism (13).
Lecithin-cholesterol acyltransferase is the major enzyme involved in the maturation of HDL particles by rapidly re-esterifying free cholesterol on HDL surface. Lecithin cholesteryl ester acyltransferase deficiency associated with NS is mainly caused by urinary loss. Albumin is also one of the free-cholesterol carriers, especially from the peripheral tissues, and hypoalbuminemia seems to contribute to fewer cholesterol ester-rich HDL particles. Cholesteryl ester transfer protein exchanges cholesterol ester with triglycerides between HDL2 and IDL, promoting the formation of IDL into LDL. High CETP serum levels have been associated with NS. This alteration contributes to impaired maturation of HDLcholesterol, therefore also affecting triglyceriderich lipoprotein metabolism (11).
SR-B1 receptors play a central role in reverse cholesterol transport, enabling the uptake of cholesterol ester from HDL by hepatocytes and thereafter returning unloaded HDL particle into circulation. Nephrotic animal studies indicate a reduction in SR-B1 receptor despite its normal mRNA expression. This alteration could be due to a significant reduction in PDZK1 protein, an adapter protein which confers stability to SR-B1 in hepatocyte plasma membrane. Acquired SRB1 deficiency alters reverse cholesterol transport, leading to a higher atherogenic risk in NS (11, 14). Unlike SR-B1, hepatic HDL endocytic receptor, which mediates apoA-I and lipid-poor HDL endocytosis, is upregulated in NS. Also, hepatic lipase deficiency contributes to HDL enrichment in triglyceride (11).
Triglyceride-rich lipoproteins, such as chylomicrons, VLDL, and their remnants, carry fatty acids (as triglycerides) between the sites of absorption, generation, storage and metabolism. Nephrotic syndrome is distinguished by high serum triglyceride, VLDL and IDL levels, increased triglyceride contents in apoB lipoproteins and prolonged postprandial lipemia. All these alterations result from impaired VLDL and chylomicron clearance due to impaired LPL (lipoprotein lipase), hepatic lipase and VLDL receptor, and up-regulation of CETP and LRP (LDL recep- tor-related protein) (9).
Consequences of lipid abnormalities in nephrotic syndrome
Systemic consequences – Accumulation of atherogenic particles such as LDL, IDL and chylomicron remnants, related to impaired reverse cholesterol transport, promotes atheroma plaque organization and increases the risk of cardiovascular disease. An elevated Lp(a) level leads to a high risk of thromboembolic events. Impaired free fatty acids transport to the muscles and adipose tissue may influence body muscle mass and diminished exercise capacity; these changes are accentuated by insulin resistance which is usually seen in any kidney disease (9).
Importantly, systemic consequences seem to amplify patients’ preexistent cardiovascular risk and are proportional to NS duration and severity. Accordingly, the therapeutic approach should take in consideration both these factors, although there are no studies addressing this issue (15).
Kidney consequences – Alterations of lipid metabolism had been thought to play an important role in the progression of kidney disease since the ‘80s (16). Since then, many animal models, which evaluated the effect of a high cholesterol diet, have been supporting this theory. Hyperlipidemia alone is not sufficient to explain this accelerate progression; therefore, a precursor condition such as hypertension, hyperfiltration, decreased nephron mass or inflammation is required (17).
Lipid nephrotoxicity is due to a direct toxic effect of lipids on the kidney, associated with an indirect effect due to atherosclerosis. The initial event involved in lipid nephrotoxicity is unclear, but it is believed that the process induced by oxidative stress initiates many of the events that accelerate the progression of renal impairment. Nephrotic syndrome specific altered lipid metabolism increases the production of reactive oxygen species (ROS) in monocytes. Increased ROS levels contribute to tissue dysfunction in multiple manners: by altering the redox-sensitive signaling pathway, by inducing oxidative molecular alteration, damaging DNA, protein and lipid structure, and macrophage activation (15).
However, in addition to proteinuria and decreased filtration rate, lipotoxicity may be associated with more subtle manifestations of lipid-induced renal tubular dysfunction. Lipid accumulation in proximal tubule decreases ammonium secretion at these levels, in part by reducing membrane Na+/H+ exchanger-3 activity and by affecting the regulation of Na+/H+ exchanger-3 by specific antagonist. This alteration is clinically manifested with an increased risk of uric acid nephrolithiasis due to lower urinary pH and impaired ammonium excretion (24).
Interpreting the relation between lipid and renal failure requires further studies in animal models and non-invasive urinary proteomics assays (urinary secretion of proinflammatory cytokines, epidermal growth factor) (25).
Current therapy of hypercholesterolemia in nephrotic syndrome
Therapeutic recommendations for nephrotic patients include diet, life-style changes, and use of pharmacological lipid-lowering agents. However, there are no controlled studies investigating the effect of therapy on cardiovascular endpoints in nephrotic patients. Accordingly, KDIGO 2021 recommended therapy guidelines for the general population, taking into account an estimated duration of more than six months for the NS, its severity (serum albumin lower than 2.5 g/dL and proteinuria over 8 g/day) and additional risks factors specific to glomerular disease, i.e., systemic inflammation. Although these recommendations aim to reduce the cardiovascular risk, some benefit in terms of kidney function could exist. However, these recommendations were mainly based on data from membranous nephropathy, where the effects of therapy on dyslipidemia were best investigated (13).
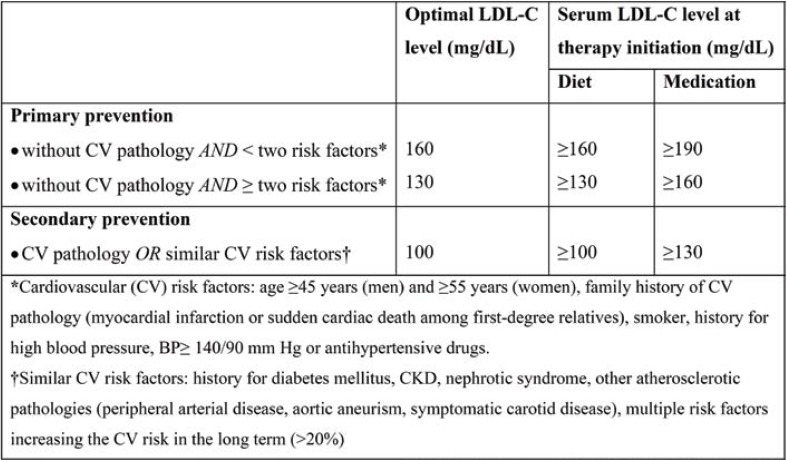
Thus, with a 10 mmol/L reduction in LDL level in the general population, there are further reductions in the incidence of major cardiovascular events (such as heart attack and ischemic stroke) and revascularization (CTT, 2010). Also, according to National Cholesterol Education Program, Kidney Disease Outcomes Quality Initiative and the American Diabetes Association for primary and secondary prevention, LDL must be maintained below 100 mg/dL in patients with coronary heart disease, atherosclerosis or diabetes (Table 1) (24).
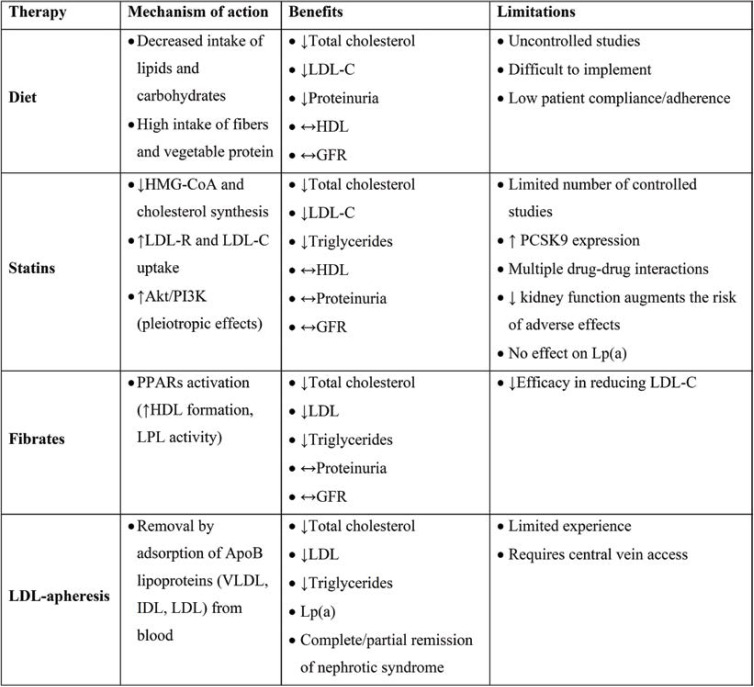
In nephrotic patients, various drugs are used to treat hyperlipidemia, but only statins, either alone or in combination with ezetimibe, prove to be effective (Table 2). Thus, the clinician should aim to reduce LDL-cholesterol at least 50% from baseline as this reduction is also associated with a considerable decrease in albuminuria and slows down renal function decline (28-30). But these therapeutic targets are difficult to achieve, even with proper treatment, and LDL-cholesterol levels are often suboptimally controlled.
However, given the central role of proteinuria in the pathogenesis of lipid metabolism disorder in NS, the therapeutic target is the reversal or at least attenuation of proteinuria by either specific (i.e., immunosuppression) or general measures. Among general anti-proteinuric measures, the control of blood pressure values is the main intervention approach, renin-angiotensin-aldosterone system inhibitors being chosen of first intention. By controlling blood pressure and improving glomerular selectivity, angiotensin-converting enzyme (ACE) inhibitor and angiotensin II receptor blocker (ARB) reduce proteinuria. The two drug classes also improve glomerulosclerosis by reducing pro-inflammatory cytokine secretion (like TGH-β) (31). ACE inhibitors seem to have an indirect effect on LDL cholesterol level, as a result of reducing proteinuria, and a direct dose dependent effect on triglycerides (32). The ARB hipolipemiant action is also indirect, due to antiproteinuric action, and depends on the interaction with PPAR-. (peroxisome proliferator-activated receptor-gamma), which is involved in carbohydrate and lipid metabolism.
Diet – Lifestyle changes and dietary adjustments appear to be a physiological measure to control NS associated hyperlipidemia with minimal side effects. Initially, a hyperprotein diet (2 g proteins/kg ideal body weight) has been proposed to avoid malnutrition, but this idea has been abandoned given the lack of improvement in serum albumin and the negative effect on glomerular hemodynamics. Thereafter, a low protein diet (0.5 g protein/kg ideal body weight) was suggested as it limits proteinuria, uremia and total cholesterol level (34-36). This idea was suggested by Richard Bright and later supported by Brenner et al, with Pedrini et al meta-analysis complementing their statements (37, 38). However, despite all benefits, a low protein diet seems to be associated with malnutrition; therefore, a normal protein diet (1 g protein/kg ideal body weight) is now recommended for most nephrotic patients (33)
The type of protein to consume is another debatable topic since the ‘90s. Beef and chicken meat significantly alter the glomerular flow, in contrast to soy proteins, which have a low postprandial effect and are the best options for diabetic patients. Soy proteins are considered the next “complete proteins” after egg albumin. Soy has a high content of fibers and complex carbohydrates as well as a good glycemic index, and it lowers insulin secretion due to its arginine/lysine ratio (39, 40). An intake of 31-47 g soy proteins/day lowers serum cholesterol levels. The mechanism appears to be a reduction in cholesterol intestinal absorption and an increase in the excretion of steroids in the stool (39, 40). In addition to lowering LDL-cholesterol and triglycerides, soy diet reduces proteinuria in nephrotic patients. Genistein and daidzein, the main soy isoflavones, limit LDL oxidation through their phenolic ring (41). Accordingly, vegetarian diets are suggested by KDIGO 2021 (13).
Statins – KDIGO 2021 recommends the use of statins as first line therapy in patients with NS. Statins are competitive inhibitors of hydroxymetyl glutaryl (HMG) CoA reductase, the rate-limiting enzyme on the mevalonate pathway of cholesterol synthesis, resulting in a low intracellular cholesterol level. Statins have a low bioavailability, which limits the interaction with extra-hepatic HMG-CoA, reducing side effects. However, associated muscle and liver toxicity may be a problem in nephrotic patients. The main reason is drug-to-drug interaction with CYP450 activity, which may increase the serum concentration of statins. Drugs that interact with CYP450 include calcineurin inhibitors (cyclosporin, tacrolimus), fibrates (gemfibrozil, bezafibrate), macrolides (erythromycin), anti-arrhythmics (amiodarone, digoxin, verapamil), protease inhibitors (amprenavir, indinavir), or anticoagulants (warfarin). Chronic or acute renal failure also increases the risk of toxicity. Particular attention should be paid to the use of rosuvastatin, which may intensify proteinuria and renal failure (44, 45). However, studies which investigated the effect of statins on hyperlipemia of NS have shown that they were well tolerated (46, 47).
In addition to the lipid-lowering effect, by blocking mevalonate pathway, statins also influence processes involved in renal dysfunction such as inflammation, mesangial proliferation (both cellular and matrix), interstitial fibrosis and others (43).
Fibrates – Unlike statins, this class of lipid-lowering drugs does not interfere with cholesterol synthesis but rather acts on the plasma levels of fatty acids ant triglycerides. The fibrate molecule has a structure that meets many characteristics of short-chain fatty acids and acts as an antagonist for peroxisome proliferator activated receptor α (PPAR α). Fibrate effect is due to LPL activation by both PPARá activity and decrease of apo CIII expression. Thus, triglyceride lipolysis from chylomicrons and VLD is accentuated, making lipoprotein residues much easier to remove from the circulation.
By activating PPARα, which are mainly present in the proximal convoluted tubule and less in the mesangium, they have a reno-protective effect by attenuating lipotoxicity, oxidative stress and inflammation. Fibrates improve increase in intracellular lipids by downregulating SREBP-1 and CHREBP-1 and limit oxidative stress and glomerulosclerosis by inhibiting the activation of PI3K-Akt-O3a pathway, a regulator of mitochondrial oxidative stress (48, 49).
The use of fibrates in patients with renal pathology has been limited due to the increase in serum creatinine, especially in those with mild to moderate renal failure and transplant patients, probably due to fibrate interference with prostaglandins generation. Hottelart et al suggest that fenofibrate-induced creatinine increase did not reflect an impairment in renal function, tubular function alteration, or muscular damage, and is likely due to an increased creatinine metabolic production (48). Observations from post-trial studies of FIELD and ACCORD trials support the opinion of Hottelart et al. However, KDOQI recommends careful prescribing of fibrates in patients with CKD (51, 52).
Second-line lipid-lowering drugs include ezetimibe, nicotinic acid and bile acid sequestrants (colestipol, cholestyramine). Bile acid sequestrants have a minimal effect on LDL-C and the side effects limit their prescription, while ezetimibe and nicotinic acid have not been shown to be effective in nephrotic patients (53).
LDL APHERESIS was developed as a treatment for refractory familial hypercholesterolemia (FH) and is currently used as a new therapeutic strategy for rapid correction dyslipidemia in refractory NS. In LDL apheresis, plasma is separated from blood cells, after which lipids are removed from plasma, which is then reinfused. There are four different techniques for removing plasma LDL: immunoadsorption, dextran sulfate cellulose adsorption, heparin extracorporeal LDL precipitation (HELP), direct adsorption of lipoprotein using hemoperfusion. Of this, dextran sulfate cellulose adsorption, which removes the positively charged apolipoprotein B-containing lipoproteins [LDL, VLD, Lp (a)] using negatively charged dextran sulfate, rapidly decrease LDL levels by 76-81% and Lp (a) level by 65-68% with minimal side effects (54).
Several mechanisms could explain the therapeutic effects of LDL-apheresis: limiting glomerular injuries by reducing macrophage hyperstimulation by improving dyslipidemia, direct adsorption and eliminating circulating “soluble factors” involved in the pathogenesis of FSGS. Recovery of cell sensitivity to lipophilic drugs such as cyclosporine (CyA), as a result of ameliorating dyslipidemia and recovery of the CyA receptor function, has also been observed. Thus, in addition to ameliorating hyperlipidemia, it also relives proteinuria, inducing remission or partial remission in refractory NS (55). Besides numerous retrospective studies, the prospective cohort study POLARIS, initiated by the Japanese Society of Kidney and Lipids, shows the effectiveness of LDL-apheresis in ameliorating proteinuria in nearly half of patients with refractory NS (56).
PCSK9 inhibitors – As mentioned earlier, the underlying mechanism of upregulation of HMG-CoA reductase and impaired LDL clearance in NS is PCSK9- and IDOL-mediated degradation of LDL receptors. Starting from the central role that PCSK9 plays in NS associated dyslipidemia, PCSK9 inhibitors may be effective in the treatment of nephrotic patients.
PKSC9 inhibitors are a novel lipid-lowering tool. Alirocumab and evolocumab are clinically available and the current evidence supports their benefits in patients who might not be eligible or unable to meet lipid targets with other lipid-lowering drugs. Bococizumab is a human monoclonal antibody to PCSK9 that was under development, but researchers have discontinued the clinical trials due to the development of antidrug antibodies. Another tested agent is Iclisiran, a small RNAs molecules designed to target PCSK9 messenger RNA (57).
However, available data supporting the efficacy and safety of monoclonal antibodies against PCSK9 in nephrotic patients are still limi- ted (58-61) – they not only support a new recommendation for these new drugs, confirming the central role of PCSK9 in pathogenesis of hypercholesterolemia of NS, but also will certify the “lipid nephrotoxicity” hypothesis (8).
Infections
Patients with NS have an increased risk of infection. Prior to the introduction of corticosteroids and antibiotics, infections were the most common cause of death, especially in nephrotic children. Despite considerable therapeutic improvements, infections remain an important cause of morbidity and mortality, mainly in children (62). In children, Streptococcus pneumoniae is known to be the main pathogen, followed by β-hemolytic streptococci, Haemophylus spp and Gram-negative bacteria. In adults with NS, infections are mainly caused by nosocomial bacteria (59, 63).
Hypogammaglobulinemia (serum IgG level under 600 mg/dL) and serum creatinine level over 2 mg/dL are independent risk factors for infection in adults with NS. Low serum IgG level is due to urinary loss without a compensatory increase in synthesis (64, 65).
Alternative complement pathway proteins I and B low serum levels were reported to increase the risk of infections with encapsulated bacteria (66). Low serum levels of transferrin, which is essential for lymphocytes growth, and zinc, relevant for normal thymic hormones activity, may also impair the normal immune response (67).
Defense mechanisms are also altered by malnutrition, mechanical factors such as edema and ascites, and immunosuppressive therapy, which may mask the typical clinical presentation of the infection and delay proper intervention.
Frequent infections can lead to repeated relapses and treatment failure, which can cause significant morbidity. Many efforts have been made to control these events worldwide. Various prophylactic regimens are used or recommended to diminish the infection risk in nephrotic patients in clinical practice. These cover pneumococcal and influenza vaccines (in patients and households), prophylactic antibiotics (trimethoprim– sulfamethoxazole in those receiving immunosuppressive therapy), and immunoglobulin replacement. However, data obtained so far are insufficient to recommend a standardized prophylactic strategy (13, 59).
Other complications of nephrotic syndrome
In addition to albumin, many binding proteins are lost in the urine but with uncertain clinical consequences. However, the associated extracellular volume expansion due to water and salt retention could significantly alter the volume of distribution of small to intermediate-proteins, decreasing their concentrations.
Thyroid binding globulin levels are low but, although total serum T3 and T4 decreases, free T3 and T4 are normal. Similarly, although cortisol and vitamin D binding proteins have low levels, cortisol proportionally decreases with serum albumin. However, ionic calcium, the physiologically significant fraction of serum calcium, was seldom reported to be low. It seems that hypocalcemia, associated with low calcitriol levels and high PTH, develops only in a small proportion of nephrotic patients (7, 68, 69).
Anemia is a complication of resistant NS, mostly in children, and in animal and human studies it was related to urinary loss of erythropoietin, transferrin and iron (65, 70).
The alteration in the volume of distribution due to hypoalbuminemia modifies drug pharmacokinetics, increasing the free circulating drug. Low levels of protein-bound drug do not necessarily indicate low effective drug levels, but on the contrary, because the level of free drug may be normal or even dangerously elevated. This should be considered when monitoring the drug plasma level.
Conflict of interests: none declared.
Financial support: none declared.
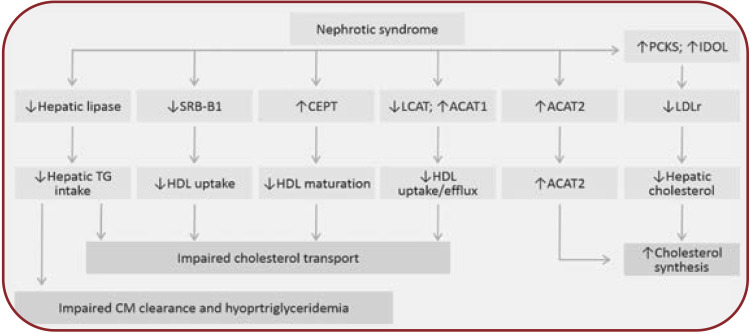
FIGURE 1.
Pathogenesis of hyperlipidemia in the nephrotic syndrome. ACAT-2=acyl-CoA cholesterol acyltransferase-2; CETP=cholesteryl ester transfer protein; CM=chylomicrons; LCAT=lecithin cholesterol acyltransferase; LDLr=LDL receptor; SR-B1=class B scavenger receptor type 1; TG=triglyceride. The main mechanisms of hypercholesterolemia include the increase in hepatic cholesterol synthesis and impaired reverse cholesterol transport, and for hypertriglyceridemia, impaired triglycerides uptake. (i) Increased hepatic cholesterol synthesis is stimulated by the low intracellular level of free cholesterol induced by the increased expression of PCSK9 and INDOL (which reduces LDL hepatic uptake by degrading the LDLr) and by the increase in cholesterol acylation by activated ACAT2. (ii) The reverse cholesterol transport to the liver is altered as a result of reduced HDL-mediated extraction of cholesterol from lipid-laden cells by heavy urinary loss of LCAT and increased ACAT-1 expression. Moreover, the marked increase in serum CETP impairs HDL maturation, and the marked reduction of SR-B1, an HDL docking receptor, limits HDL uptake by the hepatocyte. The altered reverse cholesterol transport promotes atheroma plaque organization. (iii) The reduced activity of hepatic lipoprotein lipase affects chylomicron (CM) clearance and contributes to the development of hypertriglyceridemia.
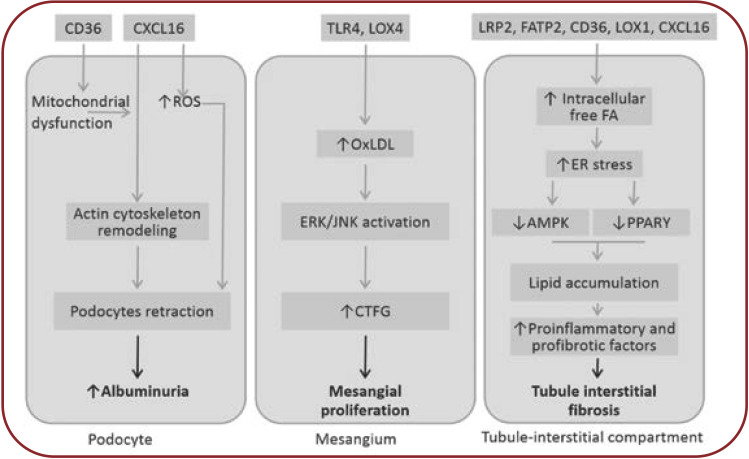
FIGURE 2.
Potential effects of hyperlipemia on kidney (18-23). AMPK=AMP activated protein kinase; CTGF=conective tissue growth factor; ER=endoplasmic reticulum; ERK=extracelular signal-regulated kinase; JNK=c-jun-NH2 terminal kinase; PPARá=peroxisome proliferator-activated receptor alpha; ROS=reactive oxygen species. Ox-LDL are taken over by “scavenger” receptors (SR-A, CD 36, CXCL-16) resulting in foam cells. Scavenger receptor CXCL-16 mediates podocytes uptake of ox-LDL, which induces loss of nephrin expression, contributing to proteinuria and glomerulosclerosis. Nephrin loss also affects insulin-stimulated glucose uptake in podocytes, which will exacerbate the endoplasmic reticulum stress initiated by podocyte lipid accumulation. Unlike other glomerular structures, the mesangial cells, being not covered by the basement membrane, will more easily meet Ox-LDL, which are taken by special “scavenger” receptors like TLR 4 (toll-like receptors) or LOX-1 (lectin-like oxidized LDL receptor). Thereafter, TGF-â/Smad signaling and PAI-1 transcription (plasminogen activator inhibitor-1) are activated, leading to progression of kidney failure. Overexpression of SREBP-1 and fatty acid synthase leads to increase in renal triglyceride contents. These alterations increase TGFâ1 and vascular endothelial growth factor expression, which contribute to increased mesangial matrix expression, glomerulosclerosis and proteinuria. Tubule-interstitial involvement is mainly due to albumin transported fatty acids and, to a lesser extent, to direct action of both native and oxidized LDL forms. Following high lipid exposure, tubular epithelial cells change their phenotype to a pro-inflammatory one, with increased permeability, chemotactic stimulation and leukocyte adherence. Cellular permeability is affected by reduced E-cadherin expression and FAK overexpression. Tubular cells exhibit ICAM-1, whose expression is MAPK-dependent, which promotes neutrophiles trafficking along the peritubular capillaries. These alterations in tubular cells may play a crucial role in the development and progression of tubular dysfunction.
TABLE 1.
LDL-C goals according to cardiovascular risk. Adapted after (26, 27)
TABLE 2.
Benefits and limitations of different lipid-lowering therapies used in the treatment of hypercholesterolemia associated with nephrotic syndrome. Adapted after (33)
Contributor Information
Ruxandra Mihaela BUSUIOC, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Dr. Carol Davila” Teaching Hospital of Nephrology, Romanian Renal Registry, Bucharest, Romania.
Gabriel MIRCESCU, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Dr. Carol Davila” Teaching Hospital of Nephrology, Romanian Renal Registry, Bucharest, Romania.
References
- 1.Chen T, Lv Y, Lin F, Zhu J. Acute kidney injury in adult idiopathic nephrotic syndrome. Ren Fail. 2011;33:144–149. doi: 10.3109/0886022X.2011.553301. [DOI] [PubMed] [Google Scholar]
- 2.Meyrier A, Niaudet P. Acute kidney injury complicating nephrotic syndrome of minimal change disease. Kidney Int. 2018;94:861–869. doi: 10.1016/j.kint.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Stefan G, Busuioc R, Stancu S, et al. Adult-onset minimal change disease: the significance of histological chronic changes for clinical presentation and outcome. Clin Exp Nephrol. 2021;25:240–250. doi: 10.1007/s10157-020-01985-7. [DOI] [PubMed] [Google Scholar]
- 4.Smith JD, Hayslett JP. Reversible renal failure in the nephrotic syndrome. Am J Kidney Dis. 1992;19:201–213. doi: 10.1016/s0272-6386(13)80001-7. [DOI] [PubMed] [Google Scholar]
- 5.Venkataseshan VS, Faraggiana T, Grishman E, et al. Renal failure due to tubular obstruction by large protein casts in patients with massive proteinuria. Clin Nephrol. 1993;39:321–326. [PubMed] [Google Scholar]
- 6.Koomans HA. Pathophysiology of acute renal failure in idiopatic nephrotic syndrome. Nephrol Dial Transplant. 2001;16:221–224. doi: 10.1093/ndt/16.2.221. [DOI] [PubMed] [Google Scholar]
- 7.Chen CL, Fang HC, Chou KJ, et al. Increased endothelin 1 expression in adult-onset minimal change nephropathy with acute renal failure. Am J Kidney Dis. 2005;45:818–825. doi: 10.1053/j.ajkd.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Busuioc RM, Covic A, Kanbay M, et al. Protein convertase subtilisin/kexin type 9 biology in nephrotic syndrome: implications for use as therapy. Nephrol Dial Transplant. 2020;35:1663–1674. doi: 10.1093/ndt/gfz108. [DOI] [PubMed] [Google Scholar]
- 9.Vaziri ND. Disorders of lipid metabolism in nephrotic syndrome: mechanisms and consequences. Kidney Int. 2016;90:41–52. doi: 10.1016/j.kint.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demers A, Samami S, Lauzier B, et al. PCSK9 Induces CD36 Degradation and Affects Long-Chain Fatty Acid Uptake and Triglyceride Metabolism in Adipocytes and in Mouse Liver. Arterioscler Thromb Vasc Biol. 2015;35:2517–2525. doi: 10.1161/ATVBAHA.115.306032. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri ND. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol. 2016;12:37–47. doi: 10.1038/nrneph.2015.180. [DOI] [PubMed] [Google Scholar]
- 12.Vaziri ND, Gollapudi P, Han S, et al. Nephrotic syndrome causes upregulation of HDL endocytic receptor and PDZK-1-dependent downregulation of HDL docking receptor. Nephrol Dial Transplant. 2011;26:3118–3123. doi: 10.1093/ndt/gfr136. [DOI] [PubMed] [Google Scholar]
- 13.Rovin BH, Adler SG, Barratt J, et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney International. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;11:1309–1311. doi: 10.1016/s0140-6736(82)91513-6. [DOI] [PubMed] [Google Scholar]
- 15.Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol. 2009;5:713–721. doi: 10.1038/nrneph.2009.184. [DOI] [PubMed] [Google Scholar]
- 16.Nosadini R, Tonolo G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21:79–85. doi: 10.1016/j.numecd.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Lay AC, Coward RJM. The Evolving Importance of Insulin Signaling in Podocyte Health and Diseas. Front Endocrinol (Lausanne) 2018. [DOI] [PMC free article] [PubMed]
- 18.Lin M, Tang SC. Toll-like receptors: sensing and reacting to diabetic injury in the kidney. Nephrol Dial Transplant. 2014;29:746–754. doi: 10.1093/ndt/gft446. [DOI] [PubMed] [Google Scholar]
- 19.Nagase M, Kaname S, Nagase T, et al. Expression of LOX-1, an oxidized low-density lipoprotein receptor, in experimental hypertensive glomerulosclerosis. J Am Soc Nephrol. 2000;11:1826–1836. doi: 10.1681/ASN.V11101826. [DOI] [PubMed] [Google Scholar]
- 20.Song CY, Kim BC, Hong HK, Lee HS. Oxidized LDL activates PAI-1 transcription through autocrine activation of TGF-beta signaling in mesangial cells. Kidney Int. 2005;67:1743–1752. doi: 10.1111/j.1523-1755.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 21.Kelly KJ, Wu P, Patterson CE, et al. LOX-1 and inflammation: a new mechanism for renal injury in obesity and diabetes. Am J Physiol Renal Physiol. 2008;294:F1136–F1145. doi: 10.1152/ajprenal.00396.2007. [DOI] [PubMed] [Google Scholar]
- 22.Bobulescu IA, Dubree M, Zhang J, et al. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294:F1315–F1322. doi: 10.1152/ajprenal.00550.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markova I, Miklankova D, Hüttl M, et al. The Effect of Lipotoxicity on Renal Dysfunction in a Nonobese Rat Model of Metabolic Syndrome: A Urinary Proteomic Approach. J Diabetes Res. 2019;8712979 doi: 10.1155/2019/8712979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olyaei A, Greer E, Delos Santos R, Rueda J. The efficacy and safety of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors in chronic kidney disease, dialysis, and transplant patients. Clin J Am Soc Nephrol. 2011;6:664–678. doi: 10.2215/CJN.09091010. [DOI] [PubMed] [Google Scholar]
- 25.Schulz R, Schlüter KD, Laufs U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (PCSK9). Basic Res Cardiol. 2015;110:4. doi: 10.1007/s00395-015-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cholesterol Treatment Trialists (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabelink AJ, Hené RJ, Erkelens DW, et al. Partial remission of nephrotic syndrome in patient on long-term simvastatin. Lancet. 1990;335:1045–1046. doi: 10.1016/0140-6736(90)91118-t. [DOI] [PubMed] [Google Scholar]
- 28.Chan PC, Robinson JD, Yeung WC, et al. Lovastatin in glomerulonephritis patients with hyperlipidaemia and heavy proteinuria. Nephrol Dial Transplant. 1992;7:93–99. doi: 10.1093/oxfordjournals.ndt.a092102. [DOI] [PubMed] [Google Scholar]
- 29.Remuzzi G. Renoprotective effect of ACE inhibitors: dissecting the molecular clues and expanding the blood pressure goal. Am J Kidney Dis. 1999;34:951–954. doi: 10.1016/S0272-6386(99)70057-0. [DOI] [PubMed] [Google Scholar]
- 30.Ruggenenti P, Mise N, Pisoni R, et al. Diverse Effects of Increasing Lisinopril Doses on Lipid Abnormalities in Chronic Nephropathies. Circulation. 2003;107:586–592. doi: 10.1161/01.cir.0000047526.08376.80. [DOI] [PubMed] [Google Scholar]
- 32.Kaysen GA, Davies RW, Hutchison FN. Effect of dietary protein intake and angiotensin converting enzyme inhibition in Heymann nephritis. Kidney Int Suppl. 1989;27:S154–S162. [PubMed] [Google Scholar]
- 33.Ascencio C, Torres N, Sandoval RL, et al. Reduced kidney branched chain aminotransferase expression in puromycin aminonucleoside-induced nephrotic syndrome. Life Sci. 1997;61:2407–2415. doi: 10.1016/s0024-3205(97)00959-4. [DOI] [PubMed] [Google Scholar]
- 34.Mansy H, Goodship TH, Tapson JS, et al. Effect of a high protein diet in patients with the nephrotic syndrome. Clin Sci (Lond) 1989;77:445–451. doi: 10.1042/cs0770445. [DOI] [PubMed] [Google Scholar]
- 35.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307:652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- 36.Pedrini MT, Levey AS, Lau J, et al. The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: a meta-analysis. Ann Intern Med. 1996;124:627–632. doi: 10.7326/0003-4819-124-7-199604010-00002. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura H, Takasawa M, Kashara S, et al. Effects of acute protein loads of different sources on renal function of patients with diabetic nephropathy. Tohoku J Exp Med. 1989;159:153–162. doi: 10.1620/tjem.159.153. [DOI] [PubMed] [Google Scholar]
- 38.Anderson JW, Blake JE, Turner J, Smith BM. Effects of soy protein on renal function and proteinuria in patients with type 2 diabetes. Am J Clin Nutr. 1989;68(Suppl):1347S–1353S. doi: 10.1093/ajcn/68.6.1347S. [DOI] [PubMed] [Google Scholar]
- 39.Bakhit RM, Klein BP, Essex-Sorlie D, et al. Intake of 25 g of soybean protein with or without soybean fiber alters plasma lipids in men with elevated cholesterol concentrations. J Nutr. 1994;124:213–222. doi: 10.1093/jn/124.2.213. [DOI] [PubMed] [Google Scholar]
- 40.Nagata Y, Ishiwaki N, Sugano M. Studies on the mechanism of anti-hypercholesterolemic action of soy protein and soy protein-type amino acid mixtures in relation to the casein counterparts in rats. J Nutr. 1982;112:1614–1625. doi: 10.1093/jn/112.8.1614. [DOI] [PubMed] [Google Scholar]
- 41.Tovar AR, Murguía F, Cruz C, et al. A soy protein diet alters hepatic lipid metabolism gene expression and reduces serum lipids and renal fibrogenic cytokines in rats with chronic nephrotic syndrome. J Nutr. 2002;132:2562–2569. doi: 10.1093/jn/132.9.2562. [DOI] [PubMed] [Google Scholar]
- 42.Epstein M, Vaziri ND. Statins in the management of dyslipidemia associated with chronic kidney disease. Nat Rev Nephrol. 2012;8:214–223. doi: 10.1038/nrneph.2012.33. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal R. Statin induced proteinuria: renal injury or renoprotection? J Am Soc Nephrol. 2004;15:2502–2503. doi: 10.1097/01.ASN.0000143720.71748.79. [DOI] [PubMed] [Google Scholar]
- 44.Olbricht CJ, Wanner C, Thiery J, Basten A. Simvastatin in nephrotic syndrome. Simvastatin in Nephrotic Syndrome Study Group. Kidney Int Suppl. 1999;71:S113–S116. doi: 10.1046/j.1523-1755.1999.07128.x. [DOI] [PubMed] [Google Scholar]
- 45.Gheith OA, Sobh MA, Mohamed Kel-S, et al. Impact of treatment of dyslipidemia on renal function, fat deposits and scarring in patients with persistent nephrotic syndrome. Nephron. 2002;91:612–619. doi: 10.1159/000065021. [DOI] [PubMed] [Google Scholar]
- 46.Guan Y. Peroxisome proliferator-activated receptor family and its relationship to renal complications of the metabolic syndrome. J Am Soc Nephrol. 2004;15:2801–2815. doi: 10.1097/01.ASN.0000139067.83419.46. [DOI] [PubMed] [Google Scholar]
- 47.Hong Yu Ah, et al. Fenofibrate Improves Renal Lipotoxicity through Activation of AMPK-PGC-1α in db/db Mice. PLoS One. 2014;9:e96147. doi: 10.1371/journal.pone.0096147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hottelart C, El Esper N, Rose F, et al. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron. 2002;92:536–541. doi: 10.1159/000064083. [DOI] [PubMed] [Google Scholar]
- 50.Jun M, Zhu B, Tonelli M, et al. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2012;60:2061–2071. doi: 10.1016/j.jacc.2012.07.049. [DOI] [PubMed] [Google Scholar]
- 51.Kong X, Yuan H, Fan J, et al. Lipid-lowering agents for nephrotic syndrome. Cochrane Database Syst Rev 2013. [DOI] [PubMed]
- 52.Raina R, Krishnappa V. An update on LDL apheresis for nephrotic syndrome. Pediatr Nephrol. 2019;34:1655–1669. doi: 10.1007/s00467-018-4061-9. [DOI] [PubMed] [Google Scholar]
- 53.Muso E. Beneficial effect of LDL-apheresis in refractory nephrotic syndrome. Clin Exp Nephrol. 2014;18:286–290. doi: 10.1007/s10157-013-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muso E, Mune M, Hirano T, et al. A Prospective Observational Survey on the Long-Term Effect of LDL Apheresis on Drug-Resistant Nephrotic Syndrome. Nephron Extra. 2015;5:58–66. doi: 10.1159/000437338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pokhrel B, Yuet WC, Levine SN. PCSK9 Inhibitors. StatPearls 2020. [PubMed]
- 56.Kohli M, Patel K, MacMahon Z, et al. Pro-protein subtilisin kexin-9 (PCSK9) inhibition in practice: lipid clinic experience in 2 contrasting UK centres. Int J Clin Pract 2017. [DOI] [PubMed]
- 57.Awanami Y, Fukuda M, Nonaka Y, et al. Successful treatment of a patient with refractory nephrotic syndrome with PCSK9 inhibitors: a case report. BMC Nephrol. 2017;18:21. doi: 10.1186/s12882-017-0644-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jatem E, Lima Joan, Montoro B, et al. Efficacy and Safety of PCSK9 Inhibitors in Hypercholesterolemia Associated With Refractory Nephrotic Syndrome. Kidney Int Rep. 2021;6:101–109. doi: 10.1016/j.ekir.2020.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu HM, Tang JL, Cao L, et al. Interventions for preventing infection in nephrotic syndrome. Cochrane Database Syst Rev 2012. [DOI] [PMC free article] [PubMed]
- 61.Park SJ, Shin JI. Complications of nephrotic syndrome. Korean J Pediatr. 2011;54:322–328. doi: 10.3345/kjp.2011.54.8.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kemper MJ, Altrogge H, Ganschow R, Müller-Wiefel DE. Serum levels of immunoglobulins and IgG subclasses in steroid sensitive nephrotic syndrome. Pediatr Nephrol. 2002;17:413–417. doi: 10.1007/s00467-001-0817-7. [DOI] [PubMed] [Google Scholar]
- 63.Ogi M, Yokoyama H, Tomosugi N, et al. Risk factors for infection and immunoglobulin replacement therapy in adult nephrotic syndrome. Am J Kidney Dis. 1994;24:427–436. doi: 10.1016/s0272-6386(12)80899-7. [DOI] [PubMed] [Google Scholar]
- 64.Matsell DG, Wyatt RJ. The role of I and B in peritonitis associated with the nephrotic syndrome of childhood. Pediatr Res. 1993;34:84–88. doi: 10.1203/00006450-199307000-00019. [DOI] [PubMed] [Google Scholar]
- 66.Vaziri ND. Erythropoietin and transferrin metabolism in nephrotic syndrome. Am J Kidney Dis. 2001;38:1–8. doi: 10.1053/ajkd.2001.25174. [DOI] [PubMed] [Google Scholar]
- 67.Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338:1202–1211. doi: 10.1056/NEJM199804233381707. [DOI] [PubMed] [Google Scholar]
- 68.Iorember F, Aviles D. Anemia in nephrotic syndrome: approach to evaluation and treatment. Pediatr Nephrol. 2017;32:1323–1330. doi: 10.1007/s00467-016-3555-6. [DOI] [PubMed] [Google Scholar]