Abstract
Purpose
High levels of red blood cell distribution width (RDW) and hypoalbuminemia are markers of poor prognosis in chronic obstructive pulmonary disease (COPD) patients. However, few studies have shown that the red blood cell distribution width–albumin ratio (RAR) is related to the mortality of COPD. This study aimed to explore the relationship between RAR and hospital mortality in COPD patients admitted to the intensive care unit (ICU).
Patients and Methods
Patients were retrospectively incorporated from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database and divided into two groups by a cutoff value of RAR. Propensity score matching (PSM) was performed to adjust for the imbalance of covariates. Logistic regression models and subgroup analyses were carried out to investigate the relationship between RAR and hospital mortality. The receiver operating characteristic (ROC) curve was used to evaluate the predictive performance of RAR and decision curve analysis (DCA) to assess the clinical utility.
Results
In total, 1174 patients were finally identified from the MIMIC-IV database. The cutoff value for RAR was 5.315%/g/dL. After PSM at a 1:1 ratio, 638 patients were included in the matched cohort. In the original and matched cohorts, the high RAR group had higher hospital mortality and longer hospital stays. Logistic regression analysis suggested that RAR was an independent risk factor for hospital mortality. The areas under the ROC curve in the original and matched cohorts were 0.706 and 0.611, respectively, which were larger than applying RDW alone (the original cohort: 0.600, the matched cohort: 0.514). The DCA indicated that RAR had a clinical utility.
Conclusion
A higher RAR (>5.315%/g/dL) was associated with hospital mortality in COPD patients admitted to ICU. As an easily available peripheral blood marker, RAR can predict hospital mortality in critically ill patients with COPD independently.
Keywords: chronic obstructive pulmonary disease, red blood cell distribution width, albumin, hospital mortality, MIMIC-IV
Graphical Abstract
Introduction
Chronic obstructive pulmonary disease (COPD), a common respiratory disease, is a progressive inflammatory disease of the airways, alveoli, and microvascular system characterized by irreversible airflow limitation.1 Epidemiological statistics show that, in 2015, estimated 299 million people worldwide suffered from COPD, and more than 3 million died of the disease.2,3 COPD is the third leading cause of death worldwide after ischemic heart disease and cerebrovascular disease.2 Many COPD patients need admission to intensive care units (ICU) due to acute respiratory failure, where the clinical course may be complicated by concurrent multiple organ dysfunction, which has a high mortality.4,5 Moreover, in the ICU, higher mortality was observed in patients with COPD compared with patients without COPD.6 Therefore, accurate assessment of prognosis is vital for the clinical management of COPD patients admitted to the ICU, and the development of a certain easily accessible marker associated with hospital mortality is also necessary, which can help clinicians detect patients with high hospital mortality early and take more aggressive action.
The red blood cell distribution width–albumin ratio (RAR, %/g/dl) is equal to the red blood cell distribution width (RDW) divided by serum albumin. RDW reflects the degree of heterogeneity in red blood cell volume and is traditionally used for the identification of anemia, while recent evidence indicates that elevated RDW levels are also common in inflammatory diseases, such as cardiovascular disease, community-acquired pneumonia, and COPD.7 Early studies have shown that elevated RDW levels are associated with the severity of stable COPD and the risk of death, as well as the prognosis after hospitalization for acute exacerbation of COPD (AECOPD).8–10 Serum albumin is commonly applied clinically to assess malnutrition status, and prior studies have demonstrated that a low level of albumin is an important predictor of increased mortality in COPD.11,12 Given this, we conjecture that RAR is a negatively correlated indicator of COPD prognosis. Previous studies have also indicated that RAR is associated with mortality in patients with stroke,13 diabetes,14 severe pneumonia,15 and acute respiratory distress syndrome.16 However, there are few studies that explore the relationship between RAR and the prognosis of COPD patients. Hence, this study aimed to investigate the association between RAR and hospital mortality of COPD patients admitted to the ICU, to provide a reference for the clinical management of critically ill patients with COPD.
Materials and Methods
Data Sources
This study was retrospective, and data were obtained from the Medical Information Mart for Intensive Care IV (MIMIC-IV) Database (version 1.0) (https://physionet.org/content/mimiciv/1.0/), an open-access single-center critical care clinical database containing information on patients admitted to Beth Israel Deaconess Medical Center (Boston, Massachusetts) from 2008 to 2019.17 This database was approved by the Institutional Review Board of the Massachusetts Institute of Technology. Our study was conducted following the Declaration of Helsinki, and the first author (Yuanjie Qiu) completed an online training course and a human research participant protection examination at the National Institutes of Health (NIH), with permission to extract data from the MIMIC-IV database (Record ID: 46544414). Given the nature of the retrospective study and the use of de-identified patient data extracted from public databases, patient informed consent was not applicable. This study was deemed exempt from review by the Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University.
Selection of Study Population
Patients diagnosed with COPD according to the tenth revision of the International Classification of Diseases (ICD10) (J44, J440, J441, J449). Patients older than 18 years were included in this study. For patients with multiple admissions, this study included only first-time admissions and first-time ICU admissions. Patients who stayed in the ICU for less than 24 hours and those with missing values for study variables were excluded.
Variable Extraction
Navicat Premium 15 (version 15.0.12) was used to build a data management platform and extract research data. STATA 14.0 software was exercised to merge data. We extracted study variables including demographic characteristics (age, gender, weight), vital signs [respiratory rate (HR), heart rate (RR), mean non-invasive blood pressure (NBPm)], initial laboratory data [peripheral leukocyte, erythrocyte, platelets, hemoglobin, serum creatinine, blood urea nitrogen (BUN), aspartate transferase (AST), RDW, albumin], and assisted respiration conditions (high-flow oxygen inhalation, non-invasive mechanical ventilation, invasive mechanical ventilation). Acute Physiological Score III (APS III), Oxford Acute Severity of Illness Score (OASIS), and Sequential Organ Failure Assessment (SOFA) scores were also extracted. RAR (%/g/dL) was calculated as RDW (%) divided by serum albumin (g/dL).
We identified comorbidities by the ninth or tenth revision of the International Classification of Diseases (ICD-9/10), including myocardial infarct, congestive heart failure, peripheral vascular disease, cerebrovascular disease, mild liver disease, moderate-to-severe liver disease, diabetes without chronic complication, diabetes with chronic complication, renal disease, malignant tumor, acute respiratory failure. Chronic hepatitis or cirrhosis without portal hypertension was considered mild liver disease, whereas cirrhosis with portal hypertension, with or without a history of variceal hemorrhage, was classified as moderate-to-severe liver disease.18 The age-adjusted Charlson comorbidity index (CCI) determined by the ICD-9/10 was an assessment tool specifically designed to predict long-term mortality.18–21
The primary outcome of this study was hospital mortality. Secondary outcomes encompassed length of hospital stay and length of ICU stay.
Outliers
In our study, values above 99% or below 1% of continuous variables were considered outliers, and we replaced outliers with 99% or 1% of the point values. Outliers were processed using STATA 14.0 software.
Statistical Analysis
Based on the principle of maximizing the sum of specificity and sensitivity of the receiver operating characteristic curve (ROC), the optimal cutoff value of RAR was determined, and the subjects were divided into the low RAR and the high RAR group. Continuous variables meeting the normal distribution and homogeneity were expressed as mean ± standard deviation, and the differences were analyzed using the t-test. Continuous variables not meeting the normal distribution or homogeneity were presented as median [interquartile range], and the Wilcoxon rank-sum test was applied to analyze the differences. Categorical variables were reported as numbers (percentage), and the chi-square test was exerted to compare the two groups’ differences.
A 1:1 propensity score matching (PSM) was applied to adjust for the imbalance of covariates between the two groups to ensure the robustness of our results. Univariate and multivariate logistic regression analyses were also performed on the original and matched cohorts to investigate the association between RAR and hospital mortality by calculating adjusted odds ratios (ORs). In Model 1, adjusted for age, gender, and weight; in Model 2, adjustments were made for the variables in Model 1 plus comorbidities; in Model 3, covariates included the variables in Model 2 plus the scoring system and comorbidity index; in Model 4, the covariates in Model 3 plus both vital signs and laboratory data were adjusted. We further carried out an exploratory subgroup analysis in the original cohort to investigate whether RAR remained a prognostic factor in certain patient subgroups.
The ROC curve was utilized to compare the predictive performance of RAR and RDW in predicting hospital mortality. Decision curve analysis (DCA) was used to assess the clinical utility of RAR. All analyses were performed using R software (version 4.1.2) and P<0.05 was considered statistically significant.
Results
Patient Characteristics
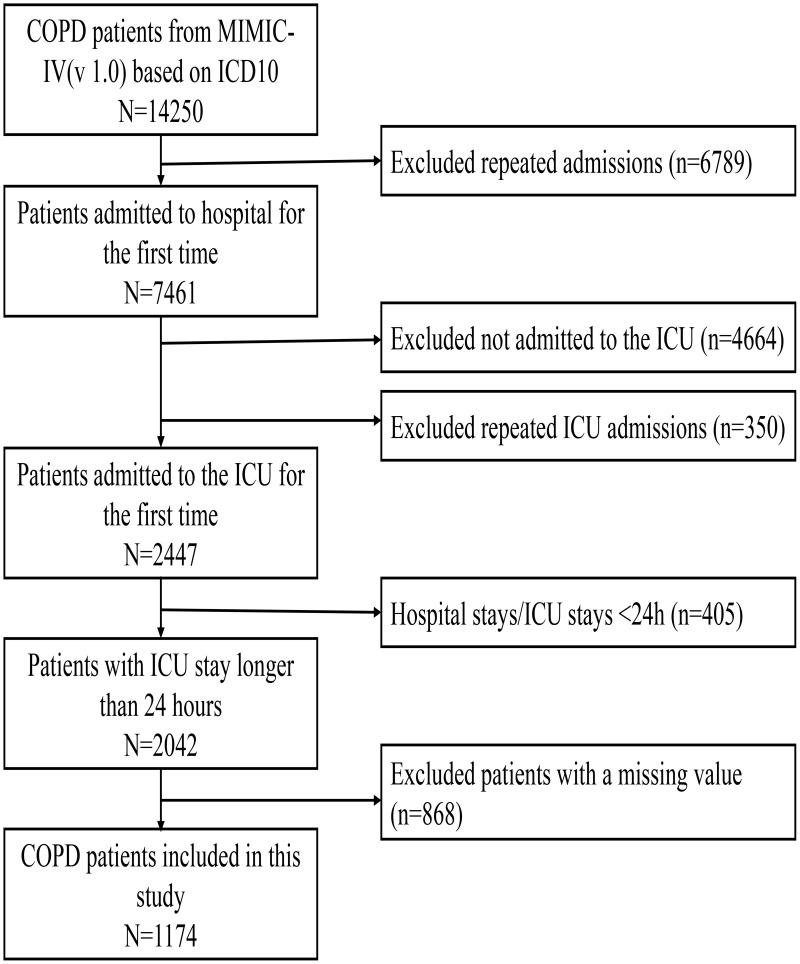
According to the above criteria, 1174 COPD patients admitted to the ICU were finally identified from the MIMIC-IV database (version 1.0) (Figure 1). Baseline clinical characteristics of patients in the original cohort before PSM are presented in Table 1 in detail. The cutoff value for RAR was 5.315%/g/dL in accordance with the maximization of the sum of specificity and sensitivity of the ROC curve. Following this, the enrolled patients were divided into the low RAR group (<5.315%/g/dL) and the high RAR group (>5.315%/g/dL). Age, gender, weight, platelet, congestive heart failure, peripheral vascular disease, cerebrovascular disease, diabetes without chronic complication, renal disease, high-flow oxygen therapy, and noninvasive ventilation did not differ significantly between the two groups. However, there were significant differences between groups in RR (P<0.001), HR (P<0.001), NBPm (P<0.001), peripheral leukocyte (P<0.001), erythrocyte (P<0.001), hemoglobin (P<0.001), serum creatinine (P<0.001), BUN (P<0.001), AST (P<0.001), myocardial infarction (P=0.009), liver disease (P<0.001), diabetes with chronic complication (P=0.036), malignant tumor (P<0.001), acute respiratory failure (P<0.001), invasive ventilation (P=0.001), and scoring system (P<0.001).
Figure 1.
Flowchart for Patient Inclusion. Note: A total of 1174 COPD patients admitted to the ICU were included in this study.
Abbreviations: COPD, chronic obstructive pulmonary disease; MIMIC-IV, Medical Information Mart for Intensive Care IV; v1.0, version 1.0; ICU, intensive care unit.
Table 1.
Baseline Characteristics from the Original Cohort of COPD Admitted to the ICU
| Characteristics | Overall | Low RAR | High RAR | P |
|---|---|---|---|---|
| N = 1174 | N = 708 | N = 466 | ||
| Demographics | ||||
| Age, year | 71.00 [63.00, 79.00] | 71.00 [63.75, 79.00] | 72.00 [63.00, 80.00] | 0.657 |
| Male | 637 (54.3) | 390 (55.1) | 247 (53.0) | 0.51 |
| Weight, kg | 79.90 [64.40, 96.47] | 81.60 [66.00, 96.43] | 77.00 [63.00, 96.52] | 0.06 |
| Vital signs | ||||
| RR, beats/min | 19.50 [16.00, 24.00] | 19.00 [16.00, 23.00] | 20.00 [16.00, 25.00] | <0.001 |
| HR, beats/min | 85.00 [75.00, 100.00] | 84.00 [74.00, 97.00] | 89.00 [76.00, 105.00] | <0.001 |
| NBPm, mmHg | 79.00 [69.00, 92.00] | 82.00 [71.75, 94.00] | 76.00 [67.00, 87.00] | <0.001 |
| Laboratory parameters | ||||
| RDW, % | 15.30 [13.90, 17.20] | 14.40 [13.40, 15.50] | 17.30 [15.60, 19.50] | <0.001 |
| Serum albumin, g/dL | 3.20 [2.70, 3.60] | 3.50 [3.20, 3.80] | 2.60 [2.40, 2.90] | <0.001 |
| Leukocyte, 109 cells/L | 10.10 [7.60, 13.90] | 9.60 [7.47, 13.00] | 11.20 [8.00, 16.10] | <0.001 |
| Erythrocyte, 1012 cells/L | 3.40 [2.86, 3.99] | 3.62 [3.10, 4.18] | 3.03 [2.67, 3.57] | <0.001 |
| Hemoglobin, g/dL | 9.90 [8.40, 11.70] | 10.80 [9.30, 12.43] | 8.70 [7.80, 10.10] | <0.001 |
| Platelet, 109 cells/L | 194.00 [140.00, 254.00] | 197.00 [149.00, 248.00] | 189.00 [109.25, 280.25] | 0.057 |
| Creatinine, mg/dL | 1.00 [0.70, 1.60] | 1.00 [0.70, 1.40] | 1.20 [0.70, 1.90] | <0.001 |
| BUN, mg/dL | 23.00 [15.00, 37.00] | 21.00 [14.00, 31.00] | 27.00 [16.00, 45.00] | <0.001 |
| AST, IU/L | 29.00 [19.00, 58.00] | 27.00 [18.00, 45.25] | 35.00 [20.00, 70.75] | <0.001 |
| Comorbidity | ||||
| Myocardial infarct | 327 (27.9) | 217 (30.6) | 110 (23.6) | 0.009 |
| Congestive heart failure | 594 (50.6) | 363 (51.3) | 231 (49.6) | 0.591 |
| Peripheral vascular disease | 220 (18.7) | 126 (17.8) | 94 (20.2) | 0.321 |
| Cerebrovascular disease | 189 (16.1) | 124 (17.5) | 65 (13.9) | 0.105 |
| Mild liver disease | 143 (12.2) | 56 (7.9) | 87 (18.7) | <0.001 |
| Moderate-to-severe liver disease | 72 (6.1) | 22 (3.1) | 50 (10.7) | <0.001 |
| DM without complication | 306 (26.1) | 177 (25.0) | 129 (27.7) | 0.309 |
| DM with complication | 210 (17.9) | 113 (16.0) | 97 (20.8) | 0.036 |
| Renal disease | 324 (27.6) | 181 (25.6) | 143 (30.7) | 0.062 |
| Malignant tumor | 171 (14.6) | 77 (10.9) | 94 (20.2) | <0.001 |
| Acute respiratory failure | 454 (38.7) | 213 (30.1) | 241 (51.7) | <0.001 |
| Assisted respiration | ||||
| High flow | 139 (11.8) | 74 (10.5) | 65 (13.9) | 0.079 |
| Non-invasive ventilation | 139 (11.8) | 91 (12.9) | 48 (10.3) | 0.197 |
| Invasive ventilation | 616 (52.5) | 344 (48.6) | 272 (58.4) | 0.001 |
| Scoring system | ||||
| CCI | 7.00 [6.00, 9.00] | 7.00 [6.00, 9.00] | 8.00 [6.00, 10.00] | <0.001 |
| SOFA | 2.00 [1.00, 5.00] | 2.00 [0.00, 4.00] | 3.00 [1.00, 6.00] | <0.001 |
| OASIS | 35.00 [29.00, 41.00] | 33.00 [28.00, 39.00] | 38.00 [31.00, 44.00] | <0.001 |
| APS III | 50.00 [37.00, 67.00] | 43.00 [32.00, 58.00] | 60.00 [47.00, 81.00] | <0.001 |
Notes: Continuous variables are represented as median [IQR]; Categorical variables are represented as n (%).
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; RAR, red blood cell distribution width–albumin ratio; RR, respiratory rate; HR, heart rate; NBPm, mean non-invasive blood pressure; RDW, red blood cell distribution width; BUN, blood urea nitrogen; AST, aspartate transferase; DM, diabetes mellitus; CCI, Charlson comorbidity index; SOFA, sequential organ failure assessment; OASIS, oxford acute severity of illness score; APS III, acute physiological score III.
We then matched the following covariates: age, gender, weight, vital signs, scoring system, CCI, comorbidities, and assisted respiration. After PSM at a 1:1 ratio, a total of 638 COPD patients were included in a matched cohort and assigned to the low RAR and the high RAR group (Table 2). Compared with the low RAR group, the peripheral blood leukocytes (9.50*10^9/L vs 11.00*10^9/L, P=0.002) and AST (28.00IU/L vs 34.00IU/L, P=0.013) in the high RAR group were significantly higher, while erythrocyte (3.51*10^12/L vs 3.10*10^12/L, P<0.001) and hemoglobin (10.50g/dL vs 8.80g/dL, P<0.001) were significantly lower.
Table 2.
Baseline Characteristics from the Matched Cohort of COPD Admitted to the ICU
| Characteristics | Overall | Low RAR | High RAR | P |
|---|---|---|---|---|
| 638 | 319 | 319 | ||
| Demographics | ||||
| Age, year | 72.00 [64.00, 80.00] | 72.00 [65.00, 80.00] | 73.00 [63.00, 81.00] | 0.716 |
| Male | 333 (52.2) | 168 (52.7) | 165 (51.7) | 0.874 |
| Weight, kg | 78.00 [62.65, 94.20] | 78.50 [63.00, 93.40] | 77.00 [62.55, 95.70] | 0.757 |
| Vital signs | ||||
| RR, beats/min | 20.00 [16.00, 24.75] | 20.00 [16.00, 25.00] | 20.00 [16.00, 24.00] | 0.886 |
| HR, beats/min | 86.50 [75.00, 102.75] | 87.00 [75.50, 102.00] | 86.00 [73.00, 103.00] | 0.859 |
| NBPm, mmHg | 78.00 [68.00, 92.00] | 80.00 [69.00, 94.00] | 77.00 [68.00, 90.00] | 0.138 |
| Laboratory parameters | ||||
| RDW, % | 15.60 [14.30, 17.60] | 14.60 [13.60, 15.70] | 17.20 [15.55, 19.30] | <0.001 |
| Serum albumin, g/dL | 3.10 [2.60, 3.50] | 3.50 [3.20, 3.80] | 2.60 [2.40, 2.90] | <0.001 |
| Leukocyte, 109 cells/L | 10.10 [7.60, 14.17] | 9.50 [7.30, 13.25] | 11.00 [8.10, 15.40] | 0.002 |
| Erythrocyte, 1012 cells/L | 3.30 [2.83, 3.86] | 3.51 [3.00, 4.12] | 3.10 [2.71, 3.63] | <0.001 |
| Hemoglobin, g/dL | 9.50 [8.30, 11.28] | 10.50 [9.00, 12.20] | 8.80 [7.90, 10.10] | <0.001 |
| Platelet, 109 cells/L | 199.00 [140.00, 265.75] | 198.00 [149.00, 252.00] | 200.00 [126.50, 288.50] | 0.905 |
| Creatinine, mg/dL | 1.00 [0.70, 1.60] | 1.00 [0.70, 1.60] | 1.00 [0.70, 1.70] | 0.896 |
| BUN, mg/dL | 24.00 [15.00, 39.00] | 24.00 [16.00, 38.00] | 24.00 [15.00, 40.50] | 0.624 |
| AST, IU/L | 31.00 [19.25, 62.00] | 28.00 [19.00, 52.50] | 34.00 [20.00, 68.00] | 0.013 |
| Comorbidities | ||||
| Myocardial infarct | 169 (26.5) | 84 (26.3) | 85 (26.6) | 1 |
| Congestive heart failure | 347 (54.4) | 178 (55.8) | 169 (53.0) | 0.525 |
| Peripheral vascular disease | 135 (21.2) | 72 (22.6) | 63 (19.7) | 0.438 |
| Cerebrovascular disease | 96 (15.0) | 49 (15.4) | 47 (14.7) | 0.912 |
| Mild liver disease | 83 (13.0) | 42 (13.2) | 41 (12.9) | 1 |
| Moderate-to-severe liver disease | 36 (5.6) | 16 (5.0) | 20 (6.3) | 0.607 |
| Diabetes without complication | 170 (26.6) | 83 (26.0) | 87 (27.3) | 0.788 |
| Diabetes with complication | 122 (19.1) | 62 (19.4) | 60 (18.8) | 0.92 |
| Renal disease | 203 (31.8) | 106 (33.2) | 97 (30.4) | 0.497 |
| Malignant cancer | 111 (17.4) | 57 (17.9) | 54 (16.9) | 0.835 |
| Acute respiratory failure | 280 (43.9) | 141 (44.2) | 139 (43.6) | 0.936 |
| Assisted respiration | ||||
| High flow | 76 (11.9) | 42 (13.2) | 34 (10.7) | 0.392 |
| Non-invasive ventilation | 76 (11.9) | 41 (12.9) | 35 (11.0) | 0.541 |
| Invasive ventilation | 350 (54.9) | 172 (53.9) | 178 (55.8) | 0.691 |
| Scoring system | ||||
| CCI | 8.00 [6.00, 10.00] | 8.00 [6.00, 10.00] | 8.00 [6.00, 10.00] | 0.249 |
| SOFA | 3.00 [1.00, 5.00] | 3.00 [1.00, 5.00] | 3.00 [1.00, 5.00] | 0.99 |
| OASIS | 35.50 [30.00, 42.00] | 35.00 [30.50, 42.00] | 36.00 [30.00, 42.00] | 0.905 |
| APSIII | 54.00 [43.00, 67.75] | 54.00 [42.00, 67.00] | 55.00 [43.00, 68.00] | 0.728 |
Notes: Continuous variables are represented as median [IQR]; Categorical variables are represented as n (%).
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; RAR, red blood cell distribution width–albumin ratio; RR, respiratory rate; HR, heart rate; NBPm, mean non-invasive blood pressure; RDW, red blood cell distribution width; BUN, blood urea nitrogen; AST, aspartate transferase; DM, diabetes mellitus; CCI, Charlson comorbidity index; SOFA, sequential organ failure assessment; OASIS, oxford acute severity of illness score; APS III, acute physiological score III.
Clinical Outcomes
The clinical outcomes of COPD patients are shown in Table 3. In the original cohort, hospital mortality was significantly higher in the high RAR group [130 (27.9%) vs 61 (8.6%), P<0.001]. Similar results were obtained in the matched cohort [72 (22.6%) vs 44 (13.8%), P=0.005]. The length of hospital stays was also longer in the high RAR group both in the original (14 days vs 9 days, P<0.001) and matched cohort (13 days vs 10 days, P<0.001). After PSM, the difference in the length of ICU stays between the two groups was not statistically significant (P=0.133).
Table 3.
Clinical Outcomes of COPD Patients Admitted to the ICU in the Original and Matched Cohorts
| Overall | Low RAR | High RAR | P | |
|---|---|---|---|---|
| Original cohort | N=1174 | N=708 | N=466 | |
| Hospital stays, day | 10.00 [6.00, 18.00] | 9.00 [5.00, 15.00] | 14.00 [8.00, 22.75] | <0.001 |
| ICU stays, day | 3.00 [1.00, 6.00] | 2.00 [1.00, 5.00] | 4.00 [2.00, 9.00] | <0.001 |
| Hospital mortality | 191 (16.3) | 61 (8.6) | 130 (27.9) | <0.001 |
| Matched cohort | N=638 | N=319 | N=319 | |
| Hospital stays, day | 12.00 [7.00, 19.00] | 10.00 [6.00, 16.50] | 13.00 [7.50, 21.50] | <0.001 |
| ICU stays, day | 3.00 [2.00, 7.00] | 3.00 [1.00, 7.00] | 3.00 [2.00, 7.00] | 0.133 |
| Hospital mortality | 116 (18.2) | 44 (13.8) | 72 (22.6) | 0.005 |
Notes: Continuous variables are represented as median [IQR]; Categorical variables are represented as n (%).
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; RAR, red blood cell distribution width–albumin ratio.
Association Between RAR and Hospital Mortality
The results of logistic regression analyses of RAR for predicting hospital mortality are exhibited in Table 4. The raw model, which did not adjust for any variables, showed that RAR was associated with hospital mortality in COPD patients (Original cohort: OR 1.51, 95% CI 1.38–1.66, P<0.001; Matched cohort: OR 1.30, 95% CI 1.15–1.46, P<0.001). Model 1, Model 2, Model 3, and Model 4 were adjusted for relevant covariates as described in the methods above. In the original cohort, the adjusted OR for Model 1, Model 2, Model 3, and Model 4 were 1.53 (95% CI 1.39–1.68, P<0.001), 1.46 (95% CI 1.32–1.62, P<0.001), 1.28 (95% CI 1.15–1.43, P<0.001), and 1.27 (95% CI 1.12–1.43, P<0.001), respectively. Likewise, the adjusted OR in the matched cohort were 1.30 (95% CI 1.16–1.47, P<0.001), 1.32 (95% CI 1.17–1.50, P<0.001), 1.32 (95% CI 1.16–1.51, P<0.001), and 1.31 (95% CI 1.13–1.52, P<0.001), respectively. These results suggested that a high level of RAR was an independent risk factor for hospital mortality in COPD patients admitted to the ICU.
Table 4.
Logistic Regression Analysis of RAR for Predicting Hospital Mortality in COPD Patients Admitted to the ICU
| Original Cohort | Matched Cohort | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Unadjusted | 1.51(1.38–1.66) | <0.001 | 1.30(1.15–1.46) | <0.001 |
| Model 1 | 1.53(1.39–1.68) | <0.001 | 1.30(1.16–1.47) | <0.001 |
| Model 2 | 1.46(1.32–1.62) | <0.001 | 1.32(1.17–1.50) | <0.001 |
| Model 3 | 1.28(1.15–1.43) | <0.001 | 1.32(1.16–1.51) | <0.001 |
| Model 4 | 1.27(1.12–1.43) | <0.001 | 1.31(1.13–1.52) | <0.001 |
Notes: Model 1 adjusted for age, gender, and weight. Model 2 adjusted for model 1 plus comorbidities. Model 3 adjusted for model 2 plus scoring system and CCI. Model 4 adjusted for model 3 plus vital signs and laboratory parameters.
Abbreviations: RAR, red blood cell distribution width–albumin ratio; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; OR, odds ratio; 95% CI, 95% confidence index; CCI, Charlson comorbidity index.
To further investigate whether RAR remained a risk factor for hospital mortality in certain disease subgroups, an exploratory subgroup analysis was performed on the original cohort. The Forest plot expressed that high RAR was a risk factor for hospital mortality in all disease subgroups except those with moderate-to-severe liver disease (Figure 2).
Figure 2.
Subgroup analysis of RAR for predicting hospital mortality.
Notes: The OR values of RAR for predicting hospital mortality in each subgroup were greater than 1 (P<0.05), indicating that RAR was an independent factor for predicting hospital mortality, except in the moderate-to-severe liver disease subgroup (OR 1.17, 95% CI 0.89–1.55, P=0.249).
Abbreviations: OR, odds ratio; CI, confidence interval.
Clinical Value of RAR
The ROC curves for RAR to predict hospital mortality are presented in Figure 3. In the original cohort (Figure 3A), the area under the ROC curve (AUC) was 0.706 (95% CI: 0.665–0.747), and the cutoff for RAR to predict hospital mortality was 5.315%/g/dL, with a specificity of 0.658 and a sensitivity of 0.681. In the matched cohort (Figure 3B), the AUC was 0.611 (95% CI 0.551–0.670), and the cut-off value of RAR was 6.165%/g/dL, with the specificity and sensitivity being 0.739 and 0.491, respectively. The AUC of RAR for predicting hospital mortality was larger than that of RDW alone [the original cohort (Figure 3A): 0.706 vs 0.600, P<0.001; the matched cohort (Figure 3B): 0.611 vs 0.514, P<0.001], whereas RAR and serum albumin alone did not significantly differ in predicting hospital mortality [the original cohort (Figure 3C): 0.706 vs 0.699, P=0.611; the matched cohort (Figure 3D): 0.611 vs 0.635, P=0.176].
Figure 3.
The ROC curves for RAR, RDW, and serum albumin to predict hospital mortality of COPD patients admitted to ICU in the original cohort (A and C) and the matched cohort (B and D).
Notes: For RAR, RDW, and serum albumin to predict hospital mortality in the original cohort, the areas under the ROC curve (AUC) were 0.706, 0.600, and 0.699, respectively. In the matched cohort, the AUCs for RAR, RDW, and serum albumin to predict hospital mortality were 0.611, 0.514, and 0.635, respectively. RAR had asubstantially larger AUC than RDW alone (P<0.001).
Abbreviations: RAR, red blood cell distribution width–albumin ratio; RDW, red blood cell distribution width; ALB, albumin; AUC, the areas under the receiver operating characteristic curve.
The DCA was used to assess the clinical utility of RAR for predicting hospital mortality. As shown in Figure 4A and B, when the risk threshold probability was in the 0–100% interval, RAR added more net benefit than the “all treatment” or “none treatment” strategy in the original cohort and the matched cohort, suggesting that RAR may have a good clinical utility performance.
Figure 4.
The DCA for assessing the clinical utility of RAR in COPD patients admitted to ICU in the original cohort (A) and the matched cohort (B).
Notes: RAR added a higher net benefit than the “all treatment” or “none treatment” approach in the original cohort and the matched cohort when the risk threshold probability was in the range of 0–100%.
Abbreviation: RAR, red blood cell distribution width–albumin ratio.
Discussion
This study included 1174 critically ill patients diagnosed with COPD in the MIMIC-IV database, and for the first time explored the relationship between RAR and hospital mortality in COPD patients admitted to the ICU. The findings suggested that RAR could be used as a marker of poor prognosis in severe COPD patients. Higher RAR levels were associated with higher hospital mortality and longer hospital stays. After PSM and subgroup analyses, the association of RAR with hospital mortality remained significant.
RAR is a combined marker of RDW and albumin. Recently, RDW was found to be a prognostic marker for multiple respiratory diseases that share the common feature of arterial hypoxemia, suggesting a correlation between RDW and arterial hypoxemia.22,23 It is well known that COPD is one of the causes of the global hypoxic burden, and an increase in RDW predicts poor outcomes in certain diseases, including many hypoxic conditions.24 Prior studies have shown higher RDW in patients requiring long-term oxygen therapy and non-invasive ventilation, and these patients tend to be sicker and have a worse prognosis, suggesting that high RDW can be used as an indicator of poor prognosis in COPD.25 The exact mechanism is still unclear, but previous research has shown that acute hypoxia can stimulate a significant increase in serum erythropoietin, thereby inducing the generation of enlarged erythrocytes, resulting in an increase in RDW, which is a possible explanation.26
Numerous studies have revealed that RDW can be tightly linked to COPD severity and mortality and that a higher level of RDW can independently predict poor COPD outcomes. Tertemiz et al found a significant difference in RDW between COPD grades (GOLD 1 13.5%; GOLD 2 13.9%; GOLD 3 14.4%; GOLD 4 15.7%; P <0.001), and that the high RDW group also had worse survival than the low RDW group (31% vs 75%).8 Ekrem Cengiz Seyhan et al also retrospectively analyzed 270 patients with stable COPD and concluded that elevated RDW levels were associated with an increased risk of death (OR 1.12; 95% CI 1.01–1.24; P = 0.01).9 Similar research findings were also found in AECOPD.10 Although the pathophysiological mechanisms underlying the relationship between high levels of RDW and poor prognosis are not fully understood, studies have demonstrated that erythrocyte sedimentation rate (ESR) and high-sensitivity C-reactive protein (Hs-CRP) can independently predict RDW.27 ESR and Hs-CRP are common markers of inflammatory activity. Based on these, we speculated that the chronic inflammatory state of COPD might be one of the mechanisms of high mortality in COPD patients with high levels of RDW.
Serum albumin plays a crucial role in maintaining physiological homeostasis and contributes to anti-inflammatory effects.28 Hypoalbuminemia is strongly associated with increased inflammation and the risk of malnutrition.29 Albumin is oxidized under conditions of oxidative stress and chronic inflammation, resulting in marked hypoalbuminemia, which may be involved in the pathogenesis of inflammatory diseases.30,31 COPD is a common chronic inflammatory disease.1 Gunen et al prospectively assessed clinical parameters at admission in 205 consecutive patients hospitalized for AECOPD and found that mortality was associated with lower serum albumin levels (RR 0.411; 95% CI 0.205–0.824; P = 0.012).11 A study by Tokgoz Akyil et al reached similar conclusions.12 Previous research also showed that malnutrition affected mortality in patients admitted to ICU.32 In view of these, a low level of serum albumin might be one of the poor prognostic markers in COPD patients admitted to ICU, possibly due to inflammatory and malnutrition mechanisms.
Recently, RAR has been gradually found to be associated with mortality from acute respiratory distress syndrome,16 stroke,13 severe pneumonia,15 and heart failure.33 However, few studies have shown that RAR is related to the mortality of COPD. Our research illustrated that RAR could predict hospital mortality in COPD patients admitted to ICU. It was found that patients in the high RAR group had significantly higher levels of peripheral blood leukocytes after PSM (9.5x10^9 cells/L vs 11.0x10^9 cells/L; P=0.002), indicating that inflammation may be one of the mechanisms of RAR. Based on the possible mechanisms of RDW and serum albumin, we speculate that the mechanism by which RAR predicts hospital mortality may be explained by the role of hypoxia, inflammation, and malnutrition. In our study, AUC intuitively showed that RAR was more effective than RDW in predicting hospital mortality in severe COPD patients (the original cohort: 0.706 vs 0.600, P<0.001; the matched cohort: 0.611 vs 0.514, P<0.001), while there was no significant difference between RAR and serum albumin (the original cohort: P=0.611; the matched cohort: P=0.176). DCA also indicated that RAR may have a good clinical utility performance. To this end, we believe it is necessary to measure RAR by adding the ratio of RDW and albumin, which is a non-invasive, reproducible, and easily accessible measurement.
The data of this study were derived from MIMIC-IV (version 1.0), and a total of 1174 patients were included, which had the advantage of large sample size, and its results had some persuasion. In retrospective studies, confounders inevitably influenced the results; however, we used PSM analysis to adjust for the imbalance of confounding factors, and after PSM, the two groups were well matched except for hematological parameters. The relationship between RAR and hospital mortality in critically ill patients with COPD was established both before and after PSM, and the results were more reliable. Moreover, the subgroup analysis also showed that the results of this study were not affected by age, gender, or some coexisting diseases.
However, there were some limitations in this study. First, the data were extracted from public databases, and some indicators had many missing values and were not included in this study. Secondly, this study was designed as a single-center, with the possibility of selection bias, and it was necessary to further validate the results of this research. Moreover, we only analyzed the initial admission values of RDW and albumin. We did not analyze the values of their dynamic evolution, ignoring the impact of dynamic changes in RAR. Finally, our research did not involve mechanistic studies, and it was necessary to conduct more in-depth mechanisms in the future.
Conclusion
In conclusion, the present study indicated that a higher RAR value (>5.315%/g/dL) was associated with a higher risk of hospital mortality in COPD patients admitted to the ICU. The RAR could be served as an independent predictor for hospital mortality in critically ill patients with COPD. Meanwhile, well-designed, prospective, multicenter studies are warranted to verify our findings in the future.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (Grant No. 81970050).
Data Sharing Statement
The data incorporated in this study were obtained from the Medical Information Mart for Intensive Care IV (MIMIC-IV) Database (version 1.0), which was an open-access database and could be visited on the website after application: https://physionet.org/content/mimiciv/1.0/.
Ethics Approval and Informed Consent
This study was deemed exempt from review by the Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University, and informed consent was not applicable given the nature of the retrospective study and the use of de-identified patient data extracted from public databases.
Consent for Publication
All authors have read this manuscript and consent to publish it.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi: 10.1016/S0140-6736(17)31222-9 [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruvuna L, Sood A. Epidemiology of chronic obstructive pulmonary disease. Clin Chest Med. 2020;41(3):315–327. doi: 10.1016/j.ccm.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995;274(23):1852–1857. doi: 10.1001/jama.1995.03530230038027 [DOI] [PubMed] [Google Scholar]
- 5.Afessa B, Morales IJ, Scanlon PD, Peters SG. Prognostic factors, clinical course, and hospital outcome of patients with chronic obstructive pulmonary disease admitted to an intensive care unit for acute respiratory failure. Crit Care Med. 2002;30(7):1610–1615. doi: 10.1097/00003246-200207000-00035 [DOI] [PubMed] [Google Scholar]
- 6.Funk GC, Bauer P, Burghuber OC, et al. Prevalence and prognosis of COPD in critically ill patients between 1998 and 2008. Eur Respir J. 2013;41(4):792–799. doi: 10.1183/09031936.00226411 [DOI] [PubMed] [Google Scholar]
- 7.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. doi: 10.3109/10408363.2014.992064 [DOI] [PubMed] [Google Scholar]
- 8.Tertemiz KC, Ozgen Alpaydin A, Sevinc C, Ellidokuz H, Acara AC, A C. Could“red cell distribution width” predict COPD severity? Rev Port Pneumol. 2016;22(4):196–201. doi: 10.1016/j.rppnen.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 9.Seyhan EC, Özgül MA, Tutar N, Ömür I, Uysal A, Altin S. Red blood cell distribution and survival in patients with chronic obstructive pulmonary disease. Copd. 2013;10(4):416–424. doi: 10.3109/15412555.2012.758697 [DOI] [PubMed] [Google Scholar]
- 10.Epstein D, Nasser R, Mashiach T, Azzam ZS, Berger G. Increased red cell distribution width: a novel predictor of adverse outcome in patients hospitalized due to acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2018;136:1–7. doi: 10.1016/j.rmed.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 11.Gunen H, Hacievliyagil SS, Kosar F, et al. Factors affecting survival of hospitalised patients with COPD. Eur Respir J. 2005;26(2):234–241. doi: 10.1183/09031936.05.00024804 [DOI] [PubMed] [Google Scholar]
- 12.Tokgoz Akyil F, Gunen H, Agca M, et al. Patient outcome after chronic obstructive pulmonary disease exacerbations requiring non-invasive ventilation during hospitalization. Arch Bronconeumol. 2016;52(9):470–476. doi: 10.1016/j.arbres.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 13.Zhao N, Hu W, Wu Z, et al. The red blood cell distribution width-albumin ratio: a promising predictor of mortality in stroke patients. Int J Gen Med. 2021;14:3737–3747. doi: 10.2147/IJGM.S322441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou D, Wang J, Li X. The red blood cell distribution width-albumin ratio was a potential prognostic biomarker for diabetic ketoacidosis. Int J Gen Med. 2021;14:5375–5380. doi: 10.2147/IJGM.S327733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong JH, Heo M, Lee SJ, Jeong YY, Lee JD, Yoo JW. Clinical usefulness of red cell distribution width/albumin ratio to discriminate 28-day mortality in critically ill patients with pneumonia receiving invasive mechanical ventilation, compared with lactate/albumin ratio: a retrospective cohort study. Diagnostics. 2021;11(12). doi: 10.3390/diagnostics11122344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo JW, Ju S, Lee SJ, Cho YJ, Lee JD, Kim HC. Red cell distribution width/albumin ratio is associated with 60-day mortality in patients with acute respiratory distress syndrome. Infect Dis. 2020;52(4):266–270. doi: 10.1080/23744235.2020.1717599 [DOI] [PubMed] [Google Scholar]
- 17.Johnson A, Bulgarelli L, Pollard T, Horng S, Celi LA, Mark R. MIMIC-IV (version 1.0). PhysioNet. 2021. doi: 10.13026/s6n6-xd98 [DOI] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson comorbidity index: a critical review of clinimetric properties. Psychother Psychosom. 2022;91(1):8–35. doi: 10.1159/000521288 [DOI] [PubMed] [Google Scholar]
- 21.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 22.Karampitsakos T, Akinosoglou K, Papaioannou O, et al. Increased red cell distribution width is associated with disease severity in hospitalized adults with sars-cov-2 infection: an observational multicentric study. Front Med. 2020;7:616292. doi: 10.3389/fmed.2020.616292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karampitsakos T, Torrisi S, Antoniou K, et al. Increased monocyte count and red cell distribution width as prognostic biomarkers in patients with idiopathic pulmonary fibrosis. Respir Res. 2021;22(1):140. doi: 10.1186/s12931-021-01725-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yčas JW. Toward a blood-borne biomarker of chronic hypoxemia: red cell distribution width and respiratory disease. Adv Clin Chem. 2017;82:105–197. doi: 10.1016/bs.acc.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 25.Karampitsakos T, Dimakou K, Papaioannou O, et al. The role of increased red cell distribution width as a negative prognostic marker in patients with COPD. Pulm Pharmacol Ther. 2020;60:101877. doi: 10.1016/j.pupt.2019.101877 [DOI] [PubMed] [Google Scholar]
- 26.Yčas JW, Horrow JC, Horne BD. Persistent increase in red cell size distribution width after acute diseases: a biomarker of hypoxemia? Clin Chim Acta. 2015;448:107–117. doi: 10.1016/j.cca.2015.05.021 [DOI] [PubMed] [Google Scholar]
- 27.Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133(4):628–632. doi: 10.5858/133.4.628 [DOI] [PubMed] [Google Scholar]
- 28.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85(4):599–610. doi: 10.1093/bja/85.4.599 [DOI] [PubMed] [Google Scholar]
- 29.Eckart A, Struja T, Kutz A, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2020;133(6):713–722.e7. doi: 10.1016/j.amjmed.2019.10.031 [DOI] [PubMed] [Google Scholar]
- 30.Danielski M, Ikizler TA, McMonagle E, et al. Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. Am J Kidney Dis. 2003;42(2):286–294. doi: 10.1016/S0272-6386(03)00653-X [DOI] [PubMed] [Google Scholar]
- 31.Magzal F, Sela S, Szuchman-Sapir A, Tamir S, Michelis R, Kristal B. In-vivo oxidized albumin- a pro-inflammatory agent in hypoalbuminemia. PLoS One. 2017;12(5):e0177799. doi: 10.1371/journal.pone.0177799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eraslan Doganay G, Cirik MO. Determinants of prognosis in geriatric patients followed in respiratory ICU; either infection or malnutrition. Medicine. 2021;100(36):e27159. doi: 10.1097/MD.0000000000027159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni Q, Wang X, Wang J, Chen P. The red blood cell distribution width-albumin ratio: a promising predictor of mortality in heart failure patients - A cohort study. Clin Chim Acta. 2022;527:38–46. doi: 10.1016/j.cca.2021.12.027 [DOI] [PubMed] [Google Scholar]