Abstract
Nasopharyngeal carcinoma (NPC) arises from the epithelial cells located in the nasopharynx and has a distinct geographic distribution. Chronic Epstein-Barr virus (EBV) infection, as its most common causative agents, can be detected in 100% of NPC types. In-depth studies of the cellular and molecular events leading to immunosuppression in NPC have revealed new therapeutic targets and diverse combinations that promise to benefit patients with highly refractory, advanced and metastatic NPC. This paper reviews the mechanisms by which NPC cells to circumvent immune surveillance and approaches being attempted to restore immunity. We integrate existing insights into anti-NPC immunity and molecular signaling pathways as well as targeting therapies in anticipation of broader applicability and effectiveness in advanced metastatic NPC.
Keywords: Nasopharyngeal carcinoma, Tumor microenvironment, Immune cell, Immunotherapy
Background
The stroma of NPC is densely infiltrated by immune cells and is therefore likely to elicit a specific adaptive antitumor response [1–4]. Conventional chemotherapy (IMRT singly or in combination with chemotherapy) is the standard treatment paradigm for early-stage NPC or locoregionally advanced NPC but is less effective in advanced recurrent or metastatic NPC (R/M NPC) due to nonspecific clinical signs and lack of early detection biomarkers [5]. Recently, immunotherapy in combination with chemotherapy is expected to be the first-line treatment option to extend progression-free survival of patients with R/M NPC [6, 7].
Recent single-cell sequencing delineates complex TME and crosstalk between cells. Persistent EBV infection decreases the immunogenicity of cancer cells and alters the tumor microenvironment [5, 8–10]. Although many factors that promote immune escape have been illuminated, treatment strategies to retrieve antitumor immunity have not been achieved. This review will systematically describe the ability of NPC to reprogram the TME through molecular and cellular interactions and shed light on the cutting-edge immunotherapies currently underway.
Immune landscape of EBV-NPC
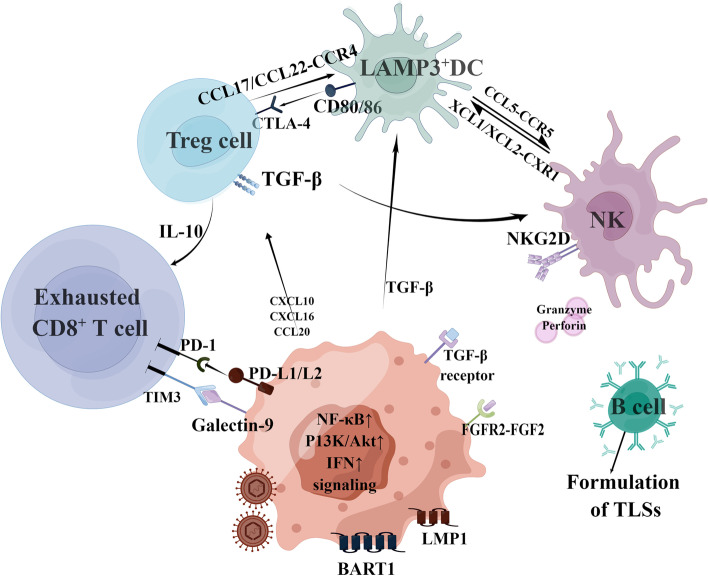
CD45 + immune cells existing in the NPC microenvironment mainly included T cells, B cells, natural killer cells (NK cells), and myeloid-derived suppressor cells (MDSCs) [11]. EBV in NPC cells exists in a type II latent state, encoding very limited non-coding RNAs and oncogenic proteins to reduce immunogenicity [12, 13], so lymphocytes fail to kill tumor cells despite the presence of massive infiltration in the tumor microenvironment (TME) (Fig. 1). Controlling persistent EBV infection and unlocking the potential of immunity requires induction of stable and effective T cell pools to prevent a recurrence. TCR analysis has confirmed the dynamic transition of T cells from activation to dysfunction [11], which is triggered early in tumor initiation, even before malignant transformation [14, 15]. IFN-induced genes are also upregulated by EBV infection, including ISG15, IFI6, IFI44L, and IFITM3. Long-term and sustained IFN activation may lead to T cell response failure [16, 17]——a mechanism that requires further investigations. EBV-infected tumor cells are usually resistant to NK cell surveillance. There have been many studies examining the role of NK cells on EBV-infected B cells, but for now, the studies on the interaction between NK cells and NPC cells is not well understood [18–20]. However, it is worth noting that tonsil NK cells may have an important effect on limiting EBV infection and subsequent carcinogenesis as the tonsils are the direct entry point for EBV [21].
Fig. 1.
Mechanisms of immune escape in nasopharyngeal carcinoma. EBV-encoding miRNA mediates the escape of the NK cell killing by down-regulating the expression of ligands to NKG2D. NPC cells also secrete factors such as TGF-β to inhibits the recruitment of APCs, and meanwhile, secret several chemokines to recruit immunosuppressive regulatory T cells (Treg). Tregs further inhibit the function of APCs through the binding of CTLA-4 to CD86 and releases suppressive cytokines such as IL-10 to activated effector T cells (Teff), prohibiting their cytotoxicity to NPC. Its expression of membrane-bound TGF-β also inhibits the action of NKs. NPC cells directly inhibit the action of Teff by expressing PD-L1 ligand, which induce T cell anergy upon binding to PD-1. Secretion of exosomes containing LMP1, miRNA, Galectin1 and Galectin 9 leads to disfunction of Teff and NK
Whole-exome sequencing studies have shown that misregulation of NF-κB signaling pathway is a major oncogenic driver of NPC. Besides, radiation-resistant nasopharyngeal carcinoma tissues often exhibit high levels of cancer-associated fibroblasts (CAFs), which produce substances that affect the biological behavior of nasopharyngeal carcinoma [22]. Fibroblast growth factor 2 (FGF2), secreted by CAFs, is the upstream molecule of the PI3K/AKT signal pathway. Aberrantly activated FGF signaling is involved in the accumulation of tumor-infiltrating MDSCs and M2-TAMs [23], remodeling the metabolism of NPC cells and promoting metastasis. Therefore, FGF2/FGFR2 may be a crucial target in NPC [24]. FGF/FGFR inhibitors promote further activation and infiltration of effector T cells into the TME by normalizing tumor vasculature. In this context, the combination of FGFR-targeted drugs with ICI may serve as an attractive therapeutic option to overcome to some extent the resistance to immunotherapy in NPC [25].
The increased B cells in NPC TME is conducive to the formulation of tertiary lymphoid structures (TLSs), in which B cells cooperate with T cells in maturation and activation [26, 27]. A high expression of B cell-related markers is associated with prolonged progression-free survival in NPC patients [11]. In addition, plasma cells are present within the TLSs, and deposits of many types of antibodies can be detected on tumor cells. TLSs have been found to produce an effective adaptive immune response like SLOs and are associated with longer survival in NPC patients [28]. Several approaches are being developed to induce TLSs formation using chemokines, cytokines, antibodies, antigen-presenting cells (APCs), or synthetic scaffolders [29].
Macrophages infiltrating the NPC microenvironment present an M1-M2 coupling paradigm and express both M1 and M2 markers [11]. Multicellular communication between macrophages and lymphocytes are prevalent but still unclear due to dynamic functional changes. Also, NPC cells can inhibit DCs maturation and IL-12 production, and promote the production of immunosuppressive factor IL-10 [30]. LAMP3 + DCs are highly mature DC cells that highly express suppressor proteins, produce multiple cytokines to recruit regulatory T cells (Tregs), and inhibit effector T cell functions [31]. The role of highly enriched MDSCs in cancer progression remains poorly understood. Functional analyses at both cellular level and animal level are necessary to clarify their modulatory potentials in NPC. Regulatory T cells (Tregs) will be recruited into the tumor microenvironment by substances secreted by the tumor and also assist the tumor to escape from immune surveillance. Tregs express immune checkpoint molecules in direct contact with other cells (e.g., LAMP3 + DCs), secret suppressive cytokines into the microenvironment, and also secret IDO to participate in metabolic reprogramming [32] (Fig. 1).
NPC crosstalk leading to immune escape
Direct cell–cell contact
-
i.
Expression of inhibitory ligands/receptors
Depleted T cells are characterized by overexpression of checkpoint molecules (e.g., PD-1, CTLA4, LAG3, TIM3, and TIGIT). Thus, depletion program might be elicited by EBV-mediated crosstalk with NPC cells [33–35]. EBER1 [36], miR-BART6-3p [37], circBART2.2 [38], and LMP1 [33] encoded by EBV activate the RIG-I pathway and upregulate PD-L1 expression on NPC, thus affecting T cell immune recognition and ultimately promoting immune escape. EBV-miR-BART11 and EBV-miR-BART17-3p promote the transcription of PD-L1 by targeting FOXP1 and PBRM1, respectively [39]. Immune checkpoint molecules expressed by NPC cells or immune cells constitute the rationale for the development of checkpoint inhibitor therapies, such as pembrolizumab, nivolumab, and camrelizumab, which are now used as standard second-line agents in the management of R/M NPC [7, 40]. Unfortunately, NPC cells can use alternative mechanisms, such as upregulating TIM-3 and LAG-3 to maintain immunosuppression [41, 42].
NKs can target EBV-infected cells that are in the lytic replication phase and maintain the homeostasis of the host immune system [43, 44]. A recent study found that LMP2A inhibited NK cell function through platelet aggregation by platelet factor 3, which offers a novel candidate for the development of NK cell immunotherapy [45]. EBV miRNAs have been confirmed to mediate the escape of NK cell killing by down-regulating NKG2D ligand and target pro-apoptotic proteins such as PUMA to resist NK cell cytotoxity [46–49]. Elevated pro-inflammatory cytokines IL-18 can also be mediated by EBV to induce PD-1 expression responsible for the functional exhaustion of NK cells [50].
-
ii.
HLA epitopes mutation
Somatic MHC class I gene aberrations occur in greater than one-third of EBV-associated NPC [51], which may allow these NPC cells to evade the immune response [52]. Moreover, these alterations were significantly associated with prognostic indicators of patients. MHC class II gene alterations may also mediate the immune escape in NPC [53]. The mechanism by which MHC-I or MHC-II aberrations or both directly contribute to immune evasion remains to be determined. EBV-encoding miRNAs impair MHC class I/II- restricted antigen presentation [54] and reduce surface expression of HLA-B molecules, which are major MHC-I restriction elements of EBV-specific CD8 + T cell responses [55]. LMP2A also downregulates HLA-A/B/C and MIC-A/B expression through promoter hypermethylation, thereby inhibiting T and NK cell responses [56].
Indirect interactions between cells
-
i.
Exosomes
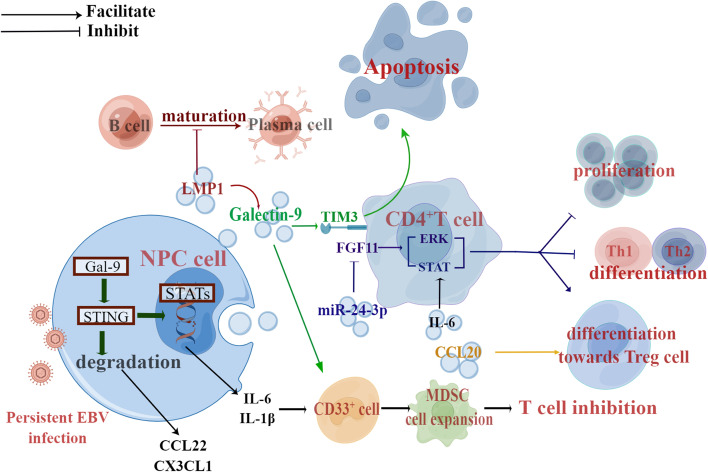
Exosomes can play a key role as messengers to mediate cellular communication and deliver components to receptor cells. NPC cells indoctrinate exosomes (NPC-Exo) containing LMP1, and miRNAs to the TME, resulting in immune escape from host surveillance [32, 57] (Fig. 2). Mrizak et al. first identified the unique immunomodulatory ability of NPC-Exos to recruit Tregs into the microenvironment via the chemokine CCL20 and to recruit conventional CD4 + T cells and induce their conversion to inhibitory Tregs [32]. Exosomal LMP1 effectively activates oncogenic NF-kB and MAPK/Akt signaling [58, 59] and leads to T cell anergy [60]. They also severely inhibit the maturation of B lymphocytes into plasma cells [61]. In addition, exosomal LMP1 activates CAFs and then promotes tumor progression through stroma-tumor inter-relationships [62]. IL-6 is an inflammatory factor that promotes the occurrence of tumors. NPC-Exos show a more robust ability to induce IL-6 secretion from macrophages than exosomes derived from normal epithelial cells, and this difference is likely due to the different cargoes loaded [63].
Fig. 2.
Pathways of exosomes involved in NPC immunosuppression. NPC affects the activities of immune effector cells by secreting exosomes to maintain persistent EBV infection and cause immunosuppression
The microenvironment characterized by hypoxia increases the level of exosomal miR-24-3p in NPC cells, thus enhancing the inhibitory effect on T cell proliferation and differentiation, and causing Treg-mediated suppression though inhibition of FGF11 [64]. In addition, MHC-I MICA and MICB ligands are likely to be removed by tumor cells into exosomes to evade recognition and clearance by NKG2D-activating receptor on natural killer cells and patrolling cytotoxic CD8 + T cells [65]. Understanding the specific substances that tumor cells select into exosomes can help reveal the mechanisms of immune evasion. As for NPC-lncRNAs, lncRNA-LINC00460 and lncRNA PVT1 have been involved in glucose metabolism and susceptibility to T cell-mediated lysis of NPC cells [66–68]. LncRNA TP73-AS1 is carried from NPC cells by extracellular vesicles and is associated with the polarization of macrophage toward the M2 phenotype [69]. TP73-AS1 is associated with a variety of cancers, so presumably, TP73-AS1 may be an NPC potential target [70–72].
Exosomal Galectin-9 produced by NPC cells stimulates the differentiation of MDSC by inhibiting STING signaling pathway and then upregulating a subset of cytokines, such as IL-1, IL-6, CX3CL1 and CCL22 [73–75]. Moreover, it can affect NK cell control of EBV and participate in M2 polarization of macrophages [76–78]. The binding of Galectin-9 to TIM-3 induces apoptosis in tumor cells, but for those PD-1-positive cells, PD-1 may inhibit this process. This is a novel mechanism of PD-1 that leads to exhausted T cell persistence [79]. High levels of soluble and exosomal Galectin-9 correlate with a higher risk of recurrence in NPC [80]. Unlocking more biological functions of the Galectin-9 will enable us to identify the its possibilities as a circulating marker and as a new target for immune checkpoint inhibitor therapy.
Recently, PD-L1 has been reported to be highly expressed in tumor-derived exosomes (Exo-PD-L1) that can directly contact T cells [81, 82]. Moreover, Exo-PD-L1 will suppress distant (e.g., spleen) T cells or even circulating T cells. It has been considered as a biomarker for HNSCC disease progression and clinical staging [83], as well as a predictor of immunotherapy [84]. It’s reasonable to assume that in addition to EBV DNA, PD-L1 in exosomes also has predictive value as a liquid biopsy method. Notably, Exo-PD-L1 can be secreted in large amounts by irradiated tumor cells, which suggests that the immunomodulatory effects of tumor-derived exosomes in response to radiotherapy and a synergistic therapeutic effect of radiotherapy and immune checkpoint inhibitors for R/M NPC [85]. Future work should identify more co-expressing molecules on PD-L1-positve exosomes to better predict the immunotherapy for NPC and other HNSCCs.
-
ii.
Cytokines and chemokines
Cytokines such as IL-6, IL-8, IP-10, TNF-α, VEGF, EGFR, and MIP-3α were found to be elevated in nasopharyngeal carcinoma [86–88]. NPC cells induce macrophage polarization toward an M2-like phenotype by secreting ISG15 [42]. In addition, EBV-infected NPCs undermine the immune response by the simulation of regulatory T cells that secrete IL-10, which inhibits T cell and NK cell infiltration and IFN-γ secretion [89]. LMP1 regulates the production of such chemokines as CXCL9, CXCL10, CX3CL1, and CCL20 through constitutive activation of the NF-κB pathway, leading to T lymphocyte infiltration, but they also inhibit cytotoxic T cells through PD-L2/PD-1 interactions [87, 90]. LMP1 also contributes to MDSC expansion through metabolic reprogramming leading to the release of IL-1β, IL-6, and GM-CSF [91]. IL-6 and TNF-α can be secreted by viral oncoproteins produced by NPC. It was found that baseline levels of IL-6 and TNF-α were adversely correlated with 2-year survival in NPC patients, which may be mediated by the immunosuppressive effects of IDO [92].
-
iii.
Metabolic mediators
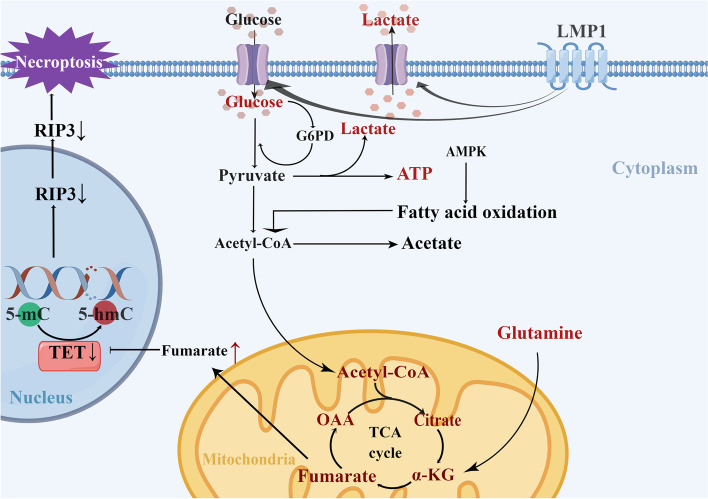
There are a growing number of studies on metabolic reprogramming and immune evasion of NPC cells, but the majority have focused only on one side of the story. Regarding the competition for nutrients, NPC cells have a greater competitive advantage over immune cells for glucose, thus inhibiting the activity of immune cells [93, 94] (Fig. 3). LMP1 activates multiple cellular signaling pathways in NPC cells [95], among which the FGF/FGFR signaling pathway is activated to increase glucose and glutamine uptake, LDHA activity, lactate generation, and the level of hypoxia-inducible factor 1 (HIF-1α) [96]. Interestingly, glutamine deprivation enhanced glucose uptake by tumor cells, thereby reducing the amount of glucose available to immune cells, suggesting that glutamine may be the limiting factor in TME, rather than glucose. Selective cellular partitioning of nutrients could be used to develop novel therapies that target specific cell types as well as more accurate imaging strategies [97].
Fig. 3.
Aerobic glycolysis in NPC cells. LMP1 secreted by EBV enhances glucose consumption and lactate generation in NPC cells. Increased glucose consumption in the tumor cells leads to immune cell starvation. EBV (LMP1) leads to the accumulation of fumarate in the tricarboxylic acid cycle (TCA), which inhibits the expression of RIP3 and protects NPC cells from TNF-induced necrotizing apoptosis. GLUT1: glucose transporter 1, OAA: Oxaloacetic acid, a-KG: a–ketoglutarate, LDH: lactate dehydrogenase, G6PD: glucose-6-phosphate dehydrogenase
Given the high metabolic rate of cancer cells, lactic acid secreted by tumors is an important metabolite in TME [98]. High concentrations of lactate interfere with the metabolism of immune effector cells, promote conversion to an immunosuppressive phenotype, inhibit the release of pro-inflammatory cytokines, and activate CAFs to produce VEGF to stimulate angiogenesis [99–102]. Similarly, lipid metabolism also affects T cell function and the expression of inhibitory receptors [103]. Fatty acids (FAs) have been reported to facilitate the malignant behavior of tumor cells [104, 105]. Virus-encoded proteins reprogram cellular metabolism and promote the optimal generation of progeny virion generation [106]. The LMP1 leads to the accumulation of the metabolite fumaric acid, inhibiting the expression of RIP3 and protecting NPC cells from TNF-induced necrosis secreted by macrophages [107]. Targeting lipid metabolism may be a novel idea worthy of further study in the treatment of NPC.
Other oncological metabolites such as adenosine, kynurenine, and tryptophan can affect tumor immunity. Elevated HIF enhances the expression of external nucleosidase (CD39 and CD37), which convert ATP to adenosine, a metabolite of immunosuppressant mediators. Extracellular adenosine attenuates T cell adhesion and cytotoxicity through A2a and A3 adenosine receptors [108, 109]. IDO is highly expressed in NPC, and as previously mentioned, IDO is an immunosuppressive metabolite that catabolizes intracellular tryptophan to produce kynurenine. The increased kynurenine in the microenvironment acts on the AHR signaling axis of the naïve T cells, causing them to differentiate into Tregs [110]. In addition, IDO can directly activate Treg cells via the PD-1/PD-L1 binding. There are clinical trials actively investigating IDO inhibitors in combination with immunotherapy (NCT03823131) or other chemotherapies [111]. Other metabolic inhibitors such as 3-bromopyruvate and oxalate inhibit hexokinase (HK) and LHDA activity and activate macrophage and T cell in animal models including NPC [112, 113]. Therefore, the combination of small molecule drugs or antibody intervention drugs that target metabolic processes with immunotherapy agents may become a potential clinical choice.
Augmenting or rescuing immunity in NPC
The combination of radiotherapy and chemotherapy (gemcitabine, cisplatin, and fluorouracil) is the conventional treatment paradigm for NPC but face obstacles in treating locoregional advanced or metastatic NPC. It is urgent to seek more effective strategies with greater efficacy and less toxicity [114, 115]. Several approaches including checkpoint inhibitors and cell-based immunotherapy have been used to revitalize the depleted immune cells in the NPC TME (Table 1).
Table 1.
Immunotherapies for NPC treatment
| Target | Effects | Treatment | NCT number |
|---|---|---|---|
| PD-1 | Bypass immune checkpoint | Nivolumab, Pembrolizumab, Toripalimab, Penpulimab, Camrelizumab, Sintilimab, Tislelizumab, Treprilimab, SHR-1701 | NCT02054806 [7], NCT02339558 [40], NCT02915432 [116], NCT03581786 [6], NCT03558191, NCT03267498, NCT03544099, NCT03734809, NCT03707509, NCT03925090, NCT03924986, NCT04282070, NCT04376866, NCT04447612, NCT04421469, NCT04534855, NCT04736810, NCT04833257, NCT04917770, NCT04944914, NCT04978012, NCT3707509, NCT05020925, NCT05097209, NCT05341193 |
| PD-L1 | Bypass immune checkpoint | SHR-1701, Avelumab | NCT04282070, NCT05020925, NCT04562441 |
| CTLA-4 & PD-1 | Bypass immune checkpoint | Ipilimumab, AK104, IBI310 | NCT03097939, NCT04220307, NCT04945421 |
| LMP2 | Direct cytotoxicity | LMP2 Antigen-specific TCR T cell | NCT03925896 |
| EBV | Direct cytotoxicity | Autologous EBV specific Cytotoxic T cell | NCT03648697, NCT02287311, NCT02578641 [117] |
| TGF-β | Direct cytotoxicity | TGF-β Resistant CTLs | NCT02065362 |
Abbreviations: CTLA-4 Cytotoxic T-lymphocyte antigen 4, PD-1 Programmed death 1, PD-L1 Programmed death ligand 1, CAR Chimeric antigen receptor
Checkpoint inhibitors
Checkpoint inhibitors act on chronically stimulated T cells to reverse the fixed “exhaustion” state. About one-third of NPC cells and half of immune cells express PD-L1 [118, 119], and the difference in expression ratios among patients correlated strongly with the clinical effect of anti-PD-1/PD-L1 immunotherapy. NCI-9742 is the first completed report on the clinical response of Nivolumab in R/M NPC patients with an ORR of 20.5% and a 1-year OS rate superior to historic data in similar populations [40]. Like Nivolumab, pembrolizumab demonstrated a favorable antitumor activity in R/M NPC patients [7]. However, more impressive is the median PFS of 11.7 months with the addition of Toripalimab to GP chemotherapy as a first-line treatment for R/M NPC patients [6]. RATIONAL-309 studies (NCT03924986, Phase III) also showed that by April 2022, Tislelizumab plus GP significantly extended the median PFS of R/M NPC patients compared to placebo plus GP (NR vs 13.9 months) after a follow-up of 15.5 months. Currently, there are lots of clinical trials testing checkpoint inhibitors alone or in combination with chemotherapy, radiotherapy and other immunotherapies (Table 1).
Another predominant regulatory checkpoint is TIM3. Inhibition of its ligand, Galectin-9, selectively revigorated infiltrating T lymphocytes by interfering with the interaction between PD-1 and TIM3 [79]. As Galectin-9 is specifically expressed by NPC cells, the TIM3/Galectin-9 interaction represents a promising approach to overcoming the resistance to the PD-1/PD-L1 inhibitors. The checkpoint receptor found on the surface of Tregs, CTLA4, binds CD80/86 on DCs to inhibit antigen presentation and secret IDO1 to induce the Treg proliferation [31]. The efficacy of the combination of ipilimumab/nivolumab in NPC are currently under investigation in phase II clinical trial (NCT03097939). Preliminary data up to November 2020 showed a partial response rate of 35% with a median response duration of 5.9 months, synergistically enhancing the efficacy of PD-1 monotherapy. Other therapeutic targets, such as LAG3, TIM3, and TIGIT, are currently immature compared to PD-1, but it is reasonable to assume that combining these molecules would be a more effective treatment option for patients with R/M NPC.
Expanding EBV/NPC-specific immune cells
An ongoing Phase III study (NCT02578641) using chemotherapy (Gemcitable & Carboplatin) followed by autologous, vitro-expanded EBV-specific cytotoxic T cell (EBVST) as first-line treatments for R/M NPC patients achieves a promising result with an overall response rate of 71.4% [117]. The individuals who received EBVST immunotherapy but showed no benefit were due to the expansion of mMDSCs leading to a dominant immune-suppressive effect, which points to a window of therapeutic opportunity [120]. Similar studies are being conducted using adoptive cellular-based immunotherapies such as EBV-TCR-T (NCT03648697) and LMP, BARF1 & EBNA1 Specific CTL (NCT02287311).
Other emerging cellular immunotherapy strategies, such as chimeric antigen receptor NK-Cell Immunotherapy (CAR-NK) and CAR-Macrophages (CAR-M), should be evaluated in NPC for their promising clinical efficacy in other cancers [121, 122]. Cancer vaccine, on the other hand, represents active immunotherapy that delivers tumor-specific antigens through APCs or viral vectors to improve immune system recognition. DCs expressing LMP2 and recombinant modified vaccinia Ankara vaccine (MVA) have been shown to be safe and well-tolerated [123]. Customized therapeutic vaccines can also be augmented by checkpoint inhibitor therapies [124]. Hence, future studies could consider combination immunotherapy to enhance the clinical responses, for example, immune checkpoint blockade combined with adoptive T-cell therapy [125, 126] or combining CAR T cells therapy combined with Ankara-oncolytic virus [127].
Targeting exosomes
There is substantial clinical evidence that exosomes can be used as biomarkers of disease, and a number of drugs have been developed to reduce the secretion of exosomes released by cancer cell and their effect on immune cells, such as RAB27A inhibitors, nSMase inhibitors, PPIs and calcium channel blockers [128]. Some of these inhibitors only affect exosomes released by tumor cells, which provides ideas for inhibiting the release of NPC-Exos. One of the most significant characteristics of NPC-Exos is the high level of miRNA content that governs the reprogramming of immune active factors and immune cell functions [129]. The use of exosome inhibitors or corresponding miRNA inhibitors might be able to block their effect of NPC cells on immune cells [130].
Besides focusing on NPC-Exos, exosomes secreted by immune cells can also serve as powerful anti-cancer weapons, such as NK-derived exosomes and Dendric cell exosomes [131, 132]. In particular, exosomes derived from phosphor-antigen-expanded Vδ2-T cells can kill EBV-infected cells more efficiently [133]. In recent years, vaccines targeting exosomes have also been exploited, for example, dual-acting exosome vaccines for melanoma and lung cancer have showen the effectiveness in mouse models. However, exosomal vaccines have not been specifically tested in NPC [134]. But there are already many EBV vaccines that can be genetically engineered by exosomes to express specific antigens or target tumor cells to increase immunogenicity [135].
Targeting cytokine and chemokine
Immunomodulatory compounds such as cytokines and chemokines are being employed to reshape the tumor immune microenvironment. EBV-NPC cells produce high levels of TGF-β to promote a more aggressive malignant phenotype. TGF-β signaling is a crucial mediator not only of suppressive phenotypic transformation of tumor-infiltrating lymphocytes but also of remodulation in the stromal environment [136–138]. Given the axial role of the TGF-β signaling pathway in tumorigenesis, it can be regarded as an attractive target for EBV-NPC therapy. Currently, a clinical trial targeting TGF-β resistant T cells is being explored (NCT02065362).
Many NPC patients are accompanied by high EGFR expression, which often predicts an aggressive phenotype. LMP-1 can induce the expression and activation of EGFR and its ligand TGF-α in epithelial cells. Numerous studies have indicated that EGFR signaling plays a vital role in NPC pathogenesis [139–145]. LMP1 can also activate the insulin-like growth factor 1 receptor (IGF1R), which promotes epithelial cell carcinogenesis and cause excessive anti-autophagic signaling to suppress anticancer immunosurveillance. It may constitute a druggable target for NPC in combination with chemo-immunotherapies [146, 147]. Altogether, further investigations on molecular targets are warranted for the treatment and management of NPC.
Conclusions
A deeper understanding of the crosstalk between the immune system and NPC that leads to suppression of anti-tumor response will greatly aid in developing more effective therapies. The identification of cytokines, immunosuppressive cell subpopulations and checkpoint pathways has allowed many immunotherapeutic drugs to be tested in clinical trials and hopefully used in clinical practice. The heterogeneity and complexity of advanced NPCs undoubtedly require a combination of these drugs to establish durable, lifelong immunity. In addition, consideration must be given to the optimal dose needed to establish durable tumor control while minimizing adverse events. Also, the unique characteristics of each NPC patient must be determined before treatment to select a balanced combination that maximizes PFS and minimizes toxicity.
Acknowledgements
We would like to thank Figdraw (www.figdraw.com) for its help in creating the figures.
Abbreviations
- R/M NPC
Recurrent or metastatic NPC
- EBV
Epstein-Barr virus
- NK cells
Natural killer cells
- MDSCs
Myeloid-derived suppressor cells
- TME
Tumor microenvironment
- ISG15
Interferon stimulating gene 15
- IFI6
Interferon-inducible-protein 6
- IFI44L
Interferon-inducible-protein 44
- IFITM3
Interferon—induced transmembrane protein 3
- FGF
Fibroblast growth factor
- TLSs
Tertiary lymphoid structures
- APCs
Antigen-presenting cells
- CAFs
Cancer-associated fibroblasts
- PD-1/PD-L1
Programmed death-1/programmed death ligand-1
- CTLA4
Cytotoxic T-lymphocyte antigen 4
- TIM3
T cell immunoglobulin domain and mucin domain-3
- LAG3
Lymphocytes activating gene 3
- TIGIT
T cell immunoreceptor with Ig and ITIM domains
- miRNAs
MicroRNAs
- lncRNA
Long non-coding RNA
- HLA
Human leucocyte antigen
- MHC
Major histocompatibility complex
- MIC
MHC class I molecular associated protein A/B
- HIF-1
Hypoxia-inducible factor-1
- EBNA
Epstein-Barr virus nuclear antigen
- LMP
Latent membrane protein
- FAs
Fatty acids
- RIP3
Receptor interacting protein 3
- IP-10
Interferon-inducible protein-10
- MIP-3α
Macrophage inflammatory protein-3α
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
Authors’ contributions
JYJ drafted the manuscript and prepared the manuscript for submission. HMY conceived this work. All authors read and approved the final draft.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. Figures were created by Figdraw (www.figdraw.com).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jianyun Jiang, Email: jjy2019531@163.com.
Hongmei Ying, Email: yinghongmei2013@163.com.
References
- 1.Li YY, Chung GTY, Lui VWY, To K-F, Ma BBY, Chow C, Woo JKS, Yip KY, Seo J, Hui EP, et al. Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nat Commun. 2017;8:14121. doi: 10.1038/ncomms14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin D-C, Meng X, Hazawa M, Nagata Y, Varela AM, Xu L, Sato Y, Liu L-Z, Ding L-W, Sharma A, et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genet. 2014;46(8):866–871. doi: 10.1038/ng.3006. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y-P, Yin J-H, Li W-F, Li H-J, Chen D-P, Zhang C-J, Lv J-W, Wang Y-Q, Li X-M, Li J-Y, et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res. 2020;30(11):1024–1042. doi: 10.1038/s41422-020-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng H, Dai W, Cheung AKL, Ko JMY, Kan R, Wong BWY, Leong MML, Deng M, Kwok TCT, Chan JY-W, et al. Whole-exome sequencing identifies multiple loss-of-function mutations of NF-κB pathway regulators in nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 2016;113(40):11283–11288. doi: 10.1073/pnas.1607606113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y-P, Chan ATC, Le Q-T, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet (London, England) 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 6.Mai H-Q, Chen Q-Y, Chen D, Hu C, Yang K, Wen J, Li J, Shi Y-R, Jin F, Xu R, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27(9):1536–1543. doi: 10.1038/s41591-021-01444-0. [DOI] [PubMed] [Google Scholar]
- 7.Hsu C, Lee S-H, Ejadi S, Even C, Cohen RB, Le Tourneau C, Mehnert JM, Algazi A, van Brummelen EMJ, Saraf S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35(36):4050–4056. doi: 10.1200/JCO.2017.73.3675. [DOI] [PubMed] [Google Scholar]
- 8.Damania B, Münz C. Immunodeficiencies that predispose to pathologies by human oncogenic γ-herpesviruses. FEMS Microbiol Rev. 2019;43(2):181–192. doi: 10.1093/femsre/fuy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangye SG, Latour S. Primary immunodeficiencies reveal the molecular requirements for effective host defense against EBV infection. Blood. 2020;135(9):644–655. doi: 10.1182/blood.2019000928. [DOI] [PubMed] [Google Scholar]
- 10.Chan KCA, Woo JKS, King A, Zee BCY, Lam WKJ, Chan SL, Chu SWI, Mak C, Tse IOL, Leung SYM, et al. Analysis of plasma epstein-barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377(6):513–522. doi: 10.1056/NEJMoa1701717. [DOI] [PubMed] [Google Scholar]
- 11.Gong L, Kwong DL-W, Dai W, Wu P, Li S, Yan Q, Zhang Y, Zhang B, Fang X, Liu L, et al. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nat Commun. 2021;12(1):1540. doi: 10.1038/s41467-021-21795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 13.Tsao SW, Yip YL, Tsang CM, Pang PS, Lau VMY, Zhang G, Lo KW. Etiological factors of nasopharyngeal carcinoma. Oral Oncol. 2014;50(5):330–338. doi: 10.1016/j.oraloncology.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545(7655):452–456. doi: 10.1038/nature22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Swiecki M, Cella M, Alber G, Schreiber RD, Gilfillan S, Colonna M. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe. 2012;11(6):631–642. doi: 10.1016/j.chom.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat P, Leggatt G, Waterhouse N, Frazer IH. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017;8(6):e2836. doi: 10.1038/cddis.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams LR, Quinn LL, Rowe M, Zuo J. Induction of the lytic cycle sensitizes epstein-barr virus-infected B cells to NK cell killing that is counteracted by virus-mediated NK cell evasion mechanisms in the late lytic cycle. J Virol. 2016;90(2):947–958. doi: 10.1128/JVI.01932-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jochum S, Moosmann A, Lang S, Hammerschmidt W, Zeidler R. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog. 2012;8(5):e1002704. doi: 10.1371/journal.ppat.1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Montanes M, Alari-Pahissa E, Sintes J, Martinez-Rodriguez JE, Muntasell A, Lopez-Botet M. Antibody-dependent NK cell activation differentially targets EBV-infected cells in lytic cycle and bystander B lymphocytes bound to viral antigen-containing particles. J Immunol. 2017;199(2):656–665. doi: 10.4049/jimmunol.1601574. [DOI] [PubMed] [Google Scholar]
- 21.Jud A, Kotur M, Berger C, Gysin C, Nadal D, Lunemann A. Tonsillar CD56brightNKG2A+ NK cells restrict primary epstein-barr virus infection in B cells via IFN-gamma. Oncotarget. 2017;8(4):6130–6141. doi: 10.18632/oncotarget.14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W, Zhang L, Yang M, Wu X, Wang X, Huang W, Yuan L, Pan H, Wang Y, Wang Z, et al. Cancer-associated fibroblasts promote the survival of irradiated nasopharyngeal carcinoma cells via the NF-κB pathway. J Exp Clin Cancer Res. 2021;40(1):87. doi: 10.1186/s13046-021-01878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol. 2019;16(2):105–122. doi: 10.1038/s41571-018-0115-y. [DOI] [PubMed] [Google Scholar]
- 24.Tay JK, Zhu C, Shin JH, Zhu SX, Varma S, Foley JW, Vennam S, Yip YL, Goh CK, Wang DY, et al. The microdissected gene expression landscape of nasopharyngeal cancer reveals vulnerabilities in FGF and noncanonical NF-κB signaling. Sci Adv. 2022;8(14):eabh2445. doi: 10.1126/sciadv.abh2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y-J, Zhou R, Zong J-F, Lin W-S, Tong S, Guo Q-J, Lin C, Lin S-J, Chen Y-X, Chen M-R, et al. Epstein-barr virus-coded miR-BART13 promotes nasopharyngeal carcinoma cell growth and metastasis via targeting of the NKIRAS2/NF-κB pathway. Cancer Lett. 2019;447:33–40. doi: 10.1016/j.canlet.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshitomi H, Kobayashi S, Miyagawa-Hayashino A, Okahata A, Doi K, Nishitani K, Murata K, Ito H, Tsuruyama T, Haga H, et al. Human Sox4 facilitates the development of CXCL13-producing helper T cells in inflammatory environments. Nat Commun. 2018;9(1):3762. doi: 10.1038/s41467-018-06187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J-P, Wu C-Y, Chen M-Y, Liu S-X, Yan S-M, Kang Y-F, Sun C, Grandis JR, Zeng M-S, Zhong Q: PD-1CXCR5CD4 Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J Immunother Can. 2021;9(7):e002101. [DOI] [PMC free article] [PubMed]
- 29.Johansson-Percival A, He B, Li Z-J, Kjellén A, Russell K, Li J, Larma I, Ganss R. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol. 2017;18(11):1207–1217. doi: 10.1038/ni.3836. [DOI] [PubMed] [Google Scholar]
- 30.Chao P-Z, Hsieh M-S, Cheng C-W, Hsu T-J, Lin Y-T, Lai C-H, Liao C-C, Chen W-Y, Leung T-K, Lee F-P, et al. Dendritic cells respond to nasopharygeal carcinoma cells through annexin A2-recognizing DC-SIGN. Oncotarget. 2015;6(1):159–170. doi: 10.18632/oncotarget.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, He S, Wang XL, Peng W, Chen QY, Chi DM, Chen JR, Han BW, Lin GW, Li YQ, et al. Tumour heterogeneity and intercellular networks of nasopharyngeal carcinoma at single cell resolution. Nat Commun. 2021;12(1):741. doi: 10.1038/s41467-021-21043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mrizak D, Martin N, Barjon C, Jimenez-Pailhes A-S, Mustapha R, Niki T, Guigay J, Pancré V, de Launoit Y, Busson P, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2015;107(1):363. doi: 10.1093/jnci/dju363. [DOI] [PubMed] [Google Scholar]
- 33.Bi X-W, Wang H, Zhang W-W, Wang J-H, Liu W-J, Xia Z-J, Huang H-Q, Jiang W-Q, Zhang Y-J, Wang L. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9(1):109. doi: 10.1186/s13045-016-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huo S, Luo Y, Deng R, Liu X, Wang J, Wang L, Zhang B, Wang F, Lu J, Li X: EBV-EBNA1 constructs an immunosuppressive microenvironment for nasopharyngeal carcinoma by promoting the chemoattraction of Treg cells. J Immunother Can. 2020;8(2):e001588. [DOI] [PMC free article] [PubMed]
- 35.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng S, Li Z, He J, Fu S, Duan Y, Zhou Q, Yan Y, Liu X, Liu L, Feng C, et al. Epstein-Barr virus noncoding RNAs from the extracellular vesicles of nasopharyngeal carcinoma (NPC) cells promote angiogenesis via TLR3/RIG-I-mediated VCAM-1 expression. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1201–1213. doi: 10.1016/j.bbadis.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Qin Z, Wang J, Zheng X, Lu J, Zhang X, Wei L, Peng Q, Zheng Y, Ou C, et al. Epstein-Barr Virus miR-BART6-3p Inhibits the RIG-I Pathway. J Innate Immun. 2017;9(6):574–586. doi: 10.1159/000479749. [DOI] [PubMed] [Google Scholar]
- 38.Ge J, Wang J, Xiong F, Jiang X, Zhu K, Wang Y, Mo Y, Gong Z, Zhang S, He Y, et al. Epstein-barr virus-encoded circular RNA CircBART2.2 promotes immune escape of nasopharyngeal carcinoma by regulating PD-L1. Cancer Res. 2021;81(19):5074–5088. doi: 10.1158/0008-5472.CAN-20-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon JW, Kong S-K, Kim BS, Kim HJ, Lim H, Noh K, Kim Y, Choi J-W, Lee J-H, Kim Y-S. IFNγ induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci Rep. 2017;7(1):17810. doi: 10.1038/s41598-017-18132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma BBY, Lim W-T, Goh B-C, Hui EP, Lo K-W, Pettinger A, Foster NR, Riess JW, Agulnik M, Chang AYC, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742) J Clin Oncol. 2018;36(14):1412–1418. doi: 10.1200/JCO.2017.77.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan JK. Virus-associated neoplasms of the nasopharynx and sinonasal tract: diagnostic problems. Mod Pathol. 2017;30(s1):S68–S83. doi: 10.1038/modpathol.2016.189. [DOI] [PubMed] [Google Scholar]
- 42.Jin S, Li R, Chen M, Yu C, Tang L, Liu Y, Li J, Liu Y, Luo Y, Zhao Y et al: Single-cell transcriptomic analysis defines the interplay between tumor cells, viral infection, and the microenvironment in nasopharyngeal carcinoma. Cell Res. 2020;30(11):950–65. [DOI] [PMC free article] [PubMed]
- 43.Chijioke O, Muller A, Feederle R, Barros MH, Krieg C, Emmel V, Marcenaro E, Leung CS, Antsiferova O, Landtwing V, et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013;5(6):1489–1498. doi: 10.1016/j.celrep.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132(3):515–525. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan X, Chen H, Zhou X, Liu P, Zhang X, Zhu Q, Zhong L, Zhang W, Zhang S, Zhang X, et al. EBV infection in epithelial malignancies induces resistance to antitumor natural killer cells via F3-mediated platelet aggregation. Can Res. 2022;82(6):1070–1083. doi: 10.1158/0008-5472.CAN-21-2292. [DOI] [PubMed] [Google Scholar]
- 46.Wong TS, Chen S, Zhang MJ, Chan JY, Gao W. Epstein-Barr virus-encoded microRNA BART7 downregulates major histocompatibility complex class I chain-related peptide a and reduces the cytotoxicity of natural killer cells to nasopharyngeal carcinoma. Oncol Lett. 2018;16(3):2887–2892. doi: 10.3892/ol.2018.9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan C, Tang Y, Wang J, Xiong F, Guo C, Wang Y, Xiang B, Zhou M, Li X, Wu X, et al. The emerging role of epstein-barr virus encoded microRNAs in nasopharyngeal carcinoma. J Cancer. 2018;9(16):2852–2864. doi: 10.7150/jca.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe. 2009;5(4):376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Choy EY, Siu KL, Kok KH, Lung RW, Tsang CM, To KF, Kwong DL, Tsao SW, Jin DY. An epstein-barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med. 2008;205(11):2551–2560. doi: 10.1084/jem.20072581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liou AK, Soon G, Tan L, Peng Y, Cher BM, Goh BC, Wang S, Lim CM. Elevated IL18 levels in nasopharyngeal carcinoma induced PD-1 expression on NK cells in TILS leading to poor prognosis. Oral Oncol. 2020;104:104616. doi: 10.1016/j.oraloncology.2020.104616. [DOI] [PubMed] [Google Scholar]
- 51.Li YY, Chung GT, Lui VW, To KF, Ma BB, Chow C, Woo JK, Yip KY, Seo J, Hui EP, et al. Exome and genome sequencing of nasopharynx cancer identifies NF-kappaB pathway activating mutations. Nat Commun. 2017;8:14121. doi: 10.1038/ncomms14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai W, Zheng H, Cheung AKL, Tang CS-m, Ko JMY, Wong BWY, Leong MML, Sham PC, Cheung F, Kwong DL-W, et al. Whole-exome sequencing identifies MST1R as a genetic susceptibility gene in nasopharyngeal carcinoma. Proc Natl Acad Sci USA. 2016;113(12):3317–3322. doi: 10.1073/pnas.1523436113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao Y, Minter HA, Chen X, Reynolds GM, Bromley M, Arrand JR. Heterogeneity of HLA and EBER expression in epstein-barr virus-associated nasopharyngeal carcinoma. Int J Cancer. 2000;88(6):949–955. doi: 10.1002/1097-0215(20001215)88:6<949::AID-IJC18>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Albanese M, Tagawa T, Bouvet M, Maliqi L, Lutter D, Hoser J, Hastreiter M, Hayes M, Sugden B, Martin L, et al. Epstein-barr virus microRNAs reduce immune surveillance by virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2016;113(42):E6467–E6475. doi: 10.1073/pnas.1605884113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of epstein-barr virus-induced disease. Annu Rev Immunol. 2015;33:787–821. doi: 10.1146/annurev-immunol-032414-112326. [DOI] [PubMed] [Google Scholar]
- 56.Singh S, Banerjee S. Downregulation of HLA-ABC expression through promoter hypermethylation and downmodulation of MIC-A/B surface expression in LMP2A-positive epithelial carcinoma cell lines. Sci Rep. 2020;10(1):5415. doi: 10.1038/s41598-020-62081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Investig. 2016;126(4):1216–1223. doi: 10.1172/JCI81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verweij FJ, de Heus C, Kroeze S, Cai H, Kieff E, Piersma SR, Jimenez CR, Middeldorp JM, Pegtel DM. Exosomal sorting of the viral oncoprotein LMP1 is restrained by TRAF2 association at signalling endosomes. J Extracell Vesicles. 2015;4:26334. doi: 10.3402/jev.v4.26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meckes DG, Shair KHY, Marquitz AR, Kung C-P, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci USA. 2010;107(47):20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, Yoshizaki T, Pagano JS, Shackelford J. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33(37):4613–4622. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai C-Y, Sakakibara S, Yasui T, Minamitani T, Okuzaki D, Kikutani H. Bystander inhibition of humoral immune responses by epstein-barr virus LMP1. Int Immunol. 2018;30(12):579–590. doi: 10.1093/intimm/dxy053. [DOI] [PubMed] [Google Scholar]
- 62.Wu X, Zhou Z, Xu S, Liao C, Chen X, Li B, Peng J, Li D, Yang L: Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Can Lett. 2020;478:93–106. [DOI] [PubMed]
- 63.Wang X, Xiang Z, Tsao GS-W, Tu W. Exosomes derived from nasopharyngeal carcinoma cells induce IL-6 production from macrophages to promote tumorigenesis. Cell Mol Immunol. 2021;18(2):501–503. doi: 10.1038/s41423-020-0420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye S-B, Zhang H, Cai T-T, Liu Y-N, Ni J-J, He J, Peng J-Y, Chen Q-Y, Mo H-Y, Jun C, et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J Pathol. 2016;240(3):329–340. doi: 10.1002/path.4781. [DOI] [PubMed] [Google Scholar]
- 65.Ashiru O, Boutet P, Fernández-Messina L, Agüera-González S, Skepper JN, Valés-Gómez M, Reyburn HT. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Can Res. 2010;70(2):481–489. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong Y-G, Cui M, Chen S-M, Xu Y, Xu Y, Tao Z-Z. LncRNA-LINC00460 facilitates nasopharyngeal carcinoma tumorigenesis through sponging miR-149-5p to up-regulate IL6. Gene. 2018;639:77–84. doi: 10.1016/j.gene.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Chen W, Lian J, Zhang H, Yu B, Zhang M, Wei F, Wu J, Jiang J, Jia Y, et al. The lncRNA PVT1 regulates nasopharyngeal carcinoma cell proliferation via activating the KAT2A acetyltransferase and stabilizing HIF-1α. Cell Death Differ. 2020;27(2):695–710. doi: 10.1038/s41418-019-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou ZW, Ma C, Medoro L, Chen L, Wang B, Gupta R, Liu T, Yang XZ, Chen TT, Wang RZ, et al. LncRNA ANRIL is up-regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells. Oncotarget. 2016;7(38):61741–61754. doi: 10.18632/oncotarget.11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yao H, Tian L, Yan B, Yang L, Li Y. LncRNA TP73-AS1 promotes nasopharyngeal carcinoma progression through targeting miR-342-3p and M2 polarization via exosomes. Cancer Cell Int. 2022;22(1):16. doi: 10.1186/s12935-021-02418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazor G, Levin L, Picard D, Ahmadov U, Carén H, Borkhardt A, Reifenberger G, Leprivier G, Remke M, Rotblat B. The lncRNA TP73-AS1 is linked to aggressiveness in glioblastoma and promotes temozolomide resistance in glioblastoma cancer stem cells. Cell Death Dis. 2019;10(3):246. doi: 10.1038/s41419-019-1477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X, Yang B, She Y, Ye Y. The lncRNA TP73-AS1 promotes ovarian cancer cell proliferation and metastasis via modulation of MMP2 and MMP9. J Cell Biochem. 2018;119(9):7790–7799. doi: 10.1002/jcb.27158. [DOI] [PubMed] [Google Scholar]
- 72.Tuo Z, Zhang J, Xue W. LncRNA TP73-AS1 predicts the prognosis of bladder cancer patients and functions as a suppressor for bladder cancer by EMT pathway. Biochem Biophys Res Commun. 2018;499(4):875–881. doi: 10.1016/j.bbrc.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 73.Kumar S, Wilkes DW, Samuel N, Blanco MA, Nayak A, Alicea-Torres K, Gluck C, Sinha S, Gabrilovich D, Chakrabarti R. ΔNp63-driven recruitment of myeloid-derived suppressor cells promotes metastasis in triple-negative breast cancer. J Clin Investig. 2018;128(11):5095–5109. doi: 10.1172/JCI99673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodríguez-Ubreva J, Català-Moll F, Obermajer N, Álvarez-Errico D, Ramirez RN, Company C, Vento-Tormo R, Moreno-Bueno G, Edwards RP, Mortazavi A, et al. Prostaglandin E2 leads to the acquisition of DNMT3A-dependent tolerogenic functions in human myeloid-derived suppressor cells. Cell Rep. 2017;21(1):154–167. doi: 10.1016/j.celrep.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 75.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185(4):2273–84 Baltimore, Md : 1950. [DOI] [PMC free article] [PubMed]
- 76.Gourzones C, Barjon C, Busson P. Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer Biol. 2012;22(2):127–136. doi: 10.1016/j.semcancer.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20(3):173–185. doi: 10.1038/s41577-019-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 79.Yang R, Sun L, Li C-F, Wang Y-H, Yao J, Li H, Yan M, Chang W-C, Hsu J-M, Cha J-H, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12(1):832. doi: 10.1038/s41467-021-21099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen T-C, Chen C-H, Wang C-P, Lin P-H, Yang T-L, Lou P-J, Ko J-Y, Wu C-T, Chang Y-L. The immunologic advantage of recurrent nasopharyngeal carcinoma from the viewpoint of Galectin-9/Tim-3-related changes in the tumour microenvironment. Sci Rep. 2017;7(1):10349. doi: 10.1038/s41598-017-10386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poggio M, Hu T, Pai C-C, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L et al: Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell. 2019;177(2):414–427.e13. [DOI] [PMC free article] [PubMed]
- 83.Theodoraki M-N, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1 exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896–905. doi: 10.1158/1078-0432.CCR-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Theodoraki M-N, Yerneni S, Gooding WE, Ohr J, Clump DA, Bauman JE, Ferris RL, Whiteside TL. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology. 2019;8(7):1593805. doi: 10.1080/2162402X.2019.1593805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Timaner M, Kotsofruk R, Raviv Z, Magidey K, Shechter D, Kan T, Nevelsky A, Daniel S, de Vries EGE, Zhang T, et al. Microparticles from tumors exposed to radiation promote immune evasion in part by PD-L1. Oncogene. 2020;39(1):187–203. doi: 10.1038/s41388-019-0971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang K-P, Chang Y-T, Wu C-C, Liu Y-L, Chen M-C, Tsang N-M, Hsu C-L, Chang Y-S, Yu J-S. Multiplexed immunobead-based profiling of cytokine markers for detection of nasopharyngeal carcinoma and prognosis of patient survival. Head Neck. 2011;33(6):886–897. doi: 10.1002/hed.21557. [DOI] [PubMed] [Google Scholar]
- 87.Huang SCM, Tsao SW, Tsang CM: Interplay of Viral Infection, Host Cell Factors and Tumor Microenvironment in the Pathogenesis of Nasopharyngeal Carcinoma. Cancers. 2018;10(4):106. [DOI] [PMC free article] [PubMed]
- 88.Liou AK-F, Soon G, Tan L, Peng Y, Cher BM, Goh BC, Wang S, Lim CM. Elevated IL18 levels in nasopharyngeal carcinoma induced PD-1 expression on NK cells in TILS leading to poor prognosis. Oral Oncol. 2020;104:104616. doi: 10.1016/j.oraloncology.2020.104616. [DOI] [PubMed] [Google Scholar]
- 89.Marshall NA, Vickers MA, Barker RN. Regulatory T cells secreting IL-10 dominate the immune response to EBV latent membrane protein 1. J Immunol. 2003;170(12):6183–9 Baltimore, Md : 1950. [DOI] [PubMed]
- 90.Tsang CM, Lui VWY, Bruce JP, Pugh TJ, Lo KW: Translational genomics of nasopharyngeal cancer. Sem Can Biol. 2020;61:84–100. [DOI] [PubMed]
- 91.Cai T-T, Ye S-B, Liu Y-N, He J, Chen Q-Y, Mai H-Q, Zhang C-X, Cui J, Zhang X-S, Busson P, et al. LMP1-mediated glycolysis induces myeloid-derived suppressor cell expansion in nasopharyngeal carcinoma. PLoS Pathog. 2017;13(7):e1006503. doi: 10.1371/journal.ppat.1006503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu W-l, Lin Y-h, Xiao H, Xing S, Chen H, Chi P-d, Zhang G. Epstein-Barr virus infection induces indoleamine 2,3-dioxygenase expression in human monocyte-derived macrophages through p38/mitogen-activated protein kinase and NF-κB pathways: impairment in T cell functions. J Virol. 2014;88(12):6660–6671. doi: 10.1128/JVI.03678-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–41. [DOI] [PMC free article] [PubMed]
- 94.Ganeshan K, Nikkanen J, Man K, Leong YA, Sogawa Y, Maschek JA, Van Ry T, Chagwedera DN, Cox JE, Chawla A: Energetic Trade-Offs and Hypometabolic States Promote Disease Tolerance. Cell. 2019;177(2):399–413.e12. [DOI] [PMC free article] [PubMed]
- 95.Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC) Semin Cancer Biol. 2012;22(2):144–153. doi: 10.1016/j.semcancer.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 96.Lo AK-F, Dawson CW, Young LS, Ko C-W, Hau P-M, Lo K-W. Activation of the FGFR1 signalling pathway by the Epstein-Barr virus-encoded LMP1 promotes aerobic glycolysis and transformation of human nasopharyngeal epithelial cells. J Pathol. 2015;237(2):238–248. doi: 10.1002/path.4575. [DOI] [PubMed] [Google Scholar]
- 97.Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, Sugiura A, Cohen AS, Ali A, Do BT, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593(7858):282–8. [DOI] [PMC free article] [PubMed]
- 98.Zhang W, Wang G, Xu Z-G, Tu H, Hu F, Dai J, Chang Y, Chen Y, Lu Y, Zeng H et al: Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 2019;178(1):176–189.e15. [DOI] [PMC free article] [PubMed]
- 99.Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, Sugiura A, Cohen AS, Ali A, Do BT, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593(7858):282–288. doi: 10.1038/s41586-021-03442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weinberg SE, Singer BD, Steinert EM, Martinez CA, Mehta MM, Martínez-Reyes I, Gao P, Helmin KA, Abdala-Valencia H, Sena LA, et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature. 2019;565(7740):495–499. doi: 10.1038/s41586-018-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zappasodi R, Serganova I, Cohen IJ, Maeda M, Shindo M, Senbabaoglu Y, Watson MJ, Leftin A, Maniyar R, Verma S, et al. CTLA-4 blockade drives loss of T stability in glycolysis-low tumours. Nature. 2021;591(7851):652–658. doi: 10.1038/s41586-021-03326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, Menk AV, Rittenhouse NL, DePeaux K, Whetstone RD, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591(7851):645–651. doi: 10.1038/s41586-020-03045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, Wang Q, Yang M, Kalady MF, Qian J et al: Cholesterol Induces CD8 T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019;30(1):143–156.e5. [DOI] [PMC free article] [PubMed]
- 104.Hirschey MD, DeBerardinis RJ, Diehl AME, Drew JE, Frezza C, Green MF, Jones LW, Ko YH, Le A, Lea MA, et al. Dysregulated metabolism contributes to oncogenesis. Semin Cancer Biol. 2015;35(Suppl):S129–S150. doi: 10.1016/j.semcancer.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheng X, Li J, Guo D. SCAP/SREBPs are central players in lipid metabolism and novel metabolic targets in cancer therapy. Curr Top Med Chem. 2018;18(6):484–493. doi: 10.2174/1568026618666180523104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thai M, Graham NA, Braas D, Nehil M, Komisopoulou E, Kurdistani SK, McCormick F, Graeber TG, Christofk HR. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19(4):694–701. doi: 10.1016/j.cmet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi F, Zhou M, Shang L, Du Q, Li Y, Xie L, Liu X, Tang M, Luo X, Fan J, et al. EBV(LMP1)-induced metabolic reprogramming inhibits necroptosis through the hypermethylation of the promoter. Theranostics. 2019;9(9):2424–2438. doi: 10.7150/thno.30941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Young A, Mittal D, Stagg J, Smyth MJ. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 2014;4(8):879–888. doi: 10.1158/2159-8290.CD-14-0341. [DOI] [PubMed] [Google Scholar]
- 109.Young A, Ngiow SF, Gao Y, Patch A-M, Barkauskas DS, Messaoudene M, Lin G, Coudert JD, Stannard KA, Zitvogel L, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Can Res. 2018;78(4):1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 110.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Investig. 2007;117(5):1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li X, Wenes M, Romero P. Huang SC-C, Fendt S-M, Ho P-C: navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat Rev Clin Oncol. 2019;16(7):425–441. doi: 10.1038/s41571-019-0203-7. [DOI] [PubMed] [Google Scholar]
- 112.Fan T, Sun G, Sun X, Zhao L, Zhong R, Peng Y: Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers. 2019;11(3):317. [DOI] [PMC free article] [PubMed]
- 113.Altinoz MA, Ozpinar A. Oxamate targeting aggressive cancers with special emphasis to brain tumors. Biomed Pharmacother. 2022;147:112686. doi: 10.1016/j.biopha.2022.112686. [DOI] [PubMed] [Google Scholar]
- 114.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018;29(1):71–83. doi: 10.1093/annonc/mdx686. [DOI] [PubMed] [Google Scholar]
- 116.Wang F-H, Wei X-L, Feng J, Li Q, Xu N, Hu X-C, Liao W, Jiang Y, Lin X-Y, Zhang Q-Y, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II Clinical Trial (POLARIS-02) J Clin Oncol. 2021;39(7):704–712. doi: 10.1200/JCO.20.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chia W-K, Teo M, Wang W-W, Lee B, Ang S-F, Tai W-M, Chee C-L, Ng J, Kan R, Lim W-T, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther. 2014;22(1):132–139. doi: 10.1038/mt.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Q, Cai M-Y, Chen C-L, Hu H, Lin H-X, Li M, Weng D-S, Zhao J-J, Guo L, Xia J-C. Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. Oncoimmunology. 2017;6(5):e1312240. doi: 10.1080/2162402X.2017.1312240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chan OSH, Kowanetz M, Ng WT, Koeppen H, Chan LK, Yeung RMW, Wu H, Amler L, Mancao C. Characterization of PD-L1 expression and immune cell infiltration in nasopharyngeal cancer. Oral Oncol. 2017;67:52–60. doi: 10.1016/j.oraloncology.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 120.Hopkins R, Xiang W, Marlier D, Au VB, Ching Q, Wu LX, Guan R, Lee B, Chia W-K, Wang W-W, et al. Monocytic myeloid-derived suppressor cells underpin resistance to adoptive T cell therapy in nasopharyngeal carcinoma. Mol Ther. 2021;29(2):734–743. doi: 10.1016/j.ymthe.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38(8):947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taylor GS, Jia H, Harrington K, Lee LW, Turner J, Ladell K, Price DA, Tanday M, Matthews J, Roberts C, et al. A recombinant modified vaccinia ankara vaccine encoding Epstein-Barr Virus (EBV) target antigens: a phase I trial in UK patients with EBV-positive cancer. Clin Cancer Res. 2014;20(19):5009–5022. doi: 10.1158/1078-0432.CCR-14-1122-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bentzen AK, Marquard AM, Lyngaa R, Saini SK, Ramskov S, Donia M, Such L, Furness AJS, McGranahan N, Rosenthal R, et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat Biotechnol. 2016;34(10):1037–1045. doi: 10.1038/nbt.3662. [DOI] [PubMed] [Google Scholar]
- 125.Smith C, McGrath M, Neller MA, Matthews KK, Crooks P, Le Texier L, Panizza B, Porceddu S, Khanna R. Complete response to PD-1 blockade following EBV-specific T-cell therapy in metastatic nasopharyngeal carcinoma. NPJ Precis Oncol. 2021;5(1):24. doi: 10.1038/s41698-021-00162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Binder DC, Schreiber H. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors–letter. Cancer Res. 2014;74(2):632. doi: 10.1158/0008-5472.CAN-13-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rezaei R, Esmaeili Gouvarchin Ghaleh H, Farzanehpour M, Dorostkar R, Ranjbar R, Bolandian M, Mirzaei Nodooshan M, Ghorbani Alvanegh A. Combination therapy with CAR T cells and oncolytic viruses: a new era in cancer immunotherapy. Cancer Gene Ther. 2022;29(6):647–60. [DOI] [PubMed]
- 128.Zhang H, Lu J, Liu J, Zhang G, Lu A. Advances in the discovery of exosome inhibitors in cancer. J Enzyme Inhib Med Chem. 2020;35(1):1322–1330. doi: 10.1080/14756366.2020.1754814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17(1):147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Catalano M, O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. 2020;9(1):1703244. [DOI] [PMC free article] [PubMed]
- 131.Fabbri M. Natural killer cell-derived vesicular miRNAs: a new anticancer approach? Can Res. 2020;80(1):17–22. doi: 10.1158/0008-5472.CAN-19-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pitt JM, André F, Amigorena S, Soria J-C, Eggermont A, Kroemer G, Zitvogel L. Dendritic cell-derived exosomes for cancer therapy. J Clin Investig. 2016;126(4):1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X, Xiang Z, Liu Y, Huang C, Pei Y, Wang X, Zhi H, Wong WH, Wei H, Ng IO et al: Exosomes derived from Vdelta2-T cells control Epstein-Barr virus-associated tumors and induce T cell antitumor immunity. Sci Transl Med. 2020;12(563):eaaz3426. [DOI] [PubMed]
- 134.Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19(1):160. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu H, Chen H, Liu Z, Le Z, Nie T, Qiao D, Su Y, Mai H, Chen Y, Liu L. Therapeutic nanovaccines sensitize EBV-associated tumors to checkpoint blockade therapy. Biomaterials. 2020;255:120158. doi: 10.1016/j.biomaterials.2020.120158. [DOI] [PubMed] [Google Scholar]
- 136.Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13(11):788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang L, MacIsaac KD, Zhou T, Huang PY, Xin C, Dobson JR, Yu K, Chiang DY, Fan Y, Pelletier M, et al. Genomic analysis of nasopharyngeal carcinoma reveals TME-based subtypes. Mol Cancer Res. 2017;15(12):1722–1732. doi: 10.1158/1541-7786.MCR-17-0134. [DOI] [PubMed] [Google Scholar]
- 138.Li JP, Wu CY, Chen MY, Liu SX, Yan SM, Kang YF, Sun C, Grandis JR, Zeng MS, Zhong Q: PD-1(+)CXCR5(-)CD4(+) Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J Immunother Cancer. 2021;9(7):e002101. [DOI] [PMC free article] [PubMed]
- 139.Xia W-X, Liang H, Lv X, Wang L, Qian C-N, Ye Y-F, Ke L-R, Qiu W-Z, Yu Y-H, Huang X-J, et al. Combining cetuximab with chemoradiotherapy in patients with locally advanced nasopharyngeal carcinoma: a propensity score analysis. Oral Oncol. 2017;67:167–174. doi: 10.1016/j.oraloncology.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 140.Li Y, Chen Q-Y, Tang L-Q, Liu L-T, Guo S-S, Guo L, Mo H-Y, Chen M-Y, Guo X, Cao K-J, et al. Concurrent chemoradiotherapy with or without cetuximab for stage II to IVb nasopharyngeal carcinoma: a case-control study. BMC Cancer. 2017;17(1):567. doi: 10.1186/s12885-017-3552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu T, Liu Y, Dou S, Li F, Guan X, Zhu G. Weekly cetuximab concurrent with IMRT aggravated radiation-induced oral mucositis in locally advanced nasopharyngeal carcinoma: results of a randomized phase II study. Oral Oncol. 2015;51(9):875–879. doi: 10.1016/j.oraloncology.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 142.You R, Hua Y-J, Liu Y-P, Yang Q, Zhang Y-N, Li J-B, Li C-F, Zou X, Yu T, Cao J-Y, et al. Concurrent chemoradiotherapy with or without Anti-EGFR-Targeted treatment for stage II-IVb nasopharyngeal carcinoma: retrospective analysis with a large cohort and long follow-up. Theranostics. 2017;7(8):2314–2324. doi: 10.7150/thno.19710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.You R, Sun R, Hua Y-J, Li C-F, Li J-B, Zou X, Yang Q, Liu Y-P, Zhang Y-N, Yu T, et al. Cetuximab or nimotuzumab plus intensity-modulated radiotherapy versus cisplatin plus intensity-modulated radiotherapy for stage II-IVb nasopharyngeal carcinoma. Int J Cancer. 2017;141(6):1265–1276. doi: 10.1002/ijc.30819. [DOI] [PubMed] [Google Scholar]
- 144.Huang J-F, Zhang F-Z, Zou Q-Z, Zhou L-Y, Yang B, Chu J-J, Yu J-H, Zhang H-W, Yuan X-P, Tai G-M, et al. Induction chemotherapy followed by concurrent chemoradiation and nimotuzumab for locoregionally advanced nasopharyngeal carcinoma: preliminary results from a phase II clinical trial. Oncotarget. 2017;8(2):2457–2465. doi: 10.18632/oncotarget.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.He X, Xu J, Guo W, Jiang X, Wang X, Zong D. Cetuximab in combination with chemoradiation after induction chemotherapy of locoregionally advanced nasopharyngeal carcinoma: preliminary results. Future Oncol. 2013;9(10):1459–1467. doi: 10.2217/fon.13.151. [DOI] [PubMed] [Google Scholar]
- 146.Tworkoski K, Raab-Traub N. LMP1 promotes expression of insulin-like growth factor 1 (IGF1) to selectively activate IGF1 receptor and drive cell proliferation. J Virol. 2015;89(5):2590–2602. doi: 10.1128/JVI.02921-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu Q, Tian A-L, Li B, Leduc M, Forveille S, Hamley P, Galloway W, Xie W, Liu P, Zhao L et al: IGF1 receptor inhibition amplifies the effects of cancer drugs by autophagy and immune-dependent mechanisms. J Immunother Can. 2021;9(6):e002722. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.