Abstract
BACKGROUND
Hypertensive disorders of pregnancy (HDP) are associated with increased risk of cardiovascular disease (CVD) 20–30 years later; however, cardiovascular (CV) risk in the decade after HDP is less studied.
OBJECTIVES
The purpose of this study was to evaluate differences in CV risk factors as well as subclinical CVD among a well-characterized group of racially diverse patients with and without a history of HDP 10 years earlier.
METHODS
This is a prospective study of patients with and without a diagnosis of HDP ≥10 years earlier (2005–2007) who underwent in-person visits with echocardiography, arterial tonometry, and flow-mediated dilation of the brachial artery.
RESULTS
A total of 135 patients completed assessments (84 with and 51 without a history of HDP); 85% self-identified as Black. Patients with a history of HDP had a 2.4-fold increased risk of new hypertension compared with those without HDP (56.0% vs. 23.5%; adjusted relative risk: 2.4; 95% CI: 1.39–4.14) with no differences in measures of left ventricular structure, global longitudinal strain, diastolic function, arterial stiffness, or endothelial function. Patients who developed hypertension, regardless of HDP history, had greater left ventricular remodeling, including greater relative wall thickness; worse diastolic function, including lower septal and lateral e’ and E/A ratio; more abnormal longitudinal strain; and higher effective arterial elastance than patients without hypertension.
CONCLUSIONS
We found a 2.4-fold increased risk of hypertension 10 years after HDP. Differences in noninvasive measures of CV risk were driven mostly by the hypertension diagnosis, regardless of HDP history, suggesting that the known long-term risk of CVD after HDP may primarily be a consequence of hypertension development.
Keywords: echocardiography, endothelial function, hypertension, preeclampsia, pregnancy, tonometry
Hypertensive disorders of pregnancy (HDP), including gestational hypertension and preeclampsia, affect up to 20% of pregnant patients and are a leading cause of maternal morbidity and mortality worldwide.1,2 Within the last decade, there has been a growing body of epidemiologic evidence demonstrating that patients with a history of HDP have a higher long-term risk of ischemic heart disease, stroke, and heart failure3–5 compared with patients without a history of HDP. Preeclampsia is considered a risk enhancer in the most recent American Heart Association guidelines for blood cholesterol assessment and management,6 and a history of adverse pregnancy outcomes is an important part of cardiovascular (CV) risk stratification in current cardiology guidelines.6–8 Both cardiovascular disease (CVD) and HDP disproportionally affect Black women,7,9,10 and these racial disparities have led national organizations to highlight the importance of further research in prevention and long-term health outcomes after HDP in this subgroup of women.7,11
Most overt CV events develop 20–30 years after the occurrence of HDP.3–5 However, there remains an intermediate time period, 5–10 years after HDP exposure, when patients are young, are asymptomatic, and might not otherwise be seeking care, yet are still at increased CV risk. Therapeutic interventions during the asymptomatic phase of CV impairment may improve overall prognosis and highlight the importance of studying this time period.12
During this intermediate time period, evaluation for both traditional clinical risk factors for CVD (eg, hypertension, metabolic syndrome) as well as assessments of noninvasive cardiac and vascular risk may identify early abnormalities before overt CVD, given the proposed mechanisms of preeclampsia as a systemic vasculotoxic disease.13 Prior studies evaluating this intermediate time period are limited by small cohort sizes and lack of racial diversity, and do not include a comprehensive assessment of a broad CV phenotyping that evaluates cardiac function, macrovasculature and microvasculature, and endothelial function all in 1 cohort.14–17 The importance of studying a more diverse population, including a larger percentage of Black patients, is of critical importance given that both HDP and CVD disproportionally affect Black women.
Our objective was to evaluate differences in the prevalence of CV risk factors as well as clinical and subclinical CVD among a well-characterized group of racially diverse patients with and without a history of HDP a decade earlier. To achieve this goal, we evaluated clinical risk factors and conducted comprehensive CV phenotyping to evaluate for subclinical dysfunction using echocardiography, arterial tonometry, and flow-mediated dilation (FMD) of the brachial artery. We hypothesized that patients with a history of HDP would have a higher rate of CV risk factors, including hypertension, as well as greater abnormalities in noninvasive CV assessments than patients without a history of HDP. Such a comprehensive evaluation of cardiac risk factors and subclinical CV phenotypes has not been performed to date in patients with a prior history of HDP, nor has it been performed in the United States in a sufficient number of Black patients, a population at greatest risk for both HDP and long-term cardiac risk.
METHODS
STUDY POPULATION AND RECRUITMENT.
We performed a prospective cross-sectional study (from April 2016 to December 2019) of patients with and without a diagnosis of HDP in a prior pregnancy at least 10 years earlier. This study recruited from a previously performed prospective observational study (parent cohort) conducted from 2005–2007 that enrolled patients with obstetrician-confirmed preeclampsia or gestational hypertension (HDP) (n = 439) and term normotensive control subjects without preeclampsia (n = 591). The objective of the original study was to evaluate the association of preeclampsia and metabolic syndrome and genetic polymorphisms at the time of preeclampsia diagnosis.18 Patients with any hypertensive disorder of pregnancy (HDP), including both mild and severe preeclampsia or gestational hypertension, were enrolled in the original parent study as cases. The original control subjects were normotensive patients presenting for delivery at term (≥37 weeks) during the same time period as the cases were recruited. There were no other criteria by which the control subjects were matched for enrollment into the original study. Patients from the parent study were contacted via an invitation to participate sent through the mail. Simultaneously, telephone calls were placed for recruitment utilizing all available phone numbers in the electronic medical record. Subjects were randomly sampled from the parent study to be invited to participate in this follow-up study. The Institutional Review Board at the University of Pennsylvania approved the study, and written consent was obtained from all participants.
COMPARISON GROUPS AND DEFINITIONS.
For the current study, only patients without a history of cardiac disease, chronic hypertension, or pregestational diabetes at the time of enrollment into the original parent study (10 years earlier) were included. However, patients that developed hypertension, diabetes, or cardiac disease since the time of enrollment into the original study were included, because they were considered outcomes (see the following text). The exposure group in the current study included the following:
HDP at the time of enrollment into the parent cohort. For the parent cohort, all cases of HDP were originally defined based on the American College of Obstetricians and Gynecologists definitions of HDP. However, because the clinical definition of preeclampsia has changed in the last decade, the cases from the parent cohort were reclassified based on the definition of HDP by current American College of Obstetricians and Gynecologists Task Force recommendations.2
Additionally, for the current study, any control from the parent study that had a subsequent pregnancy with HDP was reclassified as a case for the current study and included as an exposed group.
HDP included patients with gestational hypertension as well as preeclampsia (and superimposed pre-eclampsia) with or without severe features. Gestational hypertension was defined as 2 blood pressure (BP) measurements ≥140/90 mm Hg at least 4 hours apart with no proteinuria and no other laboratory abnormalities, while preeclampsia without severe features has an additional requirement of proteinuria. Preeclampsia (or gestational hypertension) with severe features was diagnosed after 2 BP measurements ≥160/110 mm Hg at least 4 hours apart or any of the following laboratory abnormalities or symptoms: platelets <100,000/μL, liver enzymes at least twice the normal concentration and/or severe right upper quadrant/epigastric pain, serum creatinine >1.1 mg/dL or a doubling of baseline serum creatinine, pulmonary edema, or new-onset cerebral or visual disturbances (headache, blurry vision).
STUDY VISIT PROCEDURES.
For study visits, participants were instructed to fast for 8 hours before the visit during which time blood was obtained for laboratory testing, and weight, BP (measured in a standardized manner by research staff as recommended by current guidelines),19,20 and waist circumference were obtained, along with a detailed pregnancy and medical history. Importantly, if a woman reported the development of any comorbidities or specific CV outcomes (coronary artery disease, acute coronary syndrome, stroke/transient ischemic attack, heart failure, cardiomyopathy, valvular disease, arrhythmia) since enrollment into the original parent study, they were further queried and events were confirmed by obtaining medical records. Details of the transthoracic echocardiography, arterial tonometry, and FMD of the brachial artery are noted in the following text and in the Supplemental Appendix. All echocardiograms were performed on the same day as the previously mentioned procedures. A second study visit was often required to complete the tonometry and FMD portions of the study based on timing/availability of patients and research staff.
OUTCOME DEFINITION/MEASUREMENT.
We assessed clinical risk factors for CVD in this population along with noninvasive measures of cardiac and vascular structure and function.
Clinical risk factors for CVD.
Chronic hypertension (CHTN) was defined as currently on medication for hypertension, documentation of diagnosis in medical records, or having systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg at the time of study visit (stage 2 hypertension).19 The prevalence of stage 1 hypertension with systolic BP 130–139 mm Hg or diastolic BP 80–89 mm Hg was also evaluated as a secondary outcome. Diabetes was defined as a fasting glucose ≥126mg/dL or on medication for diabetes. Metabolic syndrome (National Institute of Diabetes and Digestive and Kidney Diseases criteria for patients)21 was defined as the presence of 3 of the following: waist measurement ≥35 inches; triglycerides ≥150 mg/dL, or taking medication for elevated triglycerides; high-density lipoprotein <50 mg/dL or taking medication for low high-density lipoprotein levels; BP ≥130/85 mm Hg, or taking medication for elevated BP; and fasting glucose ≥100 mg/dL or taking medication for elevated blood glucose. The individual components of the metabolic syndrome were assessed as secondary outcomes. Lipid profiles and body mass index (BMI) were also evaluated. Impaired glucose testing (fasting glucose 110–125 mg/dL) was also evaluated.
Noninvasive measures of cardiac and vascular structure and function were measured by the following parameters.
1) Cardiac structure and function by echocardiography, including left ventricular (LV) ejection fraction; measurement of the LV intraventricular septum and posterior wall thickness; and parameters of diastolic function, including e’ average, E/e’ average, E/A, longitudinal and circumferential strain, LV-end systolic elastance, and arterial elastance. 2) Large artery and muscular artery stiffness, assessed via carotid-femoral and carotidradial pulse wave velocity (PWV), the gold-standard noninvasive method for measuring large artery stiffness, which is an independent predictor of CV events and all-cause mortality.22 3) Hemodynamic arterial function, including resistive and pulsatile hemodynamics via assessments of pressure-flow relations derived from arterial tonometry and Doppler echocardiography, as previously described.23–25 4) Endothelial function, assessed by FMD of the brachial artery.26 (See Supplemental Appendix for further methodological detail regarding these assessments.)
STATISTICAL ANALYSIS.
Demographic characteristics were compared using chi-square or Fisher exact tests where applicable for categorical variables. Continuous variables were analyzed using Student’s t-tests where normally distributed and Mann-Whitney U tests where non-normally distributed. The 1-way analysis of variance and Kruskal-Wallis tests were used when comparing continuous variables across >2 groups as appropriate. Generalized linear models were used to create relative risk (RR) and to adjust for covariates.
Confounders were chosen based on plausibility with association with HDP and cardiac disease and through statistical testing (a covariate that necessitated a change of ≥15% in the point estimate or that had a P value <0.20), and backward selection was performed during modeling. Confounders included in final models were: maternal age, BMI, race (self-reported Black vs White), and history of preterm birth. Prespecific subgroup analyses were performed stratified by severity of HDP (mild vs severe) and evaluated with a statistical test for trend. Race was found to be neither an effect modifier nor a confounder. However, in light of plausible differences in outcomes by race, supplemental data are available stratifying by race.
Exploratory analyses were performed evaluating outcomes among the following 4 groups to help explain our findings (no HDP/no CHTN; HDP only; CHTN only; HDP/CHTN). Statistical analyses were performed using STATA version 14.2 (StataCorp LLC).
Our final desired sample size was 100 (including 50 patients with and without a history of HDP). This sample size would allow us to see the following differences with prevalence based on institutional data: RR of 2.3 for chronic hypertension (assuming a baseline prevalence of 23%), RR 4.1 for diabetes (assuming a prevalence of 7.7%), and RR 1.9 for metabolic syndrome (assuming a prevalence of 35%). This sample size would also allow us to detect the following differences based on pilot data using noninvasive cardiac measurements: a 4% absolute difference in LV ejection fraction, 11% difference in strain and carotid femoral PWV, and 18% difference in FMD. We assumed a 30% loss to follow-up/incompletion of all noninvasive testing, and therefore our goal sample size was 130 subjects.
RESULTS
There were 190 patients with and 190 patients without a history of HDP randomly selected from the original study and contacted for participation in this follow-up study. Of those, 135 patients ultimately completed the blood work, physical examination, surveys, and echocardiogram (84 with and 51 without a history of HDP) and 93 of those completed arterial tonometry and FMD assessments (58 with and 35 without a history of HDP).
Demographic information for the study cohort is found in Table 1. In total, 85% of the cohort self-identified as Black, with a higher prevalence of Black patients with a history of HDP than those without a history of HDP (92% vs 78%; P = 0.037). There was no statistical difference in maternal age or BMI at study visit, tobacco use, parity, history of gestational diabetes, or years since enrollment into the original study. Of the patients with a history of HDP, 62% had severe disease and 39% had HDP in more than 1 pregnancy. There was a higher rate of preterm birth in the group with a history of HDP (50% vs 19.6%; P < 0.001).
TABLE 1.
Demographic Information for Patients With and Without a History of HDP
| No History of HDP (n = 51) |
History of HDP (n = 84) |
P Value | |
|---|---|---|---|
| Race | |||
| Black | 40 (78.4) | 77 (91.7) | 0.037 |
| White | 11 (21.6) | 7 (8.3) | |
| Age at study visit, y | 39.1 (33.2–42.6) | 35.7 (32.3–41.0) | 0.23 |
| BMI (kg/m2) at study visit | 29.9 (26.2–36.1) | 31.4 (27.2–38.4) | 0.53 |
| Current tobacco use | 11 (21.6) | 21 (25.0) | 0.74 |
| Years since enrollment in original studya | 11 (10–11) | 12 (9–13) | 0.60 |
| Obstetric history | |||
| Parity | 3 (2–4) | 3 (2–4) | 0.21 |
| History of gestational diabetes | 3 (5.9) | 7 (7.3) | 0.74 |
| Highest level of HDP | |||
| None | 51 (100.0) | 0 (0.0) | 0.001 |
| Mild: GHTN or preeclampsia | 0 (0.0) | 32 (38.1) | |
| Severe preeclampsia (including HELLP) | 0 (0.0) | 52 (61.9) | |
| Recurrent HDP | 0 (0.0) | 33 (39.3) | 0.001 |
| History of preterm birth | 10 (19.6) | 42 (50.0) | 0.001 |
Values are n (%) with Fisher exact P value or median (IQR) with Wilcoxon rank sum test P value.
For patients with a history of HDP, these numbers represent years since pregnancy complicated by HDP
BMI = body mass index; GHTN = gestational hypertension; HDP = hypertensive disorder of pregnancy; HELLP = hemolysis, elevated liver enzymes, low platelets.
Differences in the prevalence of CV risk factors between patients with and without HDP are noted in Table 2. Stage 2 chronic hypertension was noted in 56.0% of patients with a history of HDP and 23.5% of those without a history of HDP (P < 0.001), which equates to more than twice the risk of developing CHTN after HDP (relative risk: 2.38; 95% CI: 1.40–4.40) even when adjusting for race, maternal age, BMI, and history of preterm birth (adjusted relative risk: 2.40; 95% CI: 1.39–4.14). Importantly, 18% of patients with a history of HDP met criteria for a new diagnosis of hypertension identified through the study visit. There were no differences in rates of CHTN between those with and without a history of HDP when limiting the analysis to those with an established HTN diagnosis. Notably, 82.1% of patients with a history of HDP and 60.7% of patients without a history of HDP met criteria for either stage 1 or stage 2 chronic hypertension (P < 0.001).
TABLE 2.
Cardiovascular Risk Factors Between Patients With and Without a History of HDP
| No History of HDP (n = 51) |
History of HDP (n = 84) |
P Value | |
|---|---|---|---|
| Stage 2 hypertensiona | 12 (23.5) | 47 (56.0) | <0.001 |
| Established diagnosis | 7 (14.0) | 32 (39.0) | 0.003 |
| Diagnosed based on BP at study visit | 5 (9.8) | 15 (17.9) | 0.20 |
| Stage 1 hypertension | 19 (37.3) | 22 (26.2) | 0.18 |
| Systolic BP at study visit, mm Hg | 116 (108–128) | 125 (113–136) | 0.024 |
| Diastolic BP at study visit, mm Hg | 82 (75–88) | 85 (76–97) | 0.062 |
| Diabetes/impaired glucose testing | 4 (8.2) | 8 (9.6) | 0.73 |
| Diabetes | 3 (6.0) | 6 (7.3) | 1.00 |
| Impaired glucose testingb | 1 (2.0) | 2 (2.4) | 0.87 |
| BMI ≥30 kg/m2 | 24 (47.1) | 45 (54.9) | 0.48 |
| Metabolic syndromec | 15 (30.6) | 27 (35.5) | 0.70 |
| Waist circumference ≥35 inches | 32 (62.8) | 52 (62.7) | 0.99 |
| Fasting total cholesterol levels, mg/dL | 182.4 ± 37.3 | 176.3 ± 36.2 | 0.36 |
| LDL >130, mg/dL | 13 (25.5) | 16 (19.3) | 0.40 |
| TG ≥150, mg/dL | 9 (18.0) | 10 (12.5) | 0.45 |
| HDL <50, mg/dL | 26 (52.0) | 35 (43.8) | 0.37 |
| Cardiovascular event | 3d (5.9) | 7e (8.3) | 0.74 |
Values are n (%) with Fisher exact P value, median (IQR) with Wilcoxon rank sum test P value, or mean ± SD with 2-sided Student’s t-test P value.
Defined as official diagnosis based on medical records and/or on antihypertensive medications or by elevated BP at study visit.
Defined as fasting glucose 110–125 mg/dL.
Defined as having ≥3 metabolic syndrome indicators (complete markers only – excludes women missing metabolic markers).
New arrhythmia, valvular disease, stroke.
Stroke (n = 1), arrhythmia (n = 3), venous thromboembolism (n = 1), heart failure (n = 3), cardiomyopathy (n = 1), valvular (n = 1).
HDL = high-density lipoprotein; LDL = low-density lipoprotein; TG = triglycerides; other abbreviations as in Table 1.
There were no significant differences between groups in the other clinical CV risk factors. Notably, 50% of the cohort was obese (BMI ≥30 kg/m2) and more than 30% had metabolic syndrome. The prevalence of CV risk factors was similar when stratified by race (Supplemental Table 1) and severity of HDP (Supplemental Table 2). There was no difference in risk of CHTN when comparing those with mild disease (ie, without severe features) vs severe disease (62.5% vs 51.9%; P = 0.34).
Table 3 shows the echocardiographic results evaluating cardiac structure and function. Ejection fraction, relative wall thickness, and LV mass were similar between patients with and without a history of HDP, even after adjusting for age, race, BMI, history of preterm birth, and presence of CHTN diagnosis. There were no differences in diastolic function, even when excluding patients with diabetes, a known risk factor for diastolic dysfunction. Longitudinal strain was similar between patients with and without HDP, although circumferential strain was better in patients without a history of HDP compared with those with HDP (−22.8 vs −24.6; P = 0.038). Vascular elastance (stiffness) was higher in patients with a history of HDP compared with those without (1.97 vs 1.79; P = 0.044); however, this relationship was no longer significant when adjusting for demographic and clinical factors. Measures of ventricular elastance and ventricular-arterial coupling were similar between patients with and without HDP.
TABLE 3.
Echocardiographic Outcomes Evaluating Structure and Function
| No History of HDP (n = 49) |
History of HDP (n = 82) |
P Value | Adjusted P Valuea |
|
|---|---|---|---|---|
| LV structure and function | ||||
| Ejection fraction, % | 56.4 ± 6.1 | 55.4 ± 5.2 | 0.36 | 0.44 |
| IVS thickness, cm | 0.89 ± 0.18 | 0.92 ± 0.18 | 0.27 | 0.82 |
| PW thickness, cm | 0.82 ± 0.15 | 0.84 ± 0.14 | 0.53 | 0.68 |
| Relative wall thickness | 0.36 ± 0.08 | 0.38 ± 0.09 | 0.34 | 0.71 |
| LV mass | 127.06 (108.79 to 141.91) | 127.81 (108.46 to 153.27) | 0.73 | 0.79 |
| LV mass index | 65.29 (55.52 to 74.72) | 66.82 (56.43 to 79.94) | 0.41 | 0.91 |
| Diastolic function | ||||
| E/A | 1.34 (1.24 to 1.58) | 1.34 (1.17 to 1.52) | 0.73 | 0.36 |
| Septal e’, cm/s | 10 (9 to 12) | 9 (8 to 11) | 0.19 | 0.70 |
| Lateral e’, cm/s | 12 (10 to 14) | 11.5 (9 to 14) | 0.56 | 0.95 |
| E’ average, cm/s | 11 (9 to 13) | 10.5 (8.5 to 12.5) | 0.41 | 0.96 |
| E/e’ average | 7.23 (6.31 to 8.78) | 8.26 (6.33 to 9.36) | 0.19 | 0.78 |
| Strain parameters | ||||
| Longitudinal strain % | −20.6 (−22.5 to −18.9) | −20.3 (−22.2 to −18.5) | 0.36 | 0.63 |
| GLS < −18% | 39 (85) | 56 (77) | 0.29 | 0.81 |
| Circumferential strain | −22.8 (−25.8 to −20.6) | −24.6 (−28.5 to −21.9) | 0.04 | 0.03 |
| Ratio longitudinal/circumferential strain | 0.91 (0.80 to 1.01) | 0.83 (0.69 to 0.95) | 0.01 | 0.09 |
| Vascular and ventricular function | ||||
| LV Ees | 1.98 (1.59 to 2.31) | 2.01 (1.76 to 2.37) | 0.30 | 0.72 |
| Ea | 1.79 (1.46 to 1.98) | 1.97 (1.57 to 2.24) | 0.04 | 0.14 |
| VA coupling (Ees/Ea) | 1.15 ± 0.25 | 1.10 ± 0.24 | 0.25 | 0.59 |
Values are mean ± SD with 2-sided Student’s t-test P value, median (IQR) with Wilcoxon rank sum test P value, or n (%) with Fisher exact P value.
Adjusted for age, race, body mass index, history of preterm birth and presence of CHTN.
Ea = effective arterial elastance; Ees = end-systolic elastance; GLS = global longitudinal strain; HDP = hypertensive disorder of pregnancy; IVS = interventricular septum; LV = left ventricular; PW = posterior wall.
Of the 93 patients who underwent tonometry and FMD testing, 85 had results that were analyzable based on quality assessment (32 without history of HDP and 53 with history of HDP) (Table 4). There were no significant differences in measures of peripheral artery stiffness (carotid-radial PWV), large artery stiffness (carotid-femoral PWV), or FMD of the brachial artery between patients with and without HDP. No differences in measures of arterial stiffness or endothelial function were found when stratifying by severity of HDP. When evaluating hemodynamic arterial function (Table 4), there were no differences in aortic root characteristic impedance, forward and backward wave amplitudes, or reflection magnitude. There was a trend toward greater peripheral resistance in the those with a history of HDP, as well as a greater ratio of early systolic contraction to late systolic suction wave amplitude. There was no difference in any of these outcomes when the analysis was restricted to Black patients or when stratified by disease severity (data available upon request).
TABLE 4.
Tonometry and Flow-Mediated Dilation Between Patients With and Without a History of HDP
| No History of HDP (n = 32) |
History of HDP (n = 53) |
P Value | Adjusted P Valuea |
|
|---|---|---|---|---|
| PWV | ||||
| Carotid femoral PWVb | 7.7 (6.5–8.8) | 7.3 (5.2–8.2) | 0.209 | 0.55 |
| Carotid radial PWVb | 9.5 (8.5–10.6) | 9.2 (8.0–10.3) | 0.353 | 0.14 |
| Endothelial function | ||||
| Flow-mediated dilation of the brachial arteryc | 5.55 (3.65–7.70) | 4.25 (1.95–6.05) | 0.097 | 0.27 |
| Hemodynamic arterial function | ||||
| Aortic root characteristic impedance | 0.12 (0.09–0.20) | 0.15 (0.10–0.20) | 0.31 | 0.37 |
| Forward wave amplitude | 36.03 (28.24–42.37) | 35.47 (29.75–43.76) | 0.60 | 0.95 |
| Backward wave amplitude | 16.61 (12.22–20.36) | 16.41 (13.05–24.67) | 0.64 | 0.94 |
| Reflection magnitude: forward/backward wave amplitude | 0.49 (0.42–0.54) | 0.50 (0.43–0.57) | 0.60 | 0.99 |
| Early systolic forward compression wave amplitude | 0.86 (0.71–1.00) | 0.95 (0.77–1.23) | 0.19 | 0.20 |
| Late systolic forward suction/expansion wave amplitude | 0.22 (0.20–0.31) | 0.23 (0.16–0.34) | 0.66 | 0.63 |
| Ratio of early and late systolic forward suction/expansion wave amplitude | 3.45 (2.76–4.34) | 4.12 (3.10–5.50) | 0.067 | 0.07 |
| Total peripheral resistance | 1.38 (1.10–1.58) | 1.54 (1.36–1.86) | 0.064 | 0.25 |
Values are median (IQR) with P value Wilcoxon rank sum test.
Adjusted for age, race, body mass index, history of preterm birth, and presence of CHTN).
n = 78 analyzable results after quality assurance assessment). Normative mean values (±2 SD) for PWV for individuals age 30–39 years is 6.6 (4.4–8.9) with optimal blood pressure and 7.1 (4.5–7.9) with stage 1 hypertension.40
n = 60 analyzable results after quality assurance assessment).
HDP = hypertensive disorder of pregnancy; PWV = pulse wave velocity.
Exploratory analyses were performed to further explain our overall findings. We compared outcomes for the following groups: patients with no history of HDP and no CHTN at time of visit; patients with HDP alone and no CHTN; patients with CHTN and no history of HDP; and patients with a history of HDP and CHTN (Table 5). Patients with CHTN were notably different from patients without CHTN, regardless of HDP status (Central Illustration). Patients with CHTN (with HDP, n = 47; or without HDP, n=12) had greater LV remodeling, including higher intraventricular septum (P < 0.001), posterior wall (P < 0.001), and RW thickness (P < 0.001) compared with patients without CHTN. They also had worse diastolic function, including lower septal e’ (P < 0.01) and lateral e’ (P = 0.03) and E/A ratio (P = 0.02), more abnormal global longitudinal strain (P = 0.02), and higher effective arterial elastance (P = 0.03) compared with patients without CHTN, regardless of HDP history (Central Illustration).
TABLE 5.
Exploratory Analyses by Presence of HDP and Hypertension Diagnosis at Study Visit
| No Hypertension | Hypertension | ||||||
|---|---|---|---|---|---|---|---|
| No HTN/No HDP (n = 39) |
HDP Only (n = 37) |
P Value | HTN Only (n = 12) |
HTN and HDP (n = 47) |
P Value | P Valuea | |
| Echo parameters | |||||||
| Ejection fraction, % | 55.8 ± 5.6 | 55.6 ± 4.4 | 0.86 | 58.1 ± 7.2 | 55.3 ± 5.8 | 0.17 | 0.66 |
| IVS thickness, cm | 0.86 ± 0.15 | 0.84 ± 0.14 | 0.72 | 1.0 ± 0.21 | 0.98 ± 0.18 | 0.81 | <0.001 |
| PW thickness, cm | 0.81 ± 0.15 | 0.78 ± 0.11 | 0.48 | 0.89 ± 0.12 | 0.88 ± 0.14 | 0.90 | <0.001 |
| Relative wall thickness | 0.35 ± 0.08 | 0.34 ± 0.05 | 0.72 | 0.40 ± 0.07 | 0.40 ± 0.11 | 0.96 | <0.001 |
| LV mass | 124.2 (101.3 to 142.9) | 118.6 (95.7 to 147.8) | 0.59 | 132.1 (114.7 to 140.1) | 134.3 (113.6 to 164.5) | 0.87 | 0.19 |
| LV mass index | 61.5 (53.9 to 74.5) | 61.2 (54.2 to 72.1) | 0.93 | 70.5 (57.4 to 78.4) | 70.7 (59.8 to 82.5) | 0.76 | 0.08 |
| Diastolic function | |||||||
| E/A | 1.38 (1.24 to 1.64) | 1.48 (1.24 to 1.65) | 0.40 | 1.26 (1.15 to 1.43) | 1.28 (1.12 to 1.46) | 0.95 | 0.02 |
| Septal e’, cm/s | 10.5 (9 to 12) | 10 (9 to 12) | 0.77 | 9 (7–10) | 8 (7–10) | 0.89 | <0.01 |
| Lateral e’, cm/s | 12.5 (10 to 15) | 13 (10 to 15) | 0.86 | 9 (9 to 11) | 11 (9 to 13) | 0.38 | 0.03 |
| E’ average, cm/s | 11.3 (9.5 to 13.5) | 11.5 (9.5 to 13.5) | 0.86 | 9 (8 to 10) | 10 (8 to 11.5) | 0.45 | <0.01 |
| E/e’ average | 6.89 (6.19 to 8.22) | 7.74 (5.85 to 9.22) | 0.31 | 8.78 (7.75 to 9.80) | 8.32 (6.82 to 9.47) | 0.49 | 0.06 |
| Strain parameters | |||||||
| Longitudinal strain, % | −21.32 (−22.96 to −19.28) | −20.93 (−22.65 to −19.58) | 0.92 | −19.34 (−21.40 to −16.14) | −19.08 (−21.39 to −17.48) | 0.81 | 0.02 |
| GLS < −18% | 33 (92) | 29 (88) | 0.60 | 6 (60) | 27 (68) | 0.65 | 0.01 |
| Circumferential strain | −23.14 (−25.97 to −21.36) | −24.16 (−28.15 to −21.90) | 0.25 | −21.04 (−24.75 to −19.13) | −24.72 (−28.76 to −22.02) | 0.037 | 0.12 |
| Vascular and ventricular function | |||||||
| LV Ees | 1.99 (1.65 to 2.26) | 1.96 (1.68 to 2.25) | 0.96 | 1.93 (1.52 to 2.59) | 2.09 (1.81 to 2.44) | 0.58 | 0.16 |
| Ea | 1.79 (1.46 to 1.97) | 1.79 (1.51 to 2.11) | 0.60 | 1.81 (1.47 to 2.16) | 2.03 (1.82 to 2.40) | 0.15 | 0.03 |
| VA coupling (Ees/Ea) | 1.15 ± 0.25 | 1.12 ± 0.27 | 0.66 | 1.16 ± 0.26 | 1.08 ± 0.22 | 0.29 | 0.60 |
| Tonometry and FMD | |||||||
| Carotid femoral PWV | 6.9 (6.4 to 8.3) | 6.9 (4.1 to 7.5) | 0.38 | 8.4 (7.6 to 9.5) | 7.5 (5.2 to 8.3) | 0.18 | 0.26 |
| FMD brachial artery | 5.6 (3.1 to 7.8) | 4.6 (1.6 to 6.2) | 0.34 | 5.5 (4.5 to 6.7) | 4.3 (2.3 to 6.0) | 0.18 | 0.40 |
| Total peripheral resistance | 1.26 (0.96 to 1.68) | 1.44 (1.13 to 1.72) | 0.37 | 1.47 (1.26 to 1.58) | 1.66 (1.44 to 1.89) | 0.15 | 0.13 |
Values are median (IQR) with Wilcoxon rank sum test P value, mean ± SD with 2-sided t-test P value, or n (%) with Fisher exact P value.
P value comparing the groups with no hypertension to the groups with hypertension, regardless of HDP history
CENTRAL ILLUSTRATION.
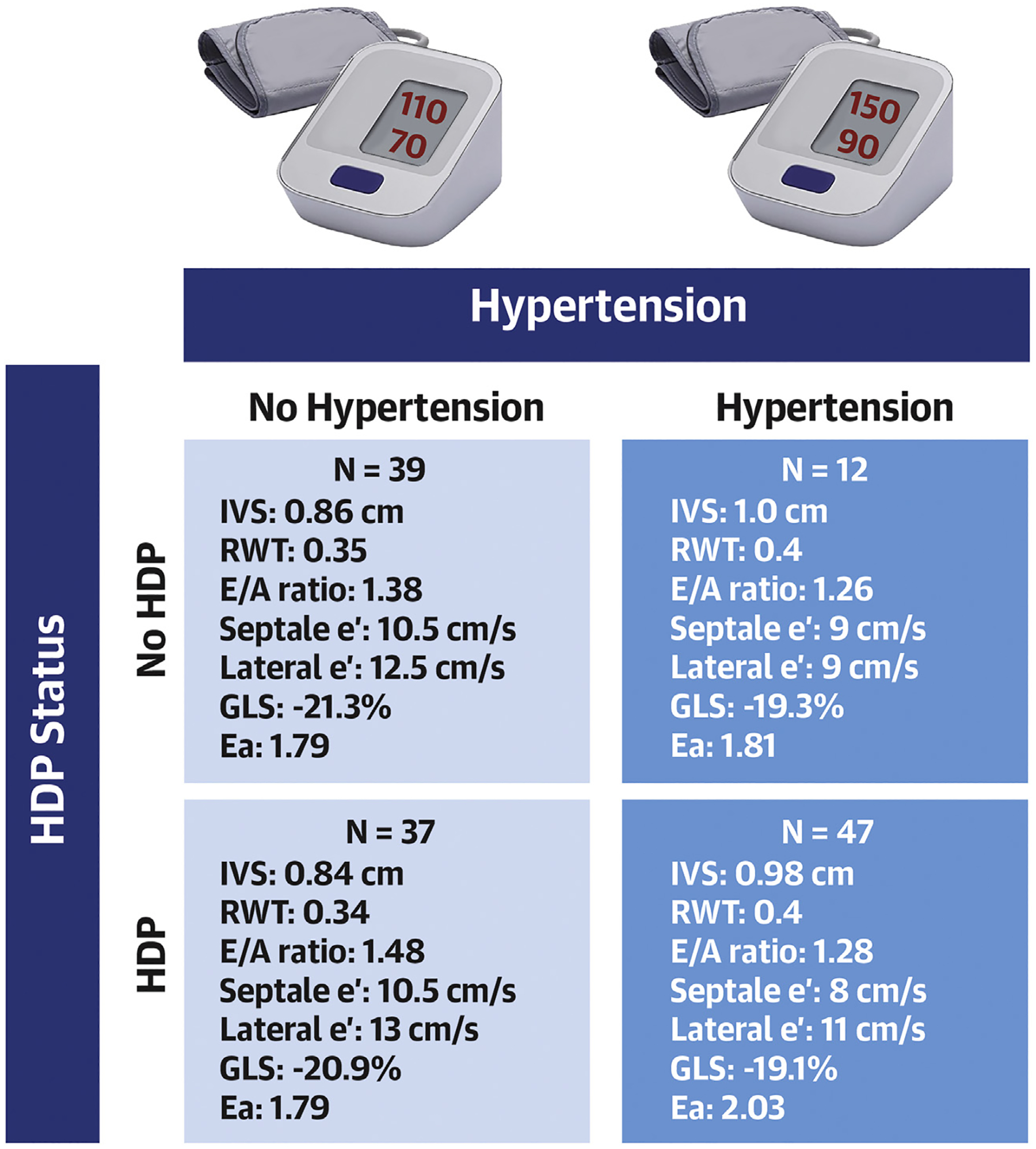
Echocardiographic Differences Stratified by History of HDP and Hypertension
The development of hypertension more clearly identifies patients with cardiovascular risk rather than history of a hypertensive disorder of pregnancy. This figure demonstrates that patients with a hypertension diagnosis (dark blue), regardless of hypertensive disorder of pregnancy history, have more abnormal echocardiographic findings than those without hypertension (light blue); P < 0.05 for all measures listed. Ea = effective arterial elastance; GLS = global longitudinal strain; HDP = hypertensive disorder of pregnancy; IVS = Interventricular septum; RWT = relative wall thickness.
DISCUSSION
Using a well-characterized prospective study with a cohort of predominantly Black women, we found that a history of HDP 10 years earlier more than doubles the risk of CHTN (adjusted relative risk: 2.4) but does not have an association with other identifiable clinical CV risk factors (including diabetes, obesity, or metabolic syndrome) or an association with differences in cardiac and vascular structure and function using multidimensional assessments. There were, however, identifiable differences in echocardiographic measures of CV risk when comparing patients that did and did not develop a diagnosis of CHTN, suggesting the known long-term risk of CVD in women with a history of HDP may primarily be a consequence of the development of HTN.
The data regarding increased risk of hypertension after HDP is not a novel finding3,4,8,27–29; however, our cohort is unique in the high baseline rate of stage 2 hypertension even among patients without a history of HDP (56% with and 23.5% without history of HDP). In fact, when looking at the diagnosis of either stage 1 or 2 hypertension, >80% of our patients with a history of HDP (and 60% without a history of HDP) met criteria for at least stage 1 hypertension, which is significantly higher than other studies using the same definition.28,30 Importantly, of those with a history of HDP, only 39% of patients with either stage 1 or 2 hypertension had a formal diagnosis, potentially missing the other half of patients in this category if screening had not otherwise been performed outside of our current study. This, along with studies with similar findings,29 further highlights the importance of routine screening for CHTN in this population. In contrast to other studies, we did not find differences in LV mass/remodeling or diastolic function,31,32 which may be a result of a higher prevalence of hypertension in the entire cohort as noted in the previous text,30 a more diverse patient population (87% Black patients),30,32 and different imaging modalities.31
Preeclampsia leads to systemic vasculotoxicity via excess of antiangiogenic factors and other mechanisms.13 Prior studies have demonstrated changes in arterial stiffness and endothelial dysfunction during pregnancy and in the early years following delivery in women with preeclampsia.16,17,33–36 Our results suggest that these changes do not persist up to 10 years after delivery and are consistent with the few small studies examining these subclinical measures a decade after HDP.15,37,38 It is plausible that our high rates of other CV risk factors in both groups (20% smokers, 30% metabolic syndrome, 50% obese) led to less notable differences in underlying vascular profiles because these risk factors are known drivers of abnormalities in the noninvasive CV measures that were assessed. This could have mitigated the inherent risk that HDP plays on future CV health. Nevertheless, our data suggest that the long-term risk of CVD after HDP may largely be borne by the high risk of developing HTN. Importantly, a history of HDP should therefore continue to be considered a risk factor for future CVD, and these patients should be followed closely postpartum given the heightened risk of hypertension development.
In exploratory analysis, 2 phenotypes of our population emerged—patients with and without hypertension, regardless of HDP history. In our cohort, hypertension alone was associated with measures of subclinical CV disease with little additional risk contributed by a history of HDP. In large prospective cohort studies, hypertension and other CV risk factors explain the majority of increased CV risk observed in women with a history of HDP.39 Haug et al39 found that hypertension and obesity mediated 77% of the excess risk of CVD in women with a history of HDP in Norway, while Honigberg et al28 demonstrated that hypertension explained 64% of the excess coronary artery disease risk associated with HDP in a U.K. population. Although these cohorts were of predominantly White race and were older than the population in our study, rates of hypertension were similar and supports the hypothesis that the high rate of hypertension (both in these study populations as well as ours) diminishes any identifiable differences in other noninvasive cardiac and vascular assessments.
STUDY STRENGTHS AND LIMITATIONS.
Strengths of this study include the use of a cohort of women who had a well-characterized diagnosis of HDP at the time of their entry into the parent study. This removes reliance on International Classification of Diseases coding or recall bias for the HDP diagnosis, which is critical given the heterogeneity of HDP disease. Additionally, we included numerous assessments of both cardiac and vascular function assessments to enable a comprehensive CV profile of all forms of HDP, including both mild and severe disease. Limitations include the lack of prepregnancy cardiac and vascular assessment to determine if prepregnancy cardiovascular abnormalities may contribute to the lack of positive findings. Although patients were randomly selected for recruitment, there may be inherent differences in the patients able to be contacted as well as the patients who agreed to enrollment compared with those who did not. If patients with more medical comorbidities were more likely to enroll, this could lead to biasing our study toward the null. Additionally, our cohort represents an urban population that included a large proportion of Black women, which may not be generalizable to other populations. However, given the unequal burden of both HDP and CVD for Black women, this remains a critical group to study to better understand the underpinnings of their associated risk. Last, although we met our target sample size for the clinical and echocardiography parameters, we were underpowered to see smaller differences that may have been more apparent with a larger cohort, such as differences in CV events and some of the noninvasive measures on echocardiography.
CONCLUSIONS
We found a 2.4-fold increased risk of hypertension among a population of predominantly Black women 10 years after a diagnosis of HDP. Importantly, a history of HDP alone did not appear to be associated with differences in noninvasive subclinical measures of CV risk. Differences in these markers were driven mostly by presence or absence of a hypertension diagnosis, regardless of HDP history, supporting the idea that hypertension itself explains a large portion of future CV risk for women with a history of HDP. Furthermore, 60% of all patients who met criteria for either stage 1 or 2 hypertension did not have a formal diagnosis. Both the prevalence of undiagnosed hypertension and the effect that hypertension has on future CV risk highlights the importance of early screening for hypertension in women postpregnancy complicated by HDP and the importance of initiating antihypertensive treatments to decrease the long-term risk of CVD. Future studies should evaluate the optimal time period to screen for postpartum hypertension and a monitoring plan for these at-risk women.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
Women developing HDP face a 2.4-fold greater risk of developing hypertension within the decade following pregnancy, accounting for a large portion of their long-term CV risk.
TRANSLATIONAL OUTLOOK:
Studies are needed to evaluate strategies for screening and treatment of hypertension in women with past HDP.
ACKNOWLEDGMENTS
The authors thank Sindhu K. Srinivas, MD, MSCE, who was the principal investigator of the parent study, and Raymond Townsend, MD, who was involved with the initial planning of the study.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This research was supported by the National Heart, Lung, and Blood Institute 1R56HL136730 (to Dr Levine), National Institutes of Health UL1TR001878 (to Drs Levine and Ky), The American Association of Obstetricians and Gynecologists Foundation (AAOGF) Bridge Funding Award (to Dr Levine), and K23 HL153667 (to Dr Lewey). Dr Chirinos is supported by National Institutes of Health grants R01-HL 121510, U01-TR003734, 3U01TR003734-01W1, U01-HL160277, R33-HL-146390, R01-HL153646, K24-AG070459, R01-AG058969, R01-HL104106, P01-HL094307, R03-HL146874, R56-HL136730, R01 HL155599, R01 HL157264, R01HL155, and 1R01HL153646-01; has recently consulted for Bayer, Sanifit, Fukuda-Denshi, Bristol Myers Squibb, Johnson and Johnson, Edwards Lifesciences, Merck, NGM Biopharmaceuticals, and the Galway-Mayo Institute of Technology; has received University of Pennsylvania research grants from National Institutes of Health, Fukuda-Denshi, Bristol Myers Squibb, Microsoft, and Abbott; is named as inventor in a University of Pennsylvania patent for the use of inorganic nitrates/nitrites for the treatment of heart failure and preserved ejection fraction and for the use of biomarkers in heart failure with preserved ejection fraction; has received payments for editorial roles from the American Heart Association, the American College of Cardiology, and Wiley; and has received research device loans from Actor Medical, Fukuda-Denshi, Uscom, Unex, NDD Medical Technologies, Microsoft, and MicroVision Medical. Dr Elovitz has served as a consultant for Mirvie; and has received support from research grants from the National Institutes of Health. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- BP
blood pressure
- CHTN
Chronic hypertension
- CV
cardiovascular
- CVD
cardiovascular disease
- FMD
flow-mediated dilation
- HDP
hypertensive disorders of pregnancy
- LV
left ventricle/ventricular
- PWV
pulse wave velocity
- RR
relative risk
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For an expanded Methods section and supplemental tables, please see the online version of this paper.
REFERENCES
- 1.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. [DOI] [PubMed] [Google Scholar]
- 2.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of pre-eclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–930. [DOI] [PubMed] [Google Scholar]
- 5.Behrens I, Basit S, Lykke JA, et al. Association between hypertensive disorders of pregnancy and later risk of cardiomyopathy. JAMA. 2016;315(10): 1026–1033. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24): e285–e350. 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 7.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123(11):1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143(18):e902–e916. [DOI] [PubMed] [Google Scholar]
- 9.Shahul S, Tung A, Minhaj M, et al. Racial disparities in comorbidities, complications, and maternal and fetal outcomes in women with pre-eclampsia/eclampsia. Hypertens Pregnancy. 2015;34(4):506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erqou S, Kip KE, Mulukutla SR, Aiyer AN, Reis SE. Endothelial dysfunction and racial disparities in mortality and adverse cardiovascular disease outcomes. Clin Cardiol. 2016;30(10): 22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maric-Bilkan C, Abrahams VM, Arteaga SS, et al. Research recommendations from the National Institutes of Health Workshop on Predicting, Preventing, and Treating Preeclampsia. Hypertension. 2019;73(4):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50–e103. 10.1016/j.jacc.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 13.Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res. 2019;124(7):1094–1112. [DOI] [PubMed] [Google Scholar]
- 14.Al-Nashi M, Eriksson MJ, Östlund E, Bremme K, Kahan T. Cardiac structure and function, and ventricular-arterial interaction 11 years following a pregnancy with preeclampsia. J Am Soc Hypertens. 2016;10(4):297–306. [DOI] [PubMed] [Google Scholar]
- 15.Weissgerber TL, Milic NM, Milin-Lazovic JS, Garovic VD. Impaired flow-mediated dilation before, during, and after preeclampsia: a systematic review and meta-analysis. Hypertension. 2016;67(2):415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orabona R, Sciatti E, Vizzardi E, et al. Endothelial dysfunction and vascular stiffness in women with a previous pregnancy complicated by early or late pre-eclampsia. Ultrasound Obstet Gynecol. 2016;26(10):15893. [DOI] [PubMed] [Google Scholar]
- 17.Yinon Y, Kingdom JC, Odutayo A, et al. Vascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction: insights into future vascular risk. Circulation. 2010;122(18):1846–1853. [DOI] [PubMed] [Google Scholar]
- 18.Srinivas SK, Sammel MD, Bastek J, et al. Evaluating the association between all components of the metabolic syndrome and pre-eclampsia. J Matern Fetal Neonatal Med. 2009;22(6):501–509. [DOI] [PubMed] [Google Scholar]
- 19.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 20.Muntner P, Einhorn PT, Cushman WC, et al. Blood pressure assessment in adults in clinical practice and clinic-based research: JACC scientific expert panel. J Am Coll Cardiol. 2019;73(3):317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.How is metabolic syndrome diagnosed? National Heart, Lung, and Blood Institute. Accessed April 3, 2017. https://www.nhlbi.nih.gov/health/metabolic-syndrome/diagnosis
- 22.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66(3):698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chirinos JA, Londono-Hoyos F, Zamani P, et al. Effects of organic and inorganic nitrate on aortic and carotid haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail. 2017;19(11):1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chirinos JA, Bhattacharya P, Kumar A, et al. Impact of diabetes mellitus on ventricular structure, arterial stiffness, and pulsatile hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2019;8(4):e011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 1: pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension. 2010;56(4):555–562. [DOI] [PubMed] [Google Scholar]
- 26.Dobbie LJ, Mackin ST, Hogarth K, et al. Validation of semi-automated flow-mediated dilation measurement in healthy volunteers. Blood Press Monit. 2020;25(4):216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behrens I, Basit S, Melbye M, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honigberg MC, Zekavat SM, Aragam K, et al. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. 2019;74(22):2743–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benschop L, Duvekot JJ, Versmissen J, van Broekhoven V, Steegers EAP, Roeters van Lennep JE. Blood pressure profile 1 year after severe preeclampsia. Hypertension. 2018;71(3):491–498. [DOI] [PubMed] [Google Scholar]
- 30.Countouris ME, Villanueva FS, Berlacher KL, Cavalcante JL, Parks WT, Catov JM. Association of hypertensive disorders of pregnancy with left ventricular remodeling later in life. J Am Coll Cardiol. 2021;77(8):1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boardman H, Lamata P, Lazdam M, et al. Variations in cardiovascular structure, function, and geometry in midlife associated with a history of hypertensive pregnancy. Hypertension. 2020;75(6):1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokslag A, Teunissen PW, Franssen C, et al. Effect of early-onset preeclampsia on cardiovascular risk in the fifth decade of life. Am J Obstet Gynecol. 2017;216(5):14. [DOI] [PubMed] [Google Scholar]
- 33.Ehrenthal DB, Goldstein ND, Wu P, Rogers S, Townsend RR, Edwards DG. Arterial stiffness and wave reflection 1 year after a pregnancy complicated by hypertension. J Clin Hypertens. 2014;16(10):695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronnback M, Lampinen K, Groop PH, Kaaja R. Pulse wave reflection in currently and previously preeclamptic women. Hypertens Pregnancy. 2005;24(2):171–180. [DOI] [PubMed] [Google Scholar]
- 35.Agatisa PK, Ness RB, Roberts JM, Costantino JP, Kuller LH, McLaughlin MK. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol. 2004;286(4):H1389–H1393. [DOI] [PubMed] [Google Scholar]
- 36.Aykas F, Solak Y, Erden A, et al. Persistence of cardiovascular risk factors in women with previous preeclampsia: a long-term follow-up study. J Investig Med. 2015;63(4):641–645. [DOI] [PubMed] [Google Scholar]
- 37.Östlund E, Al-Nashi M, Hamad RR, et al. Normalized endothelial function but sustained cardiovascular risk profile 11 years following a pregnancy complicated by preeclampsia. Hypertens Res. 2013;36(12):1081–1087. [DOI] [PubMed] [Google Scholar]
- 38.Christensen M, Kronborg CS, Eldrup N, Rossen NB, Knudsen UB. Preeclampsia and cardiovascular disease risk assessment - do arterial stiffness and atherosclerosis uncover increased risk ten years after delivery? Pregnancy Hypertens. 2016;6(2):110–114. [DOI] [PubMed] [Google Scholar]
- 39.Haug EB, Horn J, Markovitz AR, et al. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: analysis of the Nord-Trøndelag Health Study. JAMA Cardiol. 2019;4(7): 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338–2350. 10.1093/eurheartj/ehq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


